Abstract
Reactive oxygen species (ROS) are many-faceted compounds involved in cell defense against pathogens, as well as in cell signaling. Their involvement in the response to infection in epithelial cells remains poorly documented. Here, we investigated the production of ROS during infection with Chlamydia trachomatis, a strict intracellular pathogen, in HeLa cells. C. trachomatis induced a transient increase in the ROS level within a few hours, followed by a return to basal level 9 hours after infection. At this time point, the host enzyme dedicated to ROS production, NADPH oxidase, could no longer be activated by external stimuli, such as interleukin-1β. In addition, Rac, a regulatory subunit of the NADPH oxidase complex, was relocated to the membrane of the compartment in which the bacteria develop, the inclusion, while other subunits were not. Altogether, these results indicate that C. trachomatis infection elicits the production of ROS and that the bacteria rapidly target the activity of NADPH oxidase to shut it down. Prevention of ROS production at the onset of the bacterial developmental cycle might delay the host response to infection.
Reactive oxygen species (ROS), such as superoxide anion (O2−) or hydrogen peroxide (H2O2), are short-lived, very reactive compounds, that oxidize a broad variety of molecules, including proteins, lipids, and nucleic acids (35). In many cases, oxidation results in a loss of function of the target molecule. Therefore, when produced in large amounts or over extended periods of time, ROS cause irreversible damage to biological systems. ROS toxicity has been particularly exploited by phagocytic cells, which are able to produce large amounts of ROS in response to pathogen intrusion in a process known as the respiratory burst. This reaction plays a major role in pathogen killing (7). Beyond their role as toxic compounds, it became clear in the last few decades that ROS are involved in a large number of reversible regulatory processes, operating as intracellular signaling molecules (21). Under these circumstances, ROS are produced at much lower concentrations than in the respiratory burst. Our understanding of their mode of action as signaling molecules is still fragmented. The best-documented cases are their roles in activating transcription factors, like NF-κB (24), and metalloproteinases, like MMP-2 (10), and in modulating the redox-sensitive catalytic cysteine residues of tyrosine and MAPK (mitogen-activated protein kinase) phosphatases (16, 45).
In eukaryotic cells, ROS are often by-products of biological reactions that mainly occur in the mitochondria. Another source of ROS are NADPH oxidases (NOX), a family of multisubunit enzymes that reduce O2 to O2− using electrons derived from intracellular NADPH. NADPH oxidases consist of two membrane proteins (known as gp91phox and p22phox in phagocytes) and cytosolic regulatory proteins (p40phox, p47phox, p67phox, and Rac2 in phagocytes) (7). Upon stimulation, the NADPH oxidase cytosolic subunits assemble with membrane subunits into an active complex. While NADPH oxidase activity has been widely characterized in phagocytes, it is also found in nonphagocytic cell types, including epithelial cells. Nonphagocytic cells express a variety of subunits homologous to NOX2 (NOX1 to -5) and to the cytosolic regulatory subunits (6, 17).
Epithelial cells are often the first barrier met by pathogens, and production of ROS over prolonged periods of time could inflict some damage on intruders. At low concentration, NADPH oxidase-generated ROS might participate in the signaling cascades orchestrated by the cell in response to infection (25). Two reports suggest that, indeed, in nonphagocytic cells, contact with pathogens triggers ROS production. In gastric pit cells, Helicobacter pylori infection elicits production of ROS and stimulates the expression of components of NADPH oxidase (32, 34). In human colon cancer cells, flagellin from Salmonella enterica serovar Enteritidis also leads to superoxide anion production. In this case, overexpression of some of the NADPH oxidase components causes an increase in flagellin-dependent ROS production concomitant with interleukin-8 (IL-8) synthesis, suggesting that ROS may participate in the innate immune response (33).
Chlamydia trachomatis is an obligate intracellular bacterial pathogen infecting primarily epithelial cells of the eye and genital tract (40). It is the most frequent cause of sexually transmitted diseases of bacterial origin, potentially resulting in ectopic pregnancies and infertility. C. trachomatis is also the agent of trachoma, which can lead to blindness. Chlamydia has a remarkable biphasic developmental cycle. The infectious form, called the elementary body (EB), is able to induce its entry into a host cell (20). Once internalized, it differentiates into the metabolically active, noninfectious form, the reticulate body (RB), which replicates inside a membrane-bounded compartment, the inclusion. Metabolic activity is first detected shortly after bacterial entry. In particular, some bacterial proteins, called Inc proteins, are synthesized within the first 2 hours of infection and are translocated into the inclusion membrane (23). This membrane constitutes the interface between the bacteria and the host, and its composition varies during the developmental cycle, with the successive addition of different Inc proteins. At the end of a complete developmental cycle, RBs differentiate back into infectious EBs and are released from the host cell (1).
While Chlamydia-induced cell signaling and remodeling of the cytoskeleton during entry have recently received a lot of attention (9), little is known about the host response to infection in the first hours after Chlamydia intrusion. For their activities as both signaling molecules and potentially harmful components, ROS are good candidates to participate in the early host response to infection. In this study, we investigate their production within the first hours of C. trachomatis infection in epithelial cells.
MATERIALS AND METHODS
Cells, bacteria, and antibodies.
The human cervical cell line HeLa 229 was obtained from the American Type Culture Collection (ATCC) and passaged in Dulbecco's modified Eagle's medium with Glutamax (Gibco, Invitrogen) supplemented with 10% fetal calf serum (Biowest, France) (complete medium). C. trachomatis serovar L2 was from the ATCC and was prepared as previously described (11). Heat-killed EBs were prepared by incubating live EBs for 30 min at 58°C. The GPIC strain of Chlamydia caviae was obtained from Roger Rank (University of Arkansas). The polyclonal antibody against Cap1 was obtained after immunization of New Zealand White rabbits with purified recombinant Cap1 with 167 amino acids at the C terminus deleted and fused to a His tag (Agro-Bio, La Ferté Saint-Aubain, France). The monoclonal anti-EF-Tu antibody was a kind gift of You-Xun Zhang (Boston University School of Medicine, Boston, MA). The mouse anti-Flag M2 antibody was purchased from Sigma (Saint Louis, MO). The Alexa Fluor 546- and Alexa Fluor 488-conjugated goat anti-mouse secondary antibodies were from Molecular Probes, and the Fluorolink Cy3-labeled goat anti-rabbit IgG was from Amersham Biosciences.
Plasmids.
For the Flag-p22phox construct, RNA was extracted from HeLa cells using an RNeasy Minikit (Qiagen) and cDNA was generated with a Gene Amp RNA PCR Core Kit (Applied Biosystems, Branchburg, NJ) according to the manufacturers' instructions. cDNA coding for p22phox was amplified using the forward primer GGGGACAACTTTGTACAAAAAAGTTGGCATGGGGCAGATCGAGTGGGCCAT and the reverse primer GGGGACAACTTTGTACAAGAAAGTTGGTTATCACACGACCTCGTCGGTCA. The cDNA coding for p47phox was a kind gift from M. C. Dagher (Institut de Biologie Structurale Jean-Pierre Ebel, Grenoble, France) and was amplified with the primers GGGGACAACTTTGTACAAAAAAGTTGGCATGGGGGACACCTTCATCCGTCA and GGGGACAACTTTGTACAAAAAAGTTGGTTATCAGACGGCAGACGCCAGCTT. The cDNAs were cloned, using the Gateway technology (Invitrogen), into an expression vector containing a three-Flag tag in the pCINeo background (Yves Jacob, Institut Pasteur, Paris, France). The generated constructs were verified by sequencing them (Eurofins MWG Operon, Ebersberg, Germany). The plasmid coding for green fluorescent protein (GFP)-Rac1 was a kind gift from Guy Tran Van Nhieu (Institut Pasteur, Paris, France).
Measurement of ROS production.
Cells were infected with C. trachomatis serovar L2 at a multiplicity of infection (MOI) of 0.6 in complete medium in 24-well plates. The fluorescent probe 5-(and 6-)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA) (Molecular Probes, OR), was solubilized at 2 mM in dimethyl sulfoxide (DMSO) (Sigma) and then diluted to 50 μM in Hanks balanced salt solution (HBSS). The cells were washed twice with HBSS containing MgCl2 and CaCl2 (Gibco, Invitrogen), incubated with 50 μM CM-H2DCFDA in this buffer (200 μl) for 1 h at 37°C, and then washed once with complete medium and further incubated for 1 h in complete medium at 37°C. Stimulation with IL-1β (Immugenex, CA) was performed at 0.1 ng/ml in complete medium for 30 min at 37°C before cell lysis. At the indicated times following infection, the cells were washed twice in phosphate-buffered saline and lysed in 170 μl NET buffer (50 mM Tris-HCl, pH 7.4, 5 mM EDTA, 1% Triton X-100, 150 mM NaCl) with protease inhibitor cocktail (Sigma). Protein quantitation was performed on 10 μl of sample in duplicate using a BCA Protein Assay Kit (Pierce) according to the manufacturer's instructions. The remainder of the sample was used to measure the fluorescence of the oxidized product dichlorofluorescein with a Cary Eclipse fluorometer (Varian Inc., Palo Alto, CA) at a λ of 527 nm with excitation at 507 nm. For each sample, the fluorescence units were divided by the protein content. This ratio was expressed relative to its value in control cells (arbitrarily set to 1). For all points, experiments were performed with five replicates. Experiments using C. caviae or heat-killed bacteria were performed under exactly the same conditions. Statistical differences between different conditions were determined with a two-tailed Student's t test on the indicated number (n) of experiments.
Detection of ERK phosphorylation.
HeLa cells were infected or not with C. trachomatis serovar L2 at an MOI of 0.6 in complete medium in 12-well plates, centrifuged for 5 min at 385 × g, and incubated at 37°C. At the indicated times postinfection (p.i.), 10 μM diphenyleneiodonium chloride (DPI) (Sigma) or 1:1,000 DMSO (mock-treated samples) was added. Seven h p.i., the cells were lysed in 100 μl of Laemmli buffer heated to 100°C, boiled for 5 min, and analyzed by electrophoresis in 10% polyacrylamide gels in the presence of sodium dodecyl sulfate. After electrophoresis, the proteins were transferred to a polyvinylidene difluoride membrane (Millipore), and the membrane was used for blotting with anti-phospho-ERK1/2 (extracellular signal-regulated protein kinase 1/2) polyclonal antibodies (Cell Signaling Technology no. 9101). After this first revelation, the membranes were stripped and reprobed with anti-total ERK1/2 antibody (Upstate no. 06-182) to normalize them to equal ERK contents. Western blots and revelations were performed by enhanced chemifluorescence using alkaline phosphatase-linked secondary antibodies and measured on a Storm 840 fluorimager (Molecular Dynamics).
Measurement of oxidative stress.
Cells were infected with C. trachomatis serovar L2 at an MOI of 0.6 in complete medium in 24-well plates. The fluorescent probe Cell Tracker Green CMFDA (Molecular Probes, OR) was solubilized in DMSO (Sigma) at 10 mM and used at 1 μM in HBSS (0.8 ml/well). Cell lysate and fluorescence quantitation was done as for ROS measurement (see above), except that fluorescence was recorded at 517 nm with excitation at 492 nm. The level of reduced glutathione (GSH) in control noninfected cells was set to 1. Experiments were performed in triplicate.
NADPH oxidase subunit localization at early infection times.
HeLa cells were transiently transfected with GFP-Rac1, Flag-p22phox, or Flag-p47phox for 18 h using Fugene reagent (Applied Biosystems) and then infected with C. trachomatis serovar L2 in complete medium. To synchronize the infection, cells with bacteria were centrifuged for 5 min at 385 × g (Allegra 6 KR; Beckman Coulter) and incubated for 1 h at 37°C with 6% CO2. After 1 h of infection, the medium was removed and nocodazole (Sigma) was added at a final concentration of 10 μM in complete medium for some samples. In control cells, DMSO (Sigma) was added at the same concentration. After the indicated time of infection, the cells were fixed with 4% paraformaldehyde (Merck). The cells were then permeabilized with 0.05% saponin (Sigma), 1 mg/ml bovine serum albumin (PAA, Austria) in phosphate-buffered saline, and the inclusion membrane was labeled with anti-Cap1, followed by incubation with anti-rabbit Cy3 (Amersham). When appropriate, Flag-tagged proteins were stained with anti-Flag antibody followed by goat anti-mouse Alexa Fluor 488-conjugated antibody. Coverslips were mounted in Mowiol (Calbiochem) supplemented with 0.5 μg/ml of Hoechst 33342 (Molecular Probes, OR). Cells were photographed with an ApoTome microscope (Zeiss) equipped with a 63× objective and a Roper Scientific Coolsnap HQ camera, permitting optical sections of 0.7 μm.
RESULTS
Chlamydia infection elicits transient ROS production in HeLa cells.
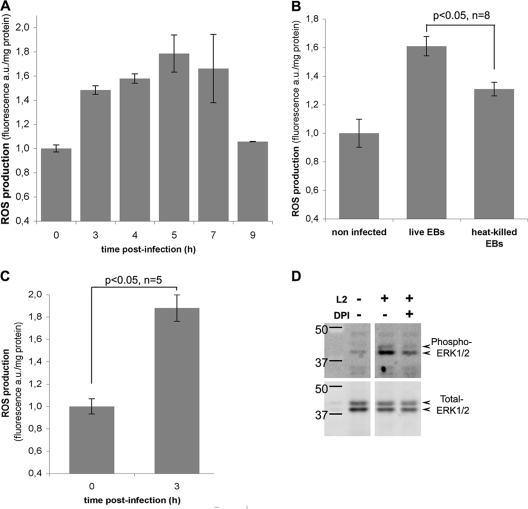
To measure intracellular ROS levels, HeLa cells infected with C. trachomatis serovar L2 for different times (0 to 9 h) were incubated with the membrane-permeant fluorescent probe CM-H2DCFDA. The cells were then lysed, and fluorescence in the cell lysates was quantified. Five h p.i., the level of intracellular ROS was about 50% higher than that measured in control noninfected cells. ROS levels remained high (between 30% and 80% above basal level, depending on the experiment) up to 5 to 7 h p.i. before returning to basal levels 9 h p.i. (Fig. 1A). The increase in ROS levels was reduced by about half when the cells were exposed to heat-killed bacteria for 3 h (data not shown) or 5 h (Fig. 1B), suggesting that bacterial activity is necessary to sustain ROS production during infection. Moreover, another Chlamydiaceae species, C. caviae, also elicited an early ROS response (Fig. 1C), showing that this phenomenon is not limited to C. trachomatis infection and might represent a common response to chlamydial invasion of epithelial cells. One of the consequences of C. trachomatis infection is the phosphorylation of ERK, which was reported at 12 h p.i. and increased further at later times of infection (14, 41). ROS have been shown to induce the ERK signaling cascade in a variety of cellular models (27, 30, 39). To investigate if ROS production contributed to the activation of the ERK signaling pathway early during infection, we looked at the effect of an inhibitor of NADPH oxidase, DPI, on ERK phosphorylation during infection. The inhibitor was introduced 2 h p.i., before the peak of ROS production (Fig. 1A). Cells were lysed 7 h p.i., and antibodies specific for the phosphorylated forms of ERK1/2 were used to assess ERK phosphorylation by Western blotting (Fig. 1D). Infected cells showed a higher level of ERK1/2 phosphorylation than noninfected cells, confirming that C. trachomatis infection induces ERK1/2 phosphorylation. In the presence of DPI, ERK1/2 phosphorylation was reduced compared to untreated infected cells, indicating that the activity of NADPH oxidase contributes to the signaling events leading to ERK1/2 phosphorylation. This result also strongly supports the hypothesis that the enzyme is responsible for the production of ROS during infection. Unfortunately, DPI interfered with the fluorimetric assay to measure ROS, and we could not test the hypothesis directly. In conclusion, this experiment indicates that ROS, produced by NADPH oxidase, act as signaling molecules and contribute to the activation of the ERK signaling cascade early during C. trachomatis infection. Altogether, our data show that the production of ROS is one of the early responses of epithelial cells to Chlamydia infection.
FIG. 1.
Transient production of ROS during Chlamydia infection. (A) ROS production was measured in HeLa cells infected with C. trachomatis serovar L2 for the indicated times. (B) ROS production measured 5 h p.i. with live or heat-killed C. trachomatis EBs. (C) ROS production measured 3 h p.i. with the species C. caviae. (A, B, and C) For each time point, incubation with CM-H2DCFDA was initiated 2 h before lysis. The cells were lysed at the indicated times, and fluorescence and protein were measured for each lysate. Fluorescence arbitrary units (a.u.) were divided by the protein content. The normalized fluorescence of control uninfected cells (zero hour time point) was set to 1. For each panel, one representative experiment of at least three is shown, and the error bars correspond to the standard deviation of the five replicates in this single experiment. Statistical analyses were conducted for the indicated number of independent experiments. (D) Phosphorylation of ERK1/2 upon C. trachomatis infection. Levels of ERK1/2 phosphorylation were measured by Western blotting using specific anti-phospho-ERK1/2 antibodies (top row) in lysates of control cells and of cells infected with C. trachomatis for 9 h. Where indicated, 10 μM DPI was added to the cells 2 h p.i. The blots were reprobed with anti-total ERK1/2 antibodies (bottom row) to verify that the total ERK contents were identical in all samples.
Chlamydia-induced ROS production does not lead to oxidative stress.
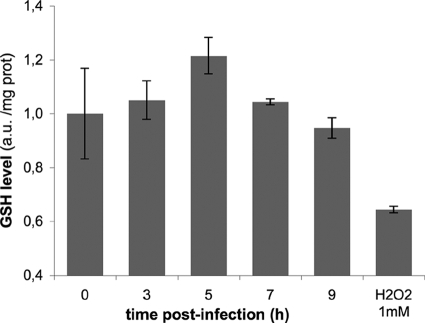
ROS generated at high levels or over long periods may cause an imbalance of the cellular redox equilibrium, leading to oxidative stress conditions. Cells have evolved several defense and repair mechanisms that would normally counteract the injurious effects of ROS (4). For instance, the thiol-reducing agent GSH acts as a major antioxidant system. Under oxidative stress, GSH, which is normally maintained in a reduced state, is oxidized to GSSG. An increase in the GSSG/GSH ratio is indicative of oxidative stress in the cells. In infected HeLa cells (0 to 9 h), the concentration of reduced GSH does not decline, as monitored by recording the fluorescence of the thiol-reactive intracellular probe CellTracker Green CMFDA (Fig. 2). Addition of 1 mM hydrogen peroxide (H2O2) for 3 h in the culture medium was used as a positive control for oxidative stress conditions. Under these circumstances, the GSH level was significantly lower than in untreated cells (Fig. 2).
FIG. 2.
Absence of oxidative stress in C. trachomatis-infected cells. Levels of reduced GSH were measured by incubating HeLa cells with 1 μM CellTracker Green CMFDA in HBSS for 1 h, 2 h before lysis. As a positive control for oxidative stress, cells were incubated with 1 mM H2O2 for 3 h. At the indicated times, cells were lysed, and the fluorescence and protein content of each lysate were measured. Fluorescence units were normalized to the protein content, and the normalized fluorescence of the control noninfected cells was set to 1 (zero hour time point). Experiments were performed in triplicate; the error bars correspond to the standard deviation of the replicates. The experiment shown is representative of two. Variations in GSH levels over the course of the infection were not statistically significant.
This result shows that infection with C. trachomatis does not elicit oxidative stress in the host cell. It is in line with our observation that ROS production remains moderate in the first hours of infection (Fig. 1).
Chlamydia infection leads to inactivation of the host NADPH oxidase.
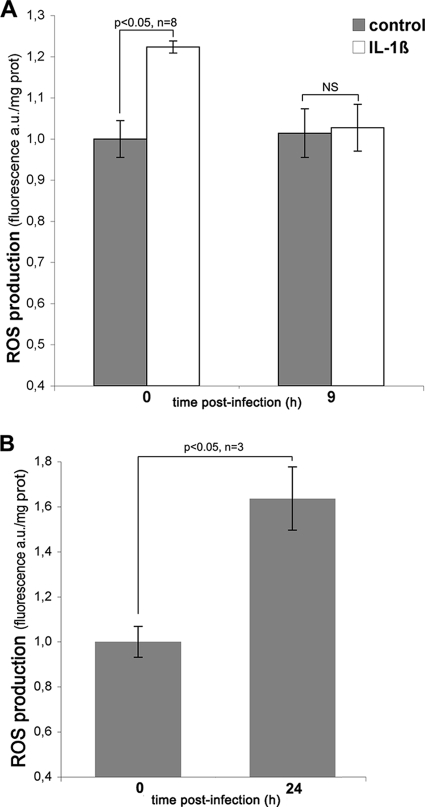
The observed transient ROS production suggests that the capacity of the host cell to produce oxygen species diminishes while the infection proceeds. If the activation of the host NADPH oxidase is responsible for ROS production during C. trachomatis infection, subsequent decrease in ROS production may be due to an impairment of this enzymatic activity later in infection. To test this hypothesis, we monitored the ability of NADPH oxidase to be activated in HeLa cells infected for 9 h. IL-1β is an inducer of NADPH oxidase-mediated ROS production in epithelial cells (31, 36). Indeed, in uninfected cells, ROS increased upon IL-1β stimulation (Fig. 3A). In contrast, in cells infected with C. trachomatis for 9 h, IL-1β stimulation failed to induce intracellular ROS production. Under these conditions, the ROS content of IL-1β-treated cells remained at basal level and did not significantly differ from the ROS content in infected untreated cells (Fig. 3A). This result shows that after the initial peak of ROS production, the host NADPH oxidase becomes unable to generate ROS upon stimulation. This might account for the concomitant return of ROS to basal levels. Finally, we asked whether the extinction of ROS production persisted over the course of the infection. In cells infected for 24 h, we observed that ROS levels were about 50% higher than in control noninfected cells (Fig. 3B), indicating a relaxation of the ROS inhibitory mechanism at this later infection time.
FIG. 3.
In C. trachomatis-infected cells, ROS production is transiently turned down. (A) HeLa cells, noninfected (zero hour time point) or infected for 7 h with C. trachomatis, were incubated with CM-H2DCFDA for 1.5 h before the addition of 0.1 ng/ml IL-1β. The cells were lysed 30 min later, and ROS were measured as described in the legend to Fig. 1. (B) ROS levels compared to noninfected cells were measured in HeLa cells infected for 24 h. For each panel, one representative experiment of at least three is shown, and the error bars correspond to the standard deviation of the five replicates in this single experiment. Statistical analyses were conducted for the indicated number of independent experiments. (NS, not significant).
The cytosolic NADPH oxidase subunit Rac is recruited to the Chlamydia inclusion after the peak of ROS production.
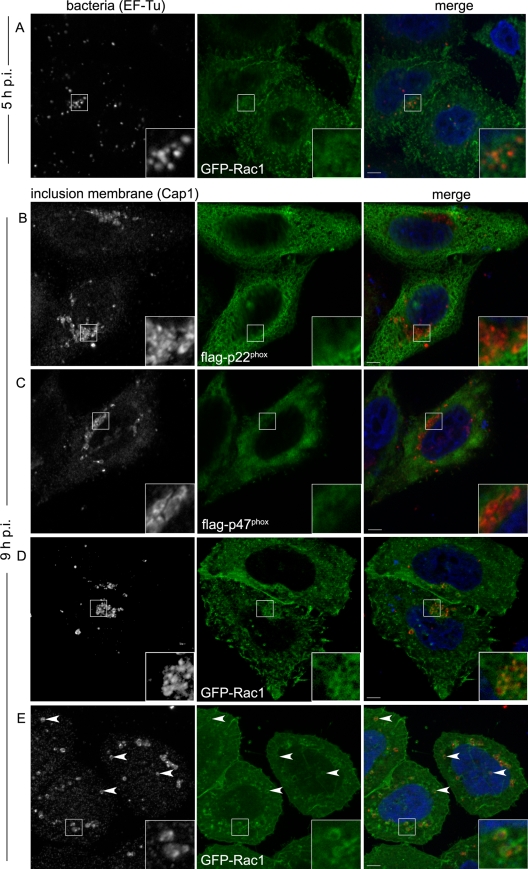
NADPH oxidase is composed of several subunits. Upon activation, the cytosolic subunits associate with the membrane components, forming the active enzyme. In phagocytes, assembly occurs both at the cell surface and on phagosomal membranes (15). To visualize the localization of different components of NADPH oxidase during Chlamydia infection, we individually overexpressed three tagged subunits of the enzyme, p22phox, p47phox, and Rac1, which is the Rac isoform present in HeLa cells. We used a high MOI (about 20 bacteria/cell) to facilitate the observation. At 5 h p.i., the distribution of GFP-Rac1, Flag-p47phox, or Flag-p22phox protein was unchanged compared to noninfected samples (Fig. 4A and data not shown). Until about 10 h p.i., the compartments in which the bacteria develop, the inclusions, mostly contain only one bacterium because multiplication has not started. In cells infected for 9 h, GFP-Rac1 appeared to localize around the bacteria, partially overlapping with an inclusion membrane marker, Cap1 (5) (Fig. 4D). In contrast, the cytosolic p47phox subunit and the p22phox component, which is mostly associated with the endoplasmic reticulum (3), did not relocalize to the inclusions (Fig. 4B and C). These observations indicate that one regulatory subunit of NADPH oxidase, Rac1, specifically relocalizes to the inclusion membrane between 5 and 9 h p.i. Early during infection, the inclusions migrate to the microtubule organizing center (19, 26), where Rac1 is predominantly found, even in uninfected cells. To rule out the possibility that GFP-Rac1 localization around the inclusions was due only to its normal localization where the inclusions are also mostly found, we used nocodazole to depolymerize microtubules. In nocodazole-treated cells, the inclusions did not cluster at the microtubule organizing center and were scattered throughout the cell cytoplasm. Under these conditions, GFP-Rac1 was clearly seen to be enriched around the inclusions (Fig. 4E), showing that it is recruited to the inclusion membranes independently of their localization in the cytoplasm.
FIG. 4.
Recruitment of NADPH oxidase subunits around maturing C. trachomatis inclusions. HeLa cells were transiently transfected with GFP-Rac1 (A, D, and E), Flag-p22phox (B), or Flag-p47phox (C) and infected with C. trachomatis for 5 h (A) or 9 h (B to E). (A) Bacteria were labeled using an anti-EF-Tu antibody and an Alexa Fluor 546-conjugated goat anti-mouse secondary antibody (left; red in merged image). (B to E) Inclusion membranes were stained with an anti-Cap1 antibody and a Cy3-conjugated goat anti-rabbit antibody (left; red in merged images); Flag-tagged proteins were labeled with anti-Flag antibody and an Alexa Fluor 488-conjugated goat anti-mouse antibody (green panels). The insets show threefold enlargements of the boxed areas. (E) To visualize inclusions scattered in the cytosol (arrowheads), 10 μM nocodazole was added 1 h p.i. In all cases, DNA was stained with Hoechst 33342 (blue in merged images). Bars, 5 μm.
These results show that the return of ROS to the basal level coincides in time with the specific recruitment of one of the subunits of NADPH oxidase to the inclusion membrane.
DISCUSSION
In this study, we examined the production of ROS in epithelial cells upon C. trachomatis infection. We observed two phases: an initial increase during the first hours of infection, followed by a decrease until the basal level was recovered, around 9 h after infection. At all time points, the level of ROS remained moderate, and there was no sign of oxidative stress in the cell. The decrease of ROS production at later time points was correlated with a lack of response of NADPH oxidase to an external stimulus, such as IL-1β. Furthermore, it temporally coincided with the specific relocalization of Rac1, a component of NADPH oxidase, to the membrane of the compartment in which the bacteria develop, the inclusion. Altogether, these observations suggest that the bacteria repress the activity of NADPH oxidase by sequestering one of its components, thus impairing one of the signaling pathways triggered by infection.
The infectious form of Chlamydia, called the EB, is metabolically inactive. The first few hours following cell invasion see dramatic changes in the pathogen: 1 h after entry, bacterial RNA and protein synthesis is detected; at 2 h p.i., neosynthesized bacterial proteins are observed on the inclusion membrane, and within a few more hours, the bacteria have fully transformed into reticulate bodies, ready to divide. Bacterial division starts around 6 to 8 h after infection, while the inclusion membrane matures, incorporating novel bacterial proteins.
All these events, starting with the invasion process itself, are expected to elicit a variety of reactions from the host cell, and in particular to initiate an early innate immune response to pathogen-associated molecular patterns (PAMPs) (37). Apart from the phosphorylation events that accompany the entry process itself (9), the signaling events that take place in the first hours of C. trachomatis infection in epithelial cells are mostly unknown. Studies of the innate immune response to C. trachomatis infection have focused on later time points (12, 18, 38). Recent studies have shown that ROS production can be an early response to pathogen invasion (43) or to contact with bacterial components (33) in epithelial cells that might potentiate proinflammatory pathways, such as NF-κB and JNK (24). We hypothesized that the epithelial cells might use ROS signaling as a response to an infection by Chlamydiaceae. Indeed, an increase in intracellular ROS was detected as early as 3 h after infection of HeLa cells with two Chlamydiaceae species, C. trachomatis and C. caviae (Fig. 1). This observation raises two immediate questions: how are ROS produced, and upon which signal?
The components of NADPH oxidase are present in HeLa cells (17). We favor the hypothesis that the enzyme was responsible for the initial ROS production for two reasons. First, we showed that DPI, often used as an inhibitor of NADPH oxidase, impaired ERK phosphorylation early during infection (Fig. 1D), indicating that the enzyme is implicated in the early response to infection. Second, we showed that the disappearance of ROS coincided with repression of NADPH oxidase activity (Fig. 3A), suggesting that the same enzyme was responsible for the initial ROS production. We could not show it directly because DPI interfered with the fluorescence assay to measure ROS. Moreover, attempts to knock down the expression of some of the subunits of NADPH oxidase by RNA silencing induced a large increase in the basal level of ROS, precluding the interpretation of the results (data not shown). Therefore, we cannot rule out the possibility that ROS produced during Chlamydia infection occur from other sources.
Another limitation of the assay for measuring the intracellular concentration of ROS was its extreme sensitivity to antibiotics. Chloramphenicol and tetracycline, which block bacterial protein synthesis, or ampicillin, which reduces Chlamydia replication, could not be used in conjunction with the probe, either because they modified its basal fluorescence or because they induced a massive production of ROS in the host cell. This prevented us from directly assessing whether bacterial protein synthesis was required for the initial ROS production. The slow kinetics of ROS appearance, which peaked only 3 to 5 h after infection, suggests that the transformation of the infectious particles into metabolically active bacteria is needed to stimulate the production of ROS. As a comparison, in HeLa cells infected with Shigella, ROS were detected as early as 45 min after infection (43). In addition, we observed a significant decrease in the ROS response when heat-killed bacteria were used, suggesting that cell contact and intrusion of the bacteria (also achieved to some extent by heat-killed bacteria) are not sufficient to trigger the ROS response. Importantly, during the whole infectious cycle, Chlamydia bacteria remain in a membrane-bounded compartment, limiting the host response to the bacterial features recognized by pattern recognition molecules located in the cytosol (such as nucleotide-binding oligomerization domain [NOD] molecules) or bound to cellular organelles. Moreover, Chlamydia lipopolysaccharide (LPS), which could potentially be sensed at the entry site or on the inclusion membrane, was shown to be about 100-fold less potent than S. enterica serovar Minnesota or Neisseria gonorrhoeae LPS in inducing an inflammatory cytokine response (29). Our observation that heat-killed bacteria induced an intermediate ROS response (Fig. 1B) also supports the hypothesis that LPS is not the main inducer of ROS production. Altogether, these data suggest that bacterial components other than PAMPs might initiate the ROS response observed in Chlamydia infection. In particular, proteins that are secreted in the host cell by a type III secretion mechanism early during infection (46) are potential triggers of the ROS response. Later in the developmental cycle, a recent study suggests that PAMPs are detected by the host cell, as the IL-8 response induced 30 h after infection is dependent on NOD1 signaling (13).
The second major finding of this study is the observation that ROS production is only transient and returns to basal levels around 9 h p.i. This means that it is turned off while the host cell is still facing an intrusive situation, and the bacteria start multiplying. One possibility is that the decrease in ROS levels results from the inactivation of NADPH oxidase. In support of this model, we observed that NADPH oxidase could no longer be activated by IL-1β 9 h after the infection (Fig. 3A). Moreover, in cells expressing GFP-tagged Rac1, this cytosolic component of NADPH oxidase was recruited to the inclusion membrane at this time point but not at earlier times, when ROS are being produced (Fig. 4). We were unable to detect endogenous Rac1 because of the high background level of the antibodies we tested. However, we can speculate that, considering endogenous levels, recruitment of this small GTPase to the inclusion membrane might result in a significant decrease in the pool of protein available for NADPH oxidase activation. In contrast, the localization of other subunits of the enzyme, such as p47phox and p22phox, did not change during infection. These data are consistent with a model in which Rac1 is specifically sequestered at the inclusion membrane, preventing the activation of the host NADPH oxidase. Targeting of this enzymatic activity by pathogens was previously illustrated in several cases, all in phagocytes. Helicobacter pylori disrupted the targeting of NADPH oxidase activity in neutrophils, preventing ROS production in the phagosome (2). Similarly, S. enterica serovar Typhimurium and Legionella pneumophila abrogated the NADPH oxidase-dependent respiratory burst in macrophages (28, 47). Interestingly, early attempts to measure ROS production upon contact between Chlamydia and phagocytes also suggested that the pathogen might disrupt the NADPH oxidase activity of phagocytes (44). However, in that case, the abrogation of the expected respiratory burst was very rapid (5 min) in neutrophils while Rac1 sequestration in epithelial cells occurred much later in infection, suggesting that different mechanisms are involved. In epithelial cells, Rac1 might be sequestered at the inclusion membrane through its association with one of the numerous Inc proteins translocated by a type III-dependent mechanism into the inclusion membrane (42). Some of these proteins are expressed only after several hours of infection, which could explain the delayed appearance of GFP-Rac1 at the inclusion membrane.
For the bacteria, the benefits of disrupting the activity of NADPH oxidase could be of two kinds. First, it prevents toxic effects that might result from prolonged ROS production. Even if ROS production remains moderate in epithelial cells, local concentrations of ROS might be sufficient to compromise the development of some of the internalized bacteria. Interestingly, transcripts of the gene coding for the bacterial superoxide dismutase SodM were detected 8 h after infection (8). This observation suggests that an increase in O2− levels might be sensed by the bacteria, which respond by increasing their antioxidant capacity. Second, ROS signaling is known to potentiate proinflammatory pathways (24). Although C. trachomatis infection elicits a proinflammatory response in epithelial cells, this response is not as immediate as with other bacteria (22). It is delayed until 20 to 24 h p.i. (38), at which time new infectious particles have already started forming. Interestingly, we observed that 24 h p.i. ROS levels had returned to elevated levels in infected cells, suggesting that the decrease in ROS levels is only transient. The early disruption of ROS production might contribute to the delay in the host innate response to infection.
Acknowledgments
We thank Valérie Malardé for excellent technical help and Paul Lazarow for his critical comments on the manuscript.
This work was supported by the Agence Nationale pour la Recherche (ANR-06-JCJC-0105) and the ERA-NET PathoGenoMics (ECIBUG).
Editor: A. Camilli
Footnotes
Published ahead of print on 26 October 2009.
REFERENCES
- 1.Abdelrahman, Y. M., and R. J. Belland. 2005. The chlamydial developmental cycle. FEMS Microbiol. Rev. 29:949-959. [DOI] [PubMed] [Google Scholar]
- 2.Allen, L. A., B. R. Beecher, J. T. Lynch, O. V. Rohner, and L. M. Wittine. 2005. Helicobacter pylori disrupts NADPH oxidase targeting in human neutrophils to induce extracellular superoxide release. J. Immunol. 174:3658-3667. [DOI] [PubMed] [Google Scholar]
- 3.Ambasta, R. K., P. Kumar, K. K. Griendling, H. H. Schmidt, R. Busse, and R. P. Brandes. 2004. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J. Biol. Chem. 279:45935-45941. [DOI] [PubMed] [Google Scholar]
- 4.Andersen, J. K. 2004. Oxidative stress in neurodegeneration: cause or consequence? Nat. Med. 10(Suppl.):S18-S25. [DOI] [PubMed] [Google Scholar]
- 5.Balsara, Z. R., N. R. Roan, L. N. Steele, and M. N. Starnbach. 2006. Developmental regulation of Chlamydia trachomatis class I accessible protein-1, a CD8+ T cell antigen. J. Infect. Dis. 193:1459-1463. [DOI] [PubMed] [Google Scholar]
- 6.Banfi, B., R. A. Clark, K. Steger, and K. H. Krause. 2003. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J. Biol. Chem. 278:3510-3513. [DOI] [PubMed] [Google Scholar]
- 7.Bedard, K., and K. H. Krause. 2007. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 87:245-313. [DOI] [PubMed] [Google Scholar]
- 8.Belland, R. J., G. Zhong, D. D. Crane, D. Hogan, D. Sturdevant, J. Sharma, W. L. Beatty, and H. D. Caldwell. 2003. Genomic transcriptional profiling of the developmental cycle of Chlamydia trachomatis. Proc. Natl. Acad. Sci. USA 100:8478-8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Betts, H. J., K. Wolf, and K. A. Fields. 2009. Effector protein modulation of host cells: examples in the Chlamydia spp. arsenal. Curr. Opin. Microbiol. 12:81-87. [DOI] [PubMed] [Google Scholar]
- 10.Binker, M. G., A. A. Binker-Cosen, D. Richards, B. Oliver, and L. I. Cosen-Binker. 2009. EGF promotes invasion by PANC-1 cells through Rac1/ROS-dependent secretion and activation of MMP-2. Biochem. Biophys. Res. Commun. 379:445-450. [DOI] [PubMed] [Google Scholar]
- 11.Boleti, H., A. Benmerah, D. M. Ojcius, N. Cerf-Bensussan, and A. Dautry-Varsat. 1999. Chlamydia infection of epithelial cells expressing dynamin and Eps15 mutants: clathrin-independent entry into cells and dynamin-dependent productive growth. J. Cell Sci. 112:1487-1496. [DOI] [PubMed] [Google Scholar]
- 12.Buchholz, K. R., and R. S. Stephens. 2006. Activation of the host cell proinflammatory interleukin-8 response by Chlamydia trachomatis. Cell Microbiol. 8:1768-1779. [DOI] [PubMed] [Google Scholar]
- 13.Buchholz, K. R., and R. S. Stephens. 2008. The cytosolic pattern recognition receptor NOD1 induces inflammatory interleukin-8 during Chlamydia trachomatis infection. Infect. Immun. 76:3150-3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buchholz, K. R., and R. S. Stephens. 2007. The extracellular signal-regulated kinase/mitogen-activated protein kinase pathway induces the inflammatory factor interleukin-8 following Chlamydia trachomatis infection. Infect. Immun. 75:5924-5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casbon, A. J., L. A. Allen, K. W. Dunn, and M. C. Dinauer. 2009. Macrophage NADPH oxidase flavocytochrome B localizes to the plasma membrane and Rab11-positive recycling endosomes. J. Immunol. 182:2325-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen, K., M. T. Kirber, H. Xiao, Y. Yang, and J. F. Keaney, Jr. 2008. Regulation of ROS signal transduction by NADPH oxidase 4 localization. J. Cell Biol. 181:1129-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng, G., Z. Cao, X. Xu, E. G. van Meir, and J. D. Lambeth. 2001. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene 269:131-140. [DOI] [PubMed] [Google Scholar]
- 18.Cheng, W., P. Shivshankar, Y. Zhong, D. Chen, Z. Li, and G. Zhong. 2008. Intracellular interleukin-1alpha mediates interleukin-8 production induced by Chlamydia trachomatis infection via a mechanism independent of type I interleukin-1 receptor. Infect. Immun. 76:942-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clausen, J. D., G. Christiansen, H. U. Holst, and S. Birkelund. 1997. Chlamydia trachomatis utilizes the host cell microtubule network during early events of infection. Mol. Microbiol. 25:441-449. [DOI] [PubMed] [Google Scholar]
- 20.Clifton, D. R., K. A. Fields, S. S. Grieshaber, C. A. Dooley, E. R. Fischer, D. J. Mead, R. A. Carabeo, and T. Hackstadt. 2004. A chlamydial type III translocated protein is tyrosine-phosphorylated at the site of entry and associated with recruitment of actin. Proc. Natl. Acad. Sci. USA 101:10166-10171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D'Autreaux, B., and M. B. Toledano. 2007. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 8:813-824. [DOI] [PubMed] [Google Scholar]
- 22.Eckmann, L., M. F. Kagnoff, and J. Fierer. 1993. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect. Immun. 61:4569-4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fields, K. A., D. J. Mead, C. A. Dooley, and T. Hackstadt. 2003. Chlamydia trachomatis type III secretion: evidence for a functional apparatus during early-cycle development. Mol. Microbiol. 48:671-683. [DOI] [PubMed] [Google Scholar]
- 24.Gloire, G., S. Legrand-Poels, and J. Piette. 2006. NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem. Pharmacol. 72:1493-1505. [DOI] [PubMed] [Google Scholar]
- 25.Grandvaux, N., A. Soucy-Faulkner, and K. Fink. 2007. Innate host defense: Nox and Duox on phox's tail. Biochimie 89:1113-1122. [DOI] [PubMed] [Google Scholar]
- 26.Grieshaber, S. S., N. A. Grieshaber, and T. Hackstadt. 2003. Chlamydia trachomatis uses host cell dynein to traffic to the microtubule-organizing center in a p50 dynamitin-independent process. J. Cell Sci. 116:3793-3802. [DOI] [PubMed] [Google Scholar]
- 27.Guyton, K. Z., Y. Liu, M. Gorospe, Q. Xu, and N. J. Holbrook. 1996. Activation of mitogen-activated protein kinase by H2O2. Role in cell survival following oxidant injury. J. Biol. Chem. 271:4138-4142. [DOI] [PubMed] [Google Scholar]
- 28.Harada, T., M. Miyake, and Y. Imai. 2007. Evasion of Legionella pneumophila from the bactericidal system by reactive oxygen species (ROS) in macrophages. Microbiol. Immunol. 51:1161-1170. [DOI] [PubMed] [Google Scholar]
- 29.Ingalls, R. R., P. A. Rice, N. Qureshi, K. Takayama, J. S. Lin, and D. T. Golenbock. 1995. The inflammatory cytokine response to Chlamydia trachomatis infection is endotoxin mediated. Infect. Immun. 63:3125-3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeon, E. S., M. J. Lee, S. M. Sung, and J. H. Kim. 2007. Sphingosylphosphorylcholine induces apoptosis of endothelial cells through reactive oxygen species-mediated activation of ERK. J. Cell Biochem. 100:1536-1547. [DOI] [PubMed] [Google Scholar]
- 31.Jung, Y., H. Kim, S. H. Min, S. G. Rhee, and W. Jeong. 2008. Dynein light chain LC8 negatively regulates NF-kappaB through the redox-dependent interaction with IkappaBalpha. J. Biol. Chem. 283:23863-23871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawahara, T., M. Kohjima, Y. Kuwano, H. Mino, S. Teshima-Kondo, R. Takeya, S. Tsunawaki, A. Wada, H. Sumimoto, and K. Rokutan. 2005. Helicobacter pylori lipopolysaccharide activates Rac1 and transcription of NADPH oxidase Nox1 and its organizer NOXO1 in guinea pig gastric mucosal cells. Am. J. Physiol. Cell Physiol. 288:C450-C457. [DOI] [PubMed] [Google Scholar]
- 33.Kawahara, T., Y. Kuwano, S. Teshima-Kondo, R. Takeya, H. Sumimoto, K. Kishi, S. Tsunawaki, T. Hirayama, and K. Rokutan. 2004. Role of nicotinamide adenine dinucleotide phosphate oxidase 1 in oxidative burst response to Toll-like receptor 5 signaling in large intestinal epithelial cells. J. Immunol. 172:3051-3058. [DOI] [PubMed] [Google Scholar]
- 34.Kawahara, T., S. Teshima, A. Oka, T. Sugiyama, K. Kishi, and K. Rokutan. 2001. Type I Helicobacter pylori lipopolysaccharide stimulates toll-like receptor 4 and activates mitogen oxidase 1 in gastric pit cells. Infect. Immun. 69:4382-4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lambeth, J. D. 2004. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 4:181-189. [DOI] [PubMed] [Google Scholar]
- 36.Li, Q., M. M. Harraz, W. Zhou, L. N. Zhang, W. Ding, Y. Zhang, T. Eggleston, C. Yeaman, B. Banfi, and J. F. Engelhardt. 2006. Nox2 and Rac1 regulate H2O2-dependent recruitment of TRAF6 to endosomal interleukin-1 receptor complexes. Mol. Cell. Biol. 26:140-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Medzhitov, R. 2001. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1:135-145. [DOI] [PubMed] [Google Scholar]
- 38.Rasmussen, S. J., L. Eckmann, A. J. Quayle, L. Shen, Y. X. Zhang, D. J. Anderson, J. Fierer, R. S. Stephens, and M. F. Kagnoff. 1997. Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. J. Clin. Invest. 99:77-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rygiel, T. P., A. E. Mertens, K. Strumane, R. van der Kammen, and J. G. Collard. 2008. The Rac activator Tiam1 prevents keratinocyte apoptosis by controlling ROS-mediated ERK phosphorylation. J. Cell Sci. 121:1183-1192. [DOI] [PubMed] [Google Scholar]
- 40.Stamm, W. E. 1999. Chlamydia trachomatis infections: progress and problems. J. Infect. Dis. 179(Suppl. 2):S380-S383. [DOI] [PubMed] [Google Scholar]
- 41.Su, H., G. McClarty, F. Dong, G. M. Hatch, Z. K. Pan, and G. Zhong. 2004. Activation of Raf/MEK/ERK/cPLA2 signaling pathway is essential for chlamydial acquisition of host glycerophospholipids. J. Biol. Chem. 279:9409-9416. [DOI] [PubMed] [Google Scholar]
- 42.Subtil, A., C. Parsot, and A. Dautry-Varsat. 2001. Secretion of predicted Inc proteins of Chlamydia pneumoniae by a heterologous type III machinery. Mol. Microbiol. 39:792-800. [DOI] [PubMed] [Google Scholar]
- 43.Tattoli, I., L. A. Carneiro, M. Jehanno, J. G. Magalhaes, Y. Shu, D. J. Philpott, D. Arnoult, and S. E. Girardin. 2008. NLRX1 is a mitochondrial NOD-like receptor that amplifies NF-kappaB and JNK pathways by inducing reactive oxygen species production. EMBO Rep. 9:293-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tauber, A. I., N. Pavlotsky, J. S. Lin, and P. A. Rice. 1989. Inhibition of human neutrophil NADPH oxidase by Chlamydia serovars E, K, and L2. Infect. Immun. 57:1108-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tonks, N. K. 2005. Redox redux: revisiting PTPs and the control of cell signaling. Cell 121:667-670. [DOI] [PubMed] [Google Scholar]
- 46.Valdivia, R. H. 2008. Chlamydia effector proteins and new insights into chlamydial cellular microbiology. Curr. Opin. Microbiol. 11:53-59. [DOI] [PubMed] [Google Scholar]
- 47.Vazquez-Torres, A., Y. Xu, J. Jones-Carson, D. W. Holden, S. M. Lucia, M. C. Dinauer, P. Mastroeni, and F. C. Fang. 2000. Salmonella pathogenicity island 2-dependent evasion of the phagocyte NADPH oxidase. Science 287:1655-1658. [DOI] [PubMed] [Google Scholar]