Abstract
Borrelia burgdorferi, the etiological agent of Lyme disease, adapts to unique host environments as a consequence of its complex life cycle that spans both arthropod and mammalian species. In this regard, B. burgdorferi must adapt to various environmental signals, pHs, temperatures, and O2 and CO2 levels to establish infectious foci. We hypothesize that the BosR protein functions as a global regulator that is required for both borrelial oxidative homeostasis and pathogenesis. To assess the role of BosR in B. burgdorferi, we constructed an IPTG (isopropyl-β-d-thiogalactopyranoside)-regulated bosR strain. The selective decrease of bosR resulted in a change in growth when cells were cultured either anaerobically or microaerobically; however, a distinct growth defect was observed for anaerobically grown B. burgdorferi relative to the growth attenuation observed for microaerobically grown B. burgdorferi. B. burgdorferi cells in which BosR levels were reduced were more sensitive to hydrogen peroxide and produced lower levels of NapA (Dps) and SodA, proteins involved in the oxidative stress response. In addition, the levels of OspC and DbpA were also induced coincident with increased BosR levels, suggesting that BosR interfaces with the RpoS regulatory cascade, which is known to modulate virulence gene expression in B. burgdorferi. Taken together, these results indicate that BosR is involved in the resistance of B. burgdorferi to oxidative stressors and affects the expression of genes, either directly or indirectly, whose products are important in borrelial pathogenesis.
Infection with Borrelia burgdorferi, the etiologic agent of Lyme disease, is the leading arthropod-borne infection in the United States and contributes to extensive morbidity in areas of endemicity, where the chronic phase of disease generally presents as arthritis. One characteristic of B. burgdorferi is its ability to adapt to both arthropod and mammalian hosts. Several studies demonstrated that B. burgdorferi responds to a number of environmental signals, including temperature, pH, and O2 and CO2 levels, as well as uncharacterized host factors (1, 2, 8, 13, 15-17, 20, 30, 36, 39, 40, 43, 46, 47, 51, 52, 55, 57). However, specific details regarding how these signals are integrated into a regulatory response are poorly understood.
The genome sequence of B. burgdorferi predicted only a few regulatory proteins (14, 22); one such regulator was annotated as Fur. However, Posey and Gherardini demonstrated that B. burgdorferi has no requirement for iron (42), suggesting that the assignment of a Fur protein in B. burgdorferi was inaccurate. In fact, examination of the primary amino acid sequence showed that borrelial Fur was most similar to PerR, an oxidative stress regulator that represses genes involved in the oxidative stress response in Bacillus spp. (9, 23, 27, 38). However, unlike PerR, BosR appears to activate expression of target borrelial genes involved in the oxidative stress response, including napA (dps) and a coenzyme A (CoA) disulfide reductase gene designated cdr (5, 7). Thus, although BosR is similar to PerR, it appears to function more like OxyR, an activator that promotes the expression of genes involved in the oxidative stress response in Escherichia coli (24, 53, 60). BosR binds in vitro to sequences upstream of genes such as napA (dps), cdr, the superoxide dismutase gene (sodA), bosR, bb0646, and oppA4, providing further support that BosR regulates the expression of many unlinked genes within the B. burgdorferi genome (5, 7, 31, 37, 48).
In this report, we describe the isolation and characterization of a conditional bosR mutant in infectious B. burgdorferi, using an IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible hybrid E. coli-B. burgdorferi promoter (25) linked to bosR (Pflac-bosR). Cells with reduced BosR have a delayed growth phenotype under all conditions but exhibit restricted cell density under microaerobic conditions. B. burgdorferi cells that do not make BosR protein exhibit a modest but significant increase in sensitivity to hydrogen peroxide, suggesting that BosR is needed for maximal resistance to oxidative stressors. Along these lines, when bosR is induced with IPTG, the levels of SodA and NapA increase, consistent with the prior contentions that BosR activates expression of napA (7) and that these proteins are important in borrelial oxidative stress homeostasis. Furthermore, BosR production coincides with the increased synthesis of OspC and DbpA, suggesting that BosR may directly or indirectly interface with the Rrp2/RpoN/RpoS regulatory machinery. These results show that in addition to affecting the expression of genes involved in the oxidative stress response, BosR may alter the production of proteins that affect the pathogenic potential of B. burgdorferi.
MATERIALS AND METHODS
Bacterial strains.
The Borrelia burgdorferi strain B31 derivatives used in this study are listed in Table 1. All B. burgdorferi strains were grown in complete BSK-II medium, either microaerobically or anaerobically, as described previously (30). For selective pressure, B. burgdorferi was grown in BSK-II medium with antibiotics, where appropriate, at the following concentrations: kanamycin at 300 μg/ml, streptomycin at 50 μg/ml, and gentamicin at 50 μg/ml. The Institutional Biosafety Committee at Texas A&M University approved the use of infectious B. burgdorferi described in this study.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype or characteristic | Reference or source |
|---|---|---|
| Strains | ||
| B. burgdorferi strains | ||
| A3-LS | Missing lp28-1; bbe02::lacI-Strr | 25 |
| JH211 | A3-LS Pflac-bosR Kanr | This study |
| ML23 | Missing lp25 | 33, 34 |
| DS102 | ML23 bb0646::Gentr | This study |
| E. coli strains | ||
| TOP10 | F−mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 nupG recA1 araD139 Δ(ara-leu)7697 galU galK rpsL(Strr) endA1 | Invitrogen |
| Mach-1-T1r | F− φ80lacZΔM15 ΔlacX74 hsdR (rK− mK+) ΔrecA1398 endA1 tonA | Invitrogen |
| Plasmids | ||
| pCR8/GW/TOPO | Spcr; Gateway PCR cloning/entry vector | Invitrogen |
| pTAflac | Kanr Ampr | 25 |
| pBSV2 | Kanr | 18 |
| pJH207 | Spcr; 1,092-bp fragment upstream of the bosR promoter region containing the 3′ domain of bb0648 | |
| pJH208 | Spcr; 1,134 bp downstream of the bosR promoter region containing the entire bosR coding sequence and a 5′ domain of the downstream bb0646 gene | |
| pJH209 | Spcr; upstream and downstream flanking regions of the bosR promoter from pJH207 and pJH208, respectively | |
| pJH209B | Spcr; flac promoter (Pflac) engineered with NdeI and NotI restriction sites | |
| pJH210 | Spcr; construct with the Pflac promoter replacing the bosR promoter region | |
| pJH210B | Kanr Spcr; Kanr cassette engineered with NotI restriction sites | |
| pJH211 | Kanr Spcr; suicide vector containing Pflac-bosR with a genetically linked Kanr cassette | |
| pDS100 | Spcr; clone of a 2,551-bp chromosomal fragment containing 1,085 bp upstream of bb0646, the 985-bp bb0646 gene, and 482 bp downstream from bb0646 | |
| pDS101 | Gentr Spcr; PflgB-Gentr cassette from pBSV2G with engineered NsiI ends for cloning into pDS100 to insertionally inactivate bb0646 | |
| pDS102 | Gentr Spcr; suicide vector containing bb0646::Gentr construct |
Escherichia coli TOP10 and Mach1-T1 cells were used for all cloning steps and were transformed with appropriate PCR-amplified products cloned into pCR8/GW/TOPO (Invitrogen Corp., Carlsbad, CA). The E. coli cells were grown with aeration in LB medium at 37°C. For experiments involving E. coli, antibiotics were used at the following concentrations: carbenicillin at 100 μg/ml, spectinomycin at 100 μg/ml, gentamicin at 5 μg/ml, and kanamycin at 50 μg/ml.
Plasmid constructs.
The assembly of pJH211, which replaces the native bosR promoter with the E. coli-B. burgdorferi hybrid flac promoter (Pflac) (25), required the production of several intermediate constructs, listed in Table 1. Briefly, to design the Pflac-bosR construct, a 1,092-bp fragment containing part of the 3′ end of bb0648 and excluding the 70 bp immediately upstream of the bosR translational start site was amplified by PCR and cloned into pCR8/GW/TOPO, and the resultant plasmid was designated pJH207. The next construct contained a 1,134-bp PCR-amplified fragment that included bosR and 605 bp of bb0646 and was engineered such that an NdeI restriction site could be used to link the Pflac promoter to the translational start of bosR. This PCR-amplified product was also cloned into pCR8/GW/TOPO, and the resultant plasmid was designated pJH208. The resulting transformants were confirmed by restriction digestion and sequencing. Both plasmids were digested at engineered NotI and EagI restriction sites to insert the 1,134-bp downstream fragment from pJH208 together with the 1,092-bp upstream fragment from pJH207 to generate pJH209. The Pflac region was amplified with NotI and NdeI engineered restriction sites from pTAflac (25) and cloned into pCR8/GW/TOPO, resulting in pJH209B. The Pflac region was cloned into pJH209 in frame with the translational start of bosR to obtain pJH210. A kanamycin resistance (Kanr) cassette was amplified from pBSV2 at NotI restriction sites and cloned into pCR8/GW/TOPO, to generate pJH210B. The final construct, pJH211, was obtained by cloning the 1,241-bp NotI fragment containing the Kanr cassette from pJH210B into pJH210 digested with NotI. The resulting final construct, containing the Kanr cassette linked to Pflac-bosR, was designated pJH211. Each construct, including pJH211, was confirmed by both restriction digestion and sequence analysis.
As the first step in genetically inactivating bb0646, a 2,551-bp fragment that included bb0646 (984 bp) plus 1,085 bp upstream (5′ of bb0646) and 482 bp downstream (3′ of bb0646) was amplified by PCR from B. burgdorferi total genomic DNA, using the oligonucleotide primers pDS100F and pDS100R (Table 2). The resulting PCR product was cloned into pCR8/GW/TOPO and was designated pDS100. The 1,014-bp gentamicin resistance cassette from pBSV2G was amplified using primers flgB_gentF-NsiI and flgB_gentR-NsiI (Table 2), followed by cloning into pCR8/GW/TOPO to generate pDS101 (Table 1). To insertionally inactivate bb0646, a unique NsiI restriction site that is located 357 bp downstream from the translational start site of bb0646 (and also unique to pDS100) was used as the target sequence to clone in the Gentr cassette. To this end, pDS100 and pDS101 were digested with NsiI separately, and the approximately 1-kb NsiI fragment containing the PflgB-Gentr cassette from pDS101 was cloned into NsiI-digested pDS100 to generate pDS102. Following ligation, pDS102 was transformed into Mach1-T1 E. coli cells (Invitrogen Corp.). Transformants containing pDS102 were then analyzed by restriction digestion and PCR and subsequently confirmed by dideoxy sequencing prior to electroporation into B. burgdorferi.
TABLE 2.
Oligonucleotides used in this study
| Designation | Oligonucleotide sequence (5′-3′) | Description |
|---|---|---|
| JH207F | GCCCTAGAAAACAACTTACAC | Amplifies a 1,092-bp region upstream of the bosR promoter region |
| JH207REagINotI | ACGCCGGCCGGCGGCCGCCCCAGTTATTAATATTTTAC | |
| JH208FNotINdeI | ACGCGCGGCCGCCATCATGAACGACAACATAATAGACG | Amplifies a 1,134-bp region downstream of the bosR promoter region |
| JH208REagI | ACGCCGGCCGCTGTTATAAGATATGCAATTTGTCGCC | |
| PflacFNotI | ACGCGCGGCCGCAATACCCGAGCTTCAAGG | Amplifies the flac promoter (Pflac) from pTAflac |
| PflacRNdeI | ACGCCATATGGAAACCTCCCTCAATTGTG | |
| PflgBkanFNotI | ACGCGCGGCCGCTAATACCCGAGCTTCAAGG | Amplifies a kanamycin resistance cassette expressed from the B. burgdorferi flgB promoter from pBSV2 |
| PflgBkanRNotI | ACGCGCCGCCGCCAACCAATTACCAATTCT | |
| bosRRPstI | CTGCAGTTTAAATGTTGAAAAAGATA | bosR-specific oligonucleotide primer (48) that, when paired with PflacFNotI, amplifies a 945-bp product in JH211 transformants |
| pDS100F | GATATCTTCTTGAATATTCAGC | Amplifies a 2,551-bp chromosomal fragment containing bb0646 |
| pDS100R | GTTTTCTGGAGGGTTTGTCG | |
| flgB-gentF-NsiI | ACGCATGCATCCGCACCGATCGCCCTTCCC | Amplifies a gentamicin resistance cassette expressed from the B. burgdorferi flgB promoter from pBSV2G |
| flgB-gentR-NsiI | ACGCATGCATCGATCTCGGCTTGAACG |
Transformation of B. burgdorferi.
B. burgdorferi strains A3-LS and ML23 were made competent and electroporated, and transformants were isolated in the presence of 5 mM IPTG, along with streptomycin and kanamycin for A3-LS and with gentamicin alone for ML23, as previously described (45, 48, 49, 56), to isolate strains JH211 and DS102, respectively.
PCR.
PCR was done using Invitrogen Supermix high-fidelity master mix as described previously, using the oligonucleotide primers listed in Table 2 (56).
SDS-PAGE and Western immunoblotting.
Anaerobic B. burgdorferi protein lysates were resolved by SDS-12.5% PAGE as previously described (30). Following SDS-PAGE, the proteins were transferred to a polyvinylidene difluoride (PVDF) membrane, and the samples were processed for Western blotting as indicated previously (30, 47-49, 56). Borrelia-specific antibodies were used at the following dilutions: murine anti-FlaB (Affinity Bioreagent [ABR], Golden, CO) at 1:20,000; rabbit anti-NapA at 1:100,000 (generously provided by Frank Gherardini); rabbit anti-OspC at 1:1,000 (generously provided by Richard Marconi); rabbit anti-DbpA at 1:10,000 (generously provided by Magnus Höök); and mouse anti-Rrp2 and anti-Hk2, each at 1:40 (generously provided by Xiaofeng Yang). For Western immunoblots that used anti-Rrp2 and anti-Hk2 as primary antibodies, an additional amplification step was incorporated, using goat anti-mouse immunoglobulin at 1:1,000 followed by anti-goat with horseradish peroxidase conjugate at 1:1,000. Appropriate secondary antibodies, e.g., anti-mouse or anti-rabbit with conjugated horseradish peroxidase, were used in conjunction with chemiluminescent substrates to detect immobilized immune complexes on PVDF membranes.
Hydrogen peroxide assays and flow cytometry analysis.
JH211 cultures were inoculated at 104 cells/ml without IPTG or treated with 3 mM IPTG after washing of cells to remove IPTG from the previous cultivation and were incubated under microaerobic conditions until reaching a cell density of 5 × 107 cells per ml. For each assay conducted, three independent cultures were grown in triplicate. Cells were pelleted and resuspended in modified BSK-II medium lacking bovine serum albumin (BSA) and normal rabbit serum. Both BSA and rabbit serum were restricted from the BSK-II medium to eliminate abundant protein-specific targets for H2O2 that might titrate out the effect of this oxidizing agent, resulting in an apparent greater level of resistance of B. burgdorferi to this oxidative stressor. A total of 5 × 107 cells were treated with 0, 1, 5, 10, 20, or 50 mM hydrogen peroxide for 4 h. Cells were pelleted, resuspended in phosphate-buffered saline (PBS) with 2% BSA, and incubated with 100 ng/μl propidium iodide (PI) in darkness for 15 min. Cells were fixed with 4% paraformaldehyde for 10 min and washed with PBS with 2% BSA. The fixed cell pellets were resuspended in 1 ml PBS with 2% BSA and analyzed by flow cytometry. The number of dead B. burgdorferi organisms was scored using the incorporation of PI as an indicator of cell death. Specifically, for each experimental replicate (experiments were done in triplicate), cells were analyzed on a FACSCalibur (Becton Dickinson Immunocytometry Systems, San Jose, CA) flow cytometer, using CellQuest (Becton Dickinson) acquisition software. Log amplification was used to collect forward scatter, side scatter, and PI fluorescence. PI fluorescence was collected through a 650-nm long-pass filter. List-mode data were acquired for each sample tested (i.e., strain, growth condition, and concentration of H2O2 imposed) for a minimum of 20,000 events per replicate (each sample was assayed in triplicate), as defined by light scatter gates. Data analysis was performed in FlowJo (versions 8.7 and 8.8; Treestar, Inc., Ashland, OR), using forward and side light scatter to gate the B. burgdorferi cells. Two gating controls were included in the analysis: a heat-killed control (>99% incorporation of propidium iodide observed) and a no-treatment control.
Statistical analyses.
The fractions of cells killed at various concentrations of hydrogen peroxide were analyzed using a logistic regression model. Analysis was performed in R, version 2.9.1 (http://CRAN.R-project.org), using the generalized linear model function with a binomial error structure. As recommended, the response data consisted of a two-column array: for each trial, column 1 contained the number of cells killed, and column 2 contained the number of live cells. The level of IPTG (none or 3 mM) was added as a group factor. The remaining explanatory variable was log10 [H2O2]. Concentrations of H2O2 required for various fractional cell kills in each IPTG group were predicted from the model, as were 95% confidence intervals around each predicted concentration. Compared with log10 [H2O2] alone, inclusion of the group factor improved the fit of the model to a highly significant extent (P < 0.001), based on χ2 analyses of deviance. This supports rejection of the null hypothesis of no difference in the lethality curves between the two IPTG groups and indicates that the two groups differed significantly (P < 0.001).
RESULTS
Construction of a conditional bosR mutant in infectious B. burgdorferi.
Numerous attempts to inactivate bosR in infectious B. burgdorferi by allelic exchange were not successful, presumably because BosR regulates genes required for the growth of pathogenic B. burgdorferi. It should be noted that a noninfectious isolate was inactivated within the bosR locus, but this strain contained a mutant variant of bosR and was missing seven plasmids (48). This lack of genetic material may have provided an enhanced background for the isolation of the bosR mutant by removing targets that, when dysregulated, are toxic to B. burgdorferi. As a part of these prior studies, the noninfectious B. burgdorferi strain was found to encode a mutant form of BosR (BosRR39K) with a mutation in a highly conserved residue that alters its activity relative to that of wild-type BosR. This point mutation may also have been instrumental in the isolation of the mutant (48). In addition, it is possible that the noninfectious B. burgdorferi strain had accumulated several point mutations that altered the cells’ regulatory response via BosR. The observation that the noninfectious B. burgdorferi strain used in the prior study was highly sensitive to oxidative stressors (low-passage, infectious B. burgdorferi strains are resistant to oxidative stressors [6, 21, 48]) suggests that one or all of these parameters were operative.
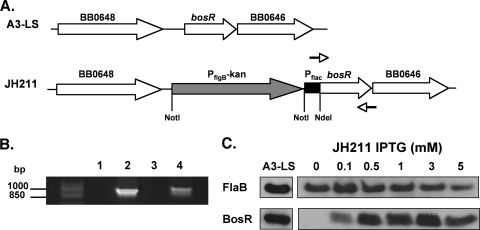
Since we were unable to isolate a knockout of bosR in infectious B. burgdorferi, we hypothesized that we could construct a conditionally lethal mutant by linking a recently developed inducible promoter system to bosR and selectively turning this gene on and off by the addition or depletion of the gratuitous inducer IPTG (25). The goal was to construct a strain that places bosR under the control of an inducible promoter that can be selected for under conditions where bosR is expressed at high levels. Based on our inability to obtain a bosR mutant in infectious B. burgdorferi, we predicted that subsequent titration of the inducer would reduce or eliminate growth. To test this hypothesis, we transformed strain A3-LS (generously supplied by Scott Samuels) with a suicide vector that contained bosR under the control of the Pflac promoter (promoter constructs were also supplied by Scott Samuels), which is a hybrid of the borrelial flaB and E. coli lac promoters (25). More importantly, Pflac is regulated by IPTG, as the strain contains constitutively expressed lacI within the infection-associated lp25 plasmid (25). The insertion of the lacI gene in the bbe02 gene of lp25 provides a regulatory protein that is responsive to IPTG and inactivates a restriction/modification gene that facilitates genetic manipulation (25, 32). Strain A3-LS was transformed with the Pflac-bosR construct (with pJH211, which contained a linked Kanr marker upstream of Pflac-bosR) (Fig. 1A), the desired transformant was selected with 5 mM IPTG together with streptomycin and kanamycin, and the putative transformants were characterized. The resulting candidates were screened by PCR (Fig. 1B), by Southern blotting (not shown), and for total plasmid content (data not shown). JH211 was isolated as a clone that contained the proper recombination event and retained all initial plasmids carried by strain A3-LS (note that A3-LS is missing lp28-1 [25]).
FIG. 1.
Construction and screening of IPTG-inducible bosR mutant JH211 in B. burgdorferi. (A) Schematic representing the A3-LS chromosomal region containing bosR and the chromosomal insertion of the IPTG-inducible promoter, Pflac, upstream of bosR, resulting in strain JH211. (B) PCR screen of the JH211 clone, specifically amplifying a 945-bp product of the Pflac-bosR region in JH211, using oligonucleotide primers PflacFNotI and bosRRPstI, which are depicted as arrows in panel A (see Table 2). Lane 1, no-template control; lane 2, pJH211; lane 3, genomic A3-LS; and lane 4, genomic JH211. Markers (in bp) are shown on the left. (C) BosR synthesis is induced in the presence of IPTG. JH211 cultures were grown with increasing concentrations of IPTG, ranging from 0 mM to 5 mM IPTG. Equivalent amounts of protein were loaded from the parent strain A3-LS. Cell lysates from each treatment condition were resolved by SDS-PAGE, immobilized on PVDF membranes, and probed with antisera for the antigens designated on the left. FlaB is constitutively synthesized and was utilized as a normalization control for equivalent loading.
Characterization of a conditional bosR mutant.
To correlate IPTG induction with BosR production, borrelial cells were incubated with various amounts of IPTG. Immunoblot analysis indicated that BosR was not produced in the absence of IPTG and that increased levels of IPTG correlated with increased production of BosR (Fig. 1C), demonstrating that this system, as expected, could be exploited to produce a wide range of BosR protein concentrations in B. burgdorferi, including levels commensurate with those seen for the parent strain A3-LS, which expresses bosR from its native promoter (Fig. 1C).
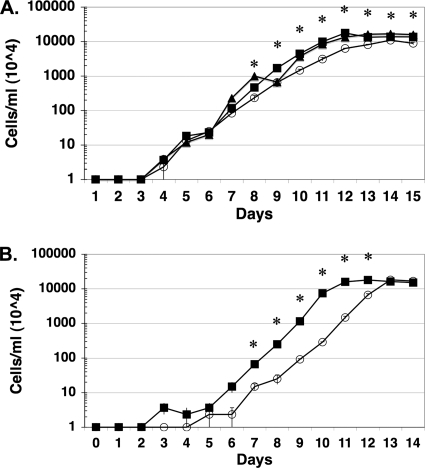
Given that we were unsuccessful in isolating a genetic knockout of bosR by using infectious B. burgdorferi, we reasoned that the elimination of IPTG from JH211 cultivated in vitro would reduce or abrogate growth. When JH211 was grown with either 0.1, 0.5, 1, 3, or 5 mM IPTG added to the BSK-II medium, we observed little difference in the growth rate relative to that of its parent strain, A3-LS; in fact, the growth curves for A3-LS and JH211 grown with 3 mM IPTG were nearly superimposable (Fig. 2A). However, if no IPTG was added, JH211 was significantly impaired in its growth, under both anaerobic and microaerobic conditions, relative to that seen for JH211 in the presence of 3 mM IPTG (Fig. 2B). Microaerobically grown JH211 lacking IPTG exhibited a reduced cell density relative to those for cultures grown with 3 mM IPTG and for anaerobically grown JH211 at either concentration of IPTG used (Fig. 2), suggesting that cells lacking BosR and grown in the presence of dissolved oxygen (i.e., microaerobically) are less capable of adapting and/or neutralizing oxidative stressors than are cells that can synthesize BosR under the same growth conditions. The fact that anaerobically grown JH211 attained similar cell densities independent of IPTG levels and grew at comparable rates (albeit with an extra 2-day lag in growth when BosR was missing) implies that microaerobically grown JH211 with reduced (or null) levels of BosR is impaired in growth because it is impaired in the response to the oxidative stressors encountered. Such an effect would be predicted to be muted under anaerobic growth conditions, independent of BosR levels, since reactive oxygen species (ROS) levels would be low and may explain the enhanced growth density observed for anaerobically grown JH211 regardless of IPTG concentration (Fig. 2).
FIG. 2.
The absence of BosR results in differential growth of JH211 lacking IPTG under anaerobic and microaerobic conditions. All cultures were grown in triplicate, and cell densities were averaged (bars indicate standard errors). The values shown on the y axis are multiplied by 104/ml to give the final culture density. (A) Strain JH211 was grown under microaerobic conditions (3.48 ppm dissolved O2, 1% CO2) without the addition of IPTG (open circles) or with 3 mM IPTG (dark squares). Microaerobic growth of strain A3-LS is also shown (dark triangles). (B) Strain JH211 was grown under anaerobic conditions (0.087 ppm dissolved O2, 5% CO2) without IPTG (open circles) or with 3 mM IPTG (dark squares). Asterisks indicate significant differences in growth density between JH211 grown without added IPTG and that grown with 3 mM IPTG added to the growth medium for the day shown (P < 0.05).
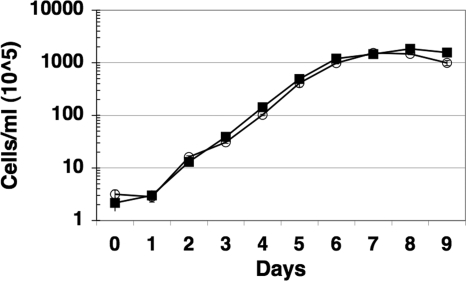
Another possible explanation for the growth phenotype observed for JH211 could be the elimination of the gene downstream from bosR, bb0646, which is predicted to encode an exported lipase (31). To address this possibility, bb0646 was inactivated in strain ML23, a low-passage-number B. burgdorferi clonal isolate that lacks lp25, which has been used previously to inactivate bbk32 and to delete dbpBA (33, 34, 49, 56). Specifically, the bb0646 gene was inactivated with a gentamicin resistance cassette (Gentr) that is constitutively expressed in B. burgdorferi (18). Following transformation, the bb0646 mutant candidates were screened by PCR and Southern blot analysis to determine whether the desired genetic lesion in bb0646 was obtained (data not shown). Several clones containing the bb0646::Gentr allele were isolated, and one was designated DS102. Following cultivation in BSK-II medium, DS102 exhibited no statistically significant defect in growth rate relative to its parent strain (ML23) (Fig. 3) or its genetic complement (not shown) and, more importantly, did not exhibit the defect observed for JH211 when IPTG was restricted (Fig. 2). These results strongly suggest that the growth defect seen for JH211 was restricted to the loss of bosR, not bb0646.
FIG. 3.
Growth of B. burgdorferi strain DS102 (bb0646::Gentr) (open circles) relative to its parent strain, ML23 (dark squares). All cultures were grown in BSK-II medium in triplicate (with 50 μg/ml gentamicin for DS102), and the average cell densities were determined. The values shown on the y axis are multiplied by 105/ml to give the final culture density. The bars indicate the standard error for each time point taken.
Sensitivity of a conditional bosR mutant to hydrogen peroxide.
The hypothesis that B. burgdorferi BosR is required for resistance to oxidative stress has been proposed based on (i) the sequence homology that BosR shares with the oxidative stress regulator PerR (7, 48), (ii) the ability of BosR to bind to operator sequences in vitro in gel shift assays (5, 7, 31, 37, 48), and (iii) the ability of recombinant BosR to transcriptionally regulate borrelial napA and cdr promoters linked to lacZ in E. coli (5, 7). To address a global response to an oxidative stressor in infectious B. burgdorferi under conditions where BosR levels vary, strain JH211 was grown either without added IPTG or with 3 mM IPTG. The 3 mM IPTG concentration was used because it represented the concentration of IPTG whereby the BosR level was increased and not further enhanced by addition of more IPTG, i.e., at or near saturation (Fig. 1C). After the B. burgdorferi cells were grown to mid-logarithmic phase, the cells were exposed to different concentrations of H2O2, ranging from 1 mM to 50 mM, for 4 h and compared to samples to which no H2O2 was added. To assess toxicity, the incorporation of propidium iodide was tracked following H2O2 treatment, since this compound is readily transported into cells whose cytoplasmic membranes have been compromised. The resulting samples were then scored by flow cytometry. This approach is advantageous because it allows for the rapid and unbiased evaluation of many cells per sample and treatment rendered. A representation of the data is depicted in Fig. 4A. Comparison of strain JH211 grown without IPTG to that grown with 3 mM IPTG following exposure to hydrogen peroxide indicated that the cells making BosR were more resistant to H2O2 (the 3 mM IPTG samples) than those that did not make BosR (no IPTG) (Fig. 4B). The statistical analyses performed indicated that a significant difference was observed across all concentrations of H2O2 tested (logit and probit analyses both yielded P values of <0.001). Based on these analyses, the 50% lethal dose (LD50) was calculated to be 13.7 mM for BosR-producing B. burgdorferi, compared to 5.2 mM for the borrelial cells that did not synthesize BosR (Fig. 4B). It should also be noted that each data point shown and the calculated regression curve were based on approximately 60,000 borrelial cells for each growth condition imposed (i.e., without or with added IPTG) and concentration of H2O2 tested and represent the compilation of three independent assays.
FIG. 4.
B. burgdorferi requires BosR for maximal response to hydrogen peroxide. (A) Flow cytometry forward scatter graphs representative of one of three independent cultures of JH211 with 0 mM IPTG or JH211 with 3 mM IPTG that were analyzed for viability after treatment with increasing concentrations of H2O2 (0 mM to 50 mM, as indicated). The number of cells labeled with propidium iodide among approximately 20,000 cells is reflected as a percentage (inset). (B) JH211 grown with 3 mM IPTG (solid squares) is significantly more resistant to killing by treatment with H2O2 than are JH211 cells lacking IPTG (open circles). The difference in total responses to H2O2 between JH211 grown with and without IPTG was significant, with a P value of <0.001.
Induction of genes involved in the oxidative stress response in B. burgdorferi.
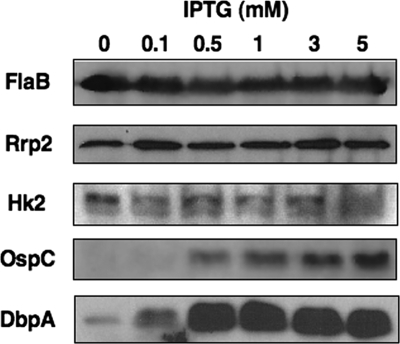
Previous studies have implicated BosR as an activator of napA (dps) and cdr, but these studies have been limited to reporter systems used in E. coli (5, 7). To test the effect of BosR on the production of proteins involved in the oxidative stress response in B. burgdorferi, we grew JH211 with increasing levels of IPTG (Fig. 5). For NapA, an increase in antigen production correlated with an increase in BosR level relative to that in cells that were grown in BSK-II medium without added IPTG (Fig. 5). Furthermore, since NapA production is important for growth of B. burgdorferi following a blood meal (35), the link of BosR to NapA synthesis suggests that BosR is important for the homeostasis of B. burgdorferi within fed ticks. Comparison of the IPTG-exposed samples to the borrelial culture grown without added IPTG indicated that the SodA level was increased when BosR production was induced (Fig. 5). Given the importance of this sole superoxide dismutase in the borrelial detoxification response and pathogenic potential in experimentally infected mice (19), the responsiveness of sodA expression and production of SodA protein commensurate with increased BosR levels suggests that BosR plays an important role in the oxidative response of B. burgdorferi. For reasons that are not entirely clear, Cdr was not detected in any of the JH211 samples tested, independent of IPTG concentration (not shown).
FIG. 5.
IPTG induction of bosR results in increased synthesis of borrelial proteins involved in the oxidative stress response. JH211 cultures were grown with increasing concentrations of IPTG, ranging from no added IPTG up to 5 mM IPTG. Cell lysates from each treatment condition were resolved by SDS-PAGE, immobilized on PVDF membranes, and probed with antisera for several different antigens, as designated on the left. As in Fig. 1, FlaB served as a normalization control.
Link of BosR to the Rrp2/RpoN/RpoS regulatory cascade.
Previously, we determined that the BosRR39K level altered the production of DbpA and dbp transcripts (29, 48). To determine whether DbpA levels were affected in JH211 following induction of bosR, the strain was exposed to different concentrations of IPTG and the resulting protein cell lysates resolved by SDS-PAGE and immunoblotted against antibodies specific for DbpA. Under conditions where no IPTG was added to the cultures, DbpA was detected in JH211 (Fig. 6). However, after exposure to as little as 0.1 mM IPTG, an increase in DbpA production was observed that appeared to become saturated at a concentration of 1 mM (Fig. 6). Previous studies demonstrated that dbp expression is dependent on the alternative sigma factor RpoS, and RpoS-dependent genes have been shown to be involved in establishing infection (3, 11, 26, 41, 50, 54, 56, 59). We therefore conducted a similar analysis with the RpoS-dependent protein OspC (Fig. 6). As with DbpA, increased synthesis of OspC was coincident with the IPTG-dependent production of BosR. Taken together, these observations imply that BosR interfaces with the Rrp2/RpoN regulatory cascade responsible for the expression of rpoS in B. burgdorferi, inasmuch as RpoS (along with Rrp2 and RpoN) is required for the expression of ospC and dbp genes (4, 10, 12, 21, 25, 28, 57, 58). Unfortunately, we were unable to determine whether RpoS levels were affected by the simultaneous induction of BosR, presumably because this protein is produced at levels that are below the threshold of detection in JH211, even following IPTG induction.
FIG. 6.
BosR level alters the production of virulence determinants regulated by the RpoN-RpoS pathway. JH211 cultures were grown with increasing concentrations of IPTG, ranging from no added IPTG up to 5 mM IPTG. Cell lysates from each treatment condition were resolved by SDS-PAGE, immobilized on PVDF membranes, and probed with antisera for several different antigens, as designated on the left. As in Fig. 1, FlaB served as a normalization control.
Since the expression of rpoS is dependent on Rrp2 function, it is possible that BosR induction results in increased levels of Rrp2 protein that accentuate the amount of functional Rrp2. Also, given that the cognate histidine kinase of Rrp2, Hk2, might be subject to the same effect as that hypothesized above for Rrp2, we determined whether Hk2 levels were altered by IPTG induction in strain JH211. The results indicated that Rrp2 and Hk2 production was unchanged and was independent of the level of IPTG (Fig. 6). This observation is consistent with the idea that the activation of these proteins does not require increased transcription or translation, since two-component regulatory proteins are activated posttranslationally via a reversible phosphorylation event.
DISCUSSION
Given that B. burgdorferi can effectively infect both arthropod and mammalian hosts, this spirochete must respond to environmental stimuli and integrate an appropriate response to modulate gene expression accordingly to adapt to these disparate environments. Along these lines, many investigators have established in vitro correlates that model various stages of the borrelial arthropod/mammalian life cycle, including temperature, pH, O2, CO2, and host-adapted conditions (1, 2, 8, 13, 15-17, 20, 30, 36, 39, 40, 43, 46, 47, 51, 52, 55, 57). From these studies, several induced and downregulated genes were identified, and due to the enhanced ability to genetically inactivate genes in virulent B. burgdorferi, this information has led to a more sophisticated molecular genetic analysis of this important pathogen (reviewed in reference 44). Despite these significant advances, there is still a dearth of knowledge relating to pathogenic mechanisms of B. burgdorferi; specifically, details of the molecular signal(s) responsible for the changes in gene expression observed are not well described.
Studies by Boylan et al. indicated that a regulatory protein, designated BosR, regulates the oxidative stress response, in part by activating the napA (dps) and cdr genes (5, 7), encoding proteins involved in movement from the tick vector into the mammalian host (35) and in cellular redox balance (5), respectively. The activator activity associated with BosR suggested that this borrelial regulatory protein shared activity more commensurate with that observed for OxyR, a regulatory protein that stimulates the expression of genes required to detoxify E. coli (24, 53, 60). An independent study referred to BB0647 as Fur and reported that the regulator functioned as a repressor, did not require any metal cofactor for activity, and recognized operator sequences of bb0647 (bosR) and bb0646 (31). All of the aforementioned studies were limited to biochemical approaches or regulatory studies in a heterologous system (i.e., E. coli [5, 7, 31]). In fact, the only genetic study on BosR in B. burgdorferi was restricted to a noninfectious isolate that carried a mutant allele of bosR (bosRR39K) (29, 48). However, given that this noninfectious strain was sensitive to ROS, whereas infectious B. burgdorferi is highly resistant to ROS, the significance of the bosRR39K results within the context of borrelial pathogenesis is limited (29, 48).
Clearly, the isolation and characterization of a bosR mutant in an infectious B. burgdorferi background are needed to link the role of this regulatory protein to a pathogenic phenotype. However, all prior attempts to obtain a bosR mutant in infectious B. burgdorferi have been unsuccessful, presumably because BosR regulates genes that are required for optimal growth of B. burgdorferi in vitro and/or the experimental conditions employed could not compensate for the loss of BosR. To circumvent these obstacles, a recently developed inducible system was exploited to regulate bosR via IPTG through the use of strain A3-LS (25) (Fig. 1). By titrating out IPTG in this strain, the amounts of bosR transcript and BosR protein would become limiting, such that a phenotypic bosR mutant could be obtained. This inducible bosR strain, designated JH211 and described herein, now allows for the characterization of BosR function in an infectious background.
Given the difficulty in isolating a conventional bosR mutant from infectious B. burgdorferi strains grown microaerobically, it was unexpected that JH211 could grow when IPTG was absent, i.e., under conditions where the borrelial cells should no longer be making BosR (Fig. 1). One possibility is that when IPTG is limiting, a small amount of BosR is made that is below the threshold of detection but is enough to sustain growth at a low level (Fig. 2). The slowed growth that ensues suggests that the reduction or absence of BosR results in the dysregulation of several genes needed for optimal growth of B. burgdorferi in vitro. To determine the complete battery of genes affected by BosR levels, array analyses are required.
The induction of bosR in strain JH211 induced the production of NapA and SodA (Fig. 5). This result for NapA is consistent with the prior contention that BosR functions as an activator for napA (7) and represents the first data directly linking BosR levels to NapA production in B. burgdorferi. How borrelial sodA is regulated is not well defined, but based on studies conducted with a noninfectious B. burgdorferi isolate, the BosR mutant protein encoded by this strain (BosRR39K) appears to act as a transcriptional repressor (29, 48). However, inasmuch as the noninfectious B. burgdorferi strain employed in the previous study was missing at least seven plasmids and encoded a mutated version of BosR (29, 48), the regulation of sodA previously described might not be representative of what occurs in infectious B. burgdorferi. Array analysis of JH211 should provide additional clarification regarding BosR regulation of sodA in virulent B. burgdorferi.
The observation that the phenotypic bosR mutant is more sensitive to H2O2 is consistent with the ability of BosR to activate genes required for detoxification, i.e., napA and sodA; however, it was somewhat surprising that the effect seen was not more dramatic (Fig. 4B). This may be due to the inability of the borrelial cells to combat the conditions imposed by the H2O2 treatment within the time frame tested. That is, if the major targets are protein or lipid species, as suggested by Boylan et al. (6), then perhaps the ability of BosR to affect genes involved in lipid and protein homeostasis function is, in part, independent of functional BosR levels. It is also conceivable that the 4-h incubation period was either too long to see a steady-state effect or not long enough to mobilize a maximal detoxification response. Alternatively, it is possible that there is another compensatory pathway that provides some level of resistance to ROS when BosR is absent or that the Pflac promoter is leaky, such that enough BosR protein was made to detoxify the cells to a lesser extent. Regardless, the differential observed was significant and was strengthened by the fact that each data point was based on data from approximately 60,000 borrelial cells per strain and H2O2 concentration evaluated (Fig. 4B).
The ability to regulate bosR by the addition and depletion of IPTG serves as a potent method to evaluate the role of BosR in borrelial pathogenesis. However, the fact that bosR is transcribed together with a downstream gene, bb0646, complicates the interpretation of the data. BB0646 is predicted to be a lipase (31); however, its exact function has not been determined. Although the phenotypes observed for JH211 grown in the absence of IPTG may be due to loss of both genes, it is more likely that the loss of a regulatory protein such as BosR would result in the phenotypes observed, given its likely ability to modulate the transcription of many unlinked genes. Along these lines, a genetic knockout of bb0646 exhibited a comparable growth rate to that of its parent, ML23 (and its respective bb0646 genetic complement [data not shown]), which was similar to the growth of JH211 in the presence of IPTG (Fig. 2 and 3). Furthermore, recent results indicated that the bb0646::Gentr mutant is capable of colonizing mice (D. K. Shaw and J. T. Skare, unpublished observations). These results suggest that the presence or absence of BB0646 does not affect the in vitro cultivation of B. burgdorferi. As such, the growth defect observed in JH211 grown in the absence of IPTG would appear to be restricted to the loss of BosR.
The most interesting characteristic of the conditional bosR mutant was the observation that the IPTG-dependent Pflac-bosR induction resulted in the increased production of OspC and DbpA (Fig. 6). Previous studies have implicated BosR in the regulation of the dbp genes, but in these reports, the BosRR39K derivative appeared to function as a repressor of the dbp genes (48). However, as indicated above, this analysis was complicated by the noninfectious status of this strain due to the absence of several plasmids and the mutant allele of bosR carried by this strain (48). The fact that OspC and DbpA levels were increased by BosR induction suggests that BosR may either directly or indirectly activate the Rrp2/RpoN/RpoS two-component regulatory system. It is well documented that RpoS is involved in the regulation of genes that are required for the successful colonization of B. burgdorferi, including ospC and the dbpBA genes (11, 12, 21, 59). Specifically, rpoS mutants do not synthesize detectable levels of OspC or DbpA protein (10, 12, 28). Separately characterized genetic knockouts or deletions of rpoS, ospC, and dbpBA result in either complete abrogation (for rpoS and ospC) or significant attenuation (for dbpBA) of borrelial infectivity (3, 11, 26, 41, 50, 54, 56, 59), indicating an important role for RpoS regulation in B. burgdorferi pathogenesis.
Alternatively, it is possible that the induction of OspC and DbpA is due entirely to a BosR-dependent regulatory effect independent of RpoS; however, the observation that both OspC and DbpA production is coincident with BosR synthesis strongly suggests that this response involves RpoS. Unfortunately, for reasons that are not evident, we were unable to track RpoS production in either the A3-LS or JH211 strain of B. burgdorferi. As such, any mechanistic linkage of BosR to the activation of the Rrp2/RpoN/RpoS regulatory cascade in these strains remains tenuous.
We were interested in determining the role that BosR played in vivo and thus carried out a number of experimental infections with A3-LS and JH211 in mice given either conventional water or water supplemented with IPTG 1 week prior to and during the infection for both immunocompetent and immunodeficient (SCID) mice. While the parent strain A3-LS was isolated from all mice infected and from the majority of tissues analyzed, all tissues from mice infected with JH211 were not infected, independent of exposure to IPTG (data not shown). The inability to “complement” JH211 from the Pflac-bosR construct by IPTG induction is similar in some respects to that seen previously for a Pflac-ospC strain. In that study, nonviable spirochetes were observed in tissues from SCID mice infected with the Pflac-ospC construct and given IPTG (25). PCR was then used to detect DNA from Pflac-ospC-containing B. burgdorferi in most of the samples tested. When a similar analysis was done for our infection study, only A3-LS-infected samples were PCR positive; no JH211 samples had detectable borrelial DNA, independent of IPTG treatment (not shown). While clearly not definitive, these results, coupled with the infectious nature of the bb0646 mutant (see above), suggest that BosR is required for experimental infection in mice.
In this report, we describe the development and characterization of a conditional bosR mutant in infectious B. burgdorferi. The results indicate that BosR is required for optimal growth of B. burgdorferi under both microaerobic and anaerobic conditions; however, the growth rate of cells cultured under anaerobic conditions is rescued following an extended lag phase of growth, suggesting that B. burgdorferi lacking BosR can mobilize an adaptive response when oxygen is limiting and when a function for BosR might be less needed. In contrast, microaerobically grown B. burgdorferi cells without BosR do not exhibit a lag phase but never grow at the same rate or to the maximum cell density, suggesting that these cells are unable to neutralize the increased levels of oxidative stressors encountered under these growth conditions. In support of this contention, microaerobically grown B. burgdorferi cells in which BosR is absent are more sensitive to H2O2, suggesting that BosR regulatory events are required for the high-level resistance to ROS seen for B. burgdorferi. Furthermore, the induction of BosR results in the expression of genes involved in both the oxidative stress response and infectivity, indicating that BosR regulation is required for homeostatic and pathogenic functions of B. burgdorferi. Certainly, the identification of the entire compendium of genes regulated by BosR will be key in determining the role of BosR in these various processes and whether the regulatory effects seen are direct or indirect. Such array-based studies, as well as the characterization of a conventional bosR mutant (along with its genetic complement), are needed to provide a clearer link of BosR to borrelial pathogenesis.
Acknowledgments
We thank Scott Samuels and Mike Gilbert for providing the B. burgdorferi strain A3-LS and the plasmid pTAflac and Patti Rosa and Philip Stewart for generously sharing the borrelial shuttle vectors pBSV2 and pBSV2G. We also gratefully acknowledge Magnus Höök, Frank Gherardini, Richard Marconi, and Xiaofeng Yang for providing borrelial antibody reagents used in this study.
This work was supported by Public Health Service grant R01-AI042345 (to J.T.S.) from the National Institute of Allergy and Infectious Diseases.
Editor: S. R. Blanke
Footnotes
Published ahead of print on 26 October 2009.
REFERENCES
- 1.Akins, D. R., K. W. Bourell, M. J. Caimano, M. V. Norgard, and J. D. Radolf. 1998. A new animal model for studying Lyme disease spirochetes in a mammalian host-adapted state. J. Clin. Invest. 101:2240-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akins, D. R., S. F. Porcella, T. G. Popova, D. Shevchenko, S. I. Baker, M. Li, M. V. Norgard, and J. D. Radolf. 1995. Evidence for in vivo but not in vitro expression of a Borrelia burgdorferi outer surface protein F (OspF) homologue. Mol. Microbiol. 18:507-520. [DOI] [PubMed] [Google Scholar]
- 3.Blevins, J. S., K. E. Hagman, and M. V. Norgard. 2008. Assessment of decorin-binding protein A to the infectivity of Borrelia burgdorferi in the murine models of needle and tick infection. BMC Microbiol. 8:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boardman, B. K., M. He, Z. Ouyang, H. Xu, X. Pang, and X. F. Yang. 2008. Essential role of the response regulator Rrp2 in the infectious cycle of Borrelia burgdorferi. Infect. Immun. 76:3844-3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boylan, J. A., C. S. Hummel, S. Benoit, J. Garcia-Lara, J. Treglown-Downey, E. J. Crane III, and F. C. Gherardini. 2006. Borrelia burgdorferi bb0728 encodes a coenzyme A disulphide reductase whose function suggests a role in intracellular redox and the oxidative stress response. Mol. Microbiol. 59:475-486. [DOI] [PubMed] [Google Scholar]
- 6.Boylan, J. A., K. A. Lawrence, J. S. Downey, and F. C. Gherardini. 2008. Borrelia burgdorferi membranes are the primary targets of reactive oxygen species. Mol. Microbiol. 68:786-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boylan, J. A., J. E. Posey, and F. C. Gherardini. 2003. Borrelia oxidative stress response regulator, BosR: a distinctive Zn-dependent transcriptional activator. Proc. Natl. Acad. Sci. USA 100:11684-11689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brooks, C. S., P. S. Hefty, S. E. Jolliff, and D. R. Akins. 2003. Global analysis of Borrelia burgdorferi genes regulated by mammalian host-specific signals. Infect. Immun. 71:3371-3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bsat, N., A. Herbig, L. Casillas-Martinez, P. Setlow, and J. D. Helmann. 1998. Bacillus subtilis contains multiple Fur homologues: identification of the iron uptake (Fur) and peroxide regulon (PerR) repressors. Mol. Microbiol. 29:189-198. [DOI] [PubMed] [Google Scholar]
- 10.Burtnick, M. N., J. S. Downey, P. J. Brett, J. A. Boylan, J. G. Frye, T. R. Hoover, and F. C. Gherardini. 2007. Insights into the complex regulation of rpoS in Borrelia burgdorferi. Mol. Microbiol. 65:277-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caimano, M. J., C. H. Eggers, K. R. Hazlett, and J. D. Radolf. 2004. RpoS is not central to the general stress response in Borrelia burgdorferi but does control expression of one or more essential virulence determinants. Infect. Immun. 72:6433-6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caimano, M. J., R. Iyer, C. H. Eggers, C. Gonzalez, E. A. Morton, M. A. Gilbert, I. Schwartz, and J. D. Radolf. 2007. Analysis of the RpoS regulon in Borrelia burgdorferi in response to mammalian host signals provides insight into RpoS function during the enzootic cycle. Mol. Microbiol. 65:1193-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carroll, J. A., C. F. Garon, and T. G. Schwan. 1999. Effects of environmental pH on membrane proteins in Borrelia burgdorferi. Infect. Immun. 67:3181-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casjens, S., N. Palmer, R. van Vugt, W. M. Huang, B. Stevenson, P. Rosa, R. Lathigra, G. Sutton, J. Peterson, R. J. Dodson, D. Haft, E. Hickey, M. Gwinn, O. White, and C. M. Fraser. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35:490-516. [DOI] [PubMed] [Google Scholar]
- 15.Cassatt, D. R., N. K. Patel, N. D. Ulbrandt, and M. S. Hanson. 1998. DbpA, but not OspA, is expressed by Borrelia burgdorferi during spirochetemia and is a target for protective antibodies. Infect. Immun. 66:5379-5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Champion, C. I., D. R. Blanco, J. T. Skare, D. A. Haake, M. Giladi, D. Foley, J. N. Miller, and M. A. Lovett. 1994. A 9.0-kilobase-pair circular plasmid of Borrelia burgdorferi encodes an exported protein: evidence for expression only during infection. Infect. Immun. 62:2653-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das, S., S. W. Barthold, S. S. Giles, R. R. Montgomery, S. R. Telford III, and E. Fikrig. 1997. Temporal pattern of Borrelia burgdorferi p21 expression in ticks and the mammalian host. J. Clin. Invest. 99:987-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elias, A. F., J. L. Bono, J. J. Kupko III, P. E. Stewart, J. G. Krum, and P. A. Rosa. 2003. New antibiotic resistance cassettes suitable for genetic studies in Borrelia burgdorferi. J. Mol. Microbiol. Biotechnol. 6:29-40. [DOI] [PubMed] [Google Scholar]
- 19.Esteve-Gassent, M. D., N. L. Elliott, and J. Seshu. 2009. sodA is essential for virulence of Borrelia burgdorferi in the murine model of Lyme disease. Mol. Microbiol. 71:594-612. [DOI] [PubMed] [Google Scholar]
- 20.Fikrig, E., S. W. Barthold, W. Sun, W. Feng, S. R. Telford III, and R. A. Flavell. 1997. Borrelia burgdorferi P35 and P37 proteins, expressed in vivo, elicit protective immunity. Immunity 6:531-539. [DOI] [PubMed] [Google Scholar]
- 21.Fisher, M. A., D. Grimm, A. K. Henion, A. F. Elias, P. E. Stewart, P. A. Rosa, and F. C. Gherardini. 2005. Borrelia burgdorferi sigma54 is required for mammalian infection and vector transmission but not for tick colonization. Proc. Natl. Acad. Sci. USA 102:5162-5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J. F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, and J. C. Venter. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580-586. [DOI] [PubMed] [Google Scholar]
- 23.Fuangthong, M., A. F. Herbig, N. Bsat, and J. D. Helmann. 2002. Regulation of the Bacillus subtilis fur and perR genes by PerR: not all members of the PerR regulon are peroxide inducible. J. Bacteriol. 184:3276-3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Georgiou, G. 2002. How to flip the (redox) switch. Cell 111:607-610. [DOI] [PubMed] [Google Scholar]
- 25.Gilbert, M. A., E. A. Morton, S. F. Bundle, and D. S. Samuels. 2007. Artificial regulation of ospC expression in Borrelia burgdorferi. Mol. Microbiol. 63:1259-1273. [DOI] [PubMed] [Google Scholar]
- 26.Grimm, D., K. Tilly, R. Byram, P. E. Stewart, J. G. Krum, D. M. Bueschel, T. G. Schwan, P. F. Policastro, A. F. Elias, and P. A. Rosa. 2004. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc. Natl. Acad. Sci. USA 101:3142-3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herbig, A. F., and J. D. Helmann. 2001. Roles of metal ions and hydrogen peroxide in modulating the interaction of the Bacillus subtilis PerR peroxide regulon repressor with operator DNA. Mol. Microbiol. 41:849-859. [DOI] [PubMed] [Google Scholar]
- 28.Hubner, A., X. Yang, D. M. Nolen, T. G. Popova, F. C. Cabello, and M. V. Norgard. 2001. Expression of Borrelia burgdorferi OspC and DbpA is controlled by a RpoN-RpoS regulatory pathway. Proc. Natl. Acad. Sci. USA 98:12724-12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hyde, J. A., J. Seshu, and J. T. Skare. 2006. Transcriptional profiling of Borrelia burgdorferi containing a unique bosR allele identifies a putative oxidative stress regulon. Microbiology 152:2599-2609. [DOI] [PubMed] [Google Scholar]
- 30.Hyde, J. A., J. P. Trzeciakowski, and J. T. Skare. 2007. Borrelia burgdorferi alters its gene expression and antigenic profile in response to CO2 levels. J. Bacteriol. 189:437-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katona, L. I., R. Tokarz, C. J. Kuhlow, J. Benach, and J. L. Benach. 2004. The fur homologue in Borrelia burgdorferi. J. Bacteriol. 186:6443-6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawabata, H., S. J. Norris, and H. Watanabe. 2004. BBE02 disruption mutants of Borrelia burgdorferi B31 have a highly transformable, infectious phenotype. Infect. Immun. 72:7147-7154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Labandeira-Rey, M., J. Seshu, and J. T. Skare. 2003. The absence of linear plasmid 25 or 28-1 of Borrelia burgdorferi dramatically alters the kinetics of experimental infection via distinct mechanisms. Infect. Immun. 71:4608-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Labandeira-Rey, M., and J. T. Skare. 2001. Decreased infectivity in Borrelia burgdorferi strain B31 is associated with loss of linear plasmid 25 or 28-1. Infect. Immun. 69:446-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li, X., U. Pal, N. Ramamoorthi, X. Liu, D. C. Desrosiers, C. H. Eggers, J. F. Anderson, J. D. Radolf, and E. Fikrig. 2007. The Lyme disease agent Borrelia burgdorferi requires BB0690, a Dps homologue, to persist within ticks. Mol. Microbiol. 63:694-710. [DOI] [PubMed] [Google Scholar]
- 36.Lybecker, M. C., and D. S. Samuels. 2007. Temperature-induced regulation of RpoS by a small RNA in Borrelia burgdorferi. Mol. Microbiol. 64:1075-1089. [DOI] [PubMed] [Google Scholar]
- 37.Medrano, M. S., Y. Ding, X. G. Wang, P. Lu, J. Coburn, and L. T. Hu. 2007. Regulators of expression of the oligopeptide permease A proteins of Borrelia burgdorferi. J. Bacteriol. 189:2653-2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mongkolsuk, S., and J. D. Helmann. 2002. Regulation of inducible peroxide stress responses. Mol. Microbiol. 45:9-15. [DOI] [PubMed] [Google Scholar]
- 39.Ojaimi, C., C. Brooks, S. Casjens, P. Rosa, A. Elias, A. Barbour, A. Jasinskas, J. Benach, L. Katona, J. Radolf, M. Caimano, J. Skare, K. Swingle, D. Akins, and I. Schwartz. 2003. Profiling of temperature-induced changes in Borrelia burgdorferi gene expression by using whole genome arrays. Infect. Immun. 71:1689-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ojaimi, C., V. Mulay, D. Liveris, R. Iyer, and I. Schwartz. 2005. Comparative transcriptional profiling of Borrelia burgdorferi clinical isolates differing in capacities for hematogenous dissemination. Infect. Immun. 73:6791-6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pal, U., X. Yang, M. Chen, L. K. Bockenstedt, J. F. Anderson, R. A. Flavell, M. V. Norgard, and E. Fikrig. 2004. OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J. Clin. Invest. 113:220-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Posey, J. E., and F. C. Gherardini. 2000. Lack of a role for iron in the Lyme disease pathogen. Science 288:1651-1653. [DOI] [PubMed] [Google Scholar]
- 43.Revel, A. T., A. M. Talaat, and M. V. Norgard. 2002. DNA microarray analysis of differential gene expression in Borrelia burgdorferi, the Lyme disease spirochete. Proc. Natl. Acad. Sci. USA 99:1562-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosa, P. A., K. Tilly, and P. E. Stewart. 2005. The burgeoning molecular genetics of the Lyme disease spirochaete. Nat. Rev. Microbiol. 3:129-143. [DOI] [PubMed] [Google Scholar]
- 45.Samuels, D. S. 1995. Electrotransformation of the spirochete Borrelia burgdorferi. Methods Mol. Biol. 47:253-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwan, T. G., J. Piesman, W. T. Golde, M. C. Dolan, and P. A. Rosa. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. USA 92:2909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seshu, J., J. A. Boylan, F. C. Gherardini, and J. T. Skare. 2004. Dissolved oxygen levels alter gene expression and antigen profiles in Borrelia burgdorferi. Infect. Immun. 72:1580-1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seshu, J., J. A. Boylan, J. A. Hyde, K. L. Swingle, F. C. Gherardini, and J. T. Skare. 2004. A conservative amino acid change alters the function of BosR, the redox regulator of Borrelia burgdorferi. Mol. Microbiol. 54:1352-1363. [DOI] [PubMed] [Google Scholar]
- 49.Seshu, J., M. D. Esteve-Gassent, M. Labandeira-Rey, J. H. Kim, J. P. Trzeciakowski, M. Hook, and J. T. Skare. 2006. Inactivation of the fibronectin-binding adhesin gene bbk32 significantly attenuates the infectivity potential of Borrelia burgdorferi. Mol. Microbiol. 59:1591-1601. [DOI] [PubMed] [Google Scholar]
- 50.Shi, Y., Q. Xu, K. McShan, and F. T. Liang. 2008. Both decorin-binding proteins A and B are critical for the overall virulence of Borrelia burgdorferi. Infect. Immun. 76:1239-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skare, J. T., D. M. Foley, S. R. Hernandez, D. C. Moore, D. R. Blanco, J. N. Miller, and M. A. Lovett. 1999. Cloning and molecular characterization of plasmid-encoded antigens of Borrelia burgdorferi. Infect. Immun. 67:4407-4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stevenson, B., T. G. Schwan, and P. A. Rosa. 1995. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect. Immun. 63:4535-4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Storz, G., and J. A. Imlay. 1999. Oxidative stress. Curr. Opin. Microbiol. 2:188-194. [DOI] [PubMed] [Google Scholar]
- 54.Tilly, K., A. Bestor, M. W. Jewett, and P. Rosa. 2007. Rapid clearance of Lyme disease spirochetes lacking OspC from skin. Infect. Immun. 75:1517-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tokarz, R., J. M. Anderton, L. I. Katona, and J. L. Benach. 2004. Combined effects of blood and temperature shift on Borrelia burgdorferi gene expression as determined by whole genome DNA array. Infect. Immun. 72:5419-5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weening, E. H., N. Parveen, J. P. Trzeciakowski, J. M. Leong, M. Hook, and J. T. Skare. 2008. Borrelia burgdorferi lacking DbpBA exhibits an early survival defect during experimental infection. Infect. Immun. 76:5694-5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang, X., M. S. Goldberg, T. G. Popova, G. B. Schoeler, S. K. Wikel, K. E. Hagman, and M. V. Norgard. 2000. Interdependence of environmental factors influencing reciprocal patterns of gene expression in virulent Borrelia burgdorferi. Mol. Microbiol. 37:1470-1479. [DOI] [PubMed] [Google Scholar]
- 58.Yang, X. F., S. M. Alani, and M. V. Norgard. 2003. The response regulator Rrp2 is essential for the expression of major membrane lipoproteins in Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA 100:11001-11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang, X. F., M. C. Lybecker, U. Pal, S. M. Alani, J. Blevins, A. T. Revel, D. S. Samuels, and M. V. Norgard. 2005. Analysis of the ospC regulatory element controlled by the RpoN-RpoS regulatory pathway in Borrelia burgdorferi. J. Bacteriol. 187:4822-4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zheng, M., F. Aslund, and G. Storz. 1998. Activation of the OxyR transcription factor by reversible disulfide bond formation. Science 279:1718-1721. [DOI] [PubMed] [Google Scholar]