Abstract
The innate immune system recognizes pathogen-associated molecular patterns (PAMPs) through pattern recognition receptors (PRR) and transduces downstream signaling to activate the host defense. Here we report that in addition to direct PAMP-PRR interactions, live Candida albicans cells can release soluble factors to actively potentiate interleukin-6 (IL-6) and IL-8 production induced in human mononuclear cells by the fungi. Although protease-activated receptor 1 (PAR1) and PAR2 ligation can moderately upregulate Toll-like receptor 4 (TLR4)-mediated IL-8 production, no effect on the C. albicans-induced cytokine was apparent. Similarly, the blockade of PAR signaling did not reverse the potentiation of cytokine production induced by soluble factors released by C. albicans. In conclusion, C. albicans releases soluble factors that potentiate cytokine release in a PAR1/2-independent manner. Thus, human PAR1 and PAR2 have a redundant role in the activation of human cells by C. albicans.
Candida albicans is a commensal fungal microorganism colonizing the skin and/or mucosa of healthy individuals. Although C. albicans colonization is usually asymptomatic, for certain categories of patients, C. albicans can cause a wide range of clinical syndromes, from oral thrush and vaginal candidiasis to systemic candidiasis. Severe C. albicans infection can cause high rates of mortality for immunocompromised patients, such as HIV and intensive care unit (ICU) patients. Host innate immunity plays a major role in the elimination of C. albicans infection. The first-line host defense is the physical barrier represented by the intact skin and mucosal surface, while the direct elimination of the fungus is executed mainly by polymorphonuclear leukocyte (PMN). A breakdown in mucosal barrier defense and decreased PMN function provides a chance for C. albicans invasion of tissues. However, to activate the host defense mechanisms, leukocytes must possess the ability to discriminate self and nonself. Host innate cells are equipped with a limited panel of germ line-encoded pattern recognition receptors (PRRs), which can recognize structures of microorganism called pathogen-associated molecular patterns (PAMPs). The main PRRs for C. albicans are the Toll-like receptors (TLRs) and the C-type lectin receptors (CLRs). In this respect, C. albicans N-linked mannan is recognized by mannose receptor (MR), and O-linked mannan is recognized by TLR4, while β-glucan is recognized by dectin-1 in cooperation with TLR2 (15). It was demonstrated that through the direct interaction between C. albicans PAMPs and host PRRs, downstream cytokines are released, recruiting more effector cells and inducing inflammation to control fungal outgrowth.
However, besides PAMPs from cell wall components, C. albicans also releases soluble factors into the surrounding environment, including metabolites, shedding PAMPs and extracellular hydrolases. Among them, the most well-studied factors are the secreted aspartic proteases (Saps). So far, 10 Sap isoenzymes encoded by the genes SAP1 to SAP10 have been identified for C. albicans (12, 13). It was previously reported that Saps have a variety of functions in C. albicans infection and are involved in phenotype switching, biofilm formation, nutrient acquisition, and tissue invasion/damage, etc. (12), and Saps have been proven to be important virulence factors. Using the reconstituted human vaginal epithelium (RHVE) model, it was previously shown that the addition of the aspartic protease inhibitor pepstatin A that neutralizes Saps strongly reduced the level of cytokine production induced by C. albicans infection (19). In in vitro models of oral (18) and cutaneous (2) candidosis, the presence of pepstatin A reduced the virulence of C. albicans.
Protease-activated receptors (PARs) are seven-transmembrane G-protein-coupled receptors with a N-terminal extracellular peptide (9). After the cleavage of the N-terminal end by a protease, a tethered ligand will be exposed, and this will bind intramolecularly to its ligand binding site and activate downstream signaling. Both host-derived proteases (e.g., PR3) (20) released in response to infection and pathogen-derived proteases (e.g., gingipains from Porphyromonas gingivalis) (21) are able to cleave PARs, thus activating downstream proinflammatory cytokine production and secretion.
A recent study suggested a role of PARs in fungal recognition in mice (11). Moretti et al. also reported cross talk interactions between PARs and TLRs in inflammation against fungal infection. It was previously proposed that the inflammation in response to C. albicans is promoted by PAR1 and PAR2 activation, which is downstream of the TLR2 signaling pathway (11). However, nothing is known regarding the potential role of PARs in the recognition of C. albicans by human cells. In this study we investigated the potential role of soluble factors released by live C. albicans cells in the induction of cytokine production in human leukocytes, and we examined the role played by PAR1 and PAR2 in mediating these effects.
MATERIALS AND METHODS
Volunteers.
Blood samples were collected from healthy, nonsmoking volunteers. After written informed consent was obtained, venipuncture was performed to collect blood into 10-ml EDTA tubes (Monoject).
Reagents.
Lipopolysaccharide (LPS) (Escherichia coli serotype O55:B5) was purchased from Sigma. LPS was repurified as described elsewhere previously (7). Synthetic Pam3Cys was purchased from EMC Microcollections, and the production of highly purified particulate β-glucan and soluble β-glucan (glucan phosphate) was described elsewhere previously (22).
C. albicans strains and conditioned medium.
C. albicans ATCC MYA-3573 (UC 820) (10) was used as the wild type. Δsap1-3 (8), Δsap4-6 (17), and Δsap9-10 (1) C. albicans strains were used and are well characterized in the literature. C. albicans organisms were grown overnight in Sabouraud broth at 37°C, and cells were thereafter harvested by centrifugation, washed twice, and resuspended in culture medium (RPMI 1640 medium; ICN Biomedicals) (23). For the preparation of heat-killed C. albicans cells, live C. albicans cells were harvested, heated for 1 h at 100°C, and resuspended in culture medium at a final concentration of 106 C. albicans yeast cells/ml. For the preparation of conditioned medium, live C. albicans cells were inoculated into RPMI medium at a concentration of 106 C. albicans yeast cells/ml and grown in an incubator at 37°C for 24 h. Conditioned medium was collected by centrifugation, and the supernatant was filtered through a 0.22-μm filter and stored at −20°C before use. The pH of conditioned medium was 6.9. The protease activity of the conditioned medium was determined by use of a QuantiCleave protease assay kit (Pierce), and no difference in protease activity was found between fresh harvested (36.5 ng/ml) and fresh thawed (35.9 ng/ml) conditioned media.
Fractionation of conditioned medium.
C. albicans conditioned media were fractionized by centrifugal filter units (Millipore) with different molecular mass cutoffs (3-, 30-, and 100-kDa cutoffs) according to the manufacturer's protocol. After centrifugation, the concentrated protein fractions were reconstituted back to the original volume.
Isolation and stimulation of PBMCs.
The separation and stimulation of peripheral blood mononuclear cells (PBMCs) were performed as described elsewhere previously (15). Cells were adjusted to 5 × 106 cells/ml and thereafter incubated at 37°C in round-bottom 96-well plates (volume, 100 μl/well) with either heat-killed C. albicans microorganisms (106 microorganisms/ml) or culture medium, with or without C. albicans conditioned medium (50 μl/well), and the final volume of each well was 200 μl. After 24 h, supernatants were collected and stored at −20°C until assayed. For the cross talk experiments between PARs and PRRs, PBMCs were stimulated simultaneously with PRR ligands, LPS (1, 10, and 100 ng/ml), Pam3Cys (0.1, 1, and 10 μg/ml), or β-glucan (1, 10, and 100 μg/ml), and 100 μM of PAR-1 agonist peptide or PAR2 agonist peptide. After 24 h, supernatants were collected and stored at −20°C until assayed.
Transwell stimulation experiments.
Live C. albicans cells (106 microorganisms/ml) were cultured in the upper well of a 24-well transwell system (pore size, 0.4 μm; Corning) to avoid direct contact between live C. albicans cells and PBMCs but allowing the free diffusion of the released soluble factors. PBMCs and other stimulants were cultured in the lower well. The culture supernatant was collected after removing the upper well and stored at −20°C before cytokine measurements.
PAR agonist and antagonist peptides.
PAR1 agonist peptide TFLLR and PAR2 agonist peptide SLIGKV were synthesized by Ansynth Service B.V., the Netherlands. PAR1 antagonist peptide FLLRN and PAR2 antagonist peptide FSLLRY were purchased from Peptides International. To determine the role of PARs in our system, both agonist peptides and antagonist peptides were added simultaneously with heat-killed C. albicans cells at a concentration of 100 μM.
Cytokine measurements.
Interleukin-6 (IL-6) and IL-8 concentrations were measured by use of commercial sandwich enzyme-linked immunosorbent assay (ELISA) kits (Pelikine Compact; CLB, Amsterdam, the Netherlands) according to the manufacturer's instructions. Measurements of human tumor necrosis factor alpha (TNF-α) were determined by specific ELISA as described elsewhere previously (6).
Quantitative PCR.
PBMCs were stimulated as described above. After 24 h, the supernatant was removed, and the cells were resuspended in 200 μl of RNAzol B RNA isolation solvent (Campro Scientific) and stored at −80°C. mRNA was isolated according to the instructions of manufacturer. cDNA was synthesized from 1 μg of total RNA by use of Superscript reverse transcriptase (Invitrogen). Relative mRNA levels were determined by using the Bio-Rad i-Cycler and the SYBR green method (Invitrogen). The following primers were used: PAR1 forward primer 5′-CAAATGCCACCTTAGATCCCC-3′ and reverse primer 5′-CTTCTGAGATGAATGCAGGAAGT-3′, PAR2 forward primer 5′-CTGGTTCCCCTTGAAGATTGC-3′ and reverse primer 5′-TCACGATGACCCAATACCTCT-3′, and β2 M forward primer 5′-ATGAGTATGCCTGCCGTGTG-3′ and reverse primer 5′-CCAAATGCGGCATCTTCAAAC-3′ (Biolegio). Values are expressed as fold increases in mRNA levels relative to those for unstimulated cells.
Statistical analysis.
Results from at least 3 sets of experiments were pooled and analyzed by using SPSS 16.0 statistical software. Data are given as means ± standard errors (SE), and the Wilcoxon signed-rank test was used to compare differences between groups (unless otherwise stated). The level of significance was set at a P value of <0.05.
RESULTS
C. albicans-released components modulate cytokine production.
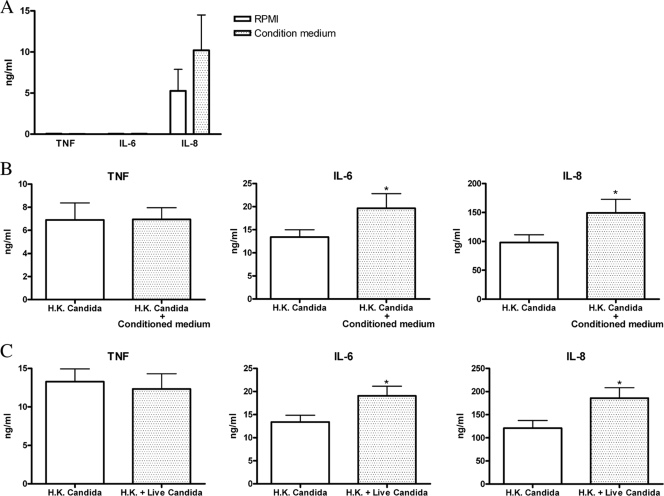
For the purpose of studying the role of the C. albicans-released components in modulating cytokine production, fresh isolated human PBMCs were cultured in the presence or absence of C. albicans conditioned medium. No significant production of TNF, IL-6, or IL-8 was induced by the conditioned medium alone (Fig. 1A). C. albicans conditioned medium induced a minor increase in IL-8 release, but this was not statistically significant.
FIG. 1.
Candida culture conditioned medium modulates host cytokine production. (A) Human PBMCs were incubated with RPMI or C. albicans culture conditioned medium. The supernatant was collected for TNF, IL-6, and IL-8 ELISA measurements after 24 h of stimulation. (B) Human PBMCs were stimulated with heat-killed (H.K.) C. albicans in the presence of RPMI or C. albicans conditioned medium. Supernatants were collected for TNF, IL-6, and IL-8 measurements after 24 h of stimulation. (C) Human PBMCs were seeded in the lower well of transwell plates and stimulated with heat-killed C. albicans cells, while RPMI medium or live C. albicans cells were added to the upper well. Supernatants were collected for TNF, IL-6, and IL-8 measurements after 24 h of stimulation. The data are cumulative results of three duplicate data from 6 different donors and are expressed as means ± SE (*, P < 0.05 compared to PBMCs incubated with heat-killed Candida alone).
To test whether conditioned medium can modulate cytokine release induced by PRR triggering, human PBMCs were stimulated with heat-killed C. albicans cells in the presence or absence of C. albicans conditioned medium. The results show that conditioned medium of C. albicans potentiates PBMC IL-6 and IL-8 secretion in response to heat-killed C. albicans stimulation (Fig. 1B). The role of the components released by C. albicans in cytokine production was also investigated in a transwell system to separate the direct contact between live C. albicans cells and PBMCs. Live C. albicans cells growing in the upper compartment released components that augmented IL-6 and IL-8 production induced by heat-killed C. albicans cells (Fig. 1C). In addition, IL-10 production was also enhanced, while interferon (IFN) release was in turn downregulated by the conditioned medium (data not shown). Taken together, the results from conditioned medium and transwell experiments demonstrated a modulatory effect of C. albicans-released soluble factors on the cytokine response of PBMCs. This was not due to differences between the pHs of RPMI medium (pH 7.0) and conditioned medium (pH 6.9).
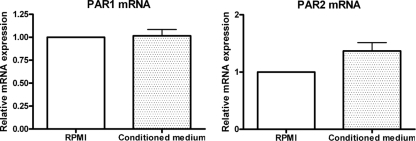
C. albicans conditioned medium has no effect on par1 and par2 expression.
The enzymatic activation of PAR1 and PAR2 was recently reported to play a role in inflammation and immunity in response to fungal infection in a murine model (11), and we hypothesized that they may play a role in cytokine modulation by C. albicans-released proteases. The incubation of human PBMCs with C. albicans conditioned medium did not influence par1 and par2 mRNA expression compared to an RPMI medium control group (Fig. 2), indicating that C. albicans-released soluble factors do not modulate par1 and par2 gene expression at the transcriptional level.
FIG. 2.
Modulation of par1 and par2 mRNA expression by C. albicans culture conditioned medium. Human PBMCs were incubated with RPMI medium, C. albicans conditioned medium, and heat-killed C. albicans cells. (A and B) par1 (A) and par2 (B) mRNA expression levels were determined by real-time PCR, normalized to the expression of the RPMI medium-incubated group.
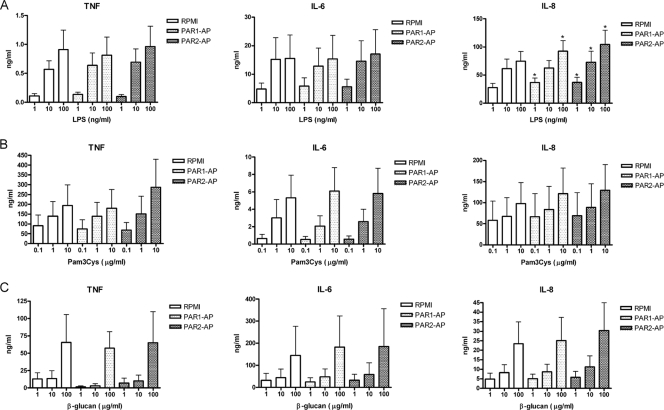
PAR1 and PAR2 agonists slightly modulate IL-8 production in combination with LPS through TLR4.
TLR2, TLR4, and dectin-1 were identified to be the major PRRs for C. albicans recognition (14), and it was previously reported that inflammation induced by C. albicans through TLR2 might be potentiated by PAR1 and PAR2 signaling in mice (11). Human PBMCs were stimulated with agonist peptides of PARs, PAR1-AP (TFLLR) and PAR2-AP (SLIGKV), together with LPS (Fig. 3A), Pam3Cys (Fig. 3B), or β-glucan (Fig. 3C), to assess the cross talk between PARs and TLR2/TLR4/dectin-1. A slight potentiation of IL-8 production when cells were stimulated with TLR4 and PAR1 ligands (23% increase) and TLR4 and PAR2 (40% increase) was observed. However, neither PAR1 nor PAR2 augmented TNF and IL-6 production in combination with all three PRR agonists used in this study.
FIG. 3.
Cross talk between TLRs and PARs. Human PBMCs were treated with LPS (A), Pam3Cys (B), and β-glucan (C) in a dose-dependent manner, in combination with RPMI medium, PAR1 agonist (PAR1-AP) (TFLLR), or PAR2-AP (SLIGRL), respectively. Supernatants were collected for TNF, IL-6, and IL-8 measurements after 24 h of stimulation. The data are cumulative results of three duplicate data from 6 different donors and are expressed as means ± SE (*, P < 0.05 compared to PBMCs stimulated with LPS alone).
PAR1 and PAR2 agonists and antagonists had no effect on modulating C. albicans-induced cytokine production.
To further assess the role of PAR1 and PAR2 in modulating cytokine production during C. albicans infection, heat-killed C. albicans was used as the source of fungal PAMPs, while PAR1-AP (TFLLR) and PAR2-AP (SLIGKV) were used to activate PAR signaling, mimicking the possible role of C. albicans-released soluble factors. However, either individual PAR agonists or PAR agonists in combination failed to potentiate heat-killed C. albicans-induced IL-6 and IL-8 production in human PBMCs (see Fig. S1 in the supplemental material).
Since PAR1 and PAR2 agonists could not modulate the cytokine profiles induced by heat-killed C. albicans, PAR1 and PAR2 antagonists were used to study the role of PAR1 and PAR2 in live C. albicans-induced cytokine upregulation. PAR1-ANT (FLLRN) and PAR2-ANT (FSLLRY) were used to block PAR1 and PAR2 signaling in the transwell system. As expected, both PAR1-ANT and PAR2-ANT could not block the IL-6 and IL-8 upregulation induced by live C. albicans-released soluble factors either respectively or in combination (data not shown). The lack of an effect of PAR stimulation on the blockade of cytokine production was also apparent when a different strain of C. albicans (NGY 152) was used or when cells were stimulated with Candida dubliniensis or Candida glabrata (see Fig. S2 in the supplemental material).
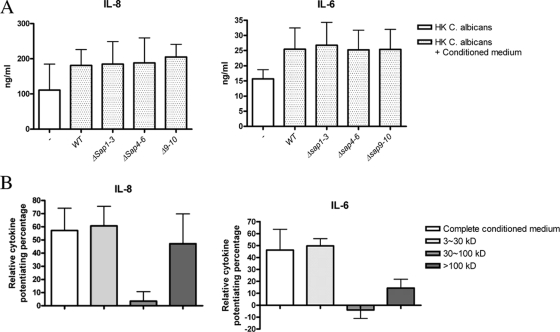
Secreted soluble factors, but not distinct Saps, modulate C. albicans-induced cytokine production.
Saps are considered to be important virulence factors for C. albicans, and they are secreted into the extracellular space from C. albicans cultures. To investigate the role of Saps in host cytokine modulation, we harvested C. albicans conditioned media from different sap mutant C. albicans strains (Δsap1-3, Δsap4-6, and Δsap9-10) and examined their capacity to modulate cytokine production (Fig. 4A). The results showed that the conditioned medium from different sap mutants induced cytokine modulation effects similar to those of wild-type C. albicans, demonstrating that neither Sap1-3, Sap4-6, nor Sap1-10 is necessary for the modulation of cytokine production.
FIG. 4.
Saps are not responsible for the cytokine modulation effect. (A) Human PBMCs were stimulated with heat-killed C. albicans cells in the presence of RPMI or conditioned medium from wild-type (WT) C. albicans or from C. albicans strains defective in different Saps: Δsap1-3, Δsap4-6, or Δsap9-10. Supernatants were collected for IL-6 and IL-8 measurements after 24 h of stimulation. (B) Human PBMCs were stimulated with heat-killed C. albicans cells in the presence of different fractions of C. albicans conditioned medium. Supernatant collected for IL-6 and IL-8 measurements after 24 h shows the relative cytokine percentages induced by conditioned medium above control stimulation with heat-killed C. albicans cells alone.
To further dissect the factors responsible for this effect, we collected the conditioned medium and divided it into different fractions according the molecular mass (small, 3 to ∼30 kDa; intermediate, 30 to ∼100 kDa; large, >100 kDa) and tested their abilities to modulate heat-killed C. albicans-induced cytokine production (Fig. 4B). We found that the small fraction contains the active components, because it can induce a cytokine modulation effect similar to that of the complete conditioned medium. Also, the large fraction can potentiate IL-8 production and, to a lesser extent, IL-6 production. However, no modulation effect was observed in the intermediate fraction, where all the Saps are located, as the molecular mass of Saps is between 34 and 45 kDa.
DISCUSSION
Through the recognition of PAMPs on the C. albicans surface, innate cells can eliminate fungi directly by phagocytosis or can activate host defense mechanisms by secreting different chemokines and cytokines. Much has been learned about the PRRs responsible for the recognition of C. albicans cell wall components such as mannan and β-glucans. However, C. albicans also releases soluble factors during infections, some of them with enzymatic properties, such as secreted aspartic proteases (Saps) (12). It was recently proposed that the activation of PAR1 and PAR2 by C. albicans-released proteases contributes to the activation of cytokine production and, in coordination with TLR signaling, is important for antifungal immunity in mice. However, nothing is known regarding the role of PAR1 and PAR2 receptors in antifungal immunity in humans. In the present study, we investigated the role of human PAR1 and PAR2 in C. albicans recognition and the activation of proinflammatory cytokines. We show that soluble factors released by live C. albicans cells potentiate IL-6 and IL-8 production induced by heat-killed C. albicans cells, but this effect is exerted mostly in a PAR-independent manner.
Regarding the cytokine profile induce by C. albicans in vitro, most previously reported studies were performed with heat-killed C. albicans cells. Two main reasons were responsible for this. First, heat-killed C. albicans cells are more immunogenic than live C. albicans cells, and this is due to the exposure of the PAMPs on the surface after heat killing. For example, β-glucan is usually masked by the outer mannoprotein layer (5) and exposed only on the budding scar (4). However, β-glucan will be exposed after heat exposure (5, 22). Second, heat-killed C. albicans cells are easier to handle, while live C. albicans cells can outgrow and kill the host cells that they are supposed to stimulate. However, there are also drawbacks of using heat-killed C. albicans. Not only is the PAMP distribution changed and different from that of live C. albicans cells, but the involvement of the soluble factors secreted by live C. albicans, which might actively shape the host immune response, is also overlooked.
To investigate the possible modulating effect elicited by factors released by live C. albicans cells, conditioned medium from C. albicans cultures was used to stimulate human PBMCs. However, conditioned medium alone could not induce TNF and IL-6 secretion yet slightly induced IL-8 secretion. It appeared, therefore, that the secreted soluble factors by themselves possess no stimulating ability to induce host cytokine production. We have further tested whether the conditioned medium can modulate the cytokine profile induced by heat-killed C. albicans cells. The production of IL-6 and IL-8 induced by heat-killed C. albicans cells was potentiated by conditioned medium. The same cytokine modulation pattern was observed when a transwell system containing both live and heat-killed fungi was used. These data demonstrate that live C. albicans cells can release soluble factors that modulate the host response induced by cell wall components. In addition, apart from proinflammatory cytokines such as IL-6 and IL-8, we found that the Th1/Th2 signature cytokines were also altered by the conditioned medium (our unpublished data), suggesting an active role of C. albicans in modulating the host immune response during infection.
A second aim of our studies was to assess the possible involvement of PARs in the modulatory effect of C. albicans-released mediators. Previous studies demonstrated the activation of cytokine release by bacterial proteases that activate PARs (21), and a role for PAR1 and PAR2 in the anti-C. albicans defense in mice was previously suggested (11). Our results indicate that PAR1/2 ligation has a slight stimulatory effect on TLR4 signaling, leading to augmented IL-8 production, which is in line with data reported previously by Rallabhandi et al. (16). However, PAR1 and PAR2 agonists failed to potentiate IL-6 or IL-8 production induced by PBMCs stimulated with heat-killed C. albicans cells despite a marginal effect on IL-6 production when PAR1 and PAR2 agonists were used in combination.
There are two possibilities to explain these results. On the one hand, PAR1 and PAR2 are not involved in C. albicans-induced cytokine production, so the addition of a PAR agonist has no effect on cytokine production. On the other hand, the modulatory effect of C. albicans might be due to the induction of host proteases, which by themselves activate PARs, and the addition of a PAR agonist has no additional effect because PARs are already activated. Therefore, PAR antagonists were applied in the transwell system to block PAR signaling to differentiate between these two different possibilities. In this way, either C. albicans PAR or host PAR signaling would be blocked. However, both PAR1 and PAR2 antagonists failed to reverse the upregulated IL-6 and IL-8 production induced by live C. albicans cells. This suggests that the soluble factors released by C. albicans modulate the human immune response largely through a PAR-independent pathway. From this point of view, it seems that human and murine PARs function differently in C. albicans infection, at least in the case of the response of PBMCs toward C. albicans. Based on the results that we have found, we suggest that human PAR1 and PAR2 are not as important in mice for the modulation of host immunity against C. albicans infection or at least that the role of PAR1/PAR2 is redundant because other pathways (e.g., TLRs and CLRs) are capable of fully compensating for their absence.
The discrepancy between the results described previously by Moretti et al. (11) and ours might be due to the different C. albicans strains used. To investigate whether this accounted for the discrepancy, we applied another clinically isolated C. albicans strain and other Candida species like C. glabrata and C. dubliniensis. However, the two different C. albicans strains and other Candida spp. showed a similar cytokine modulation phenomenon (data not shown). Therefore, this effect is not Candida strain specific but more likely a general modulation function of all Candida spp. Notwithstanding, we cannot rule out the possibility of the involvement of PAR1/2 in other experimental models such as mucosal infection, where other cell types are involved in the pathological process.
Another line of evidence is the role of Saps. Since Saps were reported to be important virulence factors for C. albicans infection, it is reasonable to hypothesize a link between Saps and the immunomodulation effect induced by live C. albicans cells. In our experiment we compared the conditioned media of wild-type C. albicans and other sap mutants. However, there was no significant difference among the wild type and sap mutants irrespective of the different Sap compositions in the conditioned medium. We also found that the major modulatory components are within the small fraction (3 to ∼30 kDa) and, to a lesser extent, the large fraction (>100 kDa). This result is in line with the results from sap mutants, because the molecular mass of Saps is between 34 and 45 kDa, which falls within the range of the intermediate fraction (30 to ∼100 kDa), suggesting that Saps are less likely to play a role in the modulation of host cytokine production. Nevertheless, other potential components released by C. albicans, such as glycans, lipases, phospholipases, or metabolites, might be involved in the modulation of the immune response. It was previously shown that certain chemotactic factors produced by C. albicans could induce the chemotaxis of PMNs and macrophages by binding to formyl peptide receptor (FPR) (3). This further demonstrates that the soluble factors secreted/produced by live C. albicans cells can be sensed by or modulate the host immune system in an active manner. Therefore, further identification of the factors responsible for this cytokine modulation effect deserves further investigation.
In conclusion, we demonstrate that live C. albicans cells can actively modulate the host immune response in the presence of fungal PAMPs by releasing soluble factors. However, in contrast to murine cells, human PAR1 and PAR2 do not play a major role in the stimulation of cytokine production by C. albicans. These data add to our understanding of the mechanism of host defense activation by fungi and open a new avenue of research in the field of immune activation by soluble components of pathogenic microorganisms.
Supplementary Material
Acknowledgments
S.-C.C. was supported by a scholarship from EU-FP7 Marie Curie grant FINSysB (PITN-GA-2008-214004). M.G.N. was supported by a Vidi grant of the Netherlands Organization for Scientific Research.
Editor: G. S. Deepe, Jr.
Footnotes
Published ahead of print on 26 October 2009.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Albrecht, A., A. Felk, I. Pichova, J. R. Naglik, M. Schaller, G. P. de, D. Maccallum, F. C. Odds, W. Schafer, F. Klis, M. Monod, and B. Hube. 2006. Glycosylphosphatidylinositol-anchored proteases of Candida albicans target proteins necessary for both cellular processes and host-pathogen interactions. J. Biol. Chem. 281:688-694. [DOI] [PubMed] [Google Scholar]
- 2.De Bernardis, F., M. Boccanera, D. Adriani, E. Spreghini, G. Santoni, and A. Cassone. 1997. Protective role of antimannan and anti-aspartyl proteinase antibodies in an experimental model of Candida albicans vaginitis in rats. Infect. Immun. 65:3399-3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edens, H. A., C. A. Parkos, T. W. Liang, A. J. Jesaitis, J. E. Cutler, and H. M. Miettinen. 1999. Non-serum-dependent chemotactic factors produced by Candida albicans stimulate chemotaxis by binding to the formyl peptide receptor on neutrophils and to an unknown receptor on macrophages. Infect. Immun. 67:1063-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gantner, B. N., R. M. Simmons, and D. M. Underhill. 2005. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J. 24:1277-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gow, N. A., M. G. Netea, C. A. Munro, G. Ferwerda, S. Bates, H. M. Mora-Montes, L. Walker, T. Jansen, L. Jacobs, V. Tsoni, G. D. Brown, F. C. Odds, J. W. Van der Meer, A. J. Brown, and B. J. Kullberg. 2007. Immune recognition of Candida albicans beta-glucan by dectin-1. J. Infect. Dis. 196:1565-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grebenchtchikov, N., J. van der Ven-Jongkrijg, G. J. Pesman, A. Geurts-Moespot, J. W. Van der Meer, and F. C. Sweep. 2005. Development of a sensitive ELISA for the quantification of human tumour necrosis factor-alpha using 4 polyclonal antibodies. Eur. Cytokine Netw. 16:215-222. [PubMed] [Google Scholar]
- 7.Hirschfeld, M., Y. Ma, J. H. Weis, S. N. Vogel, and J. J. Weis. 2000. Repurification of lipopolysaccharide eliminates signaling through both human and murine Toll-like receptor 2. J. Immunol. 165:618-622. [DOI] [PubMed] [Google Scholar]
- 8.Hube, B., D. Sanglard, F. C. Odds, D. Hess, M. Monod, W. Schafer, A. J. Brown, and N. A. Gow. 1997. Disruption of each of the secreted aspartyl proteinase genes SAP1, SAP2, and SAP3 of Candida albicans attenuates virulence. Infect. Immun. 65:3529-3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawabata, A., and R. Kuroda. 2000. Protease-activated receptor (PAR), a novel family of G protein-coupled seven trans-membrane domain receptors: activation mechanisms and physiological roles. Jpn. J. Pharmacol. 82:171-174. [DOI] [PubMed] [Google Scholar]
- 10.Lehrer, R. I., and M. J. Cline. 1969. Interaction of Candida albicans with human leukocytes and serum. J. Bacteriol. 98:996-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moretti, S., S. Bellocchio, P. Bonifazi, S. Bozza, T. Zelante, F. Bistoni, and L. Romani. 2008. The contribution of PARs to inflammation and immunity to fungi. Mucosal Immunol. 1:156-168. [DOI] [PubMed] [Google Scholar]
- 12.Naglik, J., A. Albrecht, O. Bader, and B. Hube. 2004. Candida albicans proteinases and host/pathogen interactions. Cell. Microbiol. 6:915-926. [DOI] [PubMed] [Google Scholar]
- 13.Naglik, J. R., S. J. Challacombe, and B. Hube. 2003. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol. Mol. Biol. Rev. 67:400-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Netea, M. G., G. D. Brown, B. J. Kullberg, and N. A. Gow. 2008. An integrated model of the recognition of Candida albicans by the innate immune system. Nat. Rev. Microbiol. 6:67-78. [DOI] [PubMed] [Google Scholar]
- 15.Netea, M. G., N. A. Gow, C. A. Munro, S. Bates, C. Collins, G. Ferwerda, R. P. Hobson, G. Bertram, H. B. Hughes, T. Jansen, L. Jacobs, E. T. Buurman, K. Gijzen, D. L. Williams, R. Torensma, A. McKinnon, D. M. MacCallum, F. C. Odds, J. W. Van der Meer, A. J. Brown, and B. J. Kullberg. 2006. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J. Clin. Invest. 116:1642-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rallabhandi, P., Q. M. Nhu, V. Y. Toshchakov, W. Piao, A. E. Medvedev, M. D. Hollenberg, A. Fasano, and S. N. Vogel. 2008. Analysis of proteinase-activated receptor 2 and TLR4 signal transduction: a novel paradigm for receptor cooperativity. J. Biol. Chem. 283:24314-24325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanglard, D., B. Hube, M. Monod, F. C. Odds, and N. A. Gow. 1997. A triple deletion of the secreted aspartyl proteinase genes SAP4, SAP5, and SAP6 of Candida albicans causes attenuated virulence. Infect. Immun. 65:3539-3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schaller, M., B. Hube, M. W. Ollert, W. Schafer, M. Borg-von Zepelin, E. Thoma-Greber, and H. C. Korting. 1999. In vivo expression and localization of Candida albicans secreted aspartyl proteinases during oral candidiasis in HIV-infected patients. J. Invest. Dermatol. 112:383-386. [DOI] [PubMed] [Google Scholar]
- 19.Schaller, M., H. C. Korting, C. Borelli, G. Hamm, and B. Hube. 2005. Candida albicans-secreted aspartic proteinases modify the epithelial cytokine response in an in vitro model of vaginal candidiasis. Infect. Immun. 73:2758-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uehara, A., Y. Hirabayashi, and H. Takada. 2008. Antibodies to proteinase 3 prime human oral, lung, and kidney epithelial cells to secrete proinflammatory cytokines upon stimulation with agonists to various Toll-like receptors, NOD1, and NOD2. Clin. Vaccine Immunol. 15:1060-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uehara, A., T. Imamura, J. Potempa, J. Travis, and H. Takada. 2008. Gingipains from Porphyromonas gingivalis synergistically induce the production of proinflammatory cytokines through protease-activated receptors with Toll-like receptor and NOD1/2 ligands in human monocytic cells. Cell. Microbiol. 10:1181-1189. [DOI] [PubMed] [Google Scholar]
- 22.Underhill, D. M., E. Rossnagle, C. A. Lowell, and R. M. Simmons. 2005. Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood 106:2543-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Graaf, C. A., M. G. Netea, I. Verschueren, J. W. Van der Meer, and B. J. Kullberg. 2005. Differential cytokine production and Toll-like receptor signaling pathways by Candida albicans blastoconidia and hyphae. Infect. Immun. 73:7458-7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.