Abstract
Malaria infection is initiated when a female Anopheles mosquito probing for blood injects saliva, together with sporozoites, into the skin of its mammalian host. Prior studies had suggested that saliva may enhance sporozoite infectivity. Using rodent malaria models (Plasmodium berghei and P. yoelii), we were unable to show that saliva had any detectable effect on sporozoite infectivity. This is encouraging for plans to immunize humans with washed, attenuated P. falciparum sporozoites because many individuals develop cutaneous, hypersensitivity reactions to mosquito saliva after repeated exposure. If washed sporozoites have no appreciable loss of infectivity, they likely do not have decreased immunogenicity; thus, vaccinees are unlikely to develop cutaneous reactions against mosquito saliva during attempted immunization with such sporozoites. Earlier studies also suggested that repeated prior exposure to mosquito saliva reduces infectivity of sporozoites injected by mosquitoes into sensitized hosts. However, our own studies show that prior exposure of mice to saliva had no detectable effect on numbers of sporozoites delivered by infected mosquitoes, the rate of disappearance of these sporozoites from the skin or infectivity of the sporozoites. Under natural conditions, sporozoites are delivered both to individuals who may exhibit cutaneous hypersensitivity to mosquito bite and to others who may have not yet developed such reactivity. It was tempting to hypothesize that differences in responsiveness to mosquito bite by different individuals might modulate the infectivity of sporozoites delivered into a milieu of changes induced by cutaneous hypersensitivity. Our results with rodent malaria models, however, were unable to support such a hypothesis.
The malaria infection is initiated when a female Anopheles mosquito probing for a blood meal injects saliva, together with sporozoites into the skin of its mammalian host (18, 39). Mosquito saliva is known to enhance the ability of the mosquito to locate a blood source and to inhibit hemostasis by any of several mechanisms. These include injection of an anticoagulant factor (34), inhibition of platelet aggregation by salivary apyrase (29) or a salivary factor that inhibits collagen-induced platelet aggregation (43), inhibition of thrombin activity (14), and vasodilation of host blood vessels (30). Arthropod saliva has been shown to enhance the infectivity of several different pathogens introduced into hosts by arthropods; these include sandfly transmission of Leishmania, tick transmission of viruses and spirochetes, and mosquito transmission of viruses (for a review, see reference 36). Enhancement of Plasmodium sporozoite infectivity by mosquito saliva has also been reported (12, 36) based on a prior study (41), but we felt that this study needed to be reassessed.
In addition to these studies on the direct effect of arthropod saliva on infectivity of pathogens injected by arthropods into immunologically naive hosts, studies have also been done on the role of prior exposure of hosts to arthropod saliva in modulating pathogen transmission to immunized hosts. Ever since Trager's classic study (37) showing that immunity to tick bite can lead to host protection against subsequent feedings by ticks, many workers have studied the role of host immunity to arthropod saliva in interfering with feeding by the arthropod and modulating transmission of pathogens to the host (for reviews, see references 6 and 36). Most of these studies have focused on delayed immune responses that in some cases may enhance and in other cases may control infections with arthropod-transmitted pathogens. This is an appropriate approach in circumstances when a host cellular response may interfere with feeding by the arthropod or may recruit host cells that modulate development of the pathogen at the bite site.
Mosquitoes, however, feed relatively rapidly. Thus, only an immediate hypersensitivity response is likely to be able to modulate movement of sporozoites from avascular tissue at the bite site to blood vessels, from which the sporozoite can then reach the liver for further development. Many hosts bitten by mosquitoes over a period of time develop an immediate, cutaneous, hypersensitivity response; it is relevant that this develops at the same site and within the same timeframe during which sporozoites are moving into local blood vessels. We have previously studied the kinetics of P. berghei sporozoite movement out of the skin after deposition by mosquitoes into immunologically naive mice (19). We thus set out to compare this to the kinetics of sporozoites introduced by mosquitoes into mice that we attempted to hyperimmunize against mosquito saliva by repeated mosquito bites. Our results have shown that neither the presence of mosquito saliva nor immediate hypersensitivity to saliva had any detectable effects on deposition of sporozoites by mosquitoes or the movement of these sporozoites from the bite site into the blood to induce infection.
MATERIALS AND METHODS
Sporozoites.
Anopheles stephensi mosquitoes were infected with a clone of the rodent malaria parasite, Plasmodium berghei, whose sporozoites constitutively express RedStar, an improved red fluorescent protein (15). For some studies, we used mosquitoes infected with wild-type P. berghei (strain NK65) or P. yoelii (strain 17XNL), neither of whose sporozoites expresses fluorescent protein. We used standard protocols for infecting and maintaining mosquitoes (40), which were infected by feeding upon gametocyte-carrying 6- to 8-week-old Swiss-Webster mice (Taconic Farms, Inc., Germantown, NY). Our protocols for maintenance and use of experimental animals were approved by the Institutional Animal Care and Use Committee at New York University School of Medicine, and our animal facility is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (Rockville, MD). Mosquitoes were used for sporozoite transmission studies 18 days after the infective blood meal. Prior to use of infected mosquitoes for feedings observed by intravital microscopy, live, intact mosquitoes were examined by fluorescence microscopy to establish that they had salivary gland infections (3, 35); mosquitoes found to be negative were discarded.
Detection of sporozoites after mosquito feeding on ear pinnae.
Mosquitoes were allowed to feed on mice anesthetized by intraperitoneal injection of ketamine (50 mg/kg) plus xylazine (10 mg/kg) and acepromazine (1.7 mg/kg) and placed on a warming tray. To restrict the area of sporozoite deposition for more efficient counting of sporozoites, the dorsal aspect of one ear pinna was partially masked with tape so that only its edge (8 to 10 mm long and 2 to 3 mm wide) was accessible to a feeding mosquito. Mosquitoes, previously selected for having positive salivary gland infections, were kept individually in plastic feeding tubes 2.5 cm in length and with an inside diameter of 1.5 cm; one end of the tube was covered with netting through which the mosquito was able to feed and the other end was closed with a screw cap. Each mosquito was allowed to probe and feed on the ear through the netting for 3 min from the time that probing was first observed.
At appropriate times after each feeding, the fed-upon region of the ear plus the taped adjacent area ∼2.5 mm beyond this was excised. This biopsy specimen was separated into dorsal and ventral leaflets with fine forceps (13), after which each leaflet was mounted under a coverslip and examined by fluorescence microscopy to count sporozoites and record their distribution (18). Biopsy specimens were taken either immediately after feeding or at 3 h after feeding. Parallel studies were done with mice that had been actively immunized against mosquito saliva. Fed-upon mice were kept for up to 14 days to obtain blood smears from the tip of the tail; smears were stained with Giemsa and observed by bright-field microscopy to detect patent blood infections. This is an extremely sensitive way to establish whether even a single sporozoite has left the skin to develop further in the liver and establish a blood infection.
Immunization.
For immunization against mosquito saliva, mice anesthetized as described above were exposed for 15 min to bites from uninfected mosquitoes that had been previously starved overnight. This procedure was repeated twice a week for 4 weeks. A group of age-matched mice was used as unbitten, naive controls. Assessment for immunity was done by enzyme-linked immunosorbent assay (ELISA) and by development by mice of an immediate, cutaneous hypersensitive reaction to mosquito bite, as observed and documented by intravital videomicroscopy. One week after the last exposure, mice were challenged by mosquitoes infected with red fluorescent P. berghei, as described above.
Studies assessing infectivity of sporozoites injected by syringe in the presence versus the absence of mosquito saliva.
P. berghei (strain NK65) or P. yoelii (strain 17NXL) sporozoites were prepared by dissecting out and triturating infected salivary glands collected in RPMI medium supplemented with 2% bovine serum albumin (BSA). Sporozoites were then washed twice by centrifugation at 12,000 × g for 10 min to reduce the salivary gland component. To assess reduction in salivary gland material, we assayed apyrase as an indicator for saliva and determined the presence of apyrase before and after washing, using a colorimetric method as previously described (22) to measure release of inorganic phosphate (Pi) from ADP or ATP. In brief, 1-μl aliquots of washed or unwashed sporozoites were placed in wells of a flat-bottom microplate (Immulon 2HB) and then mixed with 99 μl of 50 mM Tris-HCl buffer (pH 9.0) containing 100 mM NaCl, 5 mM CaCl2, 2 mM ADP, and 20 mM β-mercaptoethanol. Reaction buffer was used as a negative control, and the standard curve was generated by using a serial dilution of sodium phosphate (1 to 0.0625 mM). A known number of salivary glands was used to allow calculation of the amount found per inoculum before and after washing. The plate was then incubated at 37°C for 15 min. The reaction was stopped by addition of 3 μl of reducing reagent (0.02% 1-amino-2-naphthol-4-sulfonic acid, 0.12% sodium bisulfite, 0.12% sodium sulfite) and 25 μl of 1.25% ammonium molybdate in 2.5 N H2SO4. After 20 min incubation at 37°C, the plate was read at 620 nm in a Titertek Multiskan reader for determination of the optical density.
P. yoelii sporozoites were injected into BALB/c mice. Because of the significantly lower infectivity of P. berghei sporozoites (20), we injected these into C57BL/6 mice, a strain known to be significantly more susceptible to P. berghei sporozoites (24). Because of the relatively poor infectivity of P. berghei sporozoites compared to the P. yoelii strain that we used, many more sporozoites were injected by syringe than would normally be delivered by individual mosquitoes. This was done to ensure that a relatively high percentage of the injected mice developed patent blood infections. Mice were injected intravenously (100 μl/mouse) or intradermally (10 μl/mouse) with washed sporozoites; some inocula were fortified with salivary gland extract (SGE). See Tables 1 and 2 for details. To prepare SGE, mosquitoes were anesthetized on ice and then washed with 70% ethanol, followed by RPMI medium 1640 (Gibco-BRL, Grand Island, NY). Glands were dissected in RPMI containing 2% BSA and disrupted by three cycles of freezing and thawing. We injected SGE to an equivalent of glands from 0.5 mosquitoes per inoculum. Infectivity of sporozoites with or without SGE was assayed by daily Giemsa-stained smears to determine percentage of mice that developed blood infections and the prepatent period of infected mice.
TABLE 1.
Infectivity of P. berghei strain NK65 sporozoites injected into C57BL/6 micea
| Injection route and no. of sporozoites | Mice with parasitemia |
Prepatent period |
||
|---|---|---|---|---|
| % | P | Mean no. of days | P | |
| Intravenous | ||||
| 200 | 40 (6/15) | 0.45 | 6.0 | 1.0 |
| 200 + SGE | 26.7 (4/15) | 6.0 | ||
| Intradermal | ||||
| 2,000 | 100 (15/15) | 0.42 | 4.53 | 0.84 |
| 2,000 + SGE | 93.3 (14/15) | 4.57 | ||
The table presents a comparison of the routes of injection and presence versus the absence of mosquito SGE in the inoculum. SGE was added to an equivalent of glands from 0.5 mosquitoes per inoculum. P values (determined by unpaired t test) were not significant. See Materials and Methods for the statistical analysis.
TABLE 2.
Infectivity of P. yoelii strain 17NXL sporozoites injected into BALB/c micea
| Injection route and no. of sporozoites | Mice with parasitemia |
Prepatent period |
||
|---|---|---|---|---|
| % | P | Mean no. of days | P | |
| Intravenous | ||||
| 5 | 90 (18/20) | 0.54 | 4.0 | 0.14 |
| 5 + SGE | 95 (19/20) | 3.68 | ||
| Intradermal | ||||
| 10 | 65 (12/19) | 0.63 | 3.80 | 0.19 |
| 10 + SGE | 50 (10/20) | 4.20 | ||
See Table 1, footnote a.
ELISA.
Repeated exposure of mice to mosquito saliva results in production of large amounts of specific immunoglobulin G (IgG) in the serum; this inhibits binding of the relatively smaller amounts of specific IgE to salivary antigens on ELISA microplates. Thus, specific IgE in such mice cannot be measured by using an ELISA (10). Accordingly, we measured total serum IgE by coating plates with 2 μg of the purified rat anti-mouse IgE capture monoclonal antibody (BD Biosciences Pharmingen, San Jose, CA)/ml. Plates were then incubated with sera from different samples (1:100 to 1:102,400) and a standard mouse IgE (0.5 μg/ml; BD Biosciences Pharmingen, San Jose, CA).
Biotinylated rat anti-mouse IgE (BD Biosciences Pharmingen) was used as the detection antibody, streptavidin-horseradish peroxidase (BD Biosciences Pharmingen) was used as the secondary antibody, and ABTS [2,2′azinobis(3-ethylbenzthiazolinesulfonic acid); KPL, Gaithersburg, MD] was used as the substrate. The endpoint was measured as the highest dilution of serum having a change in optical density greater than the mean plus three standard deviations obtained with nonimmune sera. The results were expressed as geometric mean titers.
Microscopy.
For counting of sporozoites, we used a Leica MZ16FA fluorescence stereoscopic microscope with a ×2.0 stereoscopic objective lens. Illumination for fluorescence studies was with an EXFO X-Cite 120 F1 illumination system and with a DsRed filter set, restricting illumination to 515 to 556 nm (peak = 545 nm) and signal emission to 590 nm.
Increased vascular permeability was used to assess immediate hypersensitivity in mice. Shortly before probing of individual mosquitoes, mice were injected in a tail vein with 150 μl of fluorescein isothiocyanate (FITC)-conjugated dextran (10 mg/ml, 500-kDa dextran). Bite reaction in the ear pinnae was visualized by real-time intravital videomicroscopy observation of the extravasation of the dye using a Leica DMI 4000B inverted fluorescence microscope with a ×10 objective lens. Total observation time was 10 min with frames taken every 5 s. Illumination for fluorescence studies was with a CTR4000 illumination system and with a dual green/red filter set, restricting illumination to 480 to 500 nm (peak = 490) and 560 to 590 nm (peak = 575) and signal emission to 505 and 600 nm. Images were acquired with a Leica DFC300 FX digital camera and saved as digital files for further analysis and processing. We used Leica Application Suite software (LAS V2.7.1) for documentation and analysis.
Statistics.
The numbers of sporozoites injected by mosquitoes did not follow a normal distribution but were highly skewed with a clear floor effect. In order to approximate a normal distribution, all of the data were log transformed (ln[sporozoite count + 1]) and analyzed by using analysis of variance (ANOVA). We recorded the percentages of mice that developed parasitemia and the prepatent periods of those that developed parasitemia. We then compared these data for mice injected with sporozoites in the presence versus the absence of mosquito saliva. Similarly, we compared mice that were immunologically naive versus mice that had been hyperimmunized against saliva by repeated mosquito bite. To do these comparisons, all data were transformed by using the following equation y = √(y + 0.5), where y represents the percentage of infection. In order to test whether our data followed a Gaussian distribution, we used the Kolmogorov-Smirnov normality test (with Dallal-Wilkinson-Lillifore P value). When we found that the values were not normally distributed, we transformed them and then reconfirmed their normal distribution by using the same Kolmogorov-Smirnov test. A Student t test (unpaired, two tailed) was then used to compare the differences between the groups of mice. The analyses were performed by using GraphPad Prism version 5 software (San Diego, CA).
RESULTS
Assessment of infectivity of sporozoites injected by syringe in the presence versus the absence of mosquito saliva.
We tested for the presence of saliva in sporozoite preparations by assaying for salivary apyrase; for this, we quantified the amount of Pi released from ADP in the presence of apyrase. Our standardization curve showed that the mean amount of Pi released by the equivalent of a pair of salivary glands from 1 mosquito was 1.53 ± 0.02 mM (mean ± standard error of the mean). The mean amount of Pi released in preparations of nonwashed sporozoites was 1.19 ± 0.025 mM, which corresponds to an equivalent of 78% of the gland material from a single mosquito. There was no detectable Pi released within washed sporozoite preparations, the optical density of these aliquots being similar to what was obtained with negative controls. This confirmed that washing the sporozoites eliminated all detectable traces of apyrase and presumably mosquito saliva.
This portion of our studies, as shown in Tables 1 and 2, compared four sets of variables: (i) P. berghei (strain NK65) versus P. yoelii (strain 17NXL) sporozoites, each injected into appropriate mouse hosts; (ii) the numbers of sporozoites injected; (iii) intravenous versus intradermal inoculations; and (iv) the presence versus the absence of mosquito saliva in inocula. Assessment of sporozoite infectivity results was made by determining percentage of mice that developed patent blood infections and the mean prepatent period for those mice that developed blood infections. For both species of malaria, the presence or the absence of SGE had no significant effect on the infectivity of sporozoites under any circumstance, as determined from the percentage of infected mice or the length of the prepatent period.
Quantification of sporozoites deposited by mosquitoes in ear pinnae of mice with prior exposure to mosquito saliva.
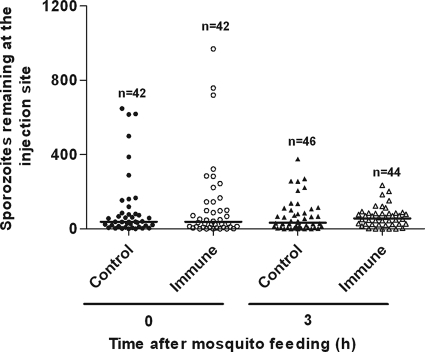
A summary of numbers of P. berghei sporozoites visualized at the bite site on the ear pinna after feedings by individual mosquitoes is presented as a scatter plot in Fig. 1. After mosquitoes fed on nonimmunized (control) mice, we found a median of 39 sporozoites in biopsy specimens taken immediately after feeding. After mosquitoes fed on mice that had been previously exposed to repeated mosquito bites, we found a median of 38.5 sporozoites in the zero-time biopsy specimens. These medians were similar to medians that we had found in other studies (18, 19). Examination of biopsy specimens from other mice at 3 h postfeeding showed medians of 33.5 sporozoites in control mice and 57.0 sporozoites in saliva-exposed mice. Because the data did not appear to follow a normal distribution, they were log transformed prior to statistical analysis by ANOVA. These analyses showed no significant difference between control and saliva-exposed mice at either 0 or 3 h postfeeding. A P. yoelii clone whose sporozoites express an intensity of fluorescence comparable to that of P. berghei RedStar sporozoites is not currently available; thus, we were able to conduct this portion of the study only with P. berghei.
FIG. 1.
Mosquito injection of P. berghei sporozoites into ear pinnae of mice immunized against mosquito saliva. Scatter plot shows numbers of sporozoites remaining at bite site on ear immediately after feeding or at 3 h postfeeding on nonimmunized (control) mice versus mice that had been actively immunized against mosquito saliva by repeated bites of noninfected mosquitoes. Each point shows number of sporozoites left by a single mosquito (n = the total number of mosquito feedings for each time point). Horizontal bars show medians. After log transformation of data, ANOVA showed no significant difference between control and immunized mice in the numbers of sporozoites deposited at zero time or in the numbers of sporozoites that remained in skin at 3 h.
Infectivity of sporozoites injected by mosquitoes into mice with prior exposure to mosquito saliva.
To test the infectivity of P. berghei sporozoites injected into mice that had been previously exposed to repeated mosquito bites, we allowed infected mosquitoes to bite saliva-exposed versus nonexposed mice (two mosquitoes per mouse) and monitored the mice via daily Giemsa smears to assess blood infections. The results showed that 10 of 16 (62.5%) of nonimmunized controls developed patent blood infections (with a mean prepatent period of 6.5 days), whereas 9 of 14 (64.3%) of the saliva-exposed mice became patent (with a mean prepatent period of 5.5 days). The differences between saliva-exposed and nonimmunized mice were not statistically significant.
Immune status of saliva-exposed mice.
Because hyperimmunized mice are known to produce large amounts of specific IgG, which inhibits binding of the relatively smaller amounts of specific IgE to salivary antigens on ELISA microplates (10), we verified the antibody status of these saliva-exposed mice by measuring total IgE. The mean total IgE level of sera taken from saliva-exposed mice on the day prior to challenge was 1:1,400 compared to 1:155 for sera from nonimmunized controls.
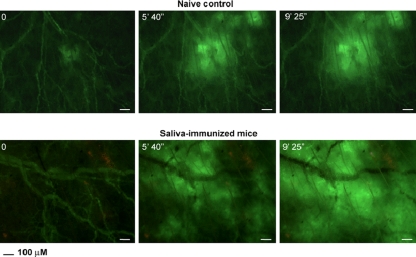
Because mice hyperimmunized against mosquito saliva are known not to show characteristic “wheal and flare” reactions upon mosquito bite challenge (10), we evaluated the development of an immediate, cutaneous, hypersensitivity reaction to mosquito bite by real-time intravital videomicroscopy of the extravasation of FITC-labeled dextran. Figure 2 shows still frames from typical videos (see Videos S1 and S2 in the supplemental material) comparing the mosquito bite challenges of a saliva-exposed versus a nonimmunized control mouse. We observed extensive extravasation of FITC-labeled dextran from blood vessels of mice immunized by repeated mosquito bites.
FIG. 2.
Demonstration of immediate, cutaneous hypersensitivity in immunized mice by observation of increased vascular permeability after mosquito bite. Still frames of typical mosquito challenges of ears of saliva-immunized and nonimmunized control mice at various times after initiation of the bite by an individual mosquito (from Videos S1 and S2 in the supplemental material prepared by intravital microscopy). Control mouse shows formation of a spreading hematoma induced by mosquito proboscis. There is release of FITC-labeled dextran from ruptured blood vessels at the bite site. The immunized mouse shows similar hematoma, followed by extensive extravasation of labeled dextran from intact blood vessels beyond the bite site.
DISCUSSION
Blood-sucking arthropods probing for a blood meal release saliva into their vertebrate hosts to aid in localization of a blood source and to inhibit hemostasis (27). In the case of arthropod vectors of disease, the saliva may be accompanied by pathogens introduced into the host with the saliva. Saliva of blood-feeding arthropods is rich in bioactive agents that enable the arthropods to successfully obtain blood (27, 28), and there is evidence that some of these agents may enhance the infectivity of arthropod-transmitted pathogens. Indeed, a recent review (36) contended that “in virtually every system analyzed, arthropod saliva has in fact enhanced infection with pathogens.” Nevertheless, the review also noted that this was not universal and cited several studies in which saliva was found to have little or no effect on transmission of Leishmania (7, 8, 25). Because Plasmodium sporozoites are the most important pathogens transmitted by arthropods, there is considerable interest in the role that mosquito saliva may play in sporozoite infectivity. Citing a prior publication (41), two other groups subsequently stated that this study (that is, reference 41) had reported that infectivity of P. berghei sporozoites is enhanced by mosquito saliva (12, 36). Nevertheless, the initial study (41) never made such a claim. The authors had merely concluded that mosquito-injected sporozoites were more infective than sporozoites injected intravenously by syringe; they had then suggested that one of the possible reasons for their results may have been the presence of saliva in the mosquito-induced infections in contrast with the greatly diluted salivary material in the intravenous injections (41).
A concern about the initially cited study (41) is that the authors had gathered data only on intravenously injected sporozoites. These data were then compared to assumptions on the numbers of sporozoites injected directly by mosquitoes; however, these mosquito injection data had been assembled from prior published studies (17, 32). We thus felt it important to investigate further the purported role of mosquito saliva in sporozoite infectivity. We attempted to better control these experiments (i) by using mice, mosquitoes, and parasites from the same cohorts within each study; (ii) by verifying and quantifying the amounts of saliva injected together with sporozoites; (iii) by comparing two different modes of syringe injection (intravenous versus intradermal); (iv) by normalizing and directly comparing the actual numbers of sporozoites used in each study; and (v) by comparing different species of parasites and different strains of mice.
We were unable to demonstrate any detectable effect of saliva on sporozoite infectivity when we added defined amounts of SGE to sporozoite preparations that had been washed free of detectable saliva. That the washing procedure did not discernibly damage sporozoites was shown by the fact that as few as five washed 17NXL P. yoelii sporozoites were able to infect close to 100% of mice injected intravenously. These results were similar to those of a prior study showing that large quantities of saliva introduced by mosquitoes into mice concomitantly injected intravenously with P. berghei sporozoites had no detectable effect on the infectivity of these sporozoites, as measured by counts of exoerythrocytic parasites that developed in the livers of the sporozoite-injected mice (38). Our findings may be relevant to attempts to immunize humans by injection of attenuated sporozoites (9). Because many individuals may develop severe cutaneous, hypersensitivity reactions to mosquito saliva after repeated mosquito bites (26), it is encouraging that purified sporozoites washed free of detectable saliva do not have an appreciable loss of infectivity. Prior studies comparing different routes of injection with viable rodent malaria sporozoites have shown that routes that result in higher infection rates, e.g., the intravenous route, were also correlated with significantly higher protective immunity after injection of radiation-attenuated sporozoites (21, 33). Thus, by extension, the high infectivity of washed, attenuated sporozoites implies that there may be a high degree of protective immunogenicity with such sporozoites against malaria without subjecting vaccinees to cutaneous reactions against mosquito saliva. Nevertheless, the possible adjuvant effect of mosquito saliva on immunogenicity of sporozoites cannot be excluded.
In addition to the question regarding the role of arthropod saliva in modulating infectivity of saliva-borne pathogens, there is a parallel question regarding the possible role of host immunity to saliva in modulating the transmission and infectivity of these pathogens. Hosts repeatedly bitten by an arthropod may develop an immune reaction against the saliva. Because mosquito bites are of relatively short duration and because Plasmodium sporozoites injected into the bite site rapidly leave the site and move into the blood for infection of the liver (4, 39), it follows that an immediate, cutaneous, hypersensitivity reaction is the only response that could play a role in mosquito deposition of sporozoites or modulation of sporozoite behavior at the injection site.
Visible immediate hypersensitivity responses induced by mosquito bite occur within 2 min of mosquito probing (16); microscopically detectable changes occur almost immediately. This may have multiple consequences. (i) Antigen-antibody complexes in the skin stimulate platelet aggregation and subsequent hemostasis. (ii) Mosquito bites on sensitized animals lead to a reduction in the local blood flow by constriction of blood vessels during local cutaneous anaphylaxis and to occlusion of the blood vessels by local edema (5, 42). Such hemostatic events may hinder mosquito feeding. However, this might also stimulate salivary secretion by the mosquito, with consequent injection of more sporozoites (31). (iii) A central event of immediate hypersensitivity reactions is antigen-induced degranulation of mast cells. This may occur after the bite of Anopheles mosquitoes, even in the absence of mediation by antibodies (11). Mast cells contain materials with multiple biological activities; a significant early effect of mast cell degranulation is a localized increase in vascular permeability and edema, permitting enhanced flow of immunoglobulins to avascular tissue. Because mosquito bite-induced mast cell degranulation can occur even in immunologically naive hosts (11), it is conceivable that mosquito saliva could directly influence the infectivity of sporozoites deposited at the bite site. Nevertheless, the results we have reported in the present study fail to support such a hypothesis. Thus, we examined the effects of prior sensitization to mosquito bite on sporozoites introduced into the bite site.
Immediate, cutaneous, hypersensitivity is associated with such rapid changes in cutaneous blood vessels and avascular tissue that it could affect the delivery of sporozoites by mosquitoes and the ability of these sporozoites to reach the blood. Because these events occur immediately after a mosquito bite, it is impossible to predict a priori whether they might enhance or inhibit sporozoite migration into blood. However, sporozoite transmission in the face of hypersensitivity to mosquito bite is how malaria is transmitted to many people in the real world, and it behooves us to consider it.
Our results showed no significant difference between hyperimmunized and control mice in either the numbers of sporozoites deposited at the bite site, the rate at which sporozoites left the bite site, or in the characteristics of blood infections resulting from challenge by mosquitoes infected with viable sporozoites. This extends previous studies that showed no differences in feeding behavior of the mosquitoes feeding on saliva-immunized versus nonimmunized mice (23). Our results differ substantially from those of others (12), who reported on studies with the A. stephensi-P. yoelii-mouse system. By assessing the “parasite burden” of the liver by reverse transcription-PCR (RT-PCR) at 40 h after mosquito challenge (a time when the liver stages are fully developed), these authors (12) reported significantly reduced parasite numbers in saliva-sensitized mice.
Most, if not all sporozoites injected by mosquitoes are introduced into avascular skin and subcutaneous tissue rather than directly into the circulation (4, 18, 39). The malaria infection may then continue when some of the injected sporozoites move from avascular tissue into dermal blood vessels and subsequently to the liver. Dramatic local changes occur at the bite site immediately after a mosquito has bitten a sensitized host; these center on degranulation of mast cells, with consequent rapid changes in cutaneous blood vessels and surrounding tissues. Considering these changes within the local environment of migrating sporozoites, it is striking that we have not been able to show any differences in the numbers of sporozoites delivered or in the ability of these sporozoites to move out of the bite area in mice hypersensitized to saliva. Obviously, sporozoites need to have evolved so that they are adapted to function efficiently in such a modified environment. Our results agree with those of other workers who reported no significant differences in sporozoite load in the ear between immunologically naive and saliva-sensitized mice at either 5 or 10 h after the mosquito bite (12). Because sporozoites in the skin become nonmotile and degraded after several hours (19, 39), it is not clear whether the reduction in sporozoite load reported in the skin of saliva-sensitized mice between 5 and 10 h by others (12) is due to a reduction in the actual numbers of sporozoites or to a degradation of the sporozoite signal as they determined by RT-PCR.
These researchers (12) do, however, report a substantial reduction in parasitemia, although not in the prepatent period, in sensitized mice after challenge by mosquito bite. These authors conclude that the protective mechanism they observed was due to a host T-helper 1 response induced by sensitization against mosquito saliva and acting against pre-erythrocytic parasites that develop within the liver. In support of their own findings, these researchers (12) commented further that an older series of studies had used repeated vaccination with large amounts of mosquito salivary gland homogenate and demonstrated that this conferred partial protection to P. berghei infection in mice (1, 2). Nevertheless, the only protection reported in these earlier studies was when both immunization and challenges were done intraperitoneally. Only 4 of 100 mice were protected, whereas none of 74 mice challenged intravenously was protected. Thus, it is simplest to ascribe these results (1, 2) to nonspecific peritoneal inflammation that led to a failure of sporozoite infectivity in several mice.
Our own results have failed to demonstrate any significant effect on sporozoite delivery or sporozoite infectivity in mice hyperimmunized to mosquito saliva. Under natural conditions, sporozoites are delivered both to individuals who may exhibit immediate, cutaneous hypersensitivity to mosquito bite and to others who either may have not yet developed such reactivity or have become desensitized. It was tempting to hypothesize that differences in responsiveness to mosquito bite by different individuals might modulate the infectivity of sporozoites delivered into a milieu of changes induced by cutaneous hypersensitivity. Our results with a rodent malaria model, however, have not been able to support such a hypothesis. One must caution, however, that even though mice exhibit a strong immediate, cutaneous hypersensitivity to mosquito bites, they do not exhibit the typical wheal and flare reaction characteristic of sensitized humans. Thus, the applicability of our results to humans remains to be confirmed.
Supplementary Material
Acknowledgments
We thank Adela Nacer for help with the statistics.
This study was supported by Public Health Service grant AI63530 from the NIH Institute of Allergy and Infectious Diseases to J.V.
Editor: J. H. Adams
Footnotes
Published ahead of print on 2 November 2009.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Alger, N. E., and J. Harant. 1976. Plasmodium berghei: sporozoite challenge, protection, and hypersensitivity in mice. Exp. Parasitol. 40:273-280. [DOI] [PubMed] [Google Scholar]
- 2.Alger, N. E., J. A. Harant, L. C. Willis, and G. M. Jorgensen. 1972. Sporozoite and normal salivary gland induced immunity in malaria. Nature 238:341. [DOI] [PubMed] [Google Scholar]
- 3.Amino, R., R. Menard, and F. Frischknecht. 2005. In vivo imaging of malaria parasites: recent advances and future directions. Curr. Opin. Microbiol. 8:407-414. [DOI] [PubMed] [Google Scholar]
- 4.Amino, R., S. Thiberge, B. Martin, S. Celli, S. Shorte, F. Frischknecht, and R. Menard. 2006. Quantitative imaging of Plasmodium transmission from mosquito to mammal. Nat. Med. 12:220-224. [DOI] [PubMed] [Google Scholar]
- 5.Bandmann, H. J., and K. Bosse. 1967. Histologie des Mückenstiches (Aedes aegypti). Arch. Klin. Exp. Dermatol. 231:59-67. [PubMed] [Google Scholar]
- 6.Benjamini, E., and B. F. Feingold. 1970. Immunity to arthropods, p. 1061-1134. In G. L. Jackson, R. Herman, and I. Singer (ed.), Immunity to parasitic animals, vol. 2. Meredith, New York, NY. [Google Scholar]
- 7.Bezerra, H. S., and M. J. Teixeira. 2001. Effect of Lutzomyia whitmani (Diptera: Psychodidae) salivary gland lysates on Leishmania (Viannia) braziliensis infection in BALB/c mice. Mem. Inst. Oswaldo Cruz 96:349-351. [DOI] [PubMed] [Google Scholar]
- 8.Castro-Sousa, F., M. Paranhos-Silva, I. Sherlock, M. S. Paixao, L. C. Pontes-de-Carvalho, and W. L. dos-Santos. 2001. Dissociation between vasodilation and Leishmania infection-enhancing effects of sand fly saliva and maxadilan. Mem. Inst. Oswaldo Cruz 96:997-999. [DOI] [PubMed] [Google Scholar]
- 9.Chattopadhyay, R., S. Conteh, M. Li, E. R. James, J. E. Epstein, and S. L. Hoffman. 2009. The Effects of radiation on the safety and protective efficacy of an attenuated Plasmodium yoelii sporozoite malaria vaccine. Vaccine 27:3675-3680. [DOI] [PubMed] [Google Scholar]
- 10.Chen, Y. L., F. E. Simons, and Z. Peng. 1998. A mouse model of mosquito allergy for study of antigen-specific IgE and IgG subclass responses, lymphocyte proliferation, and IL-4 and IFN-gamma production. Int. Arch. Allergy Immunol. 116:269-277. [DOI] [PubMed] [Google Scholar]
- 11.Demeure, C. E., K. Brahimi, F. Hacini, F. Marchand, R. Peronet, M. Huerre, P. St-Mezard, J. F. Nicolas, P. Brey, G. Delespesse, and S. Mecheri. 2005. Anopheles mosquito bites activate cutaneous mast cells leading to a local inflammatory response and lymph node hyperplasia. J. Immunol. 174:3932-3940. [DOI] [PubMed] [Google Scholar]
- 12.Donovan, M. J., A. S. Messmore, D. A. Scrafford, D. L. Sacks, S. Kamhawi, and M. A. McDowell. 2007. Uninfected mosquito bites confer protection against infection with malaria parasites. Infect. Immun. 75:2523-2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans, N. T., P. F. Naylor, and G. Rowlinson. 1981. Diffusion of oxygen through the mouse ear. Br. J. Dermatol. 105:45-56. [DOI] [PubMed] [Google Scholar]
- 14.Francischetti, I. M., J. G. Valenzuela, and J. M. Ribeiro. 1999. Anophelin: kinetics and mechanism of thrombin inhibition. Biochemistry 38: 16678-16685. [DOI] [PubMed] [Google Scholar]
- 15.Frevert, U., S. Engelmann, S. Zougbede, J. Stange, B. Ng, K. Matuschewski, L. Liebes, and H. Yee. 2005. Intravital observation of Plasmodium berghei sporozoite infection of the liver. PLoS Biol. 3:e192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gillett, J. D. 1967. Natural selection and feeding speed in a blood-sucking insect. Proc. R. Soc. Lond. B Biol. Sci. 167:316-329. [DOI] [PubMed] [Google Scholar]
- 17.Jaffe, R. I., G. H. Lowell, and D. M. Gordon. 1990. Differences in susceptibility among mouse strains to infection with Plasmodium berghei (ANKA clone) sporozoites and its relationship to protection by gamma-irradiated sporozoites. Am. J. Trop. Med. Hyg. 42:309-313. [DOI] [PubMed] [Google Scholar]
- 18.Jin, Y., C. Kebaier, and J. Vanderberg. 2007. Direct microscopic quantification of dynamics of Plasmodium berghei sporozoite transmission from mosquitoes to mice. Infect. Immun. 75:5532-5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kebaier, C., T. Voza, and J. Vanderberg. 2009. Kinetics of mosquito-injected Plasmodium sporozoites in mice: fewer sporozoites are injected into sporozoite-immunized mice. PLoS Pathog. 5:e1000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan, Z. M., and J. P. Vanderberg. 1991. Role of host cellular response in differential susceptibility of nonimmunized BALB/c mice to Plasmodium berghei and Plasmodium yoelii sporozoites. Infect. Immun. 59:2529-2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kramer, L. D., and J. P. Vanderberg. 1975. Intramuscular immunization of mice with irradiated Plasmodium berghei sporozoites. Enhancement of protection with albumin. Am. J. Trop. Med. Hyg. 24:913-916. [DOI] [PubMed] [Google Scholar]
- 22.Marinotti, O., M. de Brito, and C. K. Moreira. 1996. Apyrase and alpha-glucosidase in the salivary glands of Aedes albopictus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 113:675-679. [DOI] [PubMed] [Google Scholar]
- 23.Mathews, G. V., S. Sidjanski, and J. P. Vanderberg. 1996. Inhibition of mosquito salivary gland apyrase activity by antibodies produced in mice immunized by bites of Anopheles stephensi mosquitoes. Am. J. Trop. Med. Hyg. 55:417-423. [DOI] [PubMed] [Google Scholar]
- 24.Most, H., R. S. Nussenzweig, J. Vanderberg, R. Herman, and M. Yoeli. 1966. Susceptibility of genetically standardized (JAX) mouse strains to sporozoite- and blood-induced Plasmodium berghei infections. Milit. Med. 131(Suppl.):915-918. [PubMed] [Google Scholar]
- 25.Paranhos-Silva, M., G. G. Oliveira, E. A. Reis, R. M. de Menezes, O. Fernandes, I. Sherlock, R. B. Gomes, L. C. Pontes-de-Carvalho, and W. L. dos-Santos. 2003. A follow-up of beagle dogs intradermally infected with Leishmania chagasi in the presence or absence of sand fly saliva. Vet. Parasitol. 114:97-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reunala, T., H. Brummer-Korvenkontio, P. Lappalainen, L. Rasanen, and T. Palosuo. 1990. Immunology and treatment of mosquito bites. Clin. Exp. Allergy 20(Suppl. 4):19-24. [DOI] [PubMed] [Google Scholar]
- 27.Ribeiro, J. M. 1987. Role of saliva in blood-feeding by arthropods. Annu. Rev. Entomol. 32:463-478. [DOI] [PubMed] [Google Scholar]
- 28.Ribeiro, J. M., and I. M. Francischetti. 2003. Role of arthropod saliva in blood feeding: sialome and post-sialome perspectives. Annu. Rev. Entomol. 48:73-88. [DOI] [PubMed] [Google Scholar]
- 29.Ribeiro, J. M., P. A. Rossignol, and A. Spielman. 1984. Role of mosquito saliva in blood vessel location. J. Exp. Biol. 108:1-7. [DOI] [PubMed] [Google Scholar]
- 30.Ribeiro, J. M., R. H. Nussenzveig, and G. Tortorella. 1994. Salivary vasodilators of Aedes triseriatus and Anopheles gambiae (Diptera: Culicidae). J. Med. Entomol. 31:747-753. [DOI] [PubMed] [Google Scholar]
- 31.Rossignol, P. A., J. M. Ribeiro, and A. Spielman. 1984. Increased intradermal probing time in sporozoite-infected mosquitoes. Am. J. Trop. Med. Hyg. 33:17-20. [DOI] [PubMed] [Google Scholar]
- 32.Scheller, L. F., R. A. Wirtz, and A. F. Azad. 1994. Susceptibility of different strains of mice to hepatic infection with Plasmodium berghei. Infect. Immun. 62:4844-4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spitalny, G. L., and R. S. Nussenzweig. 1972. Effects of various routes of immunization and methods of parasite attenuation on the development of protection against sporozoite-induced rodent malaria. Milit. Med. 39(Special Issue):506-514. [Google Scholar]
- 34.Stark, K. R., and A. A. James. 1996. Salivary gland anticoagulants in culicine and anopheline mosquitoes (Diptera: Culicidae). J. Med. Entomol. 33:645-650. [DOI] [PubMed] [Google Scholar]
- 35.Tarun, A. S., K. Baer, R. F. Dumpit, S. Gray, N. Lejarcegui, U. Frevert, and S. H. Kappe. 2006. Quantitative isolation and in vivo imaging of malaria parasite liver stages. Int. J. Parasitol. 36:1283-1293. [DOI] [PubMed] [Google Scholar]
- 36.Titus, R. G., J. V. Bishop, and J. S. Mejia. 2006. The immunomodulatory factors of arthropod saliva and the potential for these factors to serve as vaccine targets to prevent pathogen transmission. Parasite Immunol. 28:131-141. [DOI] [PubMed] [Google Scholar]
- 37.Trager, W. 1939. Acquired immunity to ticks. J. Parasitol. 25:57-81. [Google Scholar]
- 38.Vanderberg, J. P. 1977. Plasmodium berghei: quantitation of sporozoites injected by mosquitoes feeding on a rodent host. Exp. Parasitol. 42:169-181. [DOI] [PubMed] [Google Scholar]
- 39.Vanderberg, J. P., and U. Frevert. 2004. Intravital microscopy demonstrating antibody-mediated immobilisation of Plasmodium berghei sporozoites injected into skin by mosquitoes. Int. J. Parasitol. 34:991-996. [DOI] [PubMed] [Google Scholar]
- 40.Vanderberg, J., and R. Gwadz. 1980. The transmission by mosquitoes of plasmodia in the laboratory p. 154-218. In J. Kreier (ed.), Malaria: pathology, vector studies and culture, vol. 2. Academic Press, Inc., New York, NY. [Google Scholar]
- 41.Vaughan, J. A., L. F. Scheller, R. A. Wirtz, and A. F. Azad. 1999. Infectivity of Plasmodium berghei sporozoites delivered by intravenous inoculation versus mosquito bite: implications for sporozoite vaccine trials. Infect. Immun. 67:4285-4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson, A. B., and A. N. Clements. 1965. The nature of the skin reaction to mosquito bites in laboratory animals. Int. Arch. Allergy Appl. Immunol. 26:294-314. [DOI] [PubMed] [Google Scholar]
- 43.Yoshida, S., T. Sudo, M. Niimi, L. Tao, B. Sun, J. Kambayashi, H. Watanabe, E. Luo, and H. Matsuoka. 2008. Inhibition of collagen-induced platelet aggregation by anopheline antiplatelet protein, a saliva protein from a malaria vector mosquito. Blood 111:2007-2014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.