Abstract
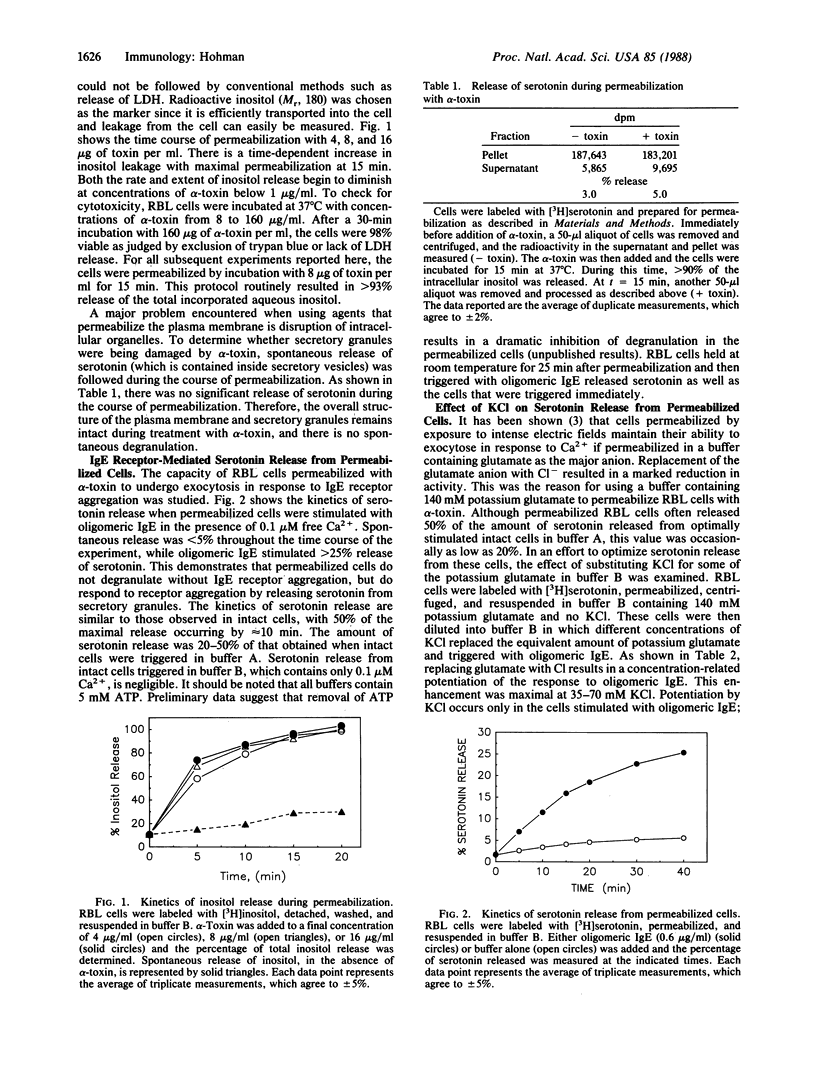
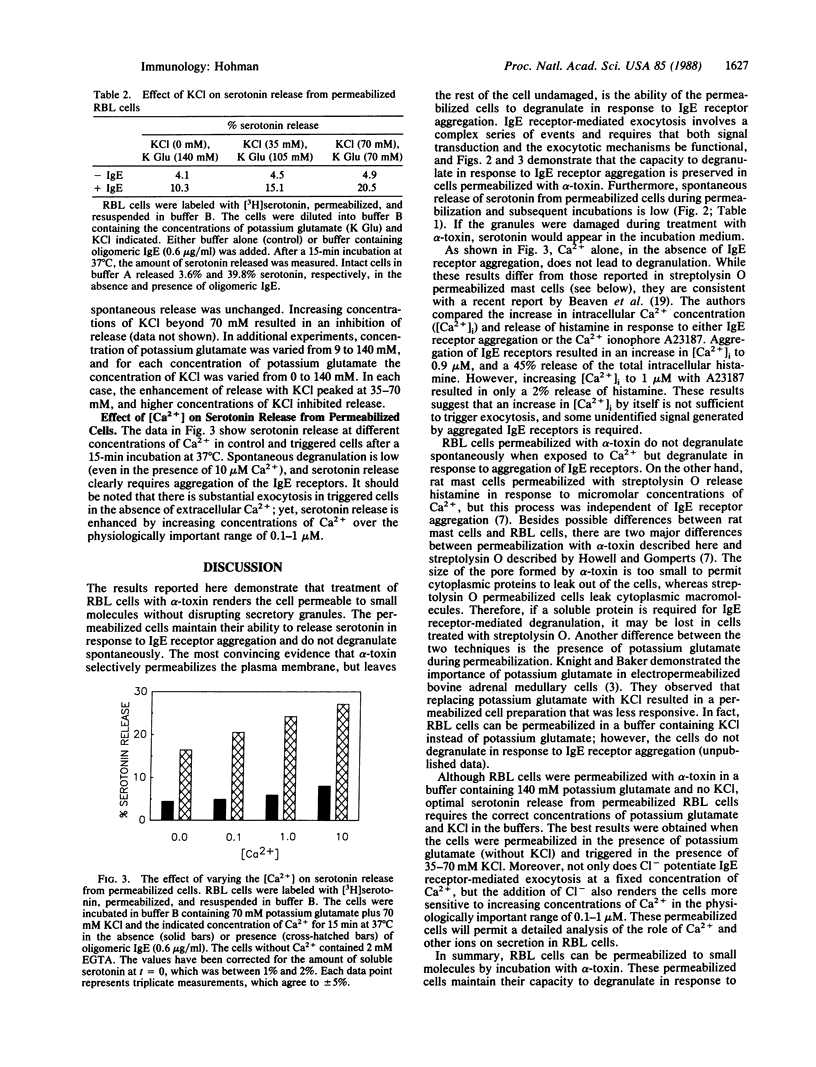
A method has been developed by which rat basophilic leukemia (RBL) cells can be permeabilized to small molecules while maintaining their ability to degranulate in response to aggregation of IgE receptors. alpha-Toxin was isolated from culture supernatants of Staphylococcus aureus by precipitation with (NH4)2SO4 and chromatography on phenyl-Sepharose. The isolated toxin binds to the plasma membrane of RBL cells and polymerizes to form a transmembrane pore that allows small molecules (Mr less than 1000), but not macromolecules, to diffuse freely across the membrane. There was no spontaneous release of the contents of RBL cell secretory granules during permeabilization or subsequent incubations. Substantial IgE receptor-mediated exocytosis occurred in the absence of Ca2+, and degranulation was maximal at 0.1-1.0 microM Ca2+, the physiologically important range of Ca2+ concentrations. Using these permeabilized cells, small molecules (i.e., substrates and inhibitors of various enzymes) normally excluded by the plasma membrane can be introduced into the cell. Moreover, the intracellular concentration of ions (such as Ca2+) can be precisely controlled. This method will allow a detailed examination of the individual biochemical events involved in degranulation of mast cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahnert-Hilger G., Bhakdi S., Gratzl M. Minimal requirements for exocytosis. A study using PC 12 cells permeabilized with staphylococcal alpha-toxin. J Biol Chem. 1985 Oct 15;260(23):12730–12734. [PubMed] [Google Scholar]
- Bader M. F., Thiersé D., Aunis D., Ahnert-Hilger G., Gratzl M. Characterization of hormone and protein release from alpha-toxin-permeabilized chromaffin cells in primary culture. J Biol Chem. 1986 May 5;261(13):5777–5783. [PubMed] [Google Scholar]
- Barsumian E. L., Isersky C., Petrino M. G., Siraganian R. P. IgE-induced histamine release from rat basophilic leukemia cell lines: isolation of releasing and nonreleasing clones. Eur J Immunol. 1981 Apr;11(4):317–323. doi: 10.1002/eji.1830110410. [DOI] [PubMed] [Google Scholar]
- Beaven M. A., Guthrie D. F., Moore J. P., Smith G. A., Hesketh T. R., Metcalfe J. C. Synergistic signals in the mechanism of antigen-induced exocytosis in 2H3 cells: evidence for an unidentified signal required for histamine release. J Cell Biol. 1987 Sep;105(3):1129–1136. doi: 10.1083/jcb.105.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. P., Cockcroft S., Gomperts B. D. Rat mast cells permeabilized with ATP secrete histamine in response to calcium ions buffered in the micromolar range. J Physiol. 1981 Aug;317:335–345. doi: 10.1113/jphysiol.1981.sp013828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Füssle R., Tranum-Jensen J. Staphylococcal alpha-toxin: oligomerization of hydrophilic monomers to form amphiphilic hexamers induced through contact with deoxycholate detergent micelles. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5475–5479. doi: 10.1073/pnas.78.9.5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks J. C., Treml S. Effect of trifluoperazine and calmodulin on catecholamine secretion by saponin-skinned cultured chromaffin cells. Life Sci. 1984 Feb 13;34(7):669–674. doi: 10.1016/0024-3205(84)90231-5. [DOI] [PubMed] [Google Scholar]
- Coleman G. A comparison of the patterns of extracellular proteins produced by the high alpha-toxin-secreting organism Staphylococcus aureus (Wood 46) during aerobic and anaerobic growth. J Gen Microbiol. 1985 Feb;131(2):405–408. doi: 10.1099/00221287-131-2-405. [DOI] [PubMed] [Google Scholar]
- Dunn L. A., Holz R. W. Catecholamine secretion from digitonin-treated adrenal medullary chromaffin cells. J Biol Chem. 1983 Apr 25;258(8):4989–4993. [PubMed] [Google Scholar]
- Füssle R., Bhakdi S., Sziegoleit A., Tranum-Jensen J., Kranz T., Wellensiek H. J. On the mechanism of membrane damage by Staphylococcus aureus alpha-toxin. J Cell Biol. 1981 Oct;91(1):83–94. doi: 10.1083/jcb.91.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. S., Kehoe M. Primary sequence of the alpha-toxin gene from Staphylococcus aureus wood 46. Infect Immun. 1984 Nov;46(2):615–618. doi: 10.1128/iai.46.2.615-618.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell T. W., Gomperts B. D. Rat mast cells permeabilised with streptolysin O secrete histamine in response to Ca2+ at concentrations buffered in the micromolar range. Biochim Biophys Acta. 1987 Feb 18;927(2):177–183. doi: 10.1016/0167-4889(87)90132-7. [DOI] [PubMed] [Google Scholar]
- Ishizaka T., Ishizaka K. Activation of mast cells for mediator release through IgE receptors. Prog Allergy. 1984;34:188–235. [PubMed] [Google Scholar]
- Knight D. E., Baker P. F. Calcium-dependence of catecholamine release from bovine adrenal medullary cells after exposure to intense electric fields. J Membr Biol. 1982;68(2):107–140. doi: 10.1007/BF01872259. [DOI] [PubMed] [Google Scholar]
- Maeyama K., Hohman R. J., Metzger H., Beaven M. A. Quantitative relationships between aggregation of IgE receptors, generation of intracellular signals, and histamine secretion in rat basophilic leukemia (2H3) cells. Enhanced responses with heavy water. J Biol Chem. 1986 Feb 25;261(6):2583–2592. [PubMed] [Google Scholar]
- McEwen B. F., Arion W. J. Permeabilization of rat hepatocytes with Staphylococcus aureus alpha-toxin. J Cell Biol. 1985 Jun;100(6):1922–1929. doi: 10.1083/jcb.100.6.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger H., Alcaraz G., Hohman R., Kinet J. P., Pribluda V., Quarto R. The receptor with high affinity for immunoglobulin E. Annu Rev Immunol. 1986;4:419–470. doi: 10.1146/annurev.iy.04.040186.002223. [DOI] [PubMed] [Google Scholar]
- Suttorp N., Seeger W., Dewein E., Bhakdi S., Roka L. Staphylococcal alpha-toxin-induced PGI2 production in endothelial cells: role of calcium. Am J Physiol. 1985 Jan;248(1 Pt 1):C127–C134. doi: 10.1152/ajpcell.1985.248.1.C127. [DOI] [PubMed] [Google Scholar]
- Wilson S. P., Kirshner N. Calcium-evoked secretion from digitonin-permeabilized adrenal medullary chromaffin cells. J Biol Chem. 1983 Apr 25;258(8):4994–5000. [PubMed] [Google Scholar]


