Abstract
Major surface protease (MSP or GP63) is the most abundant glycoprotein localized to the plasma membrane of Leishmania promastigotes. MSP plays several important roles in the pathogenesis of leishmaniasis, including but not limited to (i) evasion of complement-mediated lysis, (ii) facilitation of macrophage (Mø) phagocytosis of promastigotes, (iii) interaction with the extracellular matrix, (iv) inhibition of natural killer cellular functions, (v) resistance to antimicrobial peptide killing, (vi) degradation of Mø and fibroblast cytosolic proteins, and (vii) promotion of survival of intracellular amastigotes. MSP homologues have been found in all other trypanosomatids studied to date including heteroxenous members of Trypanosoma cruzi, the extracellular Trypanosoma brucei, unusual intraerythrocytic Endotrypanum spp., phytoparasitic Phytomonas spp., and numerous monoxenous species. These proteins are likely to perform roles different from those described for Leishmania spp. Multiple MSPs in individual cells may play distinct roles at some time points in trypanosomatid life cycles and collaborative or redundant roles at others. The cellular locations and the extracellular release of MSPs are also discussed in connection with MSP functions in leishmanial promastigotes.
Trypanosomatids are a very diverse group of protozoa including many species of medical, veterinary, and/or economical importance. Leishmania spp. cause disease on the continents of Asia, Africa, Europe, and North and South America; Trypanosoma cruzi is the etiology of Chagas' disease in South and Central America; and Trypanosoma brucei infections result in African sleeping sickness. Four genera of heteroxenous trypanosomatids transmitted by insect vectors infect a wide range of hosts including humans, animals, and plants. Six genera of monoxenous trypanosomatids parasitize numerous species of insects. Due to this diversity, the life cycle of Leishmania spp. is presented as a model to which other heteroxenous and monoxenous groups will be briefly contrasted.
During their life cycle, Leishmania spp. shuttle between a flagellated promastigote stage in the sand fly vector and a nonmotile amastigote stage in a mammalian host. During blood meals on infected hosts, the sand fly vectors ingest amastigote-laden macrophages (Mø). These amastigotes transform to procyclic promastigotes in the sand fly gut, multiply by binary fission, and eventually develop into metacyclic promastigotes, the infective stage for mammalian hosts including humans. The sand fly vectors, upon taking another blood meal, inoculate the metacyclic promastigotes into the dermis, where they are phagocytized by Mø of the mammalian hosts. Amastigotes, transformed from metacyclic promastigotes, multiply in parasitophorous vacuoles (PV) of the host's Mø. Multiplication of amastigotes eventually leads to rupture of the infected Mø and release of amastigotes, which infect additional Mø, perpetuating the cycle of replication (38).
Other trypanosomatids divert this model of the life cycle in different ways. (i) T. cruzi is transmitted to humans by the reduviid (or kissing) bug by fecal contamination of skin wounds or mucous membranes while taking a blood meal. In the human host, T. cruzi exists in amastigote and trypomastigote forms. The intracellular amastigote stage multiplies within virtually any cell. The extracellular trypomastigotes are disseminated in the blood to all parts of the body (38). (ii) T. brucei is transmitted to humans by a tsetse fly during blood meals. This parasite lives extracellularly as trypomastigotes in blood, lymph, and cerebrospinal fluid (90). (iii) Two species of Endotrypanum are parasites of forest-dwelling tree sloths in South America. Both Endotrypanum schaudinni and Endotrypanum monterogeii have an unusual intraerythrocytic stage (53, 75) as trypomastigotes and epimastigotes, respectively, even though both use sand flies as vectors (24). (iv) Phytomonas spp., transmitted between plants by phytophagous insects, infect several plants of great economic importance in widespread geographic regions (13). (v) All monoxenous trypanosomatids are parasites of various insects. They consist of six genera, including Blastocrithidia, Crithidia, Leptomonas, Herpetomonas, Rhynchoidomonas, and Phytomonas (87).
Leishmania spp. harbor a zinc metalloprotease, discovered in the mid-1980s, on their surfaces (14, 15, 36, 37, 88). This enzyme, accounting for about 1% of the total protein in promastigotes of Leishmania major and Leishmania mexicana (2, 10) and being potentially important during different stages of the life cycle, soon became the object of much investigation (8, 70, 71, 107). Although referred to here as major surface protease (MSP), this zinc metalloprotease has also been termed GP63, surface acid protease, promastigote surface protease, EC 3.4.24.36, and leishmanolysin.
MSPs of Leishmania spp. belong to the M8 family of endopeptidases, sharing several characteristics with mammalian matrix metalloproteases. Similarities include degradation of the extracellular matrix, cell surface localization, protease activity requiring Zn2+, and inhibition of protease activity by chelating agents and α-2-macroglobulin (for a review, see reference 70). Many, but not all, of the Leishmania MSPs are bound to the outer leaves of the plasma membranes of promastigotes by a glycosylphosphatidylinositol (GPI) anchor (9). MSPs are encoded by multiple MSP genes that may vary in sequence, especially in the untranslated regions. MSP genes of all Leishmania spp. studied to date are usually in tandem array and are often differentially expressed during various life cycle stages (72, 91, 92, 101). This heterogeneity in MSP genes without affecting enzymatic domains makes stage-specific regulation possible. Unlike other eukaryotic cells, trypanosomatids do not have classic promoters for RNA polymerase II. Long arrayed protein-coding genes are transcribed to polycistronic RNA precursors. Mature mRNAs derive from a coupled process in which trans splicing of a capped splicing leader at the 5′ end of one gene is coupled to polyadenylation addition of the upstream gene (20). Redundancy in certain genes including MSP genes appears to be a mechanism to affect mature mRNA production, and therefore, protein abundance.
The genomes of L. major, Leishmania infantum, and Leishmania braziliensis contain 4, 5, and 33 MSP genes, respectively, in tandem array on chromosome 10 with an additional 2, 3, and 6 MSP-like genes, respectively, located on chromosome 28 and/or 31 (49, 84). These numbers differ significantly from the earlier reported 7 and ≥18 MSP genes in tandem array detected by partial digestion of genomic DNAs and Southern blotting of L. major and the South American Leishmania chagasi, respectively (91, 101). The latter species is considered synonymous with L. infantum by many authors (60, 65, 66, 76, 89). This divergence in number of MSP genes appears related to the different parasitic strains used in these studies (MHOM/IL/81Friedlin zymodeme MON-103 versus NIH S [MHOM/SN/74/Seisman] of L. major; JPCM5 [MCAN/ES/98/LLM-877] versus MHOM/BR/00/1669 of L. infantum), although the biological significance remains to be assessed. Alternatively, variation might be caused by technical challenges in genome sequencing. Identical tandem-arrayed genes may not have been able to be correctly resolved for gene copy number. In contrast, Southern blots of the partially digested genomic DNA subjected to resolution by electrophoresis are capable of detecting gene copy numbers of identical tandem-arrayed genes.
Another GPI-anchored molecule, lipophosphoglycan (LPG), is the dominant molecule on the surfaces of Leishmania sp. promastigotes. Intriguingly, protective roles for LPG and MSP overlap, including resistance to complement-mediated lysis (CML) and facilitation of phagocytosis by host Mø. LPG also functions in the binding to and release of the parasite from the midgut of the sand fly vector, accounting for retrograde migration of the metacyclic promastigote to the sand fly proboscis (6, 16). Since roles for both MSP and LPG overlap, they may function synergistically in the pathogenesis of leishmaniasis or they may function redundantly, with the failure of one being compensated for by the other.
MSP's ROLE IN THE PATHOGENESIS OF LEISHMANIASIS
Two important roles of MSP in the pathogenesis of leishmaniasis were comprehensively reviewed (107). These include resistance to CML and facilitating phagocytosis of extracellular promastigotes by host Mø (107), briefly summarized here to avoid redundancy. MSPs of promastigotes bind to C3 with high affinity. Proteolysis of the active component C3b to the inactive C3bi inhibits CML (11, 93). MSP−/− mutant promastigotes of L. major were generated by deleting all seven tandem MSP genes on chromosome 10. Mutant cells derived from either sand flies or in vitro cultures exhibited increased sensitivity to CML. In contrast, mutant cells expressing a cloned MSP gene were significantly resistant to CML (52).
MSPs facilitate phagocytosis of promastigotes by host Mø. This is supported by observations from multiple labs collectively showing that preincubation of Mø with purified MSPs or the presence of antibodies specific to MSP significantly blocks phagocytosis of promastigotes of Leishmania amazonensis, L. braziliensis, L. mexicana, and L. chagasi (14, 63, 94, 103). Nevertheless, MSP proteolytic activity is not an absolute requirement for phagocytosis. This is supported by evidence generated from mutants lacking MSP activity (104) and MSP-low cells, overexpressing a mutated inactive form of MSP in the zinc binding motif (E265D) (12). Mø receptors known to be involved in the phagocytosis of Leishmania sp. promastigotes include (i) C3bi receptor CR3, which binds Leishmania sp. promastigotes (77, 104, 105); (ii) C3b and C3bi receptor CR1; and (iii) the scavenger receptor MARCO. The last two bind to L. major promastigotes (25, 40). In addition, mannose receptors, fibronectin receptors, TLR2 and -9, and the receptors for advanced glycoconjugates are also implicated in Leishmania recognition of host Mø (99).
The remainder of this section will focus on new developments and advancements in the last several years.
Interactions with the host extracellular matrix.
When inoculated into a subcutaneous location by a sand fly vector, metacyclic promastigotes come into contact with the host extracellular matrix. In an in vitro model system, Matrigel containing soluble extracts of extracellular matrix and basement membranes of Engelbreth-Holm-Swarm tumor cells, genetically altered L. amazonensis expressing high levels of MSP migrated more efficiently through the extracellular matrix than control promastigotes (68). Enhanced migration of promastigotes ultimately correlates with the ability of MSP to degrade components of the extracellular matrix such as fibronectin and collagen IV (68). The release of internal, but not surface-oriented, MSPs from promastigotes of L. chagasi incubated with the extracellular matrix increased at normal mammalian body temperature (37°C) but not at room temperature. Conversely, the release of surface-localized MSPs increased at room temperature but was inhibited at 37°C (106). Release of MSP at 37°C could facilitate close contact between promastigotes and host cells such as Mø and fibroblasts and thus could be favorable for parasite entry and/or long-term survival.
Both surface-localized and released MSPs of promastigotes participate in degradation of fibronectin, one component of the extracellular matrix. In contrast, degradation of fibronectin by MSPs released from amastigotes requires cysteine protease B, while degradation at the surface of amastigotes remains MSP dependent (55). This difference in degradation of extracellular matrix components between promastigote and amastigote stages may reflect their biological niches. Promastigotes are predominantly extracellular parasites, whereas amastigotes are mainly intracellular parasites. Degradation of extracellular matrix components has additional effects. Leishmania-induced degradation of fibronectin decreased production of reactive oxygen intermediates by infected Mø, facilitating survival of amastigotes in infected Mø (55).
Inhibition of NK cellular function.
Natural killer (NK) cells are a crucial component of innate immunity against diverse pathogens, including parasites, via cytotoxic activity and early cytokine production. NK cells are important in the early stages of host protective immunity against L. major infection in mouse models (61). In resistant strain C57BL/6 mice, depletion of NK1.1+ cells prior to L. major infection led to rapid parasite spreading with kinetics similar to those seen in susceptible BALB/c mice. In susceptible mice, rapid dissemination of parasites from the inoculation site in the subcutaneous footpad to the popliteal lymph node, spleen, lung, liver, and bone marrow occurred within 24 h (61). Using an MSP−/− mutant of L. major, Lieke and colleagues found that MSPs bound directly to human NK cells inhibited proliferation and suppressed gamma interferon responses (62). These authors have identified MSPs as important players in suppression of NK cells during Leishmania infection (62). By diminishing the functions of NK cells and other components of innate immunity, promastigotes of Leishmania increase their chances of survival and ability to establish a successful infection in mammalian hosts.
Resistance to AMP killing.
Antimicrobial peptides (AMPs), also called host defense peptides, are another important component of innate immunity. AMPs are short cationic peptides of diverse structure found in a wide variety of organisms including mammals. The antimicrobial activity of AMPs is capable of killing a wide spectrum of microorganisms including bacteria, fungi, parasites, enveloped viruses, and transformed cells. These peptides directly disrupt the membrane potentials of microorganisms, leading to cell death by necrosis or apoptosis (44). By comparing MSP−/− mutants to wild-type parasites of L. major, Kulkarni et al. convincingly demonstrated that the promastigotes of MSP−/− mutants were effectively killed in a dose-dependent manner by AMPs such as pexiganan whereas the wild-type parasites were not. It was found that, on reintroduction of an MSP gene into the mutant parasites, promastigote survival was comparable to that of the wild-type cells (56). These authors also showed that MSP-associated enzymatic degradation of AMPs by wild-type promastigotes resulted in inactive fragments, which led to resistance to AMP-mediated apoptotic killing (56). More recently, these same investigators defined a further mechanism of AMP-mediated apoptotic death in Leishmania spp.: calcium-dependent, caspase-independent mitochondrial toxicity. At the same time, they described a distinct group of AMPs, such as protegrin-1, that induce nonapoptotic cell death of Leishmania spp. (57).
Promoting intracellular amastigote survival in Mø.
Compared to metacyclic promastigotes, amastigotes have dramatically reduced numbers of MSPs, which account for 1% and 0.1% of the total proteins in these two stages of L. mexicana, respectively (2). It remains debatable whether MSP functions favor survival of the intracellular amastigotes inside PV of host Mø. Supporting evidence includes the following: (i) purified MSPs of L. amazonensis protect liposome-encapsulated 125I-labeled bovine serum albumin from degradation inside PV of Mø (17), (ii) genetic rescue of MSP-deficient L. amazonensis increases early survival of the parasite in Mø (67), (iii) lower levels of MSP induced by specific antisense mRNA reduce the early intracellular survival of L. amazonensis amastigotes in Mø (18), and (iv) coating MSP-low attenuated cells of L. amazonensis with proteolytically active MSP protects these cells from degradation inside Mø (97). Alternatively, data for null mutants argue that MSPs do not promote survival of amastigotes in Mø. As described earlier, an MSP−/− null mutant of L. major was generated by deletion of all seven MSP genes in tandem array on chromosome 10. MSP proteins were below detectable levels by flow cytometry (52). A second mutant of L. mexicana was developed by knocking out the gene for GPI:protein transamidase. The null mutant lacked GPI-anchored MSPs and other GPI-anchored proteins, but GPI-linked nonproteins such as LPG were unaffected (46). Both mutants were capable of surviving as amastigotes in Mø in vitro or caused lesions in mouse models in vivo, albeit with a delay in lesion formation or smaller lesions compared to the wild-type controls (46, 52). These results are unexpected but not completely surprising for the following reasons. First, two additional MSP-like genes are each located in chromosomes 28 and 31 in L. major (49). The antibody used in flow cytometry experiments may not react with the products of these MSP-like genes, leading to the conclusion that levels of MSP are undetectable. Second, it was elegantly shown that the majority of MSPs of amastigotes of L. chagasi are not GPI anchored (47). Consequently, the null mutant lacking GPI-anchored MSPs would not have had much effect on this stage. Third, Leishmania spp. exhibit reduced virulence and decreased expression of a number of virulence factors during the months of in vitro cultivation required for the generation of knockout mutants. Thus, there may have been additional undetected defects in both the wild-type and mutant parasites used in the above experiments. Finally, the data were generated for different Leishmania species, and it is possible that the role of MSP in intracellular survival of amastigotes varies between species, e.g., MSPs of L. amazonensis promote amastigote survival in host Mø and MSPs of L. major and L. mexicana do not.
Degrading cytosolic proteins of host Mø and fibroblasts.
MSP also appears to have other activities during host infection such as degradation of MARCKS-related proteins (21, 22) and the intracellular peptides presented by major histocompatibility complex class I (MHC-I) molecules (39) of infected Mø. Interestingly, these activities require the presence of MSP in the cytosol of infected Mø. How MSP localizes to the cytosol of host cells is unclear, given that amastigotes of Leishmania spp. reside inside morphologically distinct PV, unique forms of phagolysosomes (late endosomes) of infected Mø (54). Regardless, lines of evidence derived from (i) cell fractionation, (ii) radioisotope labeling, (iii) confocal microscopy, and (iv) coimmunoprecipitation suggest that leishmanial elongation factor 1α (evidence from all four lines) and fructose-1,6-bisphosphate aldolase (evidence from lines i and iv only) are exported from PV into the cytosol of infected Mø (80, 81). A model for the release of cysteine peptidase B into the cytosol of host Mø has been proposed (78). In this model, activated cysteine peptidase B is first released from flagellar pockets of amastigotes into PV. Next, vacuoles containing the peptidase bud from PV and release their contents into the cytosol of host Mø (78). Immunoelectron microscopy has shown that MSPs in amastigotes of both L. chagasi and L. mexicana are predominantly localized to the flagellar pocket (47, 73). Therefore, it is possible that MSP endopeptidase uses a similar approach to reach the cytosol of host Mø as does cysteine peptidase B.
Although Mø are the primary host cells harboring amastigotes following initial infection, fibroblasts are important host cells in chronic and persistent leishmanial infection (7). Recently, by employing cellular and in vitro assays using protease inhibitors, MSP−/− mutants, and recombinant enzymes, Halle et al. found that leishmanial MSPs degraded several signaling proteins of fibroblasts infected with L. major. These signaling proteins included the phosphorylated adaptor protein p130Cas, the protein tyrosine phosphatase PEST, cotactin, T-cell protein tyrosine phosphatase, caspase-3, and p38 (45). These authors concluded that MSPs play a central role in several molecular cascade events that likely contribute to leishmanial infectivity (45). Analogous to activity in infected Mø, degradation of these signaling proteins requires MSP localization to the cytosol of infected fibroblasts.
MSP HOMOLOGUES IN OTHER TRYPANOSOMATIDS
MSP homologues have been discovered in many trypanosomatids. These parasites infect different insect, mammalian, or plant hosts, and their life cycles differ considerably from that of the Leishmania spp. Although not as well studied, the functions of these MSPs in trypanosomatids are well adapted to sustain the unique life cycle of each parasite. The following discusses the potential roles of these MSPs homologues in trypanosomatids.
T. cruzi.
Like Leishmania, the American trypanosome T. cruzi has an intracellular amastigote stage in mammalian host cells, but T. cruzi amastigotes reside in the host cell cytosol whereas those of Leishmania spp. reside in PV. The T. cruzi genome has a minimum of 425 MSP gene homologues including 251 pseudogenes (34). The gene sequence predictions indicate that some MSPs have C-terminal hydrophobic regions that are likely replaced by a GPI anchor (23). Northern blot analyses indicate that some of these MSP genes are differentially expressed during different life cycle stages. Immunofluorescent staining and phosphatidylinositol-specific phospholipase C (PI-PLC) digestion indicate that at least one group of MSP homologues are linked by a GPI anchor to the surface at all T. cruzi life cycle stages, including metacyclic trypomastigotes, the infective stage for humans. These MSP homologues have zinc metalloprotease activity. MSP-specific antisera partially block the in vitro invasion of mammalian cells by trypomastigotes (23). Recently, Kulkarni and colleagues demonstrated that a TcMSP gene encoded a 55-kDa intracellular protein without N glycosylation and a 61-kDa ectoglycoprotein in metacyclic trypomastigotes and epimastigotes, respectively, indicating that expression was posttranslationally regulated during different life cycle stages (58). These authors further showed that antibodies against the corresponding antigens partially blocked infection of host myoblasts by trypomastigotes (58). These data collectively demonstrate that MSPs play an important role during attachment and/or entry of host cells by the American trypanosomes.
T. brucei.
The African trypanosome T. brucei is exclusively an extracellular organism, and, as such, MSP activity for host cell attachment and/or entry is irrelevant. The T. brucei genome contains 14 MSP homologues, which can be grouped into three classes based on their differential expression in various life cycle stages and the presence or absence of a GPI anchor addition site (5, 33, 59). Zinc metalloproteases encoded by T. brucei MSP B (TbMSP-B) and located on the surface at the procyclic insect stage are required to remove ectopically expressed variant surface glycoproteins (VSG) (42, 59). Recently, they have been demonstrated to participate, in concert with PLC, in the removal of the VSG coat when bloodstream stage trypanosomes differentiate to procyclic trypanosomes. TbMSP-B−/− PLC−/− double mutant bloodstream stage trypanosomes fail to differentiate into the procyclic form, with most VSG remaining on the cell surface. In contrast, TbMSP-B−/− or TbPLC−/− single mutants differentiate successfully, with VSG being released into the extracellular medium in a nontruncated form reminiscent of wild-type controls (41). A further detailed analysis provides evidence for a coordinated and inverse regulation of the activities of MSP-B and PLC during synchronous differentiation. MSP-B upregulation is inversely reflected by PLC downregulation, with the latter playing only a quantitatively minor role during differentiation (43).
Endotrypanum spp.
The trypanosomatid parasites of the forest-dwelling tree sloths in South America have an unusual intraerythrocytic stage (53, 75). As such, the Endotrypanum MSP homologues apparently cannot perform all the same functions as Leishmania MSPs, particularly those involved in Mø entry (for a review, see reference 107). MSP homologues were found in one Endotrypanum sp. using an L. mexicana MSP gene probe in Southern blots (74). Nevertheless, the role(s) of MSP homologues during the Endotrypanum life cycle and whether or not this includes attachment and/or entry into host erythrocytes remain to be elucidated.
Phytomonas spp.
Phytomonas spp. infect fruits, seeds, and plants of great economic importance in widespread and diverse geographic regions. These digenetic parasites are transmitted between plant hosts by phytophagous insects (13). A 67-kDa metalloprotease has been isolated and purified from Phytomonas françai, a parasite of cassava causing a disease known as “chochamento das raízes,” meaning “empty roots” (1). This metalloprotease, along with another 62-kDa protein, shares common epitopes with the leishmanial MSP and is localized on the cell surface. They play a role in protozoan binding to the extracted gut of Aedes aegypti, possibly through a 50-kDa ligand (64). Phytomonas serpens, the parasite of tomato fruits, has metalloprotease activity at molecular masses between 70 and 94 kDa exclusively in the extracellular stage (100). A further study shows that a 63-kDa protein reacting with MSP antibody is attached by a GPI anchor to the cell surface. An anti-MSP antibody raised against an L. amazonensis MSP significantly reduces binding of the parasite to the salivary glands of the insect Oncopeltus fasciatus, suggesting that MSP homologues in these parasites play a role in establishment of phytomonads in the insect salivary gland (28).
Monoxenous trypanosomatids.
Increasing evidence suggests that monoxenous trypanosomatids are capable of infecting and surviving in immunocompromised individuals such as AIDS patients and even immunocompetent persons, leading to visceral or cutaneous lesions resembling leishmaniasis. Monoxenous trypanosomatids from three genera, Crithidia, Herpetomonas, and Leptomonas, have been positively identified in such human hosts (19). Further, Crithidia deanei and Herpetomonas roitmani are phagocytized by and survive in mouse fibroblasts in vitro (95). These data collectively raise the question of the roles of monoxenous trypanosomatids as opportunistic pathogens in immunocompromised and even in immunocompetent populations of humans. Therefore, it is relevant to discuss these parasitic protozoa and compare them with well-recognized human pathogens.
Genes encoding MSP homologues have been cloned and sequenced in Crithidia fasciculata. They form a multicopy family comprising approximately 7 genes. The deduced amino acid sequences show a great degree of conservation compared with leishmanial MSP (48). Metalloprotease activity has been detected using fibrinogen as a substrate, suggesting that the genes are functional in these protozoa (35). Immunoreactive MSP-like proteins have been detected in Leptomonas seymouri, Crithidia luciliae (51), C. deanei (29), Crithidia guilhermei (64), and Blastocrithidia culicis (26) using antibodies to leishmanial MSPs in immunoblots. In addition, two 50- and 58-kDA metalloproteases of B. culicis possess a GPI anchor (26). Even more interesting, 67- and 62-kDa proteins in the total cellular lysates of C. deanei and C. guilhermei share common epitopes with leishmanial MSP and are localized on the cell surface (64). These MSP-like proteins function in the binding of the protozoa to the extracted gut of A. aegypti, possibly through a ligand of 50 kDa (64). Similar results are also obtained when Leptomonas collosoma, Leptomonas samueli, and Leptomonas wallacei protozoa are used (85). In experiments of Herpetomonas megaseliae adhesion to the extracted guts, it was clearly and convincingly shown that MSP-like proteins play a role in the attachment of these monoxenous parasites to the gut of their original host, Megaselia scalaris (82). Metalloproteases have also been detected in Herpetomonas samuelpessoai (35), Herpetomonas anglusteri, and H. roitmani (98), although their identities have yet to be confirmed.
The data presented so far collectively support the likelihood that all members of trypanosomatids have MSP, MSP homologues, or MSP-like proteins. The data also indicate that the protease activities of these metalloproteases contribute to the life cycles of these organisms in many different ways, either directly or indirectly. It may be that the wide substrate specificity and functionality at various pH levels of these proteases facilitate the adaptation of these protozoa to different environments.
“ABNORMAL” MSPs OF LEISHMANIAL PROMASTIGOTES: FUNCTIONS OF THE RELEASED AND INTERNAL MSPs
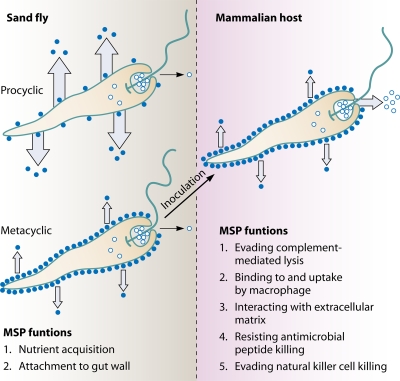
As indicated above, most of the Leishmania MSPs are bound to the plasma membranes of promastigotes through a GPI anchor (9). Several groups, including ours, have reported that some MSPs of several Leishmania spp. are intracellularly localized in promastigotes and/or are released into the culture medium (32, 51, 69, 102, 108, 109) (Fig. 1).
FIG. 1.
MSP subpopulations and possible functions of Leishmania promastigotes in the sand fly vector and the mammalian hosts. Solid and open circles represent the surface-localized and internal MSPs, respectively. Arrows depict MSP release into the extracellular environment, with their widths proportional to the amounts of MSP released. The MSP release in the procyclic and metacyclic promastigotes within the sand fly panel is depicted in the upper and the lower diagrams, respectively. A metacyclic promastigote is depicted in the mammalian host panel. (Adapted from reference 106 with permission.)
MSP release.
In contrast to the rapid loss or replacement of the VSG coat from T. brucei bloodstream forms, release of Leishmania MSPs is slow and accompanied by a loss of cell-associated MSPs (4, 108). Release of transfected L. amazonensis MSPs from the promastigote surface is dramatically reduced when MSP proteolytic activity is abrogated by site mutagenesis (E265D mutant) or inhibited with zinc chelator 1,10-phenanthroline, indicating that autoproteolysis contributes to this release (69). Two lines of evidence indicate that some released MSPs either have not acquired or have lost a GPI anchor. First, released MSPs of L. amazonensis contain neither a GPI anchor nor ethanolamine. Second, treatment of released L. amazonensis MSP with PI-PLC fails to expose the CRD motif, characteristic of PI-PLC-treated GPI anchors (69). This contrasts to L. chagasi, in which some of the released MSPs exist as either membrane-bound vesicles or large aggregated micelles (108), supporting the concept that release potentially occurs through several distinct mechanisms. MSPs lacking a GPI anchor because of defective synthesis of GPI precursors are released with a half-life (T1/2) of 2 h, which is significantly slower than the expected rate of transport of wild-type MSPs to the cell surface, 40 min (79). Similarly, MSPs lacking a GPI anchor due to deletion of the genes encoding GPI:protein transamidase are secreted in a manner dependent upon N glycosylation and are inhibited by tunicamycin (32). The mechanism of MSP release from this mutant parasite may indicate that N glycosylation is required for trafficking of MSPs forward through the endoplasmic reticulum (ER) and Golgi complex to the parasite surface, where they are usually retained via an intact membrane anchor. Release of membrane-bound N-glycosylated and GPI-anchored MSPs from wild-type promastigotes may involve autoproteolysis and/or proteolysis of a related molecule required for release.
MSP release occurs in newly isolated clinical as well as laboratory-adapted strains of Leishmania spp. A pulse-chase experiment revealed that recent clinical isolates of L. infantum and Leishmania tropica promastigotes release maximal amounts of MSP after 12 h in culture, with release continuing for at least 24 to 48 h postchase. In contrast, MSP release from L. amazonensis promastigotes in culture is detected as early as 4 h after the start of the chase, peaking at 12 h, at which time approximately 60 to 70% of the newly synthesized proteins are found in the extracellular medium (69). The release of MSPs from L. major promastigotes reaches a peak at day 4 and then decreases, whereas MSP release from Leishmania donovani promastigotes continually increases until day 6 after suspension in liquid culture (51). These observations collectively support the notion that promastigotes of different Leishmania species release MSPs at different rates. Release of MSPs may be an intrinsic property of Leishmania spp. that relates to their pathogenesis.
Many of the numerous monoxenous trypanosomatids also release MSP-like proteins or metalloproteases into the extracellular medium. These include C. guilhermei (30, 83), C. deanei (27, 29), Crithidia desouzai, Crithidia oncopelti, C, fasciculata (27), C. luciliae, Leptomonas seymouri (51), H. anglusteri and H. roitmani (98), and B. culicis (26, 31). Interestingly, significantly greater enzymatic activity of metalloproteases from an aposymbiotic strain of B. culicis than from a strain carrying bacterial endosymbionts is detected, suggesting a role for MSP-like proteins in digestion of nutrients (26). In addition, release of MSP homologues is found in plant parasites P. françai (1) and P. serpens (100).
In short, release of MSP and its homologues from the insect stages into their environments has been observed across all trypanosomatids examined to date, including monoxenous insect parasites, digenetic plant parasites, and digenetic mammalian parasites. Nutrient acquisition via degradation of gut content by the endopeptidase activity of these released MSPs is likely of major biological significance while these protozoa reside inside the guts of the insect host. MSPs may also enhance the survival of digenetic protozoa in their vectors. We have proposed a model (Fig. 1) that considers the three subpopulations of MSP in Leishmania spp. including surface-localized, intracellular, and released MSPs (106).
Internally located MSP.
In contrast to extracellular release, some MSPs remain at an intracellular location in leishmanial promastigotes. By surface biotinylation, fluorescence microscopy, and immunoelectron microscopy, approximately one-fourth of all cell-associated MSPs were shown to reside internally in L. mexicana. These internal MSPs are distributed among the tubulovesicular compartment (46%), ER (5%), the Golgi complex (1.9%), and the tubular cluster/translucent vesicle complex (3.6%) (102). They could represent both transient MSPs trafficking from ER to the plasma membrane and mature, stable MSPs situated at their final destination. To confirm the existence of the latter, L. chagasi MSPs were localized using pulse-chase metabolic labeling and immunoprecipitation to detect all MSPs and surface biotinylation to mark only surface-localized MSPs. Thus, after removal of surface MSPs with streptavidin, internal MSPs were detected in immunoprecipitates by autoradiography. These experiments revealed that one-fourth of the nascent MSPs remained internally for up to 6 days following biosynthesis, strongly supporting the existence of a stable internal subpopulation of MSPs. It appeared that only the mature 63-kDa form, not the 59-kDa protein, accounts for this subpopulation of internal MSPs (106, 109). We have not formally ruled out two less likely possibilities: (i) that some surface MSP isoforms are inaccessible to surface biotinylation due to the presence of densely packed LPG, a situation reminiscent of the failure of immunogold to label some MSPs on the cell surfaces of L. major promastigotes (86), and (ii) that MSPs are constantly internalized with biotin removal. The second explanation is also unlikely because of the following reasons: (i) one of the 11 major MSP isoforms detected on Western blots of total L. chagasi proteins separated by two-dimensional SDS-PAGE is not surface biotinylated (110), (ii) intracellular MSP does not colocalize with the endosome marker FM4-64 by confocal microscopy and is instead found in a subregion of ER (111), and (iii) quantitation of MSPs by immunoelectron and fluorescence microscopy agrees with results of surface biotinylation (102, 109). Together, these data suggest that a subpopulation of MSPs resides continuously at an internal location of promastigotes. Consistent with this possibility is the fact that the MSPC class and its homologues lack a C-terminal hydrophobic signal for GPI anchor addition. Our hypothesis is that the internal population of MSPs serves as a pool for quick release upon inoculation of metacyclic promastigotes into the mammalian host by sand fly vectors, rather than as a pool for promastigote surface MSPs in the sand flies (106). A model outlining the function of MSPs in Leishmania promastigotes is presented in Fig. 1.
CONCLUDING REMARKS
MSPs and homologues have been documented in all trypanosomatids examined to date, ranging from monoxenous insect parasites to heteroxenous plant and mammalian parasites. They are encoded by a family of genes varying from several to hundreds in number in each species. Their designations in the insect stages of promastigotes include “surface localized,” “intracellularly located,” and “released into surrounding environments,” although whether these proteins are the products of different MSP genes or different posttranslational modification of the same genes remains to be elucidated. One function conserved across the phylogenetic tree of various trypanosomatids appears to be nutrient acquisition in the gut of various insects. Provided that endopeptidases, like MSPs, do not really cleave protein substrates extensively, this very likely requires a coordinated action from other enzymes. In addition, MSPs also serve a variety of roles in protecting these parasites from innate defenses of different hosts, including insects, plants, and mammals. Consequently, collaborative efforts to develop an MSP vaccination for domestic animals and humans should continue, despite the marginal and/or moderate protection of MSP-immunized individuals against leishmaniasis in most cases. Hypothetically, a major surface protein like MSP may have been evolutionally selected to become antigenically less provocative to better allow the parasite to escape host immune surveillance. Nevertheless, liposome-encapsulated MSPs have been shown to confer significant protection against L. major infection in susceptible BALB/c mice (50). It is also worth investigating whether MSP vaccination will effectively hamper the generation of metacyclic promastigotes in the sand fly vectors, eventually leading to interruption of leishmanial transmission. Finally, the crystal structure of one L. major MSP was determined more than a decade ago (96). Structural modeling is an effective approach to search for peptidomimetic inhibitors of these endopeptidases. In fact, low micromolar concentrations of some peptidomimetics were found to be toxic for cultured bloodstream trypanosomes and inhibitory for in vitro cleavage of a synthetic peptide substrate by purified L. major MSP (3). The peptidomimetic approach has high potential for addressing the proposed MSP functions and for developing chemotherapeutic drugs against leishmaniasis.
Acknowledgments
I am grateful to Donald L. Montgomery and Kenneth W. Mills of the University of Wyoming for critically reading the manuscript.
Support for this work comes from a Veterans' Affairs Merit Review grant and a startup package from the University of Wyoming.
Biography
 Chaoqun Yao completed an M.D. from Tongji Medical University, Wuhan, People's Republic of China, and a Ph.D. from the University of Georgia, followed by postdoctoral training at Washington State University and the University of Iowa. It was in graduate school that he developed a strong interest in molecular pathogenesis and host-parasite interaction of intracellular parasites of medical/veterinary importance. Dr. Yao was promoted from an Assistant to an Associate Research Scientist at the University of Iowa and was simultaneously appointed a Research Health Science Specialist at Iowa City VA Medical Center. He is currently an Assistant Professor in the Department of Veterinary Sciences and a Parasitologist in the Wyoming State Veterinary Laboratory, University of Wyoming, and an Adjunct Assistant Professor at the University of Washington. In his work he has used two model organisms, i.e., Trichinella and Leishmania, the intracellular parasites of mammals including human beings.
Chaoqun Yao completed an M.D. from Tongji Medical University, Wuhan, People's Republic of China, and a Ph.D. from the University of Georgia, followed by postdoctoral training at Washington State University and the University of Iowa. It was in graduate school that he developed a strong interest in molecular pathogenesis and host-parasite interaction of intracellular parasites of medical/veterinary importance. Dr. Yao was promoted from an Assistant to an Associate Research Scientist at the University of Iowa and was simultaneously appointed a Research Health Science Specialist at Iowa City VA Medical Center. He is currently an Assistant Professor in the Department of Veterinary Sciences and a Parasitologist in the Wyoming State Veterinary Laboratory, University of Wyoming, and an Adjunct Assistant Professor at the University of Washington. In his work he has used two model organisms, i.e., Trichinella and Leishmania, the intracellular parasites of mammals including human beings.
Editor: J. B. Kaper
Footnotes
Published ahead of print on 26 October 2009.
REFERENCES
- 1.Almeida, F. V., M. H. Branquinha, S. Giovanni-De-Simone, and A. B. Vermelho. 2003. Extracellular metalloproteinase activity in Phytomonas françai. Parasitol. Res. 89:320-322. [DOI] [PubMed] [Google Scholar]
- 2.Bahr, V., Y. D. Stierhof, T. Ilg, M. Demar, M. Quinten, and P. Overath. 1993. Expression of lipophosphoglycan, high-molecular weight phosphoglycan and glycoprotein 63 in promastigotes and amastigotes of Leishmania mexicana. Mol. Biochem. Parasitol. 58:107-121. [DOI] [PubMed] [Google Scholar]
- 3.Bangs, J. D., D. A. Ransom, M. Nimick, G. Christie, and N. M. Hooper. 2001. In vitro cytocidal effects on Trypanosoma brucei and inhibition of Leishmania major GP63 by peptidomimetic metalloprotease inhibitors. Mol. Biochem. Parasitol. 114:111-117. [DOI] [PubMed] [Google Scholar]
- 4.Bangs, J. D., D. M. Ransom, M. A. McDowell, and E. M. Brouch. 1997. Expression of bloodstream variant surface glycoproteins in procyclic stage Trypanosoma brucei: role of GPI anchors in secretion. EMBO J. 16:4285-4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berriman, M., E. Ghedin, C. Hertz-Fowler, G. Blandin, H. Renauld, D. C. Bartholomeu, N. J. Lennard, E. Caler, N. E. Hamlin, B. Haas, U. Bohme, L. Hannick, M. A. Aslett, J. Shallom, L. Marcello, L. Hou, B. Wickstead, U. C. Alsmark, C. Arrowsmith, R. J. Atkin, A. J. Barron, F. Bringaud, K. Brooks, M. Carrington, I. Cherevach, T. J. Chillingworth, C. Churcher, L. N. Clark, C. H. Corton, A. Cronin, R. M. Davies, J. Doggett, A. Djikeng, T. Feldblyum, M. C. Field, A. Fraser, I. Goodhead, Z. Hance, D. Harper, B. R. Harris, H. Hauser, J. Hostetler, A. Ivens, K. Jagels, D. Johnson, J. Johnson, K. Jones, A. X. Kerhornou, H. Koo, N. Larke, S. Landfear, C. Larkin, V. Leech, A. Line, A. Lord, A. Macleod, P. J. Mooney, S. Moule, D. M. Martin, G. W. Morgan, K. Mungall, H. Norbertczak, D. Ormond, G. Pai, C. S. Peacock, J. Peterson, M. A. Quail, E. Rabbinowitsch, M. A. Rajandream, C. Reitter, S. L. Salzberg, M. Sanders, S. Schobel, S. Sharp, M. Simmonds, A. J. Simpson, L. Tallon, C. M. Turner, A. Tait, A. R. Tivey, S. Van Aken, D. Walker, D. Wanless, S. Wang, B. White, O. White, S. Whitehead, J. Woodward, J. Wortman, M. D. Adams, T. M. Embley, K. Gull, E. Ullu, J. D. Barry, A. H. Fairlamb, F. Opperdoes, B. G. Barrell, J. E. Donelson, N. Hall, C. M. Fraser, S. E. Melville, and N. M. El-Sayed. 2005. The genome of the African trypanosome Trypanosoma brucei. Science 309:416-422. [DOI] [PubMed] [Google Scholar]
- 6.Beverley, S. M., and S. J. Turco. 1998. Lipophosphoglycan (LPG) and the identification of virulence genes in the protozoan parasite Leishmania. Trends Microbiol. 6:35-40. [DOI] [PubMed] [Google Scholar]
- 7.Bogdan, C., N. Donhauser, R. Doring, M. Rollinghoff, A. Diefenbach, and M. G. Rittig. 2000. Fibroblasts as host cells in latent leishmaniosis. J. Exp. Med. 191:2121-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bordier, C. 1987. The promastigote surface protease of Leishmania. Parsitol. Today 3:151-153. [DOI] [PubMed] [Google Scholar]
- 9.Bordier, C., R. J. Etges, J. Ward, M. J. Turner, and M. L. Cardoso de Almeida. 1986. Leishmania and Trypanosoma surface glycoproteins have a common glycophospholipid membrane anchor. Proc. Natl. Acad. Sci. USA 83:5988-5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouvier, J., R. J. Etges, and C. Bordier. 1985. Identification and purification of membrane and soluble forms of the major surface protein of Leishmania promastigotes. J. Biol. Chem. 260:15504-15509. [PubMed] [Google Scholar]
- 11.Brittingham, A., G. Chen, B. S. McGwire, K. P. Chang, and D. M. Mosser. 1999. Interaction of Leishmania gp63 with cellular receptors for fibronectin. Infect. Immun. 67:4477-4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brittingham, A., C. J. Morrison, W. R. McMaster, B. S. McGwire, K. P. Chang, and D. M. Mosser. 1995. Role of the Leishmania surface protease gp63 in complement fixation, cell adhesion, and resistance to complement-mediated lysis. J. Immunol. 155:3102-3111. [PubMed] [Google Scholar]
- 13.Camargo, E. P. 1999. Phytomonas and other trypanosomatid parasites of plants and fruit. Adv. Parasitol. 42:29-112. [DOI] [PubMed] [Google Scholar]
- 14.Chang, C. S., and K. P. Chang. 1986. Monoclonal antibody affinity purification of a Leishmania membrane glycoprotein and its inhibition of leishmania-macrophage binding. Proc. Natl. Acad. Sci. USA 83:100-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang, C. S., T. J. Inserra, J. A. Kink, D. Fong, and K. P. Chang. 1986. Expression and size heterogeneity of a 63 kilodalton membrane glycoprotein during growth and transformation of Leishmania mexicana amazonensis. Mol. Biochem. Parasitol. 18:197-210. [DOI] [PubMed] [Google Scholar]
- 16.Chang, K. P., and B. S. McGwire. 2002. Molecular determinants and regulation of Leishmania virulence. Kinetoplastid Biol. Dis. 1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaudhuri, G., M. Chaudhuri, A. Pan, and K. P. Chang. 1989. Surface acid proteinase (gp63) of Leishmania mexicana. A metalloenzyme capable of protecting liposome-encapsulated proteins from phagolysosomal degradation by macrophages. J. Biol. Chem. 264:7483-7489. [PubMed] [Google Scholar]
- 18.Chen, D. Q., B. K. Kolli, N. Yadava, H. G. Lu, A. Gilman-Sachs, D. A. Peterson, and K. P. Chang. 2000. Episomal expression of specific sense and antisense mRNAs in Leishmania amazonensis: modulation of gp63 level in promastigotes and their infection of macrophages in vitro. Infect. Immun. 68:80-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chicharro, C., and J. Alvar. 2003. Lower trypanosomatids in HIV/AIDS patients. Ann. Trop. Med. Parasitol. 97(Suppl. 1):75-78. [DOI] [PubMed] [Google Scholar]
- 20.Clayton, C., and M. Shapira. 2007. Post-transcriptional regulation of gene expression in trypanosomes and leishmanias. Mol. Biochem. Parasitol. 156:93-101. [DOI] [PubMed] [Google Scholar]
- 21.Corradin, S., J. Mauel, A. Ransijn, C. Sturzinger, and G. Vergeres. 1999. Down-regulation of MARCKS-related protein (MRP) in macrophages infected with Leishmania. J. Biol. Chem. 274:16782-16787. [DOI] [PubMed] [Google Scholar]
- 22.Corradin, S., A. Ransijn, G. Corradin, M. A. Roggero, A. A. Schmitz, P. Schneider, J. Mauel, and G. Vergeres. 1999. MARCKS-related protein (MRP) is a substrate for the Leishmania major surface protease leishmanolysin (gp63). J. Biol. Chem. 274:25411-25418. [DOI] [PubMed] [Google Scholar]
- 23.Cuevas, I. C., J. J. Cazzulo, and D. O. Sanchez. 2003. gp63 homologues in Trypanosoma cruzi: surface antigens with metalloprotease activity and a possible role in host cell infection. Infect. Immun. 71:5739-5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cupolillo, E., E. Medina-Acosta, H. Noyes, H. Momen, and G. Grimaldi, Jr. 2000. A revised classification for Leishmania and Endotrypanum. Parasitol. Today 16:142-144. [DOI] [PubMed] [Google Scholar]
- 25.Da Silva, R. P., B. F. Hall, K. A. Joiner, and D. L. Sacks. 1989. CR1, the C3b receptor, mediates binding of infective Leishmania major metacyclic promastigotes to human macrophages. J. Immunol. 143:617-622. [PubMed] [Google Scholar]
- 26.d'Avila-Levy, C. M., F. M. Araujo, A. B. Vermelho, R. M. A. Soares, A. L. S. Santos, and M. H. Branquinha. 2005. Proteolytic expression in Blastocrithidia culicis: influence of the endosymbiont and similarities with virulence factors of pathogenic trypanosomatids. Parasitology 130:413-420. [DOI] [PubMed] [Google Scholar]
- 27.d'Avila-Levy, C. M., A. C. Melo, A. B. Vermelho, and M. H. Branquinha. 2001. Differential expression of proteolytic enzymes in endosymbiont-harboring Crithidia species. FEMS Microbiol. Lett. 202:73-77. [DOI] [PubMed] [Google Scholar]
- 28.d'Avila-Levy, C. M., L. O. Santos, F. A. Marinho, F. A. Dias, A. H. Lopes, A. L. Santos, and M. H. Branquinha. 2006. Gp63-like molecules in Phytomonas serpens: possible role in the insect interaction. Curr. Microbiol. 52:439-444. [DOI] [PubMed] [Google Scholar]
- 29.d'Avila-Levy, C. M., R. F. Souza, R. C. Gomes, A. B. Vermelho, and M. H. Branquinha. 2003. A metalloproteinase extracellularly released by Crithidia deanei. Can. J. Microbiol. 49:625-632. [DOI] [PubMed] [Google Scholar]
- 30.de Melo, A. C., C. M. d'Avila-Levy, M. H. Branquinha, and A. B. Vermelho. 2002. Crithidia guilhermei: gelatin- and haemoglobin-degrading extracellular metalloproteinases. Exp. Parasitol. 102:150-156. [DOI] [PubMed] [Google Scholar]
- 31.dos Santos, A. L., C. M. Abreu, L. M. Batista, C. S. Alviano, and R. M. de Araujo Soares. 2001. Cell-associated and extracellular proteinases in Blastocrithidia culicis: influence of growth conditions. Curr. Microbiol. 43:100-106. [DOI] [PubMed] [Google Scholar]
- 32.Ellis, M., D. K. Sharma, J. D. Hilley, G. H. Coombs, and J. C. Mottram. 2002. Processing and trafficking of Leishmania mexicana GP63: analysis using GPI8 mutants deficient in glycosylphosphatidylinositol protein anchoring. J. Biol. Chem. 277:27968-27974. [DOI] [PubMed] [Google Scholar]
- 33.El-Sayed, N. M., and J. E. Donelson. 1997. African trypanosomes have differentially expressed genes encoding homologues of the Leishmania GP63 surface protease. J. Biol. Chem. 272:26742-26748. [DOI] [PubMed] [Google Scholar]
- 34.El-Sayed, N. M., P. J. Myler, D. C. Bartholomeu, D. Nilsson, G. Aggarwal, A. N. Tran, E. Ghedin, E. A. Worthey, A. L. Delcher, G. Blandin, S. J. Westenberger, E. Caler, G. C. Cerqueira, C. Branche, B. Haas, A. Anupama, E. Arner, L. Aslund, P. Attipoe, E. Bontempi, F. Bringaud, P. Burton, E. Cadag, D. A. Campbell, M. Carrington, J. Crabtree, H. Darban, J. F. da Silveira, P. de Jong, K. Edwards, P. T. Englund, G. Fazelina, T. Feldblyum, M. Ferella, A. C. Frasch, K. Gull, D. Horn, L. Hou, Y. Huang, E. Kindlund, M. Klingbeil, S. Kluge, H. Koo, D. Lacerda, M. J. Levin, H. Lorenzi, T. Louie, C. R. Machado, R. McCulloch, A. McKenna, Y. Mizuno, J. C. Mottram, S. Nelson, S. Ochaya, K. Osoegawa, G. Pai, M. Parsons, M. Pentony, U. Pettersson, M. Pop, J. L. Ramirez, J. Rinta, L. Robertson, S. L. Salzberg, D. O. Sanchez, A. Seyler, R. Sharma, J. Shetty, A. J. Simpson, E. Sisk, M. T. Tammi, R. Tarleton, S. Teixeira, S. Van Aken, C. Vogt, P. N. Ward, B. Wickstead, J. Wortman, O. White, C. M. Fraser, K. D. Stuart, and B. Andersson. 2005. The genome sequence of Trypanosoma cruzi, etiologic agent of Chagas disease. Science 309:409-415. [DOI] [PubMed] [Google Scholar]
- 35.Etges, R. 1992. Identification of a surface metalloproteinase on 13 species of Leishmania isolated from humans, Crithidia fasciculata, and Herpetomonas samuelpessoai. Acta Trop. 50:205-217. [DOI] [PubMed] [Google Scholar]
- 36.Etges, R., J. Bouvier, and C. Bordier. 1986. The major surface protein of Leishmania promastigotes is a protease. J. Biol. Chem. 261:9098-9101. [PubMed] [Google Scholar]
- 37.Fong, D., and K. P. Chang. 1982. Surface antigenic change during differentiation of a parasitic protozoan, Leishmania mexicana: identification by monoclonal antibodies. Proc. Natl. Acad. Sci. USA 79:7366-7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia, L. S. 2001. Diagonstic medical parasitology, 4th ed. ASM Press, Washington, DC.
- 39.Garcia, M. R., S. Graham, R. A. Harris, S. M. Beverley, and P. M. Kaye. 1997. Epitope cleavage by Leishmania endopeptidase(s) limits the efficiency of the exogenous pathway of major histocompatibility complex class I-associated antigen presentation. Eur. J. Immunol. 27:1005-1013. [DOI] [PubMed] [Google Scholar]
- 40.Gomes, I. N., L. C. Palma, G. O. Campos, J. G. B. Lima, T. F. De Almeida. J. P. B. De Menezes, C. A. G. Ferreira, R. R. Dos Santos, G. A. Buck, P. A. M. Manque, L. S. Ozaki, C. M. Probst, L. A. R. De Freitas, M. A. Krieger, and P. S. T. Veras. 2009. The scavenger receptor MARCO is involved in Leishmania major infection by CBA/J macrophages. Parasite Immunol. 31:188-198. [DOI] [PubMed] [Google Scholar]
- 41.Grandgenett, P. M., K. Otsu, H. R. Wilson, M. E. Wilson, and J. E. Donelson. 2007. A function for a specific zinc metalloprotease of African trypanosomes. PLoS Pathog. 3:1432-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gruszynski, A. E., A. DeMaster, N. M. Hooper, and J. D. Bangs. 2003. Surface coat remodeling during differentiation of Trypanosoma brucei. J. Biol. Chem. 278:24665-24672. [DOI] [PubMed] [Google Scholar]
- 43.Gruszynski, A. E., F. J. van Deursen, M. C. Albareda, A. Best, K. Chaudhary, L. J. Cliffe, L. del Rio, J. D. Dunn, L. Ellis, K. J. Evans, J. M. Figueiredo, N. A. Malmquist, Y. Omosun, J. B. Palenchar, S. Prickett, G. A. Punkosdy, G. van Dooren, Q. Wang, A. K. Menon, K. R. Matthews, and J. D. Bangs. 2006. Regulation of surface coat exchange by differentiating African trypanosomes. Mol. Biochem. Parasitol. 147:211-223. [DOI] [PubMed] [Google Scholar]
- 44.Haines, L. R., J. M. Thomas, A. M. Jackson, B. A. Eyford, M. Razavi, C. N. Watson, B. Gowen, R. E. Hancock, and T. W. Pearson. 2009. Killing of trypanosomatid parasites by a modified bovine host defense peptide, BMAP-18. PLoS Negl. Trop. Dis. 3:e373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Halle, M., M. A. Gomez, M. Stuible, H. Shimizu, W. R. McMaster, M. Olivier, and M. L. Tremblay. 2009. The Leishmania surface protease GP63 cleaves multiple intracellular proteins and actively participates in p38 mitogen-activated protein kinase inactivation. J. Biol. Chem. 284:6893-6908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hilley, J. D., J. L. Zawadzki, M. J. McConville, G. H. Coombs, and J. C. Mottram. 2000. Leishmania mexicana mutants lacking glycosylphosphatidylinositol (GPI):protein transamidase provide insights into the biosynthesis and functions of GPI-anchored proteins. Mol. Biol. Cell 11:1183-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hsiao, C. H., C. Yao, P. Storlie, J. E. Donelson, and M. E. Wilson. 2008. The major surface protease (MSP or GP63) in the intracellular amastigote stage of Leishmania chagasi. Mol. Biochem. Parasitol. 157:148-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Inverso, J. A., E. Medina-Acosta, J. O'Connor, D. G. Russell, and G. A. Cross. 1993. Crithidia fasciculata contains a transcribed leishmanial surface proteinase (gp63) gene homologue. Mol. Biochem. Parasitol. 57:47-54. [DOI] [PubMed] [Google Scholar]
- 49.Ivens, A. C., C. S. Peacock, E. A. Worthey, L. Murphy, G. Aggarwal, M. Berriman, E. Sisk, M. A. Rajandream, E. Adlem, R. Aert, A. Anupama, Z. Apostolou, P. Attipoe, N. Bason, C. Bauser, A. Beck, S. M. Beverley, G. Bianchettin, K. Borzym, G. Bothe, C. V. Bruschi, M. Collins, E. Cadag, L. Ciarloni, C. Clayton, R. M. Coulson, A. Cronin, A. K. Cruz, R. M. Davies, J. De Gaudenzi, D. E. Dobson, A. Duesterhoeft, G. Fazelina, N. Fosker, A. C. Frasch, A. Fraser, M. Fuchs, C. Gabel, A. Goble, A. Goffeau, D. Harris, C. Hertz-Fowler, H. Hilbert, D. Horn, Y. Huang, S. Klages, A. Knights, M. Kube, N. Larke, L. Litvin, A. Lord, T. Louie, M. Marra, D. Masuy, K. Matthews, S. Michaeli, J. C. Mottram, S. Muller-Auer, H. Munden, S. Nelson, H. Norbertczak, K. Oliver, S. O'Neil, M. Pentony, T. M. Pohl, C. Price, B. Purnelle, M. A. Quail, E. Rabbinowitsch, R. Reinhardt, M. Rieger, J. Rinta, J. Robben, L. Robertson, J. C. Ruiz, S. Rutter, D. Saunders, M. Schafer, J. Schein, D. C. Schwartz, K. Seeger, A. Seyler, S. Sharp, H. Shin, D. Sivam, R. Squares, S. Squares, V. Tosato, C. Vogt, G. Volckaert, R. Wambutt, T. Warren, H. Wedler, J. Woodward, S. Zhou, W. Zimmermann, D. F. Smith, J. M. Blackwell, K. D. Stuart, B. Barrell, and P. J. Myler. 2005. The genome of the kinetoplastid parasite, Leishmania major. Science 309:436-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jaafari, M. R., A. Ghafarian, A. Farrokh-Gisour, A. Samiei, M. T. Kheiri, F. Mahboudi, F. Barkhordari, A. Khamesipour, and W. R. McMaster. 2006. Immune response and protection assay of recombinant major surface glycoprotein of Leishmania (rgp63) reconstituted with liposomes in BALB/c mice. Vaccine 24:5708-5717. [DOI] [PubMed] [Google Scholar]
- 51.Jaffe, C. L., and D. M. Dwyer. 2003. Extracellular release of the surface metalloprotease, gp63, from Leishmania and insect trypanosomatids. Parasitol. Res. 91:229-237. [DOI] [PubMed] [Google Scholar]
- 52.Joshi, P. B., B. L. Kelly, S. Kamhawi, D. L. Sacks, and W. R. McMaster. 2002. Targeted gene deletion in Leishmania major identifies leishmanolysin (GP63) as a virulence factor. Mol. Biochem. Parasitol. 120:33-40. [DOI] [PubMed] [Google Scholar]
- 53.Katakura, K., T. Mimori, M. Furuya, H. Uezato, S. Nonaka, M. Okamoto, L. E. Gomez, and Y. Hashiguchi. 2003. Identification of Endotrypanum species from a sloth, a squirrel and Lutzomyia sandflies in Ecuador by PCR amplification and sequencing of the mini-exon gene. J. Vet. Med. Sci. 65:649-653. [DOI] [PubMed] [Google Scholar]
- 54.Kima, P. E. 2007. The amastigote forms of Leishmania are experts at exploiting host cell processes to establish infection and persist. Int. J. Parasitol. 37:1087-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kulkarni, M. M., E. A. Jones, W. R. McMaster, and B. S. McGwire. 2008. Fibronectin binding and proteolytic degradation by Leishmania and effects on macrophage activation. Infect. Immun. 76:1738-1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kulkarni, M. M., W. R. McMaster, E. Kamysz, W. Kamysz, D. M. Engman, and B. S. McGwire. 2006. The major surface-metalloprotease of the parasitic protozoan, Leishmania, protects against antimicrobial peptide-induced apoptotic killing. Mol. Microbiol. 62:1484-1497. [DOI] [PubMed] [Google Scholar]
- 57.Kulkarni, M. M., W. R. McMaster, W. Kamysz, and B. S. McGwire. 2009. Antimicrobial peptide-induced apoptotic death of Leishmania results from calcium-dependent, caspase-independent mitochondrial toxicity. J. Biol. Chem. 284:15496-15504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kulkarni, M. M., C. L. Olson, D. M. Engman, and B. S. McGwire. 2009. Trypanosoma cruzi GP63s undergo stage-specific differential posttranslational modification and are important for host cell infection. Infect. Immun. 77:2193-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.LaCount, D. J., A. E. Gruszynski, P. M. Grandgenett, J. D. Bangs, and J. E. Donelson. 2003. Expression and function of TbMSP (GP63) genes in African trypanosomes. J. Biol. Chem. 278:24658-24664. [DOI] [PubMed] [Google Scholar]
- 60.Lainson, R., J. J. Shaw, F. T. Silveira, and R. R. Braga. 1987. American visceral leishmaniasis: on the origin of Leishmania (Leishmania) chagasi. Trans. R. Soc. Trop. Med. Hyg. 81:517. [DOI] [PubMed] [Google Scholar]
- 61.Laskay, T., A. Diefenbach, M. Rollinghoff, and W. Solbach. 1995. Early parasite containment is decisive for resistance to Leishmania major infection. Eur. J. Immunol. 25:2220-2227. [DOI] [PubMed] [Google Scholar]
- 62.Lieke, T., S. Nylen, L. Eidsmo, W. R. McMaster, A. M. Mohammadi, A. Khamesipour, L. Berg, and H. Akuffo. 2008. Leishmania surface protein gp63 binds directly to human natural killer cells and inhibits proliferation. Clin. Exp. Immunol. 153:221-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lima, A. K., C. G. Elias, J. E. Souza, A. L. Santos, and P. M. Dutra. 2009. Dissimilar peptidase production by avirulent and virulent promastigotes of Leishmania braziliensis: inference on the parasite proliferation and interaction with macrophages. Parasitology 136:1179-1191. [DOI] [PubMed] [Google Scholar]
- 64.Masini d'Avila-Levy, C., F. de Almeida Dias, A. C. Nogueira de Melo, J. L. Martins, A. H. De Carvalho Santos Lopes, A. L. Souza Dos Santos, A. B. Vermelho, and M. H. Branquinha. 2006. Insights into the role of gp63-like proteins in lower trypanosomatids. FEMS Microbiol. Lett. 254:149-156. [DOI] [PubMed] [Google Scholar]
- 65.Mauricio, I. L., M. K. Howard, J. R. Stothard, and M. A. Miles. 1999. Genomic diversity in the Leishmania donovani complex. Parasitology 119:237-246. [DOI] [PubMed] [Google Scholar]
- 66.Mauricio, I. L., J. R. Stothard, and M. A. Miles. 2000. The strange case of Leishmania chagasi. Parasitol. Today 16:188-189. [DOI] [PubMed] [Google Scholar]
- 67.McGwire, B., and K. P. Chang. 1994. Genetic rescue of surface metalloproteinase (gp63)-deficiency in Leishmania amazonensis variants increases their infection of macrophages at the early phase. Mol. Biochem. Parasitol. 66:345-347. [DOI] [PubMed] [Google Scholar]
- 68.McGwire, B. S., K. P. Chang, and D. M. Engman. 2003. Migration through the extracellular matrix by the parasitic protozoan Leishmania is enhanced by surface metalloprotease gp63. Infect. Immun. 71:1008-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McGwire, B. S., W. A. O'Connell, K. P. Chang, and D. M. Engman. 2002. Extracellular release of the glycosylphosphatidylinositol (GPI)-linked Leishmania surface metalloprotease, Gp63, is independent of GPI phospholipolysis: implications for parasite virulence. J. Biol. Chem. 277:8802-8809. [DOI] [PubMed] [Google Scholar]
- 70.McMaster, W. R., C. J. Morrison, M. H. MacDonald, and P. B. Joshi. 1994. Mutational and functional analysis of the Leishmania surface metalloproteinase GP63: similarities to matrix metalloproteinases. Parasitology 108:S29-S36. [DOI] [PubMed] [Google Scholar]
- 71.Medina-Acosta, E., S. M. Beverley, and D. G. Russell. 1993. Evolution and expression of the Leishmania surface proteinase (gp63) gene locus. Infect. Agents Dis. 2:25-34. [PubMed] [Google Scholar]
- 72.Medina-Acosta, E., R. E. Karess, and D. G. Russell. 1993. Structurally distinct genes for the surface protease of Leishmania mexicana are developmentally regulated. Mol. Biochem. Parasitol. 57:31-45. [DOI] [PubMed] [Google Scholar]
- 73.Medina-Acosta, E., R. E. Karess, H. Schwartz, and D. G. Russell. 1989. The promastigote surface protease (gp63) of Leishmania is expressed but differentially processed and localized in the amastigote stage. Mol. Biochem. Parasitol. 37:263-273. [DOI] [PubMed] [Google Scholar]
- 74.Medina-Acosta, E., S. Paul, S. Tomlinson, and L. C. Pontes-de-Carvalho. 1994. Combined occurrence of trypanosomal sialidase/trans-sialidase activities and leishmanial metalloproteinase gene homologues in Endotrypanum sp. Mol. Biochem. Parasitol. 64:273-282. [DOI] [PubMed] [Google Scholar]
- 75.Merello, S., M. T. Xavier, and A. J. Parodi. 1995. The presence of galactofuranose and ribose units in asparagine-linked oligosaccharides of the digenetic trypanosomatid Endotrypanum schaudinni. Mol. Biochem. Parasitol. 69:73-79. [DOI] [PubMed] [Google Scholar]
- 76.Momen, H., R. S. Pacheco, E. Cupolillo, and G. Grimaldi, Jr. 1993. Molecular evidence for the importation of Old World Leishmania into the Americas. Biol. Res. 26:249-255. [PubMed] [Google Scholar]
- 77.Mosser, D. M., and P. J. Edelson. 1985. The mouse macrophage receptor for C3bi (CR3) is a major mechanism in the phagocytosis of Leishmania promastigotes. J. Immunol. 135:2785-2789. [PubMed] [Google Scholar]
- 78.Mottram, J. C., G. H. Coombs, and J. Alexander. 2004. Cysteine peptidases as virulence factors of Leishmania. Curr. Opin. Microbiol. 7:375-381. [DOI] [PubMed] [Google Scholar]
- 79.Naderer, T., and M. J. McConville. 2002. Characterization of a Leishmania mexicana mutant defective in synthesis of free and protein-linked GPI glycolipids. Mol. Biochem. Parasitol. 125:147-161. [DOI] [PubMed] [Google Scholar]
- 80.Nandan, D., T. Tran, E. Trinh, J. M. Silverman, and M. Lopez. 2007. Identification of leishmania fructose-1,6-bisphosphate aldolase as a novel activator of host macrophage Src homology 2 domain containing protein tyrosine phosphatase SHP-1. Biochem. Biophys. Res. Commun. 364:601-607. [DOI] [PubMed] [Google Scholar]
- 81.Nandan, D., T. Yi, M. Lopez, C. Lai, and N. E. Reiner. 2002. Leishmania EF-1alpha activates the Src homology 2 domain containing tyrosine phosphatase SHP-1 leading to macrophage deactivation. J. Biol. Chem. 277:50190-50197. [DOI] [PubMed] [Google Scholar]
- 82.Nogueira de Melo, A. C., C. M. d'Avila-Levy, F. A. Dias, J. L. Armada, H. D. Silva, A. H. Lopes, A. L. Santos, M. H. Branquinha, and A. B. Vermelho. 2006. Peptidases and gp63-like proteins in Herpetomonas megaseliae: possible involvement in the adhesion to the invertebrate host. Int. J. Parasitol. 36:415-422. [DOI] [PubMed] [Google Scholar]
- 83.Nogueira de Melo, A. C., S. Giovanni-De-Simone, M. H. Branquinha, and A. B. Vermelho. 2001. Crithidia guilhermei: purification and partial characterization of a 62-kDa extracellular metalloproteinase. Exp. Parasitol. 97:1-8. [DOI] [PubMed] [Google Scholar]
- 84.Peacock, C. S., K. Seeger, D. Harris, L. Murphy, J. C. Ruiz, M. A. Quail, N. Peters, E. Adlem, A. Tivey, M. Aslett, A. Kerhornou, A. Ivens, A. Fraser, M. A. Rajandream, T. Carver, H. Norbertczak, T. Chillingworth, Z. Hance, K. Jagels, S. Moule, D. Ormond, S. Rutter, R. Squares, S. Whitehead, E. Rabbinowitsch, C. Arrowsmith, B. White, S. Thurston, F. Bringaud, S. L. Baldauf, A. Faulconbridge, D. Jeffares, D. P. Depledge, S. O. Oyola, J. D. Hilley, L. O. Brito, L. R. Tosi, B. Barrell, A. K. Cruz, J. C. Mottram, D. F. Smith, and M. Berriman. 2007. Comparative genomic analysis of three Leishmania species that cause diverse human disease. Nat. Genet. 39:839-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pereira, F. M., P. S. Bernardo, P. F. Dias, Jr., B. A. Silva, M. T. Romanos, C. M. d'Avila-Levy, M. H. Branquinha, and A. L. Santos. 2009. Differential influence of gp63-like molecules in three distinct Leptomonas species on the adhesion to insect cells. Parasitol. Res. 104:347-353. [DOI] [PubMed] [Google Scholar]
- 86.Pimenta, P. F., E. M. Saraiva, and D. L. Sacks. 1991. The comparative fine structure and surface glycoconjugate expression of three life stages of Leishmania major. Exp. Parasitol. 72:191-204. [DOI] [PubMed] [Google Scholar]
- 87.Podlipaev, S. 2001. The more insect trypanosomatids under study—the more diverse Trypanosomatidae appears. Int. J. Parasitol. 31:648-652. [DOI] [PubMed] [Google Scholar]
- 88.Pupkis, M. F., and G. H. Coombs. 1984. Purification and characterization of proteolytic enzymes of Leishmania mexicana mexicana amastigotes and promastigotes. J. Gen. Microbiol. 130(Pt. 9):2375-2383. [DOI] [PubMed] [Google Scholar]
- 89.Rioux, J. A., G. Lanotte, E. Serres, F. Pratlong, P. Bastien, and J. Perieres. 1990. Taxonomy of Leishmania. Use of isoenzymes. Suggestions for a new classification. Ann. Parasitol. Hum. Comp. 65:111-125. [DOI] [PubMed] [Google Scholar]
- 90.Roberts, L. S., and J. J. Janovy. 2009. Gerold D. Schmidt & Larry S. Roberts' foundation of parasitology, 8th ed. McGraw-Hill, New York, NY.
- 91.Roberts, S. C., K. G. Swihart, M. W. Agey, R. Ramamoorthy, M. E. Wilson, and J. E. Donelson. 1993. Sequence diversity and organization of the msp gene family encoding gp63 of Leishmania chagasi. Mol. Biochem. Parasitol. 62:157-171. [DOI] [PubMed] [Google Scholar]
- 92.Roberts, S. C., M. E. Wilson, and J. E. Donelson. 1995. Developmentally regulated expression of a novel 59-kDa product of the major surface protease (Msp or gp63) gene family of Leishmania chagasi. J. Biol. Chem. 270:8884-8892. [DOI] [PubMed] [Google Scholar]
- 93.Russell, D. G. 1987. The macrophage-attachment glycoprotein gp63 is the predominant C3-acceptor site on Leishmania mexicana promastigotes. Eur. J. Biochem. 164:213-221. [DOI] [PubMed] [Google Scholar]
- 94.Russell, D. G., and H. Wilhelm. 1986. The involvement of the major surface glycoprotein (gp63) of Leishmania promastigotes in attachment to macrophages. J. Immunol. 136:2613-2620. [PubMed] [Google Scholar]
- 95.Santos, D. O., S. C. Bourguignon, H. C. Castro, J. S. Silva, L. S. Franco, R. Hespanhol, M. J. Soares, and S. Corte-Real. 2004. Infection of mouse dermal fibroblasts by the monoxenous trypanosomatid protozoa Crithidia deanei and Herpetomonas roitmani. J. Eukaryot. Microbiol. 51:570-574. [DOI] [PubMed] [Google Scholar]
- 96.Schlagenhauf, E., R. Etges, and P. Metcalf. 1998. The crystal structure of the Leishmania major surface proteinase leishmanolysin (gp63). Structure 6:1035-1046. [DOI] [PubMed] [Google Scholar]
- 97.Seay, M. B., P. L. Heard, and G. Chaudhuri. 1996. Surface Zn-proteinase as a molecule for defense of Leishmania mexicana amazonensis promastigotes against cytolysis inside macrophage phagolysosomes. Infect. Immun. 64:5129-5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Souza dos Santos, A. L., A. Ferreira, V. A. Franco, C. S. Alviano, and R. M. de Araujo Soares. 1999. Characterization of proteinases in Herpetomonas anglusteri and Herpetomonas roitmani. Curr. Microbiol. 39:61-64. [DOI] [PubMed] [Google Scholar]
- 99.Ueno, N., C. L. Bratt, N. E. Rodriguez, and M. E. Wilson. 2009. Differences in human macrophage receptor usage, lysosomal fusion kinetics and survival between logarithmic and metacyclic Leishmania infantum chagasi promastigotes. Cell. Microbiol. [Epub ahead of print.] doi: 10.1111/j.1462-5822.2009.01374.x. [DOI] [PMC free article] [PubMed]
- 100.Vermelho, A. B., F. V. Almeida, L. S. Bronzato, and M. H. Branquinha. 2003. Extracellular metalloproteinases in Phytomonas serpens. Can. J. Microbiol. 49:221-224. [DOI] [PubMed] [Google Scholar]
- 101.Voth, B. R., B. L. Kelly, P. B. Joshi, A. C. Ivens, and W. R. McMaster. 1998. Differentially expressed Leishmania major gp63 genes encode cell surface leishmanolysin with distinct signals for glycosylphosphatidylinositol attachment. Mol. Biochem. Parasitol. 93:31-41. [DOI] [PubMed] [Google Scholar]
- 102.Weise, F., Y. D. Stierhof, C. Kuhn, M. Wiese, and P. Overath. 2000. Distribution of GPI-anchored proteins in the protozoan parasite Leishmania, based on an improved ultrastructural description using high-pressure frozen cells. J. Cell Sci. 113(Pt. 24):4587-4603. [DOI] [PubMed] [Google Scholar]
- 103.Wilson, M. E., and K. K. Hardin. 1988. The major concanavalin A-binding surface glycoprotein of Leishmania donovani chagasi promastigotes is involved in attachment to human macrophages. J. Immunol. 141:265-272. [PubMed] [Google Scholar]
- 104.Wilson, M. E., and K. K. Hardin. 1990. The major Leishmania donovani chagasi surface glycoprotein in tunicamycin-resistant promastigotes. J. Immunol. 144:4825-4834. [PubMed] [Google Scholar]
- 105.Wozencraft, A. O., and J. M. Blackwell. 1987. Increased infectivity of stationary-phase promastigotes of Leishmania donovani: correlation with enhanced C3 binding capacity and CR3-mediated attachment to host macrophages. Immunology 60:559-563. [PMC free article] [PubMed] [Google Scholar]
- 106.Yao, C., J. E. Donelson, and M. E. Wilson. 2007. Internal and surface-localized MSP of Leishmania and their differential release from promastigotes. Eukaryot. Cell 6:1905-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yao, C., J. E. Donelson, and M. E. Wilson. 2003. The major surface protease (MSP or GP63) of Leishmania sp. biosynthesis, regulation of expression, and function. Mol. Biochem. Parasitol. 132:1-16. [DOI] [PubMed] [Google Scholar]
- 108.Yao, C., K. G. Leidal, A. Brittingham, D. E. Tarr, J. E. Donelson, and M. E. Wilson. 2002. Biosynthesis of the major surface protease GP63 of Leishmania chagasi. Mol. Biochem. Parasitol. 121:119-128. [DOI] [PubMed] [Google Scholar]
- 109.Yao, C., J. Luo, C. Hsiao, J. E. Donelson, and M. E. Wilson. 2005. Internal and surface subpopulations of the major surface protease (MSP) of Leishmania chagasi. Mol. Biochem. Parasitol. 139:173-183. [DOI] [PubMed] [Google Scholar]
- 110.Yao, C., J. Luo, P. Storlie, J. E. Donelson, and M. E. Wilson. 2004. Multiple products of the Leishmania chagasi major surface protease (MSP or GP63) gene family. Mol. Biochem. Parasitol. 135:171-183. [DOI] [PubMed] [Google Scholar]
- 111.Zheng, Z., R. K. Tweten, and K. Mensa-Wilmot. 2005. Intracellular glycosylphosphatidylinositols accumulate on endosomes: toxicity of alpha-toxin to Leishmania major. Eukaryot. Cell 4:556-566. [DOI] [PMC free article] [PubMed] [Google Scholar]