Abstract
Vibrio cholerae O1 can cause severe watery diarrhea that can be life-threatening without treatment. Infection results in long-lasting protection against subsequent disease. Development of memory B cells of the immunoglobulin G (IgG) and IgA isotypes to V. cholerae O1 antigens, including serotype-specific lipopolysaccharide (LPS) and the B subunit of cholera toxin (CTB), after cholera infection has been demonstrated. Memory B cells of the IgM isotype may play a role in long-term protection, particularly against T-cell-independent antigens, but IgM memory has not been studied in V. cholerae O1 infection. Therefore, we assayed acute- and convalescent-phase blood samples from cholera patients for the presence of memory B cells that produce cholera antigen-specific IgM antibody upon polyclonal stimulation in in vitro culture. We also examined the development of serological and antibody-secreting cell responses following infection. Subjects developed significant IgM memory responses by day 30 after infection, both to the T-cell-independent antigen LPS and to the T-cell-dependent antigen CTB. No significant corresponding elevations in plasma IgM antibodies or circulating IgM antibody-secreting cells to CTB were detected. In 17 subjects followed to day 90 after infection, significant persistence of elevated IgM memory responses was not observed. The IgM memory response to CTB was negatively correlated with the IgG plasma antibody response to CTB, and there was a trend toward negative correlation between the IgM memory and IgA plasma antibody responses to LPS. We did not observe an association between the IgM memory response to LPS and the vibriocidal titer.
Vibrio cholerae continues to be a significant global health burden as a cause of severe secretory diarrhea, resulting in an estimated three to five million annual cases, with more than 100,000 deaths from rapid dehydration (47); cholera has recently become endemic in new regions (44, 45). V. cholerae is a noninvasive pathogen that colonizes the mucosal surface of the small intestine. Strains can be distinguished serologically by the O antigen of the lipopolysaccharide (LPS); V. cholerae O1 is the most common cause of cholera in South Asia as well as globally. The O1 serogroup has two major biotypes, El Tor and classical, and two major serotypes, Inaba and Ogawa (35). Natural infection with V. cholerae O1 El Tor induces protective immunity that lasts for at least 3 to 10 years in both areas where cholera is not endemic and areas where it is endemic (21). It remains unknown, however, what aspects of the adaptive immune response to cholera confer this long-term protection.
V. cholerae-infected patients mount immunologic responses to both protein and polysaccharide antigens, including rises in both serum immunoglobulin G (IgG) and IgA antibodies (14). A number of these serological responses have been shown to correlate with protection against reinfection; these include the complement-dependent serum vibriocidal antibody (14) and IgA (but not IgG) responses to LPS, cholera toxin B subunit (CTB), and toxin coregulated pilus A (TcpA) (17). These serological responses, however, are short-lived (4, 32), and the association of the vibriocidal titer with protection is not absolute (36), suggesting that these responses may reflect protection from more recent exposure but that other immunologic mechanisms mediate longer-term protection. In addition to serological responses, development of mucosal immune responses to intestinal antigens can be detected in the blood, when B cells activated by antigen in the gut-associated lymphoid tissues circulate transiently in the blood as antibody-secreting cells (ASCs), before homing back to intestinal mucosal surfaces (11, 26). Circulation of ASCs specific to both LPS and CTB is seen after cholera infection, peaking around the seventh day after infection and declining by day 11 (32).
Responses of the IgM isotype to cholera antigens have been less thoroughly investigated than the IgG and IgA responses. However, IgM defenses may be an important component of the overall immunologic response to cholera, since vibriocidal antibodies are principally of the IgM isotype (22) and IgM levels of pooled convalescent-phase serum samples correspond closely with vibriocidal activity (24), which in turn correlates with immunity (14). The pentameric structure of IgM facilitates strong cross-linking of antigens and activation of complement in the defense against other gram-negative enteric bacteria (2).
We have recently shown development of memory B cells of both the IgG and IgA isotypes to LPS, CTB, and TcpA; these cells persisted in the circulation beyond 1 year for the protein antigens CTB and TcpA, but were not measurably above baseline levels by 9 to 12 months after infection for the polysaccharide-containing antigen LPS (16, 18). These circulating memory B cells can be detected by ex vivo polyclonal stimulation of peripheral blood mononuclear cells (PBMCs); stimulated memory B cells mature into ASCs detectable by enzyme-linked immunospot (ELISPOT) assay. Alternatively, memory B-cell responses can be detected by measuring antigen-specific antibodies secreted by maturing ASCs during the ex vivo stimulation of PBMCs in the memory B-cell assay (18).
Memory B cells relevant for cholera immunity may include IgM+ as well as switched-memory (IgA+ and IgG+) populations. The majority of circulating IgM+ cells are naïve B cells, but some IgM+ cells bear the memory cell marker CD27+, and recent evidence suggests that these IgM+CD27+ cells are true memory B cells whose immunoglobulin variable region genes have undergone somatic hypermutation in response to antigen in early-stage germinal centers (39). IgM+ memory cells can undergo isotype switching to produce IgG, IgA, or IgE antibody, but they also have a role in producing rapid, high-affinity IgM antibody responses to acute infection (19, 37, 46). In this study, we have measured the development of memory B-cell responses of the IgA, IgG, and IgM isotypes to both a protein (CTB) and a nonprotein (LPS) antigen, and we compared these memory responses with other immunologic responses in patients after V. cholerae infection in Bangladesh.
MATERIALS AND METHODS
Study subjects and overview.
Study participants were selected from patients admitted to the International Centre for Diarrheal Disease Research in Dhaka, Bangladesh (ICDDR,B), with severe acute watery diarrhea and stool cultures positive for Vibrio cholerae O1 between April 2007 and April 2009. All were treated with intravenous fluids and with azithromycin. Acute-phase blood samples were obtained from subjects on the second day of hospitalization, and blood samples were obtained again 1 week, 1 month, and, for a subset of patients, 3 months following onset of cholera. We assayed these samples for vibriocidal antibodies, for plasma antibodies of IgA, IgG, and IgM isotypes to the V. cholerae O1 serotype-specific LPS (Inaba or Ogawa) and to CTB, and when possible, for circulating ASCs for these same antigens and isotypes, as described below. PBMCs were cultured in preparation for memory B-cell assays on study days 2, 30, and 90, as described below. For a small sample of patients, additional PBMCs at study days 2 and 30 were used for flow cytometric analysis of memory B-cell isotypes. For 26 subjects enrolled prior to the beginning of this analysis of IgM memory, IgM assays were done on frozen plasma and culture supernatants; for six of the patients enrolled prospectively, both assays on frozen samples and assays on fresh cells were completed; and for an additional nine prospectively enrolled patients, ASC and flow cytometric assays on fresh, uncultured cells were performed. All subjects provided written informed consent, and the institutional review boards of the ICDDR,B and Massachusetts General Hospital approved the study.
Bacteriological examination of patient stool samples.
Cases were confirmed by culturing stool samples onto taurocholate-tellurite-gelatin plates. After overnight incubation of the plates, suspected V. cholerae colonies were serologically confirmed by slide agglutination with specific monoclonal antibody for the Ogawa or Inaba serotype (28, 33).
PBMC isolation.
Plasma and PBMCs were isolated by centrifugation of diluted whole-blood samples on Ficoll-Isopaque (Pharmacia, Piscataway, NJ); plasma was stored at −70°C for immunological assays, and PBMCs were resuspended in RPMI complete medium containing 10% heat-inactivated fetal bovine serum (FBS) (18). Resuspended cells were placed in appropriate culture media for the memory B-cell assay or used immediately for detection of ASCs by ELISPOT assay and for flow cytometric analysis, as described below.
Serum vibriocidal assays.
Vibriocidal antibody assays were performed as previously described, using guinea pig complement and the homologous serotype of V. cholerae O1 Ogawa (X-25049) or Inaba (T19479) as the target organism (32). The vibriocidal titer was defined as the reciprocal of the highest dilution resulting in a >50% reduction of the optical density compared to that of control wells without serum.
ELISAs for LPS- and CTB-specific IgA, IgG, and IgM antibodies in plasma.
LPS- and CTB-specific IgA, IgG, and IgM responses in plasma were detected using standardized enzyme-linked immunosorbent assay (ELISA) protocols (27, 32). For this purpose, 96-well polystyrene plates (Nunc F) were coated with 2.5 μg/ml LPS or with 0.3 nmol/ml ganglioside GM1, followed by 0.5 μg/ml recombinant CTB. For each antigen, 100 μl/well of plasma, diluted 100 times for CTB and 25 times for LPS in 0.1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS)-Tween, was added and incubated for 90 min at 37°C. Horseradish peroxidase (HRP)-conjugated secondary antibodies, rabbit anti-human IgA or IgG (Jackson ImmunoResearch, West Grove, PA) or goat anti-human IgM (Southern Biotech, Birmingham, AL), were then applied. After incubation for 90 min at 37°C, the plates were washed and developed with ortho-phenylene diamine (Sigma, St. Louis, MO) in 0.1 M sodium citrate buffer (pH 4.5) and 0.015% hydrogen peroxide. Plates were read kinetically at 450 nm for 5 minutes. The maximal rate of change in optical density in milliabsorbance units per minute was normalized across plates by calculating the ratio of the test sample to a standard of pooled convalescent-phase serum from previously infected cholera patients, which was added as a positive control onto each plate, and the results were expressed as ELISA units.
Determination of ASCs by ELISPOT assay.
An ASC assay was performed as previously described (16, 32). Briefly, nitrocellulose-bottomed plates were coated with GM1 ganglioside (3 nmol/ml) followed by recombinant CTB (2.5 μg/ml), or with LPS (25 μg/ml), keyhole limpet hemocyanin (2.5 μg/ml; used as a negative control), or affinity-purified goat anti-human immunoglobulin (total Ig) (Jackson; 5 μg/ml). After blocking with RPMI complete medium, freshly isolated PBMCs were added at appropriate dilutions and incubated for 3 hours at 37°C in a 5% CO2 incubator. Plates were washed and stained using HRP-conjugated or alkaline phosphatase-conjugated goat (Southern) or mouse (Hybridoma Reagent Laboratory, Baltimore, MD) anti-human IgA, IgG, and IgM antibodies and then developed with 3-amino-9-ethyl carbazole or 5-bromo-4-chloro-3-indolyl phosphate-nitro-blue-tetrazolium (Sigma). The number of antigen-specific ASCs was expressed as the percentage of total circulating ASCs of the same antibody isotype.
Memory B-cell culture.
Memory B-cell assays were performed on days 2, 30, and 90 based on previously described methods (5, 6, 16, 18). PBMCs were cultured in a medium optimized to stimulate antigen-independent proliferation and differentiation of memory B cells into ASCs; this medium consisted of RPMI-1640, 10% fetal bovine serum, 200 units/ml penicillin, 200 μg/ml streptomycin, 2 mM l-glutamine, 50 μM β-mercaptoethanol, and a mixture of three B-cell mitogens—6 μg/ml of CpG oligonucleotide (Operon, Huntsville, AL), a 1/100,000 dilution of crude pokeweed mitogen extract, and a 1/10,000 dilution of fixed Staphylococcus aureus Cowan (Sigma, St. Louis, MO). One half million PBMCs per well were placed in 24-well cell culture plates (BD Biosciences, San Jose, CA) containing this medium; as an “unstimulated” negative control, PBMCs were also placed into wells containing this culture medium without mitogens. Plates were incubated at 37°C in a 5% CO2 incubator.
Supernatant collection and ELISPOT assays.
After 5 to 6 days of culture, contents of culture wells were collected and centrifuged. All stimulated or all unstimulated culture supernatants for a given patient day were pooled together, mixed with a protease inhibitor cocktail (18), and frozen at −70°C for immunological assays. Cells retrieved from stimulated and unstimulated culture wells were washed and added to individual ELISPOT plates for detection of total IgA, IgG, or IgM ASCs and antigen-specific ASCs of the IgA and IgG isotypes, as described above for circulating ASCs except for 5 to 6 h of incubation of cells on coated plates. For a subset of patients, some cells were also harvested after only 3 days of in vitro culture, and ELISPOT assays for total IgA, IgG, and IgM ASCs were performed with these stimulated and unstimulated cells.
ELISAs for total and LPS- and CTB-specific IgA, IgG, and IgM antibodies in supernatants of memory B-cell cultures.
Supernatants of unstimulated and stimulated cultures of PBMCs collected after 5 to 6 days were diluted twice in 0.1% BSA in PBS-Tween and assayed for LPS- and CTB-specific IgA, IgG, and IgM responses as described above for plasma. Supernatants were also assayed for total immunoglobulin. Total immunoglobulin wells were coated with affinity-purified goat anti-human IgG (for IgA and IgG ELISAs) or anti-IgM (for IgM ELISAs) immunoglobulin fragments (Jackson). HRP-conjugated secondary antibodies were added, and plates were developed with ortho-phenylene diamine, quenched after 20 min, and read at 492 nm. The concentrations of total immunoglobulin in culture supernatant, in μg/ml, were determined by comparison with serial dilutions of one of the following references added to the same plate: pooled colostrum of known IgA concentration (31), ChromPure human IgG (Jackson), or human IgM myeloma proteins (Jackson).
Flow cytometric analysis of the surface immunoglobulin isotypes of memory B cells.
PBMCs isolated on study days 2 and 30 (3 × 105 PBMCs per test) were stained in two steps at 4°C: 20 min with anti-IgD biotin (Becton Dickinson [BD], Mountain View, CA), followed by 40 min at 4°C with a mixture of anti-CD19 peridinin chlorophyll protein (BioLegend, San Diego, CA), anti-CD27 phycoerythrin (BD), streptavidin-allophycocyanin (BD), and either anti-IgA (Caltag Laboratories, Burlingame, CA), anti-IgG (BD), or anti-IgM (BD) fluorescein isothiocyanate, diluted to optimal concentrations in a buffer containing 0.1% sodium azide, 0.2% BSA, and 0.04% disodium EDTA in PBS. Cells were suspended in 2% paraformaldehyde and stored in the dark. Within 24 h, four-color data acquisition was performed with a FACSCalibur instrument (BD) by using CellQuest Pro (BD). Data were analyzed using FlowJo (Treestar, Ashland, OR) by gating on forward versus side scatter for lymphocytes, followed by gating of lymphocytes into four quadrants based on CD19 and CD27 expression, and then finally by gating of the CD19+CD27+ cells into quadrants based on the expression of IgD on one axis and that of either IgA, IgG, or IgM as appropriate on the orthogonal axis; we used negative controls to verify the placement of gating axes. Isotype fractions were calculated as percentages of the sum total of detected IgA+, IgG+, and IgM+ CD19+CD27+ cells.
Data and statistical analyses.
Antigen-specific antibody responses produced by mitogen-stimulated memory B cells were determined for LPS and CTB for each of the IgA, IgG, and IgM isotypes by dividing the difference in antigen-specific ELISA results between stimulated and unstimulated supernatant pairs by the corresponding difference in total immunoglobulin. Time points with a <20% increase in total immunoglobulin between unstimulated and stimulated culture supernatants (representing a difference falling within the typical standard deviation of our total immunoglobulin ELISA measurements) were excluded from analysis. Negative differences in antigen-specific ELISA results were treated as zero. Memory B-cell ELISPOT data were normalized by subtracting unstimulated ASC counts from the stimulated ASC counts and dividing antigen-specific results by the total ASC counts of that immunoglobulin isotype in an analogous way.
Serological responses, results of ELISA in culture supernatants, and ASC data at later study days were compared to the day 2 baseline by the use of Wilcoxon signed-rank tests. Correlations of serological and memory ELISPOT assay results with memory as measured by supernatant ELISA were evaluated using Spearman coefficients. Variables with log-normal distributions are presented as geometric means with 95% confidence intervals, and other nonnormally distributed data are presented as medians with interquartile ranges. All reported P values are two-sided. Analyses were performed using Intercooled Stata, version 9.1 (Stata Corporation, College Station, TX).
RESULTS
Study population.
To assess the development of immunologic memory, 26 cholera patients with at least a fourfold difference in total IgG ASC numbers between mitogen-stimulated and unstimulated cells after 6 days of culture (geometric mean, 20-fold difference at day 2 after infection, 62-fold at day 30) were selected for analyses of antibodies in previously obtained, frozen memory B-cell culture supernatant and plasma samples. An additional 15 patients were prospectively enrolled, as explained in Materials and Methods. Table 1 shows demographic and clinical characteristics of all 41 patients.
TABLE 1.
Demographic and clinical characteristics of study subjects
| Characteristic | Value |
|---|---|
| No. of subjects | |
| Days 2, 7, and 30; analysis of frozen samples only | 9 |
| Days 2, 7, 30, and 90; analysis of frozen samples only | 17 |
| Days 2, 7, 30, and 90; analysis of frozen samples and | |
| circulating ASC assays | 6 |
| Circulating ASC assays and flow cytometric | |
| analysis requiring fresh cells | 9 |
| Age (mean yr ± SD) | 30 ± 14 |
| Sex (no. of subjects) | |
| Female | 19 |
| Male | 22 |
| Vibrio cholerae O1 serotype (no.) | |
| Ogawa | 32 |
| Inaba | 9 |
| Dehydration status at enrollment (no.) | |
| Severe | 36 |
| Moderate | 5 |
| Duration of diarrhea at presentation (mean h ± SD) | 18 ± 12 |
| Duration of hospitalization (mean h ± SD) | 39 ± 24 |
Vibriocidal, serological, and ASC responses.
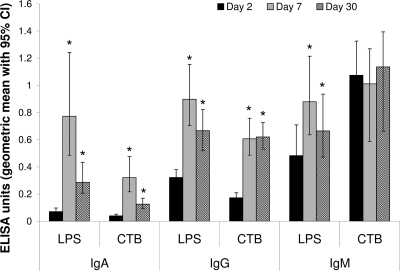
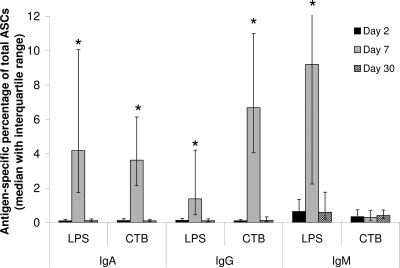
Subjects mounted strong immunologic responses to cholera infection. All 32 subjects measured had at least an eightfold increase in serum vibriocidal antibody titer for the homologous serotype at day 7, and the geometric mean of vibriocidal titer rose from 52 (95% confidence interval [CI], 29 to 92) at baseline to 3,800 (95% CI, 2,600 to 5,700; P < 0.0001) at day 7, and decreased but remained above baseline at day 30 (geometric mean, 1,400; 95% CI, 1,000 to 1,900; P < 0.0001), and for the subset of 17 patients enrolled in extended follow-up, at day 90 (geometric mean, 350; 95% CI, 170 to 720; P = 0.02). Plasma antibodies against LPS were elevated from baseline at both day 7 (P < 0.001) and day 30 (P < 0.01) for all three isotypes tested, while plasma antibodies against CTB were elevated for IgA and IgG but not IgM (Fig. 1). For cholera patients whose ASCs were assayed (n = 15), significant increases (P < 0.05) in circulating IgA and IgG ASCs were seen at day 7 for both LPS and CTB and in circulating IgM ASCs for LPS, rising to >1% of total ASCs in more than half of patients for each of these antigen-isotype combinations; cholera antigen-specific ASCs returned to baseline of <0.1% of total ASCs by day 30 (Fig. 2). No IgM ASC response to CTB was observed in these patients.
FIG. 1.
IgA, IgG, and IgM serological responses to CTB and LPS antigens in cholera patients at near baseline (day 2 after onset of illness), acute stage (day 7), and convalescence (day 30). *, Significant elevation (P < 0.002) in comparison to day 2.
FIG. 2.
Antigen-specific circulating ASCs of IgA, IgG, and IgM isotypes in cholera patients 2, 7, and 30 days after onset of illness. *, P < 0.05 in comparison to day 2; n = 15, six of whom were included in other analyses.
Memory B-cell assay results.
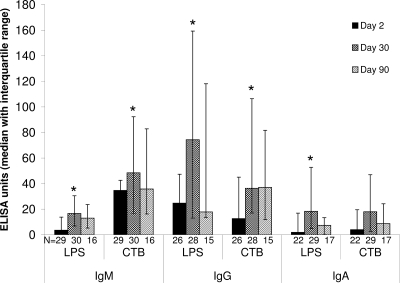
Culture supernatants from the stimulated and unstimulated memory B-cell assay were available for 32 subjects at days 2 and 30 and for 17 of those subjects at day 90. Total and antigen-specific (CTB and LPS) ELISAs were performed on all supernatants for IgM, IgG, and IgA. Two day-2 data points were excluded because of very high LPS titers in unstimulated supernatant (>50 times greater than all other unstimulated titers, and equal to 80% or more of the maximum day-2 stimulated titer); these results were found in patients who also had high numbers of circulating CTB- and LPS-specific ASCs (>2% of total ASCs) on this day of sampling and, therefore, likely reflect antibodies from circulating ASCs rather than from stimulated memory cells. Of the remaining 79 time points considered, 4, 9, and 12 were excluded for IgM, IgG, and IgA, respectively, because of inadequate stimulation as explained in Materials and Methods; the median increases for the remaining paired supernatants were 10-, 32-, and 23-fold at days 2, 30, and 90, respectively, for IgM; 2.0-, 3.7-, and 4.4-fold, respectively, for IgG; and 2.4-, 3.4-, and 3.3-fold, respectively, for IgA.
Cholera antigen-specific IgM, IgG, and IgA responses against LPS and CTB produced by mitogen stimulation in the memory B-cell assay were standardized and normalized for total immunoglobulin concentrations in the culture supernatants in stimulated cell cultures as described in Materials and Methods. For the IgM isotype, statistically significant increases in memory responses from the day-2 baseline were seen at day 30 for both LPS (P = 0.0001) and CTB (P = 0.0008) (Fig. 3). For IgG, significant increases again were seen for both LPS (P = 0.003) and CTB (P = 0.02). Within the subset of patients evaluated at day 90, only about half (38 to 58%, depending on antigen and isotype) of the patients had day-90 IgG and IgM memory responses above baseline, but 30 to 50% had elevations of >3 times above baseline, reflected by medians near baseline but with wide upper-quartile error bars in Fig. 3; these elevations in memory responses at day 90 were not statistically significant with the number of patients that could be studied. For IgA, increases in memory seen at day 30 were statistically significant only for LPS (P = 0.02) and not for CTB (P = 0.1), and responses at day 90 were not different from baseline for the patients studied.
FIG. 3.
Antigen-specific B-cell memory responses of IgA, IgG, and IgM isotypes in cholera patients 2, 30, and 90 days after onset of illness, determined from ELISA measurements of lymphocyte culture supernatant specimens. *, P < 0.05 in comparison to day 2.
Associations between immunologic markers.
Correlations between B-cell memory responses of different antigens and isotypes, as measured by antibody responses in culture supernatants at day 30, were considered. For any single isotype, memory responses to LPS and those to CT were significantly correlated with each other. On the other hand, there were no statistically significant associations between the memory responses of two different isotypes to the same antigen.
Associations of IgM memory with known surrogate markers of protection measured in plasma were also tested. The levels of IgM memory for either LPS or CTB at day 30 were not associated with the serum vibriocidal antibody, neither with the peak magnitude at day 7 (P = 0.3) nor with the percent increase from baseline (P = 0.9). For CTB, the IgG plasma response was negatively correlated with the level of day-30 IgM memory to CTB (rho = −0.5, P = 0.005) as well as with the IgM response to CTB in plasma (rho = −0.4, P = 0.01). For LPS, plasma IgA responses at day 7 were weakly negatively associated with IgM memory responses to LPS at day 30 (rho = −0.3, P = 0.08). The plasma IgA and IgG (but not IgM) responses were strongly positively correlated with each other for LPS (rho = 0.8, P < 0.0001) and for CTB (rho = 0.7, P < 0.0001).
Memory B-cell phenotypes in fresh circulating PBMCs and after ex vivo stimulation.
We utilized flow cytometry on PBMCs to measure the percentages of cells on days 2 and 30 after infection that were lymphocytes, B cells, memory B cells, and IgD+ memory B cells in our patient population (Table 2); these percentages did not differ substantially between day 2 and day 30.
TABLE 2.
Cell subsets as fractions of the parent population, detected by flow cytometry in cholera patients 2 and 30 days after hospitalization
| Day | Mean % ± SD |
|||
|---|---|---|---|---|
| Lymphocytes out of PBMCs | B cells (CD19+) out of all lymphocytes | Memory B cells (CD27+) out of all B cells | IgD+ memory B cells out of all memory B cells | |
| 2 (n = 10) | 33.6 ± 9.2 | 18.2 ± 7.7 | 32.1 ± 15.2 | 27.4 ± 9.9 |
| 30 (n = 9) | 41.0 ± 14.1 | 13.2 ± 4.8 | 35.7 ± 13.6 | 23.4 ± 9.1 |
We also assessed the percentages of CD19+CD27+ memory B cells in peripheral blood on days 2 and 30 that had IgA, IgG, or IgM on their cell surface (Table 3) and compared those to percentages of isotype-specific ASCs after 3 and 6 days of in vitro culture to determine the effect of mitogen stimulation on isotype switching in the memory B-cell assay. The percentage of ASCs producing each antibody isotype after 3 and 6 days of in vitro stimulation did not differ significantly from the original isotype distribution by flow cytometry of memory B cells in the circulation on either day 2 or day 30, although overall cell proliferation was evident by 6 days of in vitro culture.
TABLE 3.
Isotype distribution of memory B cells in circulation detected by flow cytometry, compared with isotype distribution of ASCs detected by ELISPOT assay after 3 and 6 days of in vitro stimulation (n = 7)
| Cell type | Study day | No. of cells per million PBMCs (mean ± SD) | Mean % ± SD |
||
|---|---|---|---|---|---|
| IgA+ | IgG+ | IgM+ | |||
| CD19+CD27+ memory B cells prior to stimulation, by FACS analysisa | 2 | 20,000 ± 14,000 | 21 ± 10 | 45 ± 8 | 34 ± 14 |
| 30 | 19,000 ± 14,000 | 25 ± 9 | 41 ± 8 | 33 ± 9 | |
| ASCs after 3 days of stimulated culture, by ELISPOT assay | 2 | 7,200 ± 5,900 | 32 ± 20 | 46 ± 22 | 23 ± 15 |
| 30 | 6,500 ± 6,900 | 25 ± 16 | 42 ± 17 | 33 ± 17 | |
| ASCs after 6 days of stimulated culture, by ELISPOT assay | 2 | 43,000 ± 37,000 | 18 ± 8 | 53 ± 15 | 29 ± 11 |
| 30 | 90,000 ± 86,000 | 13 ± 8 | 48 ± 11 | 39 ± 15 | |
FACS, fluorescence-activated cell sorter.
DISCUSSION
This study aimed to determine whether a memory B-cell response of the IgM isotype develops in cholera and to determine how this response differs for a T-cell-dependent protein antigen, CTB, compared to that for a T-cell-independent nonprotein antigen, LPS. Based on our results with a largely cholera-experienced population, elevated levels of circulating memory B cells of the IgM isotype, as well as those of the IgG and IgA isotypes, are present 1 month after acute infection with V. cholerae O1 for both CTB and LPS antigens.
The B cells transformed into ASCs by mitogen-stimulated culture in the memory B-cell assay bear CD27, a marker of memory B cells (5). The mitogen mixture used for this assay promotes polyclonal memory B-cell stimulation and was designed to maximize proliferation and differentiation into ASCs of memory B cells of the IgG isotype (5); nevertheless, our total immunoglobulin data show that IgM memory cells respond at least as well as do IgG cells to these mitogens. The large increases in total IgM concentrations in supernatants compared to IgG and IgA, which may be due in part to the particularly low baseline numbers of circulating IgM ASCs, provide further evidence that the IgM antibodies detected in the memory B-cell assay arose from mitogen-stimulated cells. Furthermore, the consistency between ELISPOT assay and flow cytometry determinations of the fractions of cells bearing each antibody isotype suggests that the relative numbers of ASCs of each isotype detectable at the end of the 6-day culture period are reasonably representative of the original prestimulation circulating memory B-cell population. This lack of indication of substantial isotype switching by IgM cells during in vitro culture is consistent with earlier observations using the mitogen CpG alone (3). IgM memory B cells targeting another enteric pathogen, rotavirus, have previously been demonstrated by a similar method and were shown to correspond to CD27+IgM+ B cells detectable by flow cytometry on fresh blood (34).
The majority of IgM+ B cells in humans are IgM+IgD+CD27− naïve B cells, which mount the earliest responses of the adaptive immune system, but as demonstrated by our flow cytometry results, humans also have CD27+ IgM+ cells in circulation, including a large population of CD27+ cells that express both IgD and IgM surface markers (37). These have previously been shown to be true memory cells with somatically hypermutated variable regions acquired by interaction with antigen in germinal centers (20), although they may have exited germinal centers at an earlier stage than did isotype-switched cells, without undergoing any T-cell interactions (8). IgM memory cells can produce IgM as well as IgG or IgA antibody; the CD27+IgM+IgD+ cells give rise to isotype-switched plasma cells upon reexposure to antigen, whereas CD27+ IgM+IgD− cells differentiate into IgM ASCs but do not appear to undergo isotype switching, thus playing a role in producing a rapid, high-affinity IgM antibody response to reinfection (19, 37, 46).
IgM antibody has been thought to play a role mainly with T-cell-independent nonprotein antigens that can activate B cells on their own through cross-linking, while switched-memory cells that have interacted with T cells in germinal centers are important for defense against protein antigens (9). Consistent with this dichotomy, elevations in plasma IgM anti-LPS levels have been observed after natural cholera infection (24) as well as after vaccination (30), whereas IgM antibody purified from convalescent-phase serum samples of cholera patients has shown little anti-CTB activity (24). Similarly, V. cholerae O1-infected patients develop consistent, strong IgM ASC responses to LPS, while only a fraction of patients develop positive IgM responses to CTB (32). Our serological and ASC results are consistent with these prior findings.
Despite the conventional understanding of distinct roles for IgM and isotype-switched B cells, there is considerable recent evidence for greater versatility of IgM+ memory B cells than previously assumed (39). For instance, human IgM+CD27+ memory B cells transplanted into SCID mice are capable of producing both IgM and IgG responses against both polysaccharide and protein antigens after pneumococcal immunization (25). Additionally, IgM+ memory B cells produce antibody against a wide variety of antigens, not only bacterial polysaccharides, and in fact, a greater fraction of the antibody produced by IgG+ memory B cells is specific for bacterial polysaccharide antigens than that of antibody produced by IgM+ memory B cells (40, 41). Human IgM+ and switched-memory B cells are phenotypically similar and have similarly robust responses to stimulation with both T-cell-dependent and -independent antigens (15, 23). Furthermore, generation of IgM isotype memory cells to T-cell-dependent antigens has been demonstrated following immunization against human immunodeficiency virus and hepatitis (10, 42). The existence of IgM memory cells to CTB after cholera infection is consistent with these observations. The possibility of IgM memory responses against CTB in particular, even in the absence of primary humoral IgM responses to this antigen, is also suggested by prior findings. IgM ASC responses to CTB develop following oral immunization in a small fraction of normal individuals and nearly half of IgA-deficient individuals (12), despite a near absence of anti-CTB IgM in plasma (7). Moreover, when mucosal vaccines against noncholera pathogens use CTB as an adjuvant, evidence suggests that IgM mediates the long-term protective effects of the vaccine (38, 43), although the acute response involves primarily IgA ASCs in Peyer's patches (38).
Because of the IgM predominance among vibriocidal antibodies and the importance of cross-linking in both T-cell-independent B-cell activation and the vibriocidal response, we had hypothesized that there would be a correlation between IgM memory and serum vibriocidal responses; however, no such correlation was observed. It remains possible that a correlation would be detected in a population with more-varied vibriocidal responses than ours, in which all individuals had severe clinical illness and strong (≥8-fold) increases in vibriocidal titer. We observed a negative correlation of IgM memory responses with the levels of the plasma IgG response to CTB and, more weakly, the IgA response to LPS. This would be consistent with IgM memory development and isotype-switched plasma responses representing different pathways for a naïve IgM+ B cell exposed to antigen.
In addition to demonstrating that IgM memory develops after cholera, our data suggest that it persists at 3 months after infection in some patients (16). Although the sample size for 3-month follow-up in our present study was small, we observed less persistence of IgG and IgA memory responses at day 90 by the use of our assay of culture supernatants than that seen in another study of memory B-cell responses after cholera by the use of an ELISPOT assay (16). The ELISPOT assay directly detects the total number of memory cell-derived ASCs of a given isotype at the end of 5 to 6 days of culture, while the memory cell supernatant assay used here depends both on the number of ASCs produced and on the amount of isotype-specific antibodies secreted by these ASCs into the culture supernatant; it is possible that memory B cells at differing time points following infection may mature into ASCs at different rates in vitro or secrete differing amounts of antibodies into culture supernatants. Clarification of the duration of IgM memory compared with the duration of memory in the other antibody isotypes, and to different antigens, will require follow-up for a longer time period and with a larger number of patients.
The participants in this study were mostly adults from a region where cholera is endemic, so their present illness was in most cases not their first exposure to V. cholerae. It is unclear how the baseline levels of immunity in the patients affected the responses we observed. Differences in the isotypes of responses to cholera antigens have previously been noted between naïve and previously exposed patients (13). Memory responses in a naïve or a younger population would likely be different from those seen in this study, and the antibody isotypes in the memory responses in these other populations may be important for understanding the various degrees of protection conferred by immunization (1, 29).
Our results suggest that after cholera infection, individuals in Bangladesh develop new or additional IgM memory B cells both to a T-cell-independent antigen, LPS, and to a T-cell-dependent antigen, CTB, by day 30 following infection. These IgM memory B cells are most likely of the CD19+CD27+ phenotype and appear in numbers similar to the IgG and IgA memory B cells that also develop. Despite the presence of CTB-specific IgM memory B cells, patients neither develop significant plasma IgM antibodies to CTB nor have circulating IgM-specific ASCs to this antigen. The role of IgM memory in protection against V. cholerae reinfection deserves further study.
Acknowledgments
This research was supported by the ICDDR,B Centre for Health and Population Research and by the following grants: U01 AI058935 (S.B.C.); R03 AI 063079 (F.Q.); U01 AI077883 (E.T.R.); International Research Scientist Development Award K01 TW07144 (R.C.L.); International Research Scientist Development Award K01 TW07409 (J.B.H.); and a Fogarty International Center Global Infectious Disease Research Training Program Award in Vaccine Development D43 TW05572 (M.A. and A.A.T.). E.A.K. is a recipient of a Fogarty International Clinical Research Scholars award from the Fogarty International Center at the National Institutes of Health (R24 TW007988).
We are grateful for the participation of patients and the work of the laboratory and field staff at the ICDDR,B.
Editor: V. J. DiRita
Footnotes
Published ahead of print on 26 October 2009.
REFERENCES
- 1.Ahmed, T., A. M. Svennerholm, A. Al Tarique, G. N. Sultana, and F. Qadri. 2009. Enhanced immunogenicity of an oral inactivated cholera vaccine in infants in Bangladesh obtained by zinc supplementation and by temporary withholding breast-feeding. Vaccine 27:1433-1439. [DOI] [PubMed] [Google Scholar]
- 2.Bjornson, A. B., and P. A. Detmers. 1995. The pentameric structure of IgM is necessary to enhance opsonization of Bacteroides thetaiotaomicron and Bacteroides fragilis via the alternative complement pathway. Microb. Pathog. 19:117-128. [DOI] [PubMed] [Google Scholar]
- 3.Capolunghi, F., S. Cascioli, E. Giorda, M. M. Rosado, A. Plebani, C. Auriti, G. Seganti, R. Zuntini, S. Ferrari, M. Cagliuso, I. Quinti, and R. Carsetti. 2008. CpG drives human transitional B cells to terminal differentiation and production of natural antibodies. J. Immunol. 180:800-808. [DOI] [PubMed] [Google Scholar]
- 4.Clements, M. L., M. M. Levine, C. R. Young, R. E. Black, Y. L. Lim, R. M. Robins-Browne, and J. P. Craig. 1982. Magnitude, kinetics, and duration of vibriocidal antibody responses in North Americans after ingestion of Vibrio cholerae. J. Infect. Dis. 145:465-473. [DOI] [PubMed] [Google Scholar]
- 5.Crotty, S., R. D. Aubert, J. Glidewell, and R. Ahmed. 2004. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J. Immunol. Methods 286:111-122. [DOI] [PubMed] [Google Scholar]
- 6.Crotty, S., P. Felgner, H. Davies, J. Glidewell, L. Villarreal, and R. Ahmed. 2003. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J. Immunol. 171:4969-4973. [DOI] [PubMed] [Google Scholar]
- 7.Czerkinsky, C., A. M. Svennerholm, and J. Holmgren. 1993. Induction and assessment of immunity at enteromucosal surfaces in humans: implications for vaccine development. Clin. Infect. Dis. 16(Suppl. 2):S106-S116. [DOI] [PubMed] [Google Scholar]
- 8.de Vinuesa, C. G., M. C. Cook, J. Ball, M. Drew, Y. Sunners, M. Cascalho, M. Wabl, G. G. Klaus, and I. C. MacLennan. 2000. Germinal centers without T cells. J. Exp. Med. 191:485-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fehr, T., H. Y. Naim, M. F. Bachmann, A. F. Ochsenbein, P. Spielhofer, E. Bucher, H. Hengartner, M. A. Billeter, and R. M. Zinkernagel. 1998. T-cell independent IgM and enduring protective IgG antibodies induced by chimeric measles viruses. Nat. Med. 4:945-948. [DOI] [PubMed] [Google Scholar]
- 10.Fondere, J. M., M. F. Huguet, H. Yssel, V. Baillat, J. Reynes, P. van de Perre, and J. P. Vendrell. 2003. Detection of peripheral HIV-1-specific memory B cells in patients untreated or receiving highly active antiretroviral therapy. AIDS 17:2323-2330. [DOI] [PubMed] [Google Scholar]
- 11.Forrest, B. D. 1992. Indirect measurement of intestinal immune responses to an orally administered attenuated bacterial vaccine. Infect. Immun. 60:2023-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friman, V., M. Quiding, C. Czerkinsky, I. Nordstrom, L. Larsson, D. Ericson, J. Bjorkander, K. Theman, A. Kilander, J. Holmgren, et al. 1994. Intestinal and circulating antibody-forming cells in IgA-deficient individuals after oral cholera vaccination. Clin. Exp. Immunol. 95:222-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galazka, A., D. Rymkiewicz, and J. Aleksandrowicz. 1976. Botulinum antitoxins and antibacterial IgM and IgG antibodies in sera of persons immunized with botulinum polytoxoid combined with cholera vaccine. II. Response to cholera vaccine. Arch. Immunol. Ther. Exp. (Warsz) 24:641-654. [PubMed] [Google Scholar]
- 14.Glass, R. I., A. M. Svennerholm, M. R. Khan, S. Huda, M. I. Huq, and J. Holmgren. 1985. Seroepidemiological studies of El Tor cholera in Bangladesh: association of serum antibody levels with protection. J. Infect. Dis. 151:236-242. [DOI] [PubMed] [Google Scholar]
- 15.Good, K. L., V. L. Bryant, and S. G. Tangye. 2006. Kinetics of human B cell behavior and amplification of proliferative responses following stimulation with IL-21. J. Immunol. 177:5236-5247. [DOI] [PubMed] [Google Scholar]
- 16.Harris, A. M., M. S. Bhuiyan, F. Chowdhury, A. I. Khan, A. Hossain, E. A. Kendall, A. Rahman, R. C. Larocque, J. Wrammert, E. T. Ryan, F. Qadri, S. B. Calderwood, and J. B. Harris. 2009. Antigen specific memory B-cell responses to Vibrio cholerae O1 infection in Bangladesh. Infect. Immun. 77:3850-3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris, J. B., R. C. Larocque, F. Chowdhury, A. I. Khan, T. Logvinenko, A. S. Faruque, E. T. Ryan, F. Qadri, and S. B. Calderwood. 2008. Susceptibility to Vibrio cholerae infection in a cohort of household contacts of patients with cholera in Bangladesh. PLoS Negl. Trop. Dis. 2:e221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jayasekera, C. R., J. B. Harris, S. Bhuiyan, F. Chowdhury, A. I. Khan, A. S. Faruque, R. C. Larocque, E. T. Ryan, R. Ahmed, F. Qadri, and S. B. Calderwood. 2008. Cholera toxin-specific memory B cell responses are induced in patients with dehydrating diarrhea caused by Vibrio cholerae O1. J. Infect. Dis. 198:1055-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kindler, V., and R. H. Zubler. 1997. Memory, but not naive, peripheral blood B lymphocytes differentiate into Ig-secreting cells after CD40 ligation and costimulation with IL-4 and the differentiation factors IL-2, IL-10, and IL-3. J. Immunol. 159:2085-2090. [PubMed] [Google Scholar]
- 20.Klein, U., K. Rajewsky, and R. Kuppers. 1998. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J. Exp. Med. 188:1679-1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koelle, K., X. Rodo, M. Pascual, M. Yunus, and G. Mostafa. 2005. Refractory periods and climate forcing in cholera dynamics. Nature 436:696-700. [DOI] [PubMed] [Google Scholar]
- 22.Losonsky, G. A., J. Yunyongying, V. Lim, M. Reymann, Y. L. Lim, S. S. Wasserman, and M. M. Levine. 1996. Factors influencing secondary vibriocidal immune responses: relevance for understanding immunity to cholera. Infect. Immun. 64:10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma, C. S., S. Pittaluga, D. T. Avery, N. J. Hare, I. Maric, A. D. Klion, K. E. Nichols, and S. G. Tangye. 2006. Selective generation of functional somatically mutated IgM+CD27+, but not Ig isotype-switched, memory B cells in X-linked lymphoproliferative disease. J. Clin. Invest. 116:322-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Majumdar, A. S., and A. C. Ghose. 1981. Evaluation of the biological properties of different classes of human antibodies in relation to cholera. Infect. Immun. 32:9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moens, L., M. Wuyts, I. Meyts, K. De Boeck, and X. Bossuyt. 2008. Human memory B lymphocyte subsets fulfill distinct roles in the anti-polysaccharide and anti-protein immune response. J. Immunol. 181:5306-5312. [DOI] [PubMed] [Google Scholar]
- 26.Mora, J. R., M. Iwata, B. Eksteen, S. Y. Song, T. Junt, B. Senman, K. L. Otipoby, A. Yokota, H. Takeuchi, P. Ricciardi-Castagnoli, K. Rajewsky, D. H. Adams, and U. H. von Andrian. 2006. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science 314:1157-1160. [DOI] [PubMed] [Google Scholar]
- 27.Qadri, F., F. Ahmed, M. M. Karim, C. Wenneras, Y. A. Begum, M. Abdus Salam, M. J. Albert, and J. R. McGhee. 1999. Lipopolysaccharide- and cholera toxin-specific subclass distribution of B-cell responses in cholera. Clin. Diagn. Lab. Immunol. 6:812-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qadri, F., T. Azim, A. Chowdhury, J. Hossain, R. B. Sack, and M. J. Albert. 1994. Production, characterization, and application of monoclonal antibodies to Vibrio cholerae O139 synonym Bengal. Clin. Diagn. Lab. Immunol. 1:51-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qadri, F., M. I. Chowdhury, S. M. Faruque, M. A. Salam, T. Ahmed, Y. A. Begum, A. Saha, A. Al Tarique, L. V. Seidlein, E. Park, K. P. Killeen, J. J. Mekalanos, J. D. Clemens, and D. A. Sack. 2007. Peru-15, a live attenuated oral cholera vaccine, is safe and immunogenic in Bangladeshi toddlers and infants. Vaccine 25:231-238. [DOI] [PubMed] [Google Scholar]
- 30.Qadri, F., M. I. Chowdhury, S. M. Faruque, M. A. Salam, T. Ahmed, Y. A. Begum, A. Saha, M. S. Alam, K. Zaman, L. V. Seidlein, E. Park, K. P. Killeen, J. J. Mekalanos, J. D. Clemens, and D. A. Sack. 2005. Randomized, controlled study of the safety and immunogenicity of Peru-15, a live attenuated oral vaccine candidate for cholera, in adult volunteers in Bangladesh. J. Infect. Dis. 192:573-579. [DOI] [PubMed] [Google Scholar]
- 31.Qadri, F., E. T. Ryan, A. S. Faruque, F. Ahmed, A. I. Khan, M. M. Islam, S. M. Akramuzzaman, D. A. Sack, and S. B. Calderwood. 2003. Antigen-specific immunoglobulin A antibodies secreted from circulating B cells are an effective marker for recent local immune responses in patients with cholera: comparison to antibody-secreting cell responses and other immunological markers. Infect. Immun. 71:4808-4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qadri, F., C. Wenneras, M. J. Albert, J. Hossain, K. Mannoor, Y. A. Begum, G. Mohi, M. A. Salam, R. B. Sack, and A. M. Svennerholm. 1997. Comparison of immune responses in patients infected with Vibrio cholerae O139 and O1. Infect. Immun. 65:3571-3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rahman, M., D. A. Sack, S. Mahmood, and A. Hossain. 1987. Rapid diagnosis of cholera by coagglutination test using 4-h fecal enrichment cultures. J. Clin. Microbiol. 25:2204-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rojas, O. L., C. F. Narvaez, H. B. Greenberg, J. Angel, and M. A. Franco. 2008. Characterization of rotavirus specific B cells and their relation with serological memory. Virology 380:234-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sack, D. A., R. B. Sack, G. B. Nair, and A. K. Siddique. 2004. Cholera. Lancet 363:223-233. [DOI] [PubMed] [Google Scholar]
- 36.Saha, D., R. C. LaRocque, A. I. Khan, J. B. Harris, Y. A. Begum, S. M. Akramuzzaman, A. S. Faruque, E. T. Ryan, F. Qadri, and S. B. Calderwood. 2004. Incomplete correlation of serum vibriocidal antibody titer with protection from Vibrio cholerae infection in urban Bangladesh. J. Infect. Dis. 189:2318-2322. [DOI] [PubMed] [Google Scholar]
- 37.Shi, Y., K. Agematsu, H. D. Ochs, and K. Sugane. 2003. Functional analysis of human memory B-cell subpopulations: IgD+CD27+ B cells are crucial in secondary immune response by producing high affinity IgM. Clin. Immunol. 108:128-137. [DOI] [PubMed] [Google Scholar]
- 38.Soenawan, E., I. Srivastava, S. Gupta, E. Kan, R. Janani, J. Kazzaz, M. Singh, V. Shreedhar, and M. Vajdy. 2004. Maintenance of long-term immunological memory by low avidity IgM-secreting cells in bone marrow after mucosal immunizations with cholera toxin adjuvant. Vaccine 22:1553-1563. [DOI] [PubMed] [Google Scholar]
- 39.Tangye, S. G., and K. L. Good. 2007. Human IgM+CD27+ B cells: memory B cells or “memory” B cells? J. Immunol. 179:13-19. [DOI] [PubMed] [Google Scholar]
- 40.Tiller, T., M. Tsuiji, S. Yurasov, K. Velinzon, M. C. Nussenzweig, and H. Wardemann. 2007. Autoreactivity in human IgG+ memory B cells. Immunity 26:205-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsuiji, M., S. Yurasov, K. Velinzon, S. Thomas, M. C. Nussenzweig, and H. Wardemann. 2006. A checkpoint for autoreactivity in human IgM+ memory B cell development. J. Exp. Med. 203:393-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tuaillon, E., Y. A. Tabaa, G. Petitjean, M. F. Huguet, G. Pajeaux, J. M. Fondere, B. Ponseille, J. Ducos, P. Blanc, and J. P. Vendrell. 2006. Detection of memory B lymphocytes specific to hepatitis B virus (HBV) surface antigen (HBsAg) from HBsAg-vaccinated or HBV-immunized subjects by ELISPOT assay. J. Immunol. Methods 315:144-152. [DOI] [PubMed] [Google Scholar]
- 43.Vajdy, M., and N. Lycke. 1995. Mucosal memory B cells retain the ability to produce IgM antibodies 2 years after oral immunization. Immunology 86:336-342. [PMC free article] [PubMed] [Google Scholar]
- 44.Weekly Epidemiological Record. 2001. Cholera vaccines. Wkly. Epidemiol. Rec. 76:117-124.11338983 [Google Scholar]
- 45.Weekly Epidemiological Record. 2008. Cholera, 2007. Wkly. Epidemiol. Rec. 83:269-283.18668979 [Google Scholar]
- 46.Werner-Favre, C., F. Bovia, P. Schneider, N. Holler, M. Barnet, V. Kindler, J. Tschopp, and R. H. Zubler. 2001. IgG subclass switch capacity is low in switched and in IgM-only, but high in IgD+IgM+, post-germinal center (CD27+) human B cells. Eur. J. Immunol. 31:243-249. [DOI] [PubMed] [Google Scholar]
- 47.Zuckerman, J. N., L. Rombo, and A. Fisch. 2007. The true burden and risk of cholera: implications for prevention and control. Lancet Infect. Dis. 7:521-530. [DOI] [PubMed] [Google Scholar]