Abstract
Salmonella enterica serotype Typhi, the etiological agent of typhoid fever, produces the Vi capsular antigen, a virulence factor absent in Salmonella enterica serotype Typhimurium. Previous studies suggest that the capsule-encoding viaB locus reduces inflammatory responses in intestinal tissue; however, there are currently no data regarding the in vivo expression of this locus. Here we implemented direct and indirect methods to localize and detect Vi antigen expression within polarized intestinal epithelial cells and in the bovine ileal mucosa. We report that tviB, a gene necessary for Vi production in S. Typhi, was significantly upregulated during invasion of intestinal epithelial cells in vitro. During infection of bovine ligated loops, tviB was expressed at levels significantly higher in calf tissue than those in the inoculum. The presence of the Vi capsular antigen was detected in calf ileal tissue via fluorescence microscopy. Together, these results support the concept that expression of the Vi capsular antigen is induced when S. Typhi transits from the intestinal lumen into the ileal mucosa.
Typhoid fever is an acute, systemic infection of the reticuloendothelial system caused by Salmonella enterica serotype Typhi that is annually responsible for an estimated 16 million illnesses and 600,000 deaths worldwide (6). Our knowledge of the pathogenesis of S. Typhi is limited because it infects only humans, resulting in the absence of in vivo models to study host-pathogen interactions. Many studies have relied on a murine model of human typhoid that uses S. enterica serotype Typhimurium, which causes a typhoid-like illness in mice. As a consequence, most of what is known about the pathogenicity of S. Typhi has been extrapolated from S. Typhimurium infections in mice. A significant limitation to using this murine model is that S. Typhimurium does not cause typhoid fever in humans but instead causes a localized gastroenteritis resulting in diarrhea.
Whole-genome sequencing has revealed that the S. Typhi genome contains 601 genes on 82 genetic islands that are absent from the S. Typhimurium genome (34). The largest of these islands, termed the Salmonella pathogenicity island 7 (SPI-7), contains 134 kb of S. Typhi-specific DNA and carries biosynthesis genes (viaB locus) for the production of the Vi capsular antigen, a linear polymer of α-1,4 2-deoxy-2-N-acetylgalacturonic acid that is variably O acetylated at the C-3 position (16, 18). The Vi capsular antigen is a significant virulence factor for typhoid fever, as strains positive for Vi production have higher rates of infection (20, 21), and it continues to be the focus for improvement in current treatment and prophylaxis for this disease.
The two-component-positive regulatory systems RcsBC and OmpR EnvZ, in addition to the promoter of the viaB region, located upstream of tviA, have been identified as contributors to Vi expression and are modulated by osmolarity. Under low-osmolarity conditions, the production of Sip proteins, flagellin, and Vi antigen is differentially modulated by the RcsB-RcsC regulatory system. The transcription of the iagA, invF, and sipB genes is negatively controlled by the RcsB regulator (2). The TviA protein functions as a positive regulator in cotranscribing the tviA and tviB genes (15, 50). In addition, the TviA protein may act in concert with the RcsB protein at the tviA promoter to activate transcription of the genes involved in Vi synthesis (49). S. Typhi strains harboring ompR deletions no longer agglutinate with Vi antiserum (35). The rpoS gene, which is a master regulator in stress response and required for survival under extreme conditions in S. Typhimurium, has been implicated as another regulator of Vi synthesis of S. Typhi (43). In conclusion, the regulation of Vi expression in vitro has been extensively characterized. However, gene regulation in an infected host may differ markedly from the predictions generated through in vitro and cell culture work (3, 25, 26). These considerations illustrate the need for in vivo studies to fully understand regulation of capsule expression in S. Typhi.
Understanding the role of the Vi antigen during pathogenesis is further complicated because the location of capsule expression in the host remains a matter of debate. One hypothesis predicts that osmolarity may represent an important signal for the transition of S. Typhi from the lumen into intestinal tissue (36). In the intestinal lumen, the osmolarity is high, with values considered to be equivalent to 300 mM NaCl and greater (13, 30, 45). Once S. Typhi invades the intestinal barrier, it encounters a lower-osmolarity condition equivalent to ∼150 mM (29), which has been reported to be the osmolarity of blood and plasma. It is thought that the hyperosmotic conditions within the intestinal lumen may promote a hyperinvasive phenotype while suppressing Vi expression. After invasion of epithelial cells, the expression of the Vi capsular antigen is predicted to be activated, while expression of the type III secretion system 1 (T3SS-1) is downregulated (38).
An alternative interpretation of the available data proposes that the capsule may inhibit bacterial adhesion and invasion of the intestinal epithelium (2, 31), suggesting that the Vi antigen may be produced in the intestinal lumen. Naturally occurring typhoid fever infections as well as live S. Typhi vaccines provoke a poor host protective immune response, even though Vi is a good antigen (44), which may be due to the inactivation of Vi antigen expression once the pathogen invades the intestinal epithelium and resides in macrophages. This concept has been proposed to be a possible explanation for the reduced Vi antibody responses, as decreased amounts of antigen may be presented to the host's immune system for processing (35). In conclusion, it remains unclear in which host compartment(s) the Vi capsular antigen is expressed in vivo. The purpose of this study was to investigate the expression of the Vi antigen during transition of bacteria from the intestinal lumen into mucosal tissue. We employed state-of-the-art models to study this transition in vitro, using polarized human intestinal model epithelia (an in vitro model), and in vivo, using bovine ligated ileal loops.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
S. Typhi strain Ty2 was obtained from the American Type Tissue Culture Collection (ATCC 19430; Manassas, VA). Strains and plasmids used in this study are listed in Table 1.
TABLE 1.
Strains and plasmids
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| Strains | ||
| S. Typhi | ||
| Ty2 | S. Typhi wild type | ATCC 19430 |
| QT74 | Ty2ΔompR::Kanr | This study |
| Plasmids | ||
| pCR2.1 | Cloning vector | Invitrogen |
| pBluescript SK+ | Cloning vector | Stratagene |
| pEGFP | GFPmut1 variant-expressing vector | Clontech |
| pQT13 | pGP704::ompRFR1 | This study |
| pQT17 | pGP704::ompRFR1:: ompRFR2 | This study |
| pQT19 | pGP704::ompRFR1:: ompRFR2::Kmr | This study |
| pQT27 | pEGFP::viaB promoter | This study |
| pQT28 | pBluescript SK+::egfp::viaB promoter | This study |
| pQT50 | pBluescript KS::ompR | This study |
Strains were cultured aerobically in Luria-Bertani broth containing 300 mM NaCl (LBH) for optimal Vi suppression. Strains were cultured in SOB medium (Luria Bertani [LB] broth containing 10 mM NaCl) for optimal induction of Vi antigen expression. Media was supplemented with appropriate antibiotics at the following concentrations: carbenicillin, 100 mg/liter, and kanamycin, 100 mg/liter.
For T84 cell infection experiments, each strain was grown overnight at 37°C with shaking in LBH with the appropriate antibiotics. The next day, a 1:1,000 dilution of overnight culture was made, and bacteria were grown to an optical density at 600 nm (OD600) of 1.0 to 1.5 (late log phase) for optimal Vi suppression without antibiotics. Bacteria were added at a concentration of 107 CFU/well to tissue culture plates.
For inoculation of bovine ligated ileal loops, each strain was grown overnight at 37°C with shaking in 4 ml of LBH with the appropriate antibiotics. A volume of 0.04 ml of overnight culture was used for inoculation of 4 ml of LBH without antibiotics, and bacteria were grown to an OD600 of 1.0 to 1.5 (late log phase).
Construction of a nonpolar deletion of ompR in S. Typhi and the complementation strain.
A region 1.2 kb upstream of ompR was PCR amplified using FW (5′-GACGGTTCGTGTTCCAGAGCAG-3′) and RV (5′-CTCTTGCATTGTCTGTACTCC-3′), and a 1.0-kb region downstream of ompR was PCR amplified using primers FW (5′-CGCCGTATGGTGGAAGAAG-3′) and RV (5′-ACCTGGATGCTGCCTGCCTG-3′). Both the downstream and upstream flanking regions were cloned into pCR 2.1 (Invitrogen, Carlsbad, CA). Subsequently, the upstream fragment was subcloned into the BglII/SalI site of pGP704, giving rise to pQT13. The downstream fragment was cloned into pQT13 using the XbaI/SmaI site, giving rise to pQT17. The kanamycin cassette (1.5 kb) was excised from pKIXX and inserted in the SalI site in pQT17 to give rise to pQT19. The entire construct was confirmed via nucleotide sequencing. Using the suicide plasmid pQT19, alleles were introduced into S. Typhi Ty2 with standard allelic exchange methodologies. Colonies were screened for kanamycin resistance and carbenicillin sensitivity. A single kanamycin-resistant and carbenicillin-sensitive colony was further analyzed for deletion of the ompR gene using Southern blotting with an ompR-specific DNA probe and PCR analysis. The ompR deletion was confirmed phenotypically by agglutination with rabbit anti-Vi serum (Difco, Detroit, MI). The strain was designated QT74.
For complementation, the ompR gene was amplified using FW (5′-TGCCAGCCATCAGCGGGGGCTT-3′) and RV (5′-GCCCTGATGAATCTCGGTCAG-3′) primers. The PCR fragment was cloned into pBluescript KS using EcoRV/EcoRI, creating pQT50. The plasmid was then electroporated into QT74, giving rise to QT174(pQT50). Expression of the Vi antigen by QT174(pQT50) was investigated by agglutination with rabbit anti-Vi serum (Difco, Detroit, MI).
Construction of a PtviA::egfp reporter construct.
We constructed a Ty2 strain carrying a high-copy-number plasmid encoding the viaB promoter region (PtviA) fused to a mutant variant of the gfp gene (egfp). The promoter of the viaB locus was PCR amplified using the primers FW (5′-TATACCATGGGAAGTCTCCTTATGCTGAAA-3′) and RV (5′-TATAGTCGACGCAGTCACGCACCATC-3′) flanked with restriction sites SalI and NcoI, respectively (italics indicate restriction enzyme sequences used). The resulting 600-bp PCR fragment was cloned into pCR2.1 (Invitrogen, Carlsbad, CA), and the fragment was confirmed by nucleotide sequencing. The fragment was subcloned into pEGFP, using the NcoI and SalI multiple cloning site, giving rise to plasmid pQT27. The PtviA::egfp fusion was excised using SalI and EcoRI and subsequently subcloned into pBluescript SK+ (Stratagene, La Jolla, CA), giving rise to plasmid pQT28. The plasmid was electroporated into Ty2 to produce strain Ty2(pQT28). To confirm that Vi expression is modulated by osmolarity in vitro, Ty2(pQT28) was grown in culture containing different salt concentrations, and expression of the PtviA::egfp reporter fusion was investigated by flow cytometry.
To confirm the phenotype of ompR as a regulator of Vi expression, pQT28 was electroporated into QT74, giving rise to QT74(pQT28). This strain was grown under different osmolarity conditions in broth and subjected to flow cytometric analysis.
Cell culture.
T84 cells are a human epithelial cell line of colon carcinoma cells that can be polarized in Transwell plates. T84 cells were seeded at 5 × 105 cells/well, and transepithelial resistance was monitored daily. At a transepithelial resistance of 500 to 1,500 Ω (28), bacteria were added at approximately 107 CFU/well (multiplicity of infection was approximately 10:1) to the apical compartment of polarized T84 cells for 1 h at 37°C in 5% CO2 to allow for invasion. After 1 h, the supernatant from each well was removed. To compare bacterial gene expression inside and outside mammalian cells by monitoring expression of the PtviA::egfp reporter using flow cytometry, gentamicin was not added (4). Instead, prewarmed Dulbecco's modified Eagle's medium (DMEM)-F12 medium was added to the apical side, and cells were incubated for 2 h at 37°C in 5% CO2. After this incubation period, the supernatant was collected, and extracellular bacteria were harvested via centrifugation. The monolayer was then washed three times with ice-cold phosphate-buffered saline (PBS), and T84 cells were lysed with 1% Triton X-100 on ice for 10 min to collect intracellular bacteria.
Flow cytometry.
Samples for flow cytometry were prepared as described previously (4). To characterize Ty2(pQT28) and QT74(pQT28), 1 ml of bacteria culture was harvested by centrifugation at 6,000 × g for 5 min. Bacterial cells were washed twice with PBS to remove LB media. Bacterial pellets were resuspended in 1 ml of 4% paraformaldehyde and incubated for 30 min at room temperature. Fluorescence was measured by using a FACSCalibur system (Becton Dickinson, Franklin Lakes, NJ) and the Cell quest software provided by the supplier. The bacterial population was gated by using side and forward scatter parameters and then analyzed for green fluorescence (fluorescein isothiocyanate [FITC] channel).
For infection of polarized T84 cells with Ty2(pQT28), the flow cytometry results were pooled from experimental infections of two separate Transwell plates. The bacterial population was gated with side and forward scatter parameters using samples from a Ty2(pQT28) grown under Vi-inducing conditions (SOB medium), and uninfected T84 polarized cells were treated under the same conditions as infected cells in order to set the region of fluorescence. The results were expressed as the percentage of the population which was fluorescing.
Bovine ligated ileal loop model.
Four milk-fed 3- to 4-week-old calves were obtained from a Texas A&M University cattle herd. Calves were tested for Salmonella infection by fecal swabs, enriched in tetraiodothionate broth, and plated on XLT4. To perform the ligated ileal loop experiments, calves were anesthetized using propofol induction and isoflurane maintenance for the duration of the experiment (1). A right-flank laparotomy was performed, the ileum was exposed, and loops with lengths ranging from 6 to 9 cm were ligated, leaving 1-cm loops between them. The loops were inoculated by intraluminal injection of a 4-ml suspension containing approximately 1 × 109 CFU/ml of S. Typhi strains Ty2 or QT74 grown in LBH. Loops injected with LBH served as a negative control. Loops were excised at 2 and 8 h postinfection to (i) collect the fluid that has accumulated in the lumen, (ii) harvest extracellular bacteria from 6.0-mm biopsy-punched tissue samples of the mucosa/submucosa for RNA extraction, and (iii) collect tissues for histopathology/frozen optimal cutting temperature (OCT) sections. The frozen sections were later sectioned at 10 μm thickness for fluorescence immunohistochemistry and stored at −80°C.
Histopathology.
Tissue samples were fixed in formalin, processed according to standard procedures for paraffin embedding, sectioned at 5 μm, and stained with hematoxylin and eosin. Histopathology slides were examined blindly by two board-certified veterinary pathologists.
Fluorescence immunohistochemistry.
Frozen sections of ileal tissue inoculated with S. Typhi Ty2 grown in LBH and loops inoculated with LBH (negative control) were fixed in methanol. Samples were incubated with a primary anti-Vi rabbit antibody (1:250 dilution) (Difco, Detroit, MI) overnight in 4°C. The next day, samples were incubated for 2 h at room temperature in a humidified chamber with secondary anti-rabbit conjugated to Alexa Fluor 549 (1:250 dilution) (Molecular Probes, Eugene, OR) in the dark. All antibodies were diluted in antibody dilution buffer (1% bovine serum albumin [BSA] in 0.02 M PBS-Tween). Slides were washed three times for 10 min each in 0.3% Tween-PBS. A drop of Prolong antifade DAPI (4′,6-diamidino-2-phenylindole) solution (Invitrogen, Carlsbad, CA) was added to each slide. A coverslip was placed over the top, and slides were allowed to dry overnight at room temperature. The next day, slides were stored at 4°C until ready to view. Samples were viewed and photographed with an Olympus IX-70 camera.
RT-PCR.
For analysis of intracellular and extracellular S. Typhi gene expression in vitro, bacteria were collected and stored in ethanol/phenol mRNA stop solution until RNA was extracted with a hot acid phenol-chloroform assay. For analysis of intracellular and extracellular S. Typhi gene expression during infection of bovine ileal loops, extracellular bacteria were harvested from the lumen and frozen on dry ice. Tissue samples were frozen on dry ice in TRI Reagent (Molecular Research Center, Cincinnati, OH) at the site of surgery until further processing for RNA extraction. After RNA extraction from ileal tissue, samples were processed using the microbe enrichment kit (Ambion, Austin, TX) in order to isolate bacterial RNA. All samples were DNase treated (Ambion DNA-free kit). Subsequently, 1,000 ng of each sample was reversely transcribed in a 50-μl volume (TaqMan reverse transcription reagents; Applied Biosystems, Foster City, CA), and 4 μl of cDNA was used for each real-time (RT)-PCR. RT-PCR with primers was used to detect the tviB gene in bacterial mRNA in order to measure Vi expression and rpoD, the internal control gene, in S. Typhi-infected T84 polarized cells, using SYBR green (Applied Biosystems, Foster City, CA) and the ABI 7500 real-time PCR system. The rpoD gene has been shown to have no significant variation of expression in either S. Typhi or S. Typhimurium inside macrophages (8, 9) and T84 polarized cells (this study). RNA samples were analyzed for the prgH and fliC genes to monitor the expression levels of the Salmonella pathogenicity island 1 (SPI-1) type III secretion system (T3SS) and flagellin, respectively. For each run, the calculated threshold cycle (CT) was normalized to the CT of the rpoD gene amplified from the corresponding sample, and the data were analyzed using the comparative CT method (Applied Biosystems, Foster City, CA). Levels of S. Typhi gene expression in T84 polarized cells were calculated relative to the inoculum of S. Typhi Ty2 culture grown in LBH. The levels of S. Typhi gene expression in calves were calculated for each loop inoculated with S. Typhi Ty2 relative to a loop inoculated with the Vi-negative S. Typhi ompR mutant (QT74) collected at the same time point from the same animal. A list of genes analyzed in this study with respective primers is provided in Table 2.
TABLE 2.
Primers for real-time PCR
| Gene | Primer pair sequences |
|---|---|
| fliC | 5′-CAACCTGGGCAATACCGTAAATAA-3′ |
| 5′-CTGCGCGCGAGACATG-3′ | |
| iagA | 5′-ACGGACAGGGTTATCGGTTTAAT-3′ |
| 5′-AAAAGGAAGTATCGCCAATGTATGAG-3′ | |
| tviB | 5′-ATAATAGGGATCTACGCCAATA-3′ |
| 5′-CGCTGGCAGCAAATGGA-3′ | |
| prgH | 5′-TCATAATCGCCCCTCGCTAA-3′ |
| 5′-TCTATGTCGCTGCGCAAAAT-3′ | |
| rpoD | 5′-GTATGCGTTTCGGTATC-3′ |
| 5′-GCTAGGGTGGCGCAGTTTAC-3′ |
Statistical analysis.
For statistical analysis of ratios (i.e., increases in S. Typhi gene expression or data, expressed as percentages), data were transformed logarithmically prior to performance of statistical analysis. A parametric test (paired Student's t test for T84 polarized cell samples and for calf ligated loop samples) was used to calculate whether differences were statistically significant.
RESULTS
Use of a PtviA::egfp reporter construct to monitor osmoregulation of the viaB promoter in S. Typhi.
GFP (green fluorescent protein) has been previously applied to study host pathogen interactions and Salmonella gene expression (4, 5, 46, 47). We first wanted to determine whether a plasmid-based reporter construct (pQT28) containing the viaB promoter (PtviA) fused to egfp would be responsive to changes in osmolarity when introduced into S. Typhi isolate Ty2. Ty2(pQT28) was grown under Vi-inducing (SOB medium) and Vi-suppressing (LBH medium) conditions. As a negative control, SOB medium was inoculated with the Ty2 parent strain.
When Ty2(pQT28) was grown under conditions repressing Vi antigen expression (LBH medium), the peak fluorescence intensity was comparable to that of an S. Typhi strain lacking the reporter construct (Ty2). However, growth of Ty2(pQT28) under conditions inducing expression of the Vi antigen (SOB medium) resulted in marked induction of the PtviA::egfp reporter construct, as indicated by a 10-fold increase in the peak fluorescence intensity (Fig. 1). These results were consistent with previous reports that expression of the Vi antigen of S. Typhi is affected by osmolarity (51) and validated the use of the plasmid-based PtviA::egfp reporter construct.
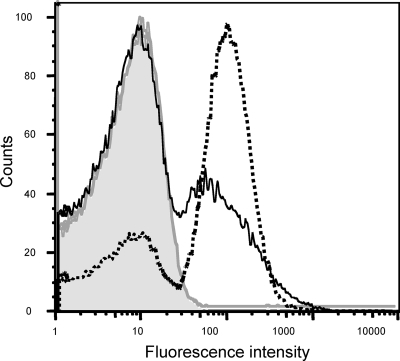
FIG. 1.
Osmoregulation of the tviA promoter (PtviA) in S. Typhi. In vitro induction of a PtviA::egfp reporter fusion carried on plasmid pQT28 in S. Typhi strain Ty2. Broken line represents Ty2(pQT28) grown under inducing conditions of low osmolarity (SOB broth). Solid line represents Ty2(pQT28) grown under noninducing conditions of high osmolarity (LBH). The shaded area under the gray line represents strain Ty2 without the reporter plasmid under inducing conditions. The results are expressed as the percentage of the population fluorescing and the mean fluorescence intensity. The number of cells used to determine fluorescence intensity and percentage of GFP-positive cells for each sample are as follows: Ty2(pQT28) in LBH, 87,759 and 28.8%; Ty2(pQT28) in SOB, 73,121 and 69.2%; and Ty2, 62,471 and 0.9%.
Expression of the Vi capsular antigen is induced after invasion of model epithelia.
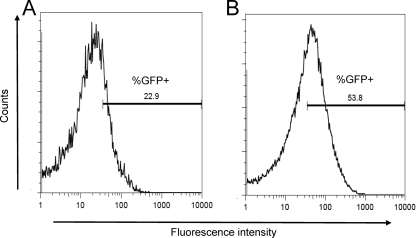
To investigate whether expression of the Vi antigen changes when S. Typhi transits from the intestinal lumen into tissue, we used intestinal model epithelia as an in vitro model. Polarized T84 intestinal epithelial cells were infected with S. Typhi strain Ty2(pQT28) grown under high osmolarity (LBH) to mimic the conditions in the intestinal lumen. The PtviA activity was monitored prior and subsequent to invasion of epithelial cells using flow cytometry. The peak fluorescence intensity was greater for Ty2(pQT28) associated with the T84 monolayer than for extracellular bacteria recovered from the apical compartment (Fig. 2). Furthermore, a larger percentage of cell-associated bacteria expressed fluorescence (53.8%) than did extracellular bacteria in the apical supernatant (22.9%).
FIG. 2.
Induction of the tviA promoter (PtviA) during S. Typhi invasion of intestinal model epithelium. Flow cytometry detection of Vi expression in T84 polarized cells using S. Typhi strain Ty2 carrying a PtviA::egfp reporter fusion (pQT28). The gate for detecting the percentage of GFP-positive counts (%GFP+) was set using control experiments shown in Fig. 1. The total numbers of particles counted were 9,704 for extracellular bacteria (A) and 49,812 for intracellular bacteria with T84 cell lysates (B).
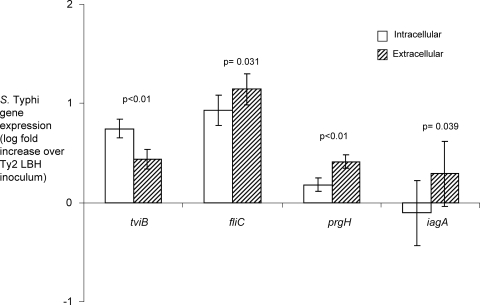
To further examine Vi expression during infection of T84 cells, we performed real-time PCR analysis. Polarized T84 cells were infected with S. Typhi Ty2 grown in LBH medium, and for each experiment, bacterial RNA was pooled from infections of 10 separate Transwells. We monitored expression of the tviB gene, the fliC gene, and genes encoded by the SPI-1 T3SS (prgH and iagA). The tviB gene was expressed at a significantly higher level (P = 0.0059) by cell-associated bacteria than by extracellular bacteria (Fig. 3). The fliC, prgH, and iagA genes were expressed at significantly higher levels by extracellular bacteria than by cell-associated bacteria. These data were consistent with previous reports indicating that the invasion-associated SPI-1 genes and the genes involved in flagellar biosynthesis are downregulated following invasion (10, 11, 12, 14, 17, 40, 41).
FIG. 3.
Transcript levels in RNA samples collected from extracellular and intracellular bacteria harvested 1 h after S. Typhi infection of polarized T84 model epithelium. Expression levels of tviB, fliC, prgH, and iagA were determined by real-time PCR. Data are shown as increases in S. Typhi gene expression relative to the inoculum culture grown under Vi-suppressing conditions. Bars represent arithmetic means (of the log-transformed means) ± margin of error. These data are the average results from three independent experiments.
The transcript levels of tviB in extracellular and cell-associated bacteria were expressed as fold increase over those measured in the inoculum culture, which was grown under Vi-suppressing conditions (300 mM NaCl). The induction of tviB expression seen in extracellular bacteria may be due to the use of tissue culture medium containing 150 mM NaCl, an osmolarity that induces Vi expression. Nonetheless, experiments with model epithelia indicated that the levels of Vi expression were significantly higher in cell-associated bacteria than in extracellular bacteria.
Deletion of ompR abrogates expression of a PtviA::egfp reporter construct.
A deletion in ompR was previously found to inhibit Vi synthesis and result in a negative Vi slide agglutination reaction (35). A deletion of the ompR gene was constructed in S. Typhi strain Ty2 (QT74), and the mutation was verified by Southern blotting (Fig. 4A). The inability of the S. Typhi ompR mutant to express the Vi antigen was verified by slide agglutination using Vi antiserum (data not shown). A plasmid carrying the intact ompR gene (plasmid pQT50) was introduced into the S. Typhi ompR mutant (QT74) by electroporation. Expression of the Vi capsular antigen in QT74(pQT50) was restored with slide agglutination using Vi antiserum (data not shown). Complementation with a low-copy-number plasmid (pWSK29) carrying the ompR gene did not restore Vi production when detected by slide agglutination (data not shown). This finding was consistent with prior ompR complementation studies (35).
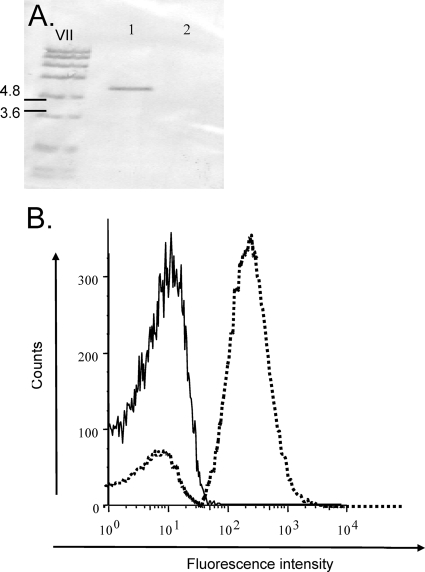
FIG. 4.
OmpR-mediated activation of the tviA promoter (PtviA). (A) Confirmation of the ompR deletion in QT74 by Southern blotting with an ompR specific DNA probe. Lane 1, S. Typhi strain Ty2; lane 2, S. Typhi ompR mutant (QT74). (B) Flow cytometric analysis of a PtviA::egfp reporter fusion in the wild type [Ty2(pQT28)] (broken line) and ompR [QT74(pQT28)] (solid line) grown under Vi-inducing conditions (SOB medium). The total numbers of counts used to determine fluorescence intensity for each sample are 17,583 and 19,413, respectively.
Vi expression by the ompR mutant was further characterized by introducing plasmid pQT28 (carrying an egfp reporter gene fused to the promoter of the viaB region). The ompR mutant [QT74(pQT28)] and S. Typhi wild type [Ty2(pQT28)] were grown under conditions inducing Vi capsule expression, and expression of the PtviA::egfp reporter construct was analyzed by flow cytometry. The fluorescence intensities emitted by S. Typhi strain Ty2 (negative control) (data not shown) and strain QT74(pQT28) grown under Vi-inducing conditions (SOB broth) were similar. In contrast, Ty2(pQT28) (positive control) grown under Vi-inducing conditions (SOB broth) exhibited a 10-fold increased peak fluorescence intensity compared to that of QT74(pQT28) (Fig. 4B). These data were consistent with previous reports that OmpR is a positive regulator required for viaB expression (35).
Expression of the Vi capsule is induced after invasion of the bovine intestinal epithelium.
Vi-suppressing conditions (300 mM NaCl) encountered in the intestinal lumen (13, 30, 45) are difficult to mimic using tissue culture models, which rely on the use of tissue culture media containing 150 mM NaCl (Fig. 3). To further study whether Vi expression is affected by the transition from the intestinal lumen (300 mM NaCl) into tissue (150 mM NaCl), we wanted to use an in vivo model suited for investigating the interaction of bacteria with intestinal tissue within hours after infection. Although cattle are resistant to oral infection, the bovine ligated ileal loop model has been implemented successfully to study early events during the interaction of S. Typhi with the intestinal mucosa (39, 40). In order to study the expression of the Vi antigen in vivo, we inoculated calf ligated ileal loops with S. Typhi strain Ty2 grown under high-osmolarity conditions (300 mM NaCl containing LB medium). Loops inoculated with the ompR mutant (QT74) served as a control, lacking expression of the Vi capsule while carrying an intact tviABCDE vexABCDE operon. To illustrate Vi expression, bacterial gene expression for each loop inoculated with the S. Typhi wild type (Ty2) was expressed as log fold increase over expression in the S. Typhi ompR mutant (QT74), collected at the same time point.
We performed real-time PCR analysis to investigate the expression of the Vi antigen by monitoring expression of the tviB gene. In addition, expression of fliC and invasion genes (prgH and iagA) was assessed in calf ileal tissue following invasion at 2 and 8 hours after infection. At 2 and 8 h, expression of tviB among intracellular bacteria within calf tissue was significantly higher than that in the inoculum, with P values of 0.001 and 0.013, respectively (Fig. 5). The level of tviB expression was also higher at 8 h than at 2 h, although this difference was not statistically significant (P = 0.06). Although no statistical significance was obtained, there was a trend that fliC, prgH, and iagA were downregulated in tissue at 2 h and 8 h compared to expression levels detected in the ompR mutant.
FIG. 5.
Expression of tviB is induced during invasion of the bovine ileal mucosa. Profile of bacterial gene expression in the inoculum and in bovine ileal tissue infected with S. Typhi Ty2 determined by real-time PCR at 2 and 8 hours postinfection. Data are expressed as increases in bacterial mRNA levels relative to loops infected with the S. Typhi ompR mutant at the same time point. Bars represent geometric means ± standard errors. These data are the average results from four different bovids.
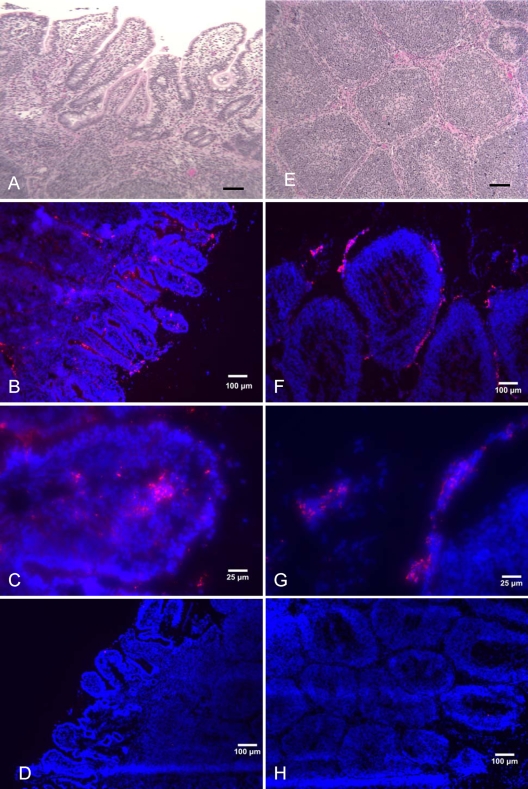
To directly detect Vi production within tissue, frozen sections of calf intestinal tissue collected at 2 h and 8 h after infection were labeled with primary anti-Vi antibody and secondary anti-rabbit goat antibody conjugated to Alexa Fluor 594 (orange-red fluorescence), and tissue was counterstained with DAPI nuclear stain (blue fluorescence). As a negative control, tissue collected at corresponding time points from loops inoculated with sterile medium (mock infection) was labeled by the same procedure. No Vi antigen production was detected in the mock-infected ileal tissue (Fig. 6D and H). Ileal loops infected with S. Typhi strain Ty2 revealed Vi-expressing bacteria along the intestinal villus tips 2 h after infection, with bacterial penetration of the intestinal epithelial barrier and entry into the lamina propria (Fig. 6B and C). Fluorescent staining of bovine Peyer's patch sections revealed large numbers of Vi-expressing bacteria in the mantle and in the area between germinal centers (Fig. 6F and G). At 8 h, our findings were similar, but the numbers of invasive bacteria expressing the capsule within the tissue were elevated. These findings were consistent with the concept that production of the Vi antigen occurs during bacterial passage through the intestinal epithelium.
FIG. 6.
Fluorescence immunohistochemistry detection of Vi antigen expression in bovine ileal tissue. Frozen calf ileal tissue in OCT medium was sectioned at 10 μm. Sections of villous intestine (A to D) and Peyer's patches (E to H) were stained with hematoxylin and eosin (H&E) (A and B) or with rabbit anti-Vi antiserum, goat anti-rabbit Alex Fluor 549 conjugate (orange-red fluorescence), and DAPI (blue fluorescence) (B, C, D, F, G, and H). Slides were viewed, and images were taken with an Olympus IX-70 at a magnification of ×100 (A, B, D, E, F, and H) or ×400 (C and G). Tissue was collected 2 h after S. Typhi infection (A, B, C, E, F, and G) or after mock infection (D and H).
The histopathology of the same sections of ileum and Peyer's patches (Fig. 6A and E, respectively) revealed moderate-to-severe changes in the epithelium infrastructure characterized by epithelial detachment, blunting, and erosion, which agrees with previous studies that demonstrate the induction of invasion genes and the destruction of the intestinal epithelium with S. Typhi under high-osmolarity growth conditions (12, 51). Only mild increases in neutrophilic counts were detected in the lamina propria and submucosa of the ileum, whereas the numbers of macrophages were markedly increased (data not shown). Sections of bovine Peyer's patches illustrated mild-to-moderate increases in neutrophil counts and markedly increased numbers of mononuclear cells (data not shown). The lack of infiltrating neutrophils and the presence of a predominate mononuclear cell infiltrate within the intestine are characteristic of typhoid fever infections (24, 32, 33).
DISCUSSION
The expression of the Vi capsular antigen in S. Typhi infection presents a possible explanation for the different clinical manifestation of typhoid fever compared to that of patients infected with nontyphoidal Salmonella serotypes. There are two major hypotheses on the regulation of Vi expression in the intestinal environment. One theory suggests that the Vi antigen is produced in the intestinal lumen and may inhibit bacterial adhesion and invasion of intestinal epithelia (2, 31). The second theory suggests that Vi is suppressed in the lumen and activated following invasion to allow the organism to evade host innate immunity (37, 39). To further test these hypotheses, we investigated the regulation of Vi antigen expression using relevant in vivo and in vitro models.
The infection of T84 polarized cells with S. Typhi grown under Vi-suppressing conditions and analyzed by flow cytometry and real-time PCR demonstrated that the Vi antigen was expressed at higher levels by cell-associated bacteria. These results, along with previous findings that a capsulated S. Typhi has a significant reduction in the amount of inflammatory cytokine secretion compared to that of a noncapsulated mutant (19, 37), support the view that the Vi antigen is activated following invasion of intestinal epithelial cells. Consistent with this concept, our analysis of T3SS-1 gene expression with real-time PCR indicated increased transcription in extracellular bacteria and a downregulation of these genes by cell-associated bacteria, suggesting that invasion gene and Vi capsule expression are regulated oppositely.
Studies of the regulatory mechanisms responsible for Vi capsule expression in culture media have revealed that expression of the biosynthesis genes is regulated by osmolarity (2). When S. Typhi is grown in LBH (Vi-suppressing medium), Vi expression is repressed, whereas the invasion-associated genes are highly expressed (2). Since the intestinal lumen is thought to be hyperosmotic, we wanted to study this aspect in vivo. We utilized the calf ligated ileal loop model, which has been used successfully to conduct S. Typhi pathogenesis studies (39, 40), to examine Vi expression among extracellular bacteria in the lumen and intracellularly within ileal tissue following invasion. We directly detected Vi production in the bovine intestinal mucosa by using anti-Vi antigen antibodies and labeling the antibodies with an orange-red fluorophore. We observed Vi-expressing bacteria during the initial stages of penetration of the intestinal epithelium, as well as following invasion in the areas of the intestinal villi and Peyer's patches. The Vi capsule is expressed inside macrophages in vitro (7) and during human infection, as indicated by the detection of anti-Vi antibodies in patient serum samples and the fact that vaccinations with the Vi antigen confer protection against typhoid fever (22, 23, 27, 42). Interestingly the Vi antigen was detected in the dendritic cell-rich areas between germinal centers.
In conclusion, we demonstrate that Vi expression is detected in S. Typhi during invasion of model epithelia in vitro and upon entry into the bovine intestinal mucosa in vivo.
Acknowledgments
Work in the laboratories of A.J.B. and L.G.A. was supported by Public Health Service grants AI040124, AI044170, AI079173, and AI076246.
Editor: F. C. Fang
Footnotes
Published ahead of print on 9 November 2009.
REFERENCES
- 1.Alves, G. E. S., S. M. Hartsfield, G. L. Carroll, S. Zhang, R. M. Tsolis, A. J. Baumler, and L. G. Adams. 2003. Use of propofol, isoflurane and morphine for prolonged anesthesia in calves. Arq. Bras. Med. Vet. Zoo. 55:411-420. [Google Scholar]
- 2.Arricau, N., D. Hermant, H. Waxin, C. Ecobichon, P. S. Duffey, and M. Y. Popoff. 1998. The RcsB-RcsC regulatory system of Salmonella typhi differentially modulates the expression of invasion proteins, flagellin and Vi antigen in response to osmolarity. Mol. Microbiol. 29:835-850. [DOI] [PubMed] [Google Scholar]
- 3.Beuzon, C. R., K. E. Unsworth, and D. W. Holden. 2001. In vivo genetic analysis indicates that PhoP-PhoQ and the Salmonella pathogenicity island 2 type III secretion system contribute independently to Salmonella enterica serovar Typhimurium virulence. Infect. Immun. 69:7254-7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bongaerts, R. J., I. Hautefort, J. M. Sidebotham, and J. C. Hinton. 2002. Green fluorescent protein as a marker for conditional gene expression in bacterial cells. Methods Enzymol. 358:43-66. [DOI] [PubMed] [Google Scholar]
- 5.Bumann, D. 2002. Examination of Salmonella gene expression in an infected mammalian host using the green fluorescent protein and two-colour flow cytometry. Mol. Microbiol. 43:1269-1283. [DOI] [PubMed] [Google Scholar]
- 6.Crump, J. A., S. P. Luby, and E. D. Mintz. 2004. The global burden of typhoid fever. Bull. World Health Organ. 82:346-353. [PMC free article] [PubMed] [Google Scholar]
- 7.Daigle, F., J. E. Graham, and R. Curtiss III. 2001. Identification of Salmonella typhi genes expressed within macrophages by selective capture of transcribed sequences (SCOTS). Mol. Microbiol. 41:1211-1222. [DOI] [PubMed] [Google Scholar]
- 8.Eriksson, S., S. Lucchini, A. Thompson, M. Rhen, and J. C. Hinton. 2003. Unravelling the biology of macrophage infection by gene expression profiling of intracellular Salmonella enterica. Mol. Microbiol. 47:103-118. [DOI] [PubMed] [Google Scholar]
- 9.Faucher, S. P., S. Porwollik, C. M. Dozois, M. McClelland, and F. Daigle. 2006. Transcriptome of Salmonella enterica serovar Typhi within macrophages revealed through the selective capture of transcribed sequences. Proc. Natl. Acad. Sci. U. S. A. 103:1906-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finlay, B. B. 1994. Molecular and cellular mechanisms of Salmonella pathogenesis. Curr. Top. Microbiol. Immunol. 192:163-185. [DOI] [PubMed] [Google Scholar]
- 11.Galan, J. E. 2001. Salmonella interactions with host cells: type III secretion at work. Annu. Rev. Cell Dev. Biol. 17:53-86. [DOI] [PubMed] [Google Scholar]
- 12.Galan, J. E., and R. Curtiss III. 1989. Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc. Natl. Acad. Sci. U. S. A. 86:6383-6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta, S., and R. Chowdhury. 1997. Bile affects production of virulence factors and motility of Vibrio cholerae. Infect. Immun. 65:1131-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hansen-Wester, I., and M. Hensel. 2001. Salmonella pathogenicity islands encoding type III secretion systems. Microbes Infect. 3:549-559. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto, Y., A. Q. Khan, and T. Ezaki. 1996. Positive autoregulation of vipR expression in ViaB region-encoded Vi antigen of Salmonella typhi. J. Bacteriol. 178:1430-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hashimoto, Y., N. Li, H. Yokoyama, and T. Ezaki. 1993. Complete nucleotide sequence and molecular characterization of ViaB region encoding Vi antigen in Salmonella typhi. J. Bacteriol. 175:4456-4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hautefort, I., A. Thompson, S. Eriksson-Ygberg, M. L. Parker, S. Lucchini, V. Danino, R. J. Bongaerts, N. Ahmad, M. Rhen, and J. C. Hinton. 2008. During infection of epithelial cells Salmonella enterica serovar Typhimurium undergoes a time-dependent transcriptional adaptation that results in simultaneous expression of three type 3 secretion systems. Cell. Microbiol. 10:958-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heyns, K., and G. Kiessling. 1967. Strukturafklarung des Vi-antigens aus Citrobacter freundii (E. coli) 5396/38. Carbohydr. Res. 3:340-353. [Google Scholar]
- 19.Hirose, K., T. Ezaki, M. Miyake, T. Li, A. Q. Khan, Y. Kawamura, H. Yokoyama, and T. Takami. 1997. Survival of Vi-capsulated and Vi-deleted Salmonella typhi strains in cultured macrophage expressing different levels of CD14 antigen. FEMS Microbiol. Lett. 147:259-265. [DOI] [PubMed] [Google Scholar]
- 20.Hone, D. M., S. R. Attridge, B. Forrest, R. Morona, D. Daniels, J. T. LaBrooy, R. C. Bartholomeusz, D. J. Shearman, and J. Hackett. 1988. A galE via (Vi antigen-negative) mutant of Salmonella typhi Ty2 retains virulence in humans. Infect. Immun. 56:1326-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hornick, R. B., S. E. Greisman, T. E. Woodward, H. L. DuPont, A. T. Dawkins, and M. J. Snyder. 1970. Typhoid fever: pathogenesis and immunologic control. N. Engl. J. Med. 283:686-691. [DOI] [PubMed] [Google Scholar]
- 22.Klugman, K. P., I. T. Gilbertson, H. J. Koornhof, J. B. Robbins, R. Schneerson, D. Schulz, M. Cadoz, and J. Armand. 1987. Protective activity of Vi capsular polysaccharide vaccine against typhoid fever. Lancet ii:1165-1169. [DOI] [PubMed] [Google Scholar]
- 23.Klugman, K. P., H. J. Koornhof, J. B. Robbins, and N. N. Le Cam. 1996. Immunogenicity, efficacy and serological correlate of protection of Salmonella typhi Vi capsular polysaccharide vaccine three years after immunization. Vaccine 14:435-438. [DOI] [PubMed] [Google Scholar]
- 24.Kraus, M. D., B. Amatya, and Y. Kimula. 1999. Histopathology of typhoid enteritis: morphologic and immunophenotypic findings. Mod. Pathol. 12:949-955. [PubMed] [Google Scholar]
- 25.Lee, S. H., and A. Camilli. 2000. Novel approaches to monitor bacterial gene expression in infected tissue and host. Curr. Opin. Microbiol. 3:97-101. [DOI] [PubMed] [Google Scholar]
- 26.Lee, S. H., D. L. Hava, M. K. Waldor, and A. Camilli. 1999. Regulation and temporal expression patterns of Vibrio cholerae virulence genes during infection. Cell 99:625-634. [DOI] [PubMed] [Google Scholar]
- 27.Lin, F. Y., V. A. Ho, H. B. Khiem, D. D. Trach, P. V. Bay, T. C. Thanh, Z. Kossaczka, D. A. Bryla, J. Shiloach, J. B. Robbins, R. Schneerson, and S. C. Szu. 2001. The efficacy of a Salmonella typhi Vi conjugate vaccine in two-to-five-year-old children. N. Engl. J. Med. 344:1263-1269. [DOI] [PubMed] [Google Scholar]
- 28.McCormick, B. A., A. M. Siber, and A. T. Maurelli. 1998. Requirement of the Shigella flexneri virulence plasmid in the ability to induce trafficking of neutrophils across polarized monolayers of the intestinal epithelium. Infect. Immun. 66:4237-4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mishra, A., R. Srivastava, C. Pruzzo, and B. S. Srivastava. 2003. Mutation in tcpR gene (Vc0832) of Vibrio cholerae O1 causes loss of tolerance to high osmolarity and affects colonization and virulence in infant mice. J. Med. Microbiol. 52:933-939. [DOI] [PubMed] [Google Scholar]
- 31.Miyake, M., L. Zhao, T. Ezaki, K. Hirose, A. Q. Khan, Y. Kawamura, R. Shima, M. Kamijo, T. Masuzawa, and Y. Yanagihara. 1998. Vi-deficient and nonfimbriated mutants of Salmonella typhi agglutinate human blood type antigens and are hyperinvasive. FEMS Microbiol. Lett. 161:75-82. [DOI] [PubMed] [Google Scholar]
- 32.Mukawi, T. J. 1978. Histopathological study of typhoid perforation of the small intestines. Southeast Asian J. Trop. Med. Public Health 9:252-255. [PubMed] [Google Scholar]
- 33.Nguyen, Q. C., P. Everest, T. K. Tran, D. House, S. Murch, C. Parry, P. Connerton, V. B. Phan, S. D. To, P. Mastroeni, N. J. White, T. H. Tran, V. H. Vo, G. Dougan, J. J. Farrar, and J. Wain. 2004. A clinical, microbiological, and pathological study of intestinal perforation associated with typhoid fever. Clin. Infect. Dis. 39:61-67. [DOI] [PubMed] [Google Scholar]
- 34.Parkhill, J., G. Dougan, K. D. James, N. R. Thomson, D. Pickard, J. Wain, C. Churcher, K. L. Mungall, S. D. Bentley, M. T. Holden, M. Sebaihia, S. Baker, D. Basham, K. Brooks, T. Chillingworth, P. Connerton, A. Cronin, P. Davis, R. M. Davies, L. Dowd, N. White, J. Farrar, T. Feltwell, N. Hamlin, A. Haque, T. T. Hien, S. Holroyd, K. Jagels, A. Krogh, T. S. Larsen, S. Leather, S. Moule, P. O'Gaora, C. Parry, M. Quail, K. Rutherford, M. Simmonds, J. Skelton, K. Stevens, S. Whitehead, and B. G. Barrell. 2001. Complete genome sequence of a multiple drug resistant Salmonella enterica serovar Typhi CT18. Nature 413:848-852. [DOI] [PubMed] [Google Scholar]
- 35.Pickard, D., J. Li, M. Roberts, D. Maskell, D. Hone, M. Levine, G. Dougan, and S. Chatfield. 1994. Characterization of defined ompR mutants of Salmonella typhi: ompR is involved in the regulation of Vi polysaccharide expression. Infect. Immun. 62:3984-3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puente, J. L., A. Verdugo-Rodriguez, and E. Calva. 1991. Expression of Salmonella typhi and Escherichia coli OmpC is influenced differently by medium osmolarity; dependence on Escherichia coli OmpR. Mol. Microbiol. 5:1205-1210. [DOI] [PubMed] [Google Scholar]
- 37.Raffatellu, M., D. Chessa, R. P. Wilson, R. Dusold, S. Rubino, and A. J. Bäumler. 2005. The Vi capsular antigen of Salmonella enterica serotype Typhi reduces Toll-like receptor-dependent interleukin-8 expression in the intestinal mucosa. Infect. Immun. 73:3367-3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raffatellu, M., D. Chessa, R. P. Wilson, C. Tukel, M. Akcelik, and A. J. Bäumler. 2006. Capsule-mediated immune evasion: a new hypothesis explaining aspects of typhoid fever pathogenesis. Infect. Immun. 74:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raffatellu, M., R. L. Santos, D. Chessa, R. P. Wilson, S. E. Winter, C. A. Rossetti, S. D. Lawhon, H. Chu, T. Lau, C. L. Bevins, L. G. Adams, and A. J. Bäumler. 2007. The capsule encoding the viaB locus reduces interleukin-17 expression and mucosal innate responses in the bovine intestinal mucosa during infection with Salmonella enterica serotype Typhi. Infect. Immun. 75:4342-4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raffatellu, M., Y. H. Sun, R. P. Wilson, Q. T. Tran, D. Chessa, H. L. Andrews-Polymenis, S. D. Lawhon, J. F. Figueiredo, R. M. Tsolis, L. G. Adams, and A. J. Bäumler. 2005. Host restriction of Salmonella enterica serotype Typhi is not caused by functional alteration of SipA, SopB, or SopD. Infect. Immun. 73:7817-7826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raffatellu, M., R. P. Wilson, D. Chessa, H. Andrews-Polymenis, Q. T. Tran, S. Lawhon, S. Khare, L. G. Adams, and A. J. Bäumler. 2005. SipA, SopA, SopB, SopD, and SopE2 contribute to Salmonella enterica serotype typhimurium invasion of epithelial cells. Infect. Immun. 73:146-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robbins, J. D., and J. B. Robbins. 1984. Reexamination of the protective role of the capsular polysaccharide (Vi antigen) of Salmonella typhi. J. Infect. Dis. 150:436-449. [DOI] [PubMed] [Google Scholar]
- 43.Santander, J., S. Y. Wanda, C. A. Nickerson, and R. Curtiss III. 2007. Role of RpoS in fine-tuning the synthesis of Vi capsular polysaccharide in Salmonella enterica serotype Typhi. Infect. Immun. 75:1382-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tacket, C. O., G. Losonsky, D. N. Taylor, L. S. Baron, D. Kopecko, S. Cryz, and M. M. Levine. 1991. Lack of immune response to the Vi component of a Vi-positive variant of the Salmonella typhi live oral vaccine strain Ty21a in human studies. J. Infect. Dis. 163:901-904. [DOI] [PubMed] [Google Scholar]
- 45.Tartera, C., and E. S. Metcalf. 1993. Osmolarity and growth phase overlap in regulation of Salmonella typhi adherence to and invasion of human intestinal cells. Infect. Immun. 61:3084-3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valdivia, R. H., and S. Falkow. 1996. Bacterial genetics by flow cytometry: rapid isolation of Salmonella typhimurium acid-inducible promoters by differential fluorescence induction. Mol. Microbiol. 22:367-378. [DOI] [PubMed] [Google Scholar]
- 47.Valdivia, R. H., A. E. Hromockyj, D. Monack, L. Ramakrishnan, and S. Falkow. 1996. Applications for green fluorescent protein (GFP) in the study of host-pathogen interactions. Gene 173:47-52. [DOI] [PubMed] [Google Scholar]
- 48.Reference deleted.
- 49.Virlogeux, I., H. Waxin, C. Ecobichon, J. O. Lee, and M. Y. Popoff. 1996. Characterization of the rcsA and rcsB genes from Salmonella typhi: rcsB through tviA is involved in regulation of Vi antigen synthesis. J. Bacteriol. 178:1691-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Virlogeux, I., H. Waxin, C. Ecobichon, and M. Y. Popoff. 1995. Role of the viaB locus in synthesis, transport and expression of Salmonella typhi Vi antigen. Microbiology 141(Pt. 12):3039-3047. [DOI] [PubMed] [Google Scholar]
- 51.Zhao, L., T. Ezak, Z. Y. Li, Y. Kawamura, K. Hirose, and H. Watanabe. 2001. Vi-suppressed wild strain Salmonella typhi cultured in high osmolarity is hyperinvasive toward epithelial cells and destructive of Peyer's patches. Microbiol. Immunol. 45:149-158. [DOI] [PubMed] [Google Scholar]