Abstract
Human cytomegalovirus (HCMV) is a major renal pathogen in congenitally infected infants and renal allograft recipients. We postulated that a specific renal cell type was involved in HCMV infection and reactivation. Human fetal kidney cortex cell cultures were assayed for their ability to support HCMV infection. Infectious center assays indicated that the low level of viral replication observed by virus yield assay occurred from a fraction of the cells in the mixed cultures. Virus-specific immunofluorescence and in situ hybridization documented the presence of HCMV-specific protein and nucleic acid, respectively, in a morphologically distinct cell type. These cells were purified, were identified as kidney mesangial cells, and were observed to support efficient HCMV replication. Our research identifies mesangial cells as a renal cell type that supports HCMV replication and provides evidence to implicate these cells in the pathogenesis of HCMV-induced renal disease.
Full text
PDF

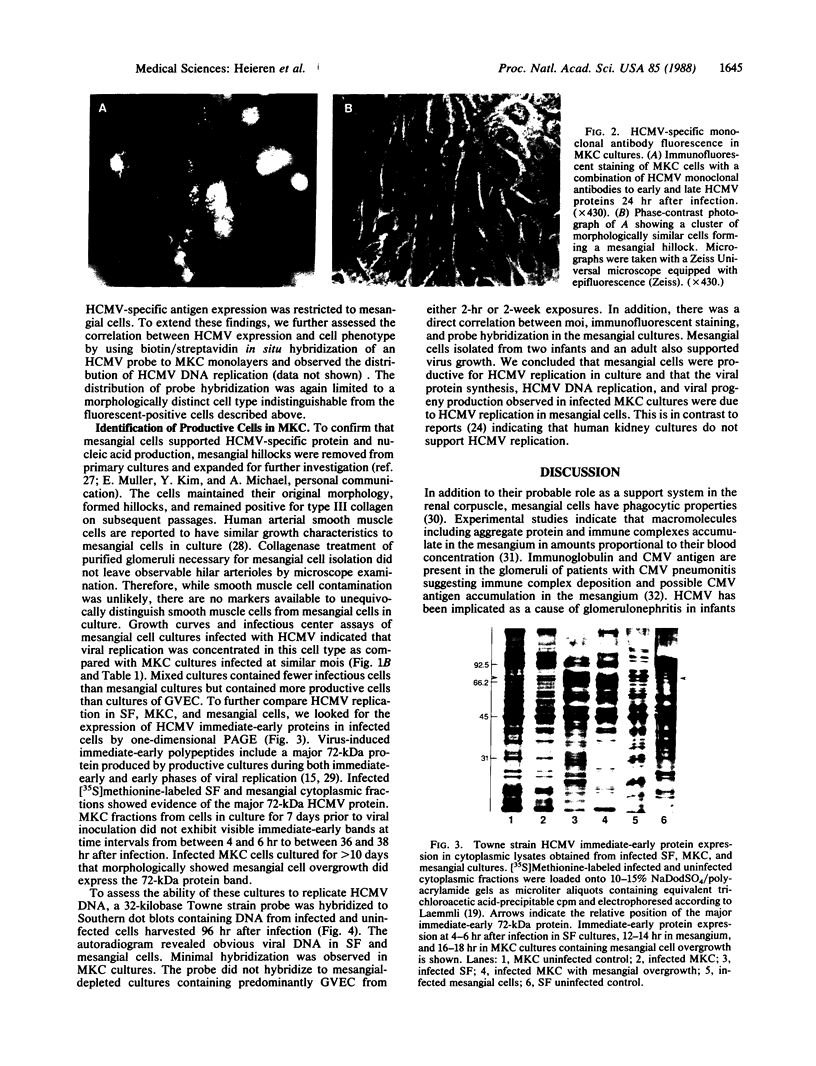

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beneck D., Greco M. A., Feiner H. D. Glomerulonephritis in congenital cytomegalic inclusion disease. Hum Pathol. 1986 Oct;17(10):1054–1059. doi: 10.1016/s0046-8177(86)80090-9. [DOI] [PubMed] [Google Scholar]
- Blanton R. A., Tevethia M. J. Immunoprecipitation of virus-specific immediate-early and early polypeptides from cells lytically infected with human cytomegalovirus strain AD 169. Virology. 1981 Jul 15;112(1):262–273. doi: 10.1016/0042-6822(81)90631-0. [DOI] [PubMed] [Google Scholar]
- Detrisac C. J., Sens M. A., Garvin A. J., Spicer S. S., Sens D. A. Tissue culture of human kidney epithelial cells of proximal tubule origin. Kidney Int. 1984 Feb;25(2):383–390. doi: 10.1038/ki.1984.28. [DOI] [PubMed] [Google Scholar]
- Gibson W., Murphy T. L., Roby C. Cytomegalovirus-infected cells contain a DNA-binding protein. Virology. 1981 May;111(1):251–262. doi: 10.1016/0042-6822(81)90669-3. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Cotran R. S. Human vascular smooth muscle in culture. Growth and ultrastructure. Lab Invest. 1975 Jul;33(1):16–27. [PubMed] [Google Scholar]
- Gönczöl E., Andrews P. W., Plotkin S. A. Cytomegalovirus replicates in differentiated but not in undifferentiated human embryonal carcinoma cells. Science. 1984 Apr 13;224(4645):159–161. doi: 10.1126/science.6322309. [DOI] [PubMed] [Google Scholar]
- Herrera G. A., Alexander R. W., Cooley C. F., Luke R. G., Kelly D. R., Curtis J. J., Gockerman J. P. Cytomegalovirus glomerulopathy: a controversial lesion. Kidney Int. 1986 Mar;29(3):725–733. doi: 10.1038/ki.1986.58. [DOI] [PubMed] [Google Scholar]
- Kreisberg J. I., Karnovsky M. J. Glomerular cells in culture. Kidney Int. 1983 Mar;23(3):439–447. doi: 10.1038/ki.1983.40. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marker S. C., Howard R. J., Simmons R. L., Kalis J. M., Connelly D. P., Najarian J. S., Balfour H. H., Jr Cytomegalovirus infection: a quantitative prospective study of three hundred twenty consecutive renal transplants. Surgery. 1981 Jun;89(6):660–671. [PubMed] [Google Scholar]
- Michael A. F., Keane W. F., Raij L., Vernier R. L., Mauer S. M. The glomerular mesangium. Kidney Int. 1980 Feb;17(2):141–154. doi: 10.1038/ki.1980.18. [DOI] [PubMed] [Google Scholar]
- Myerson D., Hackman R. C., Nelson J. A., Ward D. C., McDougall J. K. Widespread presence of histologically occult cytomegalovirus. Hum Pathol. 1984 May;15(5):430–439. doi: 10.1016/s0046-8177(84)80076-3. [DOI] [PubMed] [Google Scholar]
- Neiman P. E., Reeves W., Ray G., Flournoy N., Lerner K. G., Sale G. E., Thomas E. D. A prospective analysis interstitial pneumonia and opportunistic viral infection among recipients of allogeneic bone marrow grafts. J Infect Dis. 1977 Dec;136(6):754–767. doi: 10.1093/infdis/136.6.754. [DOI] [PubMed] [Google Scholar]
- Ozawa T., Stewart J. A. Immune-complex glomerulonephritis associated with cytomegalovirus infection. Am J Clin Pathol. 1979 Jul;72(1):103–107. doi: 10.1093/ajcp/72.1.103. [DOI] [PubMed] [Google Scholar]
- Peterson P. K., Balfour H. H., Jr, Marker S. C., Fryd D. S., Howard R. J., Simmons R. L. Cytomegalovirus disease in renal allograft recipients: a prospective study of the clinical features, risk factors and impact on renal transplantation. Medicine (Baltimore) 1980 Jul;59(4):283–300. [PubMed] [Google Scholar]
- Platt J. L., LeBien T. W., Michael A. F. Stages of renal ontogenesis identified by monoclonal antibodies reactive with lymphohemopoietic differentiation antigens. J Exp Med. 1983 Jan 1;157(1):155–172. doi: 10.1084/jem.157.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt J. L., Michael A. F. Retardation of fading and enhancement of intensity of immunofluorescence by p-phenylenediamine. J Histochem Cytochem. 1983 Jun;31(6):840–842. doi: 10.1177/31.6.6341464. [DOI] [PubMed] [Google Scholar]
- Porter K. R., Starnes D. M., Hamilton J. D. Reactivation of latent murine cytomegalovirus from kidney. Kidney Int. 1985 Dec;28(6):922–925. doi: 10.1038/ki.1985.218. [DOI] [PubMed] [Google Scholar]
- Rubin R. H., Tolkoff-Rubin N. E. Viral infection in the renal transplant patient. Proc Eur Dial Transplant Assoc. 1983;19:513–526. [PubMed] [Google Scholar]
- Schwarzenberg S. J., Sharp H. L., Manthei R. D., Seelig S. Hepatic alpha 1-antitrypsin mRNA content in cirrhosis with normal and abnormal protease inhibitor phenotypes. Hepatology. 1986 Nov-Dec;6(6):1252–1258. doi: 10.1002/hep.1840060605. [DOI] [PubMed] [Google Scholar]
- Smith R. D., Wehner R. W. Acute cytomegalovirus glomerulonephritis: an experimental model. Lab Invest. 1980 Sep;43(3):278–286. [PubMed] [Google Scholar]
- St Jeor S., Rapp F. Cytomegalovirus: conversion of nonpermissive cells to a permissive state for virus replication. Science. 1973 Sep 14;181(4104):1060–1061. doi: 10.1126/science.181.4104.1060. [DOI] [PubMed] [Google Scholar]
- Sterzel R. B., Lovett D. H., Foellmer H. G., Perfetto M., Biemesderfer D., Kashgarian M. Mesangial cell hillocks. Nodular foci of exaggerated growth of cells and matrix in prolonged culture. Am J Pathol. 1986 Oct;125(1):130–140. [PMC free article] [PubMed] [Google Scholar]
- Stinski M. F. Synthesis of proteins and glycoproteins in cells infected with human cytomegalovirus. J Virol. 1977 Sep;23(3):751–767. doi: 10.1128/jvi.23.3.751-767.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striker G. E., Killen P. D., Farin F. M. Human glomerular cells in vitro: isolation and characterization. Transplant Proc. 1980 Sep;12(3 Suppl 1):88–99. [PubMed] [Google Scholar]
- Striker G. E., Mannik M., Tung M. Y. Role of marrow-derived monocytes and mesangial cells in removal of immune complexes from renal glomeruli. J Exp Med. 1979 Jan 1;149(1):127–136. doi: 10.1084/jem.149.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka J., Kamiya S., Ogura T., Sato H., Ogura H., Hatano M. Effect of dimethyl sulfoxide on interaction of human cytomegalovirus with host cell: conversion of a nonproductive state of cell to a productive state for virus replication. Virology. 1985 Oct 30;146(2):165–176. doi: 10.1016/0042-6822(85)90001-7. [DOI] [PubMed] [Google Scholar]
- Tanaka J., Ogura T., Kamiya S., Yoshie T., Yabuki Y., Hatano M. Dexamethasone enhances human cytomegalovirus replication in human epithelial cell cultures. Virology. 1984 Jul 30;136(2):448–452. doi: 10.1016/0042-6822(84)90182-x. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen D. R., Stinski M. F. Cloning of the human cytomegalovirus genome as endonuclease XbaI fragments. Gene. 1981 Dec;16(1-3):207–216. doi: 10.1016/0378-1119(81)90077-9. [DOI] [PubMed] [Google Scholar]
- Unger E. R., Budgeon L. R., Myerson D., Brigati D. J. Viral diagnosis by in situ hybridization. Description of a rapid simplified colorimetric method. Am J Surg Pathol. 1986 Jan;10(1):1–8. doi: 10.1097/00000478-198601000-00001. [DOI] [PubMed] [Google Scholar]
- Wehner R. W., Smith R. G. Progressive cytomegalovirus glomerulonephritis - An experimental model. Am J Pathol. 1983 Sep;112(3):313–325. [PMC free article] [PubMed] [Google Scholar]
- Wentworth B. B., French L. Plaque assay of cytomegalovirus strains of human origin. Proc Soc Exp Biol Med. 1970 Nov;135(2):253–258. doi: 10.3181/00379727-135-35031. [DOI] [PubMed] [Google Scholar]