Abstract
The important veterinary pathogen Clostridium perfringens type B is unique for producing the two most lethal C. perfringens toxins, i.e., epsilon-toxin and beta-toxin. Our recent study (K. Miyamoto, J. Li, S. Sayeed, S. Akimoto, and B. A. McClane, J. Bacteriol. 190:7178-7188, 2008) showed that most, if not all, type B isolates carry a 65-kb epsilon-toxin-encoding plasmid. However, this epsilon-toxin plasmid did not possess the cpb gene encoding beta-toxin, suggesting that type B isolates carry at least one additional virulence plasmid. Therefore, the current study used Southern blotting of pulsed-field gels to localize the cpb gene to ∼90-kb plasmids in most type B isolates, although a few isolates carried a ∼65-kb cpb plasmid distinct from their etx plasmid. Overlapping PCR analysis then showed that the gene encoding the recently discovered TpeL toxin is located ∼3 kb downstream of the plasmid-borne cpb gene. As shown earlier for their epsilon-toxin-encoding plasmids, the beta-toxin-encoding plasmids of type B isolates were found to carry a tcp locus, suggesting that they are conjugative. Additionally, IS1151-like sequences were identified upstream of the cpb gene in type B isolates. These IS1151-like sequences may mobilize the cpb gene based upon detection of possible cpb-containing circular transposition intermediates. Most type B isolates also possessed a third virulence plasmid that carries genes encoding urease and lambda-toxin. Collectively, these findings suggest that type B isolates are among the most plasmid dependent of all C. perfringens isolates for virulence, as they usually carry three potential virulence plasmids.
Isolates of the Gram-positive, spore-forming anaerobe Clostridium perfringens are classified (31) into five different types (A to E), depending upon their production of four (alpha, beta, epsilon, and iota) lethal typing toxins. All C. perfringens types produce alpha-toxin; in addition, type B isolates produce both beta- and epsilon-toxins, type C isolates produce beta-toxin, type D isolates produce epsilon-toxin and type E isolates produce iota-toxin. Except for the chromosomal alpha-toxin gene (plc), all C. perfringens typing toxins are encoded by genes resident on large plasmids (11, 22, 23, 32, 33). Large plasmids can also encode other C. perfringens toxins, such as the enterotoxin (CPE) or beta2-toxin (8, 9, 14, 35), as well as other potential virulence factors such as urease (12, 23).
The large virulence plasmids of C. perfringens are only now being characterized (23, 28, 29, 33). The first analyzed, and still most studied, C. perfringens toxin plasmids are the CPE-encoding plasmids of type A isolates (14, 28). In type A isolates, most plasmids carrying the enterotoxin gene (cpe) belong to one of two families: (i) a 75.3-kb plasmid with a cpe locus containing an IS1151 element and the cpb2 gene encoding beta2-toxin or (ii) a 70.5-kb plasmid that lacks the cpb2 gene and carries a cpe locus with an IS1470-like sequence instead of an IS1151 element. Sequence comparisons (28) revealed that these two cpe plasmid families of type A isolates share a conserved region of ∼35 kb that includes the transfer of clostridial plasmid (tcp) locus, which is related to the conjugative transposon Tn916. Confirming that cpe plasmids can be conjugative, mixed mating studies have directly demonstrated transfer of the cpe plasmid from type A isolate F4969 to other C. perfringens isolates (5). A similar tcp locus is also shared by the tetracycline resistance plasmid pCW3 and several other toxin plasmids (2, 23, 28, 29, 33), as discussed below. Mutagenesis analyses demonstrated the importance of several genes in the tcp locus for conjugative transfer of pCW3 (2) and, by extension, presumably the tcp-carrying, conjugative toxin plasmids, such as the cpe plasmid of isolate F4969 (5) and some etx plasmids of type D isolates (19).
Although the sequence of an iota-toxin-encoding plasmid has not yet been published, pulsed-field gel electrophoresis (PFGE) and PCR analyses determined that these plasmids are typically larger than the cpe plasmids of type A isolates (23). Specifically, iota-toxin plasmids are often ≥100 kb in size, reaching up to a size of ∼135 kb. These plasmids of type E isolates often encode, in addition to the iota-toxin, other potential virulence factors such as lambda-toxin and urease. These plasmids also carry a tcp locus, suggesting that they may be capable of conjugative transfer. Interestingly, many iota-toxin plasmids appear to be related, sometimes extensively, to the cpe plasmids of type A isolates. Consequently, it has been suggested (3, 23) that many iota-toxin plasmids arose from insertion of an iota-toxin gene-carrying mobile genetic element near the cpe gene on a tcp-carrying type A plasmid. This insertional event apparently inactivated the cpe gene, so most or all type E isolates now carry silent cpe genes (3, 23).
The epsilon-toxin-encoding plasmids of type D isolates show considerable size variations (33), ranging from ∼48 kb to ∼110 kb. These size variations in type D etx plasmids reflect, in part, differences among their toxin gene carriage. The small 48-kb etx plasmids present in some type D isolates typically lack either the cpe gene or the cpb2 gene (encoding beta2-toxin), while the larger (>75-kb) etx plasmids found in other type D isolates can also carry the cpe gene, the cpb2 gene, or both the cpe and cpb2 genes. Consequently, some type D isolates carry a toxin plasmid encoding only etx, other type D isolates carry a toxin plasmid with up to three different functional toxin genes (etx, cpb2, and cpe), and the remaining type D isolates carry their etx, cpe, and cpb2 genes on up to three distinct plasmids.
C. perfringens type B isolates uniquely produce both beta- and epsilon-toxins, the two most lethal C. perfringens toxins (13). These bacteria are important pathogens of sheep but also cause disease in goats, calves, and foals (26). For unknown reasons, diseases caused by C. perfringens type B isolates apparently are restricted to certain geographic regions (24, 25, 26). C. perfringens type B enterotoxemias initiate when these bacteria proliferate in the gut, accompanied by toxin production. Those toxins initially affect the intestines but later are absorbed and act systemically. Studies from our group (13) showed that beta- and epsilon-toxins each contribute to lethality in a mouse model involving intravenous injection of type B culture supernatants.
There has been characterization of only one type B virulence plasmid to date. Our recent study (29) showed that most, if not all, type B isolates carry a common etx plasmid of ∼65 kb that also possesses a tcp locus and a cpb2 gene, although not the cpb gene encoding beta-toxin. Interestingly, the type B etx plasmid is highly (80%) related to the ∼75-kb cpe- and cpb2-carrying plasmid found in some type A isolates (28). The ∼65-kb etx plasmid present in most, if not all, type B isolates is also carried by a minority of type D isolates (29).
The absence of the cpb gene from their etx plasmids suggested that most type B isolates might carry additional virulence plasmids. Therefore, the current study was performed to better address virulence plasmid carriage and diversity among type B disease isolates.
MATERIALS AND METHODS
Bacterial strains, media, and reagents.
As indicated previously (13, 29), the 17 C. perfringens type B isolates examined in this study originated mainly from diseased animals (Table 1). The toxin genotypes of these isolates (13) were determined previously using multiplex PCR (15). Each type B isolate was grown overnight at 37°C under anaerobic conditions on SFP agar (Difco Laboratories) containing 0.04% d-cycloserine (Sigma Aldrich) in order to ensure culture purity. Fluid thioglycolate medium (FTG) (Difco Laboratories) or TGY medium (3% tryptic soy broth [Becton-Dickinson] containing 2% glucose [Fisher Scientific], 1% yeast extract [Becton-Dickinson], and 0.1% thioglycolate [Sigma Aldrich]) was used to grow broth cultures.
TABLE 1.
Type B plasmid sizes and plasmid-borne virulence gene locations
| Isolatea | Size(s) (kb) of plasmid(s) carrying: |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| etx | cpb | tpeL | cpb2 | tcpF/H | rep | lam | ureC | IS1151 | |
| CN1794 | 65 | 90 | 90 | 65 | 65/80/90 | 65/90 | 80 | 80 | 65/80/90 |
| CN1990b | 65 | 90 | 90 | 65 | 65/80/90 | 65/90 | 80 | 80 | 65/80/90 |
| NCTC3110b | 65c | 65 | 65 | 65 | 65 | 65 | 65 | ||
| CN677b | 65c | 65 | 65 | 65 | 65 | 65 | 65 | ||
| CN1793b | 65 | 90 | 90 | 65 | 65/80/90 | 65/90 | 80 | 80 | 65/80/90 |
| CN1795 | 65 | 90 | 90 | 65 | 65/80/90 | 65/90 | 80 | 80 | 65/80/90 |
| CN1886b | 65 | 90 | 90 | 65 | 65/80/90 | 65/90 | 80 | 80 | 65/80/90 |
| CN2003b | 65 | 90 | 90 | 65 | 65/80/90 | 65/90 | 80 | 80 | 65/8090 |
| CN2414b | 65 | 90 | 90 | 65 | 65/80/90 | 65/90 | 80 | 80 | 65/80/90 |
| CN2416b | 65 | 90 | 90 | 65 | 65/80/90 | 65/90 | 80 | 80 | 65/80/90 |
| CN3425b | 65 | 90 | 90 | 65 | 65/80/90 | 65/90 | 80 | 80 | 65/80/90 |
| CN3434b | 65 | 90 | 90 | 65 | 65/80/90 | 65/90 | 80 | 80 | 65/80/90 |
| CN3447 | 65 | 90 | 90 | 65 | 65/80/90 | 65/90 | 80 | 80 | 65/80/90 |
| NCTC8533b | 65 | 90 | 90 | 65 | 65/80/90 | 65/90 | 80 | 80 | 65/90 |
| PS49 | 65 | 90 | 90 | 65 | 65/80/90 | 65/90 | 80 | 80 | 65/90 |
| JGS1984 | 65 | 90 | 90 | 65 | 65/80/90 | 65/90 | 65/90 | ||
| Bar2 | 65 | 90 | 90 | 65 | 65/80/90 | 65/90 | 65/90 | ||
Isolates with a CN prefix originated from the Burroughs-Wellcome collection, which included a number of type B isolates typically collected from diseased animals (usually lambs) during the 1930s to 1960s. Except as otherwise indicated, the geographical and disease origins of the isolates are unknown except that Bar2 was obtained from an Australian sheep and NCTC3110 is a vaccine production strain isolated in Kenya.
Originated from the United Kingdom.
The etx and cpb genes of NCTC3110 and CN677 are located on a 65-kb plasmid different from their 65-kb plasmid carrying the cpb and tpeL genes.
Pulsed-field gel electrophoresis.
C. perfringens type B isolates were grown overnight at 37°C in FTG broth. A 0.1-ml aliquot of each starter culture was then inoculated into 10 ml of TGY and grown overnight at 37°C. The overnight TGY cultures were used to prepare genomic C. perfringens DNA-containing agarose plugs, as described previously (33).
For isolates showing Southern blot signal colocalization using probes for two different open reading frames (ORFs) (as described below), DNA plugs were digested with restriction endonuclease ApaI, AvaI, ClaI, KpnI, NcoI, SphI, NheI, or XhoI (New England Biolabs) to help distinguish whether these colocalizing signals were due to the different probes hybridizing to two genes carried on the same plasmid or to two genes located on two comigrating plasmids (9, 23, 33). In these experiments, each set of plugs was incubated, with or without a restriction enzyme, in 200 μl of the appropriate buffer solution recommended by the enzyme manufacturer.
Pulsed-field gel electrophoresis was performed with a 1% agarose gel using a CHEF-DR II system (Bio-Rad Laboratories) and 0.5× Tris-borate-EDTA buffer (Bio-Rad Laboratories) at 14°C. The running parameters for undigested DNA were as follows: initial pulse, 1 s; final pulse, 25 s; voltage, 6 V/cm; time, 24 h. The following running parameters were used for DNA digested with restriction enzymes: initial pulse, 1 s; final pulse, 12 s; voltage, 6 V/cm; time, 16 h. After pulsed-field gel electrophoresis, the gel was stained with ethidium bromide, washed with distilled water, and photographed under UV light. Mid-range or low-range PFG markers (New England Biolabs) were used as molecular size standards, as appropriate.
Southern blot analyses of pulsed field gels.
Digoxigenin (DIG)-labeled etx, cpb, cpb2, tcpH, tcpF, IS1151, replication protein gene (rep), urease C gene (ureC), and lambda-toxin gene (lam) probes were prepared with primers described previously (23, 33, 34). Using those DIG-labeled probes, Southern hybridization of pulsed-field gels was performed by our standard techniques (23, 29, 33). DIG labeling and detection reagents were obtained from Roche Applied Science. The CSPD substrate (Roche Applied Science) was used for detection of hybridized probes according to the manufacturer's instructions.
PCR analyses of lam and ureC genes in C. perfringens type B isolates.
Carriage of the lam gene or ureC gene was assessed by PCR, as described previously (23, 33). The PCR products were electrophoresed on a 1% agarose gel and stained with ethidium bromide for visualization.
PCR analyses of tpeL carriage and linkage between cpb and tpeL in type B isolates.
To evaluate the presence of the tpeL gene encoding the C. perfringens large cytotoxin (TpeL) in type B isolates, PCR was performed using the primers (TpelscF and TpelscR) listed in Table 2. Based upon the C. perfringens type B strain ATCC 3626 sequence released by the J. Craig Venter Institute (JCVI), a series of primers were then constructed (Table 2) to evaluate, by overlapping PCR, the potential linkage between tpeL and cpb in type B isolates. PCR conditions for these amplifications were as follows: 94°C for 3 min and 35 cycles of 94°C for 1 min, 55°C for 1 min, and 68°C for 1.5 min. PCR products were run on a 1% agarose gel and stained with ethidium bromide for visualization.
TABLE 2.
Primers used for internal amplification of tpeL for linkage analysis and sequencing of cpb to tpeL
| Primer | Sequence (5′ to 3′) | PCR product (size, bp) |
|---|---|---|
| TpelscF | CATTTGCCATTTATTTTCAG | (646) |
| TpelscR | TATTCATTTTGTTTGGGTTG | |
| TpelR1 | GATTATAAAGCGCAAAACTTTGAC | R1 (1,245) |
| TpelF1 | ATTTCCTATAATTATTTTATTATCC | |
| SeqR6 | GGATAATAAAATAATTATAGGAAAT | R2 (1,244) |
| SeqF6 | CTCTATATCCTATAATTGGTTCC | |
| SeqR5 | GGAACCAATTATAGGATATAGAG | R3 (1,172) |
| SeqF5 | GCATGGAGATAGAGTAGACC | |
| SeqR4 | GGTCTACTCTATCTCCATGC | R4 (1,098) |
| SeqF4 | GTTATTCTTGCAATATCCATAAG | |
| cpbR3 | GTTCTAACTGCTCCTAATGG | R5 (1,045) |
| cpbF3 | CTTATGGATATTGCAAGAATAAC | |
| N1 | TATATGAATTAGAAAATGATGGTG | —a |
| N2 | TATTGAATGATAACTCTGAAAG | — |
| N3 | TCATCTTTAGTATCTCTAAATC | — |
| N4 | TTATTCCTAAAAAGTCCTGGTG | — |
—, sequencing primer.
Long-range PCR studies were also performed to directly connect the tpeL and cpb genes, DNA was isolated from type B strains NCTC3110, CN677, CN1793, CN1795, CN3447, Bar2, and PS49 using the Master-Pure Gram-positive DNA purification kit (Epicentre). Each PCR mixture contained 1 μl of template DNA, 25 μl of TAQ Long Range Complete 2× mix (New England Biolabs), and 1 μl each of the primer pair TpelR1 and cpbF3 (Table 2) (1 μM final concentration). The reaction mixtures, with a total volume of 50 μl, were placed in a thermocycler (Techne) and subjected to the following amplification conditions: 1 cycle of 95°C for 2 min; 35 cycles of 95°C for 30 s, 56°C for 40 s, and 65°C for 5 min; and a single extension at 65°C for 10 min. PCR products were then electrophoresed on a 1% agarose gel, which was stained with ethidium bromide for product visualization. The long-range PCR products amplified from CN677 and CN1795 were PCR cloned into the pCR2.1-TOPO vector (Invitrogen), and those inserts were then sequenced at the University of Pittsburgh Core sequencing facility using primers listed in Table 2.
PCR linkage of the cpb gene to IS1151-like sequences in type B isolates.
Based upon the ATCC 3626 sequence released by JCVI, a possible linkage between the cpb gene and IS1151-2 insertion sequences in other C. perfringens type B isolates was investigated by PCR using primers cpb-F (5′-CTTGAAGAGTCAACAGATTGAT-3′) and IS1151-R (5′-GCTGCTAAAGTCTCTACTAG-3′). PCR conditions for these amplifications were as follows: 94°C for 3 min; 35 cycles of 94°C for 1 min, 55°C for 1 min, and 68°C for 4 min; and a single extension at 68°C for 10 min. PCR products were run on a 1% gel and stained with ethidium bromide for visualization.
Reverse transcription-PCR (RT-PCR) analysis of tpeL expression in type B isolates.
C. perfringens type B isolates NCTC3110, CN677, CN3447, and NCTC8533 were grown overnight at 37°C in FTG. A 0.1-ml aliquot of each of those starter cultures was transferred to 10 ml of TGY medium and grown at 37°C for either 8 h or 24 h. Total C. perfringens RNA was extracted from 2 ml of an overnight TGY culture using saturated phenol (Fisher Scientific). After centrifugation (10,000 × g at 4°C for 5 min), the nucleic acid-containing supernatant received cold ethanol. The sample was mixed well and then centrifuged (10,000 × g at 4°C) for 5 min to obtain the RNA pellet. The pellet was washed two times with cold 70% ethanol and then resuspended in 100 μl of DNase-free, RNase-free water. All RNA samples were additionally treated with 2 U of DNase I (Ambion) at 37°C for 30 min. To stop DNase I activity, a DNase I inhibitor (Ambion) was added to the reaction tube. RNA was quantified by absorbance at 260 nm and stored in 50-μl aliquots at −80°C.
RT-PCR was performed with the DNase-treated RNA samples using the AccesQuick RT-PCR system (Promega). Briefly, each RNA sample (50 ng) was reverse transcribed to cDNA at 45°C for 45 min and then used as the template for PCRs (35 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min, followed by a single cycle at 72°C for 10 min) with the gene-specific primers (TpelscF and TpelscR [Table 2]) or housekeeping gene polC using primers and conditions as described before (36) to check the quality of RNA samples (data not shown). Control RT-PCRs were similarly performed, except for the omission of reverse transcriptase.
PCR identification of possible circular transposition intermediates carrying the cpb or tpeL ORF.
Template DNAs used for these PCR studies were prepared as described previously (33). By using those template DNAs, PCR amplification of possible circular transposition intermediates containing the cpb or tpeL ORF was assessed using the primer sets TnF (5′-ATACATTAACTAACTTAGAACGTAC-3′) and BetaR (5′GAAAGAAACTGTTATTATCTTAATTG-3′) for cpb loop and LoopF (5′-AACCAATTATAGGATATAGAG-3′) and LoopR (5′GCTACTTACTTAGCTAGTGAAG-3′) for tpeL loop. PCR conditions for these amplifications were as follows: 95°C for 2 min; 35 cycles of 95°C for 30 s, 55°C for 40 s, and 68°C for 2 min; and a single cycle of 68°C for 10 min. PCR products were run on a 1% agarose gel and stained with ethidium bromide for visualization. PCR products were cloned into pCR2.1-TOPO vector (Invitrogen, Carlsbad, CA) and sequenced at the Genomics and Proteomics Core Laboratory of the University of Pittsburgh, using vector-specific primers M13F and M13R.
Nucleotide sequence accession numbers.
Results from the sequencing analyses were deposited in GenBank under accession numbers GUO54491 and GUO54492.
RESULTS
Characterization of toxin plasmid diversity among type B isolates.
To initiate these studies, pulsed-field gels were run under electrophoresis conditions that (i) allow plasmids, but not chromosomal DNA, to enter the gel (9, 12, 23, 28, 33) and (ii) provide an accurate measurement of C. perfringens plasmid size (28). We first subjected DNA that had been electrophoresed on a pulsed-field gel to Southern blot hybridization with an etx probe (Fig. 1A and Table 1), which confirmed previous observations (29) that etx is consistently present on an ∼65-kb plasmid in type B isolates.
FIG. 1.
Southern hybridization analyses of pulsed-field gels run with DNAs extracted from representative type B isolates NCTC3110, CN677, CN1793, CN1886, CN3425, and CN3447. (A) A blot hybridized with an etx-specific probe; (B) a similar blot of a different pulsed gel hybridized with a cpb2-specific probe; (C) the blot from panel B was stripped and reprobed with a cpb-specific probe; (D) overlay of the cpb-probed blot and cpb2-probed blot of panels B and C. The migration of molecular size markers is indicated on the left, and isolate designations are indicated above the lanes.
Previous experiments had also indicated that in type B isolates, a cpb2 gene is present on the ∼65-kb etx plasmid (29). To confirm that finding and (more importantly) assess for the first time whether some type B isolates might possess additional plasmids also carrying cpb2, DNA on a pulsed-field gel was Southern blotted with a cpb2 probe. However, those blots showed a single cpb2 signal at 65 kb (Fig. 1B), strongly suggesting that most, if not all, type B isolates possess only the cpb2 gene previously shown to be present on their ∼65-kb etx-carrying plasmid (29).
Plasmid carriage of the cpb gene among type B isolates has not yet been surveyed (note that, despite their similar names, cpb and cpb2 do not share sequence homology [16]). Therefore, the same Southern blot that had been hybridized with a cpb2 probe was stripped and rehybridized with a cpb probe. This analysis revealed that the cpb gene is usually present on an ∼90-kb plasmid in type B isolates (Fig. 1C and Table 1). The presence of distinct etx/cpb2 plasmids and cpb plasmids in most type B isolates was clearly demonstrated by overlaying the Southern blots shown in Fig. 1 after separate hybridizations with cpb2 or cpb probes (Fig. 1D). However, two type B isolates, NCTC3110 and CN677, did not fit this general pattern, as they carried their cpb gene on an ∼65-kb plasmid (Fig. 1C), matching the size of their etx/cpb2 plasmid.
Evaluation of whether cpb and etx/cpb2 are present on the same or different plasmids in type B isolates NCTC3110 and CN677.
As shown in Fig. 1, DNAs from type B isolates NCTC3110 and CN677 hybridized the cpb, etx, and cpb2 probes at the same 65-kb pulsed-field gel blot location, indicating that these two isolates carry these three toxin genes either on the same plasmid or (since etx and cpb2 are present on the same 65-kb plasmid in type B isolates [29]) on two distinct comigrating plasmids of similar sizes.
To discriminate between those two possibilities, DNA from NCTC3110 or CN677 was digested with a battery of restriction endonucleases. In this experiment (9, 23, 33), the migration of two genes residing on the same plasmid should consistently exhibit the same response (sensitivity or insensitivity) to digestion using each restriction endonuclease. However, if one gene showed no change in migration after digestion with a particular restriction endonuclease but migration of the second gene was affected by digestion with the same enzyme, this would indicate that the two genes are present on plasmids that are of similar size but distinct (9, 23, 33).
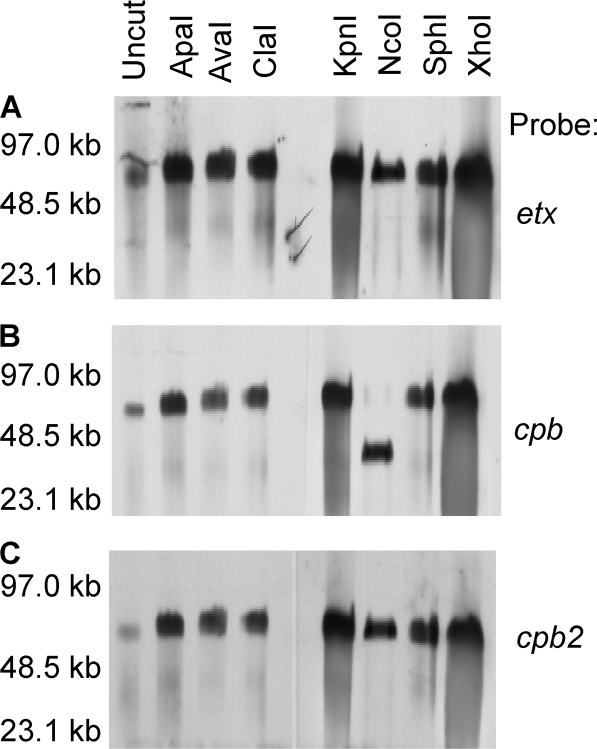
For NCTC3110 DNA, the migration of etx-containing DNA and cpb-containing DNA exhibited a different susceptibility pattern of sensitivity to restriction enzyme digestion (Fig. 2A and B). In contrast, cpb2-containing DNA showed a susceptibility to restriction enzyme digestion that was similar to that of etx-containing DNA (Fig. 2A and C), which is consistent with previous conclusions that these two toxin genes are located on the same 65-kb plasmid in type B isolates (29). These results indicated that NCTC3110 carries its cpb gene on a plasmid of similar size as, but different from, the one carrying the etx and cpb2 genes.
FIG. 2.
Southern hybridization analyses of pulsed-field gels run with restriction enzyme-digested DNA extracted from type B isolate NCTC3110. The blot was first hybridized with an etx-specific probe (A) and then stripped and reprobed with a cpb-specific probe (B) before a final stripping and reprobing with a cpb2-specific probe (C). The migration of molecular size markers is indicated on the left.
Similar analysis indicated (data not shown) that the cpb2 and etx genes of CN677 are also present together on ∼65-kb plasmid that is distinct from the ∼65-kb plasmid carrying the cpb gene in this type B isolate.
Carriage of the rep gene among plasmids of type B isolates.
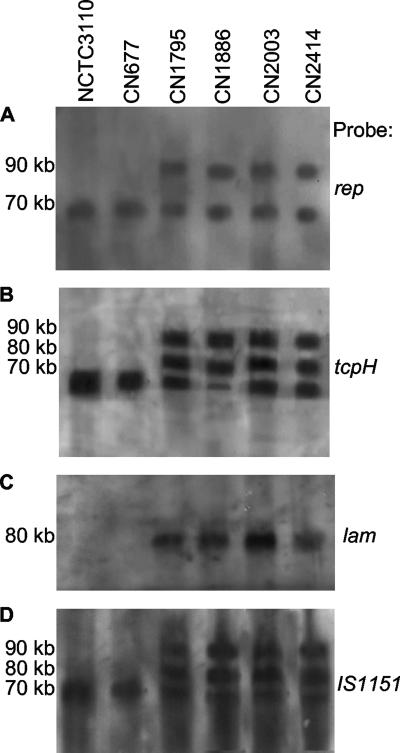
The Rep protein was shown to be important for replication of C. perfringens tetracycline resistance plasmid pCW3 (2). The gene (rep) encoding the Rep protein has also been localized to several toxin plasmids in type A, D, and E isolates (23, 28, 33), including the sequenced etx/cpb2 plasmid of type B isolates (29). Therefore, a Southern blot analysis of pulsed-field gels was performed to assess whether type B isolates might possess additional plasmids, besides their etx/cpb2 plasmid, that also carry rep sequences. This survey demonstrated that, for all surveyed type B isolates, the rep probe consistently hybridized to the same blot locations as did the cpb probe and the etx probe, suggesting that rep is commonly present on cpb plasmids, as well as on etx/cpb2 plasmids of type B isolates (Fig. 3A).
FIG. 3.
Southern hybridization analyses of pulsed-field gels run with DNAs extracted from type B isolates NCTC3110, CN677, CN1795, CN1886, CN2003, and CN2414. Probes used were a rep-specific probe (A), a tcpH-specific probe (similar results were obtained using a tcpF-specific probe [not shown]) (B), a lam-specific probe (similar results were obtained using a ureC probe [not shown]) (C), and an IS1151 probe (D). The migration of molecular size markers is indicated on the left.
Plasmid tcp locus carriage by type B isolates.
As mentioned in the introduction, tcp genes have been shown to mediate conjugative transfer of C. perfringens plasmid pCW3 and are also likely to be responsible for the demonstrated conjugative transfer of the cpe plasmids present in some type A isolates (5, 28), as well as the etx plasmids of some type D isolates (19).
Since a tcp locus was identified on the etx/cpb2 plasmid of type B isolates (29), a survey was performed to determine whether tcp genes might be associated with the cpb plasmid or other plasmids in type B isolates. For this survey, a pulsed-field gel Southern blot was hybridized with probes specific for two tcp genes (tcpF or tcpH) known to be required for pCW3 conjugative plasmid transfer (2). In this experiment, all surveyed type B isolates hybridized both tcp probes, with most type B isolates cohybridizing the tcpF and the tcpH probes at multiple blot locations (Fig. 3B and Table 1). For all surveyed type B isolates, these two tcp probes always cohybridized to the same blot location where the etx/cpb2 plasmid had migrated, as expected from previous sequencing and PCR results (29). These tcp probes also consistently cohybridized to the same blot location containing the cpb plasmid. Finally, most type B isolates hybridized the tcp probes at a third location, distinct from that for the cpb or etx/cpb2 plasmid, indicating that those type B isolates carry a third large (∼80-kb) plasmid with conjugative potential.
PCR and Southern blot analyses to evaluate the presence of plasmid-borne lambda-toxin (lam) and urease C (ureC) genes in type B isolates.
Lambda-toxin, a C. perfringens metalloprotease, can proteolytically activate epsilon-toxin (20, 30). Since the lambda-toxin gene (lam) has been detected in some type D isolates (20, 33), which resemble type B isolates in producing epsilon-toxin, our 17 type B isolates were surveyed by PCR to determine their carriage of the lam gene. This PCR testing detected the presence of the lam gene in 13 of the 17 (∼75%) surveyed type B isolates (data not shown).
To assess whether the lam gene is plasmid borne in lam+ type B isolates, a Southern blot analysis was performed after type B isolate DNA was electrophoresed on pulsed-field gels. As expected, this analysis detected no lam probe hybridization with DNAs from the four (NCTC3110, CN677, JGS1984, and Bar2) type B isolates that had tested PCR negative for carriage of the lam gene (Fig. 3C and data not shown). In contrast, DNAs from all lam PCR-positive type B isolates hybridized with the lam probe. This Southern blot analysis further revealed that all the lam+ type B isolates carried their lam gene on a plasmid of ∼80 kb (Fig. 3C and Table 1).
Urease has been considered a potential C. perfringens virulence factor, and the genes encoding urease were detected on plasmids carried by about 2% of C. perfringens isolates in a global survey (12). In several type D and E isolates, urease genes have been localized to large plasmids that also carry the etx or iota-toxin genes, respectively (12, 23, 33). While it has been shown previously that ure genes are present in a few type B isolates (12), the extent of ure gene carriage among type B isolates has not been reported, nor has it been determined whether ure genes in type B isolates are plasmid borne and, if so, whether they are carried by toxin-encoding plasmids as in type D and E isolates.
Therefore, our type B isolate collection was surveyed for ureC gene carriage by Southern blot analyses of pulsed-field gels. Those analyses found that DNAs from 13 of 17 (∼75%) isolates hybridized the ureC probe (data not shown). Furthermore, these blots demonstrated a clear association between ureC and lam carriage; i.e., isolates which were lam+ were also ureC+ and vice versa (Table 1). It was also noted that, using DNA from each lam+/ureC+ type B isolate, the ureC probe and lam probes always cohybridized to the same Southern blot location (Table 1).
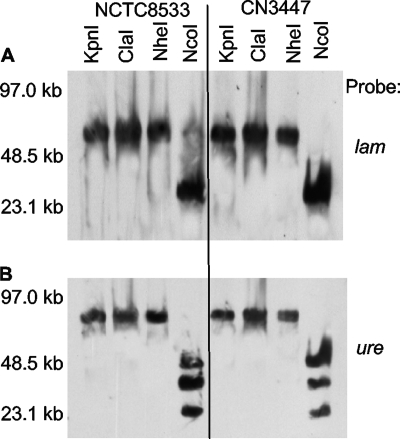
Given those results, restriction digestion analysis was conducted to test whether these two genes are present, in two representative lam+/ureC+ type B isolates, on the same plasmid or on two plasmids of similar size. Those analyses showed (Fig. 4) that lam and ureC migration were coincidently affected when DNA from type B isolate NCTC8533 or CN3447 was digested with restriction endonucleases; i.e., for these two type B isolates, if a nuclease affected migration of their ureC-carrying DNA, it also consistently affected migration of their lam-carrying DNA, and vice versa. These results suggest that the lam and ureC genes reside on the same ∼80-kb plasmid in these type B isolates.
FIG. 4.
Southern hybridization analyses of pulsed-field gels run with restriction enzyme-digested DNAs extracted from type B isolates NCTC8533 and CN3447. The blot was first hybridized with a lam-specific probe (A) before stripping and reprobing with a ureC-specific probe (B). The migration of molecular size markers is indicated on the left.
Southern blot and PCR analyses of IS1151 association with cpb-carrying plasmids in type B isolates.
IS1151 insertion sequences are closely associated with several toxin genes in C. perfringens (10, 14, 23, 28, 29, 33), including the etx genes in type B and D isolates. When analyzed by Southern blot analyses of pulsed-field gels, an IS1151 probe cohybridized with the location of the etx and cpb plasmids in all surveyed type B isolates (Fig. 3D and Table 1). In addition, all isolates, except NCTC3110 and CN677, possessed one or two other IS1151-carrying plasmids.
Bioinformatic analyses of sequence data for type B strain ATCC 3626 released by JCVI identified two IS1151-like sequences upstream of cpb. Therefore, a PCR analysis was performed using primers based upon the ATCC 3626 sequence to investigate whether IS1151-like sequences reside upstream of the cpb gene in other type B isolates. In this PCR, all four surveyed type B isolates (NCTC3110, NCTC8533, CN1793, and CN1795) supported PCR amplification of a product matching the expected size of a product from the IS1151-2-cpb region of ATCC 3626 (data not shown).
PCR evaluation of tpeL gene carriage among type B isolates.
Inspection of the JCVI ATCC 3626 sequence revealed this isolate carries the tpeL gene, which encodes the recently discovered TpeL toxin (1). Therefore, a PCR assay was performed to assess the presence of the tpeL ORF among other type B isolates. This survey determined that DNAs from all 17 surveyed type B isolates supported amplification of a tpeL PCR product (data not shown).
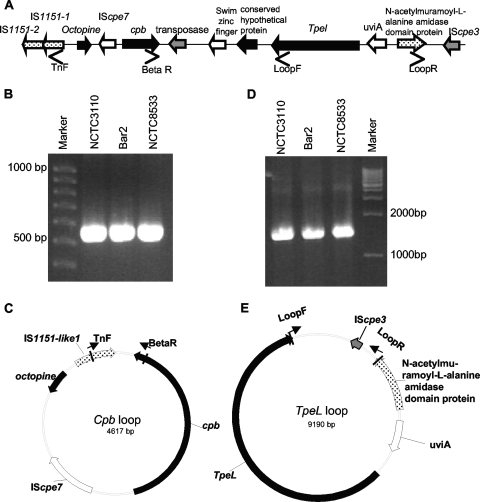
Bioinformatic analysis of the ATCC 3626 sequence also indicated that the tpeL gene in this type B isolate is located ∼3 kb downstream of its cpb gene (Fig. 5A). Therefore, an overlapping PCR assay was performed, using a five-pair set of primers (encoding PCR products R1 to R5 [Table 2]), to assess whether a similar genetic arrangement might exist between the cpb and tpeL genes in other type B isolates. Results from this overlapping PCR assay were consistent with the cpb gene being linked to the tpeL gene in all four (NCTC3110, CN677, NCTC8533, and CN3477) surveyed type B isolates (Fig. 5B and data not shown). This suggestion received further support from long-range PCR, where internal tpeL and cpb primers amplified a ∼5.7-kb product from seven different type B isolates (Fig. 5C). This ∼5.7-kb product matched the expected size of the product that these primers would amplify from ATCC 3626 DNA. In addition, sequencing confirmed that the ∼5.7-kb products from NCTC3110 and CN1795 showed >99.5% similarity to the sequence present in the tpeL-cpb region of ATCC 3626.
FIG. 5.
(A) Arrangement of the cpb and tpeL gene locus in C. perfringens type B isolate ATCC 3626 (based upon sequencing results released from JCVI). Solid black bars indicate the span of overlap PCR products. (B) Overlap PCR analysis of the region extending from cpb to tpeL in NCTC 3110 using reaction R1 to R5 primers (Table 2). (C) Long-range PCR assay using an internal cpb primer and an internal tpeL primer to link the tpeL and cpb genes.
Expression of TpeL by C. perfringens type B isolates.
In previous studies (13), we evaluated the expression of perfringolysin O, alpha-toxin, beta-toxin, beta2-toxin, and epsilon-toxin by type B isolates. However, TpeL toxin was identified (1) after that earlier study. Therefore, given the current PCR detection of the tpeL gene in all surveyed type B isolates (Fig. 5 and Table 1), RT-PCR analyses were performed to evaluate whether the tpeL gene is expressed by four representative type B isolates (NCTC3110, CN677, CN3447, and NCTC8533). This experiment showed that three of these isolates had detectable levels of tpeL transcription after either 8 h or 24 h of growth in TGY broth (Fig. 6), which opens the possibility that TpeL could contribute to type B isolate virulence.
FIG. 6.
RT-PCR analysis of tpeL expression by type B isolates NCTC3110, CN677, CN3447, and NCTC8533. tpeL expression was analyzed at 8 h (A) or 24 h (B) of growth in TGY medium at 37°C with RT (+RT) or without RT (−RT). The migration of molecular size markers is indicated on the left, and isolate designations are indicated above the lanes.
PCR identification of possible circular transposition intermediates.
Inspection of the JCVI ATCC 3626 sequence also indicated the presence of multiple transposases in the cpb-tpeL locus of that isolate. Our overlapping PCR results (Fig. 5B) strongly suggested that this arrangement is conserved in many other type B isolates as well. This finding may be significant since most other plasmid-borne C. perfringens toxin genes are also closely associated with insertion sequences (6, 14, 23, 28, 33) and since possible circular transposition intermediates containing those toxin genes have been demonstrated by PCR assays using primers capable of amplifying a PCR product only from DNA sequences that form a short circular loop (4, 23, 33).
Therefore, PCR primers in opposite orientations were designed to evaluate whether circular transposition intermediates might be formed by the transposases present in the cpb-tpeL locus (Fig. 7A). Primers BetaR and TnF consistently amplified a strong ∼0.6-kb PCR product from several type B isolates (Fig. 7B). When that product was sequenced, it was shown to contain cpb and IS1151-1 sequences, a result consistent with IS1151-mediated mobilization of a circular transposition intermediate containing cpb and several adjacent ORFs (Fig. 7C). Similarly, PCR primers LoopF and LoopR consistently amplified a strong ∼1.6-kb product (Fig. 7D) from several type B isolates. Sequencing of that product detected 3′ tpeL and downstream sequences, as well as IScpe3 sequences and sequences present immediately upstream of IScpe3 (Fig. 7E), which is consistent with IScpe3-mediated mobilization of a tpeL-containing circular transposition intermediate.
FIG. 7.
Detection of potential circular transposition intermediates carrying the cpb or tpeL gene in type B isolates. (A) Diagram of the tpeL-cpb locus in type B isolates (based on sequencing for ATCC 3626 released by JCVI). (B) PCR amplification of cpb circular intermediates using the primers BetaR and TnF. The positions of molecular size markers are indicated on the left. (C) Diagram derived from sequencing the product from panel B that depicts the putative circular transposition intermediate supporting PCR amplification with primers BetaR and TnF, with the vertical bars indicating the location in the loop of the PCR product amplified using these two primers. (D) PCR amplification of tpeL circular intermediates from NCTC8533 using primers LoopR and LoopF. (E) Diagram derived from sequencing the product from panel D that depicts the putative circular transposition intermediate supporting PCR amplification using primers LoopF and LoopR, with the vertical bars indicating the location in the loop of the PCR product amplified using these primers.
DISCUSSION
Type B isolates are unique among C. perfringens isolates in their ability to produce both beta-toxin and epsilon-toxin. Since these are the two most lethal of the 17 known C. perfringens toxins (26), type B isolates are potentially the most virulent of all C. perfringens isolates. Therefore, the limited study of the virulence genetics of type B isolates is somewhat surprising. An older pulsed-field Southern blot study (21) had determined that two type B isolates carry their etx genes on plasmids, but that previous study was unable to localize the cpb gene to plasmids in those two type B isolates. Another pulsed-field Southern blot study by the same group later identified the presence of urease genes in three different type B isolates (7, 12). The most in-depth study of type B virulence genetics to date has been our recent study demonstrating that most, if not all, type B isolates share a common ∼65-kb plasmid carrying both etx and cpb2 genes (29).
The current study provides significant new insights into the virulence genetics of type B isolates. To our knowledge, this study has demonstrated for the first time that the cpb gene is plasmid borne in most (if not all) type B isolates, as shown earlier for a few type C isolates (21, 32). While most type B isolates were found to carry an ∼90-kb cpb plasmid, type B isolates NCTC3110 and CN677 possessed ∼65-kb cpb plasmids. It might be noted these two unusual type B strains had completely independent origins, being isolated 16 years apart at different locations; i.e., CN677 came from the United Kingdom, while NCTC3110 was from Kenya.
Compared to other previously characterized C. perfringens toxin plasmids (23, 28, 29, 33), the ∼65 to 90-kb cpb plasmids in type B isolates are of medium size and show notably less size heterogeneity than the previously characterized etx plasmids of type D isolates or the iota-toxin plasmids of type E isolates (23, 33). This limited cpb plasmid diversity observed among type B isolates is reminiscent of our earlier determination that most, if not all, type B isolates carry the same 65-kb etx/cpb2 plasmid. Together, these observations raise the possibility that type B isolates are rare, at least in part, due to virulence plasmid incompatibility issues; i.e., perhaps only certain combinations of cpb and etx plasmids can be maintained in a single C. perfringens cell. This possibility will be further addressed by future studies comparing cpb plasmid size and gene content heterogeneity in type B versus type C isolates. If greater cpb plasmid heterogeneity is detected among type C isolates, future studies should directly examine potential etx and cpb virulence plasmid incompatibility issues, a subject that has not (to our knowledge) received any study to date.
Besides their cpb, etx, and cpb2 genes, most type B isolates also carry several other potential virulence genes on plasmids, including the tpeL, ureC, and lam genes. Several of these virulence genes mapped to the same plasmid in type B isolates; i.e., the cpb and tpeL genes reside on one plasmid, while the ureC and lam genes are present together on a second plasmid. Overall, most type B isolates were found to carry at least three known or potential virulence plasmids, identifying these strains as potentially among the most plasmid dependent of all C. perfringens isolates in terms of virulence. By comparison, type A isolates carrying a plasmid-borne cpe gene and type E isolates typically possess only one or two virulence plasmids (14, 23, 28); type D isolates are more varied in their virulence plasmid carriage, but only a subset of type D isolates resemble type B isolates by carrying three potential virulence plasmids (33).
Type B isolates may be able to maintain numerous known or potential plasmid-borne virulence genes because these genes are distributed onto several different virulence plasmids. However, since the virulence plasmids of type B isolates often contain homologous regions, such as the tcp locus, it is interesting that type B isolates can stably maintain multiple virulence plasmids without apparent recombination problems. Perhaps this plasmid stability reflects the relatively low recombination activity associated with pathogenic Clostridium species (18).
Insertion sequences have been previously identified in the iota-toxin gene locus of type E isolates, the cpe locus of type A isolates, and the etx locus of type B and D isolates (6, 23, 29, 33). On that basis, it was suggested (6, 23, 29, 33) that the common presence of insertion sequences near C. perfringens toxin genes facilitates mobilization of these toxin genes, possibly explaining how the etx and iota-toxin genes came to reside on several different plasmids or, in the case of cpe-positive isolates, on several different plasmids or the chromosome. Consistent with that hypothesis, possible circular transposition intermediates have been detected previously for the etx, cpe, and iota-toxin genes (6, 23, 33). Similarly, the current study detected several insertion sequences in the plasmid-borne cpb-tpeL locus of type B isolates, as well as possible circular transposition intermediates carrying those two toxin genes. Those findings are consistent with insertion sequence-mediated mobilization of tpeL and cpb, possibly helping to explain how these toxin genes came to reside on two different plasmids in type B isolates. More complete appreciation of the potential contribution of IS-mediated mobilization of cpb or tpeL genes to virulence plasmid diversity awaits the full characterization of cpb plasmids among type C isolates and of tpeL plasmids among type A and type C isolates.
As already mentioned, this study detected a tcp locus on the cpb-tpeL plasmid of type B isolates. This finding further supports the presence of a tcp locus as a common feature of C. perfringens toxin plasmids, since tcp loci have been identified previously on most iota-toxin plasmids of type E isolates, cpe plasmids of type A isolates, and etx plasmids of type B and D isolates (23, 28, 29, 33). The tcp locus has been shown to mediate transfer of C. perfringens tetracycline resistance plasmid pCW3 (2), so the presence of a tcp locus on the cpb-tpeL plasmid of type B isolates suggests that this plasmid may also conjugatively transfer among C. perfringens isolates. If a type D isolate acquired, by horizontal spread, a cpb-tpeL plasmid, it would be converted to type B. However, that process must be rare given the scarcity of B isolates among the general C. perfringens population; e.g., no type B isolates were detected in Pittsburgh area soils (24). Potential conversion of type C or D isolates to type B by conjugative acquisition of toxin plasmids is probably limited, in part, by the relatively low-level environmental presence of type C and D isolates (24), but the potential plasmid incompatibility issues raised earlier could be another factor limiting the horizontal spread of beta- or epsilon-toxin plasmids to create type B isolates.
The third potential virulence plasmid carried by most type B isolates likely encodes lambda-toxin, a metalloprotease that is capable of proteolytically activating epsilon-toxin and iota-toxin (17, 27). Consistent with proteolytic activation being the primary biologic role of lambda-toxin, it is now clear that the lam gene is relatively common among type E isolates, where it can activate iota-toxin, as well as among type B and D isolates, where it can activate epsilon-toxin (references 23 and 33 and this study). However, it is interesting that lambda-toxin gene carriage appears to be more frequent in type B (75% positive) than in type D (23% positive) isolates (reference 33 and this study). Also notable is that type B isolates carry their lam gene on a plasmid different from their etx plasmid, in contrast to lam+ type D isolates (33), where lam and etx can localize to plasmids of the same size. Similarly, the lam gene also appears to be commonly present on the iota-toxin plasmids of type E isolates (23).
The lam-carrying plasmid of type B isolates also possesses genes encoding another potential virulence factor, urease. A linkage between lam and ure genes has been noted previously; i.e., most type E isolates carry both lam and ure genes on their iota-toxin plasmid (23). Whether the common presence of lam and ure genes on the same plasmid in both type B and E isolates is indicative of a common evolutionary origin or some cooperative functional role will require further study. Interestingly, the type B plasmid carrying lam and ure lacks the rep gene, suggesting that this is a relatively unusual C. perfringens virulence plasmid. However, this lam/ure plasmid in type B isolates does carry tcp locus genes, suggesting that it may be a third conjugative plasmid in these isolates. Finally, the common ∼80-kb size of lam/ure plasmids among the surveyed type B isolates is notable, perhaps offering yet another suggestion that only certain plasmid combinations can be maintained in a single C. perfringens type B isolate due to incompatibility issues.
In summary, since (i) a recent study indicated that both epsilon-toxin and beta-toxin are important for type B virulence (13); (ii) lambda-toxin is known to activate epsilon-toxin (27); (iii) we show that TpeL, a potent cytotoxin (1), is expressed by type B isolates; and (iv) this and previous studies (29) have now shown that etx, cpb, lam, and tpeL genes are all plasmid borne in type B isolates, the pathogenicity of type B isolates is clearly heavily dependent upon virulence plasmids. Continued study of these plasmids should provide additional insights into their genetics, evolution, diversity, and role in virulence.
Acknowledgments
This research was generously supported by grant AI056177-06 from the National Institute of Allergy and Infectious Diseases.
We thank Jianming Chen and Jorge Vidal for assistance with primer design.
Editor: J. B. Bliska
Footnotes
Published ahead of print on 26 October 2009.
REFERENCES
- 1.Amimoto, K., T. Noro, E. Oishi, and M. Shimizu. 2007. A novel toxin homologous to large clostridial cytotoxins found in culture supernatant of Clostridium perfringens type C. Microbiology 153:1198-1206. [DOI] [PubMed] [Google Scholar]
- 2.Bannam, T. L., W. L. Teng, D. Bulach, D. Lyras, and J. I. Rood. 2006. Functional identification of conjugation and replication regions of the tetracycline resistance plasmid pCW3 from Clostridium perfringens. J. Bacteriol. 188:4942-4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Billington, S. J., E. U. Wieckowski, M. R. Sarker, D. Bueschel, J. G. Songer, and B. A. McClane. 1998. Clostridium perfringens type E animal enteritis isolates with highly conserved, silent enterotoxin sequences. Infect. Immun. 66:4531-4536. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Brynestad, S., and P. E. Granum. 1999. Evidence that Tn5565, which includes the enterotoxin gene in Clostridium perfringens, can have a circular form which may be a transposition intermediate. FEMS Microbiol. Lett. 170:281-286. [DOI] [PubMed] [Google Scholar]
- 5.Brynestad, S., M. R. Sarker, B. A. McClane, P. E. Granum, and J. I. Rood. 2001. The enterotoxin (CPE) plasmid from Clostridium perfringens is conjugative. Infect. Immun. 69:3483-3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brynestad, S., B. Synstad, and P. E. Granum. 1997. The Clostridium perfringens enterotoxin gene is on a transposable element in type A human food poisoning strains. Microbiology 143:2109-2115. [DOI] [PubMed] [Google Scholar]
- 7.Cole, S. T., and B. T. Canard. 1997. Structure, organization, and evolution of the genome of Clostridium perfringens, p. 49-64. In J. I. Rood, B. A. McClane, J. G. Songer, and R. W. Titball (ed.), The clostridia: molecular biology and pathogenesis. Academic Press, London, United Kingdom.
- 8.Collie, R. E., and B. A. McClane. 1998. Evidence that the enterotoxin gene can be episomal in Clostridium perfringens isolates associated with nonfoodborne human gastrointestinal diseases. J. Clin. Microbiol. 36:30-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cornillot, E., B. Saint-Joanis, G. Daube, S. Katayama, P. E. Granum, B. Carnard, and S. T. Cole. 1995. The enterotoxin gene (cpe) of Clostridium perfringens can be chromosomal or plasmid-borne. Mol. Microbiol. 15:639-647. [DOI] [PubMed] [Google Scholar]
- 10.Daube, G., P. Simon, and A. Kaeckenbeeck. 1993. IS1151, an IS-like element of Clostridium perfringens. Nucleic Acids Res. 21:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duncan, C. L., E. A. Rokos, C. M. Christenson, and J. I. Rood. 1978. Multiple plasmids in different toxigenic types of Clostridium perfringens: possible control of beta toxin production, p. 246-248. In D. Schlessinger (ed.), Microbiology—1978. ASM Press, Washington, DC.
- 12.Dupuy, B., G. Daube, M. R. Popoff, and S. T. Cole. 1997. Clostridium perfringens urease genes are plasmid-borne. Infect. Immun. 65:2313-2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez-Miyakawa, M. E., D. J. Fisher, R. Poon, S. Sayeed, V. Adams, J. I. Rood, B. A. McClane, and F. A. Uzal. 2007. Both epsilon-toxin and beta-toxin are important for the lethal properties of Clostridium perfringens type B isolates in the mouse intravenous injection model. Infect. Immun. 75:1443-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher, D. J., K. Miyamoto, B. Harrison, S. Akimoto, M. R. Sarker, and B. A. McClane. 2005. Association of beta2 toxin production with Clostridium perfringens type A human gastrointestinal disease isolates carrying a plasmid enterotoxin gene. Mol. Microbiol. 56:747-762. [DOI] [PubMed] [Google Scholar]
- 15.Garmory, H. S., N. Chanter, N. P. French, D. Busechel, J. G. Songer, and R. W. Titball. 2000. Occurence of Clostridium perfringens β2-toxin amongst animals, determined using genotyping and subtyping PCR assays. Epidemiol. Infect. 124:61-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibert, M., C. Jolivet-Reynaud, and M. R. Popoff. 1997. Beta2 toxin, a novel toxin produced by Clostridium perfringens. Gene 203:65-73. [DOI] [PubMed] [Google Scholar]
- 17.Gibert, M., L. Petit, S. Raffestin, A. Okabe, and M. R. Popoff. 2000. Clostridium perfringens iota-toxin requires activation of both binding and enzymatic components for cytopathic activity. Infect. Immun. 68:3848-3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heap, J. T., S. T. Cartman, O. J. Pennington, C. M. Cooksley, J. C. Scott, B. Blount, D. A. Burns, and N. P. Minton. 2009. Development of genetic knock-out systems for Clostridia, p. 179-198. In H. Bruggeman and G. Gottschalk (ed.) Clostridia: molecular biology in the post-genomic era. Caister Academic Press, Norfolk, United Kingdom.
- 19.Hughes, M. L., R. Poon, V. Adams, S. Sayeed, J. Saputo, F. A. Uzal, B. A. McClane, and J. I. Rood. 2007. Epsilon-toxin plasmids of Clostridium perfringens type D are conjugative. J. Bacteriol. 189:7531-7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin, F., O. Matsushita, S. Katayama, S. Jin, C. Matsushita, J. Minami, and A. Okabe. 1996. Purification, characterization, and primary structure of Clostridium perfringens lambda-toxin, a thermolysin-like metalloprotease. Infect. Immun. 64:230-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katayama, S., B. Dupuy, G. Daube, B. China, and S. T. Cole. 1996. Genome mapping of Clostridium perfringens strains with I-Ceu I shows many virulence genes to be plasmid-borne. Mol. Gen. Genet. 251:720-726. [DOI] [PubMed] [Google Scholar]
- 22.Katayama, S., O. Matsushita, J. Minami, S. Mizobuchi, and A. Okabe. 1993. Comparison of the alpha-toxin genes of Clostridium perfringens type A and C strains: evidence for extragenic regulation of transcription. Infect. Immun. 61:457-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li, J., K. Miyamoto, and B. A. McClane. 2007. Comparison of virulence plasmids among Clostridium perfringens type E isolates. Infect. Immun. 75:1811-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li, J., S. Sayeed, and B. A. McClane. 2007. Prevalence of enterotoxigenic Clostridium perfringens isolates in Pittsburgh (Pennsylvania) area soils and home kitchens. Appl. Environ. Microbiol. 73:7218-7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McClane, B. A., D. M. Lyerly, and T. D. Wilkins. 2006. Enterotoxic clostridia: Clostridium perfringens type A and Clostridium difficile, p. 703-714. In R. P. Novick. V. A. Fischetti, J. J. Ferretti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens, 3rd ed. ASM Press, Washington, DC.
- 26.McClane, B. A., F. A. Uzal, M. F. Miyakawa, D. Lyerly, and T. Wilkins. 2006. The enterotoxic clostridia, p. 688-752. In M. Dworkin, S. Falkow, E. Rosenburg, H. Schleifer, and E. Stackebrandt (ed.). The prokaryotes, 3rd ed. Springer, New York, NY.
- 27.Minami, J., S. Katayama, O. Matsushita, C. Matsushita, and A. Okabe. 1997. Lambda-toxin of Clostridium perfringens activates the precursor of epsilon-toxin by releasing its N- and C-terminal peptides. Microbiol. Immunol. 41:527-535. [DOI] [PubMed] [Google Scholar]
- 28.Miyamoto, K., D. J. Fisher, J. Li, S. Sayeed, S. Akimoto, and B. A. McClane. 2006. Complete sequencing and diversity analysis of the enterotoxin-encoding plasmids in Clostridium perfringens type A non-food-borne human gastrointestinal disease isolates. J. Bacteriol. 188:1585-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyamoto, K., J. Li, S. Sayeed, S. Akimoto, and B. A. McClane. 2008. Sequencing and diversity analyses reveal extensive similarities between some epsilon-toxin-encoding plasmids and the pCPF5603 Clostridium perfringens enterotoxin plasmid. J. Bacteriol. 190:7178-7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyata, S., J. Minami, E. Tamia, P. Matsushita, S. Shimamoto, and A. Okabe. 2002. Clostridium perfringens epsilon-toxin forms a heptameric pore within the detergent-insoluble microdomains of Madin-Darby canine kidney cells and rat synaptosomes. J. Biol. Chem. 277:39463-39468. [DOI] [PubMed] [Google Scholar]
- 31.Petit, L., M. Gilbert, and M. R. Popoff. 1999. Clostridium perfringens: toxinotype and genotype. Trends Microbiol. 7:104-110. [DOI] [PubMed] [Google Scholar]
- 32.Rokos, E. A., J. I. Rood, and C. L. Duncan. 1978. Multiple plasmids in different toxigenic types of Clostridium perfringens. FEMS Microbiol. Lett. 4:323-326. [Google Scholar]
- 33.Sayeed, S., J. Li, and B. A. McClane. 2007. Virulence plasmid diversity in Clostridium perfringens type D isolates. Infect. Immun. 75:2391-2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sayeed, S., F. A. Uzal, D. J. Fisher, J. Saputo, J. E. Vidal, Y. Chen, P. Gupta, J. I. Rood, and B. A. McClane. 2008. Beta toxin is essential for the intestinal virulence of Clostridium perfringens type C disease isolate CN3685 in a rabbit ileal loop model. Mol. Microbiol. 67:15-30. [DOI] [PubMed] [Google Scholar]
- 35.Shimizu, T., K. Ohtani, H. Hirakawa, K. Ohshima, A. Yamashita, T. Shiba, N. Ogasawara, M. Hattori, S. Kuhara, and H. Hayashi. 2002. Complete genome sequence of Clostridium perfringens, an anaerobic flesh-eater. Proc. Natl. Acad. Sci. U. S. A. 99:996-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vidal, J. E., K. Ohtani, T. Shimizu, and B. A. McClane. 2009. Contact with enterocyte-like Caco2 cells induces rapid upregulation of toxin production by Clostridium perfringens type C isolates. Cell. Microbiol. 11:1306-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]