Abstract
Dramatic alteration of surface lipoprotein profiles is a key strategy that Borrelia burgdorferi, the Lyme disease pathogen, has evolved for adapting to the diverse environments of arthropod and mammalian hosts. Several of these differentially expressed lipoproteins have been shown to play important roles in the enzootic cycle of B. burgdorferi. The BBA05 protein is a previously identified putative lipoprotein (P55 or S1 antigen) that elicits antibody responses in mammals. Recent microarray analyses indicate that the BBA05 gene is differentially expressed by many environmental factors, including temperature. However, the role of the BBA05 protein in the life cycle of B. burgdorferi has not been elucidated. Here we show that expression of the BBA05 gene was exclusively induced in feeding nymphal ticks during the spirochetal transmission from ticks to mammals. Upon generating a BBA05 mutant in an infectious strain of B. burgdorferi, we showed that the BBA05 mutant remained capable of establishing infection in mice, being acquired by ticks, persisting through tick molting, and reinfecting new mammalian hosts. These results indicate that, despite being a highly conserved and regulated antigen, the BBA05 protein has a nonessential role in the transmission cycle of B. burgdorferi, at least in the animal model.
Borrelia burgdorferi, the causative agent of Lyme disease, has an astonishing number of lipoprotein genes (∼150) in its genome (over 10% of the total genome) (9, 21); many of these lipoprotein genes are differentially expressed during the enzootic cycle of B. burgdorferi in the two diverse hosts, an arthropod tick (e.g., Ixodes scapularis) vector and a mammalian host (e.g., white-footed mice) (46, 50, 54, 59). A body of evidences indicates that these differentially regulated lipoproteins are important for B. burgdorferi's maintenance in its natural cycle. For example, OspA, an outer surface lipoprotein that is expressed chiefly in unfed ticks, functions as an adhesin essential for spirochetal survival in the tick vector (12, 41, 42, 47, 56, 70). The outer surface lipoprotein OspC, on the other hand, is induced when ticks feed, concomitant with the downregulation of OspA (17, 24, 55, 56). OspC is not required for B. burgdorferi replication in the tick vector but is essential for spirochetes to establish infection in the mammalian host (26, 51, 62, 63). Although controversial, it has been proposed that OspC may also contribute to spirochetal transmission from ticks to mice (16, 26, 48, 51, 63). In addition to OspA and OspC, several other lipoproteins, such as DbpB/A, BBK32, BB0365, and BBA64, were also shown to play a role in spirochetal colonization either in the tick vector or in the mammalian host (2, 18, 37, 45, 57, 58, 71). Thus, studying the functions and regulation of B. burgdorferi lipoproteins is significant to our understanding of how B. burgdorferi adapts to diverse hosts in its natural cycle.
In the past few years, we and others identified a regulatory pathway, the Rrp2-RpoN-RpoS pathway (also called the σ54-σS sigma factor cascade), that governs the differential expression of numerous borrelial genes during the enzootic cycle of B. burgdorferi (6-8, 19, 23, 29, 36, 60, 69). In this pathway, the two-component response regulator Rrp2, along with the alternative sigma factor RpoN (σ54 or σN), directly controls the production of a second alternative sigma factor, RpoS (σS), which in turn modulates expression of more than 145 Borrelia genes (8, 19, 44). Many of the Rrp2-RpoN-RpoS pathway-regulated genes are lipoprotein genes that are either activated (e.g., ospC, dbpA/B, BBK32, and BBA64) or repressed (e.g., ospA and lp6.6) by the pathway. This pathway is induced at the onset of nymphal feeding and is indispensable for B. burgdorferi's transmission from ticks to mice and establishment of infection in mammals.
Consistent with the essential roles of the Rrp2-RpoN-RpoS pathway in the life cycle of B. burgdorferi, this pathway controls expression of many mammalian infection-associated antigens of B. burgdorferi, including OspC (23, 29, 69). However, the Rrp2-RpoN-RpoS pathway likely controls another factor(s) essential for the enzootic cycle of B. burgdorferi, as constitutive production of OspC could not rescue the avirulent phenotype of an rrp2 mutant via either needle inoculation or tick bite (3). Although DbpB/A and BBK32 also are Rrp2-dependent antigens and have been shown to partially contribute to the infectivity of B. burgdorferi in mice upon needle inoculation, they are not required for mammalian infection upon tick feeding (2, 32, 57, 58). Therefore, the Rrp2-RpoN-RpoS pathway likely controls a yet-to-be-identified virulence determinant(s) required for mammalian infection.
In an effort to uncover new virulence factors of B. burgdorferi, we have been focusing on infection-associated antigens controlled by the Rrp2-RpoN-RpoS pathway. One such candidate is the BBA05 protein, a putative lipoprotein encoded by the BBA05 gene on endogenous linear plasmid lp54. The BBA05 protein was initially identified as an antigen (P55 or S1 antigen) that elicits antibody responses in patients with early- or late-stage Lyme disease as well as in laboratory-infected mice (14, 15). Results from several microarray analyses indicate that BBA05 expression is highly influenced by culture conditions that spirochetes likely encounter during their natural cycle (43, 52, 64). An elevated culture temperature (from 23°C to 37°C) or addition of mammalian blood into the culture medium increases levels of BBA05 expression more than nine- or sixfold, respectively (52, 64). Furthermore, most recent microarray analyses by us and others identified BBA05 as one of the top candidate genes whose expression was under the control of the Rrp2-RpoN-RpoS pathway. Mutation of rrp2, rpoN, or rpoS reduced BBA05 transcriptional levels 10- to 354-fold (3, 8, 44). Since the BBA05 protein was shown to be a mammalian infection-associated antigen and BBA05 expression is highly differentially regulated, it is tempting to speculate that the BBA05 protein may be an Rrp2-dependent virulence factor important to B. burgdorferi infection. To test this possibility, in this study, we first examined BBA05 expression in various stages of the spirochetal life cycle. We then constructed a BBA05 mutant of an infectious strain of B. burgdorferi B31 and determined its contribution to the enzootic cycle of B. burgdorferi.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Low-passage, virulent B. burgdorferi strain 5A4NP1 (a gift of H. Kawabata and S. Norris, University of Texas Health Science Center at Houston) was derived from wild-type strain B31 by inserting a kanamycin resistance marker in the restriction modification gene BBE02 on plasmid lp25 (30). Low-passage, virulent B. burgdorferi strain 297 BbAH130 and its isogenic rrp2 [rrp2(G239C)] and rpoS (rpoS) mutants were described previously (29, 70). Strain B31-13A, which lacks lp25 and lp56, was kindly provided by F. T. Liang (Louisiana State University) (65). Borreliae were cultivated in Barbour-Stoenner-Kelly (BSK-H) medium (Sigma, St. Louis, MO) supplemented with 6% normal rabbit serum (Pel Freez Biologicals, Rogers, AR) at 35°C unless indicated otherwise. the shuttle vector pBSV2 (a gift of P. Rosa, Rocky Mountain Laboratories, National Institute of Allergy and Infectious Diseases, National Institutes of Health) was maintained in Escherichia coli strain TOP10.
Generation of a BBA05 mutant.
To construct a suicide vector for homologous recombination, regions of DNA corresponding to 1.5 kb upstream and 1.3 kb downstream, respectively, of BBA05 were PCR amplified from 5A4-NP1 genomic DNA with primer pairs bba5-UF/bba5-UR and bba5-DF/bba5-DR (Table 1), respectively. The resulting DNA fragments were then cloned upstream and downstream of an aadA streptomycin resistance marker (20) within the pCR-XL-TOPO cloning vector (Invitrogen) to create suicide vector pHX05. The reconstructed suicide vector was confirmed by sequencing, and the plasmid DNA was transformed into B. burgdorferi strain 5A4NP1 as previously described (53, 70). Whole-cell lysates from positive clones were analyzed by PCR and reverse transcription-PCR (RT-PCR) to confirm corrected marker insertion and inactivation of BBA05. The plasmid profiles of the BBA05 mutant clones were determined by PCR analyses with 21 pairs of primers specific for each of the endogenous plasmids (31, 39, 49). Two of the three randomly picked clones had plasmid profiles that were identical to that of the parental strain 5A4NP1 (13). One of the clones was chosen for further study.
TABLE 1.
Primers used in the study
| Primer | Sequence (5′ → 3′) | Purpose |
|---|---|---|
| Bba5-DF | GAGCTCGGCGCGCCTGGAAAGGAATATTCTAGGTTGA | To amplify BBA05 downstream region |
| Bba5-DR | GGTACCCCTGCAGGAAGACACTATATAGCAAATCCAA | To amplify BBA05 downstream region |
| Bba5-UF | GCGGCCGCATTGTCTTTAATGTTTATACCGTA | To amplify BBA05 upstream region |
| Bba5-UR | AGATCTGCGATCGCAAATGCAATTCCTATTTTATTCA | To amplify BBA05 upstream region |
| Qbba4-F | GCTTTGACTTAATAGTAATCTCGG | qRT-PCR primer for BBA04 |
| Qbba4-R | AAAATCAGTTGAATCTGGGCTTGT | qRT-PCR primer for BBA04 |
| Qbba5-F | TTGTTGACACAAATTCATATCCA | qRT-PCR primer for BBA05 |
| Qbba5-R | TTGTCTTCTAAAATGAGCACCTT | qRT-PCR primer for BBA05 |
| Qbba6-F | TAGCTCACTTTACATACACTAAATG | qRT-PCR primer for BBA06 |
| Qbba6-R | TTAAGGCAGCTGCAAGCCTA | qRT-PCR primer for BBA06 |
| Primer A | GTGTGTGGGAGACGTATG | Used for Fig. 4 |
| Primer B | ATGAATAAAATAGGAATTGC | Used for Fig. 4 |
| Primer C | TTAACGCCTGCTAATCTC | Used for Fig. 4 |
| Primer D | TTATTTGCCGACTACCTT | Used for Fig. 4 |
| QflaB-F | ACCAGCATCACTTTCAGGGTCTCA | qRT-PCR primer for flaB |
| QflaB-R | CAGCAATAGCTTCATCTTGGTTTG | qRT-PCR primer for flaB |
| QTactin-F | CGGGACCTGACCGACTACCTGATG | qRT-PCR primer for tick actin |
| QTactin-R | CTCCTTGATGTCGCGGACAATTTC | qRT-PCR primer for tick actin |
| Probba5-F | GGATCCAATGCTAAACAGCAATTGCTACAGGTTG | To construct the BBA5-Flag fusion |
| Bba5-flag-R | CTGCAGTTATTTATCATCATCATCTTTATAATCACGCCTGCTAATCTCTTTTATCAAGCTGTTTAA | To construct the BBA5-Flag fusion |
| qbba5-cDNA-R | AAATCTCCCCACCGCTTTTTTCCTAT | cDNA synthesis for BBA05 |
| qFlaB-cDNA-R | GATTCAAGTCTATTTTGGAAAGCACC | cDNA synthesis for flaB |
Mouse infection via needle inoculation.
Three- or 4-week-old C3H/HeN mice (Harlan, Indianapolis, IN) were subcutaneously inoculated with 1 × 106 spirochetes. At 2 weeks postinoculation, ear punch biopsy samples and mouse tissue samples (skin, heart, spleen, and joint) were collected and cultured in BSK-H medium supplemented with 1× Borrelia antibiotic mixture (Sigma, St. Louis, MO). A single growth-positive culture was used as the criterion for infection of each mouse. All animal and tick protocols (see below) were approved by the Institutional Animal Care and Use Committee at Indiana University.
Tick-mouse cycle of B. burgdorferi.
The colony of Ixodes scapularis originated from females obtained from Bridgeport, CT, and was maintained in the Tick-Borne Disease Activity laboratory at the Centers for Disease Control and Prevention, Ft. Collins, CO. The tick-mouse experiments were conducted in the Vector-Borne Diseases Laboratory at Indiana University School of Medicine, Indianapolis, IN. Unfed larvae were fed on groups of mice (C3H/HeN, three mice per group, 150 to 200 larvae per mouse) that were needle infected with either 5A4NP1 or BBA05 mutant spirochetes. Ticks were allowed to feed to repletion (3 to 4 days) and then collected within 24 h. A portion of fed larvae were subjected to immunofluorescence assay (IFA), quantitative PCR (qPCR), or qRT-PCR analyses (see below). The remaining fed larvae were maintained in the tick incubator and allowed to molt to the nymphal stage (about 5 weeks). One month after molting, unfed nymphs were then allowed to feed on naive C3H/HeN mice (nine mice per group, 12 ticks per mouse). Fully engorged nymphal ticks were collected within 24 h of repletion and subjected to IFA, qPCR, and qRT-PCR analyses. Two weeks after tick feeding, mouse tissues were collected and tested for infection as described above.
IFA.
IFA was performed as reported previously (3). Briefly, the entire contents of a fed tick were smeared and fixed on silylated microscope slides (CEL Associates, Pearland, TX). The slides were incubated with BacTrace fluorescein isothiocyanate-conjugated goat anti-B. burgdorferi antibody (Kirkegaard and Perry Laboratories Gaithersburg, MD) at 37°C. Samples were observed using an Olympus BX50 fluorescence microscope. Twenty ticks from each group were examined by IFA.
qRT-PCR and qPCR.
RNA samples were extracted from either B. burgdorferi cultures or ticks using the RNeasy mini kit (Qiagen, Valencia, CA) according to the manufacturer's protocols. Mouse tissue RNAs isolated from Borrelia-infected mice were kindly provided by F. T. Liang (Louisiana State University) as previously described (66). Briefly, SCID mice were given a single intradermal injection of 104 wild-type B. burgdorferi spirochetes. Animals were sacrificed at 4 weeks postinoculation, and mouse tissues were subjected to RNA isolation (66). For RNA analysis of in vitro-cultivated B. burgdorferi, three independent culture samples were used for each strain. For RNA analysis of spirochetes in ticks, 10 groups of fed larvae (3 ticks per group), 3 groups of unfed nymphs (40 ticks per group), and 10 groups of fed nymphs (1 tick per group) were used. For RNA analysis of spirochetes in mice, four RNA samples from four mice were used. Digestion of contaminating genomic DNA in the RNA samples was performed using RNase-free DNase I (Promega, Madison, WI), and removal of DNA was confirmed by PCR amplification using primers specific for the B. burgdorferi flaB gene. For RNA samples isolated from in vitro cultures, cDNA was synthesized using SuperScript III reverse transcriptase with random primers (Invitrogen, Carlsbad, CA). For RNA samples isolated from ticks or mouse tissues, cDNA was synthesized using the primer mixes specific for the BBA05 (pbba5-cDNA-R) and flaB (qFlaB-cDNA-R) genes (Table 1).
To quantify the transcript levels of the BBA05 and flaB genes, an absolute quantitation method was used by creating a standard curve in a qPCR assay by following the manufacturer's protocol (Stratagene, La Jolla, CA). Briefly, a cloning vector containing the BBA05 gene served as a standard template. A series of 10-fold dilutions (100 to 107 copies/μl) of the standard template was prepared, and qPCR was performed in triplicate using the BBA05 primer pair. A standard curve was therefore generated by plotting the initial template quantity against the threshold cycle values for the standards. The quantities of the BBA05 and flaB genes in cDNA samples were calculated by comparing their threshold cycle values to the standard curve plot. Both standards and samples were used in triplicate on an ABI 7000 sequence detection system using Green PCR master mix (ABI, Pleasanton, CA). Levels of BBA05 transcript were reported as per 104 copies of flaB transcripts.
For qPCR analyses of B. burgdorferi DNA in ticks, DNA samples were extracted from fed nymphs (10 data points for each group, one tick per data point) using a DNeasy tissue kit (Qiagen, Valencia, CA) according to the manufacturer's protocol. qPCR was performed with primer pairs of qflaB-F/R and qTactin-F/R (Table 1). Calculations of relative DNA copy number (represented by flaB) were normalized with the copy number of the tick actin gene.
Construction of a BBA05-Flag tag fusion and proteinase K accessibility experiments.
To detect the BBA05 protein, the BBA05 gene with its native promoter region was PCR amplified from B. burgdorferi B31 genomic DNA using primers probba5-F and bba5-flag-R (Table 1). Two restriction sites, BamHI and PstI, were incorporated into the designated primers and used for insertion of the digested PCR-amplified fragment into a pBSV2-derived shuttle vector pJD55. In addition, a Flag tag sequence was also attached to the bba5-R primer so that the Flag tag sequence was fused to the C terminus of the BBA05 protein. The resulting shuttle vector, pJD55/BBA05-flag, was verified by sequencing and transformed into B31-13A (65).
Proteinase K accessibility experiments were performed as previously reported (5). Briefly, B31-13A containing pJD55/BBA05-flag was cultured at 35°C and harvested at a cell density of 1 × 108 spirochetes per ml. Spirochetes were equally divided into two samples; one sample was treated with 200 μg proteinase K (Sigma, St. Louis, MO) for 1 h at room temperature, and the other sample was incubated with 1× phosphate-buffered saline as a control. Samples were then subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting analyses.
SDS-PAGE and immunoblotting.
SDS-PAGE and immunoblotting were performed as previously described (68). Monoclonal anti-Flag tag residue was purchased from Sigma (St. Louis, MO). Monoclonal antibodies directed against OspA (14D2-27) and FlaB (8H3-33) were also described previously (67).
RESULTS
Expression of the BBA05 gene during the enzootic cycle of B. burgdorferi.
Since the expression of the BBA05 gene has been indicated to be influenced by several environmental factors that B. burgdorferi spirochetes likely encounter during their natural cycle, we sought to examine the expression of BBA05 at different stages of the tick cycle as well as during mammalian infection. Pathogen-free, unfed I. scapularis larvae were fed on mice (C3H/HeN) infected with wild-type B. burgdorferi strain B31 5A4NP1. Fed larvae were allowed to molt to the nymphal stage. Unfed nymphs were then allowed to feed on naive mice. Fed larvae, unfed nymphs, and fed nymphs were then subjected to RNA extraction and qRT-PCR analyses. As shown in Fig. 1, while the BBA05 transcript was undetectable in fed larvae and unfed nymphs, expression of BBA05 was dramatically induced in fed nymphs. Thus, BBA05 is expressed during tick feeding, exclusively in the phase of transmission, and not in the phase of acquisition.
FIG. 1.
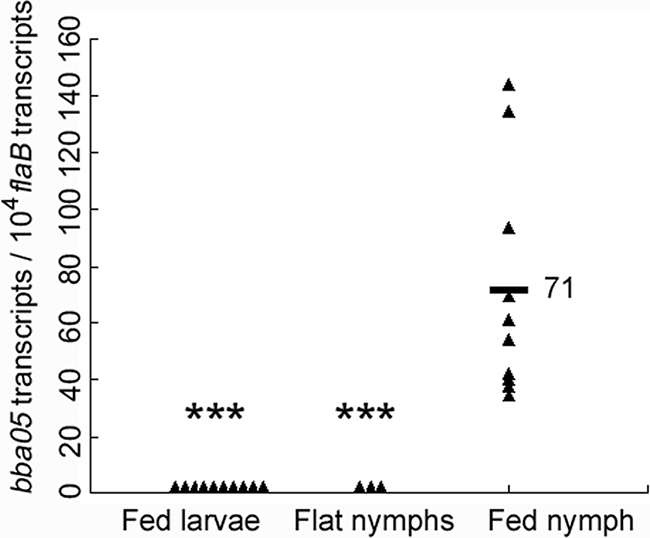
Levels of BBA05 gene expression in B. burgdorferi at different stages of the tick cycle. Relative levels of BBA05 expression in fed larvae, unfed nymphs, or fed nymphs were determined by qRT-PCR analyses and are reported as the numbers of the BBA05 transcript per 10,000 copies of the flaB transcript. Black triangles represent values from each data point, which were generated from three fed larvae (total of 10 data points), 40 unfed nymphs (3 data points), and 1 fed nymph (10 data points). The horizontal bar represents the mean value (labeled with a numerical value). ***, P < 0.001 for differences between the fed nymph group and the other groups.
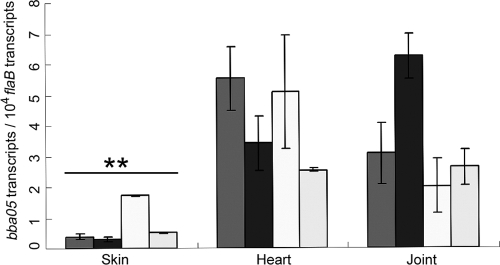
To examine the expression of BBA05 in mice, RNAs isolated from mouse tissues (skin, heart, and joint) collected after 4 weeks of infection with B. burgdorferi were subjected to qRT-PCR analyses; BBA05 transcripts were detected in all three types of tissue samples tested, with significantly higher levels in heart (P = 0.0047) and joint (P = 0.0034) tissues than that in skin samples (Fig. 2) but no obvious difference (P = 0.92) between the heart and joint tissues. In addition, levels of BBA05 expression in all mouse tissues were greatly lower than those in fed nymphs.
FIG. 2.
Levels of BBA05 expression in various mouse tissues. Relative levels of BBA05 expression in joint, heart, and skin tissues harvested from mice infected with B. burgdorferi were determined by qRT-PCR analyses and are reported as the numbers of the BBA05 transcript per 10,000 copies of the flaB transcript. Each bar represents one tissue sample from one mouse tested in triplicate. Error bars indicate standard deviations. **, P < 0.01 between skin and heart or joint.
Influence of the Rrp2-RpoN-RpoS regulatory pathway on the expression of the BBA05 gene.
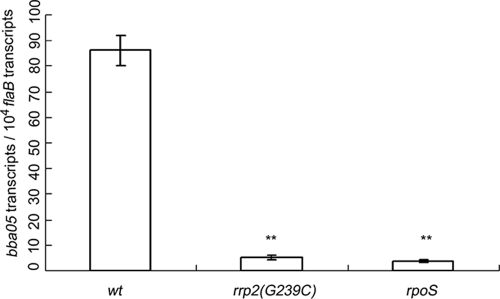
The Rrp2-RpoN-RpoS pathway has been shown to play a central role in modulating differential expression of many genes in B. burgdorferi (3, 7, 8, 10, 19, 22, 23, 25, 28, 37, 40, 44). Recent microarray analyses performed by us and others suggest that BBA05 may be an Rrp2-RpoN-RpoS pathway-controlled gene (3, 8, 44). To verify the microarray results, wild-type B. burgdorferi 297, the isogenic rrp2(G239C) mutant (3), and the rpoS mutant (29), were cultivated in BSK-H medium at 35°C. RNAs were isolated from each of the Borrelia cultures and subjected to qRT-PCR analysis. As shown in Fig. 3, mutation in rrp2 or inactivation of rpoS greatly reduced the level of BBA05 transcription, suggesting that the expression of BBA05 is governed by the Rrp2-RpoN-RpoS pathway.
FIG. 3.
Expression of BBA05 is under the control of the Rrp2-RpoN-RpoS pathway. Wild-type B. burgdorferi strain BbAH130 (wt), the rrp2 mutant [rrp2(G239C)], and the rpoS mutant were cultivated at 35°C in BSK-H medium (pH 7.5) and harvested at late-logarithmic growth. Relative levels of BBA05 expression were determined by qRT-PCR analyses and are reported as the numbers of the BBA05 transcript per 10,000 copies of the flaB transcript. Data represent three independent culture samples. Error bars indicate standard deviations. **, P < 0.01 between wt and the other groups.
Construction of the BBA05 mutant.
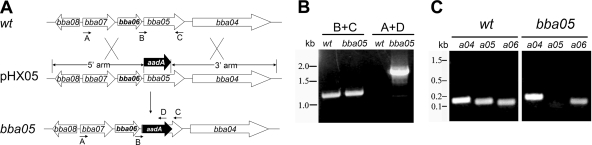
To assess the role of the BBA05 protein in the enzootic cycle of B. burgdorferi, we constructed a BBA05-deficient mutant by homologous recombination. A suicide vector, pHX5 (Fig. 4A), was constructed with an aadA gene (which confers streptomycin-resistance in B. burgdorferi) (20) flanked by upstream and downstream regions of the BBA05 gene. The pHX5 plasmid DNA was then transformed into B. burgdorferi strain B31 5A4NP1 (kanamycin resistant) (30). More than 10 streptomycin-resistant and kanamycin-resistant clones were obtained; these clones were then subjected to PCR and RT-PCR analyses for confirmation of the correct BBA05 disruption (Fig. 4B and C). All clones showed correct marker replacement and loss of the BBA05 transcript. In addition, all BBA05 mutants displayed normal growth kinetics in BSK-H medium (data not shown). Furthermore, disruption of BBA05 did not appear to affect the expression of the downstream and upstream genes, BBA04 and BBA06 (Fig. 4C). Three clones were further subjected to endogenous plasmid profile analyses; one mutant clone that had an endogenous plasmid profile identical to that of 5A4NP1 was selected for further study.
FIG. 4.
Construction of the BBA05 mutant. (A) Strategy for insertional inactivation of the BBA05 gene. wt, genomic structure of BBA05 and the surrounding regions in wild-type B. burgdorferi. pHX05, suicide plasmid used for generating the BBA05 mutant. Only the relevant portion of the plasmid is shown. BBA05, genomic structure of the BBA05 mutant. Small labeled arrows denote positions of oligonucleotide primers used for PCR analyses. (B) Confirmation of the BBA05 mutant by PCR analyses. Letter combinations denote primer pairs used for PCR. kb, DNA ladder; wt, wild-type strain; BBA05, BBA05 mutant. (C) Expression of BBA04, BBA05, and BBA06 in the wild type and the BBA05 mutant by RT-PCR analyses. Spirochetes were cultured at 35°C and harvested at late logarithmic growth. RT-PCR was performed using primers specific for BBA04, BBA05, or BBA06.
The BBA05 mutant is capable of infecting mice upon needle inoculation.
To determine whether the BBA05 protein is required for infection of mice, groups of C3H/HeN mice were inoculated with either wild-type B. burgdorferi 5A4NP1 or the BBA05 mutant. At 2 weeks after infection, all mice developed joint swelling (data not shown), and ear punch biopsy specimens were also culture positive for spirochetal growth (Table 2). Subsequent cultivation of skin, heart, bladder, joint, and spleen tissues were also positive for B. burgdorferi. These data suggest that the BBA05 protein is not essential for B. burgdorferi to establish infection in mice, colonize in various tissues, or cause Lyme arthritis.
TABLE 2.
Mouse infectivity of the BBA05 mutant
| Strain | No. of infected samples/total no. of tissue samples for indicated inoculation route |
||||
|---|---|---|---|---|---|
| Needle (ear punch) | Nymphal ticks |
||||
| Skin | Bladder | Heart | Joint | ||
| Wild type | 3/3 | 8/9 | 8/9 | 8/9 | 8/9 |
| BBA05 mutant | 3/3 | 7/9 | 7/9 | 7/9 | 7/9 |
The BBA05 mutant is capable of entering ticks, persisting through tick molting, and infecting the mammalian host.
To determine whether the BBA05 protein plays a role in the enzootic cycle of B. burgdorferi, pathogen-free I. scapularis larvae were allowed to feed on mice infected with either wild-type or BBA05 mutant spirochetes. Larval ticks were fed to repletion and collected for IFA analyses. As shown in Fig. 5A and B, both wild-type and BBA05 mutant spirochetes were readily detectable in ticks, and no obvious difference in spirochetal numbers was observed, suggesting that the BBA05 protein is not required for the process of tick acquisition.
FIG. 5.
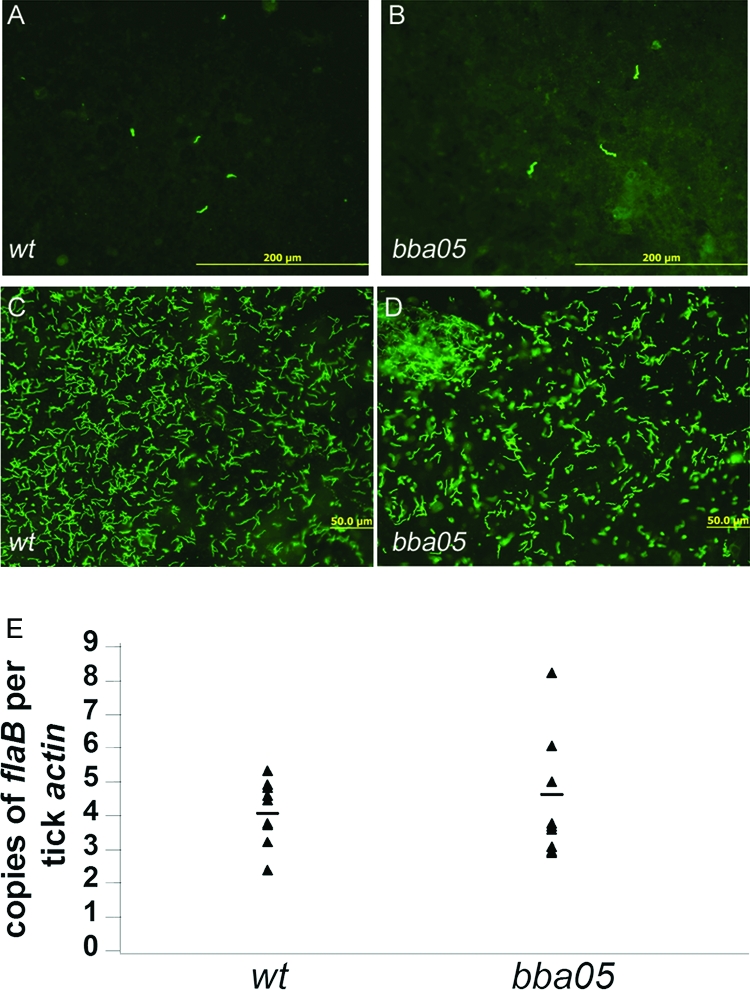
Detection of BBA05 mutant spirochetes in ticks. (A and B) Naive I. scapularis larvae were allowed to feed to repletion on mice infected with either the wild type (A) or the BBA05 mutant (B) and were subjected to IFA. Spirochetes were detected using fluorescein isothiocyanate-labeled anti-B. burgdorferi antibody. Engorged larvae were allowed to molt to nymphs. (C to E) Flat nymphs were allow to feed on naive mice, and newly engorged nymphs harboring either the wild type (C) or BBA05 (D) were subjected to IFA and qPCR analyses (E). For qPCR analyses, the copy number of flaB gene was chosen to represent the number of spirochetes. The copy numbers of flaB were normalized by the copy numbers of the tick actin gene in each DNA sample. Black triangles represent values from individual nymphs. Horizontal bars represent the mean value in each group.
To assess the ability of the BBA05 mutant to colonize and survive in the tick vector, fed larvae were maintained in the tick incubator and allowed to molt to nymphs (about 5 weeks). At 1 month after molting, unfed nymphs were then allowed to feed on naive mice. After detachment, fed nymphs were subjected to IFA analyses. Both the wild-type and mutant strains were abundantly present in fed nymphs (Fig. 5C and D). qPCR analyses indicated that there was no statistical difference in the spirochetal burdens of the fed nymphs in the two strains (Fig. 5E). These data suggest that the BBA05 protein is not essential for B. burgdorferi to colonize and survive in the tick vector.
It has been reported that needle inoculation and tick challenge of mice may have profoundly different infection outcomes (27). Therefore, we further examined the infectivity of the BBA05 mutant via tick bite. At 3 weeks after nymphal tick infestation, mice were sacrificed and tissue samples (ear, bladder, heart, and joint) were cultured for the presence of spirochetes. Similar numbers of mice were infected by wild-type and the BBA05 mutant spirochetes (Table 2). To confirm that the BBA05 mutant spirochetes retained the BBA05-deficient phenotype after going through the mouse-tick-mouse cycle, spirochetes were recovered from infected mouse tissues and subjected to PCR and RT-PCR analyses. The result showed that the BBA05 mutant phenotype was stably maintained (data not shown). Taken together, these results indicate that BBA05 is not essential for the enzootic cycle of B. burgdorferi, at least as recapitulated in the animal model.
The BBA05 protein is not surface localized.
To initially investigate a possible function of the BBA05 protein, we sought to determine whether it is a surface-localized lipoprotein, as it was reported that the BBA05 protein was recognized serologically by patients with early- or late-stage Lyme disease (15). First, a shuttle vector carrying a BBA05 gene with a Flag tag attached to the C terminus of the BBA05 protein was transformed into B. burgdorferi B31-13A (65). Transformed spirochetes were subjected to proteinase K treatment, and whole-cell lysates were subsequently subjected to immunoblotting analysis. Although OspA, an outer surface lipoprotein, was sensitive to the proteinase K treatment, BBA05-Flag remained detectable after the treatment, suggesting that the BBA05 protein is not a surface-localized lipoprotein (Fig. 6).
FIG. 6.
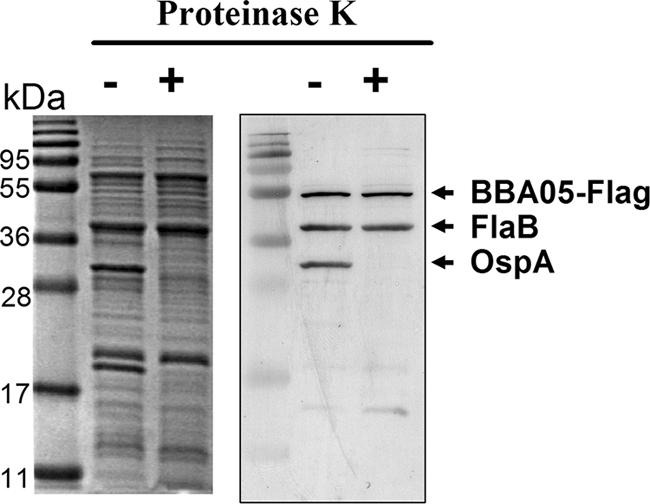
The BBA05 protein is not a surface-localized lipoprotein. B. burgdorferi strain 13A or 13A transformed with a shuttle vector that produces recombinant BBA05-Flag was incubated either with 1× PBS (−) or with proteinase K (+). Treated cells were harvested and subjected to SDS-PAGE (left panel) or to immunoblot analysis using a mixture of monoclonal antibodies against Flag epitope, FlaB, and OspA (right panel). OspA, a surface-localized lipoprotein, served as a positive control, and FlaB served as a negative control.
DISCUSSION
The B. burgdorferi genome contains very few recognizable genes involved in virulence or host-pathogen interactions (21); much work toward understanding virulence determinants of B. burgdorferi has been focused on the outer surface lipoprotein genes present in the genome. The purpose of our investigation was to determine whether BBA05, a highly differentially expressed, Rrp2-dependent lipoprotein gene, plays an important role in the enzootic cycle of B. burgdorferi. The results showed that BBA05 is induced in spirochetes exclusively during tick feeding but appears to play a nonessential role in the survival of B. burgdorferi in either the tick vector or the mammalian host.
Recent microarray analyses of the B. burgdorferi transcriptome indicate that expression of BBA05 is induced by elevated temperature or by addition of mammalian blood to the culture medium (52, 64), implying that BBA05 may be differentially expressed in the enzootic cycle of B. burgdorferi. Indeed, we found that BBA05 was not detected in unfed nymphs, but its expression was greatly induced in fed nymphs during the process of spirochetal transmission to the mammalian host (the transmission phase). We expect that the production of the BBA05 protein should be consistent with its mRNA expression, although the result has not been verified at the protein level because anti-BBA05 antibody is currently unavailable. Therefore, temperature and host blood may be the key sensing signals for BBA05 expression during tick feeding. However, other factors may be involved in regulating BBA05 expression, since BBA05 transcripts were not detectable in fed larvae even though spirochetes also encounter mammalian blood as well as a similar temperature change when bacteria enter the tick gut from mice (the acquisition phase). This pattern of expression of BBA05 during the processes of spirochetal acquisition and transmission is identical to that of ospC (55). In addition to BBA05 expression in ticks, BBA05 transcripts were also detected in spirochetes during mammalian infection. This finding is consistent with a previous report showing by microarray analysis that BBA05 is one of the lipoprotein genes expressed in infected mice (34). However, levels of BBA05 transcripts in mouse tissues appeared to be much lower than those in feeding ticks (Fig. 1 and 2). Note that the mouse tissue RNAs used were from SCID mice 4 weeks after infection with B. burgdorferi by the route of needle inoculation. Although spirochetes transmitted via tick bites likely present a different level of BBA05 in comparison with the spirochetes from needle inoculation at the early stage of mammalian infection, they likely have a similar pattern of gene expression after 4 weeks of host adaptation. Thus, our observation suggests that spirochetes express a lower level of BBA05 in the mammalian host than in the tick vector. This observation also supports the previous notion that B. burgdorferi dramatically downregulates its lipoprotein gene expression after entering the mammalians host from ticks and establishing infection in mammals as a strategy of host immune evasion (4, 34, 35).
Recent microarray analyses revealed that BBA05 expression may be controlled by the Rrp2-RpoN-RpoS pathway (3, 8, 44). The qRT-PCR results from this study further confirmed that BBA05 is an Rrp2-RpoN-RpoS-dependent lipoprotein gene. The dependence of BBA05 expression on the Rrp2-RpoN-RpoS pathway is congruent with its exclusive induction in feeding nymphs, as the Rrp2-RpoN-RpoS pathway is turned on at the onset of tick feeding (8). This pathway functions as a “gatekeeper” for B. burgdorferi's transition from the tick state to the mammalian state, by producing factors essential for spirochetal migration from ticks to mice, as well as for the establishment of infection in the mammalian host (3, 7, 8, 19, 44). We recently showed that, in addition to known virulence factors such as OspC, the Rrp2-RpoN-RpoS pathway controls a yet-to-be-identified virulence determinant(s) (3). Although the BBA05 protein appeared to be an attractive candidate, our data showed that it is not a virulence factor essential for mammalian infection. Further work will focus on investigating the roles of other Rrp2-dependent antigens in the infectious cycle of B. burgdorferi.
Previous reports showed that the BBA05 protein is an antigen (P55 or S1) that elicits antibody responses in some patients with Lyme disease as well as in mice at 90 days after B. burgdorferi infection by tick bites (14, 15). However, immunization with recombinant BBA05 proteins did not protect mice against B. burgdorferi infection by tick bites (14). The negative result of the active immunization study can be explained by the finding in this study that the BBA05 protein is not a surface-exposed lipoprotein, as demonstrated by the proteinase K treatment experiment. Note that a recent B. burgdorferi proteome array study failed to identify the BBA05 protein as an immunogen in human patients (1), and the discrepancy between the proteome array result and the previous finding remains to be further clarified.
Sequence analyses revealed that the BBA05 protein is present in many Lyme disease spirochetes, including Borrelia afzelii, Borrelia garinii, Borrelia spielmanii, and Borrelia valaisiana (data not shown), suggesting that BBA05 is a highly conserved gene among this group of bacteria. The putative function of the BBA05 protein remains unclear, as BLAST analysis did not identify any homologues with any other proteins in the database. Regardless, it is reasonable to speculate that being a highly conserved, highly regulated gene, BBA05 would play a prominent role in the enzootic cycle of B. burgdorferi. However, our results showed that inactivation of BBA05 did not affect the survival of B. burgdorferi in ticks or in mice. It should be noted that the 50% infectious dose of the BBA05 mutant was not determined in this study. Although it is possible that the BBA05 mutant may have a different level of infectivity than the wild-type strain by needle inoculation, our results showed that there is no difference in infectivity between the mutant and its parental strain via tick bites, an appropriate route of infection relevant to the natural cycle of B. burgdorferi. One possibility for the nonessential nature of BBA05 is that its function is compensated by another protein(s) present in B. burgdorferi. In this regard, the B. burgdorferi genome is highly redundant and contains more than 100 paralogous gene families. Many of the lipoprotein genes are members of these paralogous families. The loss of one of these lipoproteins of B. burgdorferi can be compensated for by the presence of other paralogous genes in the same family (11, 38). However, this appears not to be the case for BBA05, as BBA05 is one of the few lipoprotein genes that do not belong to a paralogous family. Interestingly, another nonparalogous lipoprotein gene, ospD, was also shown to be nonessential for the infectious cycle of B. burgdorferi (33, 61). Thus, either the loss of the BBA05 protein is compensated for by a Borrelia protein that has no sequence homology with the BBA05 protein or the BBA05 protein plays a nonessential, secondary role in B. burgdorferi's enzootic cycle.
Acknowledgments
We thank Margaret Bauer for comments on the manuscript. We also thank Gabrielle Dietrich for assistance with tick colony maintenance and shipment of ticks.
Funding for this work was partially provided by NIH grants R03 AR054942 and R01 AI083640 (to X.F.Y.), an American Heart Association Scientist Development Grant (to X.F.Y.), and Indiana INGEN and METACyt grants from Indiana University, funded by the Lilly Endowment, Inc. (to X.F.Y.). This investigation was partially conducted in a facility constructed with support from research facilities improvement program grant C06 RR015481-01 from the National Center for Research Resources, NIH.
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 12 October 2009.
REFERENCES
- 1.Barbour, A. G., A. Jasinskas, M. A. Kayala, D. H. Davies, A. C. Steere, P. Baldi, and P. L. Felgner. 2008. A genome-wide proteome array reveals a limited set of immunogens in natural infections of humans and white-footed mice with Borrelia burgdorferi. Infect. Immun. 76:3374-3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blevins, J., K. E. Hagman, and M. V. Norgard. 2008. Assessment of decorin-binding protein A to the infectivity of Borrelia burgdorferi in the murine models of needle and tick infection. BMC Microbiol. 8:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boardman, B. K., M. He, Z. Ouyang, H. Xu, X. Pang, and X. F. Yang. 2008. Essential role of the response regulator Rrp2 in the infectious cycle of Borrelia burgdorferi. Infect. Immun. 76:3844-3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brooks, C. S., P. S. Hefty, S. E. Jolliff, and D. R. Akins. 2003. Global analysis of Borrelia burgdorferi genes regulated by mammalian host-specific signals. Infect. Immun. 71:3371-3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brooks, C. S., S. R. Vuppala, A. M. Jett, and D. R. Akins. 2006. Identification of Borrelia burgdorferi outer surface proteins. Infect. Immun. 74:296-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burtnick, M. N., J. S. Downey, P. J. Brett, J. A. Boylan, J. G. Frye, T. R. Hoover, and F. C. Gherardini. 2007. Insights into the complex regulation of rpoS in Borrelia burgdorferi. Mol. Microbiol. 65:277-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caimano, M. J., C. H. Eggers, K. R. O. Hazlett, and J. D. Radolf. 2004. RpoS is not central to the general stress response in Borrelia burgdorferi but does control expression of one or more essential virulence determinants. Infect. Immun. 72:6433-6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caimano, M. J., R. Iyer, C. H. Eggers, C. Gonzalez, E. A. Morton, M. A. Gilbert, I. Schwartz, and J. D. Radolf. 2007. Analysis of the RpoS regulon in Borrelia burgdorferi in response to mammalian host signals provides insight into RpoS function during the enzootic cycle. Mol. Microbiol. 65:1193-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casjens, S., N. Palmer, R. van Vugt, W. M. Huang, B. Stevenson, P. Rosa, R. Lathigra, G. Sutton, J. Peterson, R. J. Dodson, D. Haft, E. Hickey, M. Gwinn, O. White, and C. M. Fraser. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35:490-516. [DOI] [PubMed] [Google Scholar]
- 10.Clifton, D. R., C. L. Nolder, J. L. Hughes, A. J. Nowalk, and J. A. Carroll. 2006. Regulation and expression of bba66 encoding an immunogenic infection-associated lipoprotein in Borrelia burgdorferi. Mol. Microbiol. 61:243-258. [DOI] [PubMed] [Google Scholar]
- 11.Coleman, A. S., X. Yang, M. Kumar, X. Zhang, K. Promnares, D. Shroder, M. R. Kenedy, J. F. Anderson, D. R. Akins, and U. Pal. 2008. Borrelia burgdorferi complement regulator-acquiring surface protein 2 does not contribute to complement resistance or host infectivity. PLoS One 3:3010e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Silva, A. M., S. R. Telford, L. R. Brunet, S. W. Barthold, and E. Fikrig. 1996. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J. Exp. Med. 183:271-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elias, A. F., P. E. Stewart, D. Grimm, M. J. Caimano, C. H. Eggers, K. Tilly, J. L. Bono, D. R. Akins, J. D. Radolf, T. G. Schwan, and P. Rosa. 2002. Clonal polymorphism of Borrelia burgdorferi strain B31 MI: implications for mutagenesis in an infectious strain background. Infect. Immun. 70:2139-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng, S., S. W. Barthold, S. R. Telford III, and E. Fikrig. 1996. P55, an immunogenic but nonprotective 55-kilodalton Borrelia burgdorferi protein in murine Lyme disease. Infect. Immun. 64:363-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng, S., S. Das, T. Lam, R. A. Flavell, and E. Fikrig. 1995. A 55-kilodalton antigen encoded by a gene on a Borrelia burgdorferi 49-kilobase plasmid is recognized by antibodies in sera from patients with Lyme disease. Infect. Immun. 63:3459-3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fingerle, V., G. Goettner, L. Gern, B. Wilske, and U. Schulte-Spechtel. 2007. Complementation of a Borrelia afzelii OspC mutant highlights the crucial role of OspC for dissemination of Borrelia afzelii in Ixodes ricinus. Int. J. Med. Microbiol. 297:97-107. [DOI] [PubMed] [Google Scholar]
- 17.Fingerle, V., S. Rauser, B. Hammer, O. Kahl, C. Heimerl, U. Schulte-Spechtel, L. Gern, and B. Wilske. 2002. Dynamics of dissemination and outer surface protein expression of different European Borrelia burgdorferi sensu lato strains in artificially infected Ixodes ricinus nymphs. J. Clin. Microbiol. 40:1456-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer, J. R., K. T. LeBlanc, and J. M. Leong. 2006. Fibronectin binding protein BBK32 of the Lyme disease spirochete promotes bacterial attachment to glycosaminoglycans. Infect. Immun. 74:435-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisher, M. A., D. Grimm, A. K. Henion, A. F. Elias, P. E. Stewart, P. A. Rosa, and F. C. Gherardini. 2005. Borrelia burgdorferi σ54 is required for mammalian infection and vector transmission but not for tick colonization. Proc. Natl. Acad. Sci. U.S.A. 102:5162-5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frank, K. L., S. F. Bundle, M. E. Kresge, C. H. Eggers, and D. S. Samuels. 2003. aadA confers streptomycin resistance in Borrelia burgdorferi. J. Bacteriol. 185:6723-6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J.-F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. Weidman, T. Utterback, L. Watthey, L. McDonald, P. Artiach, C. Bowman, S. Garland, C. Fujii, M. D. Cotton, K. Horst, K. Roberts, B. Hatch, H. O. Smith, and J. C. Venter. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580-586. [DOI] [PubMed] [Google Scholar]
- 22.Gautam, A., M. Hathaway, N. McClain, G. Ramesh, and R. Ramamoorthy. 2008. Analysis of the determinants of bba64 (P35) gene expression in Borrelia burgdorferi using a gfp reporter. Microbiology 154:275-285. [DOI] [PubMed] [Google Scholar]
- 23.Gilbert, M. A., E. A. Morton, S. F. Bundle, and D. S. Samuels. 2007. Artificial regulation of ospC expression in Borrelia burgdorferi. Mol. Microbiol. 63:1259-1273. [DOI] [PubMed] [Google Scholar]
- 24.Gilmore, R. D., and J. Piesman. 2000. Inhibition of Borrelia burgdorferi migration from the midgut to the salivary glands following feeding by ticks on OspC-immunized mice. Infect. Immun. 68:411-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilmore, R. D., Jr., R. R. Howison, V. L. Schmit, A. J. Nowalk, D. R. Clifton, C. Nolder, J. L. Hughes, and J. A. Carroll. 2007. Temporal expression analysis of the Borrelia burgdorferi paralogous gene family 54 genes BBA64, BBA65, and BBA66 during persistent infection in mice. Infect. Immun. 75:2753-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grimm, D., K. Tilly, R. Byram, P. E. Stewart, J. G. Krum, D. M. Bueschel, T. G. Schwan, P. F. Policastro, A. F. Elias, and P. A. Rosa. 2004. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc. Natl. Acad. Sci. U.S.A. 101:3142-3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hagman, K. E., X. Yang, S. K. Wikel, G. B. Schoeler, M. J. Caimano, J. D. Radolf, and M. V. Norgard. 2000. Decorin-binding protein A (DbpA) of Borrelia burgdorferi is not protective when immunized mice are challenged via tick infestation and correlates with the lack of DbpA expression by B. burgdorferi in ticks. Infect. Immun. 68:4759-4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He, M., B. K. Boardman, D. Yan, and X. F. Yang. 2007. Regulation of expression of the fibronectin-binding protein BBK32 in Borrelia burgdorferi. J. Bacteriol. 189:8377-8380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hübner, A., X. Yang, D. M. Nolen, T. G. Popova, F. C. Cabello, and M. V. Norgard. 2001. Expression of Borrelia burgdorferi OspC and DbpA is controlled by a RpoN-RpoS regulatory pathway. Proc. Natl. Acad. Sci. U.S.A. 98:12724-12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawabata, H., S. J. Norris, and H. Watanabe. 2004. BBE02 disruption mutants of Borrelia burgdorferi B31 have a highly transformable, infectious phenotype. Infect. Immun. 72:7147-7154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Labandeira-Rey, M., and J. T. Skare. 2001. Decreased infectivity in Borrelia burgdorferi strain B31 is associated with loss of linear plasmid 25 or 28-1. Infect. Immun. 69:446-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li, X., X. Liu, D. S. Beck, F. S. Kantor, and E. Fikrig. 2006. Borrelia burgdorferi lacking BBK32, a fibronectin-binding protein, retains full pathogenicity. Infect. Immun. 74:3305-3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li, X., G. Neelakanta, X. Liu, D. S. Beck, F. S. Kantor, D. Fish, J. F. Anderson, and E. Fikrig. 2007. Role of outer surface protein D in the Borrelia burgdorferi life cycle. Infect. Immun. 75:4237-4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liang, F. T., F. K. Nelson, and E. Fikrig. 2002. Molecular adaptation of Borrelia burgdorferi in the murine host. J. Exp. Med. 196:275-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liang, F. T., J. Yan, M. L. Mbow, S. L. Sviat, R. D. Gilmore, M. Mamula, and E. Fikrig. 2004. Borrelia burgdorferi changes its surface antigenic expression in response to host immune responses. Infect. Immun. 72:5759-5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lybecker, M. C., and D. S. Samuels. 2007. Temperature-induced regulation of RpoS by a small RNA in Borrelia burgdorferi. Mol. Microbiol. 64:1075-1089. [DOI] [PubMed] [Google Scholar]
- 37.Maruskova, M., M. D. Esteve-Gassent, V. L. Sexton, and J. Seshu. 2008. Role of the BBA64 locus of Borrelia burgdorferi in early stages of infectivity in a murine model of Lyme disease. Infect. Immun. 76:391-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maruskova, M., and J. Seshu. 2008. Deletion of BBA64, BBA65, and BBA66 loci does not alter the infectivity of Borrelia burgdorferi in the murine model of Lyme disease. Infect. Immun. 76:5274-5284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McDowell, J. V., S. Y. Sung, M. Labandeira-Rey, J. T. Skare, and R. T. Marconi. 2001. Analysis of mechanisms associated with loss of infectivity of clonal populations of Borrelia burgdorferi B31MI. Infect. Immun. 69:3670-3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Medrano, M. S., Y. Ding, X. G. Wang, P. Lu, J. Coburn, and L. T. Hu. 2007. Regulators of expression of the oligopeptide permease A proteins of Borrelia burgdorferi. J. Bacteriol. 189:2653-2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montgomery, R. R., S. E. Malawista, K. J. M. Feen, and L. K. Bockenstedt. 1996. Direct demonstration of antigenic substitution of Borrelia burgdorferi ex vivo: exploration of the paradox of the early immune response to outer surface proteins A and C in Lyme disease. J. Exp. Med. 183:261-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohnishi, J., J. Piesman, and A. M. de Silva. 2001. Antigenic and genetic heterogeneity of Borrelia burgdorferi populations transmitted by ticks. Proc. Natl. Acad. Sci. U.S.A. 98:670-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ojaimi, C., C. Brooks, S. Casjens, P. Rosa, A. Elias, A. Barbour, A. Jasinskas, J. Benach, L. Katona, J. Radolf, M. Caimano, J. Skare, K. Swingle, D. Akins, and I. Schwartz. 2003. Profiling of temperature-induced changes in Borrelia burgdorferi gene expression by using whole genome arrays. Infect. Immun. 71:1689-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ouyang, Z., J. S. Blevins, and M. V. Norgard. 2008. Transcriptional interplay among the regulators Rrp2, RpoN, and RpoS in Borrelia burgdorferi. Microbiology 154:2641-2658. [DOI] [PubMed] [Google Scholar]
- 45.Pal, U., J. Dai, X. Li, G. Neelakanta, P. Luo, M. Kumar, P. Wang, X. Yang, J. Anderson, and E. Fikrig. 2008. A differential role for BB0365 in the persistence of Borrelia burgdorferi in mice and ticks. J. Infect. Dis. 197:148-155. [DOI] [PubMed] [Google Scholar]
- 46.Pal, U., and E. Fikrig. 2003. Adaptation of Borrelia burgdorferi in the vector and vertebrate host. Microbes Infect. 5:659-666. [DOI] [PubMed] [Google Scholar]
- 47.Pal, U., X. Li, T. Wang, R. R. Montgomery, N. Ramamoorthi, A. M. deSilva, F. Bao, X. Yang, M. Pypaert, and D. Pradhan. 2004. TROSPA, an Ixodes scapularis receptor for Borrelia burgdorferi. Cell 119:457-468. [DOI] [PubMed] [Google Scholar]
- 48.Pal, U., X. Yang, M. Chen, L. K. Bockenstedt, J. F. Anderson, R. A. Flavell, M. V. Norgard, and E. Fikrig. 2004. OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J. Clin. Invest. 113:220-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Purser, J. E., and S. J. Norris. 2000. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA 97:13865-13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Radolf, J. D., and M. J. Caimano. 2008. The long strange trip of Borrelia burgdorferi outer-surface protein C. Mol. Microbiol. 69:1-4. [DOI] [PubMed] [Google Scholar]
- 51.Ramamoorthi, N., S. Narasimhan, U. Pal, F. Bao, X. F. Yang, D. Fish, J. Anguita, M. V. Norgard, F. S. Kantor, J. F. Anderson, R. A. Koski, and E. Fikrig. 2005. The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature 436:573-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Revel, A. T., A. M. Talaat, and M. V. Norgard. 2002. DNA microarray analysis of differential gene expression in Borrelia burgdorferi, the Lyme disease spirochete. Proc. Natl. Acad. Sci. U.S.A. 99:1562-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Samuels, D. S. 1995. Electrotransformation of the spirochete Borrelia burgdorferi, p. 253-259. In J. A. Nickoloff (ed.), Methods in molecular biology. Humana Press, Totowa, NJ. [DOI] [PMC free article] [PubMed]
- 54.Schwan, T. G. 2003. Temporal regulation of outer surface proteins of the Lyme-disease spirochete Borrelia burgdorferi. Biochem. Soc. Trans. 31:108-112. [DOI] [PubMed] [Google Scholar]
- 55.Schwan, T. G., and J. Piesman. 2000. Temporal changes in outer surface proteins A and C of the Lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J. Clin. Microbiol. 38:382-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schwan, T. G., J. Piesman, W. T. Golde, M. C. Dolan, and P. A. Rosa. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. U.S.A. 92:2909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seshu, J., M. D. Esteve-Gassent, M. Labandeira-Rey, J. H. Kim, J. P. Trzeciakowski, M. Hook, and J. T. Skare. 2006. Inactivation of the fibronectin-binding adhesin gene bbk32 significantly attenuates the infectivity potential of Borrelia burgdorferi. Mol. Microbiol. 59:1591-1601. [DOI] [PubMed] [Google Scholar]
- 58.Shi, Y., Q. Xu, K. McShan, and F. T. Liang. 2008. Both decorin-binding proteins A and B are critical for the overall virulence of Borrelia burgdorferi. Infect. Immun. 76:1239-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singh, S. K., and H. J. Girschick. 2004. Molecular survival strategies of the Lyme disease spirochete Borrelia burgdorferi. Lancet Infect. Dis. 4:575-583. [DOI] [PubMed] [Google Scholar]
- 60.Smith, A. H., J. S. Blevins, G. N. Bachlani, X. F. Yang, and M. V. Norgard. 2007. Evidence that RpoS (σS) in Borrelia burgdorferi is controlled directly by RpoN (σ54/σN). J. Bacteriol. 189:2139-2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stewart, P. E., A. Bestor, J. N. Cullen, and P. A. Rosa. 2008. A tightly regulated surface protein of Borrelia burgdorferi is not essential to the mouse-tick infectious cycle. Infect. Immun. 76:1970-1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stewart, P. E., X. Wang, D. M. Bueschel, D. R. Clifton, D. Grimm, K. Tilly, J. A. Carroll, J. J. Weis, and P. A. Rosa. 2006. Delineating the requirement for the Borrelia burgdorferi virulence factor OspC in the mammalian host. Infect. Immun. 74:3547-3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tilly, K., J. G. Krum, A. Bestor, M. W. Jewett, D. Grimm, D. Bueschel, R. Byram, D. Dorward, M. J. VanRaden, P. Stewart, and P. Rosa. 2006. Borrelia burgdorferi OspC protein required exclusively in a crucial early stage of mammalian infection. Infect. Immun. 74:3554-3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tokarz, R., J. M. Anderton, L. I. Katona, and J. L. Benach. 2004. Combined effects of blood and temperature shift on Borrelia burgdorferi gene expression as determined by whole genome DNA array. Infect. Immun. 72:5419-5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu, Q., K. McShan, and F. T. Liang. 2007. Identification of an ospC operator critical for immune evasion of Borrelia burgdorferi. Mol. Microbiol. 64:220-231. [DOI] [PubMed] [Google Scholar]
- 66.Xu, Q., K. McShan, and F. T. Liang. 2008. Verification and dissection of the ospC operator by using flaB promoter as a reporter in Borrelia burgdorferi. Microb. Pathog. 45:70-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang, X., M. S. Goldberg, T. G. Popova, G. B. Schoeler, S. K. Wikel, K. E. Hagman, and M. V. Norgard. 2000. Interdependence of environmental factors influencing reciprocal patterns of gene expression in virulent Borrelia burgdorferi. Mol. Microbiol. 37:1470-1479. [DOI] [PubMed] [Google Scholar]
- 68.Yang, X., T. G. Popova, K. E. Hagman, S. K. Wikel, G. B. Schoeler, M. J. Caimano, J. D. Radolf, and M. V. Norgard. 1999. Identification, characterization, and expression of three new members of the Borrelia burgdorferi Mlp (2.9) lipoprotein gene family. Infect. Immun. 67:6008-6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang, X. F., S. M. Alani, and M. V. Norgard. 2003. The response regulator Rrp2 is essential for the expression of major membrane lipoproteins in Borrelia burgdorferi. Proc. Natl. Acad. Sci. U.S.A. 100:11001-11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang, X. F., U. Pal, S. M. Alani, E. Fikrig, and M. V. Norgard. 2004. Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J. Exp. Med. 199:641-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang, X., A. S. Coleman, J. Anguita, and U. Pal. 2009. A chromosomally encoded virulence factor protects the Lyme disease pathogen against host-adaptive immunity. PLoS Pathog. 5:e1000326. [DOI] [PMC free article] [PubMed] [Google Scholar]