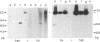Abstract
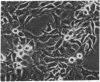
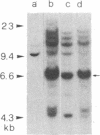
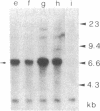
The transformed phenotype of rat FE-8 cells transfected by an activated human HRAS gene was suppressed upon fusion with normal cells. An experimental approach was developed to identify and isolate a human gene capable of suppressing the transforming activity of the HRAS oncogene in FE-8 cells. Genomic DNA from human placenta was introduced into FE-8 cells by cotransfection with the plasmid pY3 conferring hygromycin B resistance. Transfectants were selected in medium containing hygromycin B. HRAS-transformed FE-8 cells showed an increased sensitivity toward ouabain when compared to their normal counterparts. Therefore, the population of transfected hygromycin B-resistant cells was treated with ouabain to eliminate cells with a transformed phenotype. Ouabain selection resulted in a small number of cell clones exhibiting a more normal phenotype. The clones had lost the morphology of transformed cells and required anchorage for growth. The tumorigenicity of transfectants in nude mice was reduced but not completely abolished. FE-8 revertants continued to express the p21 RAS protein. Human repetitive sequences contained in the DNA of a secondary transfectant were used for isolation of the suppressor gene from reverted FE-8 cells. The cloned DNA fragment was transfected into tumorigenic FE-8 cells and conferred a partial reversion of the transformed phenotype.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andres A. C., Schönenberger C. A., Groner B., Hennighausen L., LeMeur M., Gerlinger P. Ha-ras oncogene expression directed by a milk protein gene promoter: tissue specificity, hormonal regulation, and tumor induction in transgenic mice. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1299–1303. doi: 10.1073/pnas.84.5.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R. F. DNA-mediated alteration of the reversion frequency of transformed NIH/3T3 cells. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1174–1178. doi: 10.1073/pnas.80.5.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. The molecular genetics of cancer. Science. 1987 Jan 16;235(4786):305–311. doi: 10.1126/science.3541204. [DOI] [PubMed] [Google Scholar]
- Blochlinger K., Diggelmann H. Hygromycin B phosphotransferase as a selectable marker for DNA transfer experiments with higher eucaryotic cells. Mol Cell Biol. 1984 Dec;4(12):2929–2931. doi: 10.1128/mcb.4.12.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Qasba P. K. Alkaline transfer of DNA to plastic membrane. Biochem Biophys Res Commun. 1984 Jul 18;122(1):340–344. doi: 10.1016/0006-291x(84)90480-7. [DOI] [PubMed] [Google Scholar]
- Craig R. W., Sager R. Suppression of tumorigenicity in hybrids of normal and oncogene-transformed CHEF cells. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2062–2066. doi: 10.1073/pnas.82.7.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franza B. R., Jr, Maruyama K., Garrels J. I., Ruley H. E. In vitro establishment is not a sufficient prerequisite for transformation by activated ras oncogenes. Cell. 1986 Feb 14;44(3):409–418. doi: 10.1016/0092-8674(86)90462-9. [DOI] [PubMed] [Google Scholar]
- Friend S. H., Bernards R., Rogelj S., Weinberg R. A., Rapaport J. M., Albert D. M., Dryja T. P. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986 Oct 16;323(6089):643–646. doi: 10.1038/323643a0. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Geiser A. G., Der C. J., Marshall C. J., Stanbridge E. J. Suppression of tumorigenicity with continued expression of the c-Ha-ras oncogene in EJ bladder carcinoma-human fibroblast hybrid cells. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5209–5213. doi: 10.1073/pnas.83.14.5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griegel S., Traub O., Willecke K., Schäfer R. Suppression and re-expression of transformed phenotype in hybrids of HA-ras-1-transformed rat-1 cells and early-passage rat embryonic fibroblasts. Int J Cancer. 1986 Nov 15;38(5):697–705. doi: 10.1002/ijc.2910380513. [DOI] [PubMed] [Google Scholar]
- Harris H. The genetic analysis of malignancy. J Cell Sci Suppl. 1986;4:431–444. doi: 10.1242/jcs.1986.supplement_4.23. [DOI] [PubMed] [Google Scholar]
- Jacob L., Opper M., Metzroth B., Phannavong B., Mechler B. M. Structure of the l(2)gl gene of Drosophila and delimitation of its tumor suppressor domain. Cell. 1987 Jul 17;50(2):215–225. doi: 10.1016/0092-8674(87)90217-0. [DOI] [PubMed] [Google Scholar]
- Jaggi R., Salmons B., Muellener D., Groner B. The v-mos and H-ras oncogene expression represses glucocorticoid hormone-dependent transcription from the mouse mammary tumor virus LTR. EMBO J. 1986 Oct;5(10):2609–2616. doi: 10.1002/j.1460-2075.1986.tb04541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson A. G., Jr Hereditary cancer, oncogenes, and antioncogenes. Cancer Res. 1985 Apr;45(4):1437–1443. [PubMed] [Google Scholar]
- Lee W. H., Bookstein R., Hong F., Young L. J., Shew J. Y., Lee E. Y. Human retinoblastoma susceptibility gene: cloning, identification, and sequence. Science. 1987 Mar 13;235(4794):1394–1399. doi: 10.1126/science.3823889. [DOI] [PubMed] [Google Scholar]
- Mechler B. M., McGinnis W., Gehring W. J. Molecular cloning of lethal(2)giant larvae, a recessive oncogene of Drosophila melanogaster. EMBO J. 1985 Jun;4(6):1551–1557. doi: 10.1002/j.1460-2075.1985.tb03816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Selinger Z., Scolnick E. M., Bassin R. H. Flat revertants isolated from Kirsten sarcoma virus-transformed cells are resistant to the action of specific oncogenes. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5602–5606. doi: 10.1073/pnas.80.18.5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshimura M., Gilmer T. M., Barrett J. C. Nonrandom loss of chromosome 15 in Syrian hamster tumours induced by v-Ha-ras plus v-myc oncogenes. Nature. 1985 Aug 15;316(6029):636–639. doi: 10.1038/316636a0. [DOI] [PubMed] [Google Scholar]
- Padmanabhan R., Howard T. H., Howard B. H. Specific growth inhibitory sequences in genomic DNA from quiescent human embryo fibroblasts. Mol Cell Biol. 1987 May;7(5):1894–1899. doi: 10.1128/mcb.7.5.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perucho M., Goldfarb M., Shimizu K., Lama C., Fogh J., Wigler M. Human-tumor-derived cell lines contain common and different transforming genes. Cell. 1981 Dec;27(3 Pt 2):467–476. doi: 10.1016/0092-8674(81)90388-3. [DOI] [PubMed] [Google Scholar]
- Sachs L. Cell differentiation and bypassing of genetic defects in the suppression of malignancy. Cancer Res. 1987 Apr 15;47(8):1981–1986. [PubMed] [Google Scholar]
- Sager R. Genetic suppression of tumor formation: a new frontier in cancer research. Cancer Res. 1986 Apr;46(4 Pt 1):1573–1580. [PubMed] [Google Scholar]
- Saxon P. J., Srivatsan E. S., Stanbridge E. J. Introduction of human chromosome 11 via microcell transfer controls tumorigenic expression of HeLa cells. EMBO J. 1986 Dec 20;5(13):3461–3466. doi: 10.1002/j.1460-2075.1986.tb04670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer R., Hoffmann H., Willecke K. Suppression of tumorigenicity in hybrids of tumorigenic Chinese hamster cells and diploid mouse fibroblasts: dependence on the presence of at least three different mouse chromosomes and independence of hamster genome dosage. Cancer Res. 1983 May;43(5):2240–2246. [PubMed] [Google Scholar]
- Schäfer R. Suppression of the neoplastic phenotype and "anti-oncogenes". Blut. 1987 May;54(5):257–265. doi: 10.1007/BF00320871. [DOI] [PubMed] [Google Scholar]
- Sinn E., Muller W., Pattengale P., Tepler I., Wallace R., Leder P. Coexpression of MMTV/v-Ha-ras and MMTV/c-myc genes in transgenic mice: synergistic action of oncogenes in vivo. Cell. 1987 May 22;49(4):465–475. doi: 10.1016/0092-8674(87)90449-1. [DOI] [PubMed] [Google Scholar]
- Stevenson M., Volsky D. J. Activated v-myc and v-ras oncogenes do not transform normal human lymphocytes. Mol Cell Biol. 1986 Oct;6(10):3410–3417. doi: 10.1128/mcb.6.10.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabin C. J., Bradley S. M., Bargmann C. I., Weinberg R. A., Papageorge A. G., Scolnick E. M., Dhar R., Lowy D. R., Chang E. H. Mechanism of activation of a human oncogene. Nature. 1982 Nov 11;300(5888):143–149. doi: 10.1038/300143a0. [DOI] [PubMed] [Google Scholar]
- Vogt M., Lesley J., Bogenberger J., Volkman S., Haas M. Coinfection with viruses carrying the v-Ha-ras and v-myc oncogenes leads to growth factor independence by an indirect mechanism. Mol Cell Biol. 1986 Oct;6(10):3545–3549. doi: 10.1128/mcb.6.10.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman B. E., Saxon P. J., Pasquale S. R., Jones G. R., Geiser A. G., Stanbridge E. J. Introduction of a normal human chromosome 11 into a Wilms' tumor cell line controls its tumorigenic expression. Science. 1987 Apr 10;236(4798):175–180. doi: 10.1126/science.3031816. [DOI] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978 Jul;14(3):725–731. doi: 10.1016/0092-8674(78)90254-4. [DOI] [PubMed] [Google Scholar]