Abstract
Panton-Valentine leukocidin (PVL) is a synergohymenotropic toxin (SHT) produced by Staphylococcus aureus. At present, there are conflicting reports on the leukotoxic activity of PVL and its consequent role as a virulence factor in USA300. In this work, we compared the cytolytic effects induced by wild-type PVL and those of PVL harboring a histidine-to-arginine substitution at amino acid 176 in the S. aureus USA300 strain. We also investigated the capacity of wild-type and H176R LukS-PV to recruit and form pores with the F components of other SHTs. For this purpose, we assayed polymorphonuclear neutrophils for leukotoxicity after incubation with either culture supernatants from strains bearing different PVL haplotypes or recombinant toxins from different types of SHT. We show here that the H176R variation in the PVL sequence causes no change in leukotoxicity and that the R variant is as efficient as wild-type PVL at inducing pore formation in leukocytes.
Staphylococcus aureus is an important human pathogen that expresses a variety of exoproteins. These include the synergohymenotropic toxins (SHTs), which damage host cell membranes by the synergistic action of two classes of nonassociated proteins, designated S and F (22). The SHT family is comprised of the Panton-Valentine leukocidin (PVL), gamma-hemolysin, and other leukocidins (e.g., LukE, LukD, and LukM). The F components (SHT-F: LukF-PV, LukD, and Hlg-B) and the S components (SHT-S: LukS-PV, LukE, Hlg-A, Hlg-C, and LukM) of SHT share 70 to 80% and 60 to 80% sequence identity, respectively (14). Each combination of S and F components is considered to be a biologically distinct toxin and was shown to differ in their toxicity against leukocytes in vitro and rabbits in vivo (12, 22).
Of the SHT family, only PVL has been epidemiologically linked to specific manifestations of S. aureus in humans. These include primary skin and soft tissue disease, severe necrotizing pneumonia, and severe bone and joint infection (3, 7, 10, 18, 22). These epidemiological links, as well as the detection of PVL in human samples (1), suggest that PVL plays a role in the pathophysiology of these infections. Previous publications on PVL show that it targets human immune system cells such as polymorphonuclear neutrophils (PMNs), monocytes, and macrophages (24). In vitro, PVL forms pores in human and rabbit PMNs and monocytes. Depending on the concentration, PVL pores cause cytokine release and cell death by apoptosis or necrosis, which undoubtedly contributes to the pathology induced by PVL-producing S. aureus strains (9, 16). Dose-dependent toxicity has also been observed in vivo when PVL was injected intradermally into rabbits, causing skin erythema followed by dose-dependent skin necrosis (5, 12, 27). More recently, PVL appears to play a role in pathogenesis during the early stages of bacteremic seeding of the kidney, the target organ from which bacteria were not cleared (6).
Currently, PVL is frequently detected from clinical strain because it is produced by community-acquired methicillin-resistant S. aureus (CA-MRSA) clones currently spreading throughout the world (25). One such clone, USA300, accounts for the majority of S. aureus strains responsible for skin and soft tissue infections in the United States (19). Voyich et al. did not observe differences in the leukotoxicity of isogenic PVL-positive and negative USA300 strains toward human leukocytes. They therefore questioned the importance of PVL in the virulence of these strains (26). Moreover, nucleotide polymorphisms are observed in S. aureus lukSF-PV genes. Twelve nucleotide mutations have been described, including 10 synonymous and two nonsynonymous changes (8, 21). USA300 expresses a LukS-PV variant harboring a histidine-to-arginine substitution at amino acid 176 that is not present in most other lineages. Because the substituted amino acid was localized to the surface of LukS-PV at the interaction site with SHT-F components by computer modeling, we hypothesized that pore formation and leukotoxicity might be affected (8, 15, 21). However, Berglund et al. did not observe a difference between the activities of LukS-PVR and LukS-PVH in combination with LukF-PV. However, these researchers did not examine LukS-PVR and LukS-PVH activities when combined with other class F components that could give rise to leukotoxicity as severe as that of PVL itself, raising the possibility that a LukS-PVH/R variation might have a biological impact when LukS-PV is associated not with LukF-PV but with other SHT-F components (2, 12).
The aim of the present study was to reexamine the toxicity of PVL from USA300 to human leukocytes and to determine whether the H/R variation occurring in the LukS-PV gene affects its leukotoxicity. This was accomplished using all potential combinations of LukS-PVR and LukS-PVH with all SHT class F components (LukF-PV, LukD, and Hlg-B) using leukocytes from different blood donors.
(This study was partly presented at the 18th European Congress of Clinical Microbiology and Infectious Diseases [Barcelona, Spain] and the 28ème Réunion Interdisciplinaire de Chimiothérapie Anti-Infectieuse [Paris, France].)
MATERIALS AND METHODS
Bacterial strains and culture.
Since an optimal yield of PVL is only obtainable by cultivation of strain on CCY (28), the two pairs of PVL-positive S. aureus strains and their PVL-deficient counterparts were cultured for 18 h at 37°C in 50 ml of CCY medium with vigorous shaking, as previously described (9, 17). The USA300 clinical isolate LAC and its control LACΔpvl (26) were kindly provided by F. DeLeo. LUG1515 and its control LUG1518 are LACΔpvl transcomplemented for PVL with pLUG534 (17) or with pLUG560, which carries an identical site with lukS and lukF-PV deleted but containing the lukS promoter and the lukF terminator. Bacteria were removed by centrifugation (2,800 × g for 10 min at 4°C), and culture supernatants were sterilized by filtration through a 0.20-μm-pore-size membrane (Micropore) and used immediately in the leukotoxicity assays described below. In parallel, aliquots of the tested supernatants were also used for PVL quantification by specific enzyme-linked immunosorbent assay (ELISA), as previously described (1).
Recombinant toxin production.
Toxin genes were amplified by using the primers and DNA template (LAC for LukS-PVR, LY19990053 for LukS-PVH, ATCC 49775 for LukF-PV, and Hlg-B and A870555 for LukD) described in Table 1. PCR products were codigested with the appropriate restriction enzymes (Promega), purified with the High Pure PCR product purification kit (Roche Applied Science), and ligated into the pIVEX 2.4d vector (Roche Applied Science) with T4 DNA Ligase (Roche Applied Science). Recombinant toxins expressed in Escherichia coli BL21 Star(DE3)/pLysS (Invitrogen) were purified with Ni-nitrilotriacetic acid columns. After protein purity verification by SDS-PAGE and protein quantification by the Bradford method, LukS-PVR and LukS-PVH were combined with each F component (LukF-PV, LukD, and Hlg-B) and used in the leukotoxicity assay described below on the day of their purification.
TABLE 1.
Primer sequences and bacterial strains used in this study to produce recombinant toxins
| Toxin | Strain | Source or reference | Primer pair sequencea (5′-3′) |
|---|---|---|---|
| LukS-PVH | LY19990053 | This study | TGGTACTGGCGGCCGCGAAGGTAAAATAACACCAGTC |
| ACGCGGATCCTCAATAATGTCCTTTCACTTTAATTTC | |||
| LukS-PVR | LAC | 26 | TGGTACTGGCGGCCGCGAAGGTAAAATAACACCAGTC |
| ACGCGGATCCTCAATAATGTCCTTTCACTTTAATTTC | |||
| LukF-PV | ATCC 49775 | 23 | ACCCTTAATTAAAGCTCAACATATCACACCTGTAAG |
| ACGCGGATCCTTAGCTCATAGGATTTTTTTCCTTAG | |||
| LukD | A870555 | This study | ACCCTTAATTAAAGCTCAAAATATCACACCTAAAAG |
| ACGCGGATCCTTATACTCCAGGATTAGTTTCTTTAG | |||
| Hlg-B | ATCC 49775 | 23 | TGGTACTGGCGGCCGCGAAGGTAAAATAACACCAGTC |
| ACGCGGATCCTCAATAATGTCCTTTCACTTTAATTTC |
The restriction enzymes used for LukS-PV and HlgB cloning were NotI and BamHI, whereas PacI and BamHI were used for LukF-PV and LukD.
Leukotoxicity assay.
Human PMNs collected from the blood of healthy donors were isolated by density gradient centrifugation on Polymorphprep (Nycomed Pharma). PMNs were washed twice in Hanks balanced salt solution (Sigma-Aldrich) and immediately used in experiments. Viable PMNs were determined by trypan blue exclusion and ranged from 98 to 99%. After incubation with either culture supernatants or recombinant toxins, pore formation in the cytoplasmic membrane of PMNs was assessed by the uptake of propidium iodide (PI). At 10 min, PMNs were analyzed by flow cytometry, as previously described (9).
RESULTS
Leukotoxic effect of the culture supernatants.
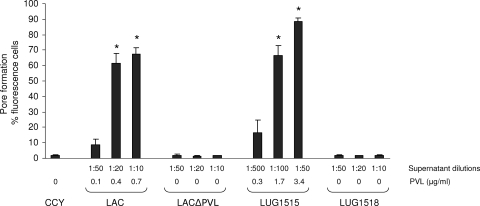
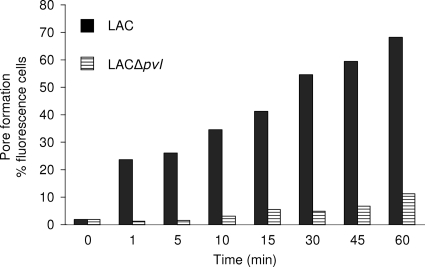
The LAC strain supernatant induced strong PI uptake in PMNs (Fig. 1 and 2). LAC PVL-mediated pore formation was concentration and time dependent. In contrast, the LAC strain with PVL deleted had a noticeably attenuated ability to induce PI uptake by PMNs. After 10 min, the level of PI uptake induced by the LAC supernatant with PVL deleted was similar to that observed with fresh CCY medium. Moreover, the level of PI uptake induced by the LACΔpvl supernatant was restored when the strain was complemented with a plasmid encoding LukS-PVH-LukF-PV (LUG1515). These results show that the PVL produced by LAC USA300 mediates pore formation in PMNs. Our observations were confirmed with four other different blood donors. After 10 min, the PI uptake with LAC supernatant (1:50 dilution) varied between blood donors from 10 to 65%.
FIG. 1.
The relationship between the formation of membrane pores in human PMNs and PVL production in USA300. Strains were grown for 18 h in CCY medium, as described in Materials and Methods. The supernatant dilutions are indicated in the key and the level of PVL in the culture medium was determined by using PVL ELISA, as indicated. The formation of membrane pores in PMNs was determined by the uptake of PI, as described elsewhere (9). The results obtained with the same blood donor, are expressed as the mean of three experiments ± the standard error of the mean. *, P < 0.05 versus CCY (analysis of variance).
FIG. 2.
Kinetics of formation of membrane pores in human PMNs induced by USA300 supernatant and USA300 Δpvl supernatant. Strains were grown for 18 h in CCY medium as described in Materials and Methods. The diluted supernatants (1:50) were added to leukocyte suspensions and incubated the times indicated. The formation of membrane pores in PMNs was determined by uptake of PI.
The level of PI uptake induced by LUG1515 was slightly higher than that observed with the wild-type LAC USA300 strain. This might reflect the high level of PVL produced from the multicopy plasmid encoding PVL in strain LUG1515 or the difference may indicate more efficient pore formation by the H haplotype, since the pvl plasmid encodes LukS-PVH-LukF-PV, whereas LAC USA300 harbors an R variant.
Leukotoxic effect of recombinant toxins.
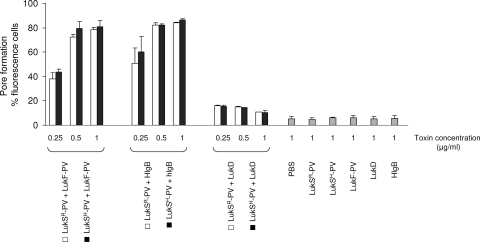
To investigate whether the H/R substitution has an impact on the efficacy of pore formation by LukS-PV, we produced LukS-PVR, LukS-PVH, LukF-PV, LukD, and Hlg-B as recombinant proteins. Both LukS-PVR and LukS-PVH synergistically enhanced PI uptake by PMNs when combined with LukF-PV, LukD, or Hlg-B (Fig. 3). These synergies were equally strong with LukF-PV and Hlg-B, but not with LukD. The synergy levels observed between LukS-PVR or LukS-PVH and each of the F components (LukF-PV, Hlg-B, and LukD) were similar, indicating that the histidine-to-arginine substitution in LukS-PV had no impact on its ability to induce pores in PMNs. In contrast, the level of PI uptake with each toxin alone (LukS-PVR, LukS-PVH, LukF-PV, LukD, or Hlg-B) was similar to that observed with the negative control (phosphate-buffered saline). These results were confirmed with two other different blood donors. Again, the level of PI uptake induced by the combination of 0.25 μg of LukS-PV/ml with 0.25 μg of either LukF-PV, Hlg-B, or LukD/ml varied from between blood donors (36 to 58%, 32 to 77%, and 11 to 17%, respectively).
FIG. 3.
The PVL histidine (H)-to-arginine (R) mutation does not affect pore formation in human PMNs. LukS-PVR, LukS-PVH, LukF-PV, LukD, and Hlg-B were produced as recombinant proteins. The indicated amounts of LukS-PVR and LukS-PVH were combined with each F component (LukF-PV, LukD, and Hlg-B) and immediately used in the leukotoxicity assay as described in Fig. 1. The results obtained with the same blood donor, are expressed as the mean of three or four experiments ± the standard error of the mean. In the analysis of variance test, P values were not significant between LukS-PVR and LukS-PVH alone or in combination with LukF-PV, LukD, or Hlg-B.
DISCUSSION
The PVL gene sequence is highly conserved in all analyzed S. aureus genomes bearing the toxin. However, two nonsynonymous nucleotide substitutions have been observed in the LukS-PV sequence: R176H in the PVL of the USA300 lineage and T157P in the PVL of several methicillin-sensitive S. aureus (MSSA) strains from different genetic background found all around the world (8, 21). The LukS H/R mutation was predicted to occur at the interaction site between the two subunits, which assemble to form the PVL pore (21). This raised the possibility that this mutation might impact the functionality of the toxin, an idea further supported by the fact that there are conflicting reports on the impact of PVL gene inactivation on the leukotoxicity of the USA300 LAC strain (4, 26). In the present study, we examined whether PVL from USA300 LAC is toxic to human leukocytes and whether the H/R variation occurring in the LukS-PV gene affects its leukotoxicity. In contrast to the published observations of Voyich et al. (26), our data show that pore formation in PMNs induced by LAC was strictly dependent on the production of PVL, using different blood donors. Moreover, using recombinant toxins we found that LukS-PVR and LukS-PVH performed similarly in leukotoxic assays regardless of the identity of the synergizing SHT-F component (LukF-PV, Hlg-B, and LukD). This indicates that the LukS-PV histidine-to-arginine substitution has no impact on its ability to induce pores in PMNs in any of the combinations tested. This is in accordance with a previous report by Berglund et al. (2); however, only purified LukS-PVR/H and LukF-PV were used in their study. Altogether, these data suggest that the PVLs produced by each CA-MRSA and MSSA lineage are equally leukotoxic. This in vitro observation is in accordance with clinical observations that PVLs impact on the severity of human skin and soft tissue infections, necrotizing pneumonia, and bone and joint infections regardless of the genetic background and the mecA status of the isolated strains (3, 11, 20).
Several points can be made to explain the discrepancy observed between Voyich et al. and our data. First, the experiments differ in the preparation of S. aureus supernatants and blood donors. We used CCY medium known to optimize PVL production, whereas Voyich et al. used YCP medium (13, 26). We used freshly made S. aureus supernatants and recombinant toxins, whereas Voyich et al. stored the supernatants before their assay, a condition that may have affected the functionality of PVL (13). An alternative hypothesis could involve differences between the donors in the sensitivity of their PMNs to PVL. We observed differences in the level of pore formation between our blood donors, but none of them was resistant to PVL. This difference could be attributable only to differences in PMN sensitivity to PVL. Identification how this has occurred would be of great interest for identifying cell pathways involved in PVL toxicity.
In a previous report, we hypothesized that the R variant has evolved toward a well-balanced host-pathogen interaction in which pathogens succeed by causing severe highly transmissible cutaneous infections and only very rarely killing the host. This could account for the “epidemic success” of USA300 CA-MRSA (8). The isofunctionality toward PMNs of the H and R LukS-PV variants used in the present study argue against this hypothesis. The special success of USA300 CA-MRSA is due to an additional factor or factors, while PVL may still be a necessary cause.
Acknowledgments
We thank F. DeLeo for providing the LAC and LACΔpvl strains used in this study and T. Greenland for editorial guidance.
This study was supported by a grant from the European Community EC 222718.
We served as consultants to bioMérieux, and the laboratory has received research grants from Pfizer and LeoPharma.
Editor: J. B. Bliska
Footnotes
Published ahead of print on 12 October 2009.
REFERENCES
- 1.Badiou, C., O. Dumitrescu, M. Croze, Y. Gillet, B. Dohin, B. Allaouchiche, J. Etienne, F. Vandenesch, and G. Lina. 2008. Panton-Valentine leukocidin is expressed at toxic levels in human skin abscesses. Clin. Microbiol. Infect. 14:1180-1183. [DOI] [PubMed] [Google Scholar]
- 2.Berglund, C., G. Prevost, B. J. Laventie, D. Keller, and B. Soderquist. 2008. The genes for Panton-Valentine leukocidin (PVL) are conserved in diverse lines of methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Microbes Infect. 10:878-884. [DOI] [PubMed] [Google Scholar]
- 3.Bocchini, C. E., K. G. Hulten, E. O. Mason, Jr., B. E. Gonzalez, W. A. Hammerman, and S. L. Kaplan. 2006. Panton-Valentine leukocidin genes are associated with enhanced inflammatory response and local disease in acute hematogenous Staphylococcus aureus osteomyelitis in children. Pediatrics 117:433-440. [DOI] [PubMed] [Google Scholar]
- 4.Brown, E. L., O. Dumitrescu, D. Thomas, C. Badiou, E. M. Koers, P. Choudhury, V. Vazquez, J. Etienne, G. Lina, F. Vandenesch, and M. G. Bowden. 2009. The Panton-Valentine leukocidin vaccine protects mice against lung and skin infections caused by Staphylococcus aureus USA300. Clin. Microbiol. Infect. 15:156-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cribier, B., G. Prevost, P. Couppie, V. Finck-Barbancon, E. Grosshans, and Y. Piemont. 1992. Staphylococcus aureus leukocidin: a new virulence factor in cutaneous infections? An epidemiological and experimental study. Dermatology 185:175-180. [DOI] [PubMed] [Google Scholar]
- 6.Diep, B. A., A. M. Palazzolo-Ballance, P. Tattevin, L. Basuino, K. R. Braughton, A. R. Whitney, L. Chen, B. N. Kreiswirth, M. Otto, F. R. DeLeo, and H. F. Chambers. 2008. Contribution of Panton-Valentine leukocidin in community-associated methicillin-resistant Staphylococcus aureus pathogenesis. PLoS ONE 3:e3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dohin, B., Y. Gillet, R. Kohler, G. Lina, F. Vandenesch, P. Vanhems, D. Floret, and J. Etienne. 2007. Pediatric bone and joint infections caused by Panton-Valentine leukocidin-positive Staphylococcus aureus. Pediatr. Infect. Dis. J. 26:1042-1048. [DOI] [PubMed] [Google Scholar]
- 8.Dumitrescu, O., A. Tristan, H. Meugnier, M. Bes, M. Gouy, J. Etienne, G. Lina, and F. Vandenesch. 2008. Polymorphism of the Staphylococcus aureus Panton-Valentine leukocidin genes and its possible link with the fitness of community-associated methicillin-resistant S. aureus. J. Infect. Dis. 198:792-794. [DOI] [PubMed] [Google Scholar]
- 9.Genestier, A. L., M. C. Michallet, G. Prevost, G. Bellot, L. Chalabreysse, S. Peyrol, F. Thivolet, J. Etienne, G. Lina, F. M. Vallette, F. Vandenesch, and L. Genestier. 2005. Staphylococcus aureus Panton-Valentine leukocidin directly targets mitochondria and induces Bax-independent apoptosis of human neutrophils. J. Clin. Investig. 115:3117-3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gillet, Y., B. Issartel, P. Vanhems, J. C. Fournet, G. Lina, M. Bes, F. Vandenesch, Y. Piemont, N. Brousse, D. Floret, and J. Etienne. 2002. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet 359:753-759. [DOI] [PubMed] [Google Scholar]
- 11.Gillet, Y., P. Vanhems, G. Lina, M. Bes, F. Vandenesch, D. Floret, and J. Etienne. 2007. Factors associated with the high fatality rate of Panton-Valentine leukocidin associated Staphylococcus aureus necrotising pneumonia. Clin. Infect. Dis. 45:315-321. [DOI] [PubMed] [Google Scholar]
- 12.Gravet, A., D. A. Colin, D. Keller, R. Girardot, H. Monteil, and G. Prevost. 1998. Characterization of a novel structural member, LukE-LukD, of the bi-component staphylococcal leucotoxins family. FEBS Lett. 436:202-208. [DOI] [PubMed] [Google Scholar]
- 13.Grojec, P. L., and J. Jeljaszewicz. 1985. Staphylococcal leukocidin, Panton-Valentine type. J. Toxicol. Toxin Rev. 4:133-189. [Google Scholar]
- 14.Guillet, V., D. Keller, G. Prevost, and L. Mourey. 2004. Crystallization and preliminary crystallographic data of a leucotoxin S component from Staphylococcus aureus. Acta Crystallogr. D Biol. Crystallogr. 60:310-313. [DOI] [PubMed] [Google Scholar]
- 15.Jayasinghe, L., and H. Bayley. 2005. The leukocidin pore: evidence for an octamer with four LukF subunits and four LukS subunits alternating around a central axis. Protein Sci. 14:2550-2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Konig, B., G. Prevost, Y. Piemont, and W. Konig. 1995. Effects of Staphylococcus aureus leukocidins on inflammatory mediator release from human granulocytes. J. Infect. Dis. 171:607-613. [DOI] [PubMed] [Google Scholar]
- 17.Labandeira-Rey, M., F. Couzon, S. Boisset, E. L. Brown, M. Bes, Y. Benito, E. M. Barbu, V. Vazquez, M. Hook, J. Etienne, F. Vandenesch, and M. G. Bowden. 2007. Staphylococcus aureus Panton-Valentine leukocidin causes necrotizing pneumonia. Science 315:1130-1133. [DOI] [PubMed] [Google Scholar]
- 18.Lina, G., Y. Piemont, F. Godail-Gamot, M. Bes, M. O. Peter, V. Gauduchon, F. Vandenesch, and J. Etienne. 1999. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin. Infect. Dis. 29:1128-1132. [DOI] [PubMed] [Google Scholar]
- 19.Moran, G. J., A. Krishnadasan, R. J. Gorwitz, G. E. Fosheim, L. K. McDougal, R. B. Carey, and D. A. Talan. 2006. Methicillin-resistant S. aureus infections among patients in the emergency department. N. Engl. J. Med. 355:666-674. [DOI] [PubMed] [Google Scholar]
- 20.Munckhof, W. J., G. R. Nimmo, J. Carney, J. M. Schooneveldt, F. Huygens, J. Inman-Bamber, E. Tong, A. Morton, and P. Giffard. 2008. Methicillin-susceptible, non-multiresistant methicillin-resistant and multiresistant methicillin-resistant Staphylococcus aureus infections: a clinical, epidemiological and microbiological comparative study. Eur. J. Clin. Microbiol. Infect. Dis. 27:355-364. [DOI] [PubMed] [Google Scholar]
- 21.O'Hara, F. P., N. Guex, J. M. Word, L. A. Miller, J. A. Becker, S. L. Walsh, N. E. Scangarella, J. M. West, R. M. Shawar, and H. Amrine-Madsen. 2008. A geographic variant of the Staphylococcus aureus Panton-Valentine leukocidin toxin and the origin of community-associated methicillin-resistant S. aureus USA300. J. Infect. Dis. 197:187-194. [DOI] [PubMed] [Google Scholar]
- 22.Prevost, G., P. Couppie, P. Prevost, S. Gayet, P. Petiau, B. Cribier, H. Monteil, and Y. Piemont. 1995. Epidemiological data on Staphylococcus aureus strains producing synergohymenotropic toxins. J. Med. Microbiol. 42:237-245. [DOI] [PubMed] [Google Scholar]
- 23.Prevost, G., B. Cribier, P. Couppie, P. Petiau, G. Supersac, V. Finck-Barbancon, H. Monteil, and Y. Piemont. 1995. Panton-Valentine leucocidin and gamma-hemolysin from Staphylococcus aureus ATCC 49775 are encoded by distinct genetic loci and have different biological activities. Infect. Immun. 63:4121-4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szmigielski, S., E. Sobiczewska, G. Prevost, H. Monteil, D. A. Colin, and J. Jeljaszewicz. 1998. Effect of purified staphylococcal leukocidal toxins on isolated blood polymorphonuclear leukocytes and peritoneal macrophages in vitro. Zentralbl. Bakteriol. 288:383-394. [DOI] [PubMed] [Google Scholar]
- 25.Tristan, A., M. Bes, H. Meugnier, G. Lina, B. Bozdogan, P. Courvalin, M. E. Reverdy, M. C. Enright, F. Vandenesch, and J. Etienne. 2007. Global distribution of Panton-Valentine leukocidin-positive methicillin-resistant Staphylococcus aureus, 2006. Emerg. Infect. Dis. 13:594-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Voyich, J. M., M. Otto, B. Mathema, K. R. Braughton, A. R. Whitney, D. Welty, R. D. Long, D. W. Dorward, D. J. Gardner, G. Lina, B. N. Kreiswirth, and F. R. DeLeo. 2006. Is Panton-Valentine leukocidin the major virulence determinant in community-associated methicillin-resistant Staphylococcus aureus disease? J. Infect. Dis. 194:1761-1770. [DOI] [PubMed] [Google Scholar]
- 27.Ward, P. D., and W. H. Turner. 1980. Identification of staphylococcal Panton-Valentine leukocidin as a potent dermonecrotic toxin. Infect. Immun. 28:393-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woodin, A. M. 1959. Fractionation of a leucocidin from Staphylococcus aureus. Biochem. J. 73:225-237. [DOI] [PMC free article] [PubMed] [Google Scholar]