Abstract
Enterotoxigenic Escherichia coli (ETEC) strains are a major cause of diarrheal disease in humans and farm animals. E. coli fimbriae, or colonization factor antigens (CFAs), and enterotoxins, including heat-labile enterotoxins (LT) and heat-stable enterotoxins (ST), are the key virulence factors in ETEC diarrhea. Unlike fimbriae or LT, STa has not often been included as an antigen in development of vaccines against ETEC diarrhea because of its poor immunogenicity. STa becomes immunogenic only after being coupled with a strongly immunogenic carrier protein. However, native or shorter STa antigens either had to retain toxic activity in order to become antigenic or elicited anti-STa antibodies that were not sufficiently protective. In this study, we genetically mutated the porcine LT (pLT) gene for a pLT192(R→G) toxoid and the porcine STa (pSTa) gene for three full-length pSTa toxoids [STa11(N→K), STa12(P→F), and STa13(A→Q)] and used the full-length pLT192 as an adjuvant to carry the pSTa toxoid for pLT192:pSTa-toxoid fusion antigens. Rabbits immunized with pLT192:pSTa12 or pLT192:pSTa13 fusion protein developed high titers of anti-LT and anti-STa antibodies. Furthermore, rabbit antiserum and antifecal antibodies were able to neutralize purified cholera toxin (CT) and STa toxin. In addition, preliminary data suggested that suckling piglets born by a sow immunized with the pLT192:pSTa13 fusion antigen were protected when challenged with an STa-positive ETEC strain. This study demonstrated that pSTa toxoids are antigenic when fused with a pLT toxoid and that the elicited anti-LT and anti-STa antibodies were protective. This fusion strategy could provide instructive information to develop effective toxoid vaccines against ETEC-associated diarrhea in animals and humans.
Enterotoxigenic Escherichia coli (ETEC) strains, which colonize host small intestines and produce one or more enterotoxins, are the major cause of diarrheal disease in humans and farm animals. The virulence determinants of ETEC in diarrhea include fimbrial adhesins and enterotoxins (1, 6, 10, 27, 28, 37, 44, 48). Fimbrial adhesins mediate attachment of bacteria to host epithelium cells and facilitate subsequent bacteria colonization. Enterotoxins, including heat-stable enterotoxins (STa and STb) and heat-labile enterotoxins (LT) (19, 20, 35), disrupt intestinal fluid homeostasis and cause fluid and electrolyte hypersecretion through activation of adenyl cyclase (by LT) or guanylate cyclase (by STa) in small intestinal epithelial cells (21, 26). ETEC strains isolated from young pigs with diarrhea express LT, STa, STb, Stx2e, and enteroaggregative E. coli ST type 1 (EAST1), alone or combined (10, 15, 50). Recent experimental studies indicated that porcine ETEC strains expressing LT, STb, or STa alone are sufficiently virulent to cause diarrhea in young pigs (6, 48, 49).
Porcine ETEC-associated diarrhea, especially postweaning diarrhea (PWD), causes a substantial economic loss to swine producers worldwide (18, 41). Currently, there are no vaccines available to effectively protect weaned pigs against ETEC infections. Experimental vaccines developed from fimbrial antigens alone showed only limited protection against ETEC strains (42). In addition, ETEC fimbriae are antigenically different. Thus, experimental vaccines developed from one specific fimbria could not provide protection against an ETEC strain expressing a different fimbria (42, 43). Moreover, recent evidence suggests that fimbriae may not function as protective antigens in the setting of naturally acquired infections and reinfections (7). Consequently, enterotoxin antigens have been reemphasized for ETEC vaccine development (43). Antitoxin vaccines currently under development largely use LT or its B subunit antigens because they are strongly immunogenic. The ST antigen cannot be used directly as a vaccine component because of its poor immunogenicity, unless it is coupled to a carrier protein and presented as a fusion or a chimeric protein (13, 22, 31, 38). Although a recent study suggested that anti-LT immunity may provide broader protection (14), experimental vaccine studies indicated that the induced anti-LT immunity provided protection only against LT-producing ETEC strains and not against ETEC strains that produce STa toxin (12, 13). As over two-thirds of human ETEC diarrhea cases and more than one-quarter of porcine ETEC diarrhea cases are caused by STa-producing ETEC strains (15, 16, 29, 30, 36, 45, 50), STa antigens must be included for developing broadly effective vaccines against ETEC infection.
Porcine STa (pSTa), a protein that consists of 18 amino acids (human STa [hSTa] consists of 19 amino acids), is poorly immunogenic (35, 41). To include pSTa as a vaccine component, we need to enhance pSTa immunogenicity. In addition, a native pSTa is not suitable to be used in developing safe vaccines because it is sufficiently toxic to cause diarrhea. Therefore, we need to attenuate the pSTa toxicity. It has been reported that shorter synthetic hSTa peptides or hSTa with disulfide bonds disrupted showed toxicity reduction (4, 5, 19, 40, 46, 47). Moreover, several shorter synthetic hSTa peptides that had the 12th, 13th, or 14th amino acid residue replaced showed a great reduction in toxicity (46, 47). However, these shorter synthetic or disulfide bond-disrupted hSTa peptides either had not been characterized for immunogenicity or failed to induce protective immunity. Only when a shorter hSTa or an hSTa mutant (with disulfide bonds disrupted) was genetically fused to a carrier protein, such as the B subunit of cholera toxin (CT) or hLT, did the hSTa antigen become immunogenic (9, 31, 32, 33). However, anti-STa immunity from these fusion antigens was not sufficiently characterized, and retention of STa toxicity in these fusion proteins could cause safety concerns for their application in vaccine development (7, 9).
No studies have been conducted to enhance pSTa immunogenicity and its potential application in vaccines against porcine ETEC infections. In this study, we mutated the porcine estA gene at nucleotides encoding the 11th, 12th, and 13th amino acids (which are homologous to the 12th, 13th, and 14th amino acids of hSTa) for three pSTa toxoids. These pSTa toxoids, which had all disulfide bonds retained, showed a great reduction in toxicity when examined in vitro (by cyclic GMP [cGMP] enzyme-linked immunosorbent assay [ELISA]) and in vivo (by porcine gut loop assay and challenge studies in a piglet model). We then genetically fused the mutated full-length porcine eltAB and estA genes for pLT192:pSTa-toxoid fusion proteins. Purified toxoid fusion antigens were used to immunize adult rabbits to assess anti-LT and anti-STa antigenicity and antibody neutralization and to immunize a pregnant sow to preliminarily evaluate anti-STa immunity in protection against infection from an STa-producing ETEC strain.
MATERIALS AND METHODS
Bacterial strains and plasmids.
A porcine E. coli field isolate, G58-2 (11), was used for constructing experimental strains in this study. Two plasmids expressing 987P fimbriae, pDMS167 (34) and pDMS158 (8), which were kindly provided by D. Schifferli of the University of Pennsylvania, were used to express 987P fimbriae in G58-2 and porcine STa constructs. Plasmid pACYC184 (Promega, Madison, WI) was used to clone and express the recombinant, each mutated porcine STa gene, and the LT and STa chimeric genes, whereas a TOPO TA cloning vector (Invitrogen, Carlsbad, CA) was used for fusion protein expression and purification. Plasmid pUC19 (Promega, Madison, WI) was also used to clone the STa gene for producing an STa challenge strain that had higher toxin expression. All constructs were cultured in LB medium supplemented with chloramphenicol (20 μg/ml) or ampicillin (50 μg/ml) (Table 1).
TABLE 1.
Escherichia coli strains and plasmids used in the studya
| Strain | Relevant properties | Plasmid | Reference |
|---|---|---|---|
| G58-2 | Nonpathogenic porcine E. coli field isolate | 11 | |
| 04-21018 | Porcine ETEC isolate | F18/STa/STb/Stx2e | 50 |
| 8221 | LT192 construct, 1836-2/pLT192 | pLT192/pBR322 | Zhang and Francis, submitted |
| 8227 | Host strain, G58-2/987P | pDMS167 | This study |
| 8795 | Host strain, G58-2/987P | pDMS158 | This study |
| 8331 | Control, G58-2/987P/pACYC184 | pACYC184 | This study |
| 8330 | pSTa recombinant, G58-2/987P/STa | pSTa/pACYC184 | This study |
| 8413 | pSTa11 mutant, G58-2/987P/STa11 | pSTa11/pACYC184 | This study |
| 8415 | pSTa12 mutant, G58-2/987P/STa12 | pSTa12/pACYC184 | This study |
| 8417 | pSTa13 mutant, G58-2/987P/STa13 | pSTa13/pACYC184 | This study |
| 8474 | pLT192:pSTa12, G58-2/987P/LT192:pSTa12 | pLT192:pSTa12/pACYC184 | This study |
| 8475 | pLT192:pSTa13, G58-2/987P/LT192:pSTa13 | pLT192:pSTa13/pACYC184 | This study |
| 8552 | pLT192:pSTa12, TOPO 10/LT192:STa12 | pLT192:pSTa12/TA vector | This study |
| 8554 | pLT192:pSTa13, TOPO 10/LT192:STa13 | pLT192:pSTa13/TA vector | This study |
| 8823 | pSTa challenge strain, G58-2/987P/pSTa | pSTa/pUC19 | This study |
The porcine E. coli field isolate G58-2 was used as a parental strain and was transformed with plasmid pDMS167 (34) or pDMA158 (8) to express 987P fimbriae (G58/987P). G58/987P was further transformed with plasmids expressing a native pSTa, a mutated pSTa, or a pLT192:STa-toxoid chimeric plasmid to give a porcine STa recombinant strain, three STa mutant strains, and pLT192:pSTa12 and pLT192:pSTa13 fusion constructs. In addition, TOPO 10 cells and TA cloning vectors (Invitrogen) were used for expression and purification of the pLT192:pSTa12 and pLT192:pSTa13 fusion proteins.
Porcine estA gene cloning and mutation.
The porcine STa gene (estA) was PCR amplified using genomic DNA from porcine ETEC field isolate 04-21018 (F18+ STa+ STb+ Stx2e+) and the designed primers STaSfcI-F (no. 5 in Fig. 1) and STaEagI-R (no. 4) (for cloning in the pACYC184 vector) or STaHindIII-F and STaBamH1-R (for cloning in pUC19 vector) (Table 2). Each forward primer contains an SfcI or a HindIII restriction site, whereas the reverse primer has an EagI or a BamHI restriction site. PCR was performed in a PTC-100 thermal cycler (Bio-Rad, Hercules, CA) in a 50-μl reaction mixture containing 1× Pfu DNA polymerase buffer (with Mg2+), 200 nM deoxynucleoside triphosphates (dNTP), 0.5 μM of each forward and reverse primer, and 1 unit of Pfu DNA polymerase (Stratagene, La Jolla, CA). Amplified products were separated by 1.5% agarose (FMC Bioproducts, Rockland, ME) gel electrophoresis and purified using a QIAquick gel extraction kit according to the manufacturer's instruction (Qiagen, Valencia, CA). Purified PCR products and plasmids pACYC184 and pUC19 were digested sequentially with SfcI and EagI or with HindIII and BamHI restriction enzymes (New England Biolabs, Ipswich, MA), respectively. Digested estA gene products and vectors were purified and then ligated with T4 DNA ligase (New England BioLabs). Two microliters of ligation product was introduced into 987P fimbrial E. coli construct 8227 (G58-2/pDMS167, to host plasmids with STa cloned in pACYC184) or 8795 (G58-2/pDMS158, to host plasmids with STa cloned in pUC19) competent cells by standard electroporation (3). Positive colonies were screened by PCR initially and then sequenced to ensure that the cloned gene was inserted in the correct reading frame.
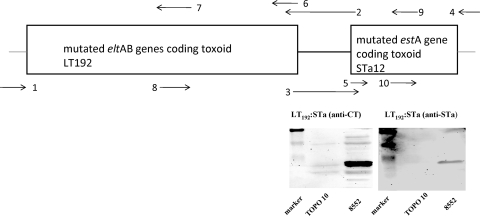
FIG. 1.
Construction of a porcine LT192:STa-toxoid genetic fusion. PCR primers 1(184EcoRV-F) and 6 (LT-R) amplified the entire porcine eltAB genes (without a stop codon), and primers 5 (STa-F) and 4 (pBREagI-R) amplified the full-length porcine estA gene (without a signal peptide). Primers 7 (LT192-R) and 8 (LT192-F; complementary to primer 7) mutated eltAB genes for LT192, and primers 10 (mSTa12-R) and 9 (mSTa12-F; complementary to primer 10) mutated the STa gene to produce an STa mutant. Primers 2 (pLT:STa-R) and 3 (pLT:STa-F) added a Gly-Pro linker and genetically fused the mutated LT genes and the mutated STa gene. The gene sizes are not proportional. The lower right panels show Western blot detection of toxoid fusions using anti-CT and anti-STa antibodies, and total protein samples from TOPO cells were used as a negative control.
TABLE 2.
PCR primers used to construct STa recombinant, STa mutant, and pLT192:pSTa12 and pLT192:pSTa12 chimeric strainsa
| Primer | Sequence (5′ to 3′) | Description |
|---|---|---|
| 184EcoRV-F | GTCAGGCACCGTGTATGAAAT | Plasmid pACYC184 sequence at the EcoRV site |
| pBREagI-R | GTCCCTGATGGTCGTCATCT | Plasmid pACYC 184 sequence downstream at EagI site |
| STaSfcI-F | GTGAAACAACCTGTAGGGA | To amplify native estA gene, cloned into pACYC184 |
| STaEagI-R | GTGGAGCCGGCCGAAACA | To amplify native estA gene, cloned into pACYC184 |
| STaHindIII-F | TGCAAAATAAGCTTAACTAATCTC | To amplify native estA gene, cloned into pUC19 |
| STaBamH1-R | GTGGAGCCGGATCCAACAG | To amplify native estA gene, cloned into pUC19 |
| pSTa11K-F | GAACTTTGTTGTAAACCTGCCTGT | Pairs with pBREagI-R to mutate the 11th amino acid |
| pSTa11K-R | ACAGGCAGGTTTACAACAAAGTTC | Pairs with 184EcoRV-F to mutate the 11th amino acid |
| pSTa12F-F | CTTTGTTGTAATTTTGCCTGTGCC | Pairs with pBREagI-R to mutate the 12th amino acid |
| pSTa12F-R | GGCACAGGCAAAATTACAACAAAG | Pairs with 184EcoRV-F to mutate the 12th amino acid |
| pSTa13Q-F | TGTTGTAATCCTCAGTGTGCTGGA | Pairs with pBREagI-R to mutate the 13th amino acid |
| pSTa13Q-R | TCCAGCACACTGAGGATTACAACA | Pairs with 184EcoRV-F to mutate the 13th amino acid |
| pLT:STa-F | GCAATCAGTGGGCCGGGGCCCATGAACAACTTTTACTGCTG | 3′ end of LT + linker + 5′ end of STa |
| pLT:STa-R | GTTGTTCATGGGCCCCGGCCCACTGATTGCCGCAATTGAATTGG | 5′ end of STa + linker + 3′ end of LT |
| STa12NS-R | ATAACATCCAGCACAGGCAAAATT | STa12 mutant without stop codon, for TA cloning |
| ST13NS-R | ATAACATCCAGCACACTGAGG | STa13 mutant without stop codon, for TA cloning |
| LT-R | GTTTTTCATACTGATTGCCCCAATTG | To amplify LTAB genes without stop codon |
| STa-F | AACAACACATTTTACTGCTGTG | To amplify the STa gene without signal peptide |
| hLT-F | ATGATTGACATCATGTTGCATATAGG | To amplify LT192:mSTa fusion genes, for TA cloning |
Bold indicates the target amino acid to be mutated for STa toxoids, underlining indicates the enzyme restriction site, and italic indicates the Gly-Pro-Gly-Pro linker. Primers pLT:STa-F and pLT:STa-R were used to fuse the mutated porcine eltAB (encoding pLT192 protein) with the mutated porcine estA (encoding STa12 or STa13 protein) genes.
To replace the 11th, 12th, and the 13th amino acids of the pSTa toxin, we designed specific PCR primers to mutate nucleotides encoding these three residues (Table 2). For an STa11 toxoid gene, we used recombinant STa strain DNA as the template and amplified the 5′ end of the estA gene in a PCR using the 184EcoRV-F and the pSTa11K-R primers (no. 9) and the 3′ end of the estA gene in another PCR with the pSTa11K-F (no. 10, complementary to the pSTa11K-R primer) and pBREagI-R primers. We then overlapped the 5′-end and the 3′-end fragments in a splice overlap extension (SOE) PCR to introduce a mutation at nucleotides encoding the 11th amino acid residue of the estA gene product (Fig. 1). Similarly, we mutated the estA gene at nucleotides encoding the 12th and 13th amino acids with the respective primers (Table 2).
STa competitive ELISA to detect STa proteins expressed by the recombinant and mutant strains.
Growth from an overnight culture of an STa recombinant, each of three mutant strains, and a negative control strain was used for STa competitive ELISA as described previously (25, 49). Briefly, each strain was cultured in LB medium overnight, and culture growth was measured by optical density (OD). Equivalent amounts of cells from each strain were used for subculture in 4 AA medium (25), and 4 AA culture supernatants were used for ELISA. An STa ELISA plate was coated with STa ovalbumin conjugate (1.25 ng per well; laboratory of D. C. Robertson) overnight at 37°C and blocked with 2.5% casein blocking buffer (2.5% casein in 0.3 N NaOH, pH 7.0). Seventy-five microliters of culture supernatant from each strain (in triplicate) and 75 μl of anti-STa serum (1:10,000; Robertson laboratory) were mixed and added to each well, followed by incubation at 37°C for 2 h on a shaker (180 rpm). After three washes, plates were blotted to dry and incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit immunoglobulin G (IgG) (1:10,000; Sigma, St. Louis, MO) at 37 C for 1 h, and the reaction of bound IgG with ABTS [2,2′-azinobis(ethylbenzthiasoline sulfonic acid)] substrate was measured at 405 nm.
Cyclic GMP ELISA to detect toxicity of STa proteins.
Toxicity of the recombinant and mutated STa proteins was tested by stimulation of intracellular cGMP levels in T-84 cells (ATCC CCL-248). Bacterial culture growth supernatant was used for cGMP ELISA with a direct cyclic GMP enzyme immunoassay (EIA) kit (Correlate EIA, acetylated version; Assay Designs, MI) according to the manufacturer's instruction. Briefly, 1 × 105 T-84 cells were seeded and cultured in each well of a 24-well plate. After removing the Dulbecco's modified Eagle medium (DMEM/F12; GIBCO/Invitrogen, Grand Island, NY), 75 μl overnight culture growth (in 4AA medium) supernatant from each strain was added to each well (in duplicate). Cells were lysed with 200 μl (per well) 0.1 M HCl after 2 h of incubation. One hundred microliters of lysis product was mixed with conjugates and antibody reagents supplied in the kit, and the mixture was added to each well of a supplied EIA plate. After incubation on a shaker (500 rpm) at room temperature for 2 h, plates were washed, blotted dry, and reacted with p-nitrophenyl phosphate (pNpp) (disodium salt) substrate solution. The OD was measured at 405 nm after 20 min of development.
Detection of STa biological activity in porcine ligated intestinal loops and animal challenge studies.
The biological activity of the STa proteins in the recombinant and each of the mutant strains was examined in a porcine ligated loop assay. Fifteen loops were made through ileum and jejunum sections, and 2 × 109 CFU of culture growth from the recombinant, each mutant strain, and a control strain was injected into each ligated loop. At 8 h postinoculation, the length of each loop (cm) and amount of fluid accumulated in each loop (g) were measured. The ratio of fluid accumulation to loop length (g/cm) was calculated as an index of enterotoxic activity.
The toxicity of each STa protein was also examined in animal challenge studies. Twenty 3-day-old gnotobiotic piglets were randomly divided into five groups. Each group was orally inoculated with 3 × 109 CFU of an overnight-grown culture of each of the three mutant strains, the STa recombinant, or the negative control strain. During the 24-h postinoculation period, piglets were closely monitored for clinical signs of disease, including vomiting, diarrhea, dehydration, lateral recumbence, and lethargy.
All animal studies in this project complied with the Animal Welfare Act, followed the Guide for the Care and Use of Laboratory Animals (21a), and were approved and supervised by South Dakota State University's Institutional Animal Care and Use committee.
Construction of pLT192:pSTa12 and pLT192:pSTa13 chimeric genes.
Recombinant porcine eltAB genes encoding pLT toxin and mutated eltAB genes encoding pLT192 variants had been cloned in our laboratory (W. Zhang and D. H. Francis, submitted for publication). A Gly-Pro-Gly-Pro (GGGCCGGGGCCC) linker (2) was used to connect the mutated eltAB and estA genes. PCR primers pLT:STa-R (no. 2) and pSTa:LT-F (no. 3) were specifically designed so that they contained nucleotides of the 3′ end of the eltAB genes (without the stop codon), the linker, and the 5′ end of the estA gene (without the signal peptide). A PCR using primer 184EcoRV-F (no. 1) and pLT:STa-R with plasmid pLT192 as the template amplified the mutated eltAB genes, the linker, and the 5′ end of the mutated estA gene. A second PCR using pSTa:LT-F and pBREagI-R (no. 4) primers and DNA from the STa mutant plasmid pSTa12 generated the fragment covering the 3′ end of the mutant eltAB genes (no stop codon), the linker, and the estA gene of mutant STa12 (no signal peptide). An SOE PCR connected the mutated eltAB and the mutated estA genes with the linker for a chimeric gene (pLT192:pSTa12) (Fig. 1). This resultant chimeric gene was further amplified with 184EcoRV-F and pBREagI-R primers and then digested with SfcI and EagI enzymes. Digested products were purified and cloned into vector pACYC184 at the SfcI and EagI sites with T4 DNA ligase. Two microliters of T4 ligation products was introduced into 8227 (G58/987P) host cells by standard electroporation. Positive colonies were screened by PCR, and then the DNA was sequenced to ensure that the cloned pLT192:pSTa12 fusion gene was inserted in the reading frame.
Similarly, a pLT192:pSTa13 chimeric gene was constructed using plasmid pSTa13 as the template. This pLT192:pSTa13 chimeric gene was also cloned into vector pACYC184 and expressed in 8227 host cells. In addition, chimeric genes pLT192:pSTa12 and LT192:pSTa13 were amplified by hLT-F paired with STa12NS-R and STa13NS-R (Table 2), cloned into the TA cloning vector pBAD-TOPO, and expressed in TOPO 10 cells for protein purification by using a His tag (Invitrogen).
Detection of LT and STa in pLT192:pSTa12 and pLT192:pSTa13 fusion proteins.
The pLT192:pSTa12 and pLT192:pSTa13 constructs were grown in Casamino Acids and yeast extract broth with lincomycin (45 μg/ml) and ampicillin (50 μg/ml) overnight at 37°C. The overnight-grown culture was centrifuged at 3,000 × g for 20 min, and pellets were collected for total protein preparation using bacterial protein extraction reagent (B-PER) (in phosphate buffer; Pierce, Rockford, IL).
Thirty microliters of each total protein sample was used to detect LT and STa by standard sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblot assay (3). The transferred membrane blot was blocked with 2% fat-free milk overnight at 4°C and then incubated with anti-CT (1:3,000; Sigma) and anti-STa (1:5,000; Robertson laboratory) sera. After three washes, the membranes were incubated with HRP-conjugated goat anti-rabbit IgG (1:5,000; Sigma) for 1 h. After a final round of washes, peroxidase bound to the fusion proteins on the membranes was detected with a SuperSignal West Pico chemiluminescent substrate kit (Pierce).
Rabbit immunization with purified pLT192:pSTa12 and pLT192:pSTa13 fusion proteins.
Fusion proteins pLT192:pSTa12 and pLT192:pSTa13 expressed in E. coli TOPO 10 cells were purified using B-PER (Pierce) and Ni-nitrilotriacetic acid (NTA) agarose (Qiagen) according to the manufacturer's protocols. Briefly, overnight culture growth (in Casamino Acids and yeast medium) was centrifuged, and the resultant pellets were lysed in B-PER reagent and sonicated. Total proteins from cell lysis were incubated with Ni-NTA resin, followed by washes and elution. The 6×His-tagged TA-cloned fusion proteins were purified and stored at −20°C until use.
Two adult rabbits were immunized intramuscularly with 100 μg of purified pLT192:pSTa12 and another two rabbits with purified pLT192:pSTa13 fusion proteins, in an equal volume of Freund's incomplete adjuvant (Sigma). Two booster injections were given at biweekly intervals. One rabbit without immunization served as the negative control. Blood and fecal samples were collected before and 14 days after each immunization. Collected serum and resuspended fecal samples were stored at −80°C until use.
Rabbit serum antibody titration.
Cholera toxin (CT) (Sigma) and STa ovalbumin conjugates were used as antigens to titrate anti-LT and anti-STa antibodies from rabbit serum and fecal samples. For anti-LT192 antibody titration, we coated an ELISA plate with GM1 (400 ng/well) for GM1 ELISA as described previously (48). Rabbit antiserum (diluted 1:50 in PBS, in triplicate) was used as the primary antibodies (in a binary dilution) and HRP-conjugated goat anti-rabbit IgG as the secondary antibodies. To titrate anti-STa antibodies, we coated an ELISA plate with STa ovalbumin conjugates (1.25 ng/well) and used rabbit antiserum or antifecal antibody samples (diluted 1:50 in STa ELISA buffer, in triplicate) as the primary antibodies and HRP-conjugated goat anti-rabbit IgG or IgA as the secondary antibodies. The OD was measured at 405 nm after 20 min of development in peroxidase substrates (KPL). The titration end point was determined as the reciprocal of the interpolated dilution giving an OD of above 0.4 after subtraction of background (23; Zhang and Francis, submitted). Antibody titers were expressed as the log10 of the reciprocal dilution.
Anti-LT and anti-STa antibody neutralization.
Neutralization of CT toxin by rabbit antiserum and antifecal antibodies was examined using a cyclic AMP (cAMP) EIA kit (Invitrogen) and T-84 cells. For anti-STa antibody neutralization, we used a cGMP EIA kit (Assay Design, Ann Arbor, MI) and T-84 cells. T-84 cells were cultured as described above. Ten nanograms of CT or 2 ng of STa (diluted in 150 μl DMEM/F12 medium) was incubated with 150 μl antiserum or antifecal antibodies (1:50 dilution in DMEM/F12 medium, in triplicate) at room temperature. After 1 h of incubation, the mixture (150 μl of diluted CT or STa toxin and 150 μl of diluted antiserum or antifecal antibodies) was added to each well, and the plate was further incubated at 37°C in 5% CO2 for 2 h. After another wash, the cells were lysed with 0.1 M HCl (200 μl per well) and then neutralized with 0.1 M NaOH. The cell lysis product was collected by centrifugation at 660 × g for 10 min at room temperature. The resultant supernatants were tested for intracellular cAMP or cGMP levels according to the manufacturer's protocols.
Immunization of a pregnant sow and piglet challenge studies.
Two pregnant sows from an isolated hog farm that had no ETEC diarrhea outbreak were used in this study. Sow 1-4 was immunized with 0.5 mg purified LT192:STa13 fusion protein in an equal volume of Freund's complete adjuvant at 6 to 8 weeks before farrowing and then given a boost injection with the same amount protein in incomplete adjuvant 4 weeks late. The other pregnant sow (25-3), which was not immunized, was used as a control. Serum samples from both sows were examined for preexisting anti-LT or anti-STa antibodies. Sows were transported from a farm to our animal research facility a few days before farrowing and were raised separately in an isolated and disinfected room. Colostrum samples were collected from each sow to test anti-LT and anti-STa antibody production. Two-day-old suckling piglets were taken away from the mother momentarily, orally inoculated with 2 × 109 CFU overnight culture growth of the STa challenge strain 8823, and brought back to the mother. Piglets were observed every 4 h for the following 72 h. At the end of 72 h, all piglets underwent necropsy, and blood and small intestinal samples were collected for antigenicity and colonization studies.
Statistical analysis.
Data were analyzed by using the mixed procedure (SAS for Windows, version 8; SAS Institute, Cary, NC), and Student's t test was used to compare the different treatment groups. Calculated P values of <0.05 were regarded as significant.
RESULTS
Twelve strains, i.e., an STa recombinant (8330), three STa mutants (8413, 8415, and 8417), four LT and STa toxoid fusion strains (8474, 8475, 8552, and 8554), two host strains (8227 and 8795), a negative control (8331), and an STa challenge strain (8823), were constructed for this study (Table 1). After confirming the expression of STa toxoid proteins and assessing the toxicity and biological activity of each toxoid, we selected STa12 and STa13 toxoids for construction of toxoid fusions (Fig. 1). Both resultant toxoid fusions were used to immunize adult rabbits but only the LT192:STa13 fusion for a pregnant sow. Elicited anti-LT and anti-STa antibodies were titrated and examined for activity in neutralizing CT and STa toxins, and anti-STa antibodies were tested preliminarily for protection against an STa-producing ETEC strain.
STa proteins were expressed by all STa recombinant and mutant strains.
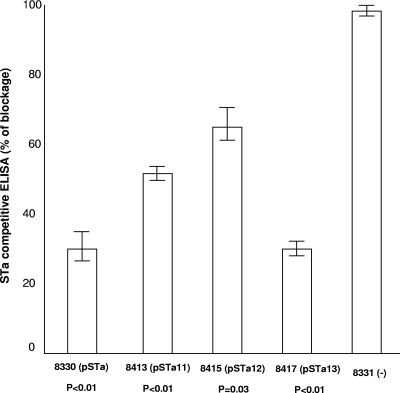
STa proteins were detected in the STa recombinant and each of the three mutant strains. Overnight-grown 4AA culture supernatant samples from the recombinant STa strain (8330); the mutant STa11 (8413), STa12 (8415), and STa13 (8417) strains; and the negative control strain (8331) were used to compete with 1.25 ng of synthetic STa-ovalbumin conjugates (in 75 μl ELISA dilution buffer, precoated in each well) for anti-STa antibodies in an STa competitive ELISA. ELISA results indicated that precoated synthetic STa-ovalbumin conjugates bound 30%, 50%, 63%, and 29.2% of the anti-STa serum after competing with STa proteins from strains 8330, 8413, 8415, and 8417; these results differed significantly from that for the negative control strain 8331 (97%) (Fig. 2). By referring to the curve generated with a series of STa synthetic peptide standards (0, 0.01, 0.05, 0.10, 0.50, 1.0, 5.0, and 10 ng in 75 μl STa ELISA buffer), STa toxoid proteins expressed in strains 8413, 8415, and 8417 were estimated to be in a range from 1.3 ng/ml to 13 ng/ml.
FIG. 2.
STa competitive ELISA to detect expression of STa proteins in the STa recombinant 8330 (STa) and mutant strains 8413 (STa11), 8415 (STa12), and 8417 (STa13). A 1.25-ng portion of STa-ovalbumin conjugate was applied to each well; anti-STa serum (1:10,000) was used as the primary antibody and horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin (IgG; 1:10,000) was used as the secondary antibody. Optical densities were measured at 405 nm. Error bars indicate standard deviations.
STa toxoids expressed by STa mutant strains are no longer toxic.
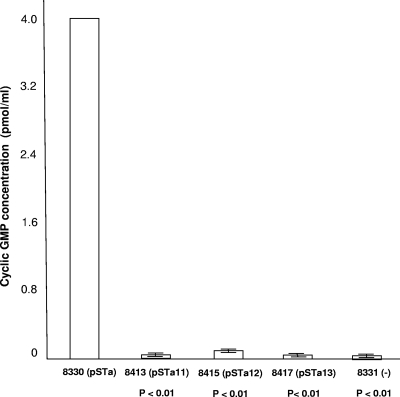
STa toxoid proteins expressed in all three mutant strains showed significant reductions in toxicity. Results from cGMP ELISA (acetylated version) indicated that intracellular cGMP concentrations in T-84 cells stimulated by 8330, 8413, 8415, 8417, and 8331 cultures were 4 ± 0, 0.0185 ± 0.0065, 0.043 ± 0.015, 0.017 ± 0.0015, and 0.012 ± 0.0005 pmol/ml, respectively (Fig. 3). All mutant strains showed low or no stimulation of cGMP levels compared to the recombinant strain, suggesting that these STa toxoid proteins had significantly reduced toxicity. Statistical analysis indicated that stimulation of intracellular cGMP in T-84 cells by the three STa toxoids was significantly lower than that by the recombinant STa (P < 0.01, P < 0.01, and P < 0.01). Among the three mutant strains, mutant STa12 (8415) showed the highest stimulation of cGMP (43 fmol/ml), whereas STa13 (8417) had the lowest stimulation of cGMP (17 fmol/ml), which was not significantly different from that for the negative control strain 8331 (12 fmol/ml; P = 0.25).
FIG. 3.
Cyclic GMP ELISA to detect toxicity of STa toxoids. Culture growth supernatant from STa toxoid strains was used to stimulate T-84 cells to produce an increase of intracellular cGMP levels by using a cGMP EIA kit (Assay Design). Error bars indicate standard deviations.
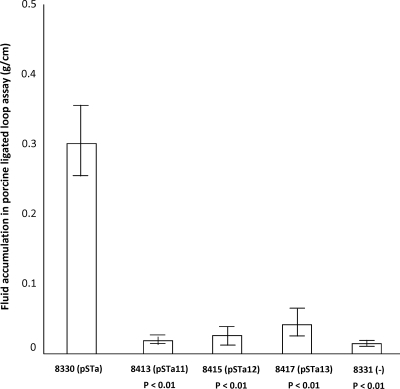
Results from the porcine ligated gut loop assay indicated that the STa toxoid proteins expressed by all three mutant strains did not stimulate fluid secretion. After 8 h of incubation, only loops incubated with the recombinant STa strain (8330) showed fluid accumulation (0.3 g/cm), whereas loops incubated with the 8413, 8415, and 8417 mutant strains had 0.02, 0.03, and 0.04 g/cm fluid accumulated, respectively. Fluid accumulation in loops incubated with the mutant strains and the negative control was significantly different from that in loops inoculated with the recombinant strain (P < 0.01) (Fig. 4).
FIG. 4.
Porcine ligated gut loop assay to detect STa toxoid toxic activity. A total of 2 × 109 CFU of 8330 (STa), 8413 (STa11), 8415 (STa12), 8417 (STa13), or negative control strain 8331 (−) was incubated in each loop (three repeats). After 8 h of incubation, fluid accumulated in each loop was measured, and a ratio of the accumulated fluid (g) to the loop length (cm) was used as an index. Error bars indicate standard deviations.
To test whether expressed STa toxoids were safe for young pigs, we challenged 3-day-old, 987P receptor-positive gnotobiotic pigs with the recombinant or each STa mutant strain. During 24 h postinoculation, only pigs in the group challenged with the STa recombinant strain developed diarrhea, whereas pigs inoculated with STa mutant strains remained completely healthy. To confirm that all piglets possessed 987P receptors, we collected small intestinal samples from each challenged pig at necropsy to prepare brush border vesicles for adherence assay. Brush border bacterial adherence assay indicated that all challenged pigs expressed 987P receptors. In addition, quantitative culture studies showed that colonization of mutant STa constructs ranged from 8.0 × 108 to 1.7 × 109 CFU per g of ileum tissue, suggesting that all mutant strains were well colonized in small intestines of the challenged pigs.
LT and STa toxoid fusions enhance STa immunogenicity.
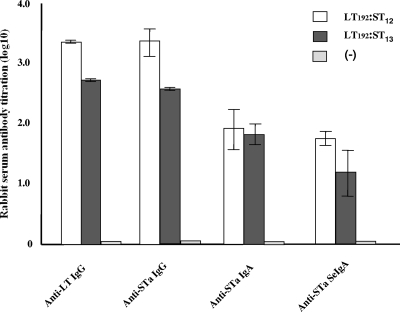
In this study, we selected toxoids pSTa12 and pSTa13 to construct LT and STa toxoid fusion proteins. The pSTa12 had the lowest recognition of anti-STa antiserum but stimulated the highest cGMP level in T-84 cells among the three toxoids. In contrast, pSTa13 was the best in recognition of anti-STa antibody and showed a lower stimulation of intracellular cGMP levels. Both LT and STa toxoids in pLT192:pSTa12 and pLT192:pSTa13 fusions were recognized by anti-CT and anti-STa antisera (Fig. 1). Antibody titration of serum samples from the rabbits immunized with purified pLT192:pSTa12 and pLT192:pSTa13 fusion antigens showed anti-STa IgG antibody titers of 3.33 ± 0.23 and 2.59 ± 0.01 (log10) and anti-STa IgA antibody titers of 1.92 ± 0.34 and 1.83 ± 0.17 (log10), respectively (Fig. 5). Anti-STa antibodies were detected in fecal samples as well. Antifecal anti-STa secretory IgA (S-IgA) antibody titers in rabbits immunized with pLT192:pSTa12 and pLT192:pSTa13 fusion antigens were 1.75 ± 0.14 and 1.26 ± 0.40, respectively. As expected, anti-LT antibodies (IgG) were detected at high titers (3.33 ± 0.02 and 2.71 ± 0.01 [log10]) (Fig. 5).
FIG. 5.
Antibody titration from serum and fecal samples of rabbits immunized with pLT192:pSTa12 or pLT192:pSTa13 fusion antigen. The titers (in log10) of anti-LT were detected in a LT GM1 ELISA using purified CT and antigen, with rabbit antiserum samples (1:50) as the primary antibody. To determine the titers of anti-STa antibody, we used STa ovalbumin conjugates as the antigen and rabbit antiserum and antifecal samples (1:50) as the primary antibodies. HRP-conjugated IgG and IgA antibodies (1:5,000) were used as the secondary antibodies. Optical densities of greater than 0.4 (after subtracting the background reading) were used to calculate antibody titers (in log10). Error bars indicate standard deviations.
Anti-LT and anti-STa antibodies neutralize CT and STa toxins.
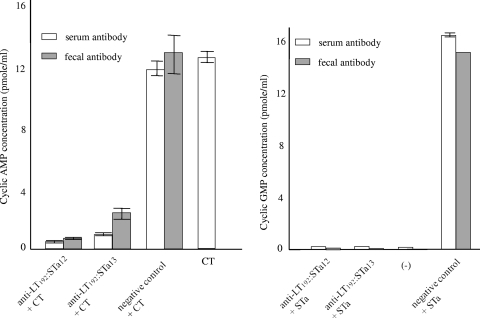
CT toxin (10 ng) was unable to stimulate an increase of intracellular cAMP levels in T-84 cell after being incubated with serum or fecal antibodies from rabbits immunized with pLT192:pSTa12 and pLT192:pSTa13 fusion antigens. In contrast, serum or fecal samples from the negative control rabbit did not prevent CT toxin from increasing cAMP levels in T-84 cells (Fig. 6). Cyclic AMP concentrations in cells treated with a mixture of CT and anti-pLT192:pSTa12 or anti-pLT192:pSTa13 serum were 0.4 ± 0.01 and 0.52 ± 0.04 pmol/ml, respectively, whereas the cAMP concentrations in cells treated with CT only or CT mixed with the serum sample from the negative control rabbit were 12 ± 0.25 and 12.7 ± 0.2 pmol/ml. Similarly, after incubation with anti-pLT192:pSTa12 and anti-pLT192:pSTa13 serum or fecal samples, 2 ng purified STa toxin did not increase intracellular cGMP levels in T-84 cells (Fig. 6). The intracellular cGMP concentration in cells treated with STa mixed with anti-pLT192:pSTa12 or anti-pLT192:pSTa13 antiserum were 0.17 ± 0.005 and 0.16 ± 0.004 pmol/ml. These cGMP levels were significantly lower than those in cells incubated with STa toxin and serum sample from the negative control rabbit (16.8 pmol/ml). Similar results were observed when STa toxin was incubated with antifecal antibodies (Fig. 6). The cGMP levels in cells treated with STa toxin and anti-pLT192:pSTa12 or anti-pLT192:pSTa13 fecal antibodies were 0.098 and 0.12 pmol/ml, respectively. That was similar to the cGMP level in cells treated with cell culture medium (0.13 ± 0.03 pmol/ml) but differed significantly from the cGMP concentration in cells incubated with STa toxin and the fecal sample of the negative control rabbit (15.6 pmol/ml).
FIG. 6.
Anti-LT and anti-STa antibody neutralization. Serum and fecal samples (1:50) from rabbits immunized with pLT192:pSTa12 or pLT192:pSTa13 fusion antigen were used to neutralize purified CT (10 ng) or STa (2 ng). The mixture was added to T-84 cells to test for an increase of intracellular cGMP (for STa; an ELISA kit from Assay Design was used) or cAMP (for CT; an ELISA kit from Invitrogen was used) levels. Cell culture medium alone was included as a negative control. CT or STa toxin alone or incubated with a serum or a fecal sample from the control rabbit was also included as a negative control. Error bars indicate standard deviations.
Suckling piglets born by an immunized sow are protected when challenged with an STa ETEC strain.
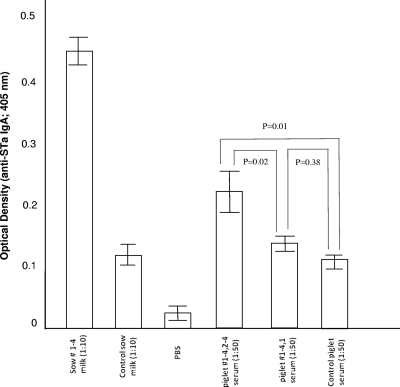
A very small litter, four piglets, was delivered from the immunized sow. After being orally challenged with strain 8823, only one piglet showed mild diarrhea during the following 72 h, whereas the other three piglets remained healthy. Anti-STa IgA ELISA showed that colostrum (1:10 dilution) from the immunized sow had an OD of 0.445 ± 0.028, which was significantly different from the OD in the colostrum from the negative control sow (0.122 ± 0.017; P = 0.01). Anti-STa IgA antibody was detected among three (of the four) piglets born by the immunized sow in an anti-STa IgA ELISA. Serum samples (1:50 dilution) from the three healthy piglets showed an OD of 0.202 ± 0.049, which is significantly different from the ODs from their diarrheal sibling (0.124 ± 0.024; P = 0.02) and the diarrheal control piglets (0.108 ± 0.025; P = 0.01) (Fig. 7).
FIG. 7.
ELISA detection of anti-STa IgA antibodies in colostrum samples from the sow immunized with LT192:STa12 antigen and a negative control sow and in serum samples from the piglets. We coated each well with 1.25 ng STa-ovalbumin conjugates and then added 100 μl of each sow colostrum sample (1:10) and piglet serum sample (1:50). HRP-conjugated goat anti-porcine IgA (1:3,000) was used as the secondary antibody. Optical densities were measured at 405 nm. Error bars indicate standard deviations.
DISCUSSION
The results from this study demonstrated for the first time that porcine STa toxoids can become immunogenic when carried by the strongly immunogenic LT toxoid LT192. Rabbits immunized with pLT192:pSTa12 and pLT192:pSTa13 fusion antigens produced neutralizing anti-LT and anti-STa antibodies. Since STa-producing ETEC strains are equivalent or even more virulent in causing diarrheal disease than LT-producing ETEC strains and since anti-LT immunity alone is not sufficiently effective in protecting against ETEC diarrhea (24), it becomes very clear that both STa and LT antigens must be included as essential components in developing broadly effective vaccines against ETEC. However, in contrast to the large and strongly immunogenic LT, STa produced by porcine or human ETEC strains is small and poorly immunogenic (35). Therefore, STa alone is unable to induce anti-STa immunity in hosts. Human STa protein became immunogenic when it was linked to a carrier protein chemically (22) or, more often, genetically (4, 9, 17, 31, 33), as the genetic fusion approach showed precise definition of the final products, easy manipulation, and low cost (9). When a native hSTa gene was genetically fused to a native human eltA gene (encoding the LTA subunit) or eltB gene (encoding the LTB subunit) or to an E. coli colonization factor antigen (CFA) gene, the fusion products were recognized by anti-STa antibodies (5, 31, 33). However, these LTA:STa and LTB:STa fusion proteins were not tested for STa immunogenicity or toxicity. Clements (9) demonstrated that antibodies obtained from mice immunized with a fusion could neutralize native STa toxin, but this fusion protein was generated by fusing the native hSTa gene at the 3′ end of the LTB gene, and whether such anti-STa antibodies were protective was not determined.
In contrast to the extensive studies of enhancement of hSTa toxin immunogenicity, no study has been conducted to facilitate pSTa immunogenicity or its potential application to induce anti-STa immunity. In addition, previous studies of hSTa antigens either did not address antigen toxicity, which could potentially compromise development of safe vaccines, or suggested that nontoxic STa antigens could not stimulate anti-STa immunity even when they were fused to a strongly immunogenic carrier protein. Batisson and Der Vartanian (4) suggested that only a native STa or STa peptides that retain toxicity were able to stimulate anti-STa antigenicity when they were jointed to the C terminus of the ClpG major subunit of E. coli CS31A fimbriae. A similar fusion protein but with a nontoxic, shorter STa peptide that had both the 13th and 14th amino acids replaced failed in stimulating anti-STa antigenicity (4). Our current study clearly demonstrated that the full-length STa toxoids with a single amino acid substitution were able to facilitate anti-STa antigenicity when they were genetically fused to a pLTAB toxoid, as rabbits immunized with the pLT192:pSTa12 or pLT192:pSTa13 fusion antigen showed high titers of anti-STa IgG (2.59 to 3.33 log10), IgA (1.83 to 1.92 log10) and S-IgA (1.26 to 1.75 log10). Furthermore, these antisera and antifecal antibodies can neutralize purified STa toxin, suggesting that the anti-STa antibodies are neutralizing. Clearly, our data did not agree with results from the study by Batisson and Der Vartanian (4), who suggested that a nontoxic STa peptide could not stimulate anti-STa immunogenicity even when fused with a CS31 major subunit. We believe that substitutions with different amino acids in a short STa peptide could resulted in a substantial alteration in STa protein structure and biological activity. In an early study, after replacement of the alanine by a leucine at the 14th amino acid, the hSTa showed the greatest reduction in toxicity and the STa14(A→L) toxoid was three times less toxic than the STa14(A→Q), which is equivalent to our pSTa13 toxoid (19). Indeed, Batisson and Der Vartanian reported that the STa toxoid that had both amino acids 13 and 14 replaced was no longer recognized by anti-STa antibodies (see Fig. 1 and 2 in reference 4). The STa toxoids pSTa12(P→F) and pSTa13(A→Q) constructed in our study had only one amino acid replaced, and both toxoids were recognized by anti-STa antibodies, indicating that these two toxoids had no major structure alterations. In addition, although our pSTa12(P→F) and pSTa13(A→Q) toxoids were unable to stimulate fluid secretion in porcine gut loops, diarrhea in gnotobiotic pigs, or an increase of cGMP levels in T-84 cells, these two toxoids likely retained very low toxicity. Hirayama (19) indicated that the hSTa12(P→F) or hSTa13(A→Q) peptide become biologically active when more than a 1,000-fold of each peptide was used. The retention of very low toxicity and maintenance of proper STa structure likely resulted in the pSTa toxoids constructed in this study having their anti-STa antigenicity facilitated when they were carried by a strongly immunogenic protein.
In contrast to the case in early studies, we fused a full-length pSTa toxoid with a single amino acid substitution and disulfide bonds retained to a full-length pLTAB toxoid in this study. It was reported that STa toxicity was retained when a native hSTa gene was fused to an hLTB gene (9, 17), and several other studies suggested that hSTa needed to be toxic in order to be antigenic (4, 38, 39). However, Sanchez and Holmgren (32) reported that a chimeric protein that resulted from a fusion between a nontoxic hSTa peptide that had a disulfide bond disrupted and a CTB subunit was able to elicit antibodies that recognized native hSTa protein. In this study, we retained all disulfide bonds but mutated nucleotides encoding a single amino acid residue of the porcine STa gene so that the pSTa toxoids would have minimum structure alteration but a sufficient reduction in toxicity. We replaced amino acids 11, 12, and 13 for pSTa toxoids because previous studies demonstrated that replacement of amino acid 12, 13, or 14 in synthetic hSTa peptides resulted in a substantial reduction in toxicity (19, 46, 47). All three constructed pSTa toxoids clearly showed toxicity reduction, as they no longer increased cyclic GMP levels in cells or stimulated fluid secretion in porcine ligated gut loops. Furthermore, our animal challenge study indicated that E. coli constructs expressing these pSTa toxoids are not diarrheagenic to young pigs, suggesting that these three STa toxoids could serve as safe antigens. Results from the STa competitive ELISA showed that pSTa13 had the least alteration in protein structure, as this toxoid recognized anti-STa antibodies at a level nearly the same as that for the native STa protein, whereas pSTa12 perhaps had the greatest relative structure alteration, as it blocked least the anti-STa antibodies that bound to the coated STa ovalbumin conjugates. Cyclic GMP ELISA suggested that pSTa13 was least toxic and pSTa12 had a relative higher toxicity to the T-84 cells, which differed from data from the porcine ligated gut loop assay that indicated that loops incubated with the strain expressing pSTa13 showed relatively more fluid accumulation. However, Hirayama (19) demonstrated that the minimum effective doses (to be biologically active) for hSTa12(N→K), hSTa13(P→F), and hST14(A→Q) are 1,300, 1,200, and 2,090 pmol, respectively. This suggests that hSTa14 is less toxic than hSTa13. Thus, we believe that pSTa13 should be less toxic than pSTa12. In this study, we targeted the pSTa12 and pSTa13 toxoids for fusion protein construction to explore their application in antigenicity enhancement. Further studies will be needed to determine enhancement in antigenicity and vaccine application for the pSTa11(N→K) toxoid, and perhaps a thorough study of other STa toxoids for structure and toxicity will identify better STa toxoid candidates for vaccine development. Our results also indicated that the pLT192:pSTa12 fusion protein facilitated a higher anti-STa antibody titer than the pLT192:pSTa13 fusion (3.33 versus 2.59 log10 for IgG, 1.92 versus 1.83 log10 for IgA, and 1.75 versus 1.26 log10 for S-IgA). These results may suggest that the remaining toxicity from an STa toxoid could indeed play a role in anti-STa antigenicity.
Our study showed that antibodies elicited from fusion antigens of the full-length pLT and pSTa toxoids can neutralize CT and STa toxins. We used CT rather than LT because CT toxin is commercially available. Although CT and LT toxins have some differences in antigenicity, they are highly homologous in structure and functions. Therefore, we believe that the anti-LT antibodies elicited from immunization of the LT and STa toxoid fusions should neutralize LT toxin.
Although our preliminary data from a animal challenge study showed that three of four piglets were protected against infection from an STa-producing ETEC strain, more challenge studies will be needed to evaluate protection from anti-STa immunity induced by LT and STa toxoid fusion antigens. Our preliminary data indicate that piglets acquire anti-STa immunity by sucking colostrums that contains anti-STa antibodies and suggest that this anti-STa immunity provides protection against STa toxin. The three piglets that remained healthy after challenge were born by natural farrowing, and the fourth piglet, which showed very mild diarrhea, was pulled out from the sow by our veterinarian 12 h late. That could have negatively affected its health and resulted in its vulnerability to ETEC infection. To further evaluate host anti-STa and anti-LT immunity, especially mucosal immunity, elicited by pLT192:pSTa12 or pLT192:pSTa13 antigens and host immunity in protection against ETEC infection, we need to conduct much larger-scale animal studies. Nevertheless, the results from this study clearly provide helpful information for future development of safe and effective toxoid vaccines against ETEC diarrhea in humans and farm animals.
Acknowledgments
We thank Eric Nelson and Craig Welbon for their help with rabbit immunization; X. Ruan, K. Mateo, M. Zhao, and D. Baker for their assistance with animal surgery and animal challenge studies; and two reviewers for their very valuable comments, which improved the manuscript.
Financial support for this study was provided by grants NIH AI068766 (W. Zhang), NIH AI083897 (W. Zhang), and NPB08-005 (W. Zhang) and by the South Dakota Agricultural Experiment Station.
Editor: V. J. DiRita
Footnotes
Published ahead of print on 26 October 2009.
REFERENCES
- 1.Alexander, T. J. L. 1994. Neonatal diarrhoea in pigs, p. 151-170. In C. L. Gyles (ed.), Escherichia coli in domestic animals and humans. CAB International, Oxon, United Kingdom.
- 2.Arakawa, R., J. Yu, and W. H. R. Langridge. 2001. Synthesis of a cholera toxin B subunit-rotavirus NSP4 fusion protein in potato. Plant Cell Rep. 20:343-348. [DOI] [PubMed] [Google Scholar]
- 3.Ausubel, F. M., R. Brent, R. K. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1999. Short protocols in molecular biology, 4th ed. John Wiley & Sons, Inc., New York, NY.
- 4.Batisson, I., and M. Der Vartinian. 2000. Contribution of defined amino acid residues to the immunogenicity of recombinant Escherichia coli heat-stable enterotoxin fusion proteins. FEMS Microbiol. Lett. 192:223-229. [DOI] [PubMed] [Google Scholar]
- 5.Batisson, I., M. Der Vartanian, B. Gaillard-Martinie, and M. Contrepois. 2000. Full capacity of recombinant Escherichia coli heat-stable enterotoxin fusion proteins for extracellular secretion, antigenicity, disulfide bond formation and activity. Infect. Immun. 68:4064-4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berberov, E. M., Y. Zhou, D. H. Francis, M. A. Scott, S. D. Kachman, and R. A. Moxley. 2004. Relative importance of heat-labile enterotoxin in the causation of severe diarrheal disease in the gnotobiotic piglet model by a strain of enterotoxigenic Escherichia coli that produces multiple enterotoxins. Infect. Immun. 72:3914-3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boedeker, E. C. 2005. Vaccines for enterotoxigenic Escherichia coli: current status. Curr. Opin. Gastroenterol. 21:15-19. [PubMed] [Google Scholar]
- 8.Choi, B. K., and D. M. Schifferli. 1999. Lysine residue 117 of the FasG adhesin of enterotoxigenic Escherichia coli is essential for binding of 987P fimbriae to sulfatide. Infect. Immun. 67:5755-5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clements, J. D. 1990. Construction of a nontoxic fusion peptide for immunization against Escherichia coli strains that produce heat-labile and heat-stable enterotoxins. Infect. Immun. 58:1159-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Francis, D. H. 2002. Enterotoxigenic Escherichia coli infection in pigs and its diagnosis. J. Swine Health Prod. 10:171-175. [Google Scholar]
- 11.Francis, D. H., and J. A. Willgohs. 1991. A live avirulent Escherichia coli vaccine for K88+ enterotoxigenic colibacillosis in weaned pigs. Am. J. Vet. Res. 52:1051-1055. [PubMed] [Google Scholar]
- 12.Frantz, J. C., and M. W. Mellencamp. 1983. Production and testing of Escherichia coli (LTb) toxoid, p. 500-517. In S. Acres (ed.), Proceedings of the Fourth International Symposium on Neonatal Diarrhea. VIDO Publications, Saskatoon, Saskatchewan, Canada.
- 13.Frantz, J. C., and D. C. Robertson. 1981. Immunological properties of Escherichia coli heat-stable enterotoxins: development of a radioimmunoassay specific for heat-stable enterotoxins with suckling mouse activity. Infect. Immun. 33:193-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frech, S. A., H. L. DuPont, A. L. Bourgeois, R. McKenzie, J. Belkind-Gerson, J. F. Figueroa, P. C. Okhuysen, N. H. Guerrero, F. G. Martinez-Sandoval, J. H. M. Melendez-Romero, Z.-D. Jiang, E. J. Asturias, J. Halpern, O. R. Torres, A. S. Hoffman, C. P. Villar, R. N. Kassem, D. C., Flyer, B. H. Andersen, K. Kazempour, S. A. Breisch, and G. M. Glenn. 2008. Use of a patch containing heat-labile toxin from Escherichia coli against travellers' diarrhea: a phase II, randomized, double-blind, placebo-controlled field trial. Lancet 371:2019-2025. [DOI] [PubMed] [Google Scholar]
- 15.Frydendahl, K. 2002. Prevalence of serogroups and virulence genes in Escherichia coli associated with postweaning diarrhoea and edema disease in pigs and a comparison of diagnostic approaches. Vet. Microbiol. 85:169-182. [DOI] [PubMed] [Google Scholar]
- 16.Girard, M. P., D. Steele, C. L. Chaignat, and M. P. Kieny. 2006. A review of vaccine research and development: human enteric infections. Vaccine 24:2732-2750. [DOI] [PubMed] [Google Scholar]
- 17.Guzman-Verduzco, L., and Y. M. Kupersztoch. 1987. Fusion of Escherichia coli heat-stable enterotoxin and heat-labile enterotoxin B subunit. J. Bacteriol. 169:5201-5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harvey, R. B., R. C. Anderson, K. J. Genovese, T. R. Callaway, and D. J. Nisbet. 2005. Use of competitive exclusion to control enterotoxigenic strains of Escherichia coli in weaned pigs. J. Anim. Sci. 83:44-47. [Google Scholar]
- 19.Hirayama, T. 1995. Heat-stable enterotoxin of Escherichia coli, p. 281-296. In J. Moss, B. Iglewski, M. Vaughan, and A. T. Tu (ed.), Bacterial toxins and virulence factors in disease. Marcel Dekker, Inc., New York, NY.
- 20.Hol, W. G. J., T. K. Sixma, and E. A. Merritt. 1995. Structure and function of E. coli heat-labile enterotoxin and cholera toxin B pentamer, p. 185-223. In J. Moss, B. Iglewski, M. Vaughan, and A. T. Tu (ed.), Bacterial toxins and virulence factors in disease. Marcel Dekker, Inc., New York, NY.
- 21.Hughes, J. M., F. Murad, B. Chang, and R. L. Guerrant. 1978. Role of cyclic GMP in the action of heat-stable enterotoxin of Escherichia coli. Nature 271:755-756. [DOI] [PubMed] [Google Scholar]
- 21a.Institute of Laboratory Animal Resources. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC.
- 22.Klipstein, F. A., R. F. Engert, and J. D. Clements. 1982. Development of a vaccine of cross-linked heat-stable and heat-labile enterotoxin that protects against Escherichia coli producing either enterotoxin. Infect. Immun. 37:550-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lapa, J. A., S. A. Sincock, M. Ananthakrishnan, C. K. Porter, F. J. Cassels, C. Brinkley, E. R. Hall, J. Hamont, J. D. Gramling, C. M. Carpenter, S. Baqar, and D. R. Tribble. 2008. Randomized clinical trial assessing the safety and immunogenicity of oral microencapsulated enterotoxigenic Escherichia coli surface antigen 6 with or without heat-labile enterotoxin with mutation R192G. Clin. Vaccine Immunol. 15:1222-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levine, M. M., D. R. Nalin, D. L. Hoover, E. J. Bergquist, R. B. Bornick, and C. R. Young. 1979. Immunity to enterotoxigenic Escherichia coli. Infect. Immun. 23:729-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lockwood, D. E., and D. C. Robertson. 1984. Development of a competitive enzyme-linked immunosorbent assay (ELISA) for Escherichia coli heat-stable enterotoxin (STa). J. Immunol. Methods 75:293-307. [DOI] [PubMed] [Google Scholar]
- 26.Moon, H. W. 1978. Mechanisms in the pathogenesis of diarrhea: a review. J. Am. Vet. Med. Assoc. 172:443-448. [PubMed] [Google Scholar]
- 27.Moon, H. W. 1990. Colonization factor antigens of enterotoxigenic Escherichia coli in animals. Curr. Top. Microbiol. Immunol. 151:147-165. [DOI] [PubMed] [Google Scholar]
- 28.Moon, H. W., and T. O. Bunn. 1993. Vaccines for preventing enterotoxigenic Escherichia coli infections in farm animals. Vaccine 11:213-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qadri, F., S. K. Das, A. S. Faruque, G. J. Fuxhs, M. J. Albert, R. B. Sack, and A.-M. Svennerholm. 2000. Prevalence of toxin types and colonization factors in enterotoxigenic Escherichia coli isolated during a 2-year period from diarrheal patients in Bangladesh. J. Clin. Microbiol. 38:27-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reischl, U., M. T. Youssef, H. Wolf, E. Hyytia-Trees, and N. A. Strockbine. 2004. Real-time fluorescense PCR assays for detection and characterization of heat-labile I and heat-stable I enterotoxin genes from enterotoxigenic Escherichia coli. J. Clin. Microbiol. 42:4092-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanchez, J., T. R. Hirst, and B. E. Uhlin. 1988. Hybrid enterotoxin LTA:STa proteins and their protection from degradation by in vivo association with B-subunits of Escherichia coli heat-labile enterotoxin. Gene 64:265-275. [DOI] [PubMed] [Google Scholar]
- 32.Sanchez, J., and J. Holmgren. 1988. Genetic fusion of a non-toxic heat-stable enterotoxin related decapeptide antigen to cholera toxin B-subunit. FEBS Lett. 241:110-114. [DOI] [PubMed] [Google Scholar]
- 33.Sanchez, J., B. E. Uhlin, T. Grundstrom, J. Holmgren, and T. R. Hirst. 1986. Immunoactive chimeric St-LT enterotoxins of Escherichia coli generated by in vitro gene fusion. FEBS Lett. 208:194-198. [DOI] [PubMed] [Google Scholar]
- 34.Schifferli, D. M., and M. A. Alrutz. 1994. Permissive linker insertion sites in the outer membrane protein of 987P fimbriae of Escherichia coli. J. Bacteriol. 176:1099-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sears, C. L., and J. B. Kaper. 1996. Enteric bacterial toxins: mechanisms of action and linkage to intestinal secretion. Microbiol. Rev. 60:167-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaheen, H. I., S. B. Khalil, M. R. Rao, R. A. Elyazeed, T. F. Wierzba, L. F. Peruski, Jr., S. Putnam, A. Navarro, B. Z. Morsy, A. Cravioto, J. D. Clemens, A.-M. Svennerholm, and S. J. Savarino. 2004. Phenotypic profiles of enterotoxigenic Escherichia coli associated with early childhood diarrhea in rural Egypt. J. Clin. Microbiol. 42:5588-5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith, H. W., and M. A. Linggood. 1971. Observation on the pathogenic properties of the K88, HIY and ENT plasmids of Escherichia coli with particular reference to porcine diarrhea. J. Med. Microbiol. 4:467-485. [DOI] [PubMed] [Google Scholar]
- 38.Svennerholm, A.-M., and J. Holmgren. 1992. Immunity to enterotoxin-producing bacteria, p. 227-246. In T. T. MacDonald (ed.), Immunology of gastrointestinal disease. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 39.Svennerholm, A.-M., and J. Holmgren. 1995. Oral B-subunit whole-cell vaccines against cholera and enterotoxigenic Escherichia coli diarrhea, p. 205-232. In D. A. A. AlaAldeen, and C. E. Hormaeche (ed.), Molecular and clinical aspects of bacterial vaccine development. John Wiley & Sons Ltd., Chicester, England.
- 40.Svennerholm, A.-M., M. Lindblad, B. Svennerholm, and J. Holmgren. 1988. Synthesis of nontoxic, antibody-binding Escherichia coli heat-stable enterotoxin (STa) peptides. FEMS Microbiol. Lett. 55:23-28. [Google Scholar]
- 41.Svensmark, B., K. Nielsen, P. Willeberg, and S. E. Jorsal. 1989. Epidemiological studies of piglet diarrhoea in intensively managed Danish sow herds. II. Post-weaning diarrhoea. Acta Vet. Scand. 30:55-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van den Broeck, W., E. Cox, and B. M. Goddeeris. 1999. Induction of immune responses in pigs following oral administration of purified F4 fimbriae. Vaccine 17:2020-2029. [DOI] [PubMed] [Google Scholar]
- 43.Walker, R. I. 2005. Considerations for development of whole cell bacterial vaccines to prevent diarrheal diseases in children in developing countries. Vaccine 23:3369-3385. [DOI] [PubMed] [Google Scholar]
- 44.Wilson, M. R., and D. H. Francis. 1986. Fimbriae and enterotoxins associated with Escherichia coli serogroups isolated from pigs with colibacillosis. Am. J. Vet. Res. 47:213-217. [PubMed] [Google Scholar]
- 45.Wolf, M. K. 1997. Occurrence, distribution, and associations of O and H serogroups, colonization factor antigens, and toxin of enterotoxigenic Escherichia coli. Clin. Microbiol. Rev. 10:569-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamasaki, S., H. Ito, T. Hirayama, Y. Takeda, and Y. Shimonishi. 1988. Effects on the activity of amino acids replacement at positions 12, 13, and 14 heat-stable enterotoxin (STh) by chemical synthesis, p. 42. Abstr. 24th Joint Conf. U.S.-Japan Cooperative Med. Sci. Program Cholera Related Diarrheal Disease Panel, Tokyo, Japan.
- 47.Yamasaki, S., T. Sato, Y. Hidaka, H. Ozaki, H. Ito, T. Hirayama, Y. Takeda, T. Sugimura, A. Tai, and Y. Shimonishi. 1990. Structure-activity relationship of Escherichia coli heat-stable enterotoxin: role of Ala residue at position 14 in toxin-receptor interaction. Bull. Chem. Soc. Jpn. 63:2063-2070. [Google Scholar]
- 48.Zhang, W., E. M. Berberov, J. Freeling, D. He, R. A. Moxley, and D. H. Francis. 2006. Significance of heat-stable and heat-labile enterotoxins in porcine colibacillosis, in an additive model for pathogenicity studies. Infect. Immun. 74:3107-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang, W., D. C. Robertson, C. Zhang, W. Bai, M. Zhao, and D. H. Francis. 2008. Escherichia coli constructs expressing human or porcine enterotoxins induce identical diarrheal diseases in a piglet infection model. Appl. Environ. Microbiol. 74:5832-5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang, W., M. Zhao, L. Ruesch, A. Omot, and D. H. Francis. 2007. Prevalence of virulence genes in Escherichia coli strains isolated from young pigs with diarrhea in the U.S. Vet. Microbiol. 123:145-152. [DOI] [PubMed] [Google Scholar]