Abstract
Yersinia pestis causes primary pneumonic plague in many mammalian species, including humans, mice, and rats. Virulent Y. pestis strains undergo frequent spontaneous deletion of a 102-kb chromosomal DNA fragment, known as the pigmentation (pgm) locus, when grown in laboratory media, yet this locus is present in every virulent isolate. The pgm locus encodes, within a high-pathogenicity island, siderophore biosynthesis genes that are required for growth in the mammalian host when inoculated by peripheral routes. Recently, higher challenge doses of nonpigmented Y. pestis were reported to cause fatal pneumonic plague in mice, suggesting a useful model for studies of virulence and immunity. In this work, we show that intranasal infection of BALB/c mice with nonpigmented Yersinia pestis does not result in pneumonic plague. Despite persistent bacterial colonization of the lungs and the eventual death of infected mice, pulmonary inflammation was generally absent, and there was no disease pathology characteristic of pneumonic plague. Iron given to mice at the time of challenge, previously shown to enhance the virulence of pgm-deficient strains, resulted in an accelerated disease course, with less time to bacteremia and lethality, but lung inflammation and pneumonia were still absent. We examined other rodent models and found differences in lung inflammatory responses, some of which led to clearance and survival even when high challenge doses were used. Together, the results suggest that the Y. pestis pgm locus encodes previously unappreciated virulence factors required for the induction of pneumonic plague that are independent of iron scavenging from the mammalian host.
Yersinia pestis is the causative agent of pneumonic plague, a disease that has killed more people in recorded history than any other disease caused by a bacterial pathogen (44). Compared to the bubonic form of the disease, pneumonic plague results in higher rates of morbidity and mortality. The deadly nature of the disease is further exacerbated by the small window for administering effective treatment after the onset of symptoms (27). Fortunately, while Y. pestis still causes endemic disease throughout the world, development of the pneumonic form is rare, largely due to improved sanitation practices and treatment (19). Bubonic plague is most commonly seen in rural areas of endemicity and is generally successfully treated with antibiotics. However, multidrug-resistant strains have been isolated from human bubonic plague patients, and their emergence, combined with the known capacity of Y. pestis for horizontal DNA exchange during its vector life cycle, make antibiotic-resistant Y. pestis outbreaks a continued threat to public health (20, 23, 25).
Pneumonic plague, whether in humans or in other susceptible mammalian hosts, has been described as severe necrohemorrhagic pneumonia that can develop in less than 72 h postinfection (1, 14, 32, 44). Late-stage disease involves dissemination from the lungs into the vascular system, allowing high levels of bacterial replication in multiple tissues and the blood. Hemorrhaging, necrosis, and inflammation are found in the liver, spleen, and other organs, with death involving multiple organ failure (26, 34). This extreme pathogenicity is caused by a number of known bacterial virulence factors, many of which are encoded on mobile genetic elements, while involvement of the host response in the development of pneumonic plague has not been formally demonstrated (26). Mice lacking genes required for the stimulation of innate immune responses, such as the gamma interferon (IFN-γ) gene, are less able to develop protective responses to vaccination, suggesting that the bacteria disable essential immune activation mechanisms in the host (31, 40). Increased resistance of some strains of mice to attenuated, nonpigmented Y. pestis strains (see below) has been reported, and though there may be multiple mechanisms for resistance, this suggests the presence of host factors that are involved in the progression of plague (13).
Nonpigmented Y. pestis strains result from spontaneous deletion of a 102-kb chromosomal DNA fragment, known as the pigmentation (pgm) locus, that is flanked by a repetitive sequence element (9, 18). Deletion of the pgm locus attenuates virulence but occurs at a high frequency in laboratory media. The locus is present in every virulent isolate of Yersinia pestis, with little sequence variation between clinical strains (7, 48). The pgm locus includes a high-pathogenicity island (HPI), which carries 11 genes involved in siderophore synthesis and the acquisition of iron during mammalian infection (3). The yersiniabactin and iron response proteins (Ybt and Irp, respectively) are required for this system and for virulence, and mutants lacking genes within the ybt/irp segment are attenuated (3). Injection of inorganic iron into mice prior to subcutaneous challenge with nonpigmented Y. pestis restores the development of lethal plague, suggesting that the primary virulence defect caused by the pgm deletion is iron deficiency (7, 10). The effect of iron injection during respiratory infections with pgm-deficient Y. pestis has not been described, but high-dose respiratory challenge can result in lethal infections of mice and nonhuman primates (39, 53). Transcription of ybt/irp has been shown to be induced in vivo during pneumonic as well as septicemic plague in mice, supporting their essential role in bacterial survival in all forms of the disease (12, 32).
In this work, we were interested in using nonpigmented Y. pestis as a model for the study of host-pathogen interactions during pneumonic plague, and we sought to gain an understanding of the disease caused by intranasal infection. We characterized disease progression in BALB/c mice following intranasal infection with Y. pestis strains lacking the pgm locus and found that despite persistent colonization of the lung, there was a general absence of inflammation and lesions characteristic of pneumonic plague. These mice instead developed lethal infections with multiorgan damage. Delivery of supplemental inorganic iron at the time of challenge, which was expected to enhance the virulence of nonpigmented Y. pestis, resulted in a faster disease course, with more consistent progression to lethal septicemic plague, but did not restore the development of pneumonia. We extended these findings to other small-animal models and found similar results in other strains of mice, though there was variability in host response and susceptibility. In contrast, five strains of rats challenged by intranasal infection following iron injection developed localized lung inflammatory responses and ultimately cleared the infection, with no lesions observed in other tissues. Together, these data support roles for both host responses and the pgm locus in causing pneumonic plague, revealing the presence of a previously undefined role in virulence encoded within this conserved genetic element.
MATERIALS AND METHODS
Bacterial strains.
All strains used were taken from frozen stocks and streaked for isolation onto heart infusion agar (HIA) plates. The plates used for Y. pestis strains CO92 and CO92Δpgm were supplemented with 0.005% Congo red and 0.2% galactose (51). Y. pestis KIM D27, a nonpigmented strain originally isolated by Robert Brubaker (7, 38), was grown aerobically at 27°C in heart infusion broth overnight and was diluted in sterile phosphate-buffered saline (PBS) just prior to intranasal or intravenous challenge. Fully virulent Y. pestis CO92 was used to produce the CO92Δpgm mutant and for pneumonic plague challenges. Bacteria were grown aerobically at 37°C in heart infusion broth with 1 mM CaCl2 and were diluted in sterile PBS for intranasal challenge. The pgm locus was detected by PCR using primers specific for ybtP (YbtP-Nde5 [AACATATGTCATCTCAATCATCCAATA] and YbtP-Bam3 [AAGGATCCTTATTGTCTTTCATTTTCC]) and upstream and downstream regions flanking the pgm locus (pgm-upstream [AAAGTCATTTTTTATCTTTACAATCTG] and pgm-downstream [AAATGGGCAACTTCCTGATTTGAA]), with genomic DNA as a template. Genomic preparations were performed using the Wizard genomic DNA purification kit (Promega, Madison, WI).
Animals.
Six- to 8-week-old female BALB/c, C57BL/6, and C3H mice (Charles River Laboratories, Wilmington, MA) were used as mouse models. Brown Norway (Charles River Laboratories, Wilmington, MA), ACI, F344, Lewis, and Wistar Furth rats (Harlan Laboratories, Indianapolis, IN) were used for rat experiments; all rats were 6- to 8-week-old females. Animals were maintained in containment facilities in accordance with the guidelines outlined by the University of Missouri Animal Care and Use Committee. All infected animals were monitored at least once daily by weight and assignment of health scores. Animals that were moribund (exhibiting neurologic signs, unable to ambulate, able to be tipped over) were euthanized by CO2 asphyxiation followed by bilateral pneumothorax, methods approved by the American Veterinary Medical Association Guidelines on Euthanasia.
Infection.
Bacteria were grown as described above, diluted in sterile PBS to the desired dose, and plated in triplicate to verify the actual CFU delivered. All animals intranasally infected with Y. pestis were lightly anesthetized by isoflurane inhalation for the procedure. The inoculum was administered to mice or rats as a 20-μl or 50-μl volume, respectively, distributed equally between the two nares. Animals infected with pgm-deficient strains received an intraperitoneal injection of ferrous chloride (FeCl2·4H2O) diluted in sterile deionized and distilled water. Mice receiving intravenous injections were not anesthetized and did not receive supplemental ferrous chloride; the inoculum was administered into the lateral tail vein as a 20-μl volume. Rats receiving intravenous injections were anesthetized by isoflurane inhalation, and the dose was administered as a 50-μl volume into the lateral saphenous vein without supplemental iron. All animals were observed until full recovery from anesthesia.
Calculation of the LD50.
Groups of mice (n = 5) were intranasally infected as described above. Serial fivefold dilutions, ranging from 30 CFU to 1.4 × 105 CFU, were made in sterile PBS. The 50% lethal dose (LD50) of 1 × 104 CFU was calculated using the method described by Reed and Muench (47) and was used to determine the challenge dose.
Quantification of bacterial loads in blood and tissues.
Immediately after euthanasia, blood was collected directly from the heart and diluted 1:100 in sterile PBS. Spleens were collected aseptically and were homogenized in sterile PBS. Prior to collection of the lungs, sterile PBS was perfused through the vasculature by catheterization of the right ventricle. When the lungs were fully perfused and free of blood, the lung lobes were removed aseptically and homogenized in sterile PBS. Serial dilutions of the diluted blood and homogenized tissues were then plated onto HIA plates for quantification of bacterial loads (CFU per milliliter or CFU per organ, respectively).
Histopathologic evaluation of tissues.
Tissues were collected promptly after euthanasia and were placed in 10% formalin; lungs were inflated with 10% formalin prior to removal. All tissues were fixed for a minimum of 48 h. Fixed tissues were embedded in paraffin, and sections were cut and stained with hematoxylin and eosin (H&E). For histologic scoring, all lung lobes were evaluated and read blinded by a veterinarian. Lesion scores were assigned as follows: 0, no lesions; 1, focal or a few multifocal (<3) areas of alveolar exudate with slightly increased inflammatory cell populations but well-defined alveolar airspaces; 2, multifocal coalescing areas of alveolar exudates with moderately increased inflammatory cell populations but well-defined alveolar airspaces; 3, focal or a few multifocal (<3) areas of fibrinopurulent consolidation with neutrophils within fibrin and cellular debris, associated alveolar exudate, and loss of alveolar integrity in areas of consolidation; 4, multifocal (>3) areas of fibrinopurulent consolidation with neutrophils within fibrin and cellular debris, associated alveolar exudate, and loss of alveolar integrity in areas of consolidation; 5, multifocal coalescing areas of inflammation as described above with 25 to 50% of lung tissue affected; 6, multifocal coalescing areas of inflammation as described above with 50 to 75% of lung tissue affected; 7, generalized inflammation affecting the entire lung section. Both the individual mouse scores and the average score for each group were determined.
Statistical analysis.
Data from time-to-death studies were statistically analyzed using the Wilcoxon rank sum test (preceded by the Kruskal-Wallis test if more than two groups were present). Histology scores for the treatment groups at various time points were analyzed and compared by nested analysis of variance (ANOVA) using R (46).
RESULTS
Iron accelerates disease progression and lethality during intranasal infection with Y. pestis KIM D27.
The attenuation of nonpigmented Y. pestis strains, such as KIM D27, when used for infection by peripheral routes, has long been attributed to inadequate iron acquisition and metabolism, in part because intraperitoneal injection of inorganic iron (20 μg to 80 μg) appeared to restore virulence (7, 10). To determine if the same is true for pneumonic plague models, we administered intraperitoneal injections of 50 μg inorganic iron to BALB/c mice just before intranasal challenge. The LD50 of Y. pestis KIM D27 following intranasal challenge of iron-supplemented BALB/c mice was measured and determined to be 10,624 CFU, calculated by the method of Reed and Muench (47). We challenged iron-treated and untreated BALB/c mice by intranasal instillation of 6.8 × 106 CFU of Y. pestis KIM D27 (640 LD50). While clinical signs were observed in all mice, iron-supplemented mice more rapidly and consistently developed lethal infection, and all succumbed to the infection within 3 to 4 days (Fig. 1). The mice that did not receive iron treatment developed various degrees of clinical signs, with some death, but this took 7 days postinfection, significantly longer than for iron-supplemented mice (P < 0.005), and 40% of these mice survived the infection. Comparisons of the effect of iron on plague lethality with other challenge doses resulted in similar responses, with iron treatment consistently resulting in a more-rapid disease course (data not shown).
FIG. 1.
Iron supplementation to the host accelerates disease progression and produces consistent lethality following intranasal infection by KIM D27. BALB/c mice were intranasally infected with 6.8 × 106 CFU (640 LD50) KIM D27. One group received intraperitoneal injections of 50 μg inorganic iron (n = 5), and the other group (n = 6) received intraperitoneal injections of sterile water, just prior to challenge. Survival and time to death were monitored over 14 days; time to death was evaluated by the Wilcoxon rank sum test, which revealed a significant decrease in the time to death for mice treated with iron (P < 0.005).
Iron does not affect the development of pulmonary pathology.
Because of the decrease in mean time to death caused by the injection of iron, we compared pathology and disease progression in more detail between mice that did or did not receive supplemental iron prior to intranasal challenge with 10 LD50 of Y. pestis KIM D27 (1 × 105 CFU). Throughout the 14-day observation period following infection, no clinical signs of illness were observed in mice that did not receive iron. Lungs from these mice, collected at the end of the observation period, were analyzed by histology, and no lesions were observed (Fig. 2A and B). In contrast, we observed the development of general clinical signs of illness, such as weight loss, unkempt fur coat, and lethargy, in all iron-supplemented mice, often progressing to severe, debilitating disease. However, none of these mice showed outward signs of respiratory disease at any time. Mice were euthanized when they became moribund (exhibited neurologic signs, were unable to ambulate, and could be tipped over). The lungs of these animals had no gross lesions, and histologic evaluation of the lung tissue showed no pathological lesions that would indicate the presence of pneumonic disease (Fig. 2C and D). Increased numbers of polymorphonuclear cells, mainly neutrophils, were found within the interstitial vasculature, but there were no identifiable areas of active inflammation within the alveoli and no tissue damage. These findings applied throughout the lungs, and all lung lobes were examined.
FIG. 2.
Iron treatment does not affect the development of pulmonary pathology. BALB/c mice were intranasally infected with approximately 10 LD50 of KIM D27 (1 × 105 CFU) (A to D) or CO92 (3 × 103 CFU) (E and F). The mice were euthanized when moribund, and lungs were perfused and fixed in 10% formalin prior to sectioning and staining with H&E. (A and B) Lungs from KIM D27-infected mice without iron treatment. Tissues shown are representative of two independent studies (n = 8). (C and D) Lungs from KIM D27-infected mice that were injected with 50 μg of iron intraperitoneally just prior to challenge. Tissues shown are representative of three independent studies (n = 29). (E and F) Lungs from moribund CO92-infected mice (n = 2).
In contrast, mice infected with virulent, pigmented Y. pestis CO92 developed obvious signs of respiratory disease, such as dyspnea and tachypnea, as early as day 2 postinfection. Lungs of moribund mice, which were dark red and difficult to inflate with formalin, showed severe necrotizing pneumonia, characterized by marked infiltration of polymorphonuclear cells, edema, hemorrhage, and bacterial aggregation (Fig. 2E and F). In most lung sections examined, the alveolar architecture was completely destroyed.
Septicemia is the apparent cause of lethality in the KIM D27 intranasal infection model.
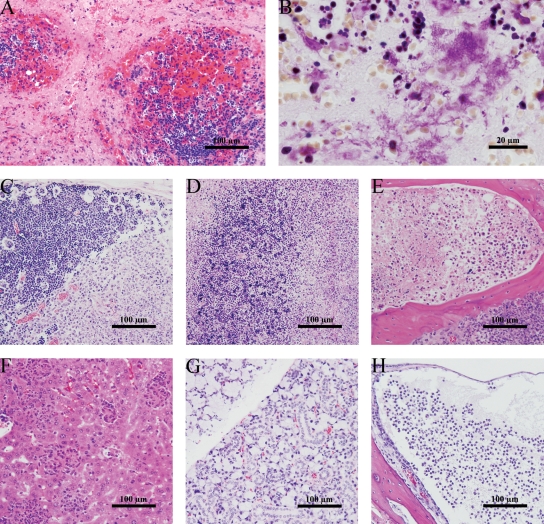
Comprehensive examination of tissues from iron-treated mice revealed extensive lesions indicative of systemic disease. Strikingly, lymphoid tissues had the most-severe lesions, including extensive necrosis and loss of multiple types of immune cells. The spleens of moribund mice were enlarged and mottled to pale peach in color. Splenic necrosis was characterized by a generalized loss of hematopoietic precursors, so that the red pulp was replaced with necrotic cellular debris (Fig. 3A). White pulp was also damaged, and even destroyed in extreme cases, with increased foci of apoptosis within the lymphoid aggregates and of necrosis around the periphery. Aggregates of Gram-negative coccobacilli were common within the necrotic red pulp (Fig. 3B).
FIG. 3.
BALB/c mice develop septicemia after intranasal infection with KIM D27 and iron treatment. Tissues were harvested from moribund animals infected with 10 LD50 of KIM D27 following intraperitoneal injection of 50 μg FeCl2. (A) H&E stain of spleen. (B) Gram stain of spleen. (C to H) H&E stains of representative sections from the mandibular lymph node (C), thymus (D), bone marrow (E), liver (F), submandibular salivary gland (G), and tympanic bulla (H). Results are representative of three independent experiments (a total of 29 mice were examined).
The thymus and mandibular lymph nodes appeared smaller than normal. Lymphocyte depletion within the nodal (Fig. 3C) and thymic (Fig. 3D) cortices was common, as were increased foci of apoptotic lymphocytes. Thymus architecture was generally nearly completely lost due to necrosis. Aggregates of Gram-negative bacilli could be seen in mandibular lymph nodes within the areas of depleted lymphocytes. Necrosis of hematopoietic precursors was found in the bone marrow of the skull (Fig. 3E) and femur (not shown), but no bacteria could be seen by Gram staining.
Pathological findings in other organs were less common, with fewer instances of tissue necrosis. As in previous studies (50), multiple areas of inflammation were observed in the liver, suggesting a systemic inflammatory response (Fig. 3F). Unexpectedly, there was marked hypertrophy of the mucous portion of the submandibular salivary gland (Fig. 3G) and sterile otitis media (Fig. 3H). It is conceivable that these resulted from the method of infection.
In a parallel experiment, we examined bacterial loads in the terminal stages of disease following intranasal infection of iron-treated mice with 1 × 107 CFU of Y. pestis KIM D27. Blood, lungs, and spleen were removed from moribund mice, and tissues were homogenized in sterile PBS and plated for enumeration of bacteria. The results showed consistent development of high titers in the spleen and lungs, with titers 2 to 3 orders of magnitude greater in the spleen than in the lungs (Fig. 4). Bacterial titers in the blood were more variable but were recovered from each mouse (n = 5), often exceeding the concentration recovered from the lungs. Additional studies were performed using a range of challenge doses, and the results were similar (data not shown). Time to disease and progression to lethal infection appeared to correlate with the challenge dose.
FIG. 4.
Bacterial titers in the blood, spleen, and lung show KIM D27 colonization of moribund mice. BALB/c mice (n = 5) were infected intranasally with 1.0 × 107 CFU of KIM D27 (1,000 LD50) and were euthanized when moribund. Tissues were homogenized in sterile PBS and were plated on HIA in triplicate for determination of bacterial loads in the blood, spleen, and lung at the time of euthanasia. Average counts are reported. Symbols with the same shape correspond to the same mouse.
Together, pathological and microbiological analyses of mice infected by intranasal instillation of Y. pestis KIM D27 show a disease characterized by invasion, damage, and destruction of lymphoid tissues. These results are consistent with the hypothesis that Y. pestis KIM D27 actively targets immune cells during infection (35). Secondary effects on multiple essential organs due to hematogenous spread are also evident. Therefore, lethality did not result from pneumonia but rather was most likely attributable to a systemic syndrome caused by septicemia, such as multiorgan dysfunction syndrome (MODS) or systemic inflammatory response syndrome (SIRS).
Y. pestis KIM D27 is unable to cause lethal pneumonia in mice.
Because we were interested in identifying any development of lung disease in this model, high challenge doses with higher doses of iron supplementation were evaluated. Given the possible toxicity of increased iron levels, we first monitored the effects of higher iron doses on the health status of BALB/c mice that were not challenged with Y. pestis. Three groups of mice received intraperitoneal injections of iron at 500-μg, 1-mg, and 2-mg doses, and we monitored their survival and condition for 14 days (Fig. 5). Acute toxicity was seen in the entire group that received 2 mg of iron, and all five mice died within 24 h (Fig. 5A). Injections of 1 mg of iron resulted in more-gradual death throughout the study period, leaving only one survivor after 14 days. Calculation of average daily weight loss compared to the original weights indicated toxicity within the whole group, whose average weight loss exceeded 30% by 7 days postinjection (Fig. 5B). In contrast, there was 100% survival in the group of mice that received 500 μg of iron, with minimal weight loss within the first 3 days postinjection, followed by a return to normal weight (Fig. 5C). These findings indicate that 500 μg of iron is the highest dose that can be used safely, without deleterious effects in the BALB/c mouse model that would complicate data analysis in infection studies.
FIG. 5.
The highest dose of inorganic iron that can be given without deleterious effects on the BALB/c mouse is 500 μg. (A) Survival curve for three different doses (500 μg, 1 mg, and 2 mg) of FeCl2 given by intraperitoneal injection to BALB/c mice (5 mice per dose). (B and C) Average weight gain or loss following intraperitoneal injection of 1 mg (B) or 500 μg (C) of iron, plotted with the corresponding survival curve.
We infected BALB/c mice with 1.0 × 107 CFU of KIM D27 (1,000 LD50), with half of the mice receiving 500 μg of iron and the other half receiving sterile water, to analyze the timing and frequency of septicemic spread following intranasal infection as well as any development of inflammation or disease in the lungs (Fig. 6). At this challenge dose, 100% of both treated and untreated mice are expected to succumb to the infection. Two groups of five mice each, one treated with iron and the other not, were euthanized at specific time points for pathological evaluation and determination of bacterial titers in the lungs and blood at 24 h (Fig. 6A and E), 48 h (Fig. 6B and F), 72 h (Fig. 6C and G), and 96 h (Fig. 6D and H) postinfection. After removal of 250 μl of blood for determination of the bacterial titer, the lung lobes were fully perfused by flushing sterile saline through the vasculature via the right ventricle. The left lung lobe was aseptically removed, homogenized in sterile PBS, serially diluted, and plated in triplicate. The remaining right lung lobes were perfused with formalin through the trachea and were removed for histologic evaluation. Multiple lung lobes from each mouse were sectioned; identities were blinded; and sections were examined histologically and assigned lesion scores. Average lesion scores were calculated for each mouse, and an average score for each group was then obtained prior to revelation of the identity of the samples. The statistical significance of differences between groups was evaluated by nested two-way ANOVA. All lesion scores and parameters are shown in Table 1. Images were also collected from tissues of BALB/c mice infected with Y. pestis CO92 at 24, 48, and 72 h postinfection (data not shown).
FIG. 6.
Iron treatment results in accelerated development of bacteremia but does not increase the degree of lesion development or the bacterial titer in the lungs. Five BALB/c mice per group were intranasally infected with 1.0 × 107 CFU (1,000 LD50) following intraperitoneal injection of 500 μg of inorganic iron (left) or an equal volume of sterile water (right). The histology of the lungs and bacterial titers in the blood and lungs were evaluated at four time points: 24 h (A and E), 48 h (B and F), 72 h (C and G), and 96 h (D and H) postinfection. The image shown at each time point for each treatment group is a representative of the average histology score for that group. Histology scores were counted following blinded evaluation of lesions; parameters, individual scores, and statistical analysis are presented in Table 1. The graph on the right side of each panel shows the bacterial titers in the lungs (CFU per tissue) and blood (CFU per milliliter) determined for each mouse in the treatment group. Data shown were collected in two independent experiments.
TABLE 1.
Histology scores of lung tissue after intranasal infection with Y. pestis KIM D27
| Group and individuala | Histological score of lung tissueb at: |
|||
|---|---|---|---|---|
| 24 h | 48 h | 72 h | 96 h | |
| Iron-treated group | ||||
| Mouse 1 | 1.00 | 3.00 | ||
| Mouse 2 | 2.33 | 3.33 | 0.67 | |
| Mouse 3 | 0.00 | 2.00 | 2.33 | 1.5 |
| Mouse 4 | 1.67 | 3.33 | 3.00 | |
| Mouse 5 | 0.33 | 1.00 | ||
| Avg | 1.07c | 2.53 | 2.00 | 1.50 |
| Non-iron-treated group | ||||
| Mouse 1 | 2.00 | 1.00 | 0.67 | 0.33 |
| Mouse 2 | 2.67 | 2.67 | 3.00 | 0.00 |
| Mouse 3 | 3.33 | 3.33 | 0.33 | 2.00 |
| Mouse 4 | 2.67 | 4.00 | 2.33 | 1.33 |
| Mouse 5 | 3.00 | 2.00 | 1.67 | 0.33 |
| Avg | 2.73c | 2.60 | 1.60 | 0.80 |
A total of 40 mice (20 with 500 μg iron and 20 without iron), 5 per group per time point, were evaluated. At each time point, 5 iron-treated mice and 5 non-iron-treated mice were euthanized, and their lungs were collected, sectioned, and examined.
Scores were calculated, as described in Materials and Methods, on the basis of blinded histologic evaluation of all individual lung lobes.
P < 0.0025.
Water-treated mice showed the greatest inflammatory response at 24 h postinfection, characterized mostly by multiple foci of fibrinopurulent lesions that often had hemorrhagic centers and accumulations of cellular debris. Despite the presence of these lesions, most of the lung tissue was still normal, and the overall histology score was 2.73. In most of the iron-treated mice at 24 h postinfection, small increases in the inflammatory cell population within alveolar airspaces were occasionally seen, though one mouse had no lesions. Overall, the histology score indicated that inflammation in iron-treated mice at 24 h postinfection was less severe (1.07). The difference in scores between iron- and water-treated mice at 24 h was statistically significant (P < 0.0025). At 48 h postinfection, inflammatory foci in iron-treated mice were more frequent, though still largely absent, and the lung tissue was similar to that of water-treated mice. Over the next 48 h, the average histologic score for the lungs declined with time in both groups, and no significant differences between iron- and water-treated groups were observed.
Bacterial titers from the blood and lungs at each time point were determined for these mice. Bacterial titers were consistently recovered from the lungs of both groups at all time points and ranged from approximately 1 × 103 to 1 × 106 CFU per homogenized tissue. In contrast, there was a noticeable correlation between consistent development of bacterial titers in the blood and survival, and these appeared to be influenced by iron treatment. Only 1 of 10 water-treated mice failed to survive until 96 h postinfection, and none of the surviving mice showed clinical signs of illness. Likewise, water-treated mice were not bacteremic at 96 h postinfection; bacteria could not be recovered from the blood of any of the mice in this group. Bacteria were, however, consistently recovered from the lungs of these mice throughout the time course, indicating that the mice had not cleared the infection after 96 h, yet there was no damage to lung tissue. In sharp contrast, all but one of the iron-treated mice examined were bacteremic by 48 h postinfection, and 9 of 10 did not survive to the 96-h time point. The surviving mouse had no detectable bacteria in the blood, while more than 1.0 × 105 CFU was still recovered from the lung.
Together, the data collected from this study suggest that development of acute and lethal disease following intranasal infection with Y. pestis KIM D27 correlates with the appearance of bacteremia. In contrast, minor lung inflammatory responses are seen only early following infection and do not result in clearance of bacteria within the first 96 h postinfection. Lethality thus appears to be independent of colonization of the lungs.
Isolation and identification of an isogenic nonpigmented mutant of strain CO92.
In order to confirm that the observed virulence defect described was caused by the absence of pgm and not by secondary mutations present in strain KIM D27, we isolated an isogenic nonpigmented variant of the virulent Y. pestis strain CO92, a strain routinely used for challenge studies (52). Loss of the pgm locus occurs at a high frequency, approximately 1 × 10−5, in laboratory media and can be readily found following plating on HIA supplemented with Congo red (HIA-CR) (24). When grown on HIA-CR, wild-type pigmented Y. pestis forms red colonies, while deletion of the pgm locus results in nonpigmented white colonies (51).
We streaked the fully virulent Y. pestis CO92 on HIA-CR and isolated a nonpigmented colony (data not shown). Two sets of primers were used to confirm the absence of the pgm locus. The first pair surrounds the pgm locus and permits PCR amplification of a 3-kb DNA fragment if the locus is missing due to recombination at the insertion sequences flanking the region. If the pgm locus is still present, no fragment is amplified, due to the large size of the locus. The second pair amplifies the ybtP open reading frame, a 1.8-kb gene that is located within the HPI and therefore will not be amplified from a nonpigmented strain. With the first set of primers, a 3-kb band was amplified from the nonpigmented CO92 isolate and also from KIM D27 (Fig. 7A). No 1.8-kb ybtP fragment could be amplified from the nonpigmented strains, but it was amplified from wild-type CO92 (Fig. 7B). Together with the absence of pigmentation on HIA-CR, these data strongly suggest that we have isolated a pgm-deficient CO92 isogenic strain.
FIG. 7.
CO92Δpgm has a deletion of the 102-kb chromosomal locus and is attenuated in the intranasal infection model. (A) Primers that flank the 102-kb pgm locus amplify a 3-kb band from the nonpigmented CO92 mutant and KIM D27 but not from the wild-type parent strain CO92. (B) YbtP-Nde5 and YbtP-Bam3 amplify the ybtP open reading frame (1.8 kb) in wild-type CO92 but not in the nonpigmented mutant or KIM D27. (C) BALB/c mice were intranasally infected with either 3.9 × 106 CFU of CO92Δpgm (solid line) (n = 6) or 2.8 × 104 CFU of wild-type CO92 (dashed line) (n = 5) and were monitored for the development of morbid disease and death. Mice infected with CO92Δpgm received an intraperitoneal injection of 50 μg of inorganic iron just prior to challenge. Time to death was evaluated by the Wilcoxon rank sum test, and differences between strains were statistically significant (P < 0.005).
Intranasal infection with the CO92Δpgm mutant also results in septicemia.
To assess possible defects in the virulence of the CO92Δpgm mutant, we intranasally infected BALB/c mice with 3.9 × 106 CFU (an expected challenge of 400 LD50). Each of these mice received an intraperitoneal injection of 50 μg of inorganic iron just before infection. A second group of mice was intranasally infected with 2.8 × 104 CFU of wild-type CO92 (100 LD50) with no supplemental iron injection. At these doses, 100% of the animals are expected to succumb to infection.
Signs of illness were observed in all of the mice by 2 days postinfection; however, these were more severe in mice infected with wild-type CO92. All of the mice in this group displayed respiratory difficulties, such as tachypnea and shallow breathing, while none of the CO92Δpgm-infected mice did. The progression of disease was more rapid in the CO92-infected group, and all of these mice were dead by 3 days postinfection (Fig. 7C). In contrast, mice infected with the CO92Δpgm mutant progressed to lethal disease with a significantly slower time course (P < 0.005), and death or euthanasia of moribund mice occurred between 4 and 7 days postinfection.
Lungs from mice infected with the CO92Δpgm mutant appeared similar to the sections from KIM D27-infected mice that were examined, and there were no signs of pneumonia (data not shown). Although there were increased populations of inflammatory cells within the vasculature, as had been observed in KIM D27-infected tissues, there was no indication of primary, active inflammation within the pulmonary tissue. Thus, the nonpigmented Y. pestis strain CO92 is unable to cause pneumonia yet is able to cause lethal disease in mice.
Infection with KIM D27 does not cause pneumonic plague in other murine models.
Previously, respiratory infection with nonpigmented Y. pestis was shown to result in bacterial colonization of the lungs, with the development of occasional lesions that were poorly described (31, 40, 43, 53). To assess if mouse strain differences may contribute to the development of pneumonia in this model, we intranasally infected three different strains of mice (BALB/c, C57BL/6, C3H) with 6.8 × 106 CFU of KIM D27 (expected challenge dose of 700 LD50), providing supplemental iron (50 μg) to all groups. The results are summarized in Table 2. As previously observed, 100% of the BALB/c mice succumbed to disease between 3 and 4 days postinfection. The C57BL/6 mice exhibited similar disease susceptibility and time to death, with 66.7% of the mice dying by 4 days postinfection (P < 0.1). The C3H mice were significantly more resistant to KIM D27 than the BALB/c mice: 80% of the C3H mice survived until the end of the study (P < 0.025). Histology of lungs recovered from moribund C3H mice (data not shown) revealed no inflammation (average histology score, zero). Moribund C57BL/6 mice (data not shown) showed no signs of pneumonia or lung tissue damage and only mild inflammatory responses in the lungs (average histology score, 1.3). For both strains, evaluation of other tissues indicated the presence of septicemia (data not shown). Together, these data suggest that different mouse strains may exhibit different susceptibilities to KIM D27 intranasal infection but not to the development of pneumonia.
TABLE 2.
Summary of susceptibilities of rodent species and strains to intranasal infection with KIM D27
| Species and strain | KIM D27 dose (CFU) | No. of animals surviving/ no. testeda | Mean time to death (days) | Inflammation in lungb |
|---|---|---|---|---|
| Mouse | 6.8 × 106 | |||
| BALB/c | 0/5 | 3.6c,d | − | |
| C57BL/6 | 2/6 | 4.0c | − | |
| C3H | 4/5 | 4.0d | − | |
| Rat | 1.0 × 107 | |||
| Brown Norway | 3/3 | NAe | + | |
| ACI | 3/3 | NA | + | |
| Fischer 344 | 3/3 | NA | + | |
| Lewis | 3/3 | NA | + | |
| Wistar Furth | 3/3 | NA | + |
At a challenge dose of approximately 1 × 107 CFU. Studies ended at 14 days postinfection.
Active inflammation in the lungs was defined as multiple foci of inflammatory cells, evidence of lymphoid tissue hyperplasia, edema, and/or loss of normal tissue architecture. See also the description of histology scoring in Materials and Methods.
The Wilcoxon rank sum test indicates no statistically significant difference between BALB/c and C57BL/6 mice (0.1 > P > 0.05).
The Wilcoxon rank sum test indicates a statistically significant difference between BALB/c and C3H mice (P < 0.025).
NA, not applicable.
Rats develop localized inflammation in the lung and clear intranasal infection of KIM D27.
Rats have been suggested to develop plague disease that is more similar to that of humans than is seen in mice (1, 2, 49). Although the Brown Norway rat is commonly used, many strains of rats, both inbred and outbred, are sensitive to virulent isolates of Y. pestis (49). To analyze host responses to KIM D27 in rats, we intranasally infected five different strains of rats (Brown Norway, ACI, Fischer 344, Lewis, Wistar Furth) with a high challenge dose (1.0 × 107 CFU) of KIM D27. All rats also received intraperitoneal injections of inorganic iron. Daily observations found that none of the rats developed any signs of illness, and all animals survived until the end of the study (data not shown). Infected rats were euthanized at 14 days postinfection; lungs from these rats were grossly normal. Histologic lesions were not consistently found, yet multifocal areas of inflammation were observed in all of the rat species (Fig. 8). The character and degree of cellular infiltration differed considerably between rat species, even though signs of illness were uniformly absent in all animals throughout the observation period. These data suggest that KIM D27 is unable to escape the innate immune responses of rats and therefore is unable to disseminate to distal tissues and cause lethal disease.
FIG. 8.
Histologic sections of lungs from rats resistant to intranasal infection with KIM D27 show multiple foci of active inflammation. Six- to 8-week-old rats (3 per strain) were intranasally infected with 1 × 107 CFU of Y. pestis KIM D27 and were sacrificed on day 14 for histologic evaluation of lungs. (A to E) H&E-stained lung sections from infected Brown Norway (A), ACI (B), Fischer 344 (C), Lewis (D), and Wistar Furth (E) rats. (F) Control lung tissue from an uninfected Brown Norway rat.
We extended these findings to determine if the pgm locus is important to septicemic plague caused by intravenous injection into rats. Brown Norway rats were found to be generally resistant to KIM D27: >1.0 × 106 CFU given by intravenous injection was required to cause illness, more than 10,000 times the amount needed for mice (data not shown). Histologic evaluation of the inguinal lymph nodes of surviving rats euthanized at 14 days postinfection found a moderate increase in mature lymphocyte populations, with many lymphocytes congested within the medullary sinuses, but there were no notable lesions in the spleen or other tissues (data not shown). Together, these data indicate that KIM D27 is severely attenuated for all forms of plague in rats.
DISCUSSION
Following the anthrax attacks of 2001, a spotlight has been cast on pathogens with potential for causing widespread harm, whether by natural occurrence or by intentional use. The resulting public and governmental concern over biological threats has since placed an emphasis on research for biodefense (17). With this increased need for research comes a need for increased regulatory compliance in work with these pathogens, requiring extensive biosafety and biosecurity programs. The extraordinary levels of effort, expense, and time needed to fully comply with regulations has strongly favored the development of surrogate models, particularly of well-characterized attenuated strains that are exempt from these regulations and are safe for use in a lower-biosafety-level laboratory. In the case of Yersinia pestis, strains lacking the pigmentation locus or the virulence plasmid pCD1 are known to be highly attenuated, are exempt from select agent status, and are commonly used laboratory strains. However, because of the many knowledge gaps concerning Yersinia-mammalian host interactions, especially regarding the development of plague, it is likely that overinterpretation of data obtained from surrogate models will occur unless the models are better understood.
We initially set out to understand respiratory disease caused by nonpigmented Y. pestis but unexpectedly found a suppressed lung inflammatory response to KIM D27 and no apparent pulmonary disease. In contrast, the pigmented and virulent Y. pestis strain CO92 causes a severe inflammatory response in the lungs, which correlates with bacterial replication to titers that typically reach 107 to 108 CFU per tissue in mice (8, 16, 32). The failure of nonpigmented Y. pestis to induce significant pulmonary disease is not due to effective clearance of the inoculum; consistent colonization of the lung tissue was found over a 96-h disease course. Instead, it appeared that the growth of pgm-deficient bacteria in the lungs is attenuated, as is the host's inflammatory response, and thus perhaps both the host and the pathogen may contribute to the development of pulmonary plague.
Examination of genes within the Y. pestis pgm locus reveals the presence of 83 potential open reading frames, many of which have previously been implicated in pathogenesis while others are unknown and do not appear to have known homologues in other organisms (9, 11). Until now, it has been assumed that virulence defects caused by deletion of this locus were due to the absence of a key iron acquisition system, one of at least eight such systems in Y. pestis (4, 21, 30, 42). While it remains possible that the failure to cause pneumonia is due to the absence of this system, the mechanism by which this is caused is most likely independent of iron acquisition. Because many of the proteins in the HPI are cell surface located, it is conceivable that they directly influence host immune responses or work in conjunction with other known virulence factors required for the invasion of lung tissue (7, 15, 33). Alternatively, proteins encoded by genes located outside the HPI, such as the hemin storage operon (hms) or ripA, may be involved in the progression of pneumonic plague (51). HmsHFRS combine to synthesize a biofilm at 21°C that is present in the flea during the vector portion of the Y. pestis life cycle (29). Deletion of the hms operon does not attenuate lethality in a subcutaneous infection model, and this, combined with the known degradation of HmsH, HmsF, and HmsR at 37°C, suggests that the biofilm is dispensable for virulence during mammalian infection (41). However, transcription of the hms operon is induced in vivo during mouse pneumonic plague, suggesting that the bacteria may be expressing at least some of these proteins in the mammalian host (32). RipA may also be involved in virulence due to its essential role in promoting the survival of bacteria within IFN-γ-activated macrophages, perhaps during early stages of infection (45). Other genes within the pgm locus encode proteins with homology to functional domains often associated with virulence, including adhesion proteins, two-component signal transduction proteins, and perhaps others (9, 45, 48).
Immunity to plague has previously been shown to involve IFN-γ and tumor necrosis factor alpha, important mediators of systemic innate and adaptive immune responses (31, 36, 40). Although the model we have described, which takes advantage of the exempt status of nonpigmented Y. pestis strains from select agent requirements, is insufficient for pneumonic plague studies, our results suggest it may be very informative for other purposes, including the identification of important host factors that can mediate resistance and susceptibility. For example, our studies suggested that BALB/c and C57BL/6 mice are more sensitive to KIM D27 than C3H mice. Among other strain differences, the gene encoding natural resistance-associated macrophage protein 1 (Nramp1) is absent in the sensitive strains and present in the resistant strain (6, 22, 37). Nramp1 encodes a phagosome-associated protein expressed in IFN-activated macrophages that is involved in the activation of innate immune responses and the destruction of intracellular pathogens (5, 28). Consistent with this hypothesis, 129 mice, which are also Nramp1+/+, have also been reported to be resistant to KIM D27 (13). Thus, at least one mechanism of host resistance to nonpigmented Y. pestis may be through Nramp1 activity. All of these strains are sensitive to virulent Y. pestis CO92, suggesting that the pgm locus encodes proteins that normally overcome or bypass this response (13, 16, 32). Thus, continued study of both host and pathogen responses using respiratory infection models of virulent and attenuated Yersinia pestis may reveal novel approaches to promoting immunity to human plague and perhaps even to other morbid respiratory infections.
Acknowledgments
This work was supported by the Midwest Regional Center of Excellence for Biodefense and Emerging Infectious Disease Research (NIH/NIAID U54 AI157160) and by a University of Missouri College of Veterinary Medicine faculty research award. H.L.-L. is supported by a Comparative Medicine Program training grant (NIH/NIAID T32 RR007004).
We thank Cynthia Besch-Wiliford and Charles Brown for helpful discussions, Craig Lewis and Loren Schultz for assistance with statistical analysis, and David Bland and Nicholas Eisele for critical comments on the manuscript. Histology was performed by the Research Animal Diagnostic Laboratory (RADIL) at the University of Missouri.
Editor: J. B. Bliska
Footnotes
Published ahead of print on 19 October 2009.
REFERENCES
- 1.Agar, S. L., J. Sha, S. M. Foltz, T. E. Erova, K. G. Walberg, W. B. Baze, G. Suarez, J. W. Peterson, and A. K. Chopra. 2009. Characterization of the rat pneumonic plague model: infection kinetics following aerosolization of Yersinia pestis CO92. Microbes Infect. 11:205-214. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, D., N. Ciletti, H. Lee-Lewis, D. Elli, J. Segal, K. Overheim, K. DeBord, M. Tretiakova, R. Brubaker, and O. Schneewind. 2009. Pneumonic plague pathogenesis and immunity in Brown Norway rats. Am. J. Pathol. 174:910-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bearden, S., J. Fetherston, and R. Perry. 1997. Genetic organization of the yersiniabactin biosynthetic region and construction of avirulent mutants in Yersinia pestis. Infect. Immun. 65:1659-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bearden, S., and R. Perry. 1999. The Yfe system of Yersinia pestis transports iron and manganese and is required for full virulence of plague. Mol. Microbiol. 32:403-414. [DOI] [PubMed] [Google Scholar]
- 5.Boyer, E., I. Bergevin, D. Malo, P. Gros, and M. Cellier. 2002. Acquisition of Mn(II) in addition to Fe(II) is required for full virulence of Salmonella enterica serovar Typhimurium. Infect. Immun. 70:6032-6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, D., W. LaFuse, and B. Zwilling. 1995. Cytokine-mediated activation of macrophages from Mycobacterium bovis BCG-resistant and -susceptible mice: differential effects of corticosterone on antimycobacterial activity and expression of the Bcg gene (candidate Nramp). Infect. Immun. 63:2983-2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brubaker, R., E. Beesley, and M. Surgalla. 1965. Pasteurella pestis: role of pesticin I and iron in experimental plague. Science 149:422-424. [DOI] [PubMed] [Google Scholar]
- 8.Bubeck, S., A. Cantwell, and P. Dube. 2007. Delayed inflammatory response to primary pneumonic plague occurs in both outbred and inbred mice. Infect. Immun. 75:697-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchrieser, C., M. Prentice, and E. Carniel. 1998. The 102-kilobase unstable region of Yersinia pestis comprises a high-pathogenicity island linked to a pigmentation segment which undergoes internal rearrangement. J. Bacteriol. 180:2321-2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burrows, T., and S. Jackson. 1956. The virulence-enhancing effect of iron on nonpigmented mutants of virulent strains of Pasteurella pestis. Br. J. Exp. Pathol. 37:577-583. [PMC free article] [PubMed] [Google Scholar]
- 11.Chain, P., E. Carniel, F. Larimer, J. Lamerdin, P. Stoutland, W. Regala, A. Georgescu, L. Vergez, M. Land, V. Motin, R. Brubaker, J. Fowler, B. Hinnebusch, M. Marceau, C. Medigue, M. Simonet, V. Chenal-Francisque, B. Souza, D. Dacheux, J. Elliott, A. Derbise, L. Hauser, and E. Garcia. 2004. Insights into the evolution of Yersinia pestis through whole-genome comparison with Yersinia pseudotuberculosis. Proc. Natl. Acad. Sci. U. S. A. 101:13826-13831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chauvaux, S., M. Rosso, L. Frangeul, C. Lacroix, L. Labarre, A. Schiavo, M. Marceau, M. Dillies, J. Foulon, J. Coppee, C. Medigue, M. Simonet, and E. Carniel. 2007. Transcriptome analysis of Yersinia pestis in human plasma: an approach for discovering bacterial genes involved in septicaemic plague. Microbiology 153:3112-3123. [DOI] [PubMed] [Google Scholar]
- 13.Congleton, Y., C. Wulff, E. Kerschen, and S. Straley. 2006. Mice naturally resistant to Yersinia pestis pgm strains commonly used in pathogenicity studies. Infect. Immun. 74:6501-6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis, K., D. Fritz, M. Pitt, S. Welkos, P. Worsham, and A. Friedlander. 1996. Pathology of experimental pneumonic plague produced by fraction 1-positive and fraction 1-negative Yersinia pestis in African green monkeys (Cercopithecus aethiops). Arch. Pathol. Lab. Med. 120:156-163. [PubMed] [Google Scholar]
- 15.de Almeida, A., A. Guiyoule, I. Guilvout, I. Iteman, G. Baranton, and E. Carniel. 1993. Chromosomal irp2 gene in Yersinia: distribution, expression, deletion and impact on virulence. Microb. Pathog. 14:9-21. [DOI] [PubMed] [Google Scholar]
- 16.DeBord, K., D. Anderson, M. Marketon, K. Overheim, R. DePaolo, N. Ciletti, B. Jabri, and O. Schneewind. 2006. Immunogenicity and protective immunity against bubonic plague and pneumonic plague by immunization of mice with the recombinant V10 antigen, a variant of LcrV. Infect. Immun. 74:4910-4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fauci, A. 2003. Biodefense on the research agenda. Nature 421:787. [DOI] [PubMed] [Google Scholar]
- 18.Fetherston, J., P. Schuetze, and R. Perry. 1992. Loss of the pigmentation phenotype in Yersinia pestis is due to the spontaneous deletion of 102 kb of chromosomal DNA which is flanked by a repetitive element. Mol. Microbiol. 6:2693-2704. [DOI] [PubMed] [Google Scholar]
- 19.Gage, K., and M. Kosoy. 2005. Natural history of plague: perspectives from more than a century of research. Annu. Rev. Entomol. 50:505-528. [DOI] [PubMed] [Google Scholar]
- 20.Galimand, M., A. Guiyoule, G. Gerbaud, B. Rasoamanana, S. Chanteau, E. Carniel, and P. Courvalin. 1997. Multidrug resistance in Yersinia pestis mediated by a transferable plasmid. N. Engl. J. Med. 337:677-680. [DOI] [PubMed] [Google Scholar]
- 21.Gao, H., D. Zhou, Y. Li, Z. Guo, Y. Han, Y. Song, J. Zhai, Z. Du, X. Wang, J. Lu, and R. Yang. 2008. The iron-responsive Fur regulon in Yersinia pestis. J. Bacteriol. 190:3063-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Govoni, G., S. Vidal, S. Gauthier, E. Skamene, D. Malo, and P. Gros. 1996. The Bcg/Ity/Lsh locus: genetic transfer of resistance to infections in C57BL/6J mice transgenic for the Nramp1 Gly169 allele. Infect. Immun. 64:2923-2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guiyoule, A., G. Gerbaud, C. Buchrieser, M. Galimand, L. Rahalison, S. Chanteau, P. Courvalin, and E. Carniel. 2001. Transferable plasmid-mediated resistance to streptomycin in a clinical isolate of Yersinia pestis. Emerg. Infect. Dis. 7:43-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hare, J., and K. McDonough. 1999. High-frequency RecA-dependent and -independent mechanisms of Congo red binding mutations in Yersinia pestis. J. Bacteriol. 181:4896-4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hinnebusch, B., M. Rosso, T. Schwan, and E. Carniel. 2002. High-frequency conjugative transfer of antibiotic resistance genes to Yersinia pestis in the flea midgut. Mol. Microbiol. 2:349-354. [DOI] [PubMed] [Google Scholar]
- 26.Huang, X., M. Nikolich, and L. Lindler. 2006. Current trends in plague research: from genomics to virulence. Clin. Med. Res. 4:189-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inglesby, T., D. Dennis, D. Henderson, J. Bartlett, M. Ascher, E. Eitzen, A. Fine, A. Friedlander, J. Hauer, J. Koerner, M. Layton, J. McDade, M. Osterholm, T. O'Toole, G. Parker, T. Perl, P. Russell, M. Schoch-Spana, and K. Tonat. 2000. Plague as a biological weapon: medical and public health management. Working Group on Civilian Biodefense. JAMA 283:2281-2290. [DOI] [PubMed] [Google Scholar]
- 28.Jabado, N., A. Jankowski, S. Dougaparsad, V. Picard, S. Grinstein, and P. Gros. 2000. Natural resistance to intracellular infections: natural resistance-associated macrophage protein 1 (NRAMP1) functions as a pH-dependent manganese transporter at the phagosomal membrane. J. Exp. Med. 192:1237-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jarrett, C., E. Deak, K. Isherwood, P. Oyston, E. Fischer, A. Whitney, S. Kobayashi, F. DeLeo, and B. Hinnebusch. 2004. Transmission of Yersinia pestis from an infectious biofilm in the flea vector. J. Infect. Dis. 190:783-792. [DOI] [PubMed] [Google Scholar]
- 30.Kirillina, O., A. Bobrov, J. Fetherston, and R. Perry. 2006. Hierarchy of iron uptake systems: Yfu and Yiu are functional in Yersinia pestis. Infect. Immun. 74:6171-6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kummer, L., F. Szaba, M. Parent, J. Adamovicz, J. Hill, L. Johnson, and S. Smiley. 2008. Antibodies and cytokines independently protect against pneumonic plague. Vaccine 26:6901-6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lathem, W., S. Crosby, V. Miller, and W. Goldman. 2005. Progression of primary pneumonic plague: a mouse model of infection, pathology, and bacterial transcriptional activity. Proc. Natl. Acad. Sci. U. S. A. 102:17786-17791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lathem, W., P. Price, V. Miller, and W. Goldman. 2007. A plasminogen activating protease specifically controls the development of primary pneumonic plague. Science 215:509-513. [DOI] [PubMed] [Google Scholar]
- 34.Lien-Teh, W. 1926. A treatise on pneumonic plague. League of Nations Health Organization, Geneva, Switzerland.
- 35.Marketon, M., R. DePaolo, K. DeBord, B. Jabri, and O. Schneewind. 2005. Plague bacteria target immune cells during infection. Science 309:1739-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakajima, R., and R. Brubaker. 1993. Association between virulence of Yersinia pestis and suppression of gamma interferon and tumor necrosis factor alpha. Infect. Immun. 61:23-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakanaga, K., S. Maeda, Y. Myojin, D. Xu, and Y. Goto. 1999. Sequence analysis and expression of Nramp-1 gene in Bcgr and Bcgs mice. J. Vet. Med. Sci. 61:717-720. [DOI] [PubMed] [Google Scholar]
- 38.Overheim, K., R. DePaolo, K. KeBord, E. Morrin, D. Anderson, N. Green, R. Brubaker, B. Jabri, and O. Schneewind. 2005. LcrV plague vaccine with altered immunomodulatory properties. Infect. Immun. 73:5152-5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parent, M., K. Berggren, I. Mullarky, F. Szaba, L. Kummer, J. Adamovicz, and S. Smiley. 2005. Yersinia pestis V protein epitopes recognized by CD4 T cells. Infect. Immun. 73:2197-2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parent, M., L. Wilhelm, L. Kummer, F. Szaba, I. Mullarky, and S. Smiley. 2006. Gamma interferon, tumor necrosis factor alpha, and nitric oxide synthase 2, key elements of cellular immunity, perform critical protective functions during humoral defense against lethal pulmonary Yersinia pestis infection. Infect. Immun. 74:3381-3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perry, R., A. Bobrov, O. Kirillina, H. Jones, L. Pedersen, J. Abney, and J. Fetherston. 2004. Temperature regulation of the hemin storage (Hms+) phenotype of Yersinia pestis is posttranscriptional. J. Bacteriol. 186:1638-1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perry, R., J. Shah, S. Bearden, J. Thompson, and J. Fetherston. 2003. Yersinia pestis TonB: role in iron, heme, and hemoprotein utilization. Infect. Immun. 71:4159-4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Philipovskiy, A., and S. Smiley. 2007. Vaccination with live Yersinia pestis primes CD4 and CD8 T cells that synergistically protect against lethal pulmonary Y. pestis infection. Infect. Immun. 75:878-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pollitzer, R. 1954. Plague. World Health Organization, Geneva, Switzerland.
- 45.Pujol, C., J. Grabenstein, R. Perry, and J. Bliska. 2005. Replication of Yersinia pestis in interferon gamma-activated macrophages requires ripA, a gene encoded in the pigmentation locus. Proc. Natl. Acad. Sci. U. S. A. 102:12909-12914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.R Development Core Team. 2008. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org.
- 47.Reed, L., and H. Muench. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 48.Schubert, S., A. Rakin, H. Karch, E. Carniel, and J. Heesemann. 1998. Prevalence of the “high-pathogenicity island” of Yersinia species among Escherichia coli strains that are pathogenic to humans. Infect. Immun. 66:480-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sebbane, F., D. Gardner, D. Long, B. Gowen, and B. Hinnebusch. 2005. Kinetics of disease progression and host response in a rat model of bubonic plague. Am. J. Pathol. 166:1427-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Straley, S., and M. L. Cibull. 1989. Differential clearance and host-pathogen interactions of YopE− and YopK− YopL− Yersinia pestis in BALB/c mice. Infect. Immun. 57:1200-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Surgalla, M., and E. Beesley. 1969. Congo red-agar plating medium for detecting pigmentation in Pasteurella pestis. Appl. Microbiol. 18:834-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Welkos, S., A. Friedlander, and K. Davis. 1997. Studies on the role of plasminogen activator in systemic infection by virulent Yersinia pestis strain CO92. Microb. Pathog. 23:211-223. [DOI] [PubMed] [Google Scholar]
- 53.Welkos, S., M. L. Pitt, M. Martinez, A. Friedlander, P. Vogel, and R. Tammariello. 2002. Determination of the virulence of the pigmentation-deficient and pigmentation-/plasminogen activator-deficient strains of Yersinia pestis in non-human primate and mouse models of pneumonic plague. Vaccine 20:2206-2214. [DOI] [PubMed] [Google Scholar]