Abstract
Upon ligand binding, G-protein-coupled receptors (GPCRs) impart the signal to heterotrimeric G proteins composed of α, β, and γ subunits, leading to dissociation of the Gα subunit from the Gβγ subunit. While the Gα subunit is imperative for downstream signaling, the Gβγ subunit, in its own right, mediates a variety of cellular responses such as GPCR desensitization via recruiting GRK to the plasma membrane and AKT stimulation. Here we report a mode of spatial regulation of the Gβγ subunit through alteration in subcellular compartmentation. RKTG (Raf kinase trapping to Golgi apparatus) is a newly characterized membrane protein specifically localized at the Golgi apparatus. The N terminus of RKTG interacts with Gβ and tethers Gβγ to the Golgi apparatus. Overexpression of RKTG impedes the interaction of Gβγ with GRK2, abrogates the ligand-induced change of subcellular distribution of GRK2, reduces isoproterenol-stimulated phosphorylation of the β2-adrenergic receptor (β2AR), and alters β2AR desensitization. In addition, RKTG inhibits Gβγ- and ligand-mediated AKT phosphorylation that is enhanced in cells with downregulation of RKTG. Silencing of RKTG also alters GRK2 internalization and compromises ligand-induced Gβ translocation to the Golgi apparatus. Taken together, our results reveal that RKTG can modulate GPCR signaling through sequestering Gβγ to the Golgi apparatus and thereby attenuating the functions of Gβγ.
Heterotrimeric G proteins are composed of distinct Gα, β, and γ subunits which relay extracellular signals from heptahelical G-protein-coupled receptors (GPCRs) to downstream effectors (16, 25, 30). Gα binds Gβγ when Gα is bound with GDP but dissociates from Gβγ after GDP is replaced with GTP upon activation of GPCRs by extracellular ligand (25). Under physiologic conditions, the Gβ and Gγ subunits form a dimer in which the two subunits are not separable (10, 30). Although Gα is the primary protein that transmits the signal of GPCRs to specific intracellular effectors, such as adenylyl cyclase and phospholipase C, emerging evidence has indicated that Gβγ is able to regulate GPCR signaling through interacting with GPCRs, the Gα subunit, and downstream effectors (30). Predominantly, Gβγ is able to directly interact with and affect the functions of a variety of membrane and intracellular effectors, such as ion channels, adenylyl cyclase, G-protein-coupled receptor kinases (GRKs), and phosphatidylinositol 3-kinase (PI3K) (30). The current model of Gβγ-mediated signaling restricts it mostly to the plasma membrane (PM) (30). In the case of membrane-bound effectors, such as adenylyl cyclases or GIRK channels, Gβγ regulates the activities of these transmembrane proteins through conformational alteration. In the case of cytosolic proteins such as PLCβ2 or GRK2, whose substrates are localized to PM, Gβγ regulates their activity by recruiting the proteins to PM. The activity of Gβγ is primarily regulated by GPCR and Gα, in which GPCR activation leads to conformational changes of Gα. Such change causes replacement of Gα-bound GDP with GTP and release of Gβγ from the heterotrimeric G proteins. The activity of Gβγ could also be regulated by interacting with cytosolic proteins such as RACK1 (7). However, how Gβγ-mediated signaling is regulated in a spatial manner via subcellular compartmentation is largely unknown.
GRK2 is a member of a family of GRKs that can phosphorylate the agonist-occupied GPCRs (4). Specific phosphorylation of activated receptors is associated with a decreased responsiveness of GPCR to prolonged stimulation by the agonist, also known as desensitization (15, 26). Gβγ regulates the activities of GRK2 and GRK3 toward several GPCRs (9). In cooperation with phosphatidylinositol 4,5-bisphosphate, Gβγ binds to the pleckstrin homology (PH) domain of GRK2 and recruits GRK2 to PM, in which it phosphorylates activated GPCRs (18, 30). The crystallographic structure of GRK2 in complex with Gβ1γ2 has been solved (20, 32). On the other hand, AKT is an intracellular target of PI3K and plays a critical role in cell growth, proliferation, and survival. It has been reported that Gβγ could activate AKT in a PI3K-dependent fashion (5), and Gβγ could mediate AKT activation at endosomes (13). Recent data also indicate that the p110β subunit of PI3K signals downstream of GPCR, and the AKT activation mediated by p110β is G protein dependent (14, 17).
PAQR3 is a member of the progestin and adipoQ receptor (PAQR) family, and the members of this family are predicted to have seven transmembrane domains similar to GPCRs (31). Recently, we demonstrated that PAQR3 is localized at the Golgi apparatus and is involved in the spatial regulation of Raf kinase, whereby this protein was named Raf kinase trapping to Golgi apparatus (RKTG) (12). Biochemical analysis of RKTG suggested that its N terminus is localized on the cytoplasmic side of the Golgi membrane (21). Using the N terminus of RKTG to screen a Saccharomyces cerevisiae two-hybrid library, we determined that RKTG is able to interact with Gβ, and detailed analyses indicate that RKTG is a spatial regulator of Gβγ signaling.
MATERIALS AND METHODS
Plasmid construction.
The full-length human Gβ1, Gβ2, Gγ2, Gαs, and β2AR cDNA were isolated from HEK293T cells by reverse transcription PCR, confirmed by DNA sequencing, and cloned into pRc/CMV-Flag vectors to fuse with one Flag epitope tag at the N terminus. Gβ1 was also subcloned into the mammalian expression vector pCS2+MT with six Myc tags at the N terminus. The Myc-tagged RKTG plasmid has been described previously (12). RKTG and its deletion mutants were cloned into pEGFP-C1 (Clontech, Mountain View, CA) to fuse with an enhanced green fluorescence protein (EGFP) at the N terminus (21). The GFP-tagged bovine GRK2 was kindly provided by Marc G. Caron (Duke University Medical Center, Durham, NC). The Flag-tagged GRK2ct that spans amino acids (aa) 501 to 689 at the C-terminal portion of GRK2 was subcloned from GFP-tagged GRK2. The RKTG short hairpin RNA (shRNA) construct was generated using a lentiviral system as previously reported (27). In short, an annealed small interfering RNA (siRNA) cassette with a targeting sequence of GGACAACCCGUACAUCACC for RKTG was inserted into the pBS-SKII-hU6 vector downstream of the hU6 promoter. The siRNA expression cassette was then subcloned into the FG12 vector and confirmed by DNA sequencing. The FG12 plasmid containing RKTG shRNA was directly used in cell transfection to silence expression of endogenous RKTG. The RKTG expression plasmid resistant to siRNA was constructed by same-sense mutations at the targeting sequence of RKTG cDNA.
Cell culture, cell transfection, RKTG retrovirus, confocal microscopy, and image analysis.
HEK293T, HeLa, and mouse embryonic fibroblast cells (MEFs) were cultured in Dulbecco modified Eagle medium containing 10% fetal bovine serum. COS7 cells were cultured in RPMI 1640 medium containing 10% fetal bovine serum. Transient transfection was performed with the polyethylenimine method for HEK293T and COS7 cells as previously reported (12). Full-length RKTG cDNA sequence was subcloned into the pR-IRES-GFP retroviral vector. Retroviruses were generated in Phoenix cells. The methods for cell fixation, immunostaining, and confocal analyses were described previously (12). The method of MEF isolation from wild-type or RKTG-deleted mouse embryos was described previously (12, 33). For determination of GRK2 internalization and Golgi translocation of Gβ1, 20 cells in each coverslip were randomly chosen and four coverslips were counted by an observer who was blind to the experiments.
Immunoblotting and immunoprecipitation.
The antibodies were purchased from the following manufacturers: total AKT and phospho-AKT(Ser473) were from Cell Signaling Technology (Danvers, MA); monoclonal anti-FLAG antibody was from Sigma-Aldrich (St. Louis, MO); monoclonal and polyclonal anti-Myc antibody and antibodies against phosphorylated β2AR (at serine residues 355 and 356), Gβ1(c-16), and GFP were from Santa Cruz Biotechnology (Santa Cruz, CA); Golgi-97 monoclonal antibody was from Invitrogen (Eugene, Oregon); GM130 polyclonal antibody, Alexa Fluor 488-conjugated donkey anti-mouse immunoglobulin G (IgG), and Alexa Fluor 546-conjugated goat anti-mouse and anti-rabbit IgG were from GE Healthcare (Chalfont St. Giles, United Kingdom); and Cy5-labeled goat anti-mouse IgG was from Jackson ImmunoResearch (West Grove, PA). The polyclonal RKTG antibody was described previously (12). Isoproterenol was from Calbiochem (San Diego, CA). Lysophosphatidic acid (LPA) was from Sigma-Aldrich. Human recombinant full-length adiponectin was from R&D Systems (Minneapolis, MN). The protocols for immunoblotting and immunoprecipitation have been previously described by us (12).
cAMP accumulation assay.
The levels of cyclic AMP (cAMP) were determined using a cAMP direct immunoassay kit (BioVision, Mountain View, CA) by following the manufacturer's instructions. The samples were diluted with 0.1 M HCl, which inactivates phosphodiesterases and lowers the concentration of immunoglobulins that may interfere with the assay.
RESULTS
Interaction of RKTG with the Gβ subunit.
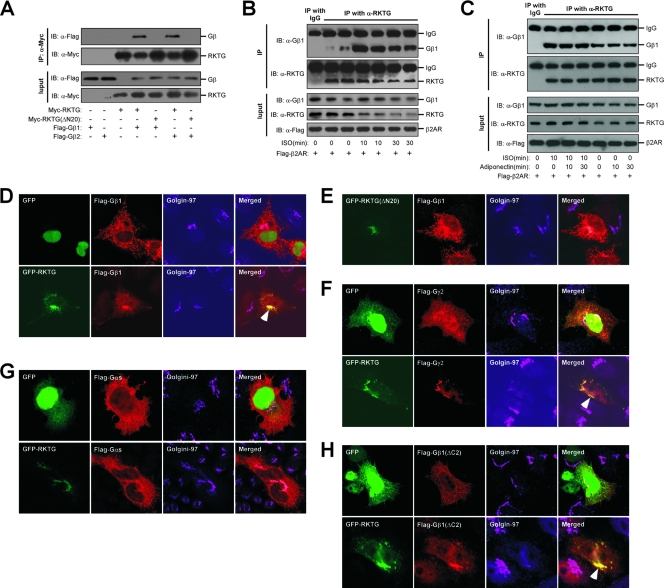
Our previous topology analysis indicates that the N terminus of RKTG is located at the cytosolic side of the Golgi apparatus (21). We used the N-terminal 71 aa as a bait to screen a human brain cDNA library using Clontech's Matchmaker yeast two-hybrid system. Out of 16 positive clones interacting with RKTG, two independent clones were found to contain the full cDNA coding region of Gβ2. We next performed a coimmunoprecipitation assay and found that overexpressed Gβ1 or Gβ2 could interact with overexpressed RKTG (Fig. 1A). The N-terminal 20 aa of RKTG appeared to be required for the interaction of RKTG with the Gβ subunit, as the interaction was lost with deletion of this region (Fig. 1A). We next investigated the interaction of endogenous Gβ1 with endogenous RKTG. As shown in Fig. 1B, a polyclonal antibody against endogenous RKTG antibody but not rabbit IgG was able to precipitate Gβ1, further confirming the interaction of RKTG with Gβ1. In addition, treatment with isoproterenol (ISO), a ligand for the β-adrenergic receptor, could enhance the interaction of endogenous RKTG with Gβ1, with the maximal interaction occurring at 10 min after ISO treatment (Fig. 1B). In addition, treatment of the cells with adiponectin, a ligand for AdipoR1 and AdipoR2 within the PAQR family, could not affect the interaction of RKTG with Gβ1 in the presence or absence of ISO (Fig. 1C).
FIG. 1.
RKTG interacts with Gβ and mobilizes Gβγ onto the Golgi apparatus. (A) Interaction of RKTG with Gβ. HEK293T cells were transiently transfected with Myc-tagged RKTG and Flag-tagged Gβ1. The cell lysate was used in immunoblotting (IB) and immunoprecipitation (IP) using the antibodies indicated. Both full-length and truncated RKTG with an N-terminal deletion of 20 aa (RKTGΔN20) were used. (B) Interaction of endogenous RKTG with endogenous Gβ1. HEK293T cells transfected with Flag-tagged β2-adrenergic receptor (β2AR) were treated with ISO at 10 μM for different lengths of time as indicated. Total cell lysate was subjected to immunoblotting and immunoprecipitation using the antibodies indicated. Rabbit IgG was used as a negative control. (C) Effect of adiponectin on the interaction of RKTG with Gβ1. HEK293T cells transfected with Flag-tagged β2-adrenergic receptor (β2AR) were treated with ISO at 10 μM or full-length adiponectin (20 μg/ml) for different lengths of time as indicated. Total cell lysate was subjected to immunoblotting and immunoprecipitation using the antibodies indicated. Rabbit IgG was used as a negative control. (D) Colocalization of Gβ with RKTG at the Golgi apparatus. HeLa cells were transiently transfected with EGFP-fused RKTG and/or Flag-tagged Gβ1 as indicated, followed by fixation, immunostaining, and confocal analysis. Golgin-97 was used as a Golgi marker. The pEGFP-C1 vector was used as a negative control. The arrowhead indicates representative colocalization of Gβ1 with RKTG at the Golgi marker. (E) Gβ1 could not colocalize with RKTGΔN20 in HeLa cells. Note that RKTGΔN20 but not Gβ1 was localized at the Golgi apparatus. (F) Colocalization of Gγ2 with RKTG but not GFP in HeLa cells. The arrowhead indicates representative colocalization of Gγ2 with RKTG and the Golgi marker. (G) Intracellular distribution of Gαs is not affected by RKTG in HeLa cells. (H) C-terminus-deleted Gβ1 (Gβ1ΔC2) could colocalize with RKTG but not GFP in HeLa cells (arrowhead).
Mobilization of Gβγ onto the Golgi apparatus by RKTG.
Since RKTG is a Golgi apparatus-localizing protein (12), we next investigated whether the interaction of Gβ with RKTG could translocate Gβ to the Golgi apparatus. The GFP-fused RKTG and Flag-tagged Gβ1 were transiently transfected into HeLa cells. In the absence of RKTG overexpression, Gβ1 was broadly distributed within the cell, with limited localization at the Golgi apparatus (Fig. 1D, top panel). Intriguingly, when RKTG was cotransfected, the majority of Gβ1 was localized at the Golgi apparatus, shown as almost complete colocalization with RKTG and the Golgi marker Golgin-97 (Fig. 1D, lower panel). In addition, Gβ2 could also be colocalized with RKTG at the Golgi apparatus (data not shown). Consistent with the result of our coimmunoprecipitation assay, deletion of the N-terminal 20 aa of RKTG could not increase Golgi localization of Gβ1, although such truncated RKTG itself was still localized at the Golgi apparatus (Fig. 1E).
As G proteins are composed of three subunits, Gα, Gβ, and Gγ, we examined whether RKTG could mobilize Gγ or Gα onto the Golgi apparatus. We found that Gγ2 was translocated to the Golgi apparatus by RKTG (Fig. 1F), consistent with the fact that Gβ and Gγ subunits are inseparable within the cell immediately after being synthesized (10, 30). However, RKTG could not elevate Golgi localization of Gαs (Fig. 1G). In addition, we analyzed whether the interaction of Gβ with RKTG is dependent on Gγ. Previous studies found that deletion of the last 2 aa in the C terminus of Gβ lost the binding of Gβ with Gγ (34). We found that such mutated Gβ1 could still be mobilized to the Golgi apparatus by RKTG (Fig. 1H), indicating that the interaction of RKTG with Gβ is not dependent on Gγ. Taken together, these data indicate that RKTG could specifically mobilize Gβγ, but not Gαs, to the Golgi apparatus through its interaction with Gβ, and such interaction is independent of Gβ binding with Gγ.
RKTG affects GRK2/Gβγ complex formation and ligand-induced GRK2 internalization.
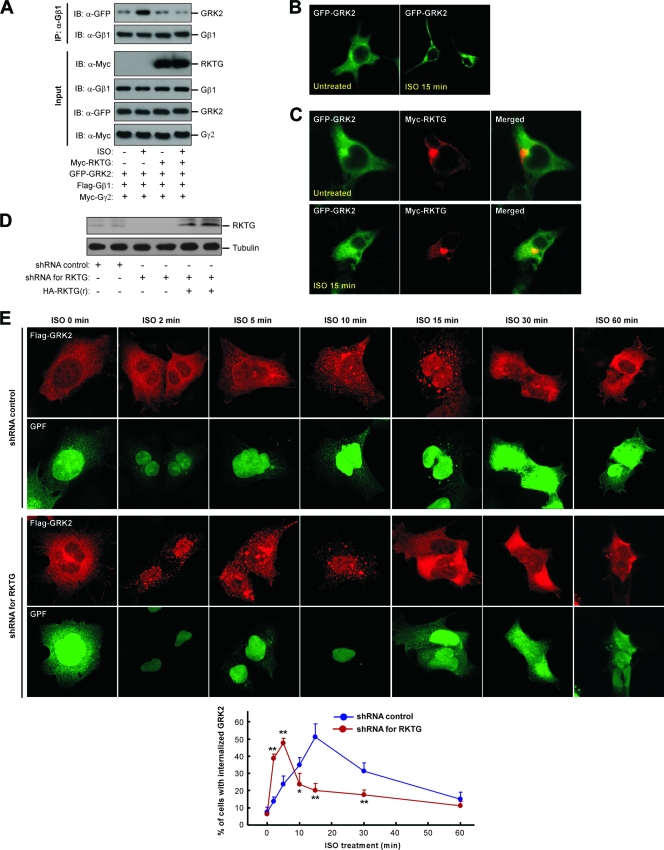
GRK2 belongs to the GPCR kinase family, which can phosphorylate and desensitize agonist-occupied GPCR (4). Gβγ interacts with GRK2 at the C-terminal PH domain (18) and recruits GRK2 to PM. As RKTG interacts with Gβ and traps Gβγ to the Golgi apparatus, we hypothesized that RKTG could disrupt the function of Gβγ on GRK2 regulation. Consistent with previous reports (8), stimulation of β2AR by ISO could enhance the interaction of GRK2 with Gβ1 (Fig. 2A). However, expression of RKTG markedly decreased ISO-stimulated GRK2/Gβ1 interaction (Fig. 2A). In the absence of ISO, GRK2 was diffusely distributed in the cytosol, and ISO could change the subcellular localization pattern of GRK2 to intracellular vesicles as well as to PM (Fig. 2B), consistent with previous observations (24, 28). However, when RKTG was cotransfected, the cytosolic distribution pattern of GRK2 was not altered by ISO stimulation (Fig. 2C), indicating that RKTG could indeed disrupt ligand-mediated GRK2 redistribution within the cell. In contrast, when endogenous RKTG was silenced by a specific shRNA (Fig. 2D), the kinetics of GRK2 internalization was markedly altered (Fig. 2E). In HEK293T cells, ISO treatment could slowly increase the percentage of cells in which GRK2 was localized in intracellular vesicles. The percentage of cells with GRK2 internalization peaked at 15 min upon ISO administration. However, downregulation of endogenous RKTG could markedly accelerate GRK2 internalization. The percentage of cells with GRK2 internalization reached the maximal level of ∼50% at 5 min upon ISO administration and rapidly declined thereafter. As GRK2 colocalizes with β2AR during agonist-induced receptor internalization (28), our data suggest that one of the physiological functions of RKTG is to modulate ligand-induced GPCR internalization through its regulation of Gβγ-mediated recruitment of GRK2 to the PM and the subsequent internalization.
FIG. 2.
RKTG affects GRK2/Gβγ complex formation. (A) Agonist-induced formation of GRK/Gβγ complex was inhibited by RKTG. COS7 cells were transiently transfected with the plasmids as indicated. The cells were serum starved for 12 h and treated with or without 10 μM of ISO for 10 min. The cell lysate was used in immunoprecipitation (IP) and immunoblotting (IB) with the antibodies indicated. (B) Agonist-induced alteration of GRK2 distribution. HEK293T cells were transiently transfected with GFP-fused GRK2 and serum starved for 12 h, followed by treatment with or without 10 μM of ISO for 15 min. The cells were used in confocal analysis. (C) RKTG alters the subcellular distribution pattern of GRK2. HEK293T cells were transiently transfected with plasmids as indicated. The cells were treated with or without ISO, followed by immunostaining and confocal analysis. (D) Analyses of shRNA efficiency for RKTG and an siRNA-resistant RKTG. HEK293T cells were transiently transfected with an FG12 control vector, an FG12 plasmid with RKTG shRNA, or a hemagglutinin (HA)-tagged siRNA-resistant RKTG [HA-RKTG(r)] as indicated. The cell lysate was used in immunoblotting with antibodies for endogenous RKTG or tubulin. (E) Silencing of RKTG affects GRK2 internalization. HEK293T cells were transiently transfected with Flag-tagged GRK2, FG12 (as an shRNA control), or FG12 containing RKTG shRNA as indicated. The cells were serum starved for 12 h and treated with ISO (10 μM) for different lengths of time, followed by confocal analysis. All cells shown here are GFP positive, as the FG12 vector contains EGFP. A diagram depicting the percentage of cells with apparent GRK2 internalization is shown at the bottom. **, P < 0.01; *, P < 0.05 by Student's t test as a comparison between the two groups at the same time point.
RKTG affects ligand-induced β2AR phosphorylation and desensitization.
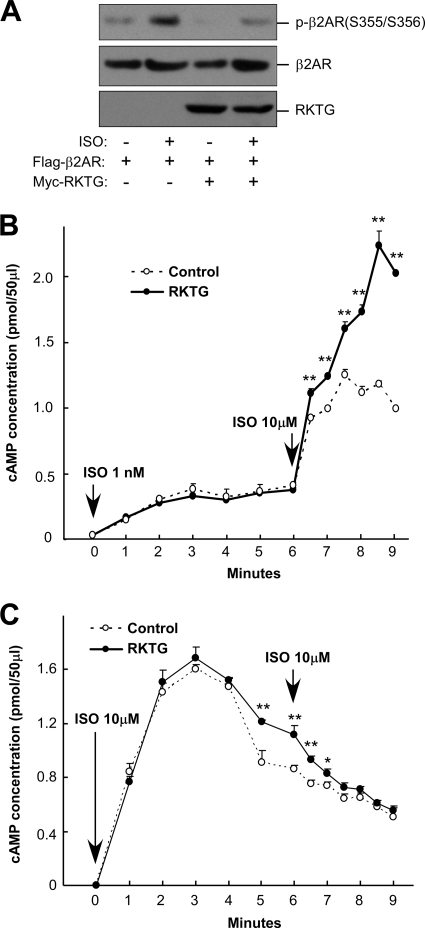
We next examined whether RKTG is implicated in the regulation of ligand-stimulated GPCR phosphorylation, which is mediated by GRK. In HEK293T cells, ISO could clearly stimulate β2AR phosphorylation at Ser355 and Ser356 (Fig. 3A). RKTG overexpression, however, largely compromised β2AR phosphorylation, consistent with our observation that RKTG could alter GRK2/Gβγ complex formation and GRK2 internalization upon ligand stimulation. As the primary consequence of GPCR phosphorylation by GRK is desensitization of GPCR signaling, we investigated desensitization of β2AR in COS7 cells with coexpression of β2AR and GRK2. Desensitization of β2AR signaling was analyzed by pretreatment of the cells with a low dose of ISO, followed by high-dose ISO challenge and cAMP measurement. In the presence of RKTG overexpression, β2AR desensitization was clearly altered, as the second challenge with high-dose ISO led to much greater response than in the cells without RKTG over expression (Fig. 3B). However, the initial response upon low-dose ISO treatment was not altered by RKTG overexpression (Fig. 3B). As a control experiment, we also analyzed cAMP accumulation in COS7 cells challenged twice with a high dose of ISO (Fig. 3C). The initial phase of cAMP rise (up to 3 min upon ISO administration) was not affected by RKTG overexpression, consistent with the notion that the initial response of β2AR upon ligand exposure is mainly mediated by Gαs and RKTG could not change the subcellular localization of Gαs (Fig. 1G). Interestingly, the cAMP accumulation at the down phase (at 5 to 7 min after ISO treatment) was slightly enhanced by RKTG overexpression (Fig. 3C), likely due to a decrease in β2AR phosphorylation (as shown in Fig. 3A) and a subsequent increase in β2AR signaling.
FIG. 3.
RKTG inhibits β2AR phosphorylation and β2AR desensitization. (A) RKTG inhibits agonist-induced β2AR phosphorylation. HEK293T cells were transiently transfected with the plasmids as indicated. The cells were treated with or without 10 μM of ISO for 2 min. The cell lysate was used in immunoblotting to detect phosphorylation of β2AR at Ser355 and Ser356. The expressions of β2AR and RKTG were determined by anti-Flag and anti-Myc antibodies, respectively. (B) RKTG reduces β2AR desensitization. COS7 cells transiently transfected with β2AR and GRK2 were coexpressed with RKTG or control vector. The cells were pretreated with 1 nM of ISO for 6 min, followed by ISO treatment at 10 μM. The accumulation of cAMP was measured (n = 3 for each group). **, P < 0.01 by Student's t test as a comparison between the two groups at the same time point. (C) The effect of RKTG on β2AR signaling with high dose of ISO treatment. COS7 cells were transfected with the plasmids as described for panel B, followed by ISO (10 μM) treatment twice and cAMP determination. **, P < 0.01; *, P < 0.05 by Student's t test as a comparison between the two groups at the same time point.
RKTG interferes with Gβγ-mediated AKT phosphorylation.
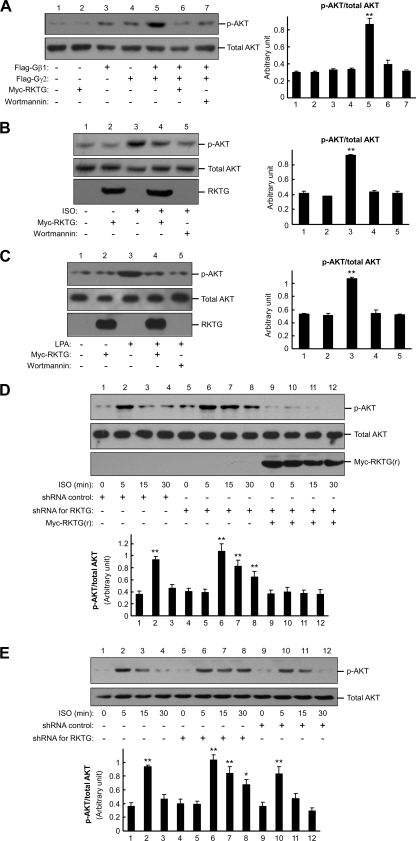
Besides modulating GRK function, Gβγ can also regulate the PI3K-AKT signaling pathway, which is implicated in cell growth and survival (5, 23). As RKTG could modulate the intracellular localization of Gβγ, we hypothesized that RKTG could also alter Gβγ-activated PI3K-AKT signaling. In COS7 cells, Gβ1γ2 overexpression markedly elevated AKT phosphorylation at Ser473, and such stimulation was abrogated by RKTG coexpression (Fig. 4A), indicating that RKTG is able to interfere with Gβγ-mediated activation of AKT. As a control experiment, we found that the Gβγ-stimulated AKT phosphorylation was decreased by wortmannin, a PI3K inhibitor (Fig. 4A). We next analyzed GPCR ligand-stimulated AKT phosphorylation. Consistently, ISO-mediated AKT stimulation was compromised by either RKTG overexpression or wortmannin (Fig. 4B). In addition, LPA, a ligand for a Gi-coupled receptor, could also stimulate AKT phosphorylation and this effect of LPA was inhibited by RKTG or wortmannin (Fig. 4C).
FIG. 4.
RKTG affects Gβγ- and GPCR-mediated AKT phosphorylation. (A) Inhibition of Gβγ-mediated AKT phosphorylation by RKTG. COS7 cells were transiently transfected with the plasmids as indicated, and the cell lysate was used in immunoblotting with antibodies specific for phosphorylated AKT (at Ser473) and total AKT. Wortmannin treatment (100 nM for 1 h) was used as a control. The relative amount of AKT phosphorylation compared with the total AKT level by density analysis is also shown as means ± standard deviations from three independent experiments. Such analysis was performed for all of the following panels based on three independent experiments. **, P < 0.01; *, P < 0.05 by Student's t test compared with the value of the first group in each data set. (B) Inhibition of ISO-induced AKT phosphorylation by RKTG. HEK293T cells were transiently transfected with β2AR and the Myc-tagged RKTG as indicated and then treated with ISO (100 μM) for 10 min, followed by an immunoblotting assay. Wortmannin treatment (100 nM for 1 h) prior to ISO stimulation was used as a control. (C) Inhibition of LPA-induced AKT phosphorylation by RKTG. The same experiment described for panel B was performed using LPA (10 μM) treatment for 10 min. (D) ISO-induced AKT phosphorylation is enhanced by RKTG shRNA. HEK293T cells were transiently transfected with the FG12 vector, FG12 containing RKTG shRNA, and HA-RKTG(s) as indicated, followed by ISO (100 μM) treatment for the times indicated. The cell lysate was used in Western blotting with the antibodies indicated. (E) LPA-induced AKT phosphorylation is enhanced in RKTG-deleted MEFs. MEFs isolated from RKTG wild-type or RKTG-deleted mouse embryos were treated with LPA (10 μM) for different lengths of time, followed by immunoblotting analysis. For a “rescue” experiment, the cells were infected with a retrovirus expressing RKTG. The lower panel depicts the ratio of phosphorylated AKT with total AKT by densitometer analysis.
We next analyzed the effect of downregulation of RKTG on ligand-mediated AKT phosphorylation. We designed a shRNA construct that could silence expression of endogenous RKTG, and such downregulation could be abrogated by expression of an siRNA-resistant RKTG expression plasmid (Fig. 2D). In HEK293T cells, ISO-stimulated AKT phosphorylation reached a maximal level at 5 min in the presence of the shRNA control (Fig. 4D). However, a high level of AKT phosphorylation remained after 30 min of ISO administration when RKTG was silenced (Fig. 4D), indicating that the ISO-stimulated AKT phosphorylation was markedly prolonged when RKTG was downregulated. Furthermore, expression of the siRNA-resistant RKTG could completely abrogate the effect of RKTG shRNA on ISO-mediated AKT phosphorylation (Fig. 4D).
We further examined LPA-mediated AKT phosphorylation in MEFs that had a targeted deletion of RKTG (12, 33). As shown in Fig. 4E, LPA treatment induced a rapid AKT phosphorylation in RKTG+/+ MEFs, reaching a maximal level at 5 min. In the absence of RKTG, LPA-stimulated AKT phosphorylation was markedly extended to at least 30 min upon ligand administration. Overexpression of RKTG via retrovirus in RKTG-deficient MEFs, on the other hand, could shorten the kinetics of AKT phosphorylation upon LPA administration (Fig. 4E). Collectively, these data indicate that RKTG can influence AKT phosphorylation upon GPCR activation.
Mutually exclusive interaction of RKTG with the Gβ subunit and Raf-1.
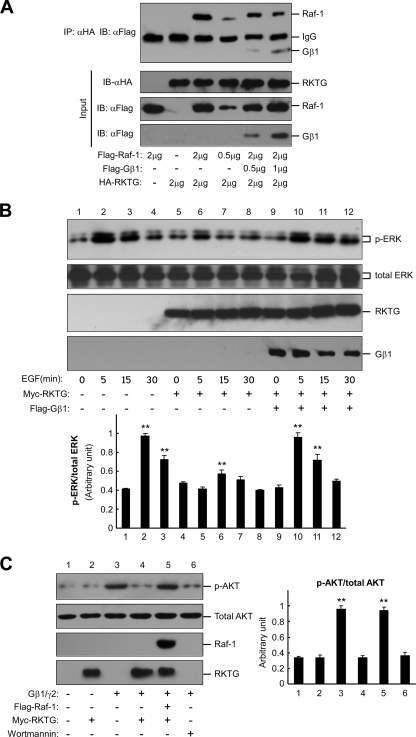
RKTG has previously been shown to interact with Raf-1 and inhibit Ras/Raf/MEK/ERK signaling (12). Based on the yeast two-hybrid experiment, the N-terminal end of RKTG was sufficient for RKTG to interact with the Gβ subunit. On the other hand, both the N terminus and the third cytosolic loop of RKTG are required for the interaction of RKTG with Raf-1 (21). We therefore hypothesized that the interactions of RKTG with the Gβ subunit and with Raf-1 were mutually exclusive. We first addressed this issue by examining whether expression of Gβ1 could reduce the interaction of RKTG with Raf-1. As expected, overexpression of Gβ1 decreased the interaction of RKTG with Raf-1 in a coimmunoprecipitation assay (Fig. 5A). Consistent with our previous report (12), RKTG overexpression reduced EGF-stimulated ERK phosphorylation (Fig. 5B). However, overexpression of Gβ1 could abrogate the inhibitory effect of RKTG on EGF-mediated ERK phosphorylation (Fig. 5B). On the other hand, the inhibitory effect of RKTG on Gβ1γ2-stimulated AKT phosphorylation was abrogated by Raf-1 overexpression (Fig. 5C). Taken together, these data suggest that RKTG interacts with the Gβ subunit or Raf-1 in a mutually exclusive manner.
FIG. 5.
Competitive interaction of Gβ and Raf-1 with RKTG. (A) Overexpression of Gβ1 reduces interaction of RKTG with Raf-1. HEK293T cells were transiently transfected with the plasmids as indicated. Note that different amounts of plasmids were used in the transfection. The cell lysate was used in immunoprecipitation and immunoblotting with the antibodies indicated. (B) Overexpression of Gβ1 abrogates RKTG-mediated inhibition of ERK phosphorylation. HEK293T cells were transiently transfected with the plasmids as indicated and treated with EGF (100 ng/ml) for different lengths of time. The cell lysate was used in immunoblotting with the antibodies indicated. The relative amounts of ERK phosphorylation compared with total ERK by density analysis are also shown with means ± standard deviations from three independent experiments. **, P < 0.01 by Student's t test compared with the value of the first group in each subgroup. (C) Overexpression of Raf-1 abrogates Gβ1γ2-mediated AKT phosphorylation. COS7 cells were transiently transfected with the plasmids as indicated, and the cell lysate was used in immunoblotting with the antibodies indicated. Wortmannin treatment (100 nM for 1 h) was used as a control. The relative amounts of AKT phosphorylation compared with total AKT by density analysis are also shown with means ± standard deviations from three independent experiments. **, P < 0.01 by Student's t test after comparison with the first group.
RKTG competes with GRK2ct to bind Gβ, and the N terminus of RKTG is sufficient for Gβ interaction.
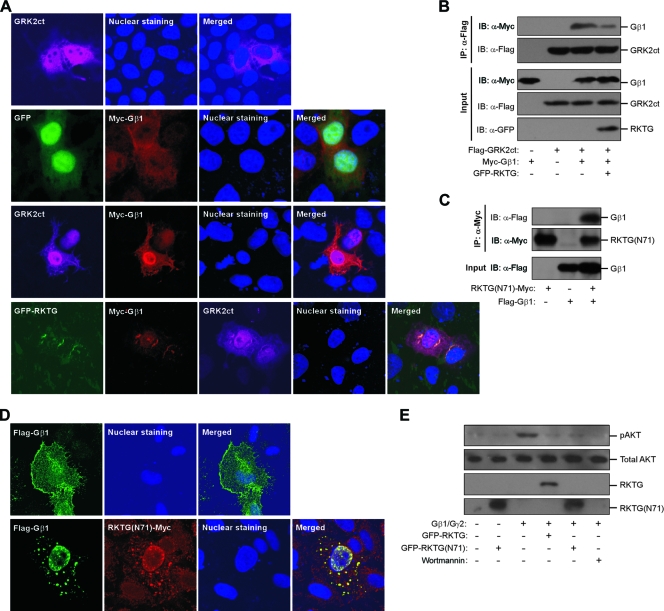
Gβγ interacts with the C-terminal end of GRK2 (GRK2ct), which contains a PH domain (18). The importance of the studies of GRK2ct interaction with Gβγ lies in the fact that GRK2ct binds the Gα/Gβγ “hot spot” interface implicated in Gβγ interaction with downstream effectors, thereby specifically disrupting the signaling of Gβγ but not Gα. Through specific inhibition of Gβγ signaling, overexpression of GRK2ct in the heart increases cardiac performance in response to βAR activation (19). As RKTG also interacts with Gβγ, we analyzed whether RKTG binds Gβγ in a region similar to the binding site of GRK2ct. We first analyzed whether RKTG could alter subcellular localization of Gβγ and GRK2ct in a cell. HeLa cells were transiently transfected with Gβ1 and GRK2ct with or without coexpression of RKTG. Gβ1 was completely colocalized with GRK2ct in the PM, the cytosol, and the nucleus (Fig. 6A). However, when RKTG was overexpressed, the majority of Gβ was translocated to the Golgi apparatus, shown as almost complete colocalization with RKTG, with only residual colocalization with GRK2ct (Fig. 6A), indicating that RKTG could disrupt the interaction of GRK2ct with Gβ. We next confirmed such finding by a coimmunoprecipitation assay. The interaction of Gβ1 with GRK2ct binding was reduced by RKTG (Fig. 6B). These data, therefore, indicate that RKTG could compete with GRK2ct for binding Gβ. Furthermore, they also suggest that RKTG binds Gβ at a region similar to that of GRK2ct, thereby masking the “hot spot” interface of Gβγ and subsequently abrogating Gβγ-mediated downstream signaling but not disrupting Gα-mediated downstream functions.
FIG. 6.
RKTG competes with GRK2ct for interaction with Gβ, and the N terminus of RKTG is sufficient for Gβ interaction. (A) RKTG disrupts colocalization of Gβ1 with GRK2ct. HeLa cells were transiently transfected with the plasmids as indicated, followed by immunostaining and confocal analysis. The cell nuclei were stained with Hoechst 33342. Note that the merged image shows a significant overlapping of Gβ1 with GRK2ct in the absence of RKTG. RKTG overexpression could sequester a majority of Gβ1 to the Golgi apparatus. (B) RKTG inhibits interaction of Gβ1 with GRK2ct. HEK293T cells were transiently transfected with the plasmid as indicated. The cell lysate was used in immunoblotting (IB) and immunoprecipitation (IP) with the antibodies indicated. (C) Interaction of RKTG(N71) with Gβ1. HEK293T cells were transiently transfected with the plasmid as indicated, and a coimmunoprecipitation assay was performed as described for panel B. (D) Colocalization of the N-terminal 71 aa of RKTG [RKTG(N71)] with Gβ1. HeLa cells were transiently transfected with the plasmids as indicated, followed by immunostaining and confocal analysis. (E) Inhibition of Gβγ-mediated AKT phosphorylation by RKTG(N71). COS7 cells were transiently transfected with the plasmids as indicated, and the cell lysate was used in immunoblotting. Wortmannin treatment (100 nM for 1 h) was used as a control.
In addition, we analyzed whether the N-terminal 71 aa (N71) located before the first transmembrane domain of RKTG is sufficient for the interaction with Gβ1. By a coimmunoprecipitation assay, we found that Gβ1 was able to interact with RKTG(N71) (Fig. 6C), consistent with the result of yeast two-hybrid screening in which the N terminus of RKTG was used as the bait. When Gβ1 was expressed alone, it was mainly localized in the cytoplasm and on the PM (Fig. 6D). However, when Gβ1 and RKTG(N71) were coexpressed, both proteins had almost complete colocalization in intracellular vesicular structures (Fig. 6D). Furthermore, RKTG(N71) could reduce AKT phosphorylation stimulated by overexpression of Gβ1γ2, similar to the full-length RKTG (Fig. 6E). These data, therefore, indicate that the N-terminal end of RKTG is sufficient for the interaction of RKTG with Gβ1, similar to the C-terminal end of GRK2 for the interaction of GRK2 with the Gβγ subunit.
RKTG affects the ISO-induced Gβ translocation to the Golgi apparatus.
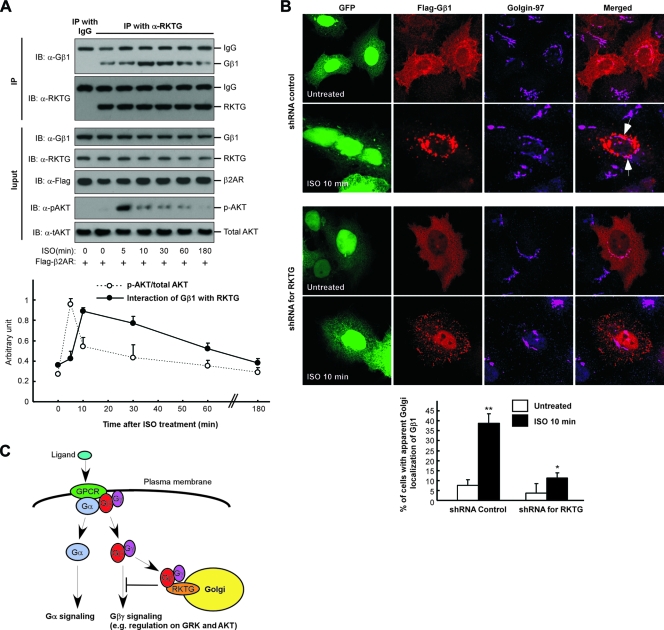
We next investigated the kinetics of RKTG/Gβ interaction and AKT phosphorylation simultaneously upon GPCR stimulation. As shown in Fig. 7A, the interaction of endogenous RKTG with endogenous Gβ was enhanced by ISO treatment, reaching a maximal level at 10 min upon ligand administration and then gradually declining to the basal level. Meanwhile, ISO-stimulated AKT phosphorylation reached a maximal level at 5 min and then rapidly decreased thereafter (Fig. 7A). Therefore, the rapid reduction of AKT phosphorylation at 10 min after ISO stimulation was coincident with the maximal RKTG/Gβ interaction at this time point, consistent with our observations that RKTG overexpression could reduce AKT phosphorylation (Fig. 4A, B, and C) and downregulation of RKTG could prolong ligand-induced AKT phosphorylation (Fig. 4D and E). Collectively, these data provided further evidence that the interaction of RKTG with Gβ is implicated in Gβγ-mediated regulation on AKT phosphorylation.
FIG. 7.
RKTG is implicated in ISO-induced Gβ1 translocation to the Golgi apparatus. (A) Studies of the kinetics of ISO-induced RKTG/Gβ1 interaction and AKT activation. HEK293T cells transfected with β2AR were cultured in serum-starved medium for 6 h and then treated with 10 μM of isoproterenol (ISO) or left untreated for various lengths of time. Total cell lysates were subjected to immunoblotting and immunoprecipitation using the antibodies indicated. Rabbit IgG was used as a negative control. The results of density analysis with immunoblotting are shown in the lower panel with means ± standard deviations from three independent experiments. (B) Silencing of RKTG reduces ISO-induced Gβ1 translocation to the Golgi apparatus. HeLa cells were transiently transfected with HA-tagged β2AR, Flag-tagged Gβ1, FG12 (as an shRNA control), or FG12 containing RKTG shRNA as indicated. The cells were serum starved for 12 h, followed by treatment with ISO (10 μM) or no treatment for 10 min and confocal analysis. All cells shown here are GFP positive, as the FG12 vector contains EGFP. The arrows indicate representative colocalization of Gβ1 with RKTG at the Golgi apparatus. A diagram representing the percentage of cells with apparent Golgi localization of Gβ1 is shown in the bottom panel. **, P < 0.01; *, P < 0.05 by Student's t test compared with the group without ISO treatment. (C) Diagram that depicts the model of RKTG regulation on Gβγ signaling. Through interacting with Gβ and tethering Gβγ to the Golgi apparatus, RKTG attenuates the signaling of Gβγ to downstream targets but has no effect on Gα-mediated specific signaling.
It has previously been reported that the Gβγ subunit is translocated from the PM to the Golgi apparatus upon GPCR activation (1, 2), although the underlying mechanism has been elusive. We hypothesized that RKTG was likely involved in ligand-induced Golgi translocation of the Gβγ subunit under physiological conditions. In HeLa cells transfected with β2AR and an shRNA control plasmid, ISO treatment for 10 min (at which there was a maximal interaction of RKTG with Gβ1 as shown in Fig. 7A) could markedly increase Golgi localization of Gβ1 (Fig. 7B), with the percentage of cells with Golgi apparatus-localized Gβ1 increasing from ∼7% at 0 min to ∼38% at 10 min. However, when endogenous RKTG was silenced by a specific shRNA, the percentage of cells with Golgi apparatus-localized Gβ1 was increased only from ∼4% at 0 min to ∼11% at 10 min. These data, collectively, indicate that one of the physiological functions of RKTG is to mediate translocation of the Gβγ subunit to the Golgi apparatus upon GPCR ligand stimulation, thereby attenuating the cellular activities of the Gβγ subunit such as its regulation on GRK2 and AKT (Fig. 7C).
DISCUSSION
Our studies reveal for the first time that Gβγ signaling is regulated in a spatial manner by alteration in subcellular compartmentation by the interaction of the Gβ subunit with RKTG. RKTG, a Golgi apparatus-specific membrane protein, can spatially segregate Gβγ-mediated signaling by interacting with Gβ and sequestering Gβγ into the Golgi apparatus, thereby interfering with the binding of Gβγ to downstream target proteins and Gβγ-mediated signaling (Fig. 6F). Two Gβγ signaling events were analyzed in this study, i.e., Gβγ-mediated regulations on GRK2 and AKT. In the study with GRK2, overexpression of RKTG could disrupt the interaction of the Gβγ subunit with GRK2, abrogate ISO-induced subcellular redistribution of GRK2, reduce ISO-stimulated β2AR phosphorylation, and subsequently decrease desensitization of β2AR. On the other hand, silencing of RKTG could accelerate GRK2 internalization upon ligand stimulation. In the study with the PI3K-AKT signaling pathway, RKTG could inhibit Gβγ- and ligand-mediated AKT phosphorylation. In contrast, the ligand-stimulated AKT activation is enhanced in cells with downregulation or deletion of RKTG. Through the studies with GRK2ct, we propose that RKTG, through its N terminus, may bind Gβ at a region or “hot spots” that are required for the interaction of Gβγ with downstream targets. Taken together, our results reveal that RKTG can negatively modulate Gβγ functions by both sequestering cytosolic Gβγ to the Golgi apparatus and blocking the interaction of Gβγ with downstream target proteins, likely through masking the effector binding site on Gβγ.
Our kinetic studies of the interaction of endogenous RKTG with Gβ1, AKT phosphorylation, and Golgi translocation of Gβ1 upon ligand stimulation indicate that RKTG is able to regulate the functions of the Gβγ subunit in a well-controlled temporal and spatial manner (Fig. 7C). We postulate that upon ligand stimulation, the Gα subunit and the Gβγ subunit are released from the heterotrimeric complex and impart downstream signaling by interaction with their individual effectors. Shortly afterward, a portion of the released Gβγ subunit is tethered to the Golgi apparatus by RKTG through its interaction with Gβ. Such an interaction is able to attenuate the specific signaling of Gβγ via abrogating the interaction of Gβγ with its downstream effectors such as GRK and PI3K. In other words, RKTG may function as a switch to shut down the signaling of Gβγ through spatial regulation. On the other hand, we also postulate that there exists a competition between the interaction of Gβγ with Gα and that with RKTG. The interaction of endogenous Gβ1 with RKTG upon ISO stimulation was reduced to the basal level by 3 h after the treatment (Fig. 7A). After GPCR signaling, the GTP-bound Gα subunit slowly returns to the basal state and the GDP-bound form Gα has a high affinity for Gβγ, thereby recruiting the cytoplasmic and Golgi apparatus-localized Gβγ subunit back into the PM.
Gβγ subunits are membrane-bound proteins through lipid modification at the C terminus of Gγ (30). The signaling of Gβγ is predominantly restricted to the PM, via actions such as direct activation of proteins on the PM and recruitment of cytosolic proteins to the PM. Intracellular trafficking of Gβγ has been observed during the synthesis of Gβγ and after GPCR activation. Gβ and Gγ are first synthesized in the cytoplasm, and upon lipid modification the Gβγ dimer is targeted to the endoplasmic reticulum (ER). With the help of Gα, Gβγ is then translocated from the ER to the PM (22). On the other hand, activation of GPCR could lead to translocation of Gβγ to intracellular membranes such as the Golgi apparatus, ER, and endosomes (1, 3, 29). However, whether the Gβγ subunits at the intracellular membrane compartment impart a subcellular signaling event remains unclear. It is worth noting that Gβγ can be selectively translocated to the Golgi apparatus upon GPCR activation, depending on the type of Gγ, and that Gβγ can traffic back to PM after receptor inactivation (2, 29). Our findings suggest that one of the physiological functions of RKTG is to modulate Golgi translocation of Gβγ upon GPCR activation, shown as an increased interaction of Gβ with RKTG upon ligand stimulation (Fig. 1B and Fig. 7A) and a decrease of ligand-induced Golgi translocation of Gβ1 when RKTG is silenced (Fig. 7B). In addition, it can be speculated that the specific Gβγ signaling is modulated by the relative level of RKTG within a particular cell. The expression level of RKTG within a specific cell could set a tone to modulate the magnitude of Gβγ-mediated signaling. A cell with a high level of RKTG expression can compromise Gβγ-mediated signaling through sequestration of Gβγ at the Golgi apparatus. By the same token, it can be envisioned that changes of RKTG expression level under either physiological or pathophysiological conditions should have an impact on specific signaling by Gβγ.
In addition to its spatial regulation of Gβγ, RKTG has been shown to sequester Raf kinase to the Golgi apparatus and consequently attenuate the signaling from Ras to ERK (11, 12). The negative regulation of RKTG on the Ras-ERK signaling pathway indicates that RKTG has a “tumor suppressor” activity, and such speculation is supported by the finding that deletion of RKTG could accelerate skin cancer formation upon carcinogen exposure (33). Interestingly, ERK and AKT signaling are two of the most important pathways involved in cell proliferation and cell survival and play major roles in tumorigenesis. RKTG blocks growth factor-mediated ERK signaling by sequestering Raf kinase and blocks GPCR-mediated AKT signaling via sequestering Gβγ. The net outcome of blocking both ERK and AKT pathways by RKTG would have a synchronized effect on cell proliferation and survival. In other words, the observed tumor-suppressive activity of RKTG in the skin cancer model could result from the regulation of RKTG on more than one signaling pathway. Considering that RKTG can sequester both Raf kinase and Gβγ to the Golgi apparatus, it is likely that RKTG may serve as a “Golgi trap” that modulates a series of signaling proteins through alteration in subcellular compartmentation.
Gβγ has been a target for therapeutic development (30). One of the seminal findings is that cardiac overexpression of GRK2ct, which blocks only Gβγ signaling but not Gα signaling, could increase cardiac performance in response to βAR stimulation (19). One characteristic of heart failure is a loss of βAR-mediated cardiac reserve. Overexpression of GRK2ct, through its interaction with Gβγ, can abrogate the activation of Gβγ downstream targets, including GRK2-mediated βAR phosphorylation and desensitization, thereby enhancing βAR actions. Recently, it was found that deletion of PI3Kγ could protect ApoE−/− mice from development of atherosclerosis, potentially through disabling macrophage migration and inflammatory functions (6). As PI3Kγ is also a downstream target of Gβγ, it can be envisioned that specific inhibition of Gβγ function may attenuate the development of inflammatory diseases, including atherosclerosis. Interestingly, RKTG can abrogate Gβγ functions, similar to the action of GRK2ct. Under such a consideration, upregulation of RKTG may have a beneficial effect by partly mimicking a Gβγ inhibitor when reduction of Gβγ function is beneficial in certain circumstances. However, this notion needs vigorous examination using different models and systems to further elucidate the biological and physiological functions of RKTG.
Acknowledgments
We thank Marc G. Caron at Duke University Medical Center for providing the plasmid.
This work was supported by research grants from the Chinese Academy of Sciences (Knowledge Innovation Program KSCX1-YW-02), the National Natural Science Foundation of China (30588002 and 30830037), the Science & Technology Commission of Shanghai Municipality (75407001), and the Ministry of Science and Technology of China (2007CB947100 and 2006CB943900) to Y.C.
Footnotes
Published ahead of print on 2 November 2009.
REFERENCES
- 1.Akgoz, M., V. Kalyanaraman, and N. Gautam. 2006. G protein betagamma complex translocation from plasma membrane to Golgi complex is influenced by receptor gamma subunit interaction. Cell Signal. 18:1758-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akgoz, M., V. Kalyanaraman, and N. Gautam. 2004. Receptor-mediated reversible translocation of the G protein betagamma complex from the plasma membrane to the Golgi complex. J. Biol. Chem. 279:51541-51544. [DOI] [PubMed] [Google Scholar]
- 3.Azpiazu, I., M. Akgoz, V. Kalyanaraman, and N. Gautam. 2006. G protein betagamma11 complex translocation is induced by Gi, Gq and Gs coupling receptors and is regulated by the alpha subunit type. Cell Signal. 18:1190-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benovic, J. L., R. H. Strasser, M. G. Caron, and R. J. Lefkowitz. 1986. Beta-adrenergic receptor kinase: identification of a novel protein kinase that phosphorylates the agonist-occupied form of the receptor. Proc. Natl. Acad. Sci. U. S. A. 83:2797-2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bommakanti, R. K., S. Vinayak, and W. F. Simonds. 2000. Dual regulation of Akt/protein kinase B by heterotrimeric G protein subunits. J. Biol. Chem. 275:38870-38876. [DOI] [PubMed] [Google Scholar]
- 6.Chang, J. D., G. K. Sukhova, P. Libby, E. Schvartz, A. H. Lichtenstein, S. J. Field, C. Kennedy, S. Madhavarapu, J. Luo, D. Wu, and L. C. Cantley. 2007. Deletion of the phosphoinositide 3-kinase p110gamma gene attenuates murine atherosclerosis. Proc. Natl. Acad. Sci. U. S. A. 104:8077-8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, S., E. J. Dell, F. Lin, J. Sai, and H. E. Hamm. 2004. RACK1 regulates specific functions of Gbetagamma. J. Biol. Chem. 279:17861-17868. [DOI] [PubMed] [Google Scholar]
- 8.Daaka, Y., J. A. Pitcher, M. Richardson, R. H. Stoffel, J. D. Robishaw, and R. J. Lefkowitz. 1997. Receptor and G betagamma isoform-specific interactions with G protein-coupled receptor kinases. Proc. Natl. Acad. Sci. U. S. A. 94:2180-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DebBurman, S. K., J. Ptasienski, J. L. Benovic, and M. M. Hosey. 1996. G protein-coupled receptor kinase GRK2 is a phospholipid-dependent enzyme that can be conditionally activated by G protein betagamma subunits. J. Biol. Chem. 271:22552-22562. [DOI] [PubMed] [Google Scholar]
- 10.Dingus, J., C. A. Wells, L. Campbell, J. H. Cleator, K. Robinson, and J. D. Hildebrandt. 2005. G Protein betagamma dimer formation: Gbeta and Ggamma differentially determine efficiency of in vitro dimer formation. Biochemistry 44:11882-11890. [DOI] [PubMed] [Google Scholar]
- 11.Fan, F., L. Feng, J. He, X. Wang, X. Jiang, Y. Zhang, Z. Wang, and Y. Chen. 2008. RKTG sequesters B-Raf to the Golgi apparatus and inhibits the proliferation and tumorigenicity of human malignant melanoma cells. Carcinogenesis 29:1157-1163. [DOI] [PubMed] [Google Scholar]
- 12.Feng, L., X. Xie, Q. Ding, X. Luo, J. He, F. Fan, W. Liu, Z. Wang, and Y. Chen. 2007. Spatial regulation of Raf kinase signaling by RKTG. Proc. Natl. Acad. Sci. U. S. A. 104:14348-14353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Regalado, A., M. L. Guzman-Hernandez, I. Ramirez-Rangel, E. Robles-Molina, T. Balla, J. Vazquez-Prado, and G. Reyes-Cruz. 2008. G protein-coupled receptor-promoted trafficking of Gbeta1gamma2 leads to AKT activation at endosomes via a mechanism mediated by Gbeta1gamma2-Rab11a interaction. Mol. Biol. Cell 19:4188-4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guillermet-Guibert, J., K. Bjorklof, A. Salpekar, C. Gonella, F. Ramadani, A. Bilancio, S. Meek, A. J. Smith, K. Okkenhaug, and B. Vanhaesebroeck. 2008. The p110beta isoform of phosphoinositide 3-kinase signals downstream of G protein-coupled receptors and is functionally redundant with p110gamma. Proc. Natl. Acad. Sci. U. S. A. 105:8292-8297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haga, K., and T. Haga. 1992. Activation by G protein beta gamma subunits of agonist- or light-dependent phosphorylation of muscarinic acetylcholine receptors and rhodopsin. J. Biol. Chem. 267:2222-2227. [PubMed] [Google Scholar]
- 16.Hamm, H. E., and A. Gilchrist. 1996. Heterotrimeric G proteins. Curr. Opin. Cell Biol. 8:189-196. [DOI] [PubMed] [Google Scholar]
- 17.Jia, S., Z. Liu, S. Zhang, P. Liu, L. Zhang, S. H. Lee, J. Zhang, S. Signoretti, M. Loda, T. M. Roberts, and J. J. Zhao. 2008. Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis. Nature 454:776-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koch, W. J., J. Inglese, W. C. Stone, and R. J. Lefkowitz. 1993. The binding site for the beta gamma subunits of heterotrimeric G proteins on the beta-adrenergic receptor kinase. J. Biol. Chem. 268:8256-8260. [PubMed] [Google Scholar]
- 19.Koch, W. J., H. A. Rockman, P. Samama, R. A. Hamilton, R. A. Bond, C. A. Milano, and R. J. Lefkowitz. 1995. Cardiac function in mice overexpressing the beta-adrenergic receptor kinase or a beta ARK inhibitor. Science 268:1350-1353. [DOI] [PubMed] [Google Scholar]
- 20.Lodowski, D. T., J. A. Pitcher, W. D. Capel, R. J. Lefkowitz, and J. J. Tesmer. 2003. Keeping G proteins at bay: a complex between G protein-coupled receptor kinase 2 and Gbetagamma. Science 300:1256-1262. [DOI] [PubMed] [Google Scholar]
- 21.Luo, X., L. Feng, X. Jiang, F. Xiao, Z. Wang, G. S. Feng, and Y. Chen. 2008. Characterization of the topology and functional domains of RKTG. Biochem. J. 414:399-406. [DOI] [PubMed] [Google Scholar]
- 22.Marrari, Y., M. Crouthamel, R. Irannejad, and P. B. Wedegaertner. 2007. Assembly and trafficking of heterotrimeric G proteins. Biochemistry 46:7665-7677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murga, C., S. Fukuhara, and J. S. Gutkind. 2000. A novel role for phosphatidylinositol 3-kinase beta in signaling from G protein-coupled receptors to Akt. J. Biol. Chem. 275:12069-12073. [DOI] [PubMed] [Google Scholar]
- 24.Murga, C., A. Ruiz-Gomez, I. Garcia-Higuera, C. M. Kim, J. L. Benovic, and F. Mayor, Jr. 1996. High affinity binding of beta-adrenergic receptor kinase to microsomal membranes. Modulation of the activity of bound kinase by heterotrimeric G protein activation. J. Biol. Chem. 271:985-994. [DOI] [PubMed] [Google Scholar]
- 25.Neer, E. J. 1995. Heterotrimeric G proteins: organizers of transmembrane signals. Cell 80:249-257. [DOI] [PubMed] [Google Scholar]
- 26.Pitcher, J. A., J. Inglese, J. B. Higgins, J. L. Arriza, P. J. Casey, C. Kim, J. L. Benovic, M. M. Kwatra, M. G. Caron, and R. J. Lefkowitz. 1992. Role of beta gamma subunits of G proteins in targeting the beta-adrenergic receptor kinase to membrane-bound receptors. Science 257:1264-1267. [DOI] [PubMed] [Google Scholar]
- 27.Qin, X. F., D. S. An, I. S. Chen, and D. Baltimore. 2003. Inhibiting HIV-1 infection in human T cells by lentiviral-mediated delivery of small interfering RNA against CCR5. Proc. Natl. Acad. Sci. U. S. A. 100:183-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruiz-Gomez, A., and F. Mayor, Jr. 1997. Beta-adrenergic receptor kinase (GRK2) colocalizes with beta-adrenergic receptors during agonist-induced receptor internalization. J. Biol. Chem. 272:9601-9604. [DOI] [PubMed] [Google Scholar]
- 29.Saini, D. K., V. Kalyanaraman, M. Chisari, and N. Gautam. 2007. A family of G protein betagamma subunits translocate reversibly from the plasma membrane to endomembranes on receptor activation. J. Biol. Chem. 282:24099-24108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smrcka, A. V. 2008. G protein betagamma subunits: central mediators of G protein-coupled receptor signaling. Cell Mol. Life Sci. 65:2191-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang, Y. T., T. Hu, M. Arterburn, B. Boyle, J. M. Bright, P. C. Emtage, and W. D. Funk. 2005. PAQR proteins: a novel membrane receptor family defined by an ancient 7-transmembrane pass motif. J. Mol. Evol. 61:372-380. [DOI] [PubMed] [Google Scholar]
- 32.Tesmer, V. M., T. Kawano, A. Shankaranarayanan, T. Kozasa, and J. J. Tesmer. 2005. Snapshot of activated G proteins at the membrane: the Galphaq-GRK2-Gbetagamma complex. Science 310:1686-1690. [DOI] [PubMed] [Google Scholar]
- 33.Xie, X., Y. Zhang, Y. Jiang, W. Liu, H. Ma, Z. Wang, and Y. Chen. 2008. Suppressive function of RKTG on chemical carcinogen-induced skin carcinogenesis in mouse. Carcinogenesis. 29:1632-1638. [DOI] [PubMed] [Google Scholar]
- 34.Yamauchi, J., Y. Kaziro, and H. Itoh. 1997. C-terminal mutation of G protein beta subunit affects differentially extracellular signal-regulated kinase and c-Jun N-terminal kinase pathways in human embryonal kidney 293 cells. J. Biol. Chem. 272:7602-7607. [DOI] [PubMed] [Google Scholar]