Abstract
Leprosy is a chronic but treatable infectious disease caused by the intracellular pathogen Mycobacterium leprae. Host immunity to M. leprae determines the diversity of clinical manifestations seen in patients, from tuberculoid leprosy with robust production of Th1-type cytokines to lepromatous disease, characterized by elevated levels of Th2-type cytokines and a suboptimal proinflammatory response. Previous reports have indicated that M. leprae is a poor activator of macrophages and dendritic cells in vitro. To understand whether M. leprae fails to elicit an optimal Th1 immune response or actively interferes with its induction, we have examined the early interactions between M. leprae and monocytes from healthy human donors. We found that, in naïve monocytes, M. leprae induced high levels of the negative regulatory molecules MCP-1 and interleukin-1 (IL-1) receptor antagonist (IL-1Ra), while suppressing IL-6 production through phosphoinositide-3 kinase (PI3K)-dependent mechanisms. In addition, low levels of proinflammatory cytokines were observed in association with reduced activation of nuclear factor-κB (NF-κB) and delayed activation of IL-1β-converting enzyme, ICE (caspase-1), in monocytes stimulated with M. leprae compared with Mycobacterium bovis BCG stimulation. Interestingly, although in itself a weak stimulator of cytokines, M. leprae primed the cells for increased production of tumor necrosis factor alpha and IL-10 in response to a strongly inducing secondary stimulus. Taken together, our results suggest that M. leprae plays an active role to control the release of cytokines from monocytes by providing both positive and negative regulatory signals via multiple signaling pathways involving PI3K, NF-κB, and caspase-1.
In recent years the World Health Organization has reported a dramatic 20% per year decrease in global numbers of new leprosy cases, reaching an estimated 250,000 cases worldwide during 2008 (44). However, the disease remains a public health problem, particularly in Brazil, Nepal, and Mozambique (44).
In spite of intensive study, it remains unclear how leprosy is acquired and what determines the development of active disease or the severity of disease in affected individuals. Nonetheless, it is clear that the nature and extent of the host immune response to Mycobacterium leprae determine the clinical manifestation of disease and various complications that can arise (34). Leprosy presents as a spectrum of disease. At one pole is tuberculoid or paucibacillary leprosy, characterized by a robust Th1 immune response, strong cellular immunity, and low bacillary numbers. At the other end of the spectrum, lepromatous or multibacillary leprosy is characterized by high levels of Th2-type cytokines, low levels of Th1-type cytokines, and high bacillary loads in skin lesions (4, 18, 45). Although humoral immunity against M. leprae is robust in lepromatous patients, T cells in these individuals are anergic to M. leprae and its antigens.
Numerous studies to elucidate the determinants of host immunity and pathogenesis of leprosy have been carried out on peripheral blood leukocytes and biopsies of lesions obtained from patients with active disease. Previous studies have implicated as mediators of the anergy seen in lepromatous leprosy patients the involvement of suppressor T cells (23, 24), the lack of production of interleukin-2 (IL-2) (14), the preferential expression of Th2 cytokines (12, 27), and the increased expression of the leukocyte immunoglobulin-like receptor (LIR), which suppresses innate immunity and blocks antimicrobial activity triggered by Toll-like receptors (TLRs) (8). However, the immune events that characterize the early interaction between M. leprae and leukocytes of the naïve host are less well understood.
Important contributions to our understanding of the regulation of host immunity to M. leprae infection have been provided by genetic linkage studies. Previous investigations focused on polymorphisms in host genes responsible for Th1 immunity, such as those for tumor necrosis factor alpha (TNF-α) or IL-12 and its receptor (2, 31, 35, 37). More recent genetic analyses have identified associations between specific polymorphisms in TLR genes and susceptibility to leprosy and/or the type of clinical presentation. We have shown that a common variant in TLR1 is associated with altered peripheral blood monocyte cytokine responses to M. leprae and with protection against reversal reaction (RR), an acute immunologic complication seen in many borderline lepromatous leprosy patients (26). Polymorphisms in the TLR2 locus have also been strongly linked with RR and with susceptibility to leprosy (5, 6). More recently, we identified TLR4 polymorphisms that are associated with susceptibility to leprosy and showed that M. leprae downregulates TLR4-mediated IL-1β and IL-6 production in monocytes (7). Taken together, these studies point to a central role for TLR-induced innate immunity in determining the progression of disease following infection with M. leprae, as well as the complications of reactional states that can occur in leprosy patients.
M. leprae is reported to be a poor activator of macrophages and dendritic cells (DCs) in vitro in studies carried out using blood cells from healthy human donors. Low levels of cytokines were induced in naïve human monocytes stimulated with live or dead M. leprae (42) while DCs stimulated with dead M. leprae did not increase expression of maturation and activation surface markers (30). To understand the basis for the poor immune activation observed in these studies, we have asked whether M. leprae passively fails to elicit a host cytokine response or actively interferes with this cellular response. We examined the early interactions between M. leprae and monocytes isolated from the blood of M. leprae-naïve healthy donors. As a positive control in our studies, we used Mycobacterium bovis bacillus Calmette-Guérin (BCG), which is a potent stimulant of innate immune response (25, 43) used widely as a vaccine for tuberculosis in humans.
Our data suggest that M. leprae plays an active role to control the release of cytokines from monocytes by providing both positive and negative regulatory signals via multiple signaling pathways, involving nuclear factor-κB (NF-κB), phosphatidylinositol 3-kinase (PI3K), and IL-1β-converting enzyme, ICE (caspase-1). We observed that M. leprae induces high levels of the negative regulatory molecules IL-1 receptor antagonist (IL-1Ra) and monocyte chemoattractant protein 1 (MCP-1) and that, although in itself a weak stimulator, M. leprae primes the cells for increased production of TNF-α and IL-10 in response to a strongly inducing secondary stimulus. IL-6 production is suppressed by M. leprae via a PI3K-dependent mechanism while a delay in caspase-1 activation together with reduced NF-κB activation appears to be responsible for the low levels of IL-1β and IL-18 produced by M. leprae-stimulated cells.
MATERIALS AND METHODS
Bacterial strains.
M. leprae strain Thai-53 stock was obtained by propagation in the footpads of nude mice and collected, as described previously (20); bacteria were then enumerated by the direct count method of Shepard and McRae (40). Highly purified bacilli, free of all contaminating mouse tissue and proteins, were prepared by treating mouse footpad suspensions with 0.1 M NaOH, neutralizing with 0.1 M HCl, and washing multiple times with phosphate-buffered saline (PBS) (20). M. leprae preparations were then irradiated by gamma irradiation (1). M. bovis BCG was obtained from the Trudeau Institute (Trudeau Mycobacterial Culture Collection no. 1011), grown in 7H9 medium (Difco), and stored at −80°C until used. M. leprae cells do not maintain viability in vitro for more than a day even at their optimal incubation temperature of 33°C, at which primary monocytes survive poorly. Also, M. leprae does not replicate in vitro in human monocytes while BCG replicates slowly over time. Thus, to ensure similar bacillary loads in monocyte cultures over the duration of each experiment, we decided to use heat-killed (HK) rather than live organisms for our studies. We carried out pilot studies to compare the results of using monocytes with live versus HK BCG or M. leprae cells, incubating the cells at 33°C or 37°C. BCG and M. leprae cells were heat killed for 10 min (30), and the mycobacterial suspensions were passed through a 28-gauge needle before use to disperse any clumps prior to use. Consistent with previous reports (42), we found no differences in the patterns of cytokines induced by live and heat-killed bacilli at either temperature although incubation at 33°C yielded more variation between experiments. Based on these results, we utilized HK M. leprae and HK BCG and incubated monocyte cultures at 37°C for the present studies.
Human monocyte and peripheral blood mononuclear cell (PBMC) infections.
PBMCs were isolated from fresh human peripheral blood (buffy coat) obtained from anonymous healthy donors (New Jersey Blood Center, East Orange, NJ) by Ficoll-Paque separation (28). Cells were plated at a density of 3 × 106 PBMCs per well in RPMI 1640 culture medium (Invitrogen, Carlsbad, CA) and 1% human serum AB (Gemini Bioproducts, Calabasas, CA) in 24-well tissue culture plates (Corning Inc., Corning NY), allowed to adhere for 2 h, and then washed to remove nonadherent cells. Adherent monocytes were cultured in 5% CO2 at 37°C in RPMI 1640 medium supplemented with 20% human serum. For each experiment, adherent monocytes from between 4 and 15 independent donors were stimulated with bacteria at a multiplicity of infection (MOI) of 5:1 (bacilli to monocyte) and incubated in 5% CO2 at 37°C. For a limited number of experiments the monocytes were stimulated with M. leprae at an MOI of 40:1. For the costimulation experiments, monocytes from healthy donors were exposed to M. leprae, BCG (MOI of 5:1, bacilli to monocyte), or LPS (100 ng/ml) alone, or M. leprae plus BCG or LPS simultaneously or primed for 5 h first with M. leprae, followed by exposure to BCG or LPS, or vice versa.
Detection of secreted cytokines by Luminex assay.
Supernatants from stimulated monocytes were probed using a multiplex human cytokine Luminex panel (Bio-Rad, Hercules, CA) according to the manufacturer's instructions. The Luminex panel used for the analysis of supernatants included IL-18, IL-1β, IL-1Ra, IL-6, IL-10, IL-12, TNF-α, and MCP-1. The results were read using a Bio-Plex 200 system (Bio-Rad, Hercules, CA), and data and statistical analyses were carried out using GraphPad Prism, version 4 (GraphPad, San Diego, CA).
Inhibition of the PI3K signaling pathway.
Adherent human peripheral blood monocytes from five independent donors were isolated as described above and incubated with the PI3K inhibitor LY294002 (20 μM final concentration) (EMD Biosciences, San Diego, CA) for 60 min and then stimulated with M. leprae or BCG. Cell culture supernatants were removed at 24 h postinfection, and cytokine levels were examined by Luminex assay as described above.
NF-κB fast-activated cell-based ELISA (FACE) assay.
For detection of phosphorylated NF-κB p65, the FACE NF-κB p65 profiler (Active Motif, Carlsbad, CA) was utilized. Adherent monocytes from two donors were plated in a 96-well culture plate at 1 × 105 cells/well and stimulated with M. leprae or BCG (MOIs of 5:1, bacteria to monocyte) or were left unstimulated, and cultures were then centrifuged at 1,000 rpm for 5 min at 4°C to bring bacilli into contact with the cells. Cells were then incubated for 30 min at 37°C before fixation with 4% paraformaldehyde in PBS. Staining with antibodies (total p65, phospho-p65 S468, and phospho-p65 S536) and with crystal violet (for cell density) was performed according to the manufacturer's instructions. The assay was read at an optical density (OD) of 450 nm for an enzyme-linked immunosorbent assay (ELISA) and 590 nm for the crystal violet stain. Relative phosphorylation of NF-κB p65 was ascertained by normalizing each ELISA (total p65, phospho-p65 S468, and phospho-p65 S536) to cell density, followed by normalization to total p65 and then normalization to unstimulated cells. Data and statistical analysis were carried out using GraphPad Prism, version 4 (GraphPad, San Diego, CA).
Caspase-1 activation in M. leprae-stimulated monocytes.
Adherent human monocytes from three to five donors were stimulated with M. leprae, BCG (MOI 5:1, bacilli to monocyte), or LPS (100 ng/ml) for 1, 2, 3, 4, 5, 24, and 48 h. Analysis of M. leprae induction of active caspase-1 was also analyzed over 4 days of culture. Aliquots of cell supernatants were removed and assayed for levels of caspase-1 p20 subunit, according to the manufacturer's instructions (Quantikine; R&D Systems, Minneapolis, MN).
Costimulation of monocytes with M. leprae and the TLR and nucleotide-binding oligomerization domain 2 (NOD2) agonists.
Adherent human monocytes from two donors were isolated as described above and stimulated with M. leprae (MOI of 5:1, bacilli to monocyte) and treated with one of the following agonists of TLR or NOD2, or monocytes were pretreated with M. leprae or TLR or NOD2 agonist, followed by secondary stimulation with the other agent. TLR and NOD2 agonists were obtained from InvivoGen (San Diego, CA) and used at the following concentrations: Pam2CSK4 (TLR2/TLR6 [TLR2/6]), 250 ng/ml; Pam3CSK4 (TLR2/1), 250 ng/ml; LPS (TLR4), 100 ng/ml; CpG DNA (TLR9), 625 ng/ml; and muramyl dipeptide ([MDP] NOD2), 100 ng/ml.
RESULTS
Effect of M. leprae on cytokine production by human monocytes.
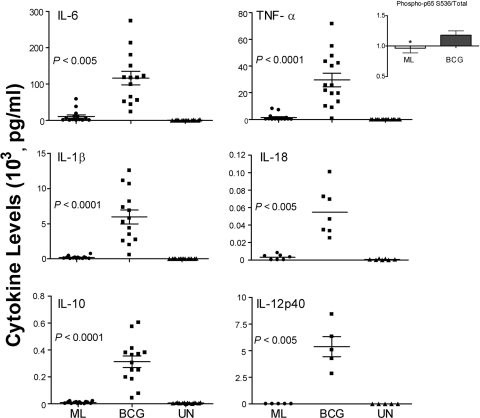
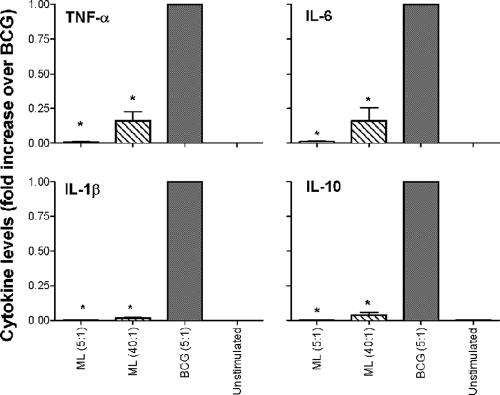
Cytokine production by peripheral blood monocytes obtained from healthy donors after exposure to M. leprae for 24 or 48 h was evaluated by a Luminex assay. In these studies, M. bovis BCG, a related mycobacterium known to induce a strong proinflammatory cytokine response in monocytes (3, 32) via TLR2 and TLR4, was used as a positive control. M. leprae elicited low levels of the proinflammatory cytokines IL-6 (mean, 10,038 ± 11,857 pg/ml), TNF-α (mean, 1,375 ± 2,582 pg/ml), and IL-1β (mean, 207 ± 214 pg/ml) and undetectable levels of IL-18, IL-10, and IL-12p40 (Fig. 1). By comparison, BCG under the same conditions induced significantly higher levels of all six cytokines (Fig. 1). Low-level cytokine induction by M. leprae was associated with reduced NF-κB activation relative to BCG-stimulated cells, as measured by NF-κBp65 phosphorylation (Fig. 1, inset). To confirm that the differential cytokine levels between M. leprae- and BCG-stimulated monocytes were not the result of differences in bacterial numbers, we stimulated monocytes with M. leprae at low and high MOIs (5:1 or 40:1) and compared the cytokine levels to those induced by BCG at the MOI of 5:1. While the monocytes stimulated with M. leprae at the high MOI of 40:1 elicited somewhat higher levels of TNF-α, IL-6, IL-1β, and IL-10 than stimulation at the low MOI of 5:1 (Fig. 2), the dramatic difference in cytokine levels observed between M. leprae- and BCG-stimulated monocytes was still maintained and remained statistically significant. This strongly suggests that the differences in cytokine responses are attributable to differential stimulation characteristics of the mycobacteria and not to changes in the M. leprae MOI (Fig. 2).
FIG. 1.
Differential cytokine production by monocytes stimulated with M. leprae or BCG. Human monocytes from healthy donors were stimulated with M. leprae (ML) or M. bovis BCG at a MOI of 5:1 (bacilli to monocyte) or left unstimulated (UN). Cell supernatants were collected at 24 and 48 h postinfection and analyzed by Luminex assay for the presence of cytokines. Results are shown for IL-1β, IL-18, IL-6, IL-10, and IL-12p40 at 48 h and for TNF-α at 24 h. Results for IL-1β, IL-6, IL-10, and TNF-α are the means ± standard error of 14 experiments (representing 14 independent donors) performed in duplicate. Results for IL-18 and IL-12p40 are the means ± standard error of 7 and 5 experiments, respectively (representing seven and five independent donors) performed in duplicate. A two-tailed paired t test was used for statistical analysis. Inset shows relative levels of NF-κB phospho-p65 S536 at 30 min postinfection (normalized to unstimulated cells) in M. leprae- or BCG-stimulated monocytes. A two-tailed paired t test was used for statistical analysis (*, P < 0.05, in comparison to BCG).
FIG. 2.
Differential cytokine production by monocytes stimulated with M. leprae at a low and high multiplicity of infection. Human monocytes were stimulated with M. leprae at a low MOI of 5:1 (bacilli to monocyte) or a high MOI of 40:1. M. bovis BCG was used only at the low MOI of 5:1. Cell supernatants were collected at 24 and 48 h poststimulation and assayed by Luminex for the presence of cytokines. Results are shown for TNF-α, IL-1β, and IL-6 at 24 h and for IL-10 at 48 h. Results are expressed as fold increase over BCG and are the means ± standard error of three experiments (representing three independent donors) performed in duplicate. A two-tailed paired t test was used for statistical analysis (*, P < 0.05, in comparison to BCG).
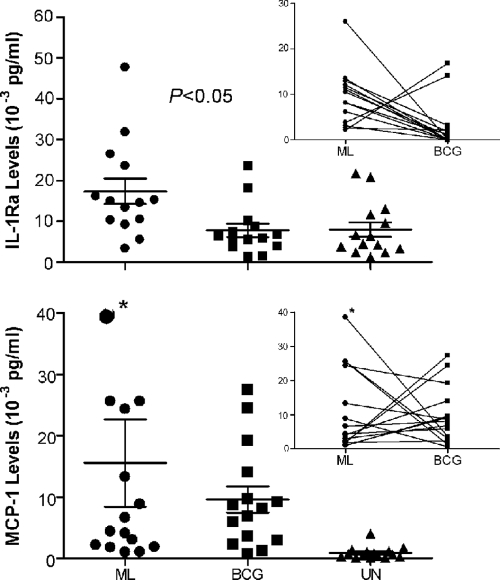
In the same set of experiments as those depicted in Fig. 1, M. leprae strongly induced the inhibitory chemokines MCP-1 and IL-1Ra (Fig. 3). The induction of MCP-1 and IL-1Ra was not affected by the increase in the M. leprae MOI to 40:1 (data not shown). When monocytes were exposed to BCG, IL-1Ra was induced at significantly lower levels while levels of MCP-1 were comparable to those induced by M. leprae (Fig. 3). Interestingly, in monocytes from most of the donors, the induction of both IL-1Ra and MCP-1 showed an inverse relationship in response to M. leprae versus BCG (Fig. 3, insets). Consistent with the reported reciprocal association between IL-1Ra and IL-1β, in the panel of 14 donors analyzed, the 2 donors showing the highest levels of IL-1Ra in response to BCG stimulation yielded the lowest IL-1β levels in response to the same stimulus (data not shown) while the three highest IL-1β BCG-stimulated responders produced low levels of IL-1Ra (data not shown).
FIG. 3.
Cytokines strongly induced by M. leprae. Human monocytes were stimulated with M. leprae or M. bovis BCG at a MOI of 5:1 (bacilli to monocyte). Cell supernatants were collected at 48 h postinfection for IL-1Ra and at 24 h postinfection for MCP-1 and analyzed by Luminex assay. Results are the means ± standard error of 14 and 15 experiments, respectively, for IL-1Ra and MCP-1 (representing 14 and 15 independent donors) performed in duplicate. The insets show an inverse correlation in donors between the responses of monocytes to M. leprae versus BCG. A two-tailed paired t test was used for statistical analysis. The asterisk (*) indicates the out-of-scale value of 110 (10−3 pg/ml).
The role of PI3K in M. leprae induction of monocyte cytokines.
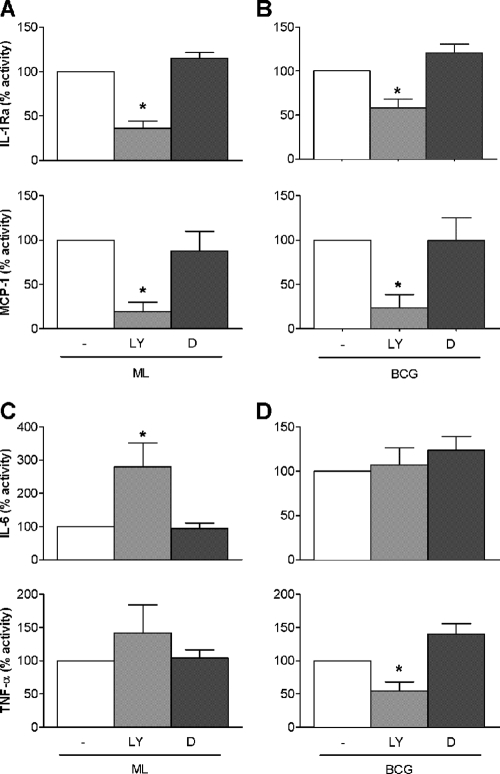
Because PI3K has been implicated in regulating the early proinflammatory cytokine response and shown to dampen IL-1β production and induce IL-1Ra in human monocytes (29), we examined its role in the M. leprae-induced monocyte response. Fresh human monocytes were pretreated with the specific PI3K inhibitor LY294002 or vehicle (0.1% dimethyl sulfoxide [DMSO]) alone or left untreated and then stimulated with M. leprae. Inhibition of PI3K signaling significantly reduced the ability of M. leprae to elicit IL-1Ra and MCP-1 production (Fig. 4A). Similarly, BCG-induced levels of both IL-1Ra and MCP-1 were reduced in the presence of the PI3K inhibitor (Fig. 4B). In the presence of the PI3K inhibitor, higher levels of IL-6 were induced in monocytes exposed to M. leprae while levels of TNF-α (Fig. 4C) and IL-1β (data not shown) were unaffected. In contrast, inhibition of PI3K in BCG-stimulated monocytes had no impact on IL-6 (Fig. 4D) or IL-1β (data not shown) but significantly reduced TNF-α production (Fig. 4D). Taken together, these results suggest that the PI3K pathway is involved in M. leprae-mediated induction of IL-1Ra and MCP-1 and suppression of IL-6 production in monocytes. In contrast, while BCG-mediated induction of TNF-α apparently depends upon PI3K signaling, induction of IL-6 by BCG does not involve this pathway. Thus, the PI3K pathway appears to play different roles in regulating the profiles of soluble immune molecules produced by monocytes in response to M. leprae versus BCG.
FIG. 4.
Role of PI3K in M. leprae cytokine induction. Monocytes from healthy donors were pretreated with the PI3K inhibitor LY294002 (LY) or vehicle (0.1% DMSO [D]) or were left untreated (−) for 60 min, followed by stimulation with M. leprae or BCG at an MOI of 5:1 (bacilli to monocyte). Cell supernatants were collected at 24 h postinfection, and cytokines were analyzed by Luminex assay. Values are expressed as percentage activity relative to the M. leprae- or BCG-stimulated untreated cells. Results are the means ± standard error of 10 experiments (representing 10 independent donors) performed in duplicate. A two-tailed paired t test was used for statistical analysis (*, P < 0.05, compared to the untreated M. leprae- or BCG-stimulated cells).
Cytokine response of monocytes costimulated with M. leprae and BCG or LPS.
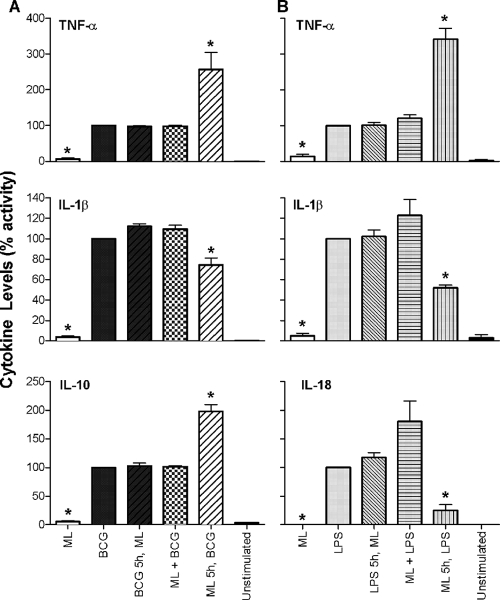
To ask whether M. leprae is simply a poor inducer of monocyte proinflammatory cytokines or actively interferes with their induction, we exposed human monocytes to M. leprae together with a strong stimulus (BCG or LPS) and determined the resulting cytokine levels. Monocytes from healthy human donors were exposed to M. leprae, BCG, or LPS alone or to M. leprae plus BCG or LPS simultaneously or primed first with M. leprae, followed by exposure to BCG or LPS, or vice versa. Preexposure of monocytes to M. leprae followed by stimulation with either BCG or LPS resulted in greatly increased levels of induced TNF-α (Fig. 5), indicating that M. leprae is exerting a priming effect on the cells that modifies their response to a strong secondary stimulus. However, when M. leprae was administered to cells simultaneously with BCG or LPS or added as a second stimulus, the levels of TNF-α released from monocytes were unaffected and comparable to those of BCG or LPS stimulation alone (Fig. 5). Similar results were obtained for the induction of IL-10 by BCG (Fig. 5A). In contrast, prestimulation of cells with M. leprae followed by the second stimulus led to significantly reduced levels of IL-1β in comparison to stimulation with BCG or LPS alone, while simultaneous addition or prestimulation with BCG or LPS showed no effect. Similar results were obtained for IL-18 with LPS as a second stimulus; however, IL-18 induction was also enhanced in cells stimulated simultaneously with M. leprae and LPS. Prestimulation of monocytes with M. leprae followed by BCG or vice versa had no effect on levels of IL-18 (data not shown). None of these costimulation experiments showed any demonstrable impact on the production of IL-1Ra, IL-6, or MCP-1 (data not shown). These results indicate that M. leprae has the capacity to actively induce both positive and negative signals in naïve human monocytes.
FIG. 5.
Cytokine response of monocytes costimulated with M. leprae, M. bovis BCG, and LPS. Monocytes from healthy human donors were stimulated with M. leprae (ML) or BCG alone or with ML and BCG simultaneously or were preexposed to ML or BCG for 5 h, followed by stimulation with the other organism (A); alternatively, monocytes were stimulated with ML or LPS alone or with ML and LPS simultaneously or were preexposed to ML or LPS for 5 h, followed by exposure to the other stimulant (B). Cell supernatants were collected at 48 h postinfection, and cytokines were analyzed by Luminex assay. Values are expressed as percent activity over BCG-stimulated (A) or LPS-stimulated (B) cells. Results are the means ± standard error of four experiments (representing four independent donors) performed in duplicate. A two-tailed, paired t test was used for statistical analysis (*, P < 0.05, in comparison to BCG- or LPS-stimulated cells).
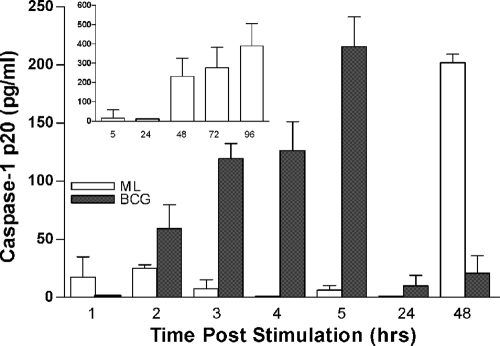
One possible explanation for the observed suppression of IL-1β and IL-18 in M. leprae-prestimulated monocytes was that the bacilli could be inhibiting activation of caspase-1, which is required for the activation of IL-1β and IL-18. To examine this possibility, we measured levels of the caspase-1 p20 subunit in culture supernatants of M. leprae-stimulated cells. Cells were exposed to M. leprae, BCG, or LPS for 1, 2, 3, 4, 5, 24, or 48 h. In BCG-stimulated cells, activated caspase-1 was detectable at 2 h poststimulation, reaching peak levels at 5 h and then falling to baseline (Fig. 6). Similarly, in LPS-stimulated cells, the levels of active caspase-1 peaked at 5 h and then fell (data not shown). In M. leprae-stimulated monocytes, the caspase-1 p20 subunit remained at basal levels for the initial 24 h and then rose sharply at 48 h, slightly increasing over 4 days of culture (Fig. 6, inset). However, in prestimulation experiments, we were unable to show an inhibitory effect of M. leprae on the activation of caspase-1 by BCG or LPS (data not shown), suggesting that the reduction of IL-1β and IL-18 in cells prestimulated with M. leprae is occurring via other regulatory pathways. These data also suggest that the low levels of IL-1β and IL-18 detected in M. leprae-stimulated cells may be attributable to the delay in activation of caspase-1.
FIG. 6.
Caspase-1 activation in human monocytes stimulated with M. leprae. Human monocytes were stimulated with M. leprae or M. bovis BCG at an MOI of 5:1 (bacilli to monocyte). Cell supernatants were collected at 1, 2, 3, 4, 5, 24, and 48 h poststimulation and analyzed for the levels of caspase-1 p20 subunit. Inset shows caspase-1 p20 levels in M. leprae-stimulated monocytes over 4 days of culture. Results are the mean ± standard error of three and five experiments (representing independent donors) minus the values of the unstimulated cells.
M. leprae primes monocytes for a robust response to TLR/NOD2 agonists.
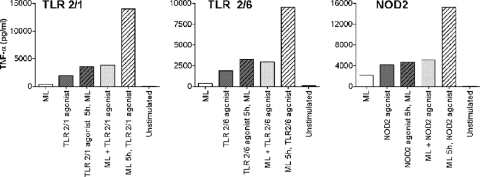
To understand whether the priming effect of M. leprae on monocyte BCG- and LPS-induced TNF-α production (Fig. 5) was mediated through TLR4 only (as indicated by the response to LPS) or via other TLRs, we carried out similar experiments using other specific agonists of different TLRs or NOD2 as the secondary stimulus. For these experiments, we utilized agonists specific for TLR2/1 (Pam3CSK4), TLR2/6 (Pam2CSK4), TLR9 (CpG DNA), and NOD2 (MDP). We found that preexposure to M. leprae resulted in a strong priming effect on monocyte TNF-α production induced by secondary stimulation with a number of TLR agonists (Fig. 7). This priming effect was observed with 5 h of prestimulation in the presence of TLR2/1, TLR2/6, or NOD2 agonist. No priming was observed when a TLR9 agonist was used as a secondary stimulus (data not shown). Exposure of monocytes to simultaneously applied M. leprae and TLR agonists or prestimulation with the TLR agonists did not increase TNF-α production in comparison to the TLR agonists alone. While prestimulation is not a true model of M. leprae infection, the results of these experiments shed light on the ability of M. leprae to manipulate the infected host's immune response to the pathogen's advantage. Thus, the exact cytokine response induced in monocytes exposed to M. leprae would depend on the presence or absence of other stimuli that the cells may be exposed to prior to or following exposure to M. leprae.
FIG. 7.
TNF-α production in monocytes stimulated with M. leprae in the presence/absence of TLR or NOD2 agonist. Monocytes were stimulated with M. leprae, TLR agonist (TLR2/1 or TLR2/6) or NOD2 agonist alone, M. leprae and TLR agonist (TLR2/1 or TLR2/6) simultaneously, or M. leprae and NOD2 agonist simultaneously or preexposed to M. leprae or TLR agonist (TLR2/1 or TLR2/6) or NOD2 agonist for 5 h, followed by exposure to the other stimulus. Cell supernatants were collected at 48 h postinfection; TNF-α levels were determined by Luminex assay. Results are representative of experiments performed in duplicate on monocytes isolated from two independent donors.
DISCUSSION
Consistent with other reports suggesting that M. leprae is a poor inducer of host innate immunity (15, 30, 42), we found that monocyte production of the proinflammatory cytokines IL-1β, IL-18, IL-6, IL-10, IL-12, and TNF-α in response to M. leprae stimulation was absent or very low compared to that in response to BCG stimulation. In contrast, we report for the first time that M. leprae induces high levels of the inhibitory chemokine MCP-1 in naïve human monocytes, and this induction involves the PI3K signaling pathway. The presence of high levels of MCP-1 may, in part, explain the lack of IL-12 in supernatants of M. leprae-stimulated cells as MCP-1 has been associated with dose-dependent suppression of mycobacteria-induced IL-12 (11). Interestingly, we found an inverse relationship between levels of MCP-1 induced by M. leprae versus BCG in different healthy donors; i.e., higher MCP-1 induction by M. leprae corresponded to lower induction by BCG, and vice versa. A recent genetic study of tuberculosis susceptibility showed that individuals with polymorphisms in the mcp-1 promoter had higher circulating levels of MCP-1 and were 5-fold more likely to develop disease (11). It is possible that, by analogy to tuberculosis, some individuals may have a genetically based tendency to produce higher levels of MCP-1 in response to M. leprae infection, which may predispose them to an elevated risk of leprosy per se or development of lepromatous versus tuberculoid disease.
In our monocyte experiments, we also found that IL-1Ra induction was significantly higher in response to M. leprae than to BCG (Fig. 3). While previous reports have described M. leprae-mediated induction of IL-1Ra in human monocytes, the regulatory pathways involved were not explored (42). In our study, the presence of a PI3K-specific inhibitor significantly reduced the ability of M. leprae to stimulate IL-1Ra production by monocytes, implicating the PI3K signaling pathway in this cytokine's induction. Similar to results for MCP-1, we found an inverse correlation in the magnitude of the IL-1Ra response stimulated by either M. leprae or BCG, suggesting that some natural variation may exist in this population of healthy donors and may contribute to the differential response. The high levels of IL-1Ra we observed may have been responsible for downregulating IL-1β to nearly undetectable levels as IL-1Ra is a negative regulator of IL-1β (13). The use of a PI3K-specific inhibitor additionally revealed that M. leprae can be a strong inducer of IL-6 induction by monocytes while simultaneously suppressing this cytokine via a PI3K-dependent mechanism. Taken together, these results support a central role for the PI3K signaling pathway in the response of human monocytes to M. leprae.
The patterns of cytokine induction we observed suggest that M. leprae interacts with cells of the innate immune response through other signaling pathways in addition to PI3K. The failure of M. leprae to induce IL-1β and IL-18, both of which require caspase-1 for activation, is likely due in part to the delay in caspase-1 activation in M. leprae-exposed monocytes. Monocytes stimulated with M. leprae also showed reduced NF-κB activation relative to BCG-stimulated cells, indicating impaired NF-κB signaling in response to M. leprae. Activation of the NF-κB transcription factor is a key event common to several pathways necessary to produce an optimal proinflammatory response. Thus, the combined effects of impaired NF-κB signaling, downregulation of IL-6 and IL-12 through PI3K-dependent mechanisms, and delayed activation of caspase-1 would be expected to dampen the production of the majority of proinflammatory cytokines by M. leprae-stimulated monocytes. Taken together, our results support the premise that the failure of naïve human monocytes to mount an effective cytokine response to M. leprae is due to an active program of suppression induced by the bacilli via multiple pathways.
An ability to hinder development of an optimal innate immune response has been described for other species of mycobacteria. We previously showed that the M. tuberculosis clinical isolate HN878 preferentially induces a Th2-type cytokine response over a Th1-type response in monocytes in vitro (21) and leads to impaired immunity and early death of infected mice (22). Mycobacterium ulcerans mycolactone has been reported to suppress the host innate immune response by interfering with activation of NF-κB (33), disrupting immune cell trafficking (9), and inhibiting cytokine production (41). Thus, a number of virulent mycobacteria are capable of subverting the immune response as a strategy for survival in the infected host.
In addition to its direct effects on host cells, M. leprae was also able to act on naïve human monocytes to modify their response to a second stimulus. Prestimulation of monocytes with M. leprae resulted in suppression of BCG- and/or LPS-induced production of IL-1β and IL-18 (Fig. 5). In similar experiments, M. leprae prestimulation primed the cells to produce increased levels of TNF-α in response to a second stimulus provided by BCG, LPS, or other TLR/NOD2 agonists. These effects were not seen in monocytes exposed simultaneously to M. leprae plus another stimulus or when M. leprae followed as the second stimulus. The observation that prestimulation of monocytes with BCG before exposure to M. leprae as a second stimulus does not affect the cytokine induction profile suggests that BCG primes the cells, rendering them refractory to the downregulatory effects of M. leprae. This may be the scenario observed in the borderline tuberculoid (BT) and polar tuberculoid (TT) leprosy patients that are clearly capable of generating a Th1 immune response in spite of infection with M. leprae (34). In these individuals, prior induction of Th1 responses by exposure to BCG may determine their subsequent response to exposure to M. leprae, ensuring development of tuberculoid-type disease. In addition, this observation may explain how BCG vaccination protects against the subsequent development of leprosy (38). The priming effect of M. leprae may provide some explanation for the development of erythema nodosum leprosum (ENL), which involves a sudden increase in circulating levels of TNF-α in lepromatous patients (10, 39). The occurrence of ENL has been associated with intralesional injections of interferon gamma (IFN-γ), BCG vaccination, purified protein derivative (PPD) skin testing, and initiation of chemotherapy in lepromatous patients (10, 36, 39). These therapeutic interventions may correspond to the second stimulus in our experimental model, triggering the burst of TNF-α production seen in patients with ENL. The ability of M. leprae to prime monocytes for an augmented response to activation via TLR1, TLR2, TLR4, or NOD2 is consistent with studies showing associations between specific polymorphisms in these genes and susceptibility to leprosy, the type of leprosy, or the complications of RR and ENL (5-7, 16, 17). While M. leprae has been shown to interact directly with TLR1/2 (19), it is not clear whether the bacilli are directly engaging TLR4 and NOD2 on the monocytes or acting indirectly by affecting downstream activities in these pathways to modify the cellular response to a second stimulus. However, no convincing evidence indicates an association between common bacterial infection and development of ENL.
Elucidation of the early cytokine activation pathways induced following exposure of cells of the naïve innate immune system to M. leprae should ultimately facilitate a better understanding of determinants underlying the progression to disease or the development of lepromatous versus tuberculoid leprosy and inflammatory complications of leprosy. In addition, these types of studies provide valuable insight that cannot be obtained through the use of clinical samples from leprosy patients.
Acknowledgments
This study was supported by NIH grant AI54631 to G.K.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 19 October 2009.
REFERENCES
- 1.Adams, L. B., N. A. Soileau, J. R. Battista, and J. L. Krahenbuhl. 2000. Inhibition of metabolism and growth of Mycobacterium leprae by gamma irradiation. Int. J. Lepr. Other Mycobact. Dis. 68:1-10. [PubMed] [Google Scholar]
- 2.Alvarado-Navarro, A., M. Montoya-Buelna, J. F. Munoz-Valle, R. I. Lopez-Roa, C. Guillen-Vargas, and M. Fafutis-Morris. 2008. The 3′UTR 1188 A/C polymorphism in the interleukin-12p40 gene (IL-12B) is associated with lepromatous leprosy in the west of Mexico. Immunol. Lett. 118:148-151. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson, S., E. Valadas, S. M. Smith, P. T. Lukey, and H. M. Dockrell. 2000. Monocyte-derived macrophage cytokine responses induced by M. bovis BCG. Tuber. Lung Dis. 80:197-207. [DOI] [PubMed] [Google Scholar]
- 4.Bleharski, J. R., H. Li, C. Meinken, T. G. Graeber, M. T. Ochoa, M. Yamamura, A. Burdick, E. N. Sarno, M. Wagner, M. Rollinghoff, T. H. Rea, M. Colonna, S. Stenger, B. R. Bloom, D. Eisenberg, and R. L. Modlin. 2003. Use of genetic profiling in leprosy to discriminate clinical forms of the disease. Science 301:1527-1530. [DOI] [PubMed] [Google Scholar]
- 5.Bochud, P. Y., T. R. Hawn, and A. Aderem. 2003. Cutting edge: a Toll-like receptor 2 polymorphism that is associated with lepromatous leprosy is unable to mediate mycobacterial signaling. J. Immunol. 170:3451-3454. [DOI] [PubMed] [Google Scholar]
- 6.Bochud, P. Y., T. R. Hawn, M. R. Siddiqui, P. Saunderson, S. Britton, I. Abraham, A. T. Argaw, M. Janer, L. P. Zhao, G. Kaplan, and A. Aderem. 2008. Toll-like receptor 2 (TLR2) polymorphisms are associated with reversal reaction in leprosy. J. Infect. Dis. 197:253-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bochud, P. Y., D. Sinsimer, A. Aderem, M. R. Siddiqui, P. Saunderson, S. Britton, I. Abraham, A. Tadesse Argaw, M. Janer, T. R. Hawn, and G. Kaplan. 9 May 2009. Polymorphisms in Toll-like receptor 4 (TLR4) are associated with protection against leprosy. Eur. J. Clin. Microbiol. Infect. Dis. doi: 10.1007/s10096-009-0746-0. [DOI] [PMC free article] [PubMed]
- 8.Borges, L., and D. Cosman. 2000. LIRs/ILTs/MIRs, inhibitory and stimulatory Ig-superfamily receptors expressed in myeloid and lymphoid cells. Cytokine Growth Factor Rev. 11:209-217. [DOI] [PubMed] [Google Scholar]
- 9.Coutanceau, E., J. Decalf, A. Martino, A. Babon, N. Winter, S. T. Cole, M. L. Albert, and C. Demangel. 2007. Selective suppression of dendritic cell functions by Mycobacterium ulcerans toxin mycolactone. J. Exp. Med. 204:1395-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuevas, J., J. L. Rodriguez-Peralto, R. Carrillo, and F. Contreras. 2007. Erythema nodosum leprosum: reactional leprosy. Semin. Cutan. Med. Surg. 26:126-130. [DOI] [PubMed] [Google Scholar]
- 11.Flores-Villanueva, P. O., J. A. Ruiz-Morales, C. H. Song, L. M. Flores, E. K. Jo, M. Montano, P. F. Barnes, M. Selman, and J. Granados. 2005. A functional promoter polymorphism in monocyte chemoattractant protein-1 is associated with increased susceptibility to pulmonary tuberculosis. J. Exp. Med. 202:1649-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goulart, I. M., J. R. Mineo, and N. T. Foss. 2000. Production of transforming growth factor-beta 1 (TGF-beta1) by blood monocytes from patients with different clinical forms of leprosy. Clin. Exp. Immunol. 122:330-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hannum, C. H., C. J. Wilcox, W. P. Arend, F. G. Joslin, D. J. Dripps, P. L. Heimdal, L. G. Armes, A. Sommer, S. P. Eisenberg, and R. C. Thompson. 1990. Interleukin-1 receptor antagonist activity of a human interleukin-1 inhibitor. Nature 343:336-340. [DOI] [PubMed] [Google Scholar]
- 14.Haregewoin, A., A. S. Mustafa, I. Helle, M. F. Waters, D. L. Leiker, and T. Godal. 1984. Reversal by interleukin-2 of the T cell unresponsiveness of lepromatous leprosy to Mycobacterium leprae. Immunol. Rev. 80:77-86. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto, K., Y. Maeda, H. Kimura, K. Suzuki, A. Masuda, M. Matsuoka, and M. Makino. 2002. Mycobacterium leprae infection in monocyte-derived dendritic cells and its influence on antigen-presenting function. Infect. Immun. 70:5167-5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawn, T. R., E. A. Misch, S. J. Dunstan, G. E. Thwaites, N. T. Lan, H. T. Quy, T. T. Chau, S. Rodrigues, A. Nachman, M. Janer, T. T. Hien, J. J. Farrar, and A. Aderem. 2007. A common human TLR1 polymorphism regulates the innate immune response to lipopeptides. Eur. J. Immunol. 37:2280-2289. [DOI] [PubMed] [Google Scholar]
- 17.Johnson, C. M., E. A. Lyle, K. O. Omueti, V. A. Stepensky, O. Yegin, E. Alpsoy, L. Hamann, R. R. Schumann, and R. I. Tapping. 2007. Cutting edge: a common polymorphism impairs cell surface trafficking and functional responses of TLR1 but protects against leprosy. J. Immunol. 178:7520-7524. [DOI] [PubMed] [Google Scholar]
- 18.Jullien, D., P. A. Sieling, K. Uyemura, N. D. Mar, T. H. Rea, and R. L. Modlin. 1997. IL-15, an immunomodulator of T cell responses in intracellular infection. J. Immunol. 158:800-806. [PubMed] [Google Scholar]
- 19.Krutzik, S. R., M. T. Ochoa, P. A. Sieling, S. Uematsu, Y. W. Ng, A. Legaspi, P. T. Liu, S. T. Cole, P. J. Godowski, Y. Maeda, E. N. Sarno, M. V. Norgard, P. J. Brennan, S. Akira, T. H. Rea, and R. L. Modlin. 2003. Activation and regulation of Toll-like receptors 2 and 1 in human leprosy. Nat. Med. 9:525-532. [DOI] [PubMed] [Google Scholar]
- 20.Lahiri, R., B. Randhawa, and J. L. Krahenbuhl. 2005. Effects of purification and fluorescent staining on viability of Mycobacterium leprae. Int. J. Lepr. Other Mycobact. Dis. 73:194-202. [PubMed] [Google Scholar]
- 21.Manca, C., M. B. Reed, S. Freeman, B. Mathema, B. Kreiswirth, C. E. Barry III, and G. Kaplan. 2004. Differential monocyte activation underlies strain-specific Mycobacterium tuberculosis pathogenesis. Infect. Immun. 72:5511-5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manca, C., L. Tsenova, A. Bergtold, S. Freeman, M. Tovey, J. M. Musser, C. E. Barry III, V. H. Freedman, and G. Kaplan. 2001. Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-alpha /beta. Proc. Natl. Acad. Sci. U. S. A. 98:5752-5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehra, V., P. J. Brennan, E. Rada, J. Convit, and B. R. Bloom. 1984. Lymphocyte suppression in leprosy induced by unique M. leprae glycolipid. Nature 308:194-196. [DOI] [PubMed] [Google Scholar]
- 24.Mehra, V., L. H. Mason, J. P. Fields, and B. R. Bloom. 1979. Lepromin-induced suppressor cells in patients with leprosy. J. Immunol. 123:1813-1817. [PubMed] [Google Scholar]
- 25.Mendez-Samperio, P., A. Vazquez, and H. Ayala. 2003. Infection of human monocytes with Mycobacterium bovis BCG induces production of CC-chemokines. J. Infect. 47:139-147. [DOI] [PubMed] [Google Scholar]
- 26.Misch, E. A., M. Macdonald, C. Ranjit, B. R. Sapkota, R. D. Wells, M. R. Siddiqui, G. Kaplan, and T. R. Hawn. 2008. Human TLR1 deficiency is associated with impaired mycobacterial signaling and protection from leprosy reversal reaction. PLoS Negl. Trop. Dis. 2:e231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Modlin, R. L. 1994. Th1-Th2 paradigm: insights from leprosy. J. Invest. Dermatol. 102:828-832. [DOI] [PubMed] [Google Scholar]
- 28.Molloy, A., P. A. Meyn, K. D. Smith, and G. Kaplan. 1993. Recognition and destruction of Bacillus Calmette-Guerin-infected human monocytes. J. Exp. Med. 177:1691-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molnarfi, N., L. Gruaz, J. M. Dayer, and D. Burger. 2007. Opposite regulation of IL-1beta and secreted IL-1 receptor antagonist production by phosphatidylinositide-3 kinases in human monocytes activated by lipopolysaccharides or contact with T cells. J. Immunol. 178:446-454. [DOI] [PubMed] [Google Scholar]
- 30.Murray, R. A., M. R. Siddiqui, M. Mendillo, J. Krahenbuhl, and G. Kaplan. 2007. Mycobacterium leprae inhibits dendritic cell activation and maturation. J. Immunol. 178:338-344. [DOI] [PubMed] [Google Scholar]
- 31.Ohyama, H., K. Ogata, K. Takeuchi, M. Namisato, Y. Fukutomi, F. Nishimura, H. Naruishi, T. Ohira, K. Hashimoto, T. Liu, M. Suzuki, Y. Uemura, and S. Matsushita. 2005. Polymorphism of the 5′ flanking region of the IL-12 receptor beta2 gene partially determines the clinical types of leprosy through impaired transcriptional activity. J. Clin. Pathol. 58:740-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oliveira, M. M., R. Charlab, and M. C. Pessolani. 2001. Mycobacterium bovis BCG but not Mycobacterium leprae induces TNF-alpha secretion in human monocytic THP-1 cells. Mem. Inst. Oswaldo Cruz 96:973-978. [DOI] [PubMed] [Google Scholar]
- 33.Pahlevan, A. A., D. J. Wright, C. Andrews, K. M. George, P. L. Small, and B. M. Foxwell. 1999. The inhibitory action of Mycobacterium ulcerans soluble factor on monocyte/T cell cytokine production and NF-kappa B function. J. Immunol. 163:3928-3935. [PubMed] [Google Scholar]
- 34.Ridley, D. S., and W. H. Jopling. 1966. Classification of leprosy according to immunity. A five-group system. Int. J. Lepr. Other Mycobact. Dis. 34:255-273. [PubMed] [Google Scholar]
- 35.Roy, S., W. McGuire, C. G. Mascie-Taylor, B. Saha, S. K. Hazra, A. V. Hill, and D. Kwiatkowski. 1997. Tumor necrosis factor promoter polymorphism and susceptibility to lepromatous leprosy. J. Infect. Dis. 176:530-532. [DOI] [PubMed] [Google Scholar]
- 36.Sampaio, E. P., A. L. Moreira, E. N. Sarno, A. M. Malta, and G. Kaplan. 1992. Prolonged treatment with recombinant interferon gamma induces erythema nodosum leprosum in lepromatous leprosy patients. J. Exp. Med. 175:1729-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santos, A. R., P. N. Suffys, P. R. Vanderborght, M. O. Moraes, L. M. Vieira, P. H. Cabello, A. M. Bakker, H. J. Matos, T. W. Huizinga, T. H. Ottenhoff, E. P. Sampaio, and E. N. Sarno. 2002. Role of tumor necrosis factor-alpha and interleukin-10 promoter gene polymorphisms in leprosy. J. Infect. Dis. 186:1687-1691. [DOI] [PubMed] [Google Scholar]
- 38.Scollard, D. M., L. B. Adams, T. P. Gillis, J. L. Krahenbuhl, R. W. Truman, and D. L. Williams. 2006. The continuing challenges of leprosy. Clin. Microbiol. Rev. 19:338-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sehgal, V. N., G. Srivastava, and J. A. Sundharam. 1988. Immunology of reactions in leprosy. Current status. Int. J. Dermatol. 27:157-162. [DOI] [PubMed] [Google Scholar]
- 40.Shepard, C. C., and D. H. McRae. 1968. A method for counting acid-fast bacteria. Int. J. Lepr. Other Mycobact. Dis. 36:78-82. [PubMed] [Google Scholar]
- 41.Simmonds, R. E., F. V. Lali, T. Smallie, P. L. Small, and B. M. Foxwell. 2009. Mycolactone inhibits monocyte cytokine production by a posttranscriptional mechanism. J. Immunol. 182:2194-2202. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki, K., Y. Fukutomi, M. Matsuoka, K. Torii, H. Hayashi, T. Takii, Y. Oomoto, and K. Onozaki. 1993. Differential production of interleukin 1 (IL-1), IL-6, tumor necrosis factor, and IL-1 receptor antagonist by human monocytes stimulated with Mycobacterium leprae and M. bovis BCG. Int. J. Lepr. Other Mycobact. Dis. 61:609-618. [PubMed] [Google Scholar]
- 43.Watkins, M. L., P. L. Semple, B. Abel, W. A. Hanekom, G. Kaplan, and S. R. Ress. 2008. Exposure of cord blood to Mycobacterium bovis BCG induces an innate response but not a T-cell cytokine response. Clin. Vaccine Immunol. 15:1666-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.World Health Organization. 16 November 2009, accession date. Leprosy today. World Health Organization, Geneva, Switzerland. http://www.who.int/lep/en/.
- 45.Yamamura, M., K. Uyemura, R. J. Deans, K. Weinberg, T. H. Rea, B. R. Bloom, and R. L. Modlin. 1991. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science 254:277-279. [DOI] [PubMed] [Google Scholar]