Abstract
Plasmodium falciparum malaria is a leading global cause of infectious disease burden. In areas in which P. falciparum transmission is holoendemic, such as western Kenya, severe malarial anemia (SMA) results in high rates of pediatric morbidity and mortality. Although the pathophysiological basis of SMA is multifactorial, we recently discovered that suppression of unexplored hematopoietic growth factors that promote erythroid and myeloid colony development, such as stem cell growth factor (SCGF) (C-type lectin domain family member 11A [CLEC11A]), was associated with enhanced development of SMA and reduced erythropoietic responses. To extend these investigations, the relationships between a novel SCGF promoter variant (−539C/T, rs7246355), SMA (hemoglobin [Hb] < 6.0 g/dl), and reduced erythropoietic responses (reticulocyte production index [RPI], <2.0) were investigated with Kenyan children (n = 486) with falciparum malaria from western Kenya. Circulating SCGF was positively correlated with hemoglobin levels (r = 0.251; P = 0.022) and the reticulocyte production index (RPI) (r = 0.268; P = 0.025). Children with SMA also had lower SCGF levels than those in the non-SMA group (P = 0.005). Multivariate logistic regression analyses controlling for covariates demonstrated that individuals with the homologous T allele were protected against SMA (odds ratio, 0.57; 95% confidence interval [95% CI] 0.34 to 0.94; P = 0.027) relative to CC (wild-type) carriers. Carriers of the TT genotype also had higher SCGF levels in circulation (P = 0.018) and in peripheral blood mononuclear cell culture supernatants (P = 0.041), as well as an elevated RPI (P = 0.005) relative to individuals with the CC genotype. The results presented here demonstrate that homozygous T at −539 in the SCGF promoter is associated with elevated SCGF production, enhanced erythropoiesis, and protection against the development of SMA in children with falciparum malaria.
Severe malarial anemia (SMA) is the primary manifestation of severe malaria in infants and young children in areas in which Plasmodium falciparum transmission is holoendemic, such as western Kenya (7, 34). SMA also accounts for the greatest worldwide proportion of malaria-associated morbidity and mortality (8, 41, 48). Causal etiologies of SMA include direct and indirect destruction of parasitized and nonparasitized red blood cells (RBCs), inefficient erythropoiesis, and dyserythropoiesis (1). Previous results from our laboratory further demonstrated that pediatric SMA in an area of western Kenya in which transmission is holoendemic is characterized by a reduced erythropoietic response (46).
Although erythropoietin (EPO), stem cell factor (SCF), interleukin 3 (IL-3), and IL-6 are important for promoting enhanced erythropoiesis in malaria and other diseases (9, 13, 18, 28, 43), previous studies suggest that insufficient production of these soluble mediators may not fully account for malaria-induced anemia (4, 10, 11, 27, 40).
Human stem cell growth factor (SCGF) (C-type lectin domain family member 11A [CLEC11A]) is a largely uncharacterized hematopoietic mediator that promotes enhanced erythroid progenitor formation from human bone marrow (16). Human SCGF cDNA encodes a 29-kDa polypeptide (15) for which there are currently two known isoforms: SCGF-α, a 323-amino-acid protein; and SCGF-β, a 245-amino-acid protein formed from cleavage of the conserved carbohydrate domain (30). In individuals undergoing stem cell transplantation, elevated serum SCGF levels are associated with enhanced hematopoietic recovery (17). Although this phenomenon has not been explored with malaria, we recently showed that reduced SCGF levels in circulation and in cultured peripheral blood mononuclear cells (PBMCs) were associated with both SMA and reduced erythropoiesis (22).
To more fully elucidate the potential importance of SCGF in human malaria, variation in the SCGF promoter was explored, a strategy we have previously used in western Kenya to identify immune response genes that condition susceptibility to pediatric SMA (3, 35, 37, 38). Although to date, no studies have described the effect of polymorphic variability in SCGF on any disease, we focused on a single nucleotide polymorphism (SNP) in the promoter region (−539C/T; rs7246355) based on the allelic distribution in the Yoruba in the Ibadan population in Nigeria examined as part of the HapMap (phase 3) project. The association between SCGF −539C/T variants and susceptibility to SMA was investigated with Kenyan children (n = 486; age, 3 to 36 months) exposed to holoendemic P. falciparum transmission. To further explore the potential importance of SCGF −539 genotypes, we determined the relationship between genotypic variants, SCGF levels (in vivo and in vitro), and erythropoietic responses in children with malaria.
MATERIALS AND METHODS
Study participants.
Children with P. falciparum malaria (age, 3 to 36 months, n = 486) presenting at hospital for their first documented visit for acute malaria were recruited at Siaya District Hospital, a rural setting in western Kenya with holoendemic P. falciparum transmission (29). Since this region has holoendemic P. falciparum transmission, sample collection was carried out throughout the year. The study was performed in a homogenous population from the Luo ethnic group. A full description of the study site and the manifestations of pediatric malaria in this region is presented in our previous report (34). Based on a longitudinal study examining the distribution of >14,000 hemoglobin (Hb) measurements in an age- and geographically matched reference population in western Kenya, SMA was defined as Hb < 6.0 g/dl with parasitemia of any density (29). This definition of SMA is appropriately defined by Hb distributions according to age, gender, and geographic context. However, children were also classified according to the World Health Organization (WHO) definition of SMA (Hb ≤ 5.0 g/dl with parasitemia of any density) (47) to place the current findings into a broader global context. Non-SMA was defined as Hb ≥ 6.0 g/dl and parasitemia of any density (modified definition) or Hb > 5.0 g/dl and parasitemia of any density (WHO definition). None of the children included in the study had cerebral malaria or malaria from non-P. falciparum species. Since our previous study, and those of others, demonstrated that HIV-1 and bacteremia impact the development and severity of malarial anemia (5, 36), all children were tested for these copathogens and results were appropriately controlled for in the statistical models (see procedures listed below). Pre- and posttest HIV counseling was provided for the parents/guardians of all study participants. Written informed consent, in the language of choice (i.e., English, Kiswahili, or Dholuo), was obtained from the parents/guardians of participating children. The study was approved by the ethical and scientific review committees at the Kenya Medical Research Institute and the Institutional Review Boards at the University of Pittsburgh and the University of New Mexico.
Laboratory procedures.
Venous blood samples (<3.0 ml) were obtained prior to administration of antimalarials and/or any other treatment interventions. Asexual malaria parasites (trophozoites) were counted against 300 leukocytes in peripheral blood smears stained with 3% Giemsa. Reticulocyte counts were determined with new methylene blue staining of thin blood films. The reticulocyte production index (RPI) was calculated as follows: RPI = reticulocyte index (RI)/maturation factor (MF), where RI = (reticulocyte count [%] × hematocrit [Hct]/0.36) and MF = b+(m)(x), where b = 1, m = 0.05, and x = (average normal population Hct − patient's Hct) (46). Parasite density was estimated using the white blood cell (WBC) count/μl of each individual. Complete hematological parameters were determined with a Beckman Coulter AcT Diff2 (Beckman-Coulter Corporation). The presence of the sickle cell trait (HbAS) was determined by cellulose acetate electrophoresis, while typing for the common African 3.7-kb α-globin deletion (α-thalassemia) was carried out by PCR. The glucose-6-phosphate dehydrogenase (G6PD) deficiency screening was determined through the fluorescent spot test (Sigma-Aldrich). HIV-1 exposure and infection were determined by serological and HIV-1 proviral DNA PCR testing, respectively, according to our published methods (36). Trimethoprim-sulfamethoxazole was administered to all children that were positive for one or both serological HIV-1 tests. At the time of sample collection, none of the HIV-1-positive study participants had been started on antiretroviral treatment. Bacteremia was determined using the Wampole Isostat Pediatric 1.5 system (Wampole Laboratories), and blood was processed according to the manufacturer's instructions. API biochemical galleries (bioMerieux, Inc.) and/or serology were used for identification of bacterial isolates.
Isolation and culture of peripheral blood mononuclear cells (PBMCs).
PBMCs were purified from venous blood (<3.0 ml; n = 37) collected in EDTA-containing vials using Ficoll/Hypaque as described previously (45). Cells were plated at 1 × 106 cells per ml in Dulbecco's modified Eagle's medium (DMEM) containing 10% pooled human serum (heat inactivated at 56°C for 30 min) and cultured for 48 h.
SCGF genotyping.
DNA was extracted from blood spotted on FTA Classic cards (Whatman Inc.) using the Gentra System (Gentra System, Inc.). SCGF −539C/T genotyping was carried out using a TaqMan 5′ allelic discrimination Assay-By-Design method according to the manufacturer's instructions (Assay ID: C_29188597_10; Applied Biosystems, Inc.). In brief, PCR was performed in a total volume of 5 μl with the following amplification protocol: 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Following PCR, the genotype of each individual was determined using allele-specific fluorescence on the ABI Prism 7900HT sequence detection system. SDS 2.1 software was then used for allelic discrimination (Applied Biosystems, Inc.).
Determination of SCGF levels.
Plasma and supernatant concentrations of SCGF (in ng/ml) were measured in triplicate at 1:5 and 1:10 dilutions by quantitative sandwich enzyme-linked immunosorbent assay (ELISA). Affinity-purified polyclonal antibody (goat anti-human) specific for SCGF-β (PeproTech, Inc.) was coated onto 96-well plates and kept overnight at room temperature followed by blocking for 1 h and subsequent washing. Samples and standards (recombinant human SCGF-β; PeproTech, Inc.) were incubated at room temperature for 2 h, washed, and then incubated with a biotin-conjugated SCGF-β-specific detection antibody (PeproTech, Inc.) for another 2 h. Plates were washed and incubated with ExtraAvidin (Sigma-Aldrich), a horseradish peroxidase-streptavidin conjugate, washed again, and then visualized with TMB Substrate Solution (BD Biosciences PharMingen) for 30 min while protected from light. Absorbance was determined at 405 nm. The detection limit for SCGF was 0.2 ng/ml.
Statistical analyses.
Statistical analyses were performed using SPSS (version 15.0). Chi-square analyses were used to examine differences between proportions. Cross-group comparisons of nonparametric data were determined by Kruskal-Wallis tests, while Mann-Whitney U tests were used for pairwise comparisons. Spearman's correlations were used to assess the association between circulating SCGF and hematological indices. Deviations from the Hardy-Weinberg equilibrium (HWE) were tested using the web-based site www.tufts.edu/∼mcourt01/Documents/Court%20lab%20-%20HW%20calculator.xls. The associations between the SCGF promoter genotypes and SMA were determined by multivariate logistic regression, controlling for the potential confounding effects of age, gender, HIV-1 status (including both HIV-1 exposed and definitively HIV-1-positive results), bacteremia, sickle cell trait (HbAS), G6PD deficiency, and α-thalassemia status. Significance was set at P ≤ 0.05.
RESULTS
Demographic, clinical, and laboratory characteristics of the study participants.
To investigate the role of variability in the SCGF promoter (i.e., −539C/T) in conditioning susceptibility to SMA, children (age, <3 to 36 months; n = 486) presenting at hospital with acute P. falciparum malaria were stratified into two groups on the basis of nonsevere malarial anemia (non-SMA, 6.0 g/dl ≤ Hb < 11.0 with parasitemia of any density; n = 302) and severe malarial anemia (SMA, Hb < 6.0 g/dl with parasitemia of any density; n = 184) (29). The demographic, clinical, and laboratory characteristics of the study participants are presented in Table 1. There were no significant differences in gender distribution between the groups (P = 0.302). Age (months) differed between the groups, with the members of the non-SMA group being significantly older than those in the SMA group (P = 0.001). Axillary temperature (°C) was comparable between the groups (P = 0.307). As expected, based on the stratification according to anemia status, Hb concentrations (g/dl) and red blood cell (RBC) counts (×1012/liter) significantly differed between the groups (P = 0.001 and P = 0.001, respectively). The reticulocyte production index (RPI) was significantly different between the groups (P = 0.007). Furthermore, white blood cell (WBC) counts (103/μl) were significantly higher in the SMA group than in the non-SMA group (P = 0.001), despite the fact that the numbers of individuals with bacteremia were comparable between the non-SMA (69/302 [22.8%]) and SMA (51/184 [27.7%]) (P = 0.105) groups. Peripheral parasitemia density (/μl) and prevalence of high-density parasitemia (HDP, ≥10,000 parasites/μl) were lower in children with SMA (P = 0.081 and P = 0.054, respectively).
TABLE 1.
Demographic, clinical, and laboratory characteristics of the study participantsa
| Characteristic | Result |
P | |
|---|---|---|---|
| Non-SMA | SMA | ||
| No. of participants | 302 | 184 | N/A |
| Gender [no. (%)] | |||
| Male | 158 (52.3) | 91 (49.5) | 0.302b |
| Female | 144 (47.7) | 93 (50.5) | |
| Age (mo) | 11.0 (10.0) | 8.0 (7.0) | 0.001c |
| Axillary temp (°C) | 37.5 (1.7) | 37.4 (1.5) | 0.307c |
| Hemoglobin (g/dl) | 8.1 (2.7) | 4.9 (1.3) | 0.001c |
| RBC count (1012/liter) | 3.71 (1.29) | 2.11 (0.89) | 0.001c |
| Reticulocyte production index, RPI | 1.51 (2.02) | 1.12 (1.77) | 0.007c |
| WBC count (103/μl) | 11.05 (5.80) | 13.95 (9.85) | 0.001c |
| Parasite density (μl) | 20,612 (49,528) | 16,287 (34,322) | 0.081c |
| HDP (no. [%]) | 197 (65.2) | 105 (57.1) | 0.054b |
Data are median (IQR) unless otherwise stated. Abbreviations: HDP, high-density parasitemia (≥10,000 parasites/μl); SMA, severe malarial anemia (Hb < 6.0 g/dl, with parasitemia of any density) (29); non-SMA, nonsevere malarial anemia (11.0 g/dl > Hb ≥ 6.0 g/dl, with parasitemia of any density); N/A, not available.
Statistical significance determined by chi-square analysis.
Statistical significance determined by Mann-Whitney U test.
Relationship between SCGF and anemia.
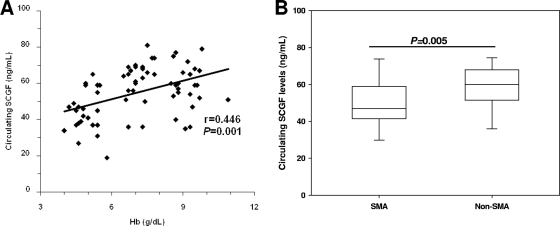
Before the influence of polymorphic variability in SCGF on malarial anemia was investigated, the relationship between circulating SCGF and Hb levels was determined. As shown in Fig. 1A, the levels of circulating SCGF were positively correlated with Hb concentrations (r = 0.446, P = 0.001). To further determine whether altered levels of SCGF were associated with anemia severity, the levels of circulating SCGF were compared between parasitemic children with and without SMA. Consistent with the positive relationship between SCGF and Hb levels, children with SMA had significantly lower SCGF concentrations (median [interquartile range {IQR}], 47.00 [43.24 to 57.48]) than those in the non-SMA group (63.00 [56.31 to 63.86]; P = 0.005) (Fig. 1B), demonstrating that reduced circulating SCGF levels are associated with more profound anemia.
FIG. 1.
Relationship between SCGF and anemia. Plasma was obtained from children with acute malaria and SCGF concentrations were determined by ELISA. (A) Correlation between SCGF concentrations and Hb levels in parasitemic children (n = 83) determined by Spearman's correlation coefficient. (B) Association between SCGF levels and anemia severity in parasitemic children with SMA (n = 25) and non-SMA (n = 58). Boxes represent the interquartile range, the line through the box is the median, and whiskers show 10th and 90th percentiles. Statistical significance was determined by the Mann-Whitney U test.
Distribution of SCGF genotypes.
After establishing that circulating SCGF levels were significantly associated with anemia severity, SCGF −539C/T genotypes were determined. Genotypic distributions of SCGF −539C/T promoter variants in the non-SMA (n = 302) and SMA (n = 184) groups are presented in Table 2. The prevalence of SCGF −539 genotypes in the population were 27.6% CC, 36.8% CT, and 35.6% TT, with overall allele frequencies of 0.46 for C and 0.54 for T. There was significant departure from the Hardy-Weinberg equilibrium (HWE) in the cohort (χ2 = 32.56; P < 0.001). The genotypic distribution of SCGF −539 in the non-SMA group was 25.2% CC, 35.8% CT, and 39.1% TT, yielding allele frequencies of 0.43 for C and 0.57 for T. The non-SMA group had a significant departure from the HWE (χ2 = 22.12; P < 0.001). Distributions of genotypes in the SMA group were 31.5% CC, 38.6% CT, and 29.9% TT, with allele frequencies of 0.51 for C and 0.49 for T. The SMA group also displayed a significant departure from the HWE (χ2 = 9.56; P < 0.019). Further analysis revealed that the distribution of the CC genotype was significantly higher in the SMA group than in the non-SMA group (P < 0.001), while the TT genotype was significantly higher in the non-SMA group (P = 0.025) than in the SMA group. However, the distribution of heterozygous individuals was comparable between the two groups (P = 0.259).
TABLE 2.
Distribution of SCGF −539C/T promoter variants in children with malariaa
| Type of anemia | No. of participants | No. (%) of participants with SCGF −539C/T variant type |
P(C) | ||
|---|---|---|---|---|---|
| CC | CT | TT | |||
| Non-SMA | 302 | 76 (25.2) | 108 (35.8) | 118 (39.1) | 0.430 |
| SMA | 184 | 58 (31.5) | 71 (38.6) | 55 (29.9) | 0.508 |
| P | <0.001b | 0.259b | 0.025b | ||
Abbreviations: SMA, severe malarial anemia (Hb < 6.0 g/dl, with parasitemia of any density) (29); non-SMA, nonsevere malarial anemia (11.0 g/dl > Hb ≥ 6.0 g/dl, with parasitemia of any density); SCGF, stem cell growth factor; P(C), frequency of the wild-type allele in the population.
Statistical significance determined by chi-square analysis. There was a nonsignificant difference in the distribution of SCGF −539 genotypes between the two groups (P = 0.099).
Association between SCGF promoter polymorphisms and SMA.
To determine the roles of SCGF −539C/T promoter variants in conditioning susceptibility to SMA, multivariate logistic regression analyses controlling for the potential confounding effects of age, gender, HIV-1 status, bacteremia, sickle cell trait, G6PD deficiency, and α-thalassemia status were conducted (2, 5, 36, 44, 50). As shown in Table 3, relative to CC carriers, children with homozygous T allele had a 43% reduction in SMA (Hb < 6.0 g/dl) (odds ratio [OR], 0.57; 95% confidence interval [95% CI], 0.34 to 0.94; P = 0.027). Additional analyses using the WHO definition of SMA (Hb ≤ 5.0 g/dl) (47) revealed that the TT genotype was associated with a 49% reduction in SMA (OR, 0.51; 95% CI, 0.33 to 0.95; P = 0.030) (Table 3) relative to homozygous C individuals. Thus, carriers of the TT genotype with falciparum malaria show significant protection against the development of SMA using both the modified and WHO definitions of SMA.
TABLE 3.
Relationship between SCGF −539C/T promoter variants and susceptibility to SMAa
| Genotype | Result according to SMA definition |
|||||
|---|---|---|---|---|---|---|
| Hb < 6.0 g/dl |
Hb < 5.0 g/dl |
|||||
| OR | 95% CI | P | OR | 95% CI | P | |
| CC | 1.00 | 1.00 | ||||
| CT | 0.84 | 0.52-1.36 | 0.483 | 0.88 | 0.50-1.53 | 0.643 |
| TT | 0.57 | 0.34-0.94 | 0.027 | 0.51 | 0.33-0.95 | 0.030 |
Parasitemic children (n = 486) were categorized according to a modified definition of SMA based on age- and geographically matched Hb concentrations (i.e., Hb < 6.0 g/dl, with parasitemia of any density) (29) and the WHO definition of SMA (i.e., Hb < 5.0 g/dl, with parasitemia of any density). Odds ratios (OR) and 95% confidence intervals (95% CI) were determined using multivariate logistic regression controlling for age, gender, HIV-1 status, bacteremia, sickle cell trait (HbAS), G6PD deficiency, and α-thalassemia status.
Prior to determining whether sickle cell trait has a negative or positive epistatic interaction with the SCGF −539C/T genotypes in conditioning SMA, multivariate logistic regression analysis was performed to determine the individual association between sickle cell trait and SMA (for both the modified and WHO definitions of SMA), controlling for the confounding effects of age, gender, HIV-1 status, bacteremia, G6PD deficiency, and α-thalassemia status. Carriers of the sickle cell trait had a 36% reduction in SMA (OR, 0.64; 95% CI, 0.23 to 0.84; P = 0.018) using the modified definition of SMA (29) and a 40% reduction in SMA (OR, 0.60; 95% CI, 0.22 to 0.87; P = 0.023), using the WHO definition of SMA (47). To test for epistasis, individuals were further stratified into the following groups: −539C/HbA, −539C/HbS, −539T/HbA, and −539T/HbS, based on the presence of allele C or T at the −539C/T locus and/or allele A or S at the HbAS locus. Multivariate logistic regression analysis controlling for age, gender, HIV-1 status, bacteremia, G6PD deficiency, and α-thalassemia status demonstrated that the −539C/HbA individuals had increased susceptibility to SMA using the modified definition (OR, 1.68; 95% CI, 1.11 to 2.56; P = 0.014) (29) and WHO definition (OR, 1.55; 95% CI, 0.94 to 2.55; P = 0.089) of SMA (47). In addition, carriage of −539T/HbS was associated with an 85% reduction in SMA with the modified definition (OR, 0.15; 95% CI, 0.11 to 0.53; P = 0.017) (29) and an 82% reduction in susceptibility to SMA using the WHO definition (OR, 0.18; 95% CI, 0.13 to 0.62; P = 0.006) (47). However, no significant associations were observed between SMA and either −539T/HbA (OR, 0.73; 95% CI, 0.48 to 1.12; P = 0.148) or −539C/HbS (OR, 1.36; 95% CI, 0.59 to 1.69; P = 0.504). Taken together, these results demonstrate that increased protection was present when the sickle cell trait was coinherited with the T allele, showing a positive epistatic interaction between the sickle cell trait and SCGF −539T alleles.
Influence of SCGF variants on in vivo and in vitro SCGF concentrations.
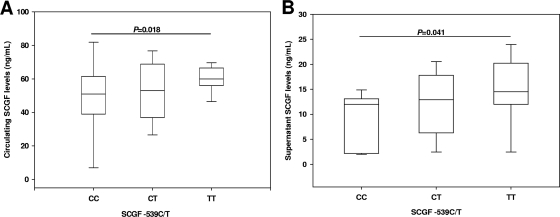
Additional experiments examined the association between SCGF −539C/T promoter variants and circulating SCGF levels. The difference in SCGF across the genotypic groups was of borderline significance (P = 0.050) (Fig. 2A). Individuals with the TT genotype (median [IQR], 60.00 [56.00 to 66.50]) had significantly higher SCGF levels relative to homozygous C carriers (49.00 [38.00 to 62.50]; P = 0.018) and nonsignificantly elevated levels compared to heterozygous children (54.00 [37.00 to 68.50]; P = 0.087) (Fig. 2A). Circulating SCGF levels were comparable between the homozygous C group and heterozygous individuals (P = 0.594) (Fig. 2A).
FIG. 2.
Functional association between SCGF −539C/T promoter variants and SCGF. (A) Circulating SCGF levels were measured using ELISA. Data are shown for the CC (n = 17), CT (n = 33), and TT (n = 33) genotypes. Data are presented as box plots, where the box represents the interquartile range, the line through the box is the median, and whiskers show the 10th and 90th percentiles. Cross-group comparisons were determined by Kruskal-Wallis tests followed by Mann-Whitney U tests for pairwise comparison. The presence of the TT genotype was associated with significantly higher circulating SCGF levels relative to individuals with CC genotype (P = 0.018). (B) PBMCs were isolated from peripheral blood (<3.0 ml) and cultured (1 × 106 cells/ml) in serum-containing media. Supernatants were obtained at 48 h for SCGF determination by ELISA. Children were stratified according to genotypes: CC (n = 11), CT (n = 13), and TT (n = 13). Data are presented as box plots, where the box represents the interquartile range, the line through the box is the median, and whiskers show the 10th and 90th percentiles. Statistical significance was determined by Mann-Whitney U test. The presence of the TT genotype was associated with significantly higher culture supernatant SCGF levels relative to individuals with the CC genotype (P = 0.041).
Consistent with the in vivo results, measurement of SCGF in PBMC culture supernatants revealed that homozygous T carriers (14.50 [10.76 to 19.00]) had significantly higher SCGF levels than those with homozygous C alleles (11.95 [5.66 to 12.59]; P = 0.041), and nonsignificantly elevated levels compared to heterozygous children (12.85 [8.20 to 15.92]; P = 0.362) (Fig. 2B). Thus, a C-to-T transition at −539 in the SCGF promoter is associated with significant changes in both in vivo and in vitro SCGF levels.
Association between SCGF variants and erythropoiesis.
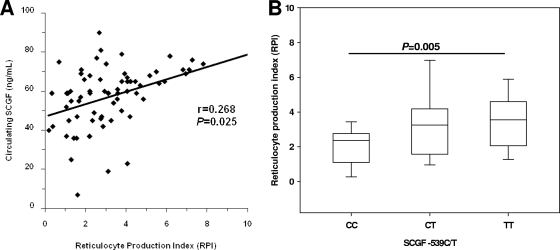
Previous in vitro studies demonstrated that SCGF promotes enhanced erythroid formation (16). An effective, noninvasive measure of the erythropoietic response, in the absence of bone marrow aspirates, is to determine the reticulocyte production index (RPI), which accounts for the production of erythrocytes corrected for the degree of anemia (24). There was a significant positive correlation between the RPI and circulating SCGF (r = 0.268; P = 0.025) (Fig. 3A), suggesting that SCGF may be an important factor for promoting erythropoiesis in children with malaria. To determine whether polymorphic variability at SCGF −539 influenced the erythropoietic response, the RPI was compared across the genotypic groups. There was a significant intergroup difference in the RPI according to SCGF promoter variants (P = 0.024). Individuals with the TT genotype (median [IQR], 3.55 [2.86 to 4.41]) had a significantly higher RPI than homozygous C carriers (2.37 [1.38 to 2.63]; P = 0.005) but reticulocyte responses that were comparable to those of heterozygous children (3.25 [2.59 to 4.24], P = 0.560, Fig. 3B). Elevated levels of SCGF in individuals with the homozygous T allele, coupled with an increased erythropoietic response and protection against SMA, suggest that SCGF may be important for augmenting erythropoiesis in children with falciparum malaria and that SCGF levels are conditioned by genotype.
FIG. 3.
Association between SCGF variants and erythropoiesis. Plasma was obtained from children with acute malaria, and SCGF concentrations were determined by ELISA. (A) Correlation between SCGF concentrations and reticulocyte production index (RPI) levels in children with anemia (n = 70). Relationships between SCGF and RPI were determined by Spearman's correlation coefficient. (B) Association between SCGF −539C/T promoter variants and the RPI in anemic children. Data are shown for the CC (n = 14), CT (n = 29), and TT (n = 27) genotypes. Boxes represent the interquartile range, the line through the box is the median, and whiskers show 10th and 90th percentiles. Cross-group comparisons were determined by Kruskal-Wallis tests followed by Mann-Whitney U tests for pairwise comparison. The presence of the TT genotype was associated with significantly higher RPI relative to individuals with the CC genotype (P = 0.005).
DISCUSSION
Studies in our laboratories using cDNA membrane arrays recently identified SCGF as one of the most differentially expressed genes in PBMCs stimulated with malarial products (i.e., P. falciparum-derived hemozoin) (22). These findings were then extended to our field studies in western Kenya, which demonstrated that in vivo and in vitro suppression of SCGF was associated with development of SMA and a reduced erythropoietic response. On the basis of these findings, we hypothesized that variations in the SCGF promoter may alter susceptibility to SMA. Cross-sectional results presented here for children with falciparum malaria illustrate that carriers of the TT (mutant) genotype are protected against SMA. The presence of the homozygous T allele in children with malaria was also associated with significantly higher SCGF levels in circulation and in cultured PBMCs. Further analyses demonstrated that the high-producing SCGF genotype (TT) was associated with protection against SMA and an enhanced erythropoietic response (i.e., an elevated RPI).
Previous hematological studies of individuals with malaria illustrate that bone marrow abnormalities, such as ineffective erythropoiesis, dyserythropoiesis, and reduced erythroblast proliferative rates, contribute to the development of severe anemia (1, 12, 42, 49). Moreover, P. falciparum malaria is defined by a suboptimal reticulocyte response for the degree of anemia, even under conditions in which there are adequate levels of erythropoietin, a critical stimulus for erythropoiesis (25, 32). Our previous results in western Kenya showing that children with SMA have a reduced erythropoietic response (RPI) are consistent with these findings (46). Although the underlying biological pathways responsible for suppression of erythropoiesis in malaria-infected individuals remain only partially defined, important causal factors include parasite-mediated erythrocyte destruction, elimination of parasitized and nonparasitized RBCs by the reticuloendothelial system, and an excessive or sustained innate immune response that polarizes adaptive T-cell responses toward the production of inflammatory mediators that suppress erythroid development (31, 32, 39). Previous results from our laboratory also demonstrate that dysregulation in cytokines, chemokines, and effector molecules is important for promoting inflammation-derived anemia in children with malaria (4, 19-21, 23, 33, 35, 46).
In this study, we extend our previous findings by investigating the impact of a growth factor (i.e., SCGF) on anemia outcomes in children with malaria. Since SCGF promotes growth of erythroid and myeloid colonies (14, 16), the associations between SCGF, Hb, and RPI were examined in children with falciparum malaria, both independently and according to SCGF promoter variants. To date, only a single polymorphic variant has been described in the SCGF gene (i.e., −539C/T). Genotyping of the C-to-T transition at position −539 in the SCGF promoter revealed C and T allele frequencies of 46% and 54%, respectively. Allele frequencies in the Kenyan cohort differ slightly from those reported in a reference African population (i.e., 52% C and 48% T, respectively) (http://www.ncbi.nlm.nih.gov/SNP/snp_ref.cgi?searchType=adhoc_search&type=rs&rs=rs7246355). Since the genotyping error rate in this assay was below 2%, differences in the distribution of SCGF alleles suggest that this locus may be subject to differential selective pressure (perhaps due to historic P. falciparum endemicity) that varies according to geographic region. Consistent with this notion, genotypic distributions showed significant departures from HWE in the non-SMA and SMA groups and in the overall cohort. These results are consistent with the fact that stratification is common, particularly in host immune response genes that mediate susceptibility to polygenic infectious diseases, such as malaria (26). Geographic and ethnic variation, along with significant differences in genotypic distributions between children with and without SMA, further suggests that variation at position −539 in SCGF may be a useful addition to the range of genetic markers utilized in future studies aimed at identifying the gene and gene pathways that condition susceptibility to severe malaria.
Multivariate modeling, controlling for the appropriate cofactors, demonstrated that carriage of the homozygous T allele was associated with a significant reduction in the development of SMA relative to the reference genotype (CC). Moreover, children with the TT genotype had significantly higher circulating and PBMC culture supernatant SCGF levels. These findings suggest that PBMC may be an important source of SCGF in circulation. Although we have previously demonstrated that acquisition of hemozoin by monocytes causes suppression of SCGF transcripts and protein (6), it remains to be determined whether phagocytosis of malarial pigment by bone marrow macrophages leads to reduced SCGF in the bone marrow milieu. Thus, it is currently unclear whether reduced SCGF from a peripheral source or in the local tissue space contributes to reduced Hb concentrations. The exact mechanism by which changes in SCGF production impact Hb levels also remains unclear. Results in the current report showing that individuals with the homologous T allele are protected against SMA, have significantly higher SCGF levels, and also have a significantly higher RPI suggest that the primary influence of SCGF on Hb concentrations is mediated by altering the maturation of RBCs in the bone marrow.
Although variation in the SCGF promoter at −539 was associated with susceptibility to SMA and functional changes in SCGF levels, it remains possible that an unidentified polymorphism(s) that is in linkage disequilibrium with this particular variant may mediate the effects described here. For example, we observed positive epistasis between the sickle cell trait and the SCGF −539 T allele. Such interactions may amplify or moderate the individual effects of the gene(s). Future studies should include examination of additional SNPs within and outside this region to test this alternative hypothesis. Furthermore, establishing how SCGF interacts with additional soluble mediators, such as SCF, IL-3, and IL-6, will be important since these factors foster efficient increases in erythropoiesis (13). In addition, to provide insight into the direct role of SCGF on the erythropoietic cascade, it will be essential to determine the effect(s) of SCGF on bone marrow progenitor cells from children with malarial anemia. Since this is the first report describing how variations at −539 in the SCGF promoter are associated with functional changes in SCGF production and anemia outcomes, it will be important to see whether this variant also conditions susceptibility to anemia in other acute and chronic inflammatory diseases.
Acknowledgments
We are indebted to the Siaya District Hospital team and the University of New Mexico/KEMRI staff for support and management: Nicholas Otieno and Martha Atandi for clinical support; Tabitha Otieno, Chris Wasonga, Joan Ochieng, Godfrey Ogulla, and Evans Raballah for laboratory support; Vincent Omanje, Jared Ondijo, and Caroline Odette for data management; Rodney Bosire for field support; and Anne On'gondo for administrative support. We are also grateful to the parents/guardians of the study participants and the children that participated in the study. These data are published with the approval of the Director, Kenya Medical Research Institute (KEMRI). The study was approved by the ethical and scientific review committees at the Kenya Medical Research Institute and the institutional review boards at the University of Pittsburgh and University of New Mexico.
This work was supported by grants from the National Institutes of Health [AI51305-02 (D.J.P.) and TW05884-02 (D.J.P.)].
There is no conflict of interest for any of the authors of the manuscript due to commercial or other affiliations.
Editor: J. H. Adams
Footnotes
Published ahead of print on 2 November 2009.
REFERENCES
- 1.Abdalla, S. H. 1990. Hematopoiesis in human malaria. Blood Cells 16:401-419. [PubMed] [Google Scholar]
- 2.Aidoo, M., D. J. Terlouw, M. S. Kolczak, P. D. McElroy, F. O. ter Kuile, S. Kariuki, B. L. Nahlen, A. A. Lal, and V. Udhayakumar. 2002. Protective effects of the sickle cell gene against malaria morbidity and mortality. Lancet 359:1311-1312. [DOI] [PubMed] [Google Scholar]
- 3.Awandare, G. A., C. Ouma, C. C. Keller, T. Were, R. Otieno, Y. Ouma, G. C. Davenport, J. B. Hittner, J. M. Ong'echa, R. Ferrell, and D. J. Perkins. 2006. A macrophage migration inhibitory factor promoter polymorphism is associated with high-density parasitemia in children with malaria. Genes Immun. 7:568-575. [DOI] [PubMed] [Google Scholar]
- 4.Awandare, G. A., Y. Ouma, C. Ouma, T. Were, R. Otieno, C. C. Keller, G. C. Davenport, J. B. Hittner, J. Vulule, R. Ferrell, J. M. Ong'echa, and D. J. Perkins. 2007. Role of monocyte-acquired hemozoin in suppression of macrophage migration inhibitory factor in children with severe malarial anemia. Infect. Immun. 75:201-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berkley, J., S. Mwarumba, K. Bramham, B. Lowe, and K. Marsh. 1999. Bacteraemia complicating severe malaria in children. Trans. R. Soc. Trop. Med. Hyg. 93:283-286. [DOI] [PubMed] [Google Scholar]
- 6.Biemba, G., V. R. Gordeuk, P. E. Thuma, G. F. Mabeza, and G. Weiss. 1998. Prolonged macrophage activation and persistent anaemia in children with complicated malaria. Trop. Med. Int. Health 3:60-65. [DOI] [PubMed] [Google Scholar]
- 7.Bloland, P. B., D. A. Boriga, T. K. Ruebush, J. B. McCormick, J. M. Roberts, A. J. Oloo, W. Hawley, A. Lal, B. Nahlen, and C. C. Campbell. 1999. Longitudinal cohort study of the epidemiology of malaria infections in an area of intense malaria transmission. II. Descriptive epidemiology of malaria infection and disease among children. Am. J. Trop. Med. Hyg. 60:641-648. [DOI] [PubMed] [Google Scholar]
- 8.Breman, J. G., A. Egan, and G. T. Keusch. 2001. The intolerable burden of malaria: a new look at the numbers. Am. J. Trop. Med. Hyg. 64:iv-vii. [DOI] [PubMed] [Google Scholar]
- 9.Burchard, G. D., P. Radloff, J. Philipps, M. Nkeyi, J. Knobloch, and P. G. Kremsner. 1995. Increased erythropoietin production in children with severe malarial anemia. Am. J. Trop. Med. Hyg. 53:547-551. [DOI] [PubMed] [Google Scholar]
- 10.Burgmann, H., S. Looareesuwan, S. Kapiotis, C. Viravan, S. Vanijanonta, U. Hollenstein, E. Wiesinger, E. Presterl, S. Winkler, and W. Graninger. 1996. Serum levels of erythropoietin in acute Plasmodium falciparum malaria. Am. J. Trop. Med. Hyg. 54:280-283. [DOI] [PubMed] [Google Scholar]
- 11.Burgmann, H., S. Looareesuwan, E. C. Wiesinger, W. Winter, and W. Graninger. 1997. Levels of stem cell factor and interleukin-3 in serum in acute Plasmodium falciparum malaria. Clin. Diagn. Lab. Immunol. 4:226-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Das, B. S., N. K. Nanda, P. K. Rath, R. N. Satapathy, and D. B. Das. 1999. Anaemia in acute, Plasmodium falciparum malaria in children from Orissa state, India. Ann. Trop. Med. Parasitol. 93:109-118. [DOI] [PubMed] [Google Scholar]
- 13.Elliott, S., E. Pham, and I. C. Macdougall. 2008. Erythropoietins: a common mechanism of action. Exp. Hematol. 36:1573-1584. [DOI] [PubMed] [Google Scholar]
- 14.Hiraoka, A., T. Ohkubo, and M. Fukuda. 1987. Production of human hematopoietic survival and growth factor by a myeloid leukemia cell line (KPB-M15) and placenta as detected by a monoclonal antibody. Cancer Res. 47:5025-5030. [PubMed] [Google Scholar]
- 15.Hiraoka, A., A. Sugimura, T. Seki, T. Nagasawa, N. Ohta, M. Shimonishi, M. Hagiya, and S. Shimizu. 1997. Cloning, expression, and characterization of a cDNA encoding a novel human growth factor for primitive hematopoietic progenitor cells. Proc. Natl. Acad. Sci. U. S. A. 94:7577-7582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiraoka, A., K. Yano Ki, N. Kagami, K. Takeshige, H. Mio, H. Anazawa, and S. Sugimoto. 2001. Stem cell growth factor: in situ hybridization analysis on the gene expression, molecular characterization and in vitro proliferative activity of a recombinant preparation on primitive hematopoietic progenitor cells. Hematol. J. 2:307-315. [DOI] [PubMed] [Google Scholar]
- 17.Ito, C. Y., C. Y. Li, A. Bernstein, J. E. Dick, and W. L. Stanford. 2003. Hematopoietic stem cell and progenitor defects in Sca-1/Ly-6A-null mice. Blood 101:517-523. [DOI] [PubMed] [Google Scholar]
- 18.Jelkmann, W. 2007. Erythropoietin after a century of research: younger than ever. Eur. J. Haematol. 78:183-205. [DOI] [PubMed] [Google Scholar]
- 19.Keller, C. C., G. C. Davenport, K. R. Dickman, J. B. Hittner, S. S. Kaplan, J. B. Weinberg, P. G. Kremsner, and D. J. Perkins. 2006. Suppression of prostaglandin E2 by malaria parasite products and antipyretics promotes overproduction of tumor necrosis factor-alpha: association with the pathogenesis of childhood malarial anemia. J. Infect. Dis. 193:1384-1393. [DOI] [PubMed] [Google Scholar]
- 20.Keller, C. C., J. B. Hittner, B. K. Nti, J. B. Weinberg, P. G. Kremsner, and D. J. Perkins. 2004. Reduced peripheral PGE2 biosynthesis in Plasmodium falciparum malaria occurs through hemozoin-induced suppression of blood mononuclear cell cyclooxygenase-2 gene expression via an interleukin-10-independent mechanism. Mol. Med. 10:45-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keller, C. C., P. G. Kremsner, J. B. Hittner, M. A. Misukonis, J. B. Weinberg, and D. J. Perkins. 2004. Elevated nitric oxide production in children with malarial anemia: hemozoin-induced nitric oxide synthase type 2 transcripts and nitric oxide in blood mononuclear cells. Infect. Immun. 72:4868-4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keller, C. C., C. Ouma, Y. Ouma, G. A. Awandare, G. C. Davenport, T. Were, J. B. Hittner, J. M. Vulule, J. M. Ong'echa, and D. J. Perkins. 2009. Suppression of a novel hematopoietic mediator in children with severe malarial anemia. Infect. Immun. 77:3864-3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keller, C. C., O. Yamo, C. Ouma, J. M. Ong'echa, D. Ounah, J. B. Hittner, J. M. Vulule, and D. J. Perkins. 2006. Acquisition of hemozoin by monocytes down-regulates interleukin-12 p40 (IL-12p40) transcripts and circulating IL-12p70 through an IL-10-dependent mechanism: in vivo and in vitro findings in severe malarial anemia. Infect. Immun. 74:5249-5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koepke, J. F., and J. A. Koepke. 1986. Reticulocytes. Clin. Lab. Haematol. 8:169-179. [DOI] [PubMed] [Google Scholar]
- 25.Kurtzhals, J. A., O. Rodrigues, M. Addae, J. O. Commey, F. K. Nkrumah, and L. Hviid. 1997. Reversible suppression of bone marrow response to erythropoietin in Plasmodium falciparum malaria. Br. J. Haematol. 97:169-174. [DOI] [PubMed] [Google Scholar]
- 26.Kwiatkowski, D. P. 2005. How malaria has affected the human genome and what human genetics can teach us about malaria. Am. J. Hum. Genet. 77:171-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyke, K. E., R. Burges, Y. Cissoko, L. Sangare, M. Dao, I. Diarra, A. Kone, R. Harley, C. V. Plowe, O. K. Doumbo, and M. B. Sztein. 2004. Serum levels of the proinflammatory cytokines interleukin-1 beta (IL-1beta), IL-6, IL-8, IL-10, tumor necrosis factor alpha, and IL-12(p70) in Malian children with severe Plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infect. Immun. 72:5630-5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDevitt, M. A., J. Xie, G. Shanmugasundaram, J. Griffith, A. Liu, C. McDonald, P. Thuma, V. R. Gordeuk, C. N. Metz, R. Mitchell, J. Keefer, J. David, L. Leng, and R. Bucala. 2006. A critical role for the host mediator macrophage migration inhibitory factor in the pathogenesis of malarial anemia. J. Exp. Med. 203:1185-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McElroy, P. D., A. A. Lal, W. A. Hawley, P. B. Bloland, F. O. Kuile, A. J. Oloo, S. D. Harlow, X. Lin, and B. L. Nahlen. 1999. Analysis of repeated hemoglobin measures in full-term, normal birth weight Kenyan children between birth and four years of age. III. The Asembo Bay Cohort Project. Am. J. Trop. Med. Hyg. 61:932-940. [DOI] [PubMed] [Google Scholar]
- 30.Mio, H., N. Kagami, S. Yokokawa, H. Kawai, S. Nakagawa, K. Takeuchi, S. Sekine, and A. Hiraoka. 1998. Isolation and characterization of a cDNA for human mouse, and rat full-length stem cell growth factor, a new member of C-type lectin superfamily. Biochem. Biophys. Res. Commun. 249:124-130. [DOI] [PubMed] [Google Scholar]
- 31.Mohan, K., and M. M. Stevenson. 1998. Dyserythropoiesis and severe anaemia associated with malaria correlate with deficient interleukin-12 production. Br J. Haematol 103:942-949. [DOI] [PubMed] [Google Scholar]
- 32.Nussenblatt, V., G. Mukasa, A. Metzger, G. Ndeezi, E. Garrett, and R. D. Semba. 2001. Anemia and interleukin-10, tumor necrosis factor alpha, and erythropoietin levels among children with acute, uncomplicated Plasmodium falciparum malaria. Clin. Diagn. Lab. Immunol. 8:1164-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ochiel, D. O., G. A. Awandare, C. C. Keller, J. B. Hittner, P. G. Kremsner, J. B. Weinberg, and D. J. Perkins. 2005. Differential regulation of beta-chemokines in children with Plasmodium falciparum malaria. Infect. Immun. 73:4190-4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ong'echa, J. M., C. C. Keller, T. Were, C. Ouma, R. O. Otieno, Z. Landis-Lewis, D. Ochiel, J. L. Slingluff, S. Mogere, G. A. Ogonji, A. S. Orago, J. M. Vulule, S. S. Kaplan, R. D. Day, and D. J. Perkins. 2006. Parasitemia, anemia, and malarial anemia in infants and young children in a rural holoendemic Plasmodium falciparum transmission area. Am. J. Trop. Med. Hyg. 74:376-385. [PubMed] [Google Scholar]
- 35.Ong'echa, J. M., A. M. Remo, J. Kristoff, J. B. Hittner, T. Were, C. Ouma, R. O. Otieno, J. M. Vulule, C. C. Keller, G. A. Awandare, and D. J. Perkins. 2008. Increased circulating interleukin (IL)-23 in children with malarial anemia: in vivo and in vitro relationship with co-regulatory cytokines IL-12 and IL-10. Clin. Immunol. 126:211-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Otieno, R. O., C. Ouma, J. M. Ong'echa, C. C. Keller, T. Were, E. N. Waindi, M. G. Michaels, R. D. Day, J. M. Vulule, and D. J. Perkins. 2006. Increased severe anemia in HIV-1-exposed and HIV-1-positive infants and children during acute malaria. AIDS 20:275-280. [DOI] [PubMed] [Google Scholar]
- 37.Ouma, C., G. C. Davenport, T. Were, M. F. Otieno, J. B. Hittner, J. M. Vulule, J. Martinson, J. M. Ong'echa, R. E. Ferrell, and D. J. Perkins. 2008. Haplotypes of IL-10 promoter variants are associated with susceptibility to severe malarial anemia and functional changes in IL-10 production. Hum. Genet. 124:515-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ouma, C., C. C. Keller, D. A. Opondo, T. Were, R. O. Otieno, M. F. Otieno, A. S. Orago, J. M. Ong'Echa, J. M. Vulule, R. E. Ferrell, and D. J. Perkins. 2006. Association of Fc gamma receptor IIA (CD32) polymorphism with malarial anemia and high-density parasitemia in infants and young children. Am. J. Trop. Med. Hyg. 74:573-577. [PubMed] [Google Scholar]
- 39.Perkins, D. J., J. B. Weinberg, and P. G. Kremsner. 2000. Reduced interleukin-12 and transforming growth factor-beta1 in severe childhood malaria: relationship of cytokine balance with disease severity. J. Infect. Dis. 182:988-992. [DOI] [PubMed] [Google Scholar]
- 40.Prakash, D., C. Fesel, R. Jain, P. A. Cazenave, G. C. Mishra, and S. Pied. 2006. Clusters of cytokines determine malaria severity in Plasmodium falciparum-infected patients from endemic areas of Central India. J. Infect. Dis. 194:198-207. [DOI] [PubMed] [Google Scholar]
- 41.Snow, R. W., M. H. Craig, U. Deichmann, and D. le Sueur. 1999. A preliminary continental risk map for malaria mortality among African children. Parasitol. Today 15:99-104. [DOI] [PubMed] [Google Scholar]
- 42.Srichaikul, T., M. Wasanasomsithi, V. Poshyachinda, N. Panikbutr, and T. Rabieb. 1969. Ferrokinetic studies and erythropoiesis in malaria. Arch. Intern. Med. 124:623-628. [PubMed] [Google Scholar]
- 43.Verhoef, H., C. E. West, J. Veenemans, Y. Beguin, and F. J. Kok. 2002. Stunting may determine the severity of malaria-associated anemia in African children. Pediatrics 110:e48. [DOI] [PubMed] [Google Scholar]
- 44.Wambua, S., J. Mwacharo, S. Uyoga, A. Macharia, and T. N. Williams. 2006. Co-inheritance of alpha+-thalassaemia and sickle trait results in specific effects on haematological parameters. Br. J. Haematol 133:206-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weinberg, S. R., E. G. McCarthy, T. J. MacVittie, and S. J. Baum. 1981. Effect of low-dose irradiation on pregnant mouse haemopoiesis. Br. J. Haematol. 48:127-135. [DOI] [PubMed] [Google Scholar]
- 46.Were, T., J. B. Hittner, C. Ouma, R. O. Otieno, A. S. Orago, J. M. Ong'echa, J. M. Vulule, C. C. Keller, and D. J. Perkins. 2006. Suppression of RANTES in children with Plasmodium falciparum malaria. Haematologica 91:1396-1399. [PubMed] [Google Scholar]
- 47.WHO. 2000. Severe falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 94(Suppl. 1):S1-S90. [PubMed] [Google Scholar]
- 48.WHO. 2005. World malaria report 2005. World Health Organization/United Nations Children's Fund, Geneva, Switzerland. http://www.rollbackmalaria.org/wmr2005/pdf/WMReport_lr.pdf.
- 49.Wickramasinghe, S. N., and S. H. Abdalla. 2000. Blood and bone marrow changes in malaria. Baillieres Best Pract. Res. Clin. Haematol. 13:277-299. [DOI] [PubMed] [Google Scholar]
- 50.Williams, T. N., T. W. Mwangi, S. Wambua, T. E. Peto, D. J. Weatherall, S. Gupta, M. Recker, B. S. Penman, S. Uyoga, A. Macharia, J. K. Mwacharo, R. W. Snow, and K. Marsh. 2005. Negative epistasis between the malaria-protective effects of alpha+-thalassemia and the sickle cell trait. Nat. Genet. 37:1253-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]