Abstract
Lyme disease is the most common tick-borne illness in the United States. In this paper we explore the contribution of Ixodes scapularis ticks to the pathogenicity of Borrelia burgdorferi in mice. Previously we demonstrated that an isolate of B. burgdorferi sensu stricto (designated N40), passaged 75 times in vitro (N40-75), was infectious but was no longer able to cause arthritis and carditis in C3H mice. We now show that N40-75 spirochetes can readily colonize I. scapularis and multiply during tick engorgement. Remarkably, tick-transmitted N40-75 spirochetes cause disease in mice. N40-75 spirochetes isolated from these animals also retained their pathogenicity when subsequently administered to mice via syringe inoculation. Array analysis revealed that several genes associated with virulence, including bba25, bba65, bba66, bbj09, and bbk32, had higher expression levels in the tick-passaged N40-75 spirochete. These data suggest that transmission of a high-passage attenuated isolate of B. burgdorferi by the arthropod vector results in the generation of spirochetes that have enhanced pathogenesis in mice.
Lyme borreliosis is the most common tick-borne infection in the United States. The disease is caused by Borrelia burgdorferi, which is transmitted by Ixodes ticks (6, 12). Spirochetes are inoculated into the skin by the bites of infected ticks and initially establish a localized infection that can cause a painless rash known as erythema migrans. Spirochetes migrate from the site of the tick bite by penetrating the blood vessels and disseminate throughout the body, establishing a persistent infection, which can result in carditis or arthritis (31). The severity of Lyme disease is multifactorial and depends on the pathogen burden, the virulence of the specific spirochete isolate, and the host immune response to infection (1).
B. burgdorferi undergoes dynamic changes within the vector during both acquisition and transmission, as well as within the vertebrate host after transmission (34). To survive, B. burgdorferi must sense the environment and synthesize appropriate proteins for interactions with the different tick and mammalian tissues (15). Replication and migration of spirochetes in the tick have been associated with physiological adaptation that prepares the spirochetes for growth in the mammalian host. During tick feeding, the spirochetes encounter environmental changes caused by the influx of mammalian blood into the gut. Some of the antigenic changes B. burgdorferi undergoes during adaptation from the tick to the host have been identified; one example is the downregulation of outer surface protein A (OspA) on B. burgdorferi during tick engorgement (32). Other genes, including ospC, bbk32, and bbk50, are specifically induced during the course of tick feeding (7, 11, 28). In the vertebrate host, several Borrelia ligands, including Bbk32, Dbp, P66, and Bgp, aid in adhesion and dissemination during infection (4, 5, 8, 24, 29). In addition, B. burgdorferi evades the mammalian immune response by recombination at the variable major protein-like sequence (vls) gene locus (13, 21).
Previous studies have shown that a clonal isolate of B. burgdorferi N40 passaged 75 times in vitro (N40-75) can infect mice when needle inoculated but does not cause carditis or arthritis. B. burgdorferi N40-75 differs from B. burgdorferi N40 in its ability to adapt to the host. The inability of N40-75 to cause arthritis and carditis in immunocompetent mice has been associated with defective upregulation of several genes that are preferentially expressed during mammalian infection, including bba24, bba25, bba64, and erpD. This defect in gene expression resulted in increased and prolonged sensitivity of B. burgdorferi N40-75 to B. burgdorferi immune sera and an inability to effectively colonize, and disseminate within, the murine host (1, 2, 36).
B. burgdorferi is exposed to different environments in the tick. Although knowledge has accumulated about the molecular mechanisms used by B. burgdorferi to regulate gene expression in response to changing environments, the extent to which passage through the vector might alter the gene expression and virulence of N40-75 has not been studied. In the present study, we have introduced N40-75 into Ixodes ticks for transmission studies in order to explore the hypothesis that interactions between N40-75 and ticks may induce changes altering the virulence of N40-75.
MATERIALS AND METHODS
B. burgdorferi.
A low-passage clonal isolate of B. burgdorferi N40 (cN40) that is infectious and pathogenic in mice was used. N40-75, as described in previous studies, was produced by in vitro passage of the clonal isolate of B. burgdorferi, cN40 (1). B. burgdorferi cN40 and N40-75 were grown in Barbour-Stoenner-Kelly (BSK) complete medium (Sigma-Aldrich) at 33°C to log phase. Spirochetes isolated from the bladders of two C3H/HeJ mice (Jackson Laboratories) fed on by B. burgdorferi N40-75-infected nymphs were designated N40-75-T.
Ixodes scapularis and the generation of infected nymphs.
Larvae were derived from an egg mass from our tick colony at Yale University. Larvae were fed on either naïve or B. burgdorferi-infected mice to generate naïve or infected nymphs, respectively. Briefly, severe combined immunodeficient (SCID) mice (Jackson Laboratories) were infected by intradermal needle injection with 104 N40 or N40-75 spirochetes. Fourteen days later, I. scapularis larvae were fed to repletion on these mice. Larvae were collected and allowed to molt, and the degree of B. burgdorferi infection in the unfed nymphs was determined by quantitative PCR. Transmission of B. burgdorferi was studied by feeding infected nymphs on naïve C3H/HeJ mice. All ticks were incubated in a humidified chamber maintained at 25°C.
Microinjection of B. burgdorferi N40-75 into ticks.
N40-75 spirochetes were microinjected into naïve nymphs and used for in vivo infection studies. This method ensured the loading of equal numbers of spirochetes into the tick guts and eliminated the possibility that N40-75 could become altered in the mice used as a blood meal host to feed larvae. The process of microinjecting B. burgdorferi into ticks has been established in our lab and has been used in previous studies (23, 28). For microinjection, N40-75 spirochetes were grown in BSK medium and concentrated to a density of 1 × 109/ml. By using a femtojet microinjector system (Eppendorf) and a stereo dissecting binocular scope (Olympus), roughly 103 spirochetes were injected into nymphs via anal pore injection. This technique has been shown to produce minimal mortality in injected nymphs. After microinjection, ticks were reared in a humid chamber for several days. Groups of nymphs were sacrificed and assessed for spirochete burden by quantitative PCR. These infected nymphs were used for in vivo invasion studies. Transmission to the murine host was assessed by comparing the B. burgdorferi burdens in mice fed upon by nymphs harboring microinjected N40-75 or N40 spirochetes. Tissue samples were collected 21 days postinfection for quantitative analyses of the spirochete burden by quantitative PCR.
Nucleic acid isolation and quantitative PCR.
DNA was isolated from tissue samples and borrelial cultures using a DNeasy tissue kit (Qiagen) according to the manufacturer's instructions. Total RNA was isolated from ground tissues using Trizol reagent (Invitrogen). Contaminating DNA was digested with DNase I (Roche), and RNA was isolated using an RNeasy minikit (Qiagen). RNA was converted into first-strand cDNA using random hexamers and SuperScript III reverse transcriptase (Invitrogen).
Quantitative PCR assays were performed using an iCycler system (Bio-Rad Laboratories) with gene-specific primers and TaqMan probes as published previously (18). Genomic borrelial, mouse, and tick DNAs were used as standards for flaB, the mouse actin gene, and the tick actin gene, respectively.
Experimental mouse infection.
In the needle transmission model, pathogen-free C3H/HeJ mice (5 mice/group) were injected with ∼104 N40-75 or N40 spirochetes intradermally. In the tick transmission model, N40-75- or N40-infected nymphs (10 nymphs/mouse) were fed on C3H/HeJ mice (5 mice/group). After 7, 14, and 21 days of infection, mice were euthanized, and blood, bladders, skin, hearts, joints, and spleens were harvested for spirochete quantification. Bladders were cultured in BSK medium and were examined for the presence of B. burgdorferi. DNA isolated from tissue samples using a DNeasy tissue kit (Qiagen) was used to quantify the levels of spirochetes by quantitative PCR. To determine the levels of B. burgdorferi in murine tissue, mouse beta-actin was used to normalize the amount of DNA in the samples, and the borrelial flaB gene was used to quantify the spirochete DNA. The relative abundance of spirochetes in mice was calculated as the number of copies of the flaB gene per mouse beta-actin gene. To determine the levels of B. burgdorferi in ticks, tick actin was used to normalize the amount of DNA in the samples, and the borrelial flaB gene was used to quantify the spirochete DNA. The relative abundance of spirochetes in ticks was calculated as the number of copies of the flaB gene per tick actin gene.
For histologic analysis, hearts and bilateral hindlimb joints (knee and tibiotarsal joints) were fixed in 10% neutral buffered formalin, limbs were decalcified (Decalcifying Solution; Richard-Allen Scientific, Kalamazoo, MI), and tissues were processed and stained with hematoxylin and eosin at the Section of Comparative Medicine, Yale School of Medicine. The hearts were evaluated for carditis, and the knee and tibiotarsal joints were scored for arthritis severity, in a blinded fashion as described previously (3).
Microarray.
Glass microarrays consisting of 140 genes coding for lipoproteins in the B. burgdorferi B31 genome were made according to previously described methods (20). Total RNA was isolated from experimental and control samples by using Trizol (Invitrogen) according to the manufacturer's instructions. mRNA samples were purified and DNase treated using the RNeasy minikit (Qiagen). Enriched borrelial mRNA was amplified by an in vitro transcription method with a commercially available kit (MessageAmp II-Bacteria; Ambion). Subsequent differential labeling reactions, hybridization, staining, washing, and scanning were performed according to the gene chip expression analysis technical manual (Affymetrix) at the W. M. Keck facility (Yale University). The expression of the genes in B. burgdorferi N40-75 in in vitro culture (with Cy3) was compared with that in tick-passaged N40-75 (N40-75-T) (with Cy5). Genes with a minimum of a twofold increase or decrease in expression were analyzed using GenePix Pro, version 6.0, and were validated by quantitative reverse transcription-PCR (RT-PCR). Quantitative RT-PCR was performed for bba04, bba07, bba25, bba65, and bb0365 with the iQ Sybr Green Supermix (Bio-Rad) using the following primers: for bba04, 5′-CTT TGT GGT TTT AAT CCT AGG GTG TAA-3′ and 5′-CCC GCC TAT CTG ATC CAC TTT-3′; for bba07, 5′-AAA GAA ATG CTT GTT ACT GTT GTT AAG TG-3′ and 5′-AGT TTT TTC AAC CTG TAG CAA TTG C-3′; for bba25, 5′-AGA ATC GCT AGA AGA CGT TGG AA-3′ and 5′-TAG CCG CAA GCA ATC TTT CA-3′; for bba65, 5′-ACT GAT ATT TTC AAT TTA GCA GAG ATT GTA A-3′ and 5′-TGT TGG ATT CGT ATA CCA CCT GAT ATT-3′; and for bb0365, 5′-TCT GAA GAA GAT GGA CAT TTT CAA AC-3′ and 5′-CTA ATT GGA GCC TCT CCT GCA T-3′.
Statistical analysis.
Differences in data sets were analyzed using Prism, version 4. Significant differences between mean values for groups were evaluated using Student's t test.
RESULTS
Pathogenicity of N40-75 in needle-challenged mice.
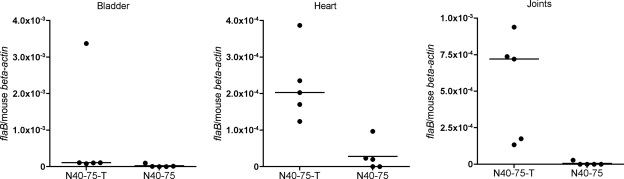
N40-75, as described in previous studies, was produced by in vitro passage of a clonal isolate of B. burgdorferi cN40 (1). We confirmed the infectivity and pathogenicity of N40-75 spirochetes when needle inoculated into groups of 5 C3H/HeJ mice. Mice were sacrificed 21 days after challenge, and the spirochete burdens were determined by quantitative PCR. The spirochete loads were low or absent in the bladders (P = 0.004), hearts (P = 0.44), and joints (P < 0.001) of N40-75-infected mice compared to those for mice infected with cN40 (Fig. 1). In accordance with published studies (1, 36), N40-75 was infectious for all 5 mice, but arthritis and carditis were not evident in any of the animals. In contrast, all 5 mice infected with cN40 were infected and had disease (see Fig. 4). Carditis was present in all mice infected with cN40 via needle inoculation, and the average inflammation score for the joints was 1.8 ± 1.0 (mean ± standard error of the mean [SEM]).
FIG. 1.
B. burgdorferi burdens in needle-challenged C3H/HeJ mice. Spirochete burdens in the bladders, hearts, and joints of mice infected with cN40 or N40-75 by needle challenge are shown. The relative spirochete burden is expressed as the number of copies of the B. burgdorferi flaB gene per murine beta-actin gene. Individual circles represent individual samples. Horizontal bars indicate the median for each set of data. Data shown are from one of three representative experiments.
FIG. 4.
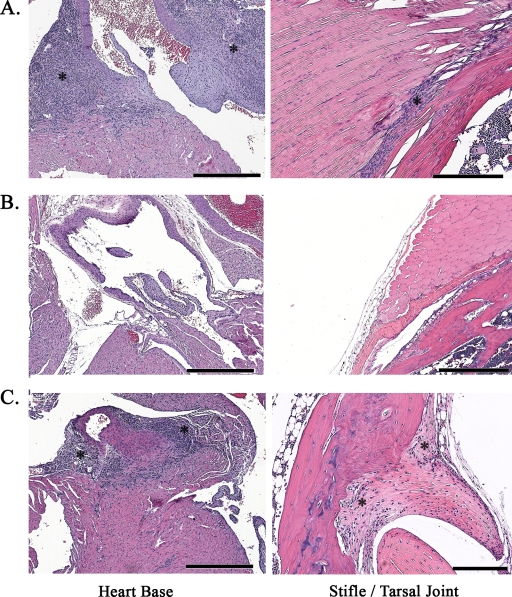
Histopathology of hearts and joints infected with B. burgdorferi. Representative hematoxylin-and-eosin-stained sections from mice infected with cN40 via needle inoculation (A), with N40-75 via needle inoculation (B), or with N40-75 via tick bite (C) are shown. Asterisks indicate area of inflammation. Bars, 500 μm.
Transmission of N40-75 by Ixodes scapularis nymphs.
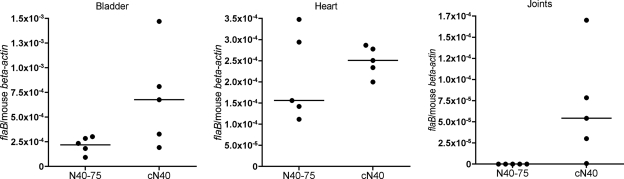
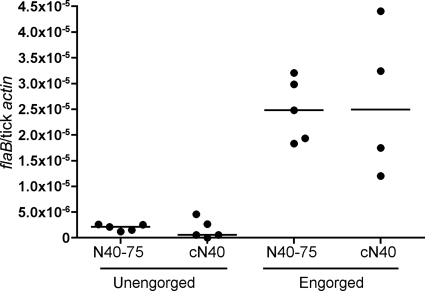
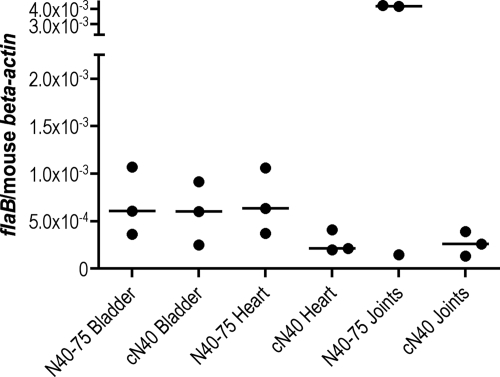
In nature, B. burgdorferi infects mammals via a tick bite. Hence, to investigate whether the pathogenicity of N40-75 is influenced by Ixodes scapularis, tick transmission studies were performed. SCID mice were infected by intradermal needle injection with 104 cN40 or N40-75 spirochetes. Fourteen days later, I. scapularis larvae were fed to repletion on these mice. Larvae were collected and allowed to molt, and the degree of B. burgdorferi infection in the unfed nymphs was examined by quantitative PCR. All cN40- and N40-75-infected ticks were positive for spirochetes (Fig. 2). N40-75- or cN40-infected nymphs (10 nymphs/mouse) were fed on two groups of C3H/HeJ mice (5 mice/group). Bladders, hearts, and joints were harvested, and infectivity and pathogenicity were assessed by quantitative PCR for the presence of B. burgdorferi in these mice at 21 days. There was no significant difference between the spirochete loads in the joints (P = 0.5), hearts (P = 0.2), or bladders (P = 0.6) of mice infected with cN40 and those in mice infected with tick-passaged N40-75 (Fig. 3). Examination of the heart and joint tissues by histopathology (Fig. 4) revealed that both cN40- and tick-passaged N40-75-infected mice had inflammatory carditis (5/5 cN40-infected mice; 5/5 mice infected with tick-passaged N40-75) and arthritis (3/5 cN40-infected mice; 3/6 mice infected with tick-passaged N40-75). Joints from both groups of mice had an average inflammatory score of 1 ± 0 (mean ± SEM).
FIG. 2.
B. burgdorferi burdens in nymphs. The numbers of spirochetes in N40-75- and cN40-infected nymphal ticks before and after engorgement are shown. The relative spirochete burden is expressed as the number of copies of the B. burgdorferi flaB gene per tick actin gene. Each individual circle represents data from a pool of 10 ticks. Horizontal bars indicate the median for each set of data. Data shown are from one of three experiments.
FIG. 3.
B. burgdorferi burdens in C3H/HeJ mice infected by tick transmission. Spirochete burdens in bladder, heart, and joint samples of mice infected with either N40-75 or cN40 via tick transmission are shown. The relative spirochete burden is expressed as the number of copies of the B. burgdorferi flaB gene per murine actin gene. Each individual circle represents a mouse tissue sample. Horizontal bars indicate the median for each set of data. Results from one of three representative studies are shown.
Tick-passaged N40-75 retains its pathogenicity when needle inoculated.
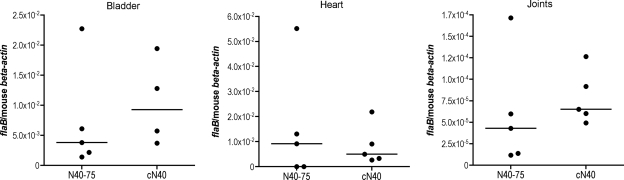
To determine whether tick-transmitted N40-75 reverts to its original phenotype when needle injected or retains its pathogenicity, C3H/HeJ mice were needle challenged with 104 N40-75 or N40-75-T (isolated from the bladders of N40-75 tick-infected mice) spirochetes. Surprisingly, N40-75-T was pathogenic, as well as infectious, when inoculated by needle challenge, while N40-75 was infectious but did not cause disease. N40-75-T spirochete burdens in the hearts and joints of these mice were similar to the spirochete burdens in cN40-infected mice (Fig. 5). In addition, the frequencies of carditis (5/5 cN40-infected mice; 5/5 N40-75-T-infected mice) and arthritis (3/5 cN40-infected mice; 2/5 N40-75-T-infected mice) were similar in the two groups of mice, with an average joint inflammation score of 1.0 ± 0.5 (mean ± SEM).
FIG. 5.
Needle inoculation of N40-75 isolated from tick-infected mouse bladders. Spirochete burdens in bladder, heart, and joint samples of mice infected by needle challenge are shown. The relative spirochete burden is expressed as the number of copies of the B. burgdorferi flaB gene per murine actin gene. Each individual circle represents a mouse tissue sample. Horizontal bars indicate the median for each set of data.
Transmission of N40-75 by microinjected nymphal ticks.
The Borrelia-infected nymphs used in the studies discussed above were generated by feeding naïve larvae on infected mice and allowing the larvae to molt into nymphs. It can be argued that B. burgdorferi N40-75 may have undergone changes in the mice used as blood meal hosts to feed larvae. In order to eliminate any effect that passage through mice may have on the pathogenicity of N40-75, a microinjection method was used to introduce the spirochetes into the guts of unfed nymphs, which were used for transmission studies. The transmission of B. burgdorferi to C3H mice fed upon by nymphs harboring microinjected N40-75 or cN40 spirochetes was compared. Tissues were collected 21 days following tick engorgement, and levels of B. burgdorferi were assessed by quantitative PCR (Fig. 6). Comparable levels of tick-passaged N40-75 and N40 were detected in the bladders, hearts, and joints of infected mice (3 mice/group). These data further indicate that B. burgdorferi N40-75 transmitted by the tick can persist in mice. The joint inflammation score for both groups of mice was 1.0 ± 0 (mean ± SEM).
FIG. 6.
B. burgdorferi burdens in C3H/HeJ mice infected by microinjected ticks. Spirochete burdens in bladder, heart, and joint samples of mice infected by microinjected ticks are shown. The relative spirochete burden is expressed as the number of copies of the B. burgdorferi flaB gene per murine actin gene. Each individual circle represents a mouse tissue sample. Horizontal bars indicate the median for each set of data.
Gene expression profile of N40-75.
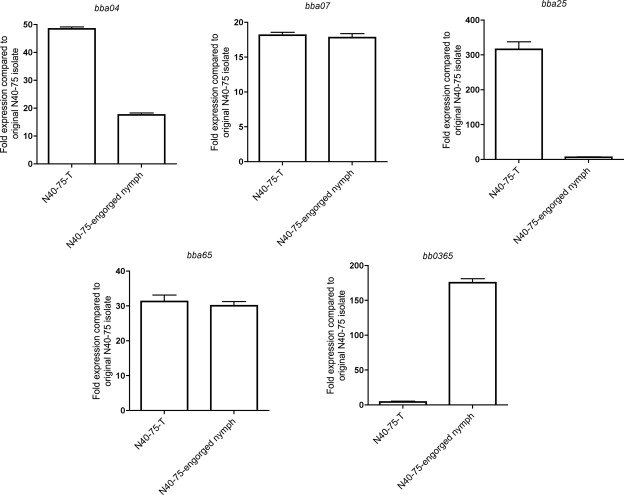
N40-75 has been shown to be defective in the upregulation of several genes that are preferentially expressed during mammalian infection and may be involved in colonization or dissemination (1). The lack of pathogenicity of N40-75 may be due to differences in the expression of genes affecting colonization, persistence, or dissemination in the mammalian host. To gain global insight into the genes expressed by N40-75, we used microarray technology to study the expression of 140 genes. We found that the expression profiles of several genes in N40-75-T isolated from the bladders of tick-infected mice and cultured in vitro were significantly altered from those in N40-75. The genes upregulated included bba04, bba07, bba25, bba66, bbg01, bbj09, bbj23, bbk07, bbk17, and bbk32 (Table 1). Real-time quantitative RT-PCR analysis to validate the differential expression of select genes from the microarray data confirmed that these genes were upregulated in N40-75-T (Fig. 7).
TABLE 1.
Genes upregulated in the tick-passaged N40-75 isolatea
| Gene | Predicted function/gene product | Mean fold upregulationb in: |
|
|---|---|---|---|
| B. burgdorferi N40-75-T | B. burgdorferi N40 | ||
| bbk32 | Fibronectin-binding protein | 9.5 | 35.24 |
| bbk17 | Adenine deaminase | 6.25 | 17.06 |
| bbj09 | Outer surface protein D (OspD) | 5.19 | 4.32 |
| bba66 | Outer surface protein | 4.97 | 26.85 |
| bba25 | Decorin-binding protein B (DbpB) | 4.78 | NC |
| bbk07 | Putative lipoprotein | 4.23 | 2.08 |
| bba36 | Lipoprotein | 4.13 | NC |
| bba07 | Putative ChpAI protein | 3.85 | NC |
| bbk19 | Putative lipoprotein | 2.80 | 19.35 |
| bbe31 | Putative surface protein | 2.60 | 41.82 |
| bba04 | S2 antigen | 2.39 | 3.1 |
| bbo39 | ErpL protein | 2.36 | 4.93 |
| bbg01 | Putative lipoprotein | 2.35 | NC |
| bbj23 | Tetratricopeptide repeat domain protein | 2.33 | 2.98 |
| bbj24 | Conserved hypothetical protein | 1.95 | 7.65 |
| bbg25 | Borrelia Orf-D family | 1.83 | 16.94 |
| bbb19 | Outer surface protein C (OspC) | 1.79 | NC |
| bba42 | Conserved hypothetical protein | 1.76 | 56.71 |
| bba73 | Putative antigen P35 | 1.65 | NC |
| bbj34 | Putative lipoprotein | 1.56 | 9.84 |
| bbk50 | Putative immunogenic protein P37 | 1.48 | 3.63 |
| bbk47 | Conserved hypothetical protein | 1.45 | 10.76 |
| bbh37 | Hypothetical protein | 1.32 | 14.88 |
| bba65 | Putative lipoprotein | 1.3 | 92.69 |
| bbk45 | Immunogenic protein P37 | 1.29 | 65.5 |
| bb0365 | Outer surface lipoprotein | 1.1 | NC |
As determined by microarray analyses. Results from two separate experiments were compared (P ≤ 0.05).
Relative to the expression of the gene in the original N40-75 isolate. NC, no change.
FIG. 7.
Quantitative RT-PCR analysis of gene expression. The expression of bba04, bba07, bba25, bb0365, and bba65 in engorged N40-75-infected nymphs and in N40-75-T (isolated from mice infected with N40-75 via tick transmission) was assessed by quantitative RT-PCR using the 2−ΔΔCT method. Bars represent the fold change in gene expression from that in the original N40-75 spirochetes grown in vitro.
DISCUSSION
Tick-borne pathogens share an intimate relationship with their vector (22). In the present study, we introduced a nonpathogenic isolate of B. burgdorferi (N40-75) into Ixodes ticks and followed the changes undergone by N40-75 as it journeyed through the tick to the murine vertebrate host. Previous work by Anguita et al. had shown that, when needle transmitted, B. burgdorferi N40-75 is infectious but does not cause arthritis or carditis in immunocompetent C3H mice (1). It was hypothesized that needle-transmitted N40-75 could not cause disease due to an inability to upregulate genes necessary for effective colonization of specific host tissues in sufficient numbers to cause disease (1).
B. burgdorferi undergoes dynamic changes within the vector during both acquisition and transmission, and likewise within the vertebrate host after transmission. Factors associated with tick/host tissues may provide the cues that cause significant selection or induction of changes in the pathogen population. A small proportion of spirochetes acquired during the larval or nymphal blood meal persist in the gut through the molt. Once the tick starts feeding again, the spirochetes become activated, multiply, and penetrate the gut wall to invade other tissues, including the salivary glands. The ability to invade the salivary glands and the specific genes expressed in response to the vector environment represent a powerful selective force directly linked to vertebrate infectivity and pathogenicity (22). Hence we explored the hypothesis that interactions between B. burgdorferi N40-75 and the tick may induce changes altering the virulence of B. burgdorferi N40-75 and may select for the transmission of B. burgdorferi spirochetes that are virulent in the vertebrate host. Our results show that transmission of B. burgdorferi N40-75 by a nymphal stage of tick led to mice that were infected and had disease. The replication and migration of B. burgdorferi N40-75 through the tick were very similar to those of the parent strain, B. burgdorferi cN40. When isolated and subsequently needle injected, tick-passaged N40-75 caused disease in C3H mice, indicating that the pathogenic phenotype was retained. Interestingly, ticks microinjected with N40 or N40-75 transmitted spirochetes efficiently resulting in comparable borrelial loads in mice. All these data suggest that when spirochetes replicate in the tick, a subpopulation of virulent B. burgdorferi spirochetes is transmitted to the host.
The cues to which spirochetes respond and the determinants of infectivity and pathogenicity expressed when spirochetes are in the tick have not been completely defined. We used microarray technology as a tool to identify genes modulated in tick-passaged B. burgdorferi N40-75. Several genes not expressed by B. burgdorferi N40-75 when grown in vitro in culture medium, including dbpA, dbpB, bba64, erpD, and genes that map to the cp32 family of plasmids, were expressed in the tick-passaged isolate (N40-75-T). Genes identified as upregulated in B. burgdorferi N40-75-T include bba04, bba07, bba25, bbg01, bba65, bba66, bb0365, bbj09, bbj23, bbk07, bbk17, and bbk32. The expression profiles of some of these genes in ticks and mice and their roles in borrelial infection have already been defined by using mutants, indicating that they are important in borrelial persistence and disease (7, 9, 10, 13, 14, 16, 18, 19, 25, 27, 35). The Rrp2-RpoN-RpoS sigma factor gene regulatory pathway in B. burgdorferi governs the expression of numerous spirochete genes important in mammalian infection and vector transmission (26). Interestingly, several genes reported here have previously been shown to be regulated by the Rrp2-RpoN-RpoS pathway (37). On the other hand, some of the genes identified in our study—bba04 (S2 antigen), bba07 (putative ChpAI protein), bbg01, and bbj23—are hypothetical genes, and their roles in borrelial infection in the vector or vertebrate host are unknown.
In vector-borne diseases, vector-specific barriers could impose severe bottlenecks on populations of invading pathogens. The levels at which the existence of such bottlenecks has been most documented in Ixodes ticks are colonization of the midgut, migration to the salivary glands, and transmission to the vertebrate host. Bottlenecks in the vertebrate host include dissemination from the site of infection and persistence. Various evolutionary forces, such as genetic bottlenecks and selection, imposed by the host during the infection process can alter the genetic composition of the pathogen population. These changes in genetic composition can play a key role in microbial evolution, contributing to the emergence of a virulent, disease-causing pathogen (31, 33). This phenomenon has been well studied in viruses where host bottlenecks affect selection, resulting in the generation of quasispecies with varied pathogenicity (17, 30). The environment that B. burgdorferi N40-75 encounters during its life cycle in the tick may thus trigger changes in the spirochete that result in a virulent phenotype. Defining the cellular and molecular factors in the tick that dictate borrelial virulence will be the next step. In summary, we have shown that passage of a previously nonpathogenic borrelial isolate in ticks leads to the selection of a phenotype with increased fitness and virulence in mice.
Acknowledgments
This work was supported by grants from the National Institutes of Health.
We thank Xin Li for the microarray slides and Deborah Beck and Kathleen DePonte for technical assistance.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 12 October 2009.
REFERENCES
- 1.Anguita, J., S. Samanta, B. Revilla, K. Suk, S. Das, S. W. Barthold, and E. Fikrig. 2000. Borrelia burgdorferi gene expression in vivo and spirochete pathogenicity. Infect. Immun. 68:1222-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anguita, J., V. Thomas, S. Samanta, R. Persinski, C. Hernanz, S. W. Barthold, and E. Fikrig. 2001. Borrelia burgdorferi-induced inflammation facilitates spirochete adaptation and variable major protein-like sequence locus recombination. J. Immunol. 167:3383-3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barthold, S. W., S. Feng, L. K. Bockenstedt, E. Fikrig, and K. Feen. 1997. Protective and arthritis-resolving activity in sera of mice infected with Borrelia burgdorferi. Clin. Infect. Dis. 25(Suppl. 1):S9-S17. [DOI] [PubMed] [Google Scholar]
- 4.Blevins, J. S., K. E. Hagman, and M. V. Norgard. 2008. Assessment of decorin-binding protein A to the infectivity of Borrelia burgdorferi in the murine models of needle and tick infection. BMC Microbiol. 8:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Exner, M. M., X. Wu, D. R. Blanco, J. N. Miller, and M. A. Lovett. 2000. Protection elicited by native outer membrane protein Oms66 (p66) against host-adapted Borrelia burgdorferi: conformational nature of bactericidal epitopes. Infect. Immun. 68:2647-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feder, H. M., Jr., B. J. Johnson, S. O'Connell, E. D. Shapiro, A. C. Steere, G. P. Wormser, W. A. Agger, H. Artsob, P. Auwaerter, J. S. Dumler, J. S. Bakken, L. K. Bockenstedt, J. Green, R. J. Dattwyler, J. Munoz, R. B. Nadelman, I. Schwartz, T. Draper, E. McSweegan, J. J. Halperin, M. S. Klempner, P. J. Krause, P. Mead, M. Morshed, R. Porwancher, J. D. Radolf, R. P. Smith, Jr., S. Sood, A. Weinstein, S. J. Wong, and L. Zemel. 2007. A critical appraisal of “chronic Lyme disease.” N. Engl. J. Med. 357:1422-1430. [DOI] [PubMed] [Google Scholar]
- 7.Fikrig, E., W. Feng, S. W. Barthold, S. R. Telford III, and R. A. Flavell. 2000. Arthropod- and host-specific Borrelia burgdorferi bbk32 expression and the inhibition of spirochete transmission. J. Immunol. 164:5344-5351. [DOI] [PubMed] [Google Scholar]
- 8.Fischer, J. R., K. T. LeBlanc, and J. M. Leong. 2006. Fibronectin binding protein BBK32 of the Lyme disease spirochete promotes bacterial attachment to glycosaminoglycans. Infect. Immun. 74:435-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilmore, R. D., Jr., R. R. Howison, V. L. Schmit, and J. A. Carroll. 2008. Borrelia burgdorferi expression of the bba64, bba65, bba66, and bba73 genes in tissues during persistent infection in mice. Microb. Pathog. 45:355-360. [DOI] [PubMed] [Google Scholar]
- 10.Gilmore, R. D., Jr., R. R. Howison, V. L. Schmit, A. J. Nowalk, D. R. Clifton, C. Nolder, J. L. Hughes, and J. A. Carroll. 2007. Temporal expression analysis of the Borrelia burgdorferi paralogous gene family 54 genes BBA64, BBA65, and BBA66 during persistent infection in mice. Infect. Immun. 75:2753-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grimm, D., K. Tilly, R. Byram, P. E. Stewart, J. G. Krum, D. M. Bueschel, T. G. Schwan, P. F. Policastro, A. F. Elias, and P. A. Rosa. 2004. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc. Natl. Acad. Sci. U. S. A. 101:3142-3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hengge, U. R., A. Tannapfel, S. K. Tyring, R. Erbel, G. Arendt, and T. Ruzicka. 2003. Lyme borreliosis. Lancet Infect. Dis. 3:489-500. [DOI] [PubMed] [Google Scholar]
- 13.Hodzic, E., S. Feng, K. J. Freet, and S. W. Barthold. 2003. Borrelia burgdorferi population dynamics and prototype gene expression during infection of immunocompetent and immunodeficient mice. Infect. Immun. 71:5042-5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hodzic, E., S. Feng, K. J. Freet, D. L. Borjesson, and S. W. Barthold. 2002. Borrelia burgdorferi population kinetics and selected gene expression at the host-vector interface. Infect. Immun. 70:3382-3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hovius, J. W., A. P. van Dam, and E. Fikrig. 2007. Tick-host-pathogen interactions in Lyme borreliosis. Trends Parasitol. 23:434-438. [DOI] [PubMed] [Google Scholar]
- 16.Hughes, J. L., C. L. Nolder, A. J. Nowalk, D. R. Clifton, R. R. Howison, V. L. Schmit, R. D. Gilmore, Jr., and J. A. Carroll. 2008. Borrelia burgdorferi surface-localized proteins expressed during persistent murine infection are conserved among diverse Borrelia spp. Infect. Immun. 76:2498-2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuss, S. K., C. A. Etheredge, and J. K. Pfeiffer. 2008. Multiple host barriers restrict poliovirus trafficking in mice. PLoS Pathog. 4:e1000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li, X., X. Liu, D. S. Beck, F. S. Kantor, and E. Fikrig. 2006. Borrelia burgdorferi lacking BBK32, a fibronectin-binding protein, retains full pathogenicity. Infect. Immun. 74:3305-3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li, X., G. Neelakanta, X. Liu, D. S. Beck, F. S. Kantor, D. Fish, J. F. Anderson, and E. Fikrig. 2007. Role of outer surface protein D in the Borrelia burgdorferi life cycle. Infect. Immun. 75:4237-4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang, F. T., F. K. Nelson, and E. Fikrig. 2002. DNA microarray assessment of putative Borrelia burgdorferi lipoprotein genes. Infect. Immun. 70:3300-3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDowell, J. V., S. Y. Sung, L. T. Hu, and R. T. Marconi. 2002. Evidence that the variable regions of the central domain of VlsE are antigenic during infection with Lyme disease spirochetes. Infect. Immun. 70:4196-4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munderloh, U. G., and T. J. Kurtti. 1995. Cellular and molecular interrelationships between ticks and prokaryotic tick-borne pathogens. Annu. Rev. Entomol. 40:221-243. [DOI] [PubMed] [Google Scholar]
- 23.Neelakanta, G., X. Li, U. Pal, X. Liu, D. S. Beck, K. DePonte, D. Fish, F. S. Kantor, and E. Fikrig. 2007. Outer surface protein B is critical for Borrelia burgdorferi adherence and survival within Ixodes ticks. PLoS Pathog. 3:e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norman, M. U., T. J. Moriarty, A. R. Dresser, B. Millen, P. Kubes, and G. Chaconas. 2008. Molecular mechanisms involved in vascular interactions of the Lyme disease pathogen in a living host. PLoS Pathog. 4:e1000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ojaimi, C., V. Mulay, D. Liveris, R. Iyer, and I. Schwartz. 2005. Comparative transcriptional profiling of Borrelia burgdorferi clinical isolates differing in capacities for hematogenous dissemination. Infect. Immun. 73:6791-6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ouyang, Z., J. S. Blevins, and M. V. Norgard. 2008. Transcriptional interplay among the regulators Rrp2, RpoN and RpoS in Borrelia burgdorferi. Microbiology 154:2641-2658. [DOI] [PubMed] [Google Scholar]
- 27.Pal, U., J. Dai, X. Li, G. Neelakanta, P. Luo, M. Kumar, P. Wang, X. Yang, J. F. Anderson, and E. Fikrig. 2008. A differential role for BB0365 in the persistence of Borrelia burgdorferi in mice and ticks. J. Infect. Dis. 197:148-155. [DOI] [PubMed] [Google Scholar]
- 28.Pal, U., X. Yang, M. Chen, L. K. Bockenstedt, J. F. Anderson, R. A. Flavell, M. V. Norgard, and E. Fikrig. 2004. OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J. Clin. Invest. 113:220-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parveen, N., K. A. Cornell, J. L. Bono, C. Chamberland, P. Rosa, and J. M. Leong. 2006. Bgp, a secreted glycosaminoglycan-binding protein of Borrelia burgdorferi strain N40, displays nucleosidase activity and is not essential for infection of immunodeficient mice. Infect. Immun. 74:3016-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfeiffer, J. K., and K. Kirkegaard. 2005. Increased fidelity reduces poliovirus fitness and virulence under selective pressure in mice. PLoS Pathog. 1:e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosa, P. A., K. Tilly, and P. E. Stewart. 2005. The burgeoning molecular genetics of the Lyme disease spirochaete. Nat. Rev. Microbiol. 3:129-143. [DOI] [PubMed] [Google Scholar]
- 32.Schwan, T. G., and J. Piesman. 2000. Temporal changes in outer surface proteins A and C of the Lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J. Clin. Microbiol. 38:382-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scully, L. R., and M. J. Bidochka. 2006. The host acts as a genetic bottleneck during serial infections: an insect-fungal model system. Curr. Genet. 50:335-345. [DOI] [PubMed] [Google Scholar]
- 34.Singh, S. K., and H. J. Girschick. 2004. Molecular survival strategies of the Lyme disease spirochete Borrelia burgdorferi. Lancet Infect. Dis. 4:575-583. [DOI] [PubMed] [Google Scholar]
- 35.Stewart, P. E., A. Bestor, J. N. Cullen, and P. A. Rosa. 2008. A tightly regulated surface protein of Borrelia burgdorferi is not essential to the mouse-tick infectious cycle. Infect. Immun. 76:1970-1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas, V., J. Anguita, S. Samanta, P. A. Rosa, P. Stewart, S. W. Barthold, and E. Fikrig. 2001. Dissociation of infectivity and pathogenicity in Borrelia burgdorferi. Infect. Immun. 69:3507-3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang, X. F., S. M. Alani, and M. V. Norgard. 2003. The response regulator Rrp2 is essential for the expression of major membrane lipoproteins in Borrelia burgdorferi. Proc. Natl. Acad. Sci. U. S. A. 100:11001-11006. [DOI] [PMC free article] [PubMed] [Google Scholar]