Abstract
Lineage-specific responses from the effector T-cell repertoire form a critical component of adaptive immunity. The recent identification of Th17 cells—a third, distinct lineage of helper T cells—collapses the long-accepted paradigm in which Th1 and Th2 cells distinctly mediate cellular and humoral immunity, respectively. In this minireview, we discuss the involvement of the Th17 lineage during infection by extracellular bacteria, intracellular bacteria, and fungi. Emerging trends suggest that the Th17 population bridges innate and adaptive immunity to produce a robust antimicrobial inflammatory response. However, because Th17 cells mediate both host defense and pathological inflammation, elucidation of mechanisms that attenuate but do not completely abolish the Th17 response may have powerful implications for therapy.
Different classes of microbes elicit lineage-specific responses from the effector T-cell repertoire. Whereas helper T1 (Th1) cells are involved in cellular immunity against intracellular bacteria and viruses, Th2 cells mediate the humoral response to parasitic infection. Regulating the activation and expansion of these lineages are regulatory T cells (Tregs), a third subset of CD4+ T cells that holds the inflammatory response in check. Recently, the identification of a novel lineage of helper T cells, Th17, has collapsed the long-held paradigm that three lineages (Th1, Th2, and Treg) derive from naïve CD4+ T cells (Thp). Distinguished by the production of interleukin-17 (IL-17), these Th17 cells develop from Thp progenitors along a fourth distinct pathway under the influence of a network of inflammatory cytokines, including IL-1, IL-6, and transforming growth factor beta (TGF-β), which support commitment to this lineage, and IL-23, which stabilizes the phenotype of differentiated Th17 cells (27, 46, 70, 94). Studies of infectious disease highlight the critical role of the Th17 response in host defense against extracellular pathogens, particularly Gram-negative bacteria that colonize mucosal surfaces. More recent observations implicate Th17 cells in the immune response to facultative and obligate intracellular pathogens, including intracellular bacteria and fungi. However, whether Th17 cells mediate essential antimicrobial mechanisms or drive tissue destruction in these contexts remains unclear. This minireview will focus first on the general mechanistic paradigm associated with IL-17-mediated host defense and then discuss findings on the involvement of the Th17 response during infection by extracellular and intracellular bacteria and fungi. Emerging trends highlight the capacity of Th17 cells to bridge innate and adaptive immunity, while balancing the host on the precarious precipice between protective immunity and pathological inflammation.
PROTECTIVE MECHANISMS OF THE IL-17 AXIS
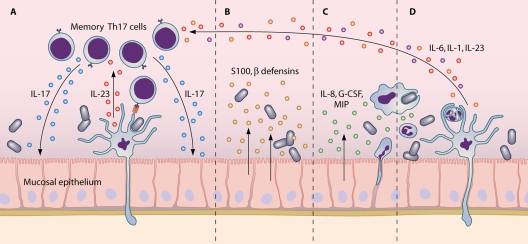
Seminal studies linked increased morbidity during mucosal infection to dysfunctional IL-17 signaling (22, 61, 99). Consequently, interest grew in the protective mechanisms that characterize the Th17 response (Fig. 1). Production of IL-17 by CD4+ T cells has been demonstrated during the first 24 h of infection with extracellular bacteria (12, 26). Such activity is consistent with peripheral populations of constitutively activated IL-17-producing T cells observed in uninfected mice and implicates Th17 cells in immune surveillance and the first line of host defense (74). Additionally, IL-17 production has been observed from CD4+ T cells during a later time frame that coincides with the elaboration of adaptive immunity (89, 105). Evidence suggests that efficient Th17 differentiation requires high-dose antigen (Ag) stimulation through the T-cell receptor and additional stimulation through the costimulatory surface receptor CD40. (36, 73) Stimuli associated with severe infection—like coincidental activation of complement and Toll-like receptors (TLRs) or significant apoptosis in the intestinal epithelium—favor Th17 differentiation at the expense of the Th1 lineage (21, 89). Such stimuli promote proinflammatory cytokine release from antigen-presenting cells: of these, dendritic cell (DC)-derived IL-6 is critical for Th17 differentiation in murine models (21, 73, 90). In addition to traditional Th17 cells, CD8+ T cells (26), activated memory γδ T cells (66, 81, 85), and invariant natural killer T cells (19, 56) have been identified as IL-17 producers during the early response to extracellular infection. However, the extent to which IL-17 production from these alternative sources is crucial, compensatory, or redundant likely varies with each infection (26, 81).
FIG. 1.
Mucosal infection engages the Th17 axis of host defense. (A) Peripheral dendritic cells activated by pathogens produce IL-23, which upregulates IL-17 and IL-22 production from resident memory Th17 cells. (B) Synergy between IL-17 and IL-22 induces secretion of antimicrobial peptides from epithelial cells. (C) IL-17 also drives epithelial expression of granulopoeitic and chemotactic factors. (D) As innate immune cells accumulate at the mucosal surface, dendritic cells that have phagocytosed infected, apoptotic neutrophils respond by secreting proinflammatory cytokines that support Th17 differentiation from uncommitted infiltrating CD4+ T cells.
The near-ubiquitous expression of the IL-17 receptor facilitates the pleiotropic effector mechanisms of IL-17. Alone or in synergy, the Th17 effector cytokines IL-17 and IL-22 induce an array of antimicrobial peptides, including β-defensin-2, lipocalin 2, and members of the S100 family from colonic, skin, and airway epithelia (4, 11, 41, 50, 105, 106). Cytokines involved in the Th17 response, IL-1 and IL-6, are implicated in protecting the integrity of the colonic epithelium during bacterial infection (17, 48). IL-17 also induces chemokine expression in nonimmune cells. Upregulation of neutrophil-chemotactic IL-8 (CXCL8), macrophage inflammatory proteins 1 and 2 (MIP-1 and -2), and macrophage/monocyte-chemotactic protein 1 (MCP-1) (CCL20) by IL-17 turns infected sites into an innate immune cell sink (45, 61, 99, 105). IL-17 enhances the function and survival of recruited macrophages (80), and its effects on granulocytes appear even more pronounced. In addition to mediating neutrophil recruitment, IL-17 promotes granulopoiesis via the induction of granulocyte colony-stimulating factor (G-CSF) and stem cell factor (SCF) and potentiates neutrophilic cytotoxicity and phagocytosis (32, 53, 99). It is tempting to speculate that this neutrophil influx reinforces the involvement of the IL-17 axis by providing stimuli involved in Th17 differentiation. As recruited neutrophils apoptose and are phagocytosed by resident activated antigen-presenting cells, naïve-T-cell fate may be further biased toward the Th17 lineage (89), thus giving rise to Th17-associated adaptive immunity. Although IL-17 primarily galvanizes the innate branch of host defense, recent evidence suggests a role for this cytokine in initiating the B-cell response, an aspect of immunity typically associated with Th2-related follicular T cells and Th2 cytokines (75, 101). In an ovalbumin-induced murine model of airway inflammation, adoptive transfer of Th17 clones leads to the induction of IgA and IgM and the recruitment of B cells (38), a function that may be supported by the preferential expression of B-cell chemoattractant CXCL13 on Th17 cells (88, 101).
The mechanisms by which the Th17 response is attenuated postinfection remain ill defined. Binding of IL-17 to its receptor has been proposed to play a role in clearance of this cytokine (85), and the induction of Th17 cells that coexpress the anti-inflammatory cytokine IL-10 may lead to a subsequent stage of dampened inflammation (89). As bacterial burden decreases, phagocytosis of apoptotic but increasingly uninfected neutrophils by DCs may downregulate the Th17-stabilizing cytokine IL-23 (86). Notably, the vaccine-induced kinetics of Th1 and Th17 populations suggest that activation of the IL-17 axis may partially induce a subsequent Th1 response that then attenuates IL-17 production through inhibitory effects of the Th1-produced cytokine gamma interferon (IFN-γ) (43, 65, 74). On the other hand, abundance of the antiapoptotic protein c-FLIP in Th17 cells decreases their susceptibility to activation-induced cell death, a known mechanism of clearance of Th1 cells (100). The Th17 subset also appears to subvert Treg-driven immunosuppression; unlike IFN-γ, Th17-associated IL-6 inhibits rather than enhances tryptophan catabolism, which is a process associated with Treg induction (18). In the absence of attenuation, Th17-associated effector mechanisms can mediate tissue injury through protracted local inflammation, a feature of certain chronic biofilm infections (20, 29, 78, 84).
EXTRACELLULAR BACTERIAL PATHOGENS
According to the Th1/Th2 paradigm, different subsets of helper T cells protect against distinct types of infections; while Th1 cells primarily respond to intracellular pathogens, Th2 cells are principally associated with humoral immunity. Similarly, Th17 cells are thought to mediate immunity against another class of pathogen: extracellular bacteria, particularly those that colonize exposed surfaces like the airways, skin, and intestinal lumen. Mice infected by Klebsiella pneumoniae (25, 99), Bordetella pertussis (30), or Streptococcus pneumoniae (53, 105), for example, mount a Th17 response, and disruption of IL-17 signaling increases susceptibility in these models. Intact signaling appears particularly critical for the induction of a chemokine gradient that facilitates the recruitment of innate immune cells to the site of infection; however, the extent to which monocytes/macrophages or neutrophils account for the bulk of the IL-17-mediated cellular infiltrate varies with the infection (81, 98, 99, 105). Deficiency in IL-23, a cytokine that induces IL-17 production from memory cells and stabilizes the Th17 phenotype in effector cells (2, 87), also exacerbates pathology, but at least in the case of K. pneumoniae infection, exogenous IL-17 can compensate for the absence of IL-23 (25, 81). A critical role for the IL-23-IL-17 axis in these infectious models is supported by vaccination studies; activation of the Th17 lineage by pertussis toxin and pneumococcal antigen is required to confer full protection against subsequent infection (30, 53, 55).
Although host defense against extracellular bacteria is widely considered the Th17 domain, some evidence indicates that efficient protection requires synergy between the Th1 and Th17 lineages. For example, challenge by Bordetella pertussis induces both transient IFN-γ production that coincides with the peak phase of infection and extended IL-17 production that precedes the Th1 response and persists until clearance. Continuous neutralization of IL-17 modestly increases early bacterial load, but neither delays nor impedes ultimate resolution (3). This finding supports other work that suggests a role for the Th1 response in clearance (3, 57). Similarly, IL-12- and IL-23-deficient mice have targeted defects in the Th1 and Th17 networks, respectively, but both experience increased though comparable susceptibility to Klebsiella pneumoniae. Administration of IL-17 more effectively attenuates pathology in IL-23-deficient than in IL-23-/IL-12-deficient mice, suggesting a unique protective role for the Th1 axis (25). Thus, whether it would be more accurate to classify extracellular bacterial pathogens under a shared Th1/Th17 domain of host defense remains to be elucidated. Further contributing to this uncertainty are indications of the potentially detrimental nature of the Th17 response elicited by certain extracellular pathogens. Th17 cell infiltrates contribute to pathological abscess formation in response to Bacteroides fragilis (12), and modulation of macrophage function toward a Th17-conducive phenotype may facilitate persistent colonization by Bordetella bronchiseptica (82).
Chronic microbial infections in the setting of human disease underscore the precarious balance between inadequate host defense and unregulated inflammation. Immunocompromised hosts with defects in Th17 expansion, including HIV/AIDS patients and individuals with hyperimmunoglobulin E syndrome (HIES, or Job's syndrome), are susceptible to opportunistic infections by Staphylococcus aureus, Haemophilus influenzae, and Streptococcus pneumoniae, among others (15, 31, 59, 72). In HIV infection, Th17 cells are preferentially depleted from the gastrointestinal lymphocyte compartment, despite the unresponsiveness of this subset to viral antigens (9). Because the Th17 lineage is critical for mucosal homeostasis, their selective depletion compromises intestinal integrity and facilitates the translocation of microbial products across the epithelial barrier during progressive disease (10). However, there is some indication that infection does not similarly deplete the bronchoalveolar Th17 population (9), so the precise link between defects in the IL-17 axis and the high incidence of pneumonia remains unclear. In the case of HIES, mutations in the signal transducer and activator of transcription-3 (STAT3) gene preclude the generation observed in healthy patients of Th17 memory cells against common microbes, such as Staphylococcus aureus and Candida albicans (54, 58). Although HIES is characterized by a systemic deficiency in IL-17 production, recurrent staphylococcal infections are typically confined to the skin and lung. However, the bronchial and dermal epithelium appears particularly sensitive to synergy between IL-17 and classical proinflammatory cytokines, and such dependence may account for this apparent contradiction (59).
Excessive activation of the Th17 lineage may help precipitate and sustain inflammatory diseases in which microbial antigens contribute to disease etiology and/or pathogenesis. For example, deficiency in IL-17 inhibits the development of a canonical form of hypersensitivity pneumonitis (40, 84), a set of pulmonary disorders that results from chronic exposure to inhaled antigen, including Saccharopolyspora rectivirgula and other pathogenic bacteria (5). Likewise, animal models of allergen-induced airway and contact hypersensitivity suggest that the hyperreactivity of the Th17 lineage toward microbial antigens and homologous endogenous ligands contributes to pathology (64, 71, 92, 96). This has potential implications for our understanding of their human counterparts—severe asthma and allergen contact dermatitis, in which elevated levels of IL-17 have been observed (39, 47, 52, 62). Thus, establishing the mechanisms that regulate the Th17 response without entirely abolishing it will have important implications for therapy development.
FUNGAL PATHOGENS
Studies examining T-cell polarization in response to pathogen-associated molecular patterns (PAMPs) have identified an array of fungal components that preferentially induce the Th17 lineage (1, 9), suggesting a role for Th17 cells in fungus-induced host defense. Interestingly, recognition of these fungal PAMPs can occur independently of signaling by Toll-like receptors (TLRs), the primary but not exclusive receptors for PAMPs (93). For example, deficiency in the adaptor TRIF (TIR domain-containing adapter-inducing IFN-β), a downstream mediator of TLR signaling, does not preclude Th17 development in response to infection by Candida albicans (18). Indeed, under certain conditions, TRIF deficiency even enhances IL-17 production by dampening signals from the Th1 and Treg lineages that inhibit Th17 development (6, 18). Accordingly, alternative pathogen recognition receptors have been identified that bypass TLR signaling to activate the Th17 lineage. One such cascade engages dectin-1, a β-glucan receptor on antigen-presenting cells that can signal in concert with or independently of TLR2. In response to certain fungal glucans, dectin-1 sidesteps synergy with TLR2 and activates DCs by engaging the kinase Syk and adaptor CARD9, ultimately resulting in the induction of Th17 cells (49). In another example, monocytes activated through the mannose receptor by C. albicans mannan drive IL-17 production during coculture with human memory T cells (93). Such findings may have important implications not only for understanding the host response to fungal pathogens, but also in the context of autoimmunity. Further studies of these alternative PAMP receptors may suggest new targets for adjuvants that preferentially skew the T-cell repertoire toward certain lineages and may also reveal homologous endogenous ligands that drive autoimmune pathology (7).
Consistent with the ability of fungal components to drive IL-17 production, some animal models of opportunistic fungal infections suggest a positive, and in some cases essential, role for Th17 cells. Neutralization of or deficiency in IL-23 or IL-17 during disseminated and oral candidiasis and pulmonary aspergillus, among others, exacerbates pathology characterized by inadequate neutrophil recruitment, increased fungal burden, and reduced chemokine and antimicrobial expression (13, 35, 77, 95). In support of these findings, reexposure to antigen drives IL-17 production by human memory T cells specific for C. albicans antigen (1, 93, 95). However, vaccination models of Ag-induced protection and infection by C. albicans in TRIF−/− mice have linked the Th17 lineage to deleterious inflammation rather than protective immunity and buttress the prior emphasis on the Th1 lineage for host defense (7, 18, 68). Several of the fungal antigens identified as nonprotective in vaccine drive DCs toward an alternative activation state characterized by IL-23 rather than IL-12 production, thus supporting the expansion of the Th17 (sometimes concomitantly with the Th2) lineage at the expense of the Th1 and Treg lineages (18). Such bias results in tissue injury and reduced fungal clearance, in part by impairing the killing activity while extending the viability of recruited neutrophils (8, 102). Furthermore, expansion of the Th17 subset establishes a positive feedback loop that favors the Th17 response (18, 102) and inhibits the induction of Tregs required for homeostatic tolerance to commensal but opportunistic fungal pathogens (6, 63).
INTRACELLULAR BACTERIAL PATHOGENS
Infection by intracellular bacteria is classically considered the Th1 domain of host defense. However, current knowledge of the immune response during listeriosis illustrates the degree of uncertainty that remains about the role of IL-17 in host defense against this class of pathogen. A facultative intracellular bacterium, Listeria monocytogenes, tends to colonize phagocytes and induces a robust response associated with the prototypical Th1 cytokine IFN-γ (23, 33, 34). However, the essential role of IFN-γ has been called into question by studies demonstrating that IFN-γ deficiency does not preclude the generation of Ag-specific CD4+ T cells (28). Indeed, additional factors unassociated with the Th1 lineage, including IL-1 and IL-6, have been increasingly recognized for their protective role (37, 51, 67, 76). Additionally, production of IL-6 is linked to early neutrophilia, a phenomenon required for resolution of L. monocytogenes infection (16).
At the time of these studies, Th17 cells had not yet been distinguished as a distinct subset of helper T cells. Given that IL-6 and IL-1 are cytokines intimately associated with the Th17 lineage, which is known to mediate granulopoeisis and neutrophil chemotaxis, the possibility emerges that the Th17 lineage may be critical for the early host response to L. monocytogenes. Recent studies verify this link, with some evidence arguing for an essential role for IL-17. IL-17 production is localized to a third of hepatic γδ T cells and increases during the first 5 days of L. monocytogenes infection (24). IL-17A−/− mice experience higher morbidity than their wild-type counterparts, and infection in knockouts is characterized by increased bacterial burden, decreased neutrophilic induction, and malformed granulomatous lesions (24, 60). However, other evidence suggests that the contribution of IL-17 may serve a more compensatory function under unfavorable conditions. The absence of type I and II interferon signaling abrogates the Th1 response; in this situation, a low-magnitude Th17 response to Listeria is observed. This shift in CD4+ T-cell lineage commitment does not preclude resolution of infection (69). Cross-protection may be another method by which the Th17 lineage is recruited for anti-L. monocytogenes responses. Mice infected with Mycoplasma pulmonis exhibit enhanced protection against secondary infection with L. monocytogenes resulting from IL-17-induced neutrophil mobilization (83).
The lingering doubt about the role of IL-17 during L. monocytogenes infection reflects our generally incomplete picture of the Th17 lineage during host defense against intracellular bacteria. Studies of other intracellular infections, such as Salmonella enterica serovar Enteritidis (79), Toxoplasma gondii (42), and Chlamydia trachomatis (103), indicate a nonredundant role for IL-17 in complete bacterial clearance. Synergy between the Th1 and Th17 lineages has also been observed in response to postvaccination Mycobacterium tuberculosis challenge and Chlamydia muridarum, with some suggestion that deficiency in IL-17 impairs the expansion of antigen-specific Th1 cells (43, 91). Additionally, studies of primary infection by Mycobacterium tuberculosis indicate that the IL-23/IL-17 axis accounts for the modest protective response observed in the absence of Th1-associated IL-12 (14, 44). However, others suggest that the induction of a robust Th17 response is a detrimental supplement or even a malignant subversion of the protective Th1 response (97, 107). Another important consideration in evaluating the protection afforded by the Th17 lineage is the stage of infection. Some reports identify IL-17 function as part of the innate γδ T-cell response and neutrophil induction during the early response to intracellular bacteria (24, 79). However, findings diverge on the involvement of classical CD4+ Th17 cells and the Th17 adaptive immune response to these infections (79, 104). Some evidence indicates that IL-17 may bridge the innate and adaptive immune responses by supporting the generation of Th1 adaptive immunity; indeed, a murine model of vaccination against Mycobacterium tuberculosis suggests that IL-17 may play a crucial albeit indirect role in the recruitment of the Th1 cells required for efficient recall responses (43). It will be interesting to see whether underlying these diverging observations there are as yet undiscovered mechanisms that dictate the role of the IL-17 axis during infection by intracellular pathogens.
SUMMARY
A population that bridges innate and adaptive immunity, Th17 cells are uniquely primed to mediate surveillance and early defense during mucosal infections by extracellular bacteria. However, the intensity of IL-17-driven inflammation may undermine the Th1 lineage and subvert more sustainable antimicrobial responses during intracellular infection by certain bacterial and fungal pathogens. Thus, elucidation of mechanisms that govern the amplification and attenuation of the Th17 lineage will have important implications for therapy. Particularly in the context of chronic infectious diseases that afflict both immune-compromised and immunocompetent hosts, strategies that restrain but do not entirely abolish the Th17 response will likely be critical to avoid magnifying susceptibility or pathological inflammation.
Editor: J. B. Kaper
Footnotes
Published ahead of print on 9 November 2009.
REFERENCES
- 1.Acosta-Rodriguez, E. V., L. Rivino, J. Geginat, D. Jarrossay, M. Gattorno, A. Lanzavecchia, F. Sallusto, and G. Napolitani. 2007. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 8:639-646. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal, S., N. Ghilardi, M. H. Xie, F. J. de Sauvage, and A. L. Gurney. 2003. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 278:1910-1914. [DOI] [PubMed] [Google Scholar]
- 3.Andreasen, C., D. A. Powell, and N. H. Carbonetti. 2009. Pertussis toxin stimulates IL-17 production in response to Bordetella pertussis infection in mice. PLoS One 4:e7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aujla, S. J., Y. R. Chan, M. Zheng, M. Fei, D. J. Askew, D. A. Pociask, T. A. Reinhart, F. McAllister, J. Edeal, K. Gaus, S. Husain, J. L. Kreindler, P. J. Dubin, J. M. Pilewski, M. M. Myerburg, C. A. Mason, Y. Iwakura, and J. K. Kolls. 2008. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat. Med. 14:275-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogaert, P., K. G. Tournoy, T. Naessens, and J. Grooten. 2009. Where asthma and hypersensitivity pneumonitis meet and differ: noneosinophilic severe asthma. Am. J. Pathol. 174:3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonifazi, P., T. Zelante, C. D'Angelo, A. De Luca, S. Moretti, S. Bozza, K. Perruccio, R. G. Iannitti, G. Giovannini, C. Volpi, F. Fallarino, P. Puccetti, and L. Romani. 2009. Balancing inflammation and tolerance in vivo through dendritic cells by the commensal Candida albicans. Mucosal Immunol. 2:362-374. [DOI] [PubMed] [Google Scholar]
- 7.Bozza, S., C. Clavaud, G. Giovannini, T. Fontaine, A. Beauvais, J. Sarfati, C. D'Angelo, K. Perruccio, P. Bonifazi, S. Zagarella, S. Moretti, F. Bistoni, J.-P. Latge, and L. Romani. 2009. Immune sensing of Aspergillus fumigatus proteins, glycolipids, and polysaccharides and the impact on Th immunity and vaccination. J. Immunol. 183:2407-2414. [DOI] [PubMed] [Google Scholar]
- 8.Bozza, S., T. Zelante, S. Moretti, P. Bonifazi, A. DeLuca, C. D'Angelo, G. Giovannini, C. Garlanda, L. Boon, F. Bistoni, P. Puccetti, A. Mantovani, and L. Romani. 2008. Lack of Toll IL-1R8 exacerbates Th17 cell responses in fungal infection. J. Immunol. 180:4022-4031. [DOI] [PubMed] [Google Scholar]
- 9.Brenchley, J. M., M. Paiardini, K. S. Knox, A. I. Asher, B. Cervasi, T. E. Asher, P. Scheinberg, D. A. Price, C. A. Hage, L. M. Kholi, A. Khoruts, I. Frank, J. Else, T. Schacker, G. Silvestri, and D. C. Douek. 2008. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood 112:2826-2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brenchley, J. M., D. A. Price, T. W. Schacker, T. E. Asher, G. Silvestri, S. Rao, Z. Kazzaz, E. Bornstein, O. Lambotte, D. Altmann, B. R. Blazar, B. Rodriguez, L. Teixeira-Johnson, A. Landay, J. N. Martin, F. M. Hecht, L. J. Picker, M. M. Lederman, S. G. Deeks, and D. C. Douek. 2006. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 12:1365-1371. [DOI] [PubMed] [Google Scholar]
- 11.Chan, Y. R., J. S. Liu, D. A. Pociask, M. Zheng, T. A. Mietzner, T. Berger, T. W. Mak, M. C. Clifton, R. K. Strong, P. Ray, and J. K. Kolls. 2009. Lipocalin 2 is required for pulmonary host defense against Klebsiella infection. J. Immunol. 182:4947-4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung, D. R., D. L. Kasper, R. J. Panzo, T. Chitnis, M. J. Grusby, M. H. Sayegh, and A. O. Tzianabos. 2003. CD4+ T cells mediate abscess formation in intra-abdominal sepsis by an IL-17-dependent mechanism. J. Immunol. 170:1958-1963. [DOI] [PubMed] [Google Scholar]
- 13.Conti, H. R., F. Shen, N. Nayyar, E. Stocum, J. N. Sun, M. J. Lindemann, A. W. Ho, J. H. Hai, J. J. Yu, J. W. Jung, S. G. Filler, P. Masso-Welch, M. Edgerton, and S. L. Gaffen. 2009. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J. Exp. Med. 206:299-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper, A. M., A. Kipnis, J. Turner, J. Magram, J. Ferrante, and I. M. Orme. 2002. Mice lacking bioactive IL-12 can generate protective, antigen-specific cellular responses to mycobacterial infection only if the IL-12 p40 subunit is present. J. Immunol. 168:1322-1327. [DOI] [PubMed] [Google Scholar]
- 15.Crum-Cianflone, N., J. Weekes, and M. Bavaro. 2009. Recurrent community-associated methicillin-resistant Staphylococcus aureus infections among HIV-infected persons: incidence and risk factors. AIDS Patient Care STDs 23:499-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dalrymple, S. A., L. A. Lucian, R. Slattery, T. McNeil, D. M. Aud, S. Fuchino, F. Lee, and R. Murray. 1995. Interleukin-6-deficient mice are highly susceptible to Listeria monocytogenes infection: correlation with inefficient neutrophilia. Infect. Immun. 63:2262-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dann, S. M., M. E. Spehlmann, D. C. Hammond, M. Iimura, K. Hase, L. J. Choi, E. Hanson, and L. Eckmann. 2008. IL-6-dependent mucosal protection prevents establishment of a microbial niche for attaching/effacing lesion-forming enteric bacterial pathogens. J. Immunol. 180:6816-6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Luca, A., C. Montagnoli, T. Zelante, P. Bonifazi, S. Bozza, S. Moretti, C. D'Angelo, C. Vacca, L. Boon, F. Bistoni, P. Puccetti, F. Fallarino, and L. Romani. 2007. Functional yet balanced reactivity to Candida albicans requires TRIF, MyD88, and IDO-dependent inhibition of Rorc. J. Immunol. 179:5999-6008. [DOI] [PubMed] [Google Scholar]
- 19.Doisne, J. M., C. Becourt, L. Amniai, N. Duarte, J. B. Le Luduec, G. Eberl, and K. Benlagha. 2009. Skin and peripheral lymph node invariant NKT cells are mainly retinoic acid receptor-related orphan receptor (gamma)t+ and respond preferentially under inflammatory conditions. J. Immunol. 183:2142-2149. [DOI] [PubMed] [Google Scholar]
- 20.Dubin, P. J., and J. K. Kolls. 2007. IL-23 mediates inflammatory responses to mucoid Pseudomonas aeruginosa lung infection in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 292:L519-L528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang, C., X. Zhang, T. Miwa, and W. C. Song. 2009. Complement promotes the development of inflammatory T-helper 17 cells through synergistic interaction with Toll-like receptor signaling and interleukin-6 production. Blood 114:1005-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freitas, A., J. C. Alves-Filho, T. Victoni, T. Secher, H. P. Lemos, F. Sonego, F. Q. Cunha, and B. Ryffel. 2009. IL-17 receptor signaling is required to control polymicrobial sepsis. J. Immunol. 182:7846-7854. [DOI] [PubMed] [Google Scholar]
- 23.Geginat, G., M. Lalic, M. Kretschmar, W. Goebel, H. Hof, D. Palm, and A. Bubert. 1998. Th1 cells specific for a secreted protein of Listeria monocytogenes are protective in vivo. J. Immunol. 160:6046-6055. [PubMed] [Google Scholar]
- 24.Hamada, S., M. Umemura, T. Shiono, K. Tanaka, A. Yahagi, M. D. Begum, K. Oshiro, Y. Okamoto, H. Watanabe, K. Kawakami, C. Roark, W. K. Born, R. O'Brien, K. Ikuta, H. Ishikawa, S. Nakae, Y. Iwakura, T. Ohta, and G. Matsuzaki. 2008. IL-17A produced by gammadelta T cells plays a critical role in innate immunity against Listeria monocytogenes infection in the liver. J. Immunol. 181:3456-3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Happel, K. I., P. J. Dubin, M. Zheng, N. Ghilardi, C. Lockhart, L. J. Quinton, A. R. Odden, J. E. Shellito, G. J. Bagby, S. Nelson, and J. K. Kolls. 2005. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J. Exp. Med. 202:761-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Happel, K. I., M. Zheng, E. Young, L. J. Quinton, E. Lockhart, A. J. Ramsay, J. E. Shellito, J. R. Schurr, G. J. Bagby, S. Nelson, and J. K. Kolls. 2003. Cutting edge: roles of Toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J. Immunol. 170:4432-4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrington, L. E., R. D. Hatton, P. R. Mangan, H. Turner, T. L. Murphy, K. M. Murphy, and C. T. Weaver. 2005. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6:1123-1132. [DOI] [PubMed] [Google Scholar]
- 28.Harty, J. T., and M. J. Bevan. 1995. Specific immunity to Listeria monocytogenes in the absence of IFN gamma. Immunity 3:109-117. [DOI] [PubMed] [Google Scholar]
- 29.He, R., M. K. Oyoshi, H. Jin, and R. S. Geha. 2007. Epicutaneous antigen exposure induces a Th17 response that drives airway inflammation after inhalation challenge. Proc. Natl. Acad. Sci. U. S. A. 104:15817-15822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higgins, S. C., A. G. Jarnicki, E. C. Lavelle, and K. H. Mills. 2006. TLR4 mediates vaccine-induced protective cellular immunity to Bordetella pertussis: role of IL-17-producing T cells. J. Immunol. 177:7980-7989. [DOI] [PubMed] [Google Scholar]
- 31.Hirschtick, R. E., J. Glassroth, M. C. Jordan, T. C. Wilcosky, J. M. Wallace, P. A. Kvale, N. Markowitz, M. J. Rosen, B. T. Mangura, and P. C. Hopewell. 1995. Bacterial pneumonia in persons infected with the human immunodeficiency virus. Pulmonary Complications of HIV Infection Study Group. N Engl. J. Med. 333:845-851. [DOI] [PubMed] [Google Scholar]
- 32.Hoshino, H., M. Laan, M. Sjostrand, J. Lotvall, B. E. Skoogh, and A. Linden. 2000. Increased elastase and myeloperoxidase activity associated with neutrophil recruitment by IL-17 in airways in vivo. J. Allergy Clin. Immunol. 105:143-149. [DOI] [PubMed] [Google Scholar]
- 33.Hsieh, B., M. D. Schrenzel, T. Mulvania, H. D. Lepper, L. DiMolfetto-Landon, and D. A. Ferrick. 1996. In vivo cytokine production in murine listeriosis. Evidence for immunoregulation by gamma delta+ T cells. J. Immunol. 156:232-237. [PubMed] [Google Scholar]
- 34.Hsieh, C. S., S. E. Macatonia, C. S. Tripp, S. F. Wolf, A. O'Garra, and K. M. Murphy. 1993. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science 260:547-549. [DOI] [PubMed] [Google Scholar]
- 35.Huang, W., L. Na, P. L. Fidel, and P. Schwarzenberger. 2004. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J. Infect. Dis. 190:624-631. [DOI] [PubMed] [Google Scholar]
- 36.Iezzi, G., I. Sonderegger, F. Ampenberger, N. Schmitz, B. J. Marsland, and M. Kopf. 2009. CD40-CD40L cross-talk integrates strong antigenic signals and microbial stimuli to induce development of IL-17-producing CD4+ T cells. Proc. Natl. Acad. Sci. U. S. A. 106:876-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iizawa, Y., R. D. Wagner, and C. J. Czuprynski. 1993. Analysis of cytokine mRNA expression in Listeria-resistant C57BL/6 and Listeria-susceptible A/J mice during Listeria monocytogenes infection. Infect. Immun. 61:3739-3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaffar, Z., M. E. Ferrini, L. A. Herritt, and K. Roberts. 2009. Cutting edge: lung mucosal Th17-mediated responses induce polymeric Ig receptor expression by the airway epithelium and elevate secretory IgA levels. J. Immunol. 182:4507-4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jatakanon, A., C. Uasuf, W. Maziak, S. Lim, K. F. Chung, and P. J. Barnes. 1999. Neutrophilic inflammation in severe persistent asthma. Am. J. Respir. Crit. Care Med. 160:1532-1539. [DOI] [PubMed] [Google Scholar]
- 40.Joshi, A. D., D. J. Fong, S. R. Oak, G. Trujillo, K. R. Flaherty, F. J. Martinez, and C. M. Hogaboam. 2009. Interleukin-17-mediated immunopathogenesis in experimental hypersensitivity pneumonitis. Am. J. Respir. Crit. Care Med. 179:705-716. [DOI] [PubMed] [Google Scholar]
- 41.Kao, C. Y., Y. Chen, P. Thai, S. Wachi, F. Huang, C. Kim, R. W. Harper, and R. Wu. 2004. IL-17 markedly up-regulates beta-defensin-2 expression in human airway epithelium via JAK and NF-kappaB signaling pathways. J. Immunol. 173:3482-3491. [DOI] [PubMed] [Google Scholar]
- 42.Kelly, M. N., J. K. Kolls, K. Happel, J. D. Schwartzman, P. Schwarzenberger, C. Combe, M. Moretto, and I. A. Khan. 2005. Interleukin-17/interleukin-17 receptor-mediated signaling is important for generation of an optimal polymorphonuclear response against Toxoplasma gondii infection. Infect. Immun. 73:617-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khader, S. A., G. K. Bell, J. E. Pearl, J. J. Fountain, J. Rangel-Moreno, G. E. Cilley, F. Shen, S. M. Eaton, S. L. Gaffen, S. L. Swain, R. M. Locksley, L. Haynes, T. D. Randall, and A. M. Cooper. 2007. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol. 8:369-377. [DOI] [PubMed] [Google Scholar]
- 44.Khader, S. A., J. E. Pearl, K. Sakamoto, L. Gilmartin, G. K. Bell, D. M. Jelley-Gibbs, N. Ghilardi, F. deSauvage, and A. M. Cooper. 2005. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-{gamma} responses if IL-12p70 is available. J. Immunol. 175:788-795. [DOI] [PubMed] [Google Scholar]
- 45.Laan, M., Z. H. Cui, H. Hoshino, J. Lotvall, M. Sjostrand, D. C. Gruenert, B. E. Skoogh, and A. Linden. 1999. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J. Immunol. 162:2347-2352. [PubMed] [Google Scholar]
- 46.Langrish, C. L., Y. Chen, W. M. Blumenschein, J. Mattson, B. Basham, J. D. Sedgwick, T. McClanahan, R. A. Kastelein, and D. J. Cua. 2005. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201:233-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Larsen, J. M., C. M. Bonefeld, S. S. Poulsen, C. Geisler, and L. Skov. 2009. IL-23 and T(H)17-mediated inflammation in human allergic contact dermatitis. J. Allergy Clin. Immunol. 123:486-492. [DOI] [PubMed] [Google Scholar]
- 48.Lebeis, S. L., K. R. Powell, D. Merlin, M. A. Sherman, and D. Kalman. 2009. Interleukin-1 receptor signaling protects mice from lethal intestinal damage caused by the attaching and effacing pathogen Citrobacter rodentium. Infect. Immun. 77:604-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.LeibundGut-Landmann, S., O. Gross, M. J. Robinson, F. Osorio, E. C. Slack, S. V. Tsoni, E. Schweighoffer, V. Tybulewicz, G. D. Brown, J. Ruland, and C. Reis e Sousa. 2007. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat. Immunol. 8:630-638. [DOI] [PubMed] [Google Scholar]
- 50.Liang, S. C., X. Y. Tan, D. P. Luxenberg, R. Karim, K. Dunussi-Joannopoulos, M. Collins, and L. A. Fouser. 2006. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 203:2271-2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu, Z., R. J. Simpson, and C. Cheers. 1995. Interaction of interleukin-6, tumour necrosis factor and interleukin-1 during Listeria infection. Immunology 85:562-567. [PMC free article] [PubMed] [Google Scholar]
- 52.Lowes, M. A., T. Kikuchi, J. Fuentes-Duculan, I. Cardinale, L. C. Zaba, A. S. Haider, E. P. Bowman, and J. G. Krueger. 2008. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J. Invest. Dermatol. 128:1207-1211. [DOI] [PubMed] [Google Scholar]
- 53.Lu, Y. J., J. Gross, D. Bogaert, A. Finn, L. Bagrade, Q. Zhang, J. K. Kolls, A. Srivastava, A. Lundgren, S. Forte, C. M. Thompson, K. F. Harney, P. W. Anderson, M. Lipsitch, and R. Malley. 2008. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog. 4:e1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma, C. S., G. Y. Chew, N. Simpson, A. Priyadarshi, M. Wong, B. Grimbacher, D. A. Fulcher, S. G. Tangye, and M. C. Cook. 2008. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J. Exp. Med. 205:1551-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Malley, R., A. Srivastava, M. Lipsitch, C. M. Thompson, C. Watkins, A. Tzianabos, and P. W. Anderson. 2006. Antibody-independent, interleukin-17A-mediated, cross-serotype immunity to pneumococci in mice immunized intranasally with the cell wall polysaccharide. Infect. Immun. 74:2187-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Michel, M. L., A. C. Keller, C. Paget, M. Fujio, F. Trottein, P. B. Savage, C. H. Wong, E. Schneider, M. Dy, and M. C. Leite-de-Moraes. 2007. Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J. Exp. Med. 204:995-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mills, K. H., A. Barnard, J. Watkins, and K. Redhead. 1993. Cell-mediated immunity to Bordetella pertussis: role of Th1 cells in bacterial clearance in a murine respiratory infection model. Infect. Immun. 61:399-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Milner, J. D., J. M. Brenchley, A. Laurence, A. F. Freeman, B. J. Hill, K. M. Elias, Y. Kanno, C. Spalding, H. Z. Elloumi, M. L. Paulson, J. Davis, A. Hsu, A. I. Asher, J. O'Shea, S. M. Holland, W. E. Paul, and D. C. Douek. 2008. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature 452:773-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Minegishi, Y., M. Saito, M. Nagasawa, H. Takada, T. Hara, S. Tsuchiya, K. Agematsu, M. Yamada, N. Kawamura, T. Ariga, I. Tsuge, and H. Karasuyama. 2009. Molecular explanation for the contradiction between systemic Th17 defect and localized bacterial infection in hyper-IgE syndrome. J. Exp. Med. 206:1291-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miyamoto, M., M. Emoto, Y. Emoto, V. Brinkmann, I. Yoshizawa, P. Seiler, P. Aichele, E. Kita, and S. H. Kaufmann. 2003. Neutrophilia in LFA-1-deficient mice confers resistance to listeriosis: possible contribution of granulocyte-colony-stimulating factor and IL-17. J. Immunol. 170:5228-5234. [DOI] [PubMed] [Google Scholar]
- 61.Miyamoto, M., O. Prause, M. Sjostrand, M. Laan, J. Lotvall, and A. Linden. 2003. Endogenous IL-17 as a mediator of neutrophil recruitment caused by endotoxin exposure in mouse airways. J. Immunol. 170:4665-4672. [DOI] [PubMed] [Google Scholar]
- 62.Molet, S., Q. Hamid, F. Davoine, E. Nutku, R. Taha, N. Page, R. Olivenstein, J. Elias, and J. Chakir. 2001. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J. Allergy Clin. Immunol. 108:430-438. [DOI] [PubMed] [Google Scholar]
- 63.Montagnoli, C., A. Bacci, S. Bozza, R. Gaziano, P. Mosci, A. H. Sharpe, and L. Romani. 2002. B7/CD28-dependent CD4+CD25+ regulatory T cells are essential components of the memory-protective immunity to Candida albicans. J. Immunol. 169:6298-6308. [DOI] [PubMed] [Google Scholar]
- 64.Nakae, S., Y. Komiyama, A. Nambu, K. Sudo, M. Iwase, I. Homma, K. Sekikawa, M. Asano, and Y. Iwakura. 2002. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity 17:375-387. [DOI] [PubMed] [Google Scholar]
- 65.Nakagome, K., K. Okunishi, M. Imamura, H. Harada, T. Matsumoto, R. Tanaka, J. Miyazaki, K. Yamamoto, and M. Dohi. 2009. IFN-{gamma} attenuates antigen-induced overall immune response in the airway as a Th1-type immune regulatory cytokine. J. Immunol. 183:209-220. [DOI] [PubMed] [Google Scholar]
- 66.Nakamura, R., K. Shibata, H. Yamada, K. Shimoda, K. Nakayama, and Y. Yoshikai. 2008. Tyk2-signaling plays an important role in host defense against Escherichia coli through IL-23-induced IL-17 production by gammadelta T cells. J. Immunol. 181:2071-2075. [DOI] [PubMed] [Google Scholar]
- 67.Nakane, A., A. Numata, and T. Minagawa. 1992. Endogenous tumor necrosis factor, interleukin-6, and gamma interferon levels during Listeria monocytogenes infection in mice. Infect. Immun. 60:523-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Netea, M. G., A. G. Vonk, M. van den Hoven, I. Verschueren, L. A. Joosten, J. H. van Krieken, W. B. van den Berg, J. W. Van der Meer, and B. J. Kullberg. 2003. Differential role of IL-18 and IL-12 in the host defense against disseminated Candida albicans infection. Eur. J. Immunol. 33:3409-3417. [DOI] [PubMed] [Google Scholar]
- 69.Orgun, N. N., M. A. Mathis, C. B. Wilson, and S. S. Way. 2008. Deviation from a strong Th1-dominated to a modest Th17-dominated CD4 T cell response in the absence of IL-12p40 and type I IFNs sustains protective CD8 T cells. J. Immunol. 180:4109-4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Park, H., Z. Li, X. O. Yang, S. H. Chang, R. Nurieva, Y. H. Wang, Y. Wang, L. Hood, Z. Zhu, Q. Tian, and C. Dong. 2005. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 6:1133-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Park, S. J., K. S. Lee, S. R. Kim, K. H. Min, Y. H. Choe, H. Moon, H. J. Chae, W. H. Yoo, and Y. C. Lee. 2009. Peroxisome proliferator-activated receptor {gamma} agonist down-regulates IL-17 expression in a murine model of allergic airway inflammation. J. Immunol. 183:3259-3267. [DOI] [PubMed] [Google Scholar]
- 72.Paulson, M. L., A. F. Freeman, and S. M. Holland. 2008. Hyper IgE syndrome: an update on clinical aspects and the role of signal transducer and activator of transcription 3. Curr. Opin. Allergy Clin. Immunol. 8:527-533. [DOI] [PubMed] [Google Scholar]
- 73.Perona-Wright, G., S. J. Jenkins, R. A. O'Connor, D. Zienkiewicz, H. J. McSorley, R. M. Maizels, S. M. Anderton, and A. S. MacDonald. 2009. A pivotal role for CD40-mediated IL-6 production by dendritic cells during IL-17 induction in vivo. J. Immunol. 182:2808-2815. [DOI] [PubMed] [Google Scholar]
- 74.Potzl, J., C. Botteron, E. Tausch, X. Pedre, A. M. Mueller, D. N. Mannel, and A. Lechner. 2008. Tracing functional antigen-specific CCR6 Th17 cells after vaccination. PLoS One 3:e2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reinhardt, R. L., H. E. Liang, and R. M. Locksley. 2009. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat. Immunol. 10:385-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rogers, H. W., K. C. Sheehan, L. M. Brunt, S. K. Dower, E. R. Unanue, and R. D. Schreiber. 1992. Interleukin 1 participates in the development of anti-Listeria responses in normal and SCID mice. Proc. Natl. Acad. Sci. U. S. A. 89:1011-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rudner, X. L., K. I. Happel, E. A. Young, and J. E. Shellito. 2007. Interleukin-23 (IL-23)-IL-17 cytokine axis in murine Pneumocystis carinii infection. Infect. Immun. 75:3055-3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saito, H., N. Tsurikisawa, T. Tsuburai, C. Oshikata, and K. Akiyama. 2009. Cytokine production profile of CD4+ T cells from patients with active Churg-Strauss syndrome tends toward Th17. Int. Arch. Allergy Immunol. 149(Suppl. 1):61-65. [DOI] [PubMed] [Google Scholar]
- 79.Schulz, S. M., G. Kohler, C. Holscher, Y. Iwakura, and G. Alber. 2008. IL-17A is produced by Th17, gammadelta T cells and other CD4− lymphocytes during infection with Salmonella enterica serovar Enteritidis and has a mild effect in bacterial clearance. Int. Immunol. 20:1129-1138. [DOI] [PubMed] [Google Scholar]
- 80.Sergejeva, S., S. Ivanov, J. Lotvall, and A. Linden. 2005. Interleukin-17 as a recruitment and survival factor for airway macrophages in allergic airway inflammation. Am. J. Respir. Cell Mol. Biol. 33:248-253. [DOI] [PubMed] [Google Scholar]
- 81.Shibata, K., H. Yamada, H. Hara, K. Kishihara, and Y. Yoshikai. 2007. Resident Vdelta1+ gammadelta T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J. Immunol. 178:4466-4472. [DOI] [PubMed] [Google Scholar]
- 82.Siciliano, N. A., J. A. Skinner, and M. H. Yuk. 2006. Bordetella bronchiseptica modulates macrophage phenotype leading to the inhibition of CD4+ T cell proliferation and the initiation of a Th17 immune response. J. Immunol. 177:7131-7138. [DOI] [PubMed] [Google Scholar]
- 83.Sieve, A. N., K. D. Meeks, S. Bodhankar, S. Lee, J. K. Kolls, J. W. Simecka, and R. E. Berg. 2009. A novel IL-17-dependent mechanism of cross protection: respiratory infection with mycoplasma protects against a secondary listeria infection. Eur. J. Immunol. 39:426-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Simonian, P. L., C. L. Roark, F. Wehrmann, A. K. Lanham, F. Diaz del Valle, W. K. Born, R. L. O'Brien, and A. P. Fontenot. 2009. Th17-polarized immune response in a murine model of hypersensitivity pneumonitis and lung fibrosis. J. Immunol. 182:657-665. [PMC free article] [PubMed] [Google Scholar]
- 85.Simonian, P. L., C. L. Roark, F. Wehrmann, A. M. Lanham, W. K. Born, R. L. O'Brien, and A. P. Fontenot. 2009. IL-17A-expressing T cells are essential for bacterial clearance in a murine model of hypersensitivity pneumonitis. J. Immunol. 182:6540-6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stark, M. A., Y. Huo, T. L. Burcin, M. A. Morris, T. S. Olson, and K. Ley. 2005. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity 22:285-294. [DOI] [PubMed] [Google Scholar]
- 87.Stritesky, G. L., N. Yeh, and M. H. Kaplan. 2008. IL-23 promotes maintenance but not commitment to the Th17 lineage. J. Immunol. 181:5948-5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Takagi, R., T. Higashi, K. Hashimoto, K. Nakano, Y. Mizuno, Y. Okazaki, and S. Matsushita. 2008. B cell chemoattractant CXCL13 is preferentially expressed by human Th17 cell clones. J. Immunol. 181:186-189. [DOI] [PubMed] [Google Scholar]
- 89.Torchinsky, M. B., J. Garaude, A. P. Martin, and J. M. Blander. 2009. Innate immune recognition of infected apoptotic cells directs T(H)17 cell differentiation. Nature 458:78-82. [DOI] [PubMed] [Google Scholar]
- 90.Uematsu, S., K. Fujimoto, M. H. Jang, B. G. Yang, Y. J. Jung, M. Nishiyama, S. Sato, T. Tsujimura, M. Yamamoto, Y. Yokota, H. Kiyono, M. Miyasaka, K. J. Ishii, and S. Akira. 2008. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat. Immunol. 9:769-776. [DOI] [PubMed] [Google Scholar]
- 91.Umemura, M., A. Yahagi, S. Hamada, M. D. Begum, H. Watanabe, K. Kawakami, T. Suda, K. Sudo, S. Nakae, Y. Iwakura, and G. Matsuzaki. 2007. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J. Immunol. 178:3786-3796. [DOI] [PubMed] [Google Scholar]
- 92.van der Fits, L., S. Mourits, J. S. A. Voerman, M. Kant, L. Boon, J. D. Laman, F. Cornelissen, A.-M. Mus, E. Florencia, E. P. Prens, and E. Lubberts. 2009. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 182:5836-5845. [DOI] [PubMed] [Google Scholar]
- 93.van de Veerdonk, F. L., R. J. Marijnissen, B. J. Kullberg, H. J. Koenen, S. C. Cheng, I. Joosten, W. B. van den Berg, D. L. Williams, J. W. van der Meer, L. A. Joosten, and M. G. Netea. 2009. The macrophage mannose receptor induces IL-17 in response to Candida albicans. Cell Host Microbe 5:329-340. [DOI] [PubMed] [Google Scholar]
- 94.Veldhoen, M., R. J. Hocking, C. J. Atkins, R. M. Locksley, and B. Stockinger. 2006. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24:179-189. [DOI] [PubMed] [Google Scholar]
- 95.Werner, J. L., A. E. Metz, D. Horn, T. R. Schoeb, M. M. Hewitt, L. M. Schwiebert, I. Faro-Trindade, G. D. Brown, and C. Steele. 2009. Requisite role for the dectin-1 beta-glucan receptor in pulmonary defense against Aspergillus fumigatus. J. Immunol. 182:4938-4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wilson, R. H., G. S. Whitehead, H. Nakano, M. E. Free, J. K. Kolls, and D. N. Cook. 2009. Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness. Am. J. Respir. Crit. Care Med. 180:720-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Woolard, M. D., L. L. Hensley, T. H. Kawula, and J. A. Frelinger. 2008. Respiratory Francisella tularensis live vaccine strain infection induces Th17 cells and prostaglandin E2, which inhibits generation of gamma interferon-positive T cells. Infect. Immun. 76:2651-2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ye, P., P. B. Garvey, P. Zhang, S. Nelson, G. Bagby, W. R. Summer, P. Schwarzenberger, J. E. Shellito, and J. K. Kolls. 2001. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am. J. Respir. Cell Mol. Biol. 25:335-340. [DOI] [PubMed] [Google Scholar]
- 99.Ye, P., F. H. Rodriguez, S. Kanaly, K. L. Stocking, J. Schurr, P. Schwarzenberger, P. Oliver, W. Huang, P. Zhang, J. Zhang, J. E. Shellito, G. J. Bagby, S. Nelson, K. Charrier, J. J. Peschon, and J. K. Kolls. 2001. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 194:519-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yu, Y., C. Iclozan, T. Yamazaki, X. Yang, C. Anasetti, C. Dong, and X. Z. Yu. 2009. Abundant c-Fas-associated death domain-like interleukin-1-converting enzyme inhibitory protein expression determines resistance of T helper 17 cells to activation-induced cell death. Blood 114:1026-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zaretsky, A. G., J. J. Taylor, I. L. King, F. A. Marshall, M. Mohrs, and E. J. Pearce. 2009. T follicular helper cells differentiate from Th2 cells in response to helminth antigens. J. Exp. Med. 206:991-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zelante, T., A. De Luca, P. Bonifazi, C. Montagnoli, S. Bozza, S. Moretti, M. L. Belladonna, C. Vacca, C. Conte, P. Mosci, F. Bistoni, P. Puccetti, R. A. Kastelein, M. Kopf, and L. Romani. 2007. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur. J. Immunol. 37:2695-2706. [DOI] [PubMed] [Google Scholar]
- 103.Zhang, F., G. Meng, and W. Strober. 2008. Interactions among the transcription factors Runx1, RORgammat and Foxp3 regulate the differentiation of interleukin 17-producing T cells. Nat. Immunol. 9:1297-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang, X., L. Gao, L. Lei, Y. Zhong, P. Dube, M. T. Berton, B. Arulanandam, J. Zhang, and G. Zhong. 2009. A MyD88-dependent early IL-17 production protects mice against airway infection with the obligate intracellular pathogen Chlamydia muridarum. J. Immunol. 183:1291-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang, Z., T. B. Clarke, and J. N. Weiser. 2009. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J. Clin. Invest. 119:1899-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zheng, Y., P. A. Valdez, D. M. Danilenko, Y. Hu, S. M. Sa, Q. Gong, A. R. Abbas, Z. Modrusan, N. Ghilardi, F. J. de Sauvage, and W. Ouyang. 2008. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 14:282-289. [DOI] [PubMed] [Google Scholar]
- 107.Zhou, X., Q. Chen, J. Moore, J. K. Kolls, S. Halperin, and J. Wang. 2009. Critical role of the interleukin-17/interleukin-17 receptor axis in regulating host susceptibility to respiratory infection with Chlamydia species. Infect. Immun. 77:5059-5070. [DOI] [PMC free article] [PubMed] [Google Scholar]