Abstract
T-helper 17 (Th17) cells are characterized by their production of interleukin-17 (IL-17) and have a role in the protection against infections and in certain inflammatory diseases. Humans who lack Th17 cells are more susceptible to Staphylococcus aureus infections compared to individuals having Th17 cells. S. aureus is part of the commensal skin microflora and also colonize the infant gut. To investigate whether UV-killed S. aureus would be more capable of inducing IL-17 than other commensal bacteria, we stimulated mononuclear cells from adults, infants, and newborns with various gram-positive and gram-negative commensal bacteria. IL-17 was produced from adult memory Th17 cells after stimulation with superantigen-producing S. aureus but not nonsuperantigenic S. aureus or other common commensal gut bacteria. Cells from newborns were poor IL-17 producers after stimulation with S. aureus, whereas in some cases IL-17 was secreted from cells isolated from infants at the age of 4 and 18 months. These results suggest that superantigenic S. aureus are particularly efficient in stimulating IL-17 production and that the cytokine is produced from memory T cells.
The recently discovered T-helper 17 (Th17) cell, characterized by the ability to produce interleukin-17 (IL-17) and IL-22, is a distinct T-helper subset with the lineage-specific transcription factor RORγt (10, 20). The factors required for driving the differentiation of naive cells to Th17 cells in mice are transforming growth factor β (TGF-β) and IL-6, whereas in humans IL-1β and IL-23 are needed in addition to TGF-β and IL-6 (20, 28). The combination of these cytokines induces the expression of RORγt in a STAT3-dependent manner (19). Th17 cells have been shown to be involved in the pathogenesis of several inflammatory diseases such as rheumatoid arthritis, psoriasis, and inflammatory bowel disease (IBD) (9, 15, 24). For example, patients suffering from Crohn's disease display higher levels of IL-17 in serum, as well as stronger expression of IL-17 in the colonic mucosa, than healthy subjects (9). The IL-17-producing T cells found in the gut of Crohn's patients are likely to be Th17 cells, based on their expression of CCR6, IL-23R, and RORγt (5). Furthermore, IL-17 has been ascribed a role in protective immunity against both intracellular and extracellular bacteria, as well as fungi, although this has not been fully elucidated (reviewed in reference 22). The mechanisms behind the protective effects are varied; for example, IL-17 is able to induce the production of antibacterial peptides such as β-defensins in human airway epithelial cells (12). Moreover, IL-17 attracts neutrophils by regulating chemokines such as CXCL8/IL-8 (14). Mice deficient in the IL-17 receptor have been shown to be sensitive to intranasal infection with the gram-negative bacterium Klebsiella pneumoniae and displayed an impaired neutrophil recruitment compared to wild-type mice (29). However, humans deficient in Th17 cells are highly susceptible to infections caused by the gram-positive bacterium Staphylococcus aureus. Such Th17 deficiency has been discovered in patients with hyper-immunoglobulin E syndrome, which in most cases is caused by mutations in the DNA-binding domain of STAT3 (19, 25). These findings make it reasonable to suggest that Th17 cells might have a role in the host protection against S. aureus.
In adults, S. aureus primarily colonize the skin in the nasal area but in newborns S. aureus has emerged as a major early colonizer of the gut (17, 18). The gastrointestinal tract is the major site of colonization by commensal bacteria and as many of these bacterial populations are acquired shortly after birth, they are important for the development and maturation of the immune system early in life (26). About half of the S. aureus strains colonizing infants by 1 week of life produce one or more toxins with superantigenic functions, most commonly staphylococcal enterotoxin C (SEC) (54%), followed by a combination of toxic shock syndrome toxin 1 (TSST-1) and staphylococcal enterotoxin A (SEA) (46%) (18). Each of these superantigenic toxins stimulate 10 to 30% of all T cells without being processed by antigen-presenting cells (APC) (21). Superantigens bind directly to specific regions of major histocompatibility complex class II molecules on the APC and to the variable region of the β chain (Vβ) of the T-cell receptor (TCR) (6).
The aim of the present study was to evaluate the capacity of S. aureus, compared to other fecal commensal bacteria isolated from healthy infants, to stimulate production of IL-17 in mononuclear cells from adults, infants, and newborns and to determine the cellular origin of IL-17. We show that superantigenic S. aureus are strong inducers of IL-17 from adult memory Th17 cells, in contrast to nonsuperantigenic S. aureus and other commensal intestinal bacterial strains. However, superantigenic S. aureus are poor inducers of IL-17 in T cells from infants and newborns.
MATERIALS AND METHODS
Cell isolation and culture.
Venous blood samples were obtained from healthy volunteers and collected in sterile heparinized tubes. Cord blood samples were drawn from the umbilical cord of healthy babies born at the Sahlgrenska University Hospital (Mölndal, Sweden). Blood samples were also provided from healthy 4- and 18-month-old children who were included in the prospective FARMFLORA study. The study was approved by the Human Research Ethics Committee of the Medical Faculty at the Sahlgrenska Academy, Gothenburg University. Peripheral blood mononuclear cells (PBMC), as well as cord blood mononuclear cells (CBMC), were isolated by density gradient centrifugation on Lymphoprep (Fresenius Kabi, Oslo, Norway). In the CD4-positive cell separation experiment, CD4-positive cells were purified by using Dynabead CD4-positive isolation kit (Dynal Biotech ASA, Oslo, Norway). The purity of the CD4-positive cells was typically >94%. Cells were cultured (37°C, 5% CO2) in the serum-free medium X-vivo 15 (Lonza, Verviers, Belgium) supplemented with 1% l-glutamine (Sigma-Aldrich, St. Louis, MO) in 96-well culture plates (2 × 106 cells/ml) for 72 h. PBMC and CBMC were stimulated in duplicate with various UV-killed bacteria (5 × 107/ml), as shown in Table 1, or with 2 ng of purified SEA, SEB, or TSST-1 (Sigma-Aldrich)/ml. Supernatants were collected on day 3 and stored at −20°C until analysis.
TABLE 1.
Bacterial strains
| Straina | Sourceb |
|---|---|
| Gram-positive bacteria | |
| Enterococcus faecalis | AF 137.1.7 |
| Bifidobacterium bifidum | CCUG 18364 |
| Lactobacillus paracasei | AF 36.4.15 |
| Lactobacillus rhamnosus | AF 15.5.23 |
| Clostridium difficile | AF 140.6.21 |
| Clostridium perfringens | AF 137.4.22 |
| Staphylococcus aureus nonsuperantigenic 1 | AF 122.1.3 |
| Staphylococcus aureus nonsuperantigenic 2 | AF 7.0.1 |
| Staphylococcus aureus SEA 1 | AF 18.2.7 |
| Staphylococcus aureus SEA 2 | AF 21.2.6 |
| Staphylococcus aureus SEB 1 | AF 14.0.5 |
| Staphylococcus aureus SEB 2 | AFI 12:4:5 |
| Staphylococcus aureus SEC | AF 8.0.7 |
| Staphylococcus aureus SEC 1 | AF 137.1.4 |
| Staphylococcus aureus SEC 2 | AF 39.0.3 |
| Staphylococcus aureus TSST-1 1 | AF 181.1.5 |
| Staphylococcus aureus TSST-1 2 | AF 27.1.3 |
| Staphylococcus aureus TSST-1/SEA 1 | AF 11.1.6 |
| Staphylococcus aureus TSST-1/SEA 2 | AF 72.0.5 |
| Gram-negative bacteria | |
| Bacteroides fragilis | AF 137.6.12 |
| Escherichia coli | AF 120.4.1 |
All strains were obtained from infant fecal samples.
AF, Allergyflora Study, in collaboration with the Department of Clinical Bacteriology, University of Gothenburg; CCUG, Culture Collection of the University of Gothenburg; AFI, Allergyflora Study Italy, in collaboration with the Department of Clinical Bacteriology, University of Gothenburg.
Intestinal bacterial strains.
The bacterial strains used in the present study were isolated from healthy infants in the Allergyflora study and cultured from stool samples obtained at 1, 2, 4, and 8 weeks and at 6 and 12 months of age as previously described in detail (2). Bacteria were identified to the species level by using biochemical or molecular methods (2, 4, 18). S. aureus isolates were tested for the production of enterotoxins A, B, C, and D (SET-RPLA kit) and TSST-1 (TST-RPLA kit) (both from Oxoid, Hampshire, Great Britain) (18). Prior to use in cell culture the bacteria were inactivated by exposure to UV light (280 to 315 nm) for 15 to 20 min. Complete UV inactivation was confirmed by negative viable count, i.e., the inability of the bacteria to grow when cultured on horse blood agar for 24 h. Bacteria were stored at −70°C until use.
Cytokine measurements.
Secreted IL-17, gamma interferon (IFN-γ), IL-6, and tumor necrosis factor (TNF) in culture supernatants were quantified by in-house sandwich enzyme-linked immunosorbent assays (ELISAs), as described previously (13). Briefly, Costar plates (1/2 area; Invitrogen, San Diego, CA) were coated with monoclonal anti-human IL-17, IFN-γ, IL-6, or TNF antibodies; secreted cytokines were subsequently detected with biotinylated anti-human antibodies; and recombinant human IL-17, IFN-γ, IL-6, or TNF were used as standards. Antibodies and standards for the IFN-γ, IL-6, and TNF ELISAs were purchased from BD Pharmingen (San Jose, CA), and antibodies and standards for the IL-17 ELISA were purchased from R&D Systems (Minneapolis, MN). Samples, standards, and biotinylated antibodies were diluted in high-performance ELISA buffer (HPE; Sanquin, Amsterdam, The Netherlands). Absorbance was measured at 450 nm by using a SpectraMax Plus spectrophotometer (Molecular Devices, Downingtown, PA). Raw data were processed, and standard curves generated with the computer software SoftMax Pro (Molecular Devices) and Deltasoft (BioMetallics, Inc., Princeton, NJ). The detection limits for the assays were as follows: 20 pg/ml for IL-17, 0.1 ng/ml for IFN-γ, 0.6 ng/ml for IL-6, and 0.025 ng/ml for TNF.
Surface and intracellular staining for flow cytometry analysis.
After 3 days of culture, cells were reactivated with phorbol myristate acetate (50 ng/ml; Sigma-Aldrich) and ionomycin (1 μg/ml; Sigma-Aldrich) in the presence of Golgistop (BD Biosciences, Franklin Lakes, NJ) for at least 3 h. Cells were labeled for surface markers using anti-CD3 PerCP, anti-CD8 Pacific Blue, anti-CD45RA fluorescein isothiocyanate, or anti-CD45RO PE (BD Biosciences) for approximately 20 min in 8°C, followed by fixation in FIX/PERM buffer (eBioscience, San Diego, CA), permeabilization with PERM buffer (eBioscience), blocking by the addition of 2% normal rat serum (eBioscience), and intracellular staining with anti-IL-17 Alexa fluor 647 (eBioscience) for 30 min in 8°C. Fluorescence-minus-one or isotype controls were used as controls. Cells were analyzed by using FACSCantoII (BD Biosciences), and data were further processed by using FlowJo software (Three Star, Inc., Ashland, VA).
Statistical analysis.
For statistical evaluations, the Kruskal-Wallis test, followed by post-hoc analysis, was used for comparisons between all groups. A P value of <0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Superantigen-producing S. aureus are potent inducers of IL-17 production from adult memory Th17 cells.
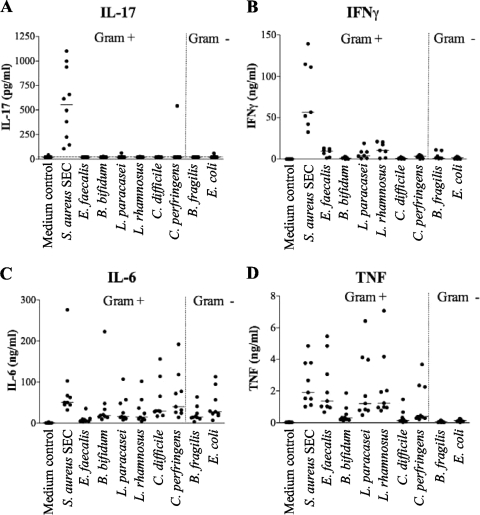
To investigate whether commensal intestinal bacteria would induce IL-17 production, we stimulated PBMC for 72 h with a selection of UV-killed gram-positive and gram-negative bacteria, as shown in detail in Table 1 (S. aureus producing SEC, Enterococcus faecalis, Bifidobacterium bifidum, Lactobacillus paracasei, Lactobacillus rhamnosus, Clostridium difficile, Clostridium perfringens, Bacteroides fragilis, and Escherichia coli) and determined the concentration of secreted IL-17 in the supernatant. We found that SEC-producing S. aureus, but none of the other bacteria, was able to consistently induce IL-17 secretion from PBMC (Fig. 1A). On the other hand, all of the gram-positive and gram-negative bacterial strains induced IFN-γ production, but similar to IL-17 production, stimulation with SEC-producing S. aureus resulted in the highest levels of IFN-γ (Fig. 1B). Furthermore, IL-6 was secreted after stimulation by any of the bacterial strains tested (Fig. 1C), whereas TNF was secreted upon stimulation with gram-positive but not gram-negative strains (Fig. 1D), as previously shown (11, 13). Thus, all bacteria were capable of evoking an inflammatory response from PBMC, but IL-17 was only consistently induced by SEC-producing S. aureus.
FIG. 1.
Production of IL-17, IFN-γ, IL-6, and TNF by human PBMC after stimulation with commensal bacteria. The concentrations of IL-17 (A), IFN-γ (B), IL-6 (C), and TNF (D) in the supernatants of PBMC cultured for 72 h, together with UV-killed gram-positive (Gram+) bacteria, i.e., S. aureus SEC, E. faecalis, B. bifidum, L. paracasei, L. rhamnosus, C. difficile, C. perfringens, or the gram-negative (gram-) bacteria B. fragilis or E. coli, were determined. Each dot represents one individual, with at least nine different individuals in each group, and the horizontal bar represents the median.
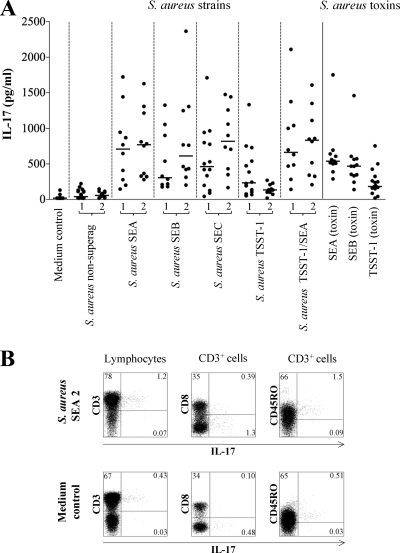
To investigate whether the induction of IL-17 was caused by the S. aureus bacterium itself or the superantigen, we stimulated PBMC with a selection of both UV-killed nonsuperantigenic and superantigen-producing strains of S. aureus (SEA, SEB, SEC, and TSST-1), which were obtained from infant fecal samples (Table 1). In Fig. 2A we show that superantigen-producing S. aureus were significantly more efficient in stimulating IL-17 production from PBMC compared to the nonsuperantigenic strains (S. aureus nonsuperantigenic 1 or 2 versus S. aureus SEA 1 or 2, S. aureus SEB 1 or 2, S. aureus SEC 1 or 2, S. aureus TSST-1 1, or S. aureus TSST-1/SEA 1 or 2; P < 0.001). Interestingly, the S. aureus strains only producing TSST-1 seemed less IL-17 stimulatory compared to the other strains producing SEA, SEB, SEC, or TSST-1/SEA (S. aureus nonsuperantigenic 1 or 2 versus S. aureus TSST-1 2; P < 0.05). Stimulation with the purified staphylococcal toxins TSST-1, SEA, and SEB induced increased IL-17 production compared to the medium control (P < 0.001), and TSST-1 tended to induce lower levels of IL-17 than SEA and SEB (Fig. 2A). To determine which cells were responsible for the IL-17 secretion induced by superantigenic S. aureus, we stimulated PBMC with SEA-producing S. aureus and stained for intracellular IL-17 (Fig. 2B). We found that the IL-17-secreting cells stimulated by superantigenic S. aureus were CD4-positive memory T cells, since the majority of the IL-17-producing cells were CD3 positive and CD8 negative, and also expressed CD45RO.
FIG. 2.
Production of IL-17 from adult PBMC in response to S. aureus strains and superantigens. (A) PBMC from at least 10 different blood donors were cultured for 72 h in the presence of nonsuperantigenic strains of S. aureus, superantigenic strains (producing SEA, SEB, SEC, TSST-1, or SEA/TSST-1), or pure staphylococcal toxins (SEA, SEB, or TSST-1). (B) After coculture with SEA-producing S. aureus, PBMC were restimulated with phorbol myristate acetate and ionomycin in the presence of Golgistop, and the intracellular expression of IL-17 was analyzed by flow cytometry, as well as the surface expression of CD3, CD8, and CD45RO. Percentages of all memory CD3+ CD45RO+ IL-17+ T cells are shown in the upper right quadrant in the right panel.
Superantigens activate T cells by directly cross-linking the conserved regions of the variable domains of the TCR β-chain with major histocompatibility complex class II molecules on the APC. In order to investigate the effect of APC on CD4-positive cells upon S. aureus stimulation, CD4-positive cells were isolated by using magnetic bead separation and stimulated with superantigenic and nonsuperantigenic strains of S. aureus, as well as purified S. aureus toxins (Table 2). We show that the stimulated CD4-positive cells induced considerably lower levels of IL-17 than the cultures with stimulated PBMC. The reductions in median levels of IL-17 were >67% when we compared PBMC and purified CD4-positive cells after stimulation with the different superantigen-producing S. aureus strains (Table 2 and Fig. 2A). The purity of CD4-positive cells after the separation is typically >94%; therefore, it is likely that APC are still present in the separated cell fraction and can enable the minor production of IL-17 from the CD4-positive cells observed in Table 2. In Fig. 2A, analysis of the IL-17 responses from individual donors revealed large variations depending on the superantigenic strain used, i.e., it is possibly not the number of Th17 cells in an individual donor that decides the magnitude of the IL-17 response, but rather the type of TCR β-chain on the cells. Altogether, these results indicate that the IL-17-inducing capacity of S. aureus is associated with the superantigen-producing abilities of the bacteria. However, the bacteria were UV killed and therefore no longer able to actively secrete toxins, as confirmed by testing the bacterium-stimulated cell culture supernatants for presence of toxins (data not shown). The absence of soluble toxins in the culture suggests that the bacteria harbor membrane-bound toxins, which may be sufficient to stimulate IL-17 secretion. Confirming our in vitro results, a study by Eyerich et al. recently showed that application of SEB, together with allergen, in an atopy patch test in vivo induced IL-17 production, which was not induced by the allergen alone (7).
TABLE 2.
Production of IL-17 from purified CD4-positive cells in response to stimulation with S. aureus strains or toxins
| Stimulation | IL-17 concn (pg/ml) (n = 3) |
|
|---|---|---|
| Median | Rangea | |
| Medium control | 20 | 20-20 |
| S. aureus nonsuperantigenic 1 | 20 | 20-33 |
| S. aureus nonsuperantigenic 2 | 20 | 20-26 |
| S. aureus SEA 1 | 156 | 92-417 |
| S. aureus SEA 2 | 173 | 133-334 |
| S. aureus SEB 1 | 70 | 43-96 |
| S. aureus SEB 2 | 203 | 122-373 |
| S. aureus SEC 1 | 96 | 55-224 |
| S. aureus SEC 2 | 115 | 53-136 |
| S. aureus TSST-1 1 | 20 | 20-62 |
| S. aureus TSST-1 2 | 38 | 20-40 |
| S. aureus TSST-1/SEA 1 | 174 | 74-211 |
| S. aureus TSST-1/SEA 2 | 209 | 121-400 |
| SEA (toxin) | 39 | 20-52 |
| SEB (toxin) | 20 | 20-20 |
| TSST-1 (toxin) | 20 | 20-379 |
Detection limit, 20 pg/ml.
It has recently been shown that several gram-positive and gram-negative bacterial strains from the commensal flora, as well as various Toll-like receptor (TLR) ligands, are able to induce IL-17 production from memory T cells via dendritic cells (27). However, in that study SEB was added in the final step of the cultures, which might explain the IL-17 stimulatory ability of bacterial species that were nonstimulatory in our hands. Furthermore, it has been proposed that peptidoglycan is more efficient than other TLR ligands in inducing Th17 cells, by stimulating APC to secrete cytokines that favor Th17 differentiation (1, 27). The significance of these results is now questioned since it has been shown that commercial peptidoglycan preparations made from S. aureus and S. pyogenes are contaminated with superantigens, which stimulate the production of IL-17 independently of TLR-2 signaling (16).
Superantigenic S. aureus and the IL-17 production from mononuclear cells in newborns and infants.
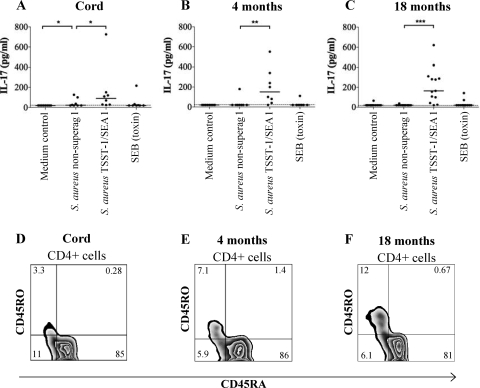
To investigate whether superantigenic S. aureus or purified S. aureus toxins would induce IL-17 production in cells from newborns and infants, we stimulated mononuclear cells from cord blood and 4- and 18-month-old infants. We found that CBMC were poor producers of IL-17, whereas PBMC from some 4- and 18-month-old children produced IL-17, although at much lower levels than adult cells (Fig. 3A to C). Interestingly, and in contrast to the results with cells from adults, purified SEB did not induce levels of IL-17 comparable to the levels obtained with superantigenic S. aureus in cells from infants. Thus, when the threshold for IL-17 production is higher, as in cells from infants, UV-killed S. aureus seem to be a more efficient stimulus than purified staphylococcal enterotoxin. CBMC were also stimulated with purified SEA and TSST-1, but none of the toxins induced any IL-17 production from CBMC (data not shown). The capacity of IL-17 secretion after S. aureus stimulation also seemed to be related to the fraction of memory Th17 cells in the newborns and infants (Fig. 3D to F). We were not able to detect intracellular IL-17 in cells from the children due to the low IL-17 production. The lack of IL-17-producing memory cells might contribute to the increased frequency of staphylococcal skin infections in newborn children.
FIG. 3.
S. aureus-induced IL-17 production in mononuclear cells from neonates and infants. (A to C) CBMC from newborns (n = 8) or PBMC from 4- and 18-month-old infants (n = 8 and n = 13, respectively) were stimulated with nonsuperantigenic S. aureus, TSST-1/SEA-producing S. aureus, or pure SEB for 72 h, and secreted IL-17 was detected by ELISA. Statistical analysis was performed by using Kruskal-Wallis test followed by post-hoc analysis: *, P < 0.05; **, P < 0.01; ***, P < 0.001. (D to F) CBMC from newborns or PBMC from infants were freshly isolated and immediately stained with fluorescing anti-CD4, anti-CD45RA, and anti-CD45RO and analyzed by flow cytometry.
Many newborn infants in the Western world have been shown to be early colonized in the intestine by toxin-producing S. aureus (3, 18). Thus, our results suggesting that superantigenic S. aureus is not capable of inducing IL-17 in newborns are reassuring, since such stimulation could otherwise lead to inflammation in the infant gut. The commensal intestinal microflora is not only an important immunostimulatory agent during maturation of the immune system but could also play a role in the pathogenesis of IBD (8). IL-17 has been postulated to play a role in the pathogenesis of IBD since the levels and expression of IL-17 are higher in the sera and colonic mucosa of Crohn's disease patients than in healthy subjects (5, 9). Perhaps an imbalance of the intestinal microflora of the adult IBD patient might be able to facilitate an overgrowth of superantigenic S. aureus, which in turn could stimulate the secretion of IL-17 that induces inflammation in the colonic mucosa. Supporting this hypothesis, superantigens have been implicated in the pathogenesis of IBD since blood and gut-derived lymphocytes from patients with Crohn's disease have been shown to contain an expanded population of T cells bearing the Vβ regions of TCRs (23).
In conclusion, we show that superantigenic S. aureus are strong inducers of IL-17 from adult human memory Th17 cells, in contrast to nonsuperantigenic S. aureus and other commensal intestinal bacterial strains. However, superantigenic S. aureus are poor inducers of IL-17 in T cells from infants, which is reassuring since many infants in the Western world are now being colonized with superantigenic S. aureus within a few days after birth.
Acknowledgments
This study was supported by the Swedish Research council (grant K2006-745X-IH455-05-3), by Medi-SAM (ALF), by the Swedish Foundation for Health Care Sciences and Allergy Research, by Adlerbert Research Foundation, by Stiftelsen Konrad och Helfrid Johanssons Forskningsfond, by Stiftelserna Sigurd och Elsa Goljes Minne, and by Magnus Bergvalls Stiftelse.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 12 October 2009.
REFERENCES
- 1.Acosta-Rodriguez, E. V., G. Napolitani, A. Lanzavecchia, and F. Sallusto. 2007. Interleukins 1β and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat. Immunol. 8:942-949. [DOI] [PubMed] [Google Scholar]
- 2.Adlerberth, I., E. Lindberg, N. Aberg, B. Hesselmar, R. Saalman, I. L. Strannegard, and A. E. Wold. 2006. Reduced enterobacterial and increased staphylococcal colonization of the infantile bowel: an effect of hygienic lifestyle? Pediatr. Res. 59:96-101. [DOI] [PubMed] [Google Scholar]
- 3.Adlerberth, I., D. P. Strachan, P. M. Matricardi, S. Ahrne, L. Orfei, N. Aberg, M. R. Perkin, S. Tripodi, B. Hesselmar, R. Saalman, A. R. Coates, C. L. Bonanno, V. Panetta, and A. E. Wold. 2007. Gut microbiota and development of atopic eczema in 3 European birth cohorts. J. Allergy Clin. Immunol. 120:343-350. [DOI] [PubMed] [Google Scholar]
- 4.Ahrne, S., E. Lonnermark, A. E. Wold, N. Aberg, B. Hesselmar, R. Saalman, I. L. Strannegard, G. Molin, and I. Adlerberth. 2005. Lactobacilli in the intestinal microbiota of Swedish infants: microbes and infection. Inst. Pasteur. 7:1256-1262. [DOI] [PubMed] [Google Scholar]
- 5.Annunziato, F., L. Cosmi, V. Santarlasci, L. Maggi, F. Liotta, B. Mazzinghi, E. Parente, L. Fili, S. Ferri, F. Frosali, F. Giudici, P. Romagnani, P. Parronchi, F. Tonelli, E. Maggi, and S. Romagnani. 2007. Phenotypic and functional features of human Th17 cells. J. Exp. Med. 204:1849-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi, Y. W., A. Herman, D. DiGiusto, T. Wade, P. Marrack, and J. Kappler. 1990. Residues of the variable region of the T-cell-receptor beta-chain that interact with Staphylococcus aureus toxin superantigens. Nature 346:471-473. [DOI] [PubMed] [Google Scholar]
- 7.Eyerich, K., D. Pennino, C. Scarponi, S. Foerster, F. Nasorri, H. Behrendt, J. Ring, C. Traidl-Hoffmann, C. Albanesi, and A. Cavani. 2009. IL-17 in atopic eczema: linking allergen-specific adaptive and microbial-triggered innate immune response. J. Allergy Clin. Immunol. 123:59-66. [DOI] [PubMed] [Google Scholar]
- 8.Frank, D. N., A. L. St. Amand, R. A. Feldman, E. C. Boedeker, N. Harpaz, and N. R. Pace. 2007. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 104:13780-13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujino, S., A. Andoh, S. Bamba, A. Ogawa, K. Hata, Y. Araki, T. Bamba, and Y. Fujiyama. 2003. Increased expression of interleukin 17 in inflammatory bowel disease. Gut 52:65-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrington, L. E., R. D. Hatton, P. R. Mangan, H. Turner, T. L. Murphy, K. M. Murphy, and C. T. Weaver. 2005. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6:1123-1132. [DOI] [PubMed] [Google Scholar]
- 11.Hessle, C. C., B. Andersson, and A. E. Wold. 2005. gram-positive and gram-negative bacteria elicit different patterns of pro-inflammatory cytokines in human monocytes. Cytokine 30:311-318. [DOI] [PubMed] [Google Scholar]
- 12.Kao, C. Y., Y. Chen, P. Thai, S. Wachi, F. Huang, C. Kim, R. W. Harper, and R. Wu. 2004. IL-17 markedly up-regulates beta-defensin-2 expression in human airway epithelium via JAK and NF-κB signaling pathways. J. Immunol. 173:3482-3491. [DOI] [PubMed] [Google Scholar]
- 13.Karlsson, H., C. Hessle, and A. Rudin. 2002. Innate immune responses of human neonatal cells to bacteria from the normal gastrointestinal flora. Infect. Immun. 70:6688-6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laan, M., Z. H. Cui, H. Hoshino, J. Lotvall, M. Sjostrand, D. C. Gruenert, B. E. Skoogh, and A. Linden. 1999. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J. Immunol. 162:2347-2352. [PubMed] [Google Scholar]
- 15.Langrish, C. L., Y. Chen, W. M. Blumenschein, J. Mattson, B. Basham, J. D. Sedgwick, T. McClanahan, R. A. Kastelein, and D. J. Cua. 2005. IL-23 drives a pathogenic T-cell population that induces autoimmune inflammation. J. Exp. Med. 201:233-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li, H., M. M. Nooh, M. Kotb, and F. Re. 2008. Commercial peptidoglycan preparations are contaminated with superantigen-like activity that stimulates IL-17 production. J. Leukoc. Biol. 83:409-418. [DOI] [PubMed] [Google Scholar]
- 17.Lindberg, E., I. Adlerberth, B. Hesselmar, R. Saalman, I. L. Strannegard, N. Aberg, and A. E. Wold. 2004. High rate of transfer of Staphylococcus aureus from parental skin to infant gut flora. J. Clin. Microbiol. 42:530-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindberg, E., F. Nowrouzian, I. Adlerberth, and A. E. Wold. 2000. Long-time persistence of superantigen-producing Staphylococcus aureus strains in the intestinal microflora of healthy infants. Pediatr. Res. 48:741-747. [DOI] [PubMed] [Google Scholar]
- 19.Ma, C. S., G. Y. Chew, N. Simpson, A. Priyadarshi, M. Wong, B. Grimbacher, D. A. Fulcher, S. G. Tangye, and M. C. Cook. 2008. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J. Exp. Med. 205:1551-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manel, N., D. Unutmaz, and D. R. Littman. 2008. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORγt. Nat. Immunol. 9:641-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marrack, P., and J. Kappler. 1990. The staphylococcal enterotoxins and their relatives. Science 248:1066. [PubMed] [Google Scholar]
- 22.Matsuzaki, G., and M. Umemura. 2007. Interleukin-17 as an effector molecule of innate and acquired immunity against infections. Microbiol. Immunol. 51:1139-1147. [DOI] [PubMed] [Google Scholar]
- 23.McKay, D. M. 2001. Bacterial superantigens: provocateurs of gut dysfunction and inflammation? Trends Immunol. 22:497-501. [DOI] [PubMed] [Google Scholar]
- 24.Pene, J., S. Chevalier, L. Preisser, E. Venereau, M. H. Guilleux, S. Ghannam, J. P. Moles, Y. Danger, E. Ravon, S. Lesaux, H. Yssel, and H. Gascan. 2008. Chronically inflamed human tissues are infiltrated by highly differentiated Th17 lymphocytes. J. Immunol. 180:7423-7430. [DOI] [PubMed] [Google Scholar]
- 25.Renner, E. D., S. Rylaarsdam, S. Anover-Sombke, A. L. Rack, J. Reichenbach, J. C. Carey, Q. Zhu, A. F. Jansson, J. Barboza, L. F. Schimke, M. F. Leppert, M. M. Getz, R. A. Seger, H. R. Hill, B. H. Belohradsky, T. R. Torgerson, and H. D. Ochs. 2008. Novel signal transducer and activator of transcription 3 (STAT3) mutations, reduced T(H)17 cell numbers, and variably defective STAT3 phosphorylation in hyper-IgE syndrome. J. Allergy Clin. Immunol. 122:181-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shroff, K. E., K. Meslin, and J. J. Cebra. 1995. Commensal enteric bacteria engender a self-limiting humoral mucosal immune response while permanently colonizing the gut. Infect. Immun. 63:3904-3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Beelen, A. J., Z. Zelinkova, E. W. Taanman-Kueter, F. J. Muller, D. W. Hommes, S. A. Zaat, M. L. Kapsenberg, and E. C. de Jong. 2007. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity 27:660-669. [DOI] [PubMed] [Google Scholar]
- 28.Volpe, E., N. Servant, R. Zollinger, S. I. Bogiatzi, P. Hupe, E. Barillot, and V. Soumelis. 2008. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat. Immunol. 9:650-657. [DOI] [PubMed] [Google Scholar]
- 29.Ye, P., F. H. Rodriguez, S. Kanaly, K. L. Stocking, J. Schurr, P. Schwarzenberger, P. Oliver, W. Huang, P. Zhang, J. Zhang, J. E. Shellito, G. J. Bagby, S. Nelson, K. Charrier, J. J. Peschon, and J. K. Kolls. 2001. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 194:519-527. [DOI] [PMC free article] [PubMed] [Google Scholar]