Abstract
Maintenance of genomic stability is needed for cells to survive many rounds of division throughout their lifetime. Key to the proper inheritance of intact genome is the tight temporal and spatial coordination of cell cycle events. Moreover, checkpoints are present that function to monitor the proper execution of cell cycle processes. For instance, the DNA damage and spindle assembly checkpoints ensure genomic integrity by delaying cell cycle progression in the presence of DNA or spindle damage, respectively. A checkpoint that has recently been gaining attention is the antephase checkpoint that acts to prevent cells from entering mitosis in response to a range of stress agents. We review here what is known about the pathway that monitors the status of the cells at the brink of entry into mitosis when cells are exposed to insults that threaten the proper inheritance of chromosomes. We highlight issues which are unresolved in terms of our understanding of the antephase checkpoint and provide some perspectives on what lies ahead in the understanding of how the checkpoint functions.
Segregation of sister chromosomes during the metaphase-to-anaphase transition is a dramatic event that results in the inheritance of a complete set of chromosomes by each daughter cell undergoing cell division. This process, which occurs during mitosis, requires the temporal and spatial coordination of a myriad of proteins. As many excellent reviews on the process of chromosome segregation have been published (9, 37, 84, 97, 136), we give here an overview of the process.
In essence, duplicated chromosomes are condensed and then lined up at the metaphase plate, where the sister chromatids are subsequently pulled apart by microtubules attached to the kinetochores. The duplicated chromosomes are condensed by condensin I and II complexes that function to pack interphase chromatin so that it can then be neatly divided into daughter cells (6, 48, 50) (see below). Yet other protein complexes essential for ensuring genomic integrity during nuclear separation are the cohesins which maintain cohesion between sister chromatids (17, 85). The cohesins are loaded onto the duplicated chromosomes toward the end of mitosis in the preceding round of cell division or in late G1/early S phase in the new round of cell division (9, 90, 111, 130). The presence of the cohesins helps keep the sister chromatids together until the kinetochores are correctly attached to spindle microtubules emanating from both microtubule-organizing centers (i.e., the spindle pole bodies in Saccharomyces cerevisiae or the centrosomes in higher eukaryotes) in a process known as bi-orientation (122). Upon proper attachment of the mitotic spindles to the kinetochores, the sister chromatids separate as cohesins are destroyed through proteolysis by separase, a CD clan protease (129). Chromosome separation occurs as the spindle microtubules pull the chromosomes toward opposite ends of the dividing cells. This process of chromosome segregation is highly complex and requires tight regulation in order that genomic stability is maintained over successive rounds of cell division (1).
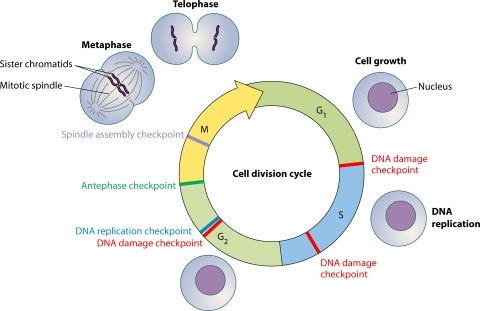
In addition to the tight coordination of events during chromosome segregation, the genomic integrity of dividing cells is kept in check by the presence of checkpoints (Fig. 1) that are needed to prevent the propagation of transformed cells (44). In mitosis, the spindle assembly checkpoint pathway plays a critical role in the surveillance of spindle integrity and elicits a delay in the metaphase-to-anaphase transition in the presence of spindle damage (83). The requirement for an intact spindle assembly checkpoint to maintain genomic integrity as cells undergo division is underscored by the correlations between mutations in the spindle assembly checkpoint genes and chromosome instability (15, 16, 72). Key players at the spindle assembly checkpoint include MAD2 and BUB1 (83).
FIG. 1.
Cell cycle checkpoint pathways impinging upon the cell division cycle. The cell division cycle is monitored throughout by various checkpoints, including the DNA replication (blue box) and DNA damage (red box) checkpoints, as well as the spindle assembly checkpoint (gray box). In addition, the antephase checkpoint (green box) plays an important role in preventing mitotic entry in the presence of various stress conditions (see text).
Of late, interest has been gathering around a checkpoint that is presumably present in antephase and delays entry into mitosis. This checkpoint, named the “antephase checkpoint” by Matsusaka and Pines (71), is distinct from the G2 checkpoints, which are activated in response to DNA damage (4, 5) and unreplicated DNA (100, 101). Also, a decatenation checkpoint that monitors the status of chromosome decatenation by topoisomerase II appears to act in a manner distinct from that of the antephase checkpoint (24). The antephase checkpoint has been proposed to function in response to a range of stress agents to delay entry into mitosis (97).
In this review, we highlight the initial experiments which led to the idea of the existence of an antephase checkpoint which functions to prevent chromosome condensation, thereby safeguarding entry into mitosis in the presence of perturbations as cells prepare for chromosome condensation and segregation. We also review the players implicated in the checkpoint, such as CHFR (checkpoint with FHA and RING domains) (109) and p38 stress kinase (66), and discuss their roles in modulating the antephase checkpoint and the correlations between mutations or alterations in these genes with tumor formation. Lastly, we look at how the antephase checkpoint is likely to function, based on the current understanding of entry into mitosis and chromosome condensation.
SAFEGUARDING ENTRY INTO MITOSIS
The term “antephase” refers to the time in late G2 phase when signs of chromosome condensation first become visible until commitment to mitosis (13, 74, 96). More significantly, cells in antephase appear able to reversibly delay mitotic entry when exposed to certain stress conditions. Initial observations made with grasshopper neuroblasts at early or mid-prophase (as judged by visual observation of faint threads of chromosomes using light microscopy) exposed to X-ray irradiation showed a decrease in prophase cells with time (19). Avian and mammalian cells similarly exposed in early prophase also showed a reduction in the number of cells progressing into mitosis (18). One possible interpretation of these observations was that the irradiated cells proceeded into mitosis but were subsequently blocked at the next interphase. Alternatively, the irradiation treatment could have resulted in the cells delaying entry into mitosis and reverting to an earlier phase of the cell division cycle, thereby showing a net decrease in the number of mitotic cells. By careful observations of living neuroblasts using phase-contrast microscopy, Carlson noted that in fact the irradiated cells did revert from prophase to an earlier stage where the chromosomes were less defined or appeared more diffused (18, 19). This reversion was not permanent, as cells were subsequently able to resume progression into mitosis.
Using neuroblasts, it was further observed that the treatment of early or mid-prophase cells with colchicine led to a slowing down of entry into mitosis and, in some cases, a reversion of the prophase stage (79). This effect of microtubule poison was also seen when nontumorigenic human cells were used, whereas the passage of tumorigenic cells through cell division appeared unperturbed by the treatment (58). Another report showed that prophase rat kangaroo Ptk1 cells exposed to microtubule poisons decondensed their chromosomes and returned to G2 (104). These cells were subsequently able to reenter mitosis, indicating that the decondensation of chromosomes and delayed entry into mitosis were reversible. Yet other studies showed that cells treated with other agents of stress, such as low temperatures (102) or chromosome damage (105), resulted in chromosome decondensation and delayed entry into mitosis.
The finding that cells are capable of reversibly delaying mitotic entry when exposed to stress conditions in early prophase supports the idea that a checkpoint mechanism exists in normal cells to prevent passage into mitosis in the presence of stress agents or a suboptimal condition. However, the window for decondensation and delayed entry into mitosis appears to close before prometaphase, as cells are no longer able to respond to mitotic stresses after prometaphase (18, 19, 58, 79, 105). Presumably, cells are unable to revert once they progress past a “point of no return,” when they commit themselves to mitosis (see below).
KEY PLAYERS
While much is known about the components that function at the classical checkpoints, it remains unresolved what the players and pathways are that are involved in the antephase checkpoint. However, some headway has been made in the identification of components that may play a part in the antephase checkpoint. Below, we highlight the recent findings on the main components implicated in the antephase checkpoint.
CHFR IN THE ANTEPHASE CHECKPOINT
CHFR, a key player recently shown (71) to function in the antephase checkpoint, was initially identified from a database search for expressed sequence tags with forkhead-associated (FHA) domains (109). CHFR also carries a RING finger domain at its N terminus and a cysteine-rich region at its C terminus (109) and is ubiquitously expressed in normal human tissues (109). Orthologues in other model systems, including mice and fission and budding yeasts, have been identified from sequence comparisons. These orthologues have been found to function during mitosis. For instance, in the fission yeast, an orthologue of CHFR, Dma1p, has been shown to play a role in regulating mitotic events such as spindle assembly and septum formation (39, 80). In the budding yeast, Dma1p and Dma2p have been linked to the positioning of mitotic spindles (31).
Early studies on CHFR implicated it in delaying mitotic entry in cells exposed to various stress agents, although no clear connection of its function to the antephase checkpoint was made. It was observed initially in tumor cell lines that express nonfunctional CHFR accumulated a high mitotic index (defined as the percentage of cells with condensed chromosomes) when exposed to nocodazole while wild-type cells treated similarly showed a low mitotic index (109). The finding that normal but not tumor cells progressed more slowly through mitosis when treated with a microtubule poison is reminiscent of previous observations described above (58, 103). In addition, transfection of wild-type CHFR into CHFR-null DLD1 cells or U2OS cells carrying inactive CHFR restored the cells' ability to delay mitotic entry when these cells were exposed to microtubule poisons (109), indicating that CHFR is needed for response to mitotic stress.
Interestingly, the CHFR-dependent delay in mitotic entry could only be detected in cells treated with mitotic stress 12 h after release from a G1/S block but not if the stress was applied at 14 h after G1/S (109), which suggested that there is a period during the progression from S to mitosis in which a mitotic stress could activate a pathway that would prolong the time taken to pass into mitosis. This was again similar to the observations made in the original studies on reversion of chromosome condensation (18, 19, 58, 79, 105). It should be noted that Scolnick and Halazonetis (109) did not link CHFR to the antephase checkpoint but rather referred to it as part of a mitotic stress checkpoint that delays entry into metaphase. In subsequent studies on CHFR, however, Halazonetis and colleagues acknowledged that the delay caused by such a mitotic stress checkpoint in early prophase shares similarities with the delay caused by the antephase checkpoint and that these checkpoints may be one and the same (116).
In another study (59), it was found that the presence of CHFR in Xenopus extracts abolished the ability of a nondestructible form of cyclin B to promote mitotic entry, further implicating CHFR in the negative regulation of entry into mitosis. Also, in human cell lines expressing CHFR, exposure to mitotic stress agents such as taxol gave rise to a delay in mitotic progression (20). The function of CHFR at the mitotic stress checkpoint appears to be important for cells, as the presence of CHFR in cells gave rise to better viability when they were exposed to taxol (20). While these authors made no reference to the term antephase checkpoint, they proposed that CHFR is involved in a pathway needed to prevent mitotic entry as a response to mitotic stress.
Matsusaka and Pines (71) directly examined the requirement of CHFR for the ability of cells to reverse chromosome condensation, a hallmark of the antephase checkpoint. The authors noted a direct correlation between cells carrying wild-type CHFR, such as Ptk1 cells, and their ability to trigger the antephase checkpoint upon exposure to nocodazole or colcemid. Moreover, the injection of a dominant-negative form of CHFR into Ptk1 cells led to failure of the cells to activate the antephase checkpoint. Conversely, cells that lack functional CHFR, such as HeLa and U2OS cells, failed to prevent mitotic entry when exposed to the microtubule poisons. In U2OS cells that are defective in CHFR function (109), the antephase checkpoint was restored in stable clones expressing CHFR from the tet-off inducible plasmid (71). Taken together, these experiments linked CHFR to the antephase checkpoint.
MOLECULAR CHARACTERIZATION OF CHFR
The mode of action of CHFR at the antephase checkpoint remains to be elucidated. Crystallographic data indicate that CHFR possibly binds to phosphorylated proteins via the FHA domain (113). In vitro and in vivo biochemical and mutational analyses revealed that CHFR is a ubiquitin ligase which depends upon its RING finger domain (10, 20, 59) but not its FHA domain (20, 59) for its ubiquitination activity. Moreover, the ubiquitination activity, which includes autoubiquitination (10, 20, 29), appears to be needed for the checkpoint function in regulating mitotic entry. Indeed, CHFR with mutations in the RING finger domain failed to delay mitotic entry when expressed in various cell lines (10, 20) or when added to Xenopus extracts (59). Methyl-ubiquitin, which blocks ubiquitination, delayed mitotic entry in a CHFR-dependent manner when added to Xenopus extracts (59), while in Ptk1 cells, injection of methyl-ubiquitin abolished the antephase response (71). Taken together, these observations lend support to the idea that ubiquitination activity is critical for CHFR function at the checkpoint.
Conflicting data, however, have been reported as to whether the ubiquitination of substrates by CHFR leads to the ubiquitin-mediated destruction of substrates. Chaturvedi and coworkers had proposed that the autoubiquitination of CHFR likely contributes to its fluctuating protein levels over the cell cycle (20). Contrary to this, Bothos and coworkers (10) reported that CHFR was likely to be a noncanonical ubiquitin ligase and therefore may be involved in signaling pathways that rely on ubiquitination rather than in promoting the destruction of specific substrates by the 26S proteasome (135). In fact, CHFR, together with the ubiquitin-conjugating E2 heterodimer Ub13-Mms2, autoubiquitinates at lysine-63 (10), which has been implicated in signaling during stress rather than in degradation (135). In line with this idea, autoubiquitinated CHFR was found not to undergo cell cycle degradation (10), which contradicts the earlier report (20). Instead, CHFR was observed to undergo phosphorylation over the cell cycle (10). It was suggested (10) that the different observations could be due to the fact that the antibodies used in the earlier study (20) were insensitive to the phosphorylated forms and hence could have given rise to the idea that CHFR is cell cycle regulated. Direct examination of CHFR turnover upon autoubiquitination is needed to directly address this issue.
In Xenopus extracts, CHFR targets other substrates, such as polo-like kinase 1 (Plk1), for ubiquitination and destruction (59). Mouse embryonic fibroblasts (MEFs) from CHFR−/− mice showed higher levels of Plk1 (140), supporting the idea that CHFR affects Plk1 stability. Plk1 is needed for regulating mitotic entry via its function in the regulation of cyclin-dependent kinase activity (see below), and presumably downregulation of Plk1 causes the delay in progression into mitosis. In contrast to the Xenopus data, CHFR overexpressed in HCT116 cells did not destabilize Plk1 (116). The disparity observed may reflect differences in the various model systems studied. The apparent inconsistencies could also be due to the nature of the experimental setup. For instance, in experiments involving Xenopus extracts (59), the authors assayed the function of recombinant human CHFR. Such a system could have resulted in CHFR functioning differently than in human cells. Indeed, human CHFR acted to delay mitotic entry in Xenopus extracts in the absence of stress factors. It is further unclear if the antephase checkpoint exists in Xenopus. In fact, it was previously observed in Xenopus that the spindle assembly checkpoint functions only in the presence of a large amount of nuclei in combination with nocodazole (75). The discrepancies in these observations require further examination in order that a clearer idea of CHFR function can emerge.
CHFR also interacts with Aurora A (89, 98, 140), a key kinase that is needed in cell division (reviewed in reference 131). The interaction occurs via the cysteine-rich C-terminal region of CHFR and the N-terminal region of Aurora A (140) and appears to be required for the ubiquitination of Aurora A in vitro and in vivo (140). Interestingly, higher Aurora A levels were observed in CHFR−/− MEFs, indicating that CHFR influences Aurora A levels, perhaps by ubiquitin-mediated destruction (140). However, no evidence showing CHFR ubiquitination of Aurora A directly leading to instability was demonstrated (140). Nonetheless, several other studies showed that downregulation or loss of CHFR in cells is closely associated with elevated Aurora A expression (89, 98, 125). However, CHFR overexpression in HCT116 cells did not destabilize Aurora A (116). It remains to be seen if the lowered Aurora A levels are due directly to ubiquitination by CHFR.
More recently, CHFR was found to interact directly with HDAC1, a histone deacetylase, in standard pull-down experiments both in vitro and in vivo (89). HDAC1 was ubiquitinated by CHFR both in vitro and in vivo, and the levels of HDAC1 protein correlated inversely with those of CHFR when examined in CHFR-null HeLa cells transfected with wild-type but not mutant forms of CHFR that lacked ubiquitin ligase activity. Treatment of CHFR-transfected cells with MG132, a proteasome inhibitor, protected HDAC1 from destruction, indicating that ubiquitinated HDAC1 was unstable. In HEK293T cells, small interfering RNA (siRNA) of CHFR led to increased HDAC1 levels, which corroborates other findings that CHFR regulates its substrates via ubiquitin-mediated destruction.
The significance of ubiquitin-mediated destruction at the antephase checkpoint was further complicated by the observation that the presence of proteosomal inhibitors did not abolish the antephase checkpoint in Ptk1 cells (71). These observations are different from that in Xenopus extracts, where the addition of a proteosomal inhibitor disrupted the activation of the antephase checkpoint (59). The discrepancy could again be due to the differences in how the checkpoint functions in Xenopus and mammalian cells and could be resolved in detailed studies on the checkpoint in these and other systems.
In addition to the FHA and RING finger domains, a recent analysis of eukaryotic proteins involved in the DNA damage response and checkpoint regulation identified a single novel poly(ADP-ribose)-binding zinc finger (PBZ) motif in CHFR (2). During poly(ADP-ribosyl)ation, long chains of ADP-ribose units linked by glycosidic ribose-ribose bonds are added to substrates (45). Recombinant wild-type CHFR binds to poly(ADP-ribose) (PAR), whereas mutating the cysteine residues in the PBZ domain of CHFR abrogated its ability to bind to PAR. The interaction between CHFR and PAR was also seen in pull-downs of flag-tagged-CHFR transfected into HEK293 cells. Mutations in the PBZ motif in CHFR abolished the antephase checkpoint function of CHFR. In the presence of cysteine mutations in the PBZ motif in the CHFR-ΔFHA mutant, the dominant-negative effect of CHFR lacking the amino-terminal FHA domain (71) was abolished (2). Also, the authors found that in HeLa cells lacking CHFR (71), the presence of wild-type CHFR restored the antephase checkpoint but that of the form with mutations in the PBZ motif did not. Only the antephase checkpoint function was disrupted by the mutations, as the ubiquitinylation function was not affected. Nonetheless, the data indicated that poly(ADP-ribosyl)ation, which plays an important part in other checkpoint functions (45), is also needed during antephase checkpoint activation.
Related to its role in regulating mitotic events in the nucleus, CHFR was found to localize to the nucleus, where it colocalized with promyelocytic leukemia protein (PML) bodies (25). Mutational analysis confirmed that a lysine-rich stretch at positions 257 to 259 of CHFR is needed for its nuclear localization but not its ubiquitination activity (65). Interestingly, the association of CHFR with PML bodies appears to be important for its checkpoint role in response to microtubule poisons, as the CHFRΔFHA mutant that did not colocalize with PML (25) showed a dominant-negative effect with respect to the antephase checkpoint (25, 109). PML−/− MEFs failed to delay mitotic entry in the presence of mitotic stress (25), indicating that the association of CHFR with PML bodies is important for its antephase checkpoint function, though how the localization of CHFR to PML bodies affects its function awaits further elucidation.
POSSIBLE ROLE OF p38 IN THE ANTEPHASE CHECKPOINT
Several lines of evidence point toward the possible involvement of yet another player, p38, in the antephase checkpoint. p38 is a key kinase of the stress pathway that has been previously implicated in the response to a variety of stress agents in G2/M. For instance, UV radiation, which induces DNA double-stranded break and oxidative stress, triggers a signaling cascade including the ATM/ATR, JNK (jun amino-terminal kinase), and p38 kinase pathways (141). Activation of these pathways leads to a complex transcriptional response of the cells, resulting in regulation of DNA damage repair, cell cycle arrest, and apoptosis (35). For example, the p38 kinase pathway was reported to be the key player involved in G2/M arrest after UV radiation. Bulavin and coworkers found that the cell cycle arrest in G2/M after UV radiation is dependent on p38 but is ATM/ATR independent because inhibitors of ionizing radiation signaling proteins, such as caffeine (which inhibits ATM/ATR) and UCN-01 (which inhibits Chk1), had no effect on the UV-induced G2/M checkpoint initiation (12). Only the inhibition of p38 kinase by SB202190 (a potent p38-specific inhibitor) abrogated rapid initiation of the G2/M checkpoint after UV radiation. These observations support the idea that delaying mitotic entry is dependent on p38.
Cells are also very sensitive to osmolality changes in their environment. When cells are treated with NaCl and sucrose, the hypertonic environment causes the cell membrane to ruffle and to be enriched with actin, resulting in recruitment of the RAC-OSM-MEKK3-MKK3 complex (128) and activation of p38 (28, 69, 86, 94, 128). Dmitrieva and colleagues (28) showed that in renal inner medullary epithelial cells, NaCl treatment caused DNA damage, which, when sensed by ATM, led to the activation of p38 and G2 arrest. Under hypo-osmotic conditions, cells swell, which causes membrane tension to change (86). This defect sensed by integrins can lead to the activation of focal adhesion kinase/Src (46) and the p38 pathway (14, 46, 62, 86, 123). As a result, the cell volume returns to normal (14).
Yet another type of stress that triggers a p38-dependent delay in mitotic entry is alteration of chromatin structure. Mikhailov and colleagues found that treating various cell lines in culture with topoisomerase II inhibitors that produce changes in chromatin structure led to a G2/M delay (73). By their accounts, this was similar to a delay in antephase. Interestingly, they showed that treatment of cells with the topoisomerase II inhibitor aclarubicin, which does not cause double-stranded breaks in DNA and therefore does not activate the ATM DNA damage checkpoint at G2/M, can result in an antephase delay. The authors noted that exposure of cells to other topoisomerase II inhibitors such as adriamycin and merbarone could induce double-strand breaks and hence activate ATM (73). However, in the presence of caffeine or wortmannin, which both inhibit ATM, cells treated with adriamycin and merbarone failed to overcome the antephase delay. Indeed, this and a previous study (58) using ATM−/− cells exposed to mitotic stresses showed that the antephase checkpoint is independent of ATM. More importantly, it was found that exposure of cells to anisomycin, which triggers p38 activity, resulted in an antephase delay (71, 73). Conversely, treatment of cells with inhibitors of p38 such as SB203580 led to a failure of cells to activate the antephase checkpoint (71, 73). Furthermore, it was found that p38 likely functions downstream of CHFR, as treatment of U2OS cells (in which CHFR is nonfunctional) or Ptk1 cells expressing the dominant-negative form of CHFR with anisomycin could trigger the antephase checkpoint (71).
It was previously observed that inhibiting histone deacetylase with apicidin, which alters chromatin structure (38), could lead to an arrest at G2/M via an undefined checkpoint (99). Similar to the treatment of cells with topoisomerase II inhibitors, the antephase delay in the cells was dependent on p38, indicating that the antephase checkpoint is also functional when the global chromatin structure is disrupted (73). While previous studies indicated that p38 plays a role in a G2/M checkpoint that responds to different stresses, these studies (71, 73) demonstrated direct links between p38 function and the antephase checkpoint.
CORRELATION BETWEEN DOWNREGULATION OF CHFR FUNCTION AND CANCERS
The significance of CHFR function in mitosis is reflected in the findings that CHFR is absent or nonfunctional in several transformed cell lines and tumors (Table 1). For instance, genetic mutations in the CHFR gene have been found in U20S cells (109). In lung tumors, newly identified polymorphisms at codons 270 and 497 with amino acid substitutions and silent polymorphisms at codons 295 and 569 were identified (76). Also, the authors identified two heterozygotes for a G-to-A transition at codon 580. This has been reported to be a potential missense mutation with functional impairment of CHFR in the U2OS osteosarcoma cell line (109). The A allele has been reported to confer reduced function. However, in the two cases of lung tumors, loss of heterozygosity resulted in loss of the A allele in one of the cases. These observations imply that, in fact, genetic alteration of the CHFR gene might not be a key factor in lung cancers. In addition to the genetic mutations documented, cancer samples from lung tumors (76), as well as from various tissue types, show a downregulation of CHFR, mostly as a result of the hypermethylation of its promoter region (Table 1).
TABLE 1.
Cancers and cancer cell lines with genetic or epigenetic modifications in the CHFR gene
| Tissue and/or cell type | Mutation | Reference(s) |
|---|---|---|
| Lung cancer tissue and cell lines | Genetic mutation, hypermethylation leading to downregulation of CHFR expression | 23, 77 |
| Esophageal primary cancer and cell lines | Hypermethylation | 78, 110 |
| Gastric cancer and cell lines | Hypermethylation | 51, 52, 60, 78 |
| Brain tumors | Hypermethylation | 23 |
| Bone tumors | Hypermethylation | 23 |
| Colorectal cancers and adenomas | Hypermethylation | 23, 127 |
| Head and neck cancers | Hypermethylation | 127 |
| Breast cancer cells | Hypermethylation | 30 |
| Gallbladder carcinomas | Hypermethylation | 119 |
| T-cell lymphoma | Hypermethylation | 132 |
| Nasopharyngeal carcinomas | Hypermethylation | 21 |
| Hepatocellular carcinoma | Hypermethylation | 108 |
| Malignant peripheral nerve sheath tumors | Reduced expression | 64 |
| Endometrial cancer | Hypermethylation | 139 |
Correlations between the role of CHFR and tumor formation can also be seen in CHFR knockout mice (140). Indeed, the authors suggested the possibility that CHFR functions as a tumor suppressor. Interestingly, a CHFR−/− mouse is able to undergo proper embryonic development, indicating that CHFR does not play a major role during mouse embryogenesis. However, CHFR-null mice developed lymphomas that invaded various organs such as the thymus, spleen, liver, and ovary after 40 days. Moreover, CHFR+/− mice also died in the same period from lymphomas, indicating that CHFR normally acts to prevent aberrant mitosis. While these mutations and downregulation of CHFR may not be the causative agents that initiate the tumorigenic state in cells or tissues, they may well contribute to a multistep progression toward tumor formation via a loss of CHFR function in its key role in safeguarding mitotic entry.
POSSIBLE PATHWAYS LEADING TO ANTEPHASE CHECKPOINT ACTIVATION
A typical checkpoint pathway consists of three key components, namely, the sensor or surveillance system, the signal transduction pathway, and the effectors (82). Essentially, in a checkpoint pathway, the sensor detects the presence of a defect during the cell division cycle. A signal will be transmitted via the signal transduction pathway, which will then trigger effectors that will delay cell cycle progression and turn on the necessary pathway for repair. In these regards, several important questions about the antephase checkpoint remain.
SENSORS
First, it is unknown what the sensors of the antephase checkpoint may be, given that cells appear to respond to diverse stress factors (e.g., UV radiation, disruption of chromatin topology, microtubule disassembly, and osmotic shock) to block entry into mitosis. Multiple sensors are likely to be involved, perhaps impinging upon p38 as a central player. For example, in the case of a disruption of chromatin structure, Mikhailov and coworkers (73) proposed that in cells treated with inhibitors of topoisomerase II and histone deacetylase, the dissociation of a factor from chromatin activates c-Abl and the DNA-PK pathway (61), which then activates p38 through the mitogen-activated protein (MAP) kinase kinase cascade, thereby triggering the antephase checkpoint. Given the complexity of the process of chromosome condensation (6, 48), it would require further effort to elucidate how the antephase checkpoint is triggered upon the exposure of cells to various insults.
TRANSDUCTION PATHWAY
A second question relates to what the signal transduction pathway of the antephase checkpoint is that prevents mitotic entry. One of the key downstream factors needed for mitotic entry is the cyclin B1-Cdk1 complex (reviewed recently in reference 68). As alluded to in the review in reference 68, the cyclin B1-Cdk1 complex is under tight regulation and it is conceivable that the cyclin B1-Cdk1 complex is targeted by the antephase checkpoint at specific regulatory points to prevent activation of the complex and hence entry into mitosis. Cdk1 is inhibited by phosphorylation at threonine-14 (T-14) and tyrosine-15 (Y-15) by Myt1 and Wee1, respectively (88). Reversal of phosphorylation on T-14 and Y-15 depends upon the Cdc25 phosphatase, of which there are three isoforms, Cdc25A, Cdc25B, and Cdc25C (11, 88). The antephase checkpoint possibly impinges upon Wee1 and Myt1, as well as Cdc25, so as to control activation of the cyclin B1-Cdk1 complex and hence entry into mitosis.
Indeed, CHFR has been implicated in the ubiquitination and destruction of polo-like kinase 1 (Plk1) (59). Plk1 is needed for the destruction of Wee1, as well as inhibition of Myt1 activity (reviewed in reference 133). In addition, the activity of the Cdc25C phosphatase appears to be activated by Plk1 (133). Destruction of Plk1 prior to mitosis will lead to persistence of the inhibitory phosphorylation on T-14 and Y-15 of Cdk1, resulting in failure of mitotic entry. In Xenopus extracts, as well as in in vitro reconstitution assays, Plx1, Xenopus Plk1, was ubiquitinated and degraded in a CHFR-dependent manner (59). As a result, both the inactivation of Wee1 and the activation of Cdc25C were delayed, with the consequence that Cdk1 was kept inactive and mitotic entry was delayed (59).
In CHFR-defective HCT116 cells transfected with a CHFR construct, it was proposed that the cells were able to delay progression to metaphase when exposed to microtubule poisons, likely by preventing the nuclear localization of cyclin B1 and keeping cyclin B1-Cdk1 inactive (116). As the forced expression of cyclin B1 with a mutated nuclear export sequence was able to override the delay in early prophase upon activation of CHFR, the authors proposed that in the presence of microtubule poisons, CHFR acts to inhibit the cyclin B1-Cdk1 complex to prevent progression through prophase to metaphase (116).
However, several lines of evidence support the idea that cyclin A-Cdk2 may, in fact, be the initial target of the antephase checkpoint. Cyclin A was shown to function in human cells not just during S phase but in mitosis as well (95). Also, cyclin A localizes to the nuclei of HeLa cells from S phase until prophase (96), after which cyclin A is destroyed in prometaphase in a manner dependent on the anaphase-promoting complex/cyclosome (34) and 26S proteasome (27). Unlike cyclin A, cyclin B1 only localizes to the nucleus during prophase (96).
In addition, downregulation of cyclin A by short hairpin RNA leads to a G2 arrest in HeLa cells (32) while a dominant-negative form of Cdk2 when expressed in U2OS cells can delay cells in G2/M (53). Furthermore, cyclin A-Cdk2 activity can promote entry into mitosis (26, 33, 76), specifically for mitotic progression until late prophase (33). The notion that cyclin A-Cdk2 is important for the antephase checkpoint is also supported by the finding that the overexpression of a constitutively active cyclin A-Cdk2AF complex can bypass the antephase checkpoint in the presence of stress inducers (71). It is therefore likely that cyclin A-Cdk2 activity is normally downregulated by the antephase checkpoint via the p38-Cdc25 pathway in the presence of stress factors.
The three isoforms of Cdc25 phosphatase are thought to perform overlapping functions during the different cell cycle stages (11). For instance, Cdc25A, previously implicated in the G1/S transition (87), can cause an increase in cyclin A-Cdk activities at G2/M when overexpressed in U2OS cells (124). Of note is the authors' finding that the activity of Cdc25A occurs prior to Cdc25B activity during mitosis. Given that cyclin A-Cdk2 likely plays an important role in the activation of cyclin B-Cdk1 (32, 22), presumably via activation of Cdc25B and Cdc25C at G2/M in U2OS cells (76), it is conceivable that upon activation, the antephase checkpoint targets Cdc25A for export from the nuclei and away from cyclin A-Cdk2 (11). In so doing, it tips the balance toward the inhibition of Cdk2 by phosphorylation of Y-15 by the Wee1 kinase, leading to a downregulation of cyclin A-Cdk2 activity. Without active cyclin A-Cdk2, cyclin B1-Cdk1, whose activation requires cyclin A-Cdk2, will be kept inactive and the cells will be prevented from entering mitosis. However, a functional interaction between p38 and Cdc25A has yet to be demonstrated.
The idea that cyclin A-Cdk2 is the target of the antephase checkpoint is disputed by Summers and coworkers, who proposed that cyclin B1-Cdk1 is the target (116; see above). In their study, they noted that during activation of the antephase, the cyclin A-Cdk2 complex was active, as judged by the mobility shift of Cdk2 in Western blot analysis. They contended that the block in mitotic entry upon antephase checkpoint activation was not likely due to inactivation of the cyclin A-Cdk2 complex. Rather, they suggested that cyclin B1-Cdk1 was inactive because cyclin B1 was sequestered in the cytoplasm.
It should be noted, however, that Cdk2 mobility is affected not by T-14 or Y-15 but by phosphorylation on T-160 (106) and therefore does not serve well as an indication of cyclin A-Cdk2 activity. The inactive cyclin B1-Cdk1 noted by Summers and colleagues (116) could therefore be a consequence of the antephase checkpoint acting to downregulate cyclin A-Cdk2 activity. Their observation that the CHFR-mediated delay in mitotic entry led to a failure to import cyclin B1 into the nuclei may not be a direct effect of the antephase checkpoint. In fact, cyclin B1 is normally localized to the cytoplasm during interphase due to a higher rate of nuclear export compared to nuclear import (43, 126, 137). In prophase, it is the activation of cyclin B1-Cdk1 in the cytoplasm (42, 55) that results in the reduced rate of nuclear export and accumulation of cyclin B in the nucleus (43, 126, 137). The lack of nuclear accumulation of cyclin B1 could again be a result of the antephase checkpoint acting to downregulate cyclin A-Cdk2 activity. Furthermore, the finding that overexpression of a cyclin B1 export mutant was able to override the antephase checkpoint could simply mean that cyclin B1-Cdk1 has a function that overlaps that of cyclin A-Cdk2 in promoting the early stages of mitosis.
While it remains a speculation, the likelihood that cyclin A-Cdk2 is a target of the antephase checkpoint could account for the reversibility of antephase in cells exposed to stress factors given that cyclin A-Cdk2 is an upstream effector of mitosis. Once the cells pass antephase, cyclin B1-Cdk1 would be activated. At such a point, the cells are committed to mitosis and would pass the point of no return and proceed to mitosis even in the presence of stress factors. Further studies to resolve the issue of whether cyclin A-Cdk2 or cyclin B1-Cdk1 is relevant in the antephase checkpoint pathway will help provide a clearer picture of how cells respond to insults as they approach mitosis.
However, the pathway from CHFR to the cyclin-Cdk complexes remains unclear. As alluded to above, p38 appears to function downstream of CHFR (71), though the exact links between CHFR and p38 have yet to be established. Interestingly, Matsusaka and Pines isolated in a yeast two-hybrid screen an interactor of CHFR known as TRAF6-binding protein (T6BP) (71 and references therein). TRAF6 is a ubiquitin ligase that activates C-TAK1 (a MAP kinase kinase kinase). C-TAK is a MAP kinase kinase kinase that functions in the p38 pathway in response to interferon. The authors proposed that CHFR, in combination with T6BP, could activate C-TAK1. C-TAK1 is known to be able to phosphorylate Cdc25 and, as a result, creates a 14-3-3 binding site on Cdc25 (11). The binding of 14-3-3 sequesters Cdc25 away from cyclin-Cdk complexes, thereby abolishing the ability of the phosphatase to dephosphorylate cyclin-Cdk complexes on T-14 and Y-15. Without active cyclin-Cdk complexes, cells fail to trigger mitotic events and are delayed at antephase. Much of this scheme requires experimental verification at this stage.
CHFR also has been shown to downregulate HDAC1 (89). More significantly, HDAC1 normally represses the expression of p21, a CDK inhibitor (134). The downregulation of HDAC1 by CHFR increased p21 mRNA and protein levels (89). Interestingly, microinjection of cells with the N-terminal fragment of p21 inhibited cyclin A-Cdk2 and caused a reversal of chromosome condensation in cells at prophase (33). It was previously proposed that p21 binds to and inhibits the cyclin A-Cdk 2 complex (29), likely by preventing the binding of the Cdk-activating kinase to Cdk2 (3). It is possible that this constitutes a slow-response arm of the antephase checkpoint (74) that can cause a sustained antephase delay via p21-mediated inactivation of cyclin-Cdk complexes.
TARGETS OF THE ANTEPHASE CHECKPOINT
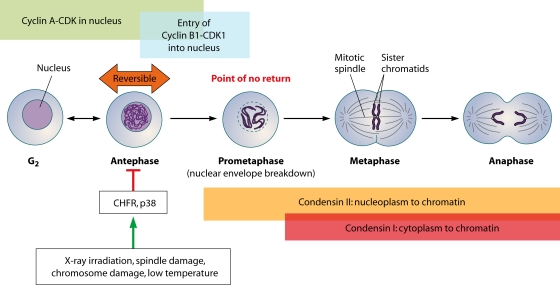
Last but not least, it is currently unclear how the antephase checkpoint signaling pathway impinges upon its targets to cause reversible chromosome condensation. Chromosome condensation begins in prophase and continues until cells enter mitosis, when segregation of condensed chromosomes occurs (48). Given the complex nature of chromosome condensation, it is still unclear mechanistically how the chromatin is eventually condensed into chromosomes (6, 118). Nonetheless, chromosome condensation is critical for the proper segregation of sister chromatids, as is evident from studies of various systems showing that mutations in components of the condensin complex result in defective chromosome segregation and the formation of chromosome bridges and lagging chromosomes (7, 8, 22, 36, 40, 54, 67, 92, 93, 107, 112, 114, 115, 117). Such events can contribute to chromosomal instability, which is a prelude to malignant tumor formation (56). To date, two complexes, condensins I and II, are known to be involved in chromosome condensation. The eventual targets of the antephase checkpoint are likely the condensin complexes, but of these two complexes, which is the more important during checkpoint activation remains to be seen (Fig. 2).
FIG. 2.
Key players at the antephase checkpoint. As cells progress from G2 into mitosis, there is a phase between these phases called antephase where chromosome condensation is reversible. The antephase appears to depend upon both p38 and CHFR for reversing chromosome condensation when the cells are exposed to stress agents, including spindle damage and low temperature.
The canonical condensin I complex is made up of two core subunits belonging to a family of ATPases known as structural maintenance of chromosomes proteins (SMC2/CAP-E and SMC4/CAP-C) and three non-SMC proteins (CAP-D2, CAP-G, and CAP-H) (41, 47, 57). The action of the condensin complex, in cooperation with topoisomerases I and II, is ATP dependent and results in supercoiling and hence compaction of the chromatin into chromosomes. The condensin II complex consists of the SMC2 and SMC4 subunits and non-SMC subunits hCAP-D3, hCAP-G2, and hCAP-H2 (91, 138) and appears to function earlier in prophase, while the condensin I complex is needed in prometaphase (48).
Direct links between condensins and the antephase checkpoint have yet to be established. However, it was seen in Xenopus extracts that the phosphorylation of the non-SMC2 subunits by Cdc2 in mitosis is important for localizing the complex to the chromosomes and for regulating its positive supercoiling activity (49). Conversely, immunodepletion of Cdc2 in Xenopus extracts decreased the phosphorylation of condensin I subunits and abolished the condensation activity (63). In HeLa cells, the phosphorylation of condensin I complexes also leads to their localization in the nucleus (120). Peptide analysis using mass spectrometry identified several potential Cdc2 phosphorylation sites on hCAP-G from HeLa cells (81). Mutation of these sites, such as T-308 and T-322, to alanine affects the chromosomal localization of hCAP-G in mitotic cells, indicating that phosphorylation of hCAP-G by Cdc2 is indeed important for its proper chromosome localization.
Condensin I non-SMC subunits may also be phosphorylated by another mitotic kinase, Aurora B (131), in vitro and in vivo (70, 120, 121). Also, the chromosomal localization of enhanced green fluorescent protein (EGFP)-hCAP-H was greatly reduced after treatment of cells with Hesperadin, an Aurora B inhibitor. A reduction of EGFP-hCAP-H chromosome association was also observed in Aurora B siRNA HeLa cells (70). The condensin II subunits, however, appear not to be phosphorylated by the Aurora kinase (70).
Based on the timing of condensin II complex localization, it may well be that the antephase checkpoint targets the condensin II subunits during checkpoint activation to cause reversal of the condensation process. The condensin II complex is nuclear throughout the cell division cycle and is localized to chromatin prior to the condensin I complex (50, 91). Time-lapse imaging revealed that the condensin II subunit hCAP-H2 colocalized with cyclin A in the nucleus during interphase until prophase (91). Cyclin B1 is normally found in the nucleus only in prophase (96), and its colocalization with the condensin I complex occurs only after nuclear envelope breakdown (50, 91).
Although a subunit of the condensin II complex, hCAP-D3, is phosphorylated during mitosis (138), the significance of this phosphorylation is undetermined. It may well be that the phosphorylation of condensin II subunits by cyclin A-Cdk complexes during prophase helps localize the complex to the chromatin for the condensation of the chromosomes by the complex (48). It is conceivable that when the antephase checkpoint acts to downregulate cyclin A-Cdk activation (73), the condensin II complex is not maintained in the active form and chromosome condensation is reversed. However, no evidence of phosphorylation of the condensin II subunits by cyclin A-Cdk complexes has been shown to date. It is also currently unknown what the phosphatase is that could dephosphorylate the condensin subunits during antephase checkpoint activation to reverse condensation.
PERSPECTIVES
The presence of an antephase checkpoint that halts mitotic entry puts in place yet another safeguard for cells to prevent commitment to mitosis under adverse conditions. The key downstream events targeted by the antephase checkpoint in the presence of insults include activation of the cyclin-Cdk complexes, as well as chromosome condensation. By delaying mitotic entry and thereby promoting reversal of chromosome condensation, the antephase checkpoint ensures that cells exposed to stressful situations that may cause errors during chromosome segregation do not to execute mitotic events until the conditions are favorable.
The two key factors CHFR and p38 have been linked directly to the antephase checkpoint. A clear working model of how the antephase checkpoint functions is not available, although data are slowly emerging as to the connections these factors have with the major components regulating the progression of the cell division cycle. Interesting challenges are to elucidate the molecular mechanism by which CHFR acts and to reconcile the data from various labs. Furthermore, the finding that p38 plays a central role in stress responses would mean that the mechanisms by which the various stresses that activate the antephase checkpoint can be fairly complex and would require substantial effort to identify. Also, an important question that remains to be answered is how the antephase checkpoint causes chromosomes to undergo a reversal of condensation. Nonetheless, it is important for detailed investigations into the workings of the antephase checkpoint so that we obtain a better view of how cells respond to insults prior to segregating chromosomes.
Acknowledgments
We thank Phuay-Yee Goh and Alice Tay for critical reading of the manuscript. We are also grateful to the reviewers for their insightful comments.
We are supported by the NUS, YIA/LSI grant R183-000-608-101.
Footnotes
Published ahead of print on 19 October 2009.
REFERENCES
- 1.Adames, N. R., J. R. Oberle, and J. A. Cooper. 2001. The surveillance mechanism of the spindle position checkpoint in yeast. J. Cell Biol. 153:159-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahel, I., D. Ahel, T. Matsusaka, A. J. Clark, J. Pines, S. J. Boulton, and S. C. West. 2008. Poly(ADP-ribose)-binding zinc finger motifs in DNA repair/checkpoint proteins. Nature 451:81-85. [DOI] [PubMed] [Google Scholar]
- 3.Aprelikova, O., Y. Xiong, and E. T. Liu. 1995. Both p16 and p21 families of cyclin-dependent kinase (CDK) inhibitors block the phosphorylation of cyclin-dependent kinases by the CDK-activating kinase. J. Biol. Chem. 270:18195-18197. [DOI] [PubMed] [Google Scholar]
- 4.Bartek, J., C. Lukas, and J. Lukas. 2004. Checking on DNA damage in S phase. Nat. Rev. Mol. Cell Biol. 5:792-804. [DOI] [PubMed] [Google Scholar]
- 5.Bartek, J., and J. Lukas. 2001. Mammalian G1- and S-phase checkpoints in response to DNA damage. Curr. Opin. Cell Biol. 13:738-747. [DOI] [PubMed] [Google Scholar]
- 6.Belmont, A. S. 2006. Mitotic chromosome structure and condensation. Curr. Opin. Cell Biol. 18:632-638. [DOI] [PubMed] [Google Scholar]
- 7.Bhalla, N., S. Biggins, and A. W. Murray. 2002. Mutation of YCS4, a budding yeast condensin subunit, affects mitotic and nonmitotic chromosome behavior. Mol. Biol. Cell 13:632-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhat, M. A., A. V. Philp, D. M. Glover, and H. J. Bellen. 1996. Chromatid segregation at anaphase requires the barren product, a novel chromosome-associated protein that interacts with topoisomerase II. Cell 87:1103-1114. [DOI] [PubMed] [Google Scholar]
- 9.Blow, J. J., and T. U. Tanaka. 2005. The chromosome cycle: coordinating replication and segregation. Second in the cycles review series. EMBO Rep. 6:1028-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bothos, J., M. K. Summers, M. Venere, D. M. Scolnick, and T. D. Halazonetis. 2003. The Chfr mitotic checkpoint protein functions with Ubc13-Mms2 to form Lys63-linked polyubiquitin chains. Oncogene 22:7101-7107. [DOI] [PubMed] [Google Scholar]
- 11.Boutros, R., C. Dozier, and B. Ducommun. 2006. The when and wheres of CDC25 phosphatases. Curr. Opin. Cell Biol. 18:185-191. [DOI] [PubMed] [Google Scholar]
- 12.Bulavin, D. V., Y. Higashimoto, I. J. Popoff, W. A. Gaarde, V. Basrur, O. Potapova, E. Appella, and A. J. J. Fornace. 2001. Initiation of a G2/M checkpoint after ultraviolet radiation requires p38 kinase. Nature 411:102-107. [DOI] [PubMed] [Google Scholar]
- 13.Bullough, W. S., and M. Johnson. 1951. The energy relations of mitotic activity in adult mouse epidermis. Proc. R. Soc. Lond. B Biol. Sci. 138:562-575. [DOI] [PubMed] [Google Scholar]
- 14.Bustamante, M., F. Roger, M. L. Bochaton-Piallat, G. Gabbiani, P. Y. Martin, and E. Feraille. 2003. Regulatory volume increase is associated with p38 kinase-dependent actin cytoskeleton remodeling in rat kidney MTAL. Am. J. Physiol. Renal Physiol. 285:F336-F347. [DOI] [PubMed] [Google Scholar]
- 15.Cahill, D. P., L. T. da Costa, E. B. Carson-Walter, K. W. Kinzler, B. Vogelstein, and C. Lengauer. 1999. Characterization of MAD2B and other mitotic spindle checkpoint genes. Genomics 58:181-187. [DOI] [PubMed] [Google Scholar]
- 16.Cahill, D. P., C. Lengauer, J. Yu, G. J. Riggins, J. K. Willson, S. D. Markowitz, K. W. Kinzler, and B. Vogelstein. 1998. Mutations of mitotic checkpoint genes in human cancers. Nature 392:300-303. [DOI] [PubMed] [Google Scholar]
- 17.Campbell, J. L., and O. Cohen-Fix. 2002. Chromosome cohesion: ring around the sisters? Trends Biochem. Sci. 27:492-495. [DOI] [PubMed] [Google Scholar]
- 18.Carlson, J. G. 1969. X-ray-induced prophase delay and reversion of selected cells in certain avian and mammalian tissues in culture. Radiat. Res. 37:15-30. [PubMed] [Google Scholar]
- 19.Carlson, J. G. 1969. A detailed analysis of X-ray-induced prophase delay and reversion of grasshopper neuroblasts in culture. Radiat. Res. 37:1-14. [PubMed] [Google Scholar]
- 20.Chaturvedi, P., V. Sudakin, M. L. Bobiak, P. W. Fisher, M. R. Mattern, S. A. Jablonski, M. R. Hurle, Y. Zhu, T. J. Yen, and B. B. Zhou. 2002. Chfr regulates a mitotic stress pathway through its RING-finger domain with ubiquitin ligase activity. Cancer Res. 62:1797-1801. [PubMed] [Google Scholar]
- 21.Cheung, H. W., Y. P. Ching, J. M. Nicholls, M. T. Ling, Y. C. Wong, N. Hui, A. Cheung, S. W. Tsao, Q. Wang, P. W. Yeun, K. W. Lo, D. Y. Jin, and X. Wang. 2005. Epigenetic inactivation of CHFR in nasopharyngeal carcinoma through promoter methylation. Mol. Carcinog. 43:237-245. [DOI] [PubMed] [Google Scholar]
- 22.Coelho, P. A., J. Queiroz-Machado, and C. E. Sunkel. 2003. Condensin-dependent localisation of topoisomerase II to an axial chromosomal structure is required for sister chromatid resolution during mitosis. J. Cell Sci. 116:4763-4776. [DOI] [PubMed] [Google Scholar]
- 23.Corn, P. G., M. K. Summers, F. Fogt, A. K. Virmani, A. F. Gazdar, T. D. Halazonetis, and W. S. El-Deiry. 2003. Frequent hypermethylation of the 5′ CpG island of the mitotic stress checkpoint gene Chfr in colorectal and non-small cell lung cancer. Carcinogenesis 24:47-51. [DOI] [PubMed] [Google Scholar]
- 24.Damelin, M., and T. H. Bestor. 2007. The decatenation checkpoint. Br. J. Cancer 96:201-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daniels, M. J., A. Marson, and A. R. Venkitaraman. 2004. PML bodies control the nuclear dynamics and function of the CHFR mitotic checkpoint protein. Nat. Struct. Mol. Biol. 11:1114-1121. [DOI] [PubMed] [Google Scholar]
- 26.De Boer, L., V. Oakes, H. Beamish, N. Giles, F. Stevens, M. Somodevilla-Torres, C. Desouza, and B. Gabrielli. 2008. Cyclin A/cdk2 coordinates centrosomal and nuclear mitotic events. Oncogene 27:4261-4268. [DOI] [PubMed] [Google Scholar]
- 27.den Elzen, N., and J. Pines. 2001. Cyclin A is destroyed in prometaphase and can delay chromosome alignment and anaphase. J. Cell Biol. 153:121-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dmitrieva, N. I., D. V. Bulavin, A. J. J. Fornace, and M. B. Burg. 2002. Rapid activation of G2/M checkpoint after hypertonic stress in renal inner medullary epithelial (IME) cells is protective and requires p38 kinase. Proc. Natl. Acad. Sci. U. S. A. 99:184-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dulić, V., G. H. Stein, D. F. Far, and S. I. Reed. 1998. Nuclear accumulation of p21Cip1 at the onset of mitosis: a role at the G2/M-phase transition. Mol. Cell. Biol. 18:546-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erson, A. E., and E. M. Petty. 2004. CHFR-associated early G2/M checkpoint defects in breast cancer cells. Mol. Carcinog. 39:26-33. [DOI] [PubMed] [Google Scholar]
- 31.Fraschini, R., D. Bilotta, G. Lucchini, and S. Piatti. 2004. Functional characterization of Dma1 and Dma2, the budding yeast homologues of Schizosaccharomyces pombe Dma1 and human Chfr. Mol. Biol. Cell 15:3796-3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fung, T. K., H. T. Ma, and R. Y. Poon. 2007. Specialized roles of the two mitotic cyclins in somatic cells: cyclin A as an activator of M phase-promoting factor. Mol. Biol. Cell 18:1861-1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furuno, N., N. den Elzen, and J. Pines. 1999. Human cyclin A is required for mitosis until mid prophase. J. Cell Biol. 147:295-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geley, S., E. Kramer, C. Gieffers, J. Gannon, J. M. Peters, and T. Hunt. 2001. Anaphase-promoting complex/cyclosome-dependent proteolysis of human cyclin A starts at the beginning of mitosis and is not subject to the spindle assembly checkpoint. J. Cell Biol. 153:137-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gentile, M., L. Latonen, and M. Laiho. 2003. Cell cycle arrest and apoptosis provoked by UV radiation-induced DNA damage are transcriptionally highly divergent responses. Nucleic Acids Res. 31:4779-4790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gerlich, D., T. Hirota, B. Koch, J. M. Peters, and J. Ellenberg. 2006. Condensin I stabilizes chromosomes mechanically through a dynamic interaction in live cells. Curr. Biol. 16:333-344. [DOI] [PubMed] [Google Scholar]
- 37.Ghosh, S. K., S. Hajra, A. Paek, and M. Jayaram. 2006. Mechanisms for chromosome and plasmid segregation. Annu. Rev. Biochem. 75:211-241. [DOI] [PubMed] [Google Scholar]
- 38.Grunstein, M. 1997. Molecular model for telomeric heterochromatin in yeast. Curr. Opin. Cell Biol. 9:383-387. [DOI] [PubMed] [Google Scholar]
- 39.Guertin, D. A., S. Venkatram, K. L. Gould, and D. McCollum. 2002. Dma1 prevents mitotic exit and cytokinesis by inhibiting the septation initiation network (SIN). Dev. Cell 3:779-790. [DOI] [PubMed] [Google Scholar]
- 40.Hagstrom, K. A., V. F. Holmes, N. R. Cozzarelli, and B. J. Meyer. 2002. C. elegans condensin promotes mitotic chromosome architecture, centromere organization, and sister chromatid segregation during mitosis and meiosis. Genes Dev. 16:729-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hagstrom, K. A., and B. J. Meyer. 2003. Condensin and cohesin: more than chromosome compactor and glue. Nat. Rev. Genet. 4:520-534. [DOI] [PubMed] [Google Scholar]
- 42.Hagting, A., M. Jackman, K. Simpson, and J. Pines. 1999. Translocation of cyclin B1 to the nucleus at prophase requires a phosphorylation-dependent nuclear import signal. Curr. Biol. 9:680-689. [DOI] [PubMed] [Google Scholar]
- 43.Hagting, A., C. Karlsson, P. Clute, M. Jackman, and J. Pines. 1998. MPF localization is controlled by nuclear export. EMBO J. 17:4127-4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hartwell, L. H., and M. B. Kastan. 1994. Cell cycle control and cancer. Science 266:1821-1828. [DOI] [PubMed] [Google Scholar]
- 45.Hassa, P. O., S. S. Haenni, M. Elser, and M. O. Hottiger. 2006. Nuclear ADP-ribosylation reactions in mammalian cells: where are we today and where are we going? Microbiol. Mol. Biol. Rev. 70:789-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Häussinger, D., A. K. Kurz, M. Wettstein, D. Graf, S. Vom Dahl, and F. Schliess. 2003. Involvement of integrins and Src in tauroursodeoxycholate-induced and swelling-induced choleresis. Gastroenterology 124:1476-1487. [DOI] [PubMed] [Google Scholar]
- 47.Hirano, T. 2002. The ABCs of SMC proteins: two-armed ATPases for chromosome condensation, cohesion, and repair. Genes Dev. 16:399-414. [DOI] [PubMed] [Google Scholar]
- 48.Hirano, T. 2005. Condensins: organizing and segregating the genome. Curr. Biol. 15:R265-R275. [DOI] [PubMed] [Google Scholar]
- 49.Hirano, T., R. Kobayashi, and M. Hirano. 1997. Condensins, chromosome condensation protein complexes containing XCAP-C, XCAP-E and a Xenopus homolog of the Drosophila Barren protein. Cell 89:511-521. [DOI] [PubMed] [Google Scholar]
- 50.Hirota, T., D. Gerlich, B. Koch, J. Ellenberg, and J. M. Peters. 2004. Distinct functions of condensin I and II in mitotic chromosome assembly. J. Cell Sci. 117:6435-6445. [DOI] [PubMed] [Google Scholar]
- 51.Homma, N., G. Tamura, T. Honda, Z. Jin, K. Ohmura, S. Kawata, and T. Motoyama. 2005. Hypermethylation of Chfr and hMLH1 in gastric noninvasive and early invasive neoplasias. Virchows Arch. 446:120-126. [DOI] [PubMed] [Google Scholar]
- 52.Honda, T., G. Tamura, T. Waki, S. Kawata, S. Nishizuka, and T. Motoyama. 2004. Promoter hypermethylation of the Chfr gene in neoplastic and non-neoplastic gastric epithelia. Br. J. Cancer 90:2013-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hu, B., J. Mitra, S. van den Heuvel, and G. H. Enders. 2001. S and G2 phase roles for Cdk2 revealed by inducible expression of a dominant-negative mutant in human cells. Mol. Cell. Biol. 21:2755-2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hudson, D. F., P. Vagnarelli, R. Gassmann, and W. C. Earnshaw. 2003. Condensin is required for nonhistone protein assembly and structural integrity of vertebrate mitotic chromosomes. Dev. Cell 5:323-336. [DOI] [PubMed] [Google Scholar]
- 55.Jackman, M., C. Lindon, E. A. Nigg, and J. Pines. 2003. Active cyclin B1-Cdk1 first appears on centrosomes in prophase. Nat. Cell Biol. 5:143-148. [DOI] [PubMed] [Google Scholar]
- 56.Jallepalli, P. V., and C. Lengauer. 2001. Chromosome segregation and cancer: cutting through the mystery. Nat. Rev. Cancer 1:109-117. [DOI] [PubMed] [Google Scholar]
- 57.Jessberger, R. 2002. The many functions of SMC proteins in chromosome dynamics. Nat. Rev. Mol. Cell Biol. 3:767-778. [DOI] [PubMed] [Google Scholar]
- 58.Jha, M. N., J. R. Bamburg, and J. S. Bedford. 1994. Cell cycle arrest by colcemid differs in human normal and tumor cells. Cancer Res. 54:5011-5015. [PubMed] [Google Scholar]
- 59.Kang, D., J. Chen, J. Wong, and G. Fang. 2002. The checkpoint protein Chfr is a ligase that ubiquitinates Plk1 and inhibits Cdc2 at the G2 to M transition. J. Cell Biol. 156:249-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kang, H. C., I. J. Kim, J. H. Park, Y. Shin, H. W. Park, J. L. Ku, H. K. Yang, K. U. Lee, K. J. Choe, and J. G. Park. 2004. Promoter hypermethylation and silencing of CHFR mitotic stress checkpoint gene in human gastric cancers. Oncol. Rep. 12:129-133. [PubMed] [Google Scholar]
- 61.Kharbanda, S., P. Pandey, S. Jin, S. Inoue, A. Bharti, Z. M. Yuan, R. Weichselbaum, D. Weaver, and D. Kufe. 1997. Functional interaction between DNA-PK and c-Abl in response to DNA damage. Nature 386:732-735. [DOI] [PubMed] [Google Scholar]
- 62.Kim, R. D., C. E. Darling, H. Cerwenka, and R. S. Chari. 2000. Hypoosmotic stress activates p38, ERK 1 and 2, and SAPK/JNK in rat hepatocytes. J. Surg. Res. 90:58-66. [DOI] [PubMed] [Google Scholar]
- 63.Kimura, K., M. Hirano, R. Kobayashi, and T. Hirano. 1998. Phosphorylation and activation of 13S condensin by Cdc2 in vitro. Science 282:487-490. [DOI] [PubMed] [Google Scholar]
- 64.Kobayashi, C., Y. Oda, T. Takahira, T. Izumi, K. Kawaguchi, H. Yamamoto, S. Tamiya, T. Yamada, Y. Iwamoto, and M. Tsuneyoshi. 2006. Aberrant expression of CHFR in malignant peripheral nerve sheath tumors. Mod. Pathol. 19:524-532. [DOI] [PubMed] [Google Scholar]
- 65.Kwon, Y. E., Y. S. Kim, Y. M. Oh, and J. H. Seol. 2009. Nuclear localization of Chfr is crucial for its checkpoint function. Mol. Cells 27:359-363. [DOI] [PubMed] [Google Scholar]
- 66.Kyriakis, J. M., and J. Avruch. 2001. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 81:807-869. [DOI] [PubMed] [Google Scholar]
- 67.Lavoie, B. D., E. Hogan, and D. Koshland. 2002. In vivo dissection of the chromosome condensation machinery: reversibility of condensation distinguishes contributions of condensin and cohesin. J. Cell Biol. 156:805-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lindqvist, A., V. Rodriguez-Bravo, and R. H. Medema. 2009. The decision to enter mitosis: feedback and redundancy in the mitotic entry network. J. Cell Biol. 185:193-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liovic, M., B. Lee, M. Tomic-Canic, M. D'Alessandro, V. N. Bolshakov, and E. B. Lane. 2008. Dual-specificity phosphatases in the hypo-osmotic stress response of keratin-defective epithelial cell lines. Exp. Cell Res. 314:2066-2075. [DOI] [PubMed] [Google Scholar]
- 70.Lipp, J. J., T. Hirota, I. Poser, and J. M. Peters. 2007. Aurora B controls the association of condensin I but not condensin II with mitotic chromosomes. J. Cell Sci. 120:1245-1255. [DOI] [PubMed] [Google Scholar]
- 71.Matsusaka, T., and J. Pines. 2004. Chfr acts with the p38 stress kinases to block entry to mitosis in mammalian cells. J. Cell Biol. 166:507-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Michel, L. S., V. Liberal, A. Chatterjee, R. Kirchwegger, B. Pasche, W. Gerald, M. Dobles, P. K. Sorger, V. V. Murty, and R. Benezra. 2001. MAD2 haplo-insufficiency causes premature anaphase and chromosome instability in mammalian cells. Nature 409:355-359. [DOI] [PubMed] [Google Scholar]
- 73.Mikhailov, A., M. Shinohara, and C. L. Rieder. 2004. Topoisomerase II and histone deacetylase inhibitors delay the G2/M transition by triggering the p38 MAPK checkpoint pathway. J. Cell Biol. 166:517-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mikhailov, A., M. Shinohara, and C. L. Rieder. 2005. The p38-mediated stress-activated checkpoint. A rapid response system for delaying progression through antephase and entry into mitosis. Cell Cycle 4:57-62. [DOI] [PubMed] [Google Scholar]
- 75.Minshull, J., H. Sun, N. K. Tonks, and A. W. Murray. 1994. A MAP kinase-dependent spindle assembly checkpoint in Xenopus egg extracts. Cell 79:475-486. [DOI] [PubMed] [Google Scholar]
- 76.Mitra, J., and G. H. Enders. 2004. Cyclin A/Cdk2 complexes regulate activation of Cdk1 and Cdc25 phosphatases in human cells. Oncogene 23:3361-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mizuno, K., H. Osada, H. Konishi, Y. Tatematsu, Y. Yatabe, T. Mitsudomi, Y. Fujii, and T. Takahashi. 2002. Aberrant hypermethylation of the CHFR prophase checkpoint gene in human lung cancers. Oncogene 21:2328-2333. [DOI] [PubMed] [Google Scholar]
- 78.Morioka, Y., K. Hibi, M. Sakai, M. Koike, M. Fujiwara, Y. Kodera, K. Ito, and A. Nakao. 2006. Aberrant methylation of the CHFR gene in digestive tract cancer. Anticancer Res. 26:1791-1795. [PubMed] [Google Scholar]
- 79.Mueller, G. A., M. E. Gaulden, and W. Drane. 1971. The effects of varying concentrations of colchicine on the progression of grasshopper neuroblasts into metaphase. J. Cell Biol. 48:253-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Murone, M., and V. Simanis. 1996. The fission yeast dma1 gene is a component of the spindle assembly checkpoint, required to prevent septum formation and premature exit from mitosis if spindle function is compromised. EMBO J. 15:6605-6616. [PMC free article] [PubMed] [Google Scholar]
- 81.Murphy, L. A., and K. D. Sarge. 2008. Phosphorylation of CAP-G is required for its chromosomal DNA localization during mitosis. Biochem. Biophys. Res. Commun. 377:1007-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Murray, A. W. 1995. The genetics of cell cycle checkpoints. Curr. Opin. Genet. Dev. 5:5-11. [DOI] [PubMed] [Google Scholar]
- 83.Musacchio, A., and E. D. Salmon. 2007. The spindle-assembly checkpoint in space and time. Nat. Rev. Mol. Cell Biol. 8:379-393. [DOI] [PubMed] [Google Scholar]
- 84.Nasmyth, K. 2002. Segregating sister genomes: the molecular biology of chromosome separation. Science 297:559-565. [DOI] [PubMed] [Google Scholar]
- 85.Nasmyth, K. 2005. How might cohesin hold sister chromatids together? Philos. Trans. R. Soc. Lond. B Biol. Sci. 360:483-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Niisato, N., M. Post, W. Van Driessche, and Y. Marunaka. 1999. Cell swelling activates stress-activated protein kinases, p38 MAP kinase and JNK, in renal epithelial A6 cells. Biochem. Biophys. Res. Commun. 266:547-550. [DOI] [PubMed] [Google Scholar]
- 87.Nilsson, I., and I. Hoffmann. 2000. Cell cycle regulation by the Cdc25 phosphatase family. Prog. Cell Cycle Res. 4:107-114. [DOI] [PubMed] [Google Scholar]
- 88.O'Farrell, P. H. 2001. Triggering the all-or-nothing switch into mitosis. Trends Cell Biol. 11:512-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Oh, Y. M., Y. E. Kwon, J. M. Kim, S. J. Bae, B. K. Lee, S. J. Yoo, C. H. Chung, R. J. Deshaies, and J. H. Seol. 2009. Chfr is linked to tumour metastasis through the downregulation of HDAC1. Nat. Cell Biol. 11:295-302. [DOI] [PubMed] [Google Scholar]
- 90.Onn, I., J. M. Heidinger-Pauli, V. Guacci, E. Unal, and D. E. Koshland. 2008. Sister chromatid cohesion: a simple concept with a complex reality. Annu. Rev. Cell Dev. Biol. 24:105-129. [DOI] [PubMed] [Google Scholar]
- 91.Ono, T., Y. Fang, D. L. Spector, and T. Hirano. 2004. Spatial and temporal regulation of condensins I and II in mitotic chromosome assembly in human cells. Mol. Biol. Cell 15:3296-3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ono, T., A. Losada, M. Hirano, M. P. Myers, A. F. Neuwald, and T. Hirano. 2003. Differential contributions of condensin I and condensin II to mitotic chromosome architecture in vertebrate cells. Cell 115:109-121. [DOI] [PubMed] [Google Scholar]
- 93.Ouspenski, I. I., O. A. Cabello, and B. R. Brinkley. 2000. Chromosome condensation factor Brn1p is required for chromatid separation in mitosis. Mol. Biol. Cell 11:1305-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Padda, R., A. Wamsley-Davis, M. C. Gustin, R. Ross, C. Yu, and D. Sheikh-Hamad. 2006. MEKK3-mediated signaling to p38 kinase and TonE in hypertonically stressed kidney cells. Am. J. Physiol. Renal Physiol. 291:F874-F881. [DOI] [PubMed] [Google Scholar]
- 95.Pagano, M., R. Pepperkok, F. Verde, W. Ansorge, and G. Draetta. 1992. Cyclin A is required at two points in the human cell cycle. EMBO J. 11:961-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pines, J., and T. Hunter. 1991. Human cyclins A and B1 are differentially located in the cell and undergo cell cycle-dependent nuclear transport. J. Cell Biol. 115:1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pines, J., and C. L. Rieder. 2001. Re-staging mitosis: a contemporary view of mitotic progression. Nat. Cell Biol. 3:E3-E6. [DOI] [PubMed] [Google Scholar]
- 98.Privette, L. M., J. F. Weier, H. N. Nguyen, X. Yu, and E. M. Petty. 2008. Loss of CHFR in human mammary epithelial cells causes genomic instability by disrupting the mitotic spindle assembly checkpoint. Neoplasia 10:643-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Qiu, L., A. Burgess, D. P. Fairlie, H. Leonard, P. G. Parsons, and B. G. Gabrielli. 2000. Histone deacetylase inhibitors trigger a G2 checkpoint in normal cells that is defective in tumor cells. Mol. Biol. Cell 11:2069-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rhind, N., and P. Russell. 1998. Mitotic DNA damage and replication checkpoints in yeast. Curr. Opin. Cell Biol. 10:749-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rhind, N., and P. Russell. 2000. Chk1 and Cds1: linchpins of the DNA damage and replication checkpoint pathways. J. Cell Sci. 113:3889-3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rieder, C. L. 1981. Effect of hypothermia (20-25 degrees C) on mitosis in PtK1 cells. Cell Biol. Int. Rep. 5:563-573. [DOI] [PubMed] [Google Scholar]
- 103.Rieder, C. L., and R. Cole. 2000. Microscopy-induced radiation damage, microtubules, and progression through the terminal stage of G2 (prophase) in vertebrate somatic cells. Cold Spring Harb. Symp. Quant. Biol. 65:369-376. [DOI] [PubMed] [Google Scholar]
- 104.Rieder, C. L., and R. Cole. 2000. Microtubule disassembly delays the G2-M transition in vertebrates. Curr. Biol. 10:1067-1070. [DOI] [PubMed] [Google Scholar]
- 105.Rieder, C. L., and R. W. Cole. 1998. Entry into mitosis in vertebrate somatic cells is guarded by a chromosome damage checkpoint that reverses the cell cycle when triggered during early but not late prophase. J. Cell Biol. 142:1013-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rosenblatt, J., Y. Gu, and D. O. Morgan. 1992. Human cyclin-dependent kinase 2 is activated during the S and G2 phases of the cell cycle and associates with cyclin A. Proc. Natl. Acad. Sci. U. S. A. 89:2824-2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Saka, Y., T. Sutani, Y. Yamashita, S. Saitoh, M. Takeuchi, Y. Nakaseko, and M. Yanagida. 1994. Fission yeast cut3 and cut14, members of a ubiquitous protein family, are required for chromosome condensation and segregation in mitosis. EMBO J. 13:4938-4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sakai, M., K. Hibi, N. Kanazumi, S. Nomoto, S. Inoue, S. Takeda, and A. Nakao. 2005. Aberrant methylation of the CHFR gene in advanced hepatocellular carcinoma. Hepatogastroenterology 52:1854-1857. [PubMed] [Google Scholar]
- 109.Scolnick, D. M., and T. D. Halazonetis. 2000. Chfr defines a mitotic stress checkpoint that delays entry into metaphase. Nature 406:430-435. [DOI] [PubMed] [Google Scholar]
- 110.Shibata, Y., N. Haruki, Y. Kuwabara, H. Ishiguro, N. Shinoda, A. Sato, M. Kimura, H. Koyama, T. Toyama, T. Nishiwaki, J. Kudo, Y. Terashita, S. Konishi, H. Sugiura, and Y. Fujii. 2002. Chfr expression is downregulated by CpG island hypermethylation in esophageal cancer. Carcinogenesis 23:1695-1699. [DOI] [PubMed] [Google Scholar]
- 111.Skibbens, R. V. 2005. Unzipped and loaded: the role of DNA helicases and RFC clamp-loading complexes in sister chromatid cohesion. J. Cell Biol. 169:841-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Somma, M. P., B. Fasulo, G. Siriaco, and G. Cenci. 2003. Chromosome condensation defects in barren RNA-interfered Drosophila cells. Genetics 165:1607-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stavridi, E. S., Y. Huyen, I. R. Loreto, D. M. Scolnick, T. D. Halazonetis, N. P. Pavletich, and P. D. Jeffrey. 2002. Crystal structure of the FHA domain of the Chfr mitotic checkpoint protein and its complex with tungstate. Structure 10:891-899. [DOI] [PubMed] [Google Scholar]
- 114.Steffensen, S., P. A. Coelho, N. Cobbe, S. Vass, M. Costa, B. Hassan, S. N. Prokopenko, H. Bellen, M. M. Heck, and C. E. Sunkel. 2001. A role for Drosophila SMC4 in the resolution of sister chromatids in mitosis. Curr. Biol. 11:295-307. [DOI] [PubMed] [Google Scholar]
- 115.Strunnikov, A. V., E. Hogan, and D. Koshland. 1995. SMC2, a Saccharomyces cerevisiae gene essential for chromosome segregation and condensation, defines a subgroup within the SMC family. Genes Dev. 9:587-599. [DOI] [PubMed] [Google Scholar]
- 116.Summers, M. K., J. Bothos, and T. D. Halazonetis. 2005. The CHFR mitotic checkpoint protein delays cell cycle progression by excluding cyclin B1 from the nucleus. Oncogene 24:2589-2598. [DOI] [PubMed] [Google Scholar]
- 117.Sutani, T., T. Yuasa, T. Tomonaga, N. Dohmae, K. Takio, and M. Yanagida. 1999. Fission yeast condensin complex: essential roles of non-SMC subunits for condensation and Cdc2 phosphorylation of Cut3/SMC4. Genes Dev. 13:2271-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Swedlow, J. R., and T. Hirano. 2003. The making of the mitotic chromosome: modern insights into classical questions. Mol. Cell 11:557-569. [DOI] [PubMed] [Google Scholar]
- 119.Takahashi, T., N. Shivapurkar, E. Riquelme, H. Shigematsu, J. Reddy, M. Suzuki, K. Miyajima, X. Zhou, B. N. Bekele, A. F. Gazdar, and I. I. Wistuba. 2004. Aberrant promoter hypermethylation of multiple genes in gallbladder carcinoma and chronic cholecystitis. Clin. Cancer Res. 10:6126-6133. [DOI] [PubMed] [Google Scholar]
- 120.Takemoto, A., K. Kimura, S. Yokoyama, and F. Hanaoka. 2004. Cell cycle-dependent phosphorylation, nuclear localization, and activation of human condensin. J. Biol. Chem. 279:4551-4559. [DOI] [PubMed] [Google Scholar]
- 121.Takemoto, A., A. Murayama, M. Katano, T. Urano, K. Furukawa, S. Yokoyama, J. Yanagisawa, F. Hanaoka, and K. Kimura. 2007. Analysis of the role of Aurora B on the chromosomal targeting of condensin I. Nucleic Acids Res. 35:2403-2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tanaka, T. U. 2005. Chromosome bi-orientation on the mitotic spindle. Philos. Trans. R. Soc. Lond. B Biol. Sci. 360:581-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tilly, B. C., M. Gaestel, K. Engel, M. J. Edixhoven, and H. R. de Jonge. 1996. Hypo-osmotic cell swelling activates the p38 MAP kinase signalling cascade. FEBS Lett. 395:133-136. [DOI] [PubMed] [Google Scholar]
- 124.Timofeev, O., O. Cizmecioglu, E. Hu, T. Orlik, and I. Hoffmann. 2009. Human Cdc25A phosphatase has a non-redundant function in G2 phase by activating cyclin A-dependent kinases. FEBS Lett. 583:841-847. [DOI] [PubMed] [Google Scholar]
- 125.Tomita, M., M. Toyota, C. Ishikawa, T. Nakazato, T. Okudaira, T. Matsuda, J. N. Uchihara, N. Taira, K. Ohshiro, M. Senba, Y. Tanaka, K. Ohshima, H. Saya, T. Tokino, and N. Mori. 2009. Overexpression of Aurora A by loss of CHFR gene expression increases the growth and survival of HTLV-1-infected T cells through enhanced NF-kappaB activity. Int. J. Cancer 124:2607-2615. [DOI] [PubMed] [Google Scholar]
- 126.Toyoshima, F., T. Moriguchi, A. Wada, M. Fukuda, and E. Nishida. 1998. Nuclear export of cyclin B1 and its possible role in the DNA damage-induced G2 checkpoint. EMBO J. 17:2728-2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Toyota, M., Y. Sasaki, A. Satoh, K. Ogi, T. Kikuchi, H. Suzuki, H. Mita, N. Tanaka, F. Itoh, J. P. Issa, K. W. Jair, K. E. Schuebel, K. Imai, and T. Tokino. 2003. Epigenetic inactivation of CHFR in human tumors. Proc. Natl. Acad. Sci. U. S. A. 100:7818-7823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Uhlik, M. T., A. N. Abell, N. L. Johnson, W. Sun, B. D. Cuevas, K. E. Lobel-Rice, E. A. Horne, M. L. Dell'Acqua, and G. L. Johnson. 2003. Rac-MEKK3-MKK3 scaffolding for p38 MAPK activation during hyperosmotic shock. Nat. Cell Biol. 5:1104-1110. [DOI] [PubMed] [Google Scholar]
- 129.Uhlmann, F. 2003. Separase regulation during mitosis. Biochem. Soc. Symp. 2003:243-251. [DOI] [PubMed] [Google Scholar]
- 130.Uhlmann, F. 2004. The mechanism of sister chromatid cohesion. Exp. Cell Res. 296:80-85. [DOI] [PubMed] [Google Scholar]
- 131.Vader, G., and S. M. Lens. 2008. The Aurora kinase family in cell division and cancer. Biochim. Biophys. Acta 1786:60-72. [DOI] [PubMed] [Google Scholar]
- 132.van Doorn, R., W. H. Zoutman, R. Dijkman, R. X. de Menezes, S. Commandeur, A. A. Mulder, P. A. van der Velden, M. H. Vermeer, R. Willemze, P. S. Yan, T. H. Huang, and C. P. Tensen. 2005. Epigenetic profiling of cutaneous T-cell lymphoma: promoter hypermethylation of multiple tumor suppressor genes including BCL7a, PTPRG, and p73. J. Clin. Oncol. 23:3886-3896. [DOI] [PubMed] [Google Scholar]
- 133.van Vugt, M. A., and R. H. Medema. 2005. Getting in and out of mitosis with Polo-like kinase-1. Oncogene 24:2844-2859. [DOI] [PubMed] [Google Scholar]
- 134.Vidal, A., and A. Koff. 2000. Cell-cycle inhibitors: three families united by a common cause. Gene 247:1-15. [DOI] [PubMed] [Google Scholar]
- 135.Welchman, R. L., C. Gordon, and R. J. Mayer. 2005. Ubiquitin and ubiquitin-like proteins as multifunctional signals. Nat. Rev. Mol. Cell Biol. 6:599-609. [DOI] [PubMed] [Google Scholar]
- 136.Yanagida, M. 2005. Basic mechanism of eukaryotic chromosome segregation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 360:609-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yang, J., E. S. Bardes, J. D. Moore, J. Brennan, M. A. Powers, and S. Kornbluth. 1998. Control of cyclin B1 localization through regulated binding of the nuclear export factor CRM1. Genes Dev. 12:2131-2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yeong, F. M., H. Hombauer, K. S. Wendt, T. Hirota, I. Mudrak, K. Mechtler, T. Loregger, A. Marchler-Bauer, K. Tanaka, J. M. Peters, and E. Ogris. 2003. Identification of a subunit of a novel Kleisin-beta/SMC complex as a potential substrate of protein phosphatase 2A. Curr. Biol. 13:2058-2064. [DOI] [PubMed] [Google Scholar]
- 139.Yoshida, K., Y. Hamai, T. Suzuki, Y. Sanada, N. Oue, and W. Yasui. 2006. DNA methylation of CHFR is not a predictor of the response to docetaxel and paclitaxel in advanced and recurrent gastric cancer. Anticancer Res. 26:49-54. [PubMed] [Google Scholar]
- 140.Yu, X., K. Minter-Dykhouse, L. Malureanu, W. M. Zhao, D. Zhang, C. J. Merkle, I. M. Ward, H. Saya, G. Fang, J. van Deursen, and J. Chen. 2005. Chfr is required for tumor suppression and Aurora A regulation. Nat. Genet. 37:401-406. [DOI] [PubMed] [Google Scholar]
- 141.Zhou, B. B., P. Chaturvedi, K. Spring, S. P. Scott, R. A. Johanson, R. Mishra, M. R. Mattern, J. D. Winkler, and K. K. Khanna. 2000. Caffeine abolishes the mammalian G(2)/M DNA damage checkpoint by inhibiting ataxia-telangiectasia-mutated kinase activity. J. Biol. Chem. 275:10342-10348. [DOI] [PubMed] [Google Scholar]