Abstract
Mature glycoprotein spikes are inserted in the Lassa virus envelope and consist of the distal subunit GP-1, the transmembrane-spanning subunit GP-2, and the signal peptide, which originate from the precursor glycoprotein pre-GP-C by proteolytic processing. In this study, we analyzed the oligomeric structure of the viral surface glycoprotein. Chemical cross-linking studies of mature glycoprotein spikes from purified virus revealed the formation of trimers. Interestingly, sucrose density gradient analysis of cellularly expressed glycoprotein showed that in contrast to trimeric mature glycoprotein complexes, the noncleaved glycoprotein forms monomers and oligomers spanning a wide size range, indicating that maturation cleavage of GP by the cellular subtilase SKI-1/S1P is critical for formation of the correct oligomeric state. To shed light on a potential relation between cholesterol and GP trimer stability, we performed cholesterol depletion experiments. Although depletion of cholesterol had no effect on trimerization of the glycoprotein spike complex, our studies revealed that the cholesterol content of the viral envelope is important for the infectivity of Lassa virus. Analyses of the distribution of viral proteins in cholesterol-rich detergent-resistant membrane areas showed that Lassa virus buds from membrane areas other than those responsible for impaired infectivity due to cholesterol depletion of lipid rafts. Thus, derivation of the viral envelope from cholesterol-rich membrane areas is not a prerequisite for the impact of cholesterol on virus infectivity.
Lassa virus (LASV) is a member of the family Arenaviridae, of which Lymphocytic choriomeningitis virus (LCMV) is the prototype. Arenaviruses comprise more than 20 species, divided into the Old World and New World virus complexes (19). The Old World arenaviruses include the human pathogenic LASV strains, Lujo virus, which was first identified in late 2008 and is associated with an unprecedented high case fatality rate in humans, the nonhuman pathogenic Ippy, Mobala, and Mopeia viruses, and the recently described Kodoko virus (10, 30, 49). The New World virus complex contains, among others, the South American hemorrhagic fever-causing viruses Junín virus, Machupo virus, Guanarito virus, Sabiá virus, and the recently discovered Chapare virus (22).
Arenaviruses contain a bisegmented single-stranded RNA genome encoding the polymerase L, matrix protein Z, nucleoprotein NP, and glycoprotein GP. The bipartite ribonucleoprotein of LASV is surrounded by a lipid envelope derived from the plasma membrane of the host cell. The matrix protein Z has been identified as a major budding factor, which lines the interior of the viral lipid membrane, in which GP spikes are inserted (61, 75). The glycoprotein is synthesized as precursor protein pre-GP-C and is cotranslationally cleaved by signal peptidase into GP-C and the signal peptide, which exhibits unusual length, stability, and topology (3, 27, 28, 33, 70, 87). Moreover, the arenaviral signal peptide functions as trans-acting maturation factor (2, 26, 33). After processing by signal peptidase, GP-C of both New World and Old World arenaviruses is cleaved by the cellular subtilase subtilisin kexin isozyme-1/site-1 protease (SKI-1/S1P) into the distal subunit GP-1 and the membrane-anchored subunit GP-2 within the secretory pathway (5, 52, 63). For LCMV, it has been shown that GP-1 subunits are linked to each other by disulfide bonds and are noncovalently connected to GP-2 subunits (14, 24, 31). GP-1 is responsible for binding to the host cell receptor, while GP-2 mediates fusion between the virus envelope and the endosomal membrane at low pH due to a bipartite fusion peptide near the amino terminus (24, 36, 44). Sequence analysis of the LCMV GP-2 ectodomain revealed two heptad repeats that most likely form amphipathic helices important for this process (34, 86).
In general, viral class I fusion proteins have triplets of α-helical structures in common, which contain heptad repeats (47, 73). In contrast, class II fusion proteins are characterized by β-sheets that form dimers in the prefusion status and trimers in the postfusion status (43). The class III fusion proteins are trimers that, unlike class I fusion proteins, were not proteolytically processed N-terminally of the fusion peptide, resulting in a fusion-active membrane-anchored subunit (39, 62). Previous studies with LCMV described a tetrameric organization of the glycoprotein spikes (14), while more recent data using a bacterially expressed truncated ectodomain of the LCMV GP-2 subunit pointed toward a trimeric spike structure (31). Due to these conflicting data regarding the oligomerization status of LCMV GP, it remains unclear to which class of fusion proteins the arenaviral glycoproteins belong.
The state of oligomerization and the correct conformation of viral glycoproteins are crucial for membrane fusion during virus entry. The early steps of infection have been shown for several viruses to be dependent on the cholesterol content of the participating membranes (i.e., either the virus envelope or the host cell membrane) (4, 9, 15, 20, 21, 23, 40, 42, 53, 56, 76, 78, 79). In fact, it has been shown previously that entry of both LASV and LCMV is susceptible to cholesterol depletion of the target host cell membrane using methyl-β-cyclodextrin (MβCD) treatment (64, 71). Moreover, cholesterol not only plays an important role in the early steps during entry in the viral life cycle but also is critical in the virus assembly and release process. Several viruses of various families, including influenza virus, human immunodeficiency virus type 1 (HIV-1), measles virus, and Ebola virus, use the ordered environment of lipid raft microdomains. Due to their high levels of glycosphingolipids and cholesterol, these domains are characterized by insolubility in nonionic detergents under cold conditions (60, 72). Recent observations have suggested that budding of the New World arenavirus Junin virus occurs from detergent-soluble membrane areas (1). Assembly and release from distinct membrane microdomains that are detergent soluble have also been described for vesicular stomatitis virus (VSV) (12, 38, 68). At present, however, it is not known whether LASV requires cholesterol in its viral envelope for successful virus entry or whether specific membrane microdomains are important for LASV assembly and release.
In this study, we first investigated the oligomeric state of the premature and mature LASV glycoprotein complexes. Since it has been shown for several membrane proteins that the oligomerization and conformation are dependent on cholesterol (58, 59, 76, 78), we further analyzed the dependence of the cholesterol content of the virus envelope on glycoprotein oligomerization and virus infectivity. Finally, we characterized the lipid membrane areas from which LASV is released.
MATERIALS AND METHODS
Cell culture and viruses.
Vero E6 (green monkey kidney) and Huh 7 (human hepatoma) cells were cultured in Dulbecco's modified Eagle Medium (DMEM). For CHO-K1 (Chinese Hamster Ovary) cells, DMEM-F12 (Invitrogen) was used. All cell cultures were grown under standard conditions (37°C, 5% CO2) in the presence of penicillin (100 U ml−1), streptomycin (100 μg ml−1), glutamine (2 mmol liter−1), and 10% fetal calf serum (FCS) (Pan Biotech). Drosophila melanogaster Schneider II cells were cultured in Schneider's Drosophila medium (Invitrogen) under standard conditions (27°C).
Virus stocks of Lassa virus (LASV) (strain Josiah) were obtained by propagation of LASV in CHO-K1 cells as described earlier (52). Titers of LASV stocks were determined from 50% tissue culture infectious doses (TCID50) and stored at −80°C until further use. All experiments involving LASV-infected samples were performed under biological safety level 4 conditions at the Philipps University Marburg, Marburg, Germany.
The vesicular stomatitis virus (VSV) (Indiana serotype) reverse-genetics system was kindly provided by J. K. Rose (Department of Pathology, Yale University School of Medicine, New Haven, CT) and was described in detail earlier (46, 48, 65). Recombinant VSV expressing the LASV glycoprotein (VSVΔG/LASV-GP) was propagated as described previously (35). Infectious experiments were performed with CHO-K1 or Vero E6 cells by infection at a multiplicity of infection (MOI) of 1. After 1 h of virus inoculation in serum-free cell culture medium, infected cells were further incubated in medium containing 2% FCS for 7 days for LASV and 2 days for VSVΔG/LASV-GP production. To concentrate viruses, the cell culture medium was pelleted through a 20% sucrose cushion for 2.5 h at 125,000 × g in an SW32 rotor (Beckman). Virus pellets derived from the supernatant of a 162-cm2 flask were dissolved in 100 μl PBSdef (PBS deficient in Ca2+ and Mg2+) and stored at 4°C until further use.
Antibodies.
The monoclonal antibody anti-AC1 binds to GP-1 and was kindly provided by M. C. Georges-Courbot (Unit of Biology of Viral Emerging Infections, Institute Pasteur, Lyon, France). Rabbit antisera anti-GP-2-N, anti-GP-2-C, anti-NP, and anti-Z were used as described previously (29, 51). Polyclonal rabbit antibodies recognizing VSV G and influenza virus hemagglutinin (HA) were purchased from Sigma-Aldrich. Secondary antibodies against mouse and rabbit were labeled with IRDye 800 (Invitrogen).
Treatment with glycosidase, SDS-PAGE, and immunoblotting.
Samples for deglycosylation were treated with PNGase F (New England Biolabs) according to the instructions of the manufacturer. Protein samples were then boiled in reducing sample buffer and subjected to SDS-PAGE followed by Western blotting as described previously (28). Protein bands on immunoblots were visualized and quantified using the Odyssey infrared imaging system (LI-COR).
Cell surface biotinylation.
Cells were washed twice with cold PBS and incubated twice for 20 min at 4°C with PBS containing 1 mg ml−1 sulfo-N-hydroxysuccinimido-biotin (sulfo-NHS-biotin) (Pierce). Thereafter, cells were washed with PBS and incubated with 0.1 M glycine for 5 min. After 3 washes with PBS, cells were lysed in PBSdef containing 1% NP-40 and complete protease inhibitor (1 tablet per 50 ml lysis buffer [Calbiochem]). After sonication (40 W, 2 min), lysates were clarified by centrifugation (20,000 × g, 4°C, 20 min). Biotinylated proteins present in the supernatants were bound to streptavidin-coupled Sepharose beads (Pierce) at 4°C overnight. After three washing steps, biotinylated proteins were mixed with sample buffer, denatured (95°C, 10 min), and subjected to SDS-PAGE, followed by immunoblotting using protein-specific antibodies.
Expression and preparation of viral glycoprotein. (i) Mammalian cells.
Wild-type LASV glycoprotein and influenza virus hemagglutinin were expressed in CHO-K1 cells using the expression vector pCAGGS and Lipofectamine 2000 (Invitrogen) (8, 28). The VSV glycoprotein open reading frame was expressed using the pcDNA3.1 vector (kindly provided by G. Herrler, Institute of Virology, University of Hannover, Hannover, Germany).
(ii) Insect cells.
Drosophila melanogaster Schneider II cells were generated that stably express the GP-2 ectodomain (amino acid [aa] G260 to aa P427) using the pMT/Bip/V5 His A plasmid and pCoHygro vectors (Drosophila Expression System; Invitrogen). Expression was induced by the addition of 500 μM copper sulfate. Soluble GP-2 released into the supernatant of the cell culture was affinity purified using concanavalin A Sepharose (GE Healthcare) after preclearance of the cell supernatant by low-speed centrifugation (3,000 × g, 15 min, 4°C).
Glycoprotein analysis by sucrose gradient ultracentrifugation. (i) GP from total cells.
LASV glycoprotein-expressing CHO-K1 cells were lysed in MNT buffer (25 mM morpolineethanesulfonic acid [MES] [pH 5.0], 30 mM Tris-HCl [pH 7.4], 100 mM NaCl, 1 mM phenylmethylsulfonylfluoride, complete protease inhibitor, and 150 mM iodoacetamide) containing 1% Triton X-100 for 16 h at 4°C. The precleared lysate (centrifugation at 10 000 × g, 15 min, 4°C) was ultracentifuged in an SW60 rotor (Beckman) at 165,000 × g for 18 h through a 5 to 35% (wt/wt) sucrose gradient also containing 1% Triton X-100. Marker proteins (bovine serum albumin, catalase, and thyroglobulin [GE Healthcare]) were used as controls. After fractionation from the top, samples were analyzed by SDS-PAGE and immunoblotting.
(ii) GP on cell surface.
Proteins on the cell surface were labeled by biotinylation as described above. After cell lysis with 1% Triton X-100, samples were ultracentrifuged as described above. Then, biotinylated proteins were precipitated using streptavidin beads and subjected to SDS-PAGE and subsequent immunoblotting.
(iii) Spikes of recombinant VSVΔG/LASV-GP.
Purified VSVΔG/LASV-GP virions were lysed in an equal volume of MNT buffer containing 1% Triton X-100 at 4°C for 5 h. Samples were subjected to sucrose gradient ultracentrifugation and analyzed as described above.
(iv) Secreted ectodomain of GP-2.
Soluble GP-2 expressed in insect cells was concentrated, dialyzed against PBSdef, precleared by low-speed centrifugation (3,000 × g for 15 min at 4°C), and then subjected to ultracentrifugtion in an SW60 rotor (Beckman) at 165,000 × g for 18 h through a 0 to 20% (wt/wt) sucrose gradient without addition of any detergent. Aliquots of each gradient fraction were analyzed by SDS-PAGE followed by immunoblotting. The marker proteins (RNase, chymotrypsinogen A, ovalbumin, bovine serum albumin, and aldolase) for gradient ultracentrifugation were purchased from GE Healthcare.
Chemical cross-linking.
Purified LASV, VSVΔG/LASV-GP particles, or purified, soluble GP-2 was incubated in the presence of the indicated concentrations of N-γ-maleimidobutyryloxy-sulfo-succinimide-ester (sulfo-GMBS; Pierce) or ethylene-glycol-disuccinate-di(N-succinimidyl)-ester (EGS; Pierce) at room temperature (RT) for 30 min. To deplete cholesterol from the viral envelope of VSVΔG/LASV-GP, particles were treated with 10 mM MβCD (Sigma-Aldrich) at RT for 1 h prior to cross-linking. All cross-linked proteins were subjected to SDS-PAGE followed by immunoblotting.
Infection assay using MβCD-treated virions.
Aliquots containing 106 PFU in a 10-μl volume were mixed with 1 μl PBSdef containing different concentrations of MβCD and incubated at RT for 30 min. Cells were infected at an MOI of 1 with pretreated virions diluted in 1 ml serum-free cell culture medium. At 1 h postinfection, the inoculum was removed and cells were incubated for either 10 h (for VSVΔG/LASV-GP) or 48 h (for LASV) in cell culture medium containing 2% FCS. For cholesterol replenishment experiments, virions pretreated with 10 mM MβCD were further incubated at RT for an additional 30 min in the presence of the indicated concentrations of cholesterol (Sigma-Aldrich), dissolved in absolute ethanol and then analyzed by infection assay.
Detergent extraction of proteins.
Detergent-resistant membrane microdomains (DRMs) of CHO-K1 cells expressing either LASV glycoprotein, influenza virus hemagglutinin (HA), or VSV glycoprotein were isolated by flotation according to a method described previously (11, 45). Briefly, glycoprotein-expressing cells were lysed in 800 μl TBS buffer (10 mM Tris-HCl [pH 7.4], 150 mM NaCl, 5 mM EDTA, complete protease inhibitor) containing 1% Triton X-100 (TBST) at 4°C for 20 min. After cell homogenization via 15 passes through a 26-gauge needle, cell lysate was adjusted to 48% (wt/vol) sucrose in TBST, placed at the bottom of an ultracentrifuge tube, overlaid with 2 ml 35% (wt/vol) sucrose in TBST and topped with 500 μl 5% sucrose (wt/vol) in TBST. The gradient was ultracentrifuged (137,000 × g, 18 h, 4°C) in an SW55 rotor (Beckman) and divided into nine equal fractions collected from the top, which were then subjected to SDS-PAGE and immunoblot analyses.
Alternatively, detergent-soluble and -insoluble membranes were separated into two fractions by centrifugation following Triton X-100 treatment. For this, cells were scraped in PBSdef and pelleted at 3,000 × g for 5 min. Pellets were resuspended and incubated in ice-cold TNE buffer (25 mM Tris-HCl [pH 7.5], 150 mM NaCl, and 15 mM EDTA) containing 0.5% Triton X-100 at 4°C. Detergent-soluble and -insoluble material was fractioniated by centrifugation (20,000 × g, 4°C, 30 min). After insoluble material was resuspended in RIPA buffer (20 mM Tris [pH 8.5], 150 mM sodium chloride, 10 mM EDTA, 1% Triton X-100, 1% deoxycholate, 0.1% SDS, 5% aprotinin), aliquots were taken, separated by SDS-PAGE, and analyzed by immunoblotting. For detection of the DRM marker GM1, a small aliquot of each fraction was dropped onto a nitrocellulose membrane. The dried membrane was blocked with 10% skim milk in PBSdef and subsequently incubated with cholera toxin B conjugated to horseradish peroxidase (HRP) (1:20,000; Sigma-Aldrich) in 1% skim milk in PBSdef with 0.1% Tween 20. GM1 was detected using enhanced chemiluminescence (GE Healthcare) by exposure to autoradiography film (Kodak Biomax).
RESULTS
Oligomers of Lassa virus glycoprotein spikes.
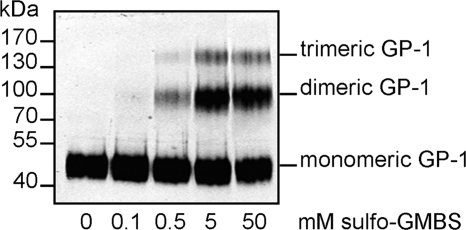
First, we investigated the oligomeric state of glycoprotein spikes on authentic LASV virions. At 7 days postinfection, viral particles released into the cell culture supernatant from infected Vero cells were purified over a 20% sucrose cushion. The oligomeric status of glycoprotein spikes on the surface of LASV virions was examined by cross-linking analyses and subsequent immunoblotting. Increasing cross-linker concentrations resulted in the appearance of three GP-1 species, while uncrosslinked virus contained only GP-1 monomers (Fig. 1). The observed molecular masses correlated well with the calculated masses of monomers (46 kDa), dimers (92 kDa), and trimers (138 kDa). Nevertheless, even at high cross-linker concentrations, we still observed monomers in addition to oligomeric GP-1. This might be explained by incomplete cross-linking, which would leave a certain amount of monomeric GP present on the virus surface. Alternatively, detection of higher oligomers by the antibody may be inefficient.
FIG. 1.
Identification of GP-1 oligomers by chemical cross-linking. Lassa virus virions purified by ultracentrifugation through a sucrose cushion were treated with increasing concentrations of sulfo-GMBS as indicated. The cross-linked proteins were separated by SDS-PAGE on an 8% acrylamide gel and analyzed by immunoblotting. GP was detected using an anti-GP-1 antibody.
It should be noted that cross-linking of oligomeric forms of GP-2 failed, most likely because GP-2 is masked by GP-1 and is therefore unaccessible (data not shown). In conclusion, these data demonstrate that the authentic LASV spike complex exhibits a trimeric organization.
Oligomerization of Lassa virus glycoprotein expressed in mammalian cells.
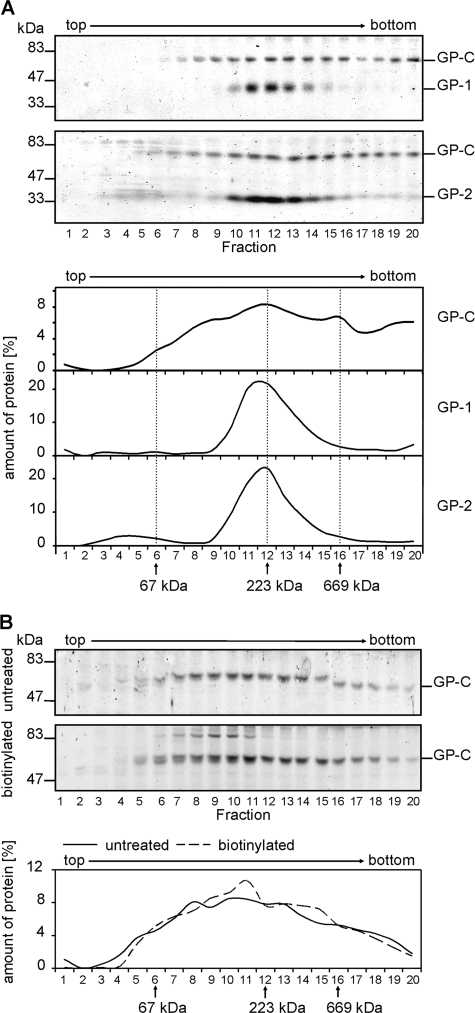
Since only cleaved glycoprotein subunits are incorporated into virus particles (52), we next wanted to know whether cleaved and noncleaved glycoproteins differ in their oligomeric state. To determine this, CHO-K1 cells expressing plasmid-encoded LASV GP were solubilized 48 h posttransfection with Triton X-100 in the presence of iodoacetamide to prevent artificial aggregation of GP. Cell lysates were separated by gradient ultracentrifugation, and the distribution of GP across the gradient was determined by SDS-PAGE and immunoblot analysis of gradient fractions. The majority of cleaved GP-1/GP-2 complexes were found in fractions 11 and 12, corresponding to the molecular mass of a GP trimer (228 kDa) as determined by comparison with the molecular mass marker protein catalase (230 kDa) (Fig. 2A). In contrast, noncleaved GP-C is widely distributed over the sucrose gradient, indicating that noncleaved GP-C exhibits various oligomeric forms (Fig. 2A).
FIG. 2.
Oligomeric state of LASV glycoprotein expressed in mammalian cells. (A) LASV glycoprotein present in cells analyzed by sucrose gradient ultracentrifugation. LASV glycoprotein-expressing cells were lysed with 1% Triton X-100, and cell lysate was subjected to sucrose gradient ultracentrifugation. Aliquots of each gradient fraction were subjected to SDS-PAGE and immunoblotting using the antibody anti-GP-1 (panel 1) or an anti-GP-2-C antiserum (panel 2). GP-C, GP-1, and GP-2 on the immunoblots were quantified (panels 3 to 5). The positions of the gradient marker proteins bovine serum albumin (67 kDa), catalase (232 kDa), and thyroglobulin (669 kDa) are indicated. (B) Comparison of oligomeric forms of GP-C on the cell surface with total GP-C. Cell surface proteins were biotinylated before cell lysis. Lysates of biotinylated and nonbiotinylated cells were subjected to sucrose gradient ultracentrifugation. Biotinylated GP-C was precipitated from gradient fractions using streptavidin-coupled Sepharose beads. Both protein samples, the biotinylated sample (broken line) and the nonbiotinylated control (solid line), were subjected to SDS-PAGE and immunoblotting using anti-GP-2-C antiserum.
Both the noncleaved and cleaved forms of GP are transported to the cell surface of both LASV-infected and GP-transfected cells (25, 52). We wanted to determine the oligomeric organization of GP-C at the cell surface. To address this question, we performed a cell surface biotinylation assay followed by sucrose gradient ultracentrifugation. As shown in Fig. 2B, GP-C at the cell surface revealed an oligomerization pattern similar to the distribution of totally expressed GP-C.
Taken together, these results demonstrate a trimeric state for mature LASV GP-1/GP-2 complexes in contrast to nonmature GP-C, which forms a variety of oligomers. It is most likely that after formation of various oligomers of GP-C in the endoplasmic reticulum (ER), the cleaved GP-1/GP-2 complexes efficiently form trimeric complexes after maturation cleavage in the early secretory pathway. In addition to the mature GP-1/GP-2 complexes, noncleaved GP-C is also transported to the plasma membrane, where it is present in a variety of oligomeric states.
Characterization of glycoprotein spikes in VSVΔG/LASV-GP virions.
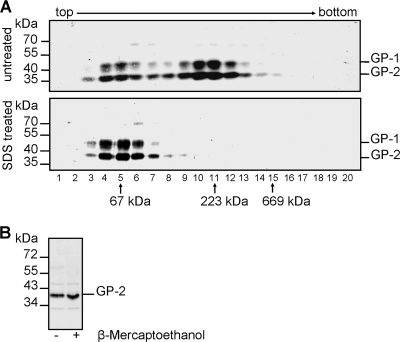
In order to characterize the formation of trimers, we utilized recombinant VSV expressing the glycoprotein of LASV, designated VSVΔG/LASV-GP (35). Since this virus is replication competent and LASV GP represents the only surface glycoprotein, the glycoprotein spikes are functional and should thus possess the same conformation as authentic LASV glycoprotein. Here, we wanted to address the question of whether the interactions within GP-1 trimers and within GP-2 trimers are stabilized by disulfide bonds. For this, purified VSVΔG/LASV-GP particles were lysed with Triton X-100 and were either denatured by SDS and heat treatment or left untreated. The samples were subjected to sucrose gradient ultracentrifugation, and GP distribution in the gradient was analyzed by SDS-PAGE and immunoblotting. The analysis of untreated VSVΔG/LASV-GP spikes revealed an accumulation of GP-1/GP-2 complexes in fractions 10 and 11 (Fig. 3A). Consistent with the presence of the molecular mass marker protein catalase (230 kDa) in these fractions, this result confirms the trimeric nature of mature spike complexes. A minor amount of monomers was detected in fractions 4 to 6, comigrating with the marker protein bovine serum albumin (BSA) (67 kDa). These represent either disintegrated trimers due to the experimental procedure or GP molecules that are present as monomers on the surfaces of virions. After denaturation by SDS and heat treatment, trimeric spikes dissociated into monomeric GP-1 and GP-2, respectively, migrating in fractions 4 to 6 (Fig. 3A, lower panel). GP-1 and GP-2 are present in the same fractions, a finding that could be due either to the similar molecular masses, about 40 kDa, of the two glycoprotein subunits or because the GP-1/GP-2 monomers are still connected by disulfide bridges, resulting in a protein complex with a molecular mass of 76 kDa. To analyze the latter possibility, VSVΔG/LASV-GP virions were separated by SDS-PAGE under reducing and nonreducing conditions, respectively. To avoid reduction due to diffusion of the reducing agent, the two samples were run on opposite sides of the SDS gel. The analysis of the GP-2 subunit revealed that independently of treatment by reducing agents, GP-2 is separated as a monomer during electrophoresis, exhibiting a molecular mass of about 40 kDa (Fig. 3B). These data demonstrate that GP-1 and GP-2 are not connected through disulfide bridges.
FIG. 3.
LASV glycoprotein spike formation on recombinant VSVΔG/LASV-GP virions. (A) Absence of intermolecular disulfide bridges. Virions purified by ultracentrifugation through a 20% sucrose cushion were solubilized with 0.5% Triton X-100. Samples were either treated with 0.2% SDS at 95°C for 10 min or left untreated and subjected to sucrose gradient analysis as performed for Fig. 1. GP-1 and GP-2 were detected using the specific antibodies anti-GP-1 and anti-GP-2-C. The three gradient marker proteins bovine serum albumin (67 kDa), catalase (232 kDa,) and thyroglobulin (669 kDa) are indicated. (B) Noncovalent bonding between GP-1 and GP-2. Purified virions were separated by SDS-PAGE under nonreducing and reducing conditions. GP-2 was immunochemically detected on the resulting immunoblot using anti-GP-2-C antiserum. Several lanes were cut and removed.
The obtained results clearly show a trimeric status of cleaved LASV GP-1/GP-2 complexes on the surfaces of recombinant VSV virions. The examination of these complexes indicates that unlike LCMV glycoprotein, the LASV GP1/GP2 heterodimers are not linked by disulfide bonds to form trimeric complexes (14, 31). However, consistent with results described for LCMV, GP-1 is noncovalently associated with GP-2 (14, 24, 31, 57).
Quarternary structure of native GP-2 ectodomain.
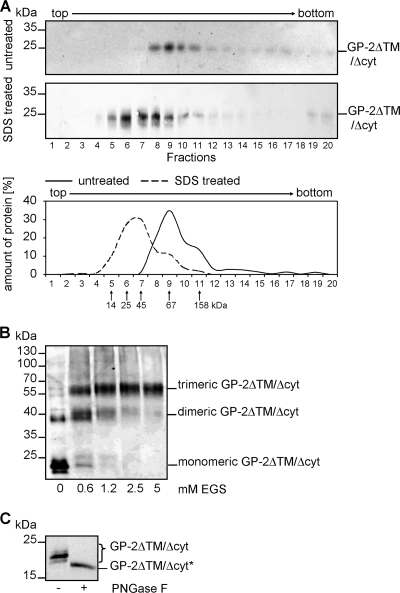
The membrane-anchored subunit GP-2 is responsible for membrane fusion during virus entry. Its quaternary structure was predicted to be trimeric, which has been demonstrated for a truncated and nonglycosylated form of the LCMV glycoprotein ectodomain expressed in Escherichia coli (31). In order to determine the quaternary structure of a full-length N-glycosylated ectodomain of LASV GP-2, we used sucrose gradient ultracentrifugation and chemical cross-linking analysis. For this, we generated a eukaryotic expression system using D. melanogaster Schneider II cells in which native GP-2 ectodomains (designated GP-2ΔTM/Δcyt) were secreted as an N-glycosylated form in an amount sufficient to allow further analysis. Oligomers of GP-2ΔTM/Δcyt were separated in a sucrose gradient under physiological conditions without detergent. Trimers of GP-2 were the predominant oligomeric form, which was found in fraction 9 (Fig. 4A). The trimeric ectodomain of GP-2 expressed in D. melanogaster cells has a calculated molecular mass of around 70 kDa, and it closely comigrated with the marker protein BSA (67 kDa). After heat treatment in the presence of SDS under nonreducing conditions, only monomers with a size of 22 kDa were detected, indicating that GP-2 ectodomain trimers are not connected through disulfide linkage (Fig. 4A). Independently, the oligomeric state of the affinity-purified GP-2 ectodomains was examined by cross-linking analysis, confirming the trimeric state of GP-2ΔTM/Δcyt (Fig. 4B). The somewhat diffuse bands of the GP-2 ectodomain are due to incomplete glycosylation in insect cells. Thus, a distinct protein band was observed only after treatment of GP-2 ectodomain with PNGase F (Fig. 4C). Altogether, these observations indicate that the complete and N-glycosylated ectodomain of the GP-2 subunit forms trimers under native conditions.
FIG. 4.
Oligomeric state of the ectodomain of the GP-2 subunit. (A) Sucrose gradient analysis without detergent. Soluble GP-2 ectodomain (GP-2ΔTM/Δcyt) was secreted from Drosophila melanogaster cells and affinity purified. Next, GP-2ΔTM/Δcyt was separated in a 0 to 20% sucrose gradient without any detergents. Fractions were collected from the top, and aliquots of each fraction were subjected to SDS-PAGE and immunoblotting using an anti-GP-2-N antiserum (upper panel). GP-2 ectodomain was quantified (lower panel, solid line). Monomeric GP-2ΔTM/Δcyt was obtained by boiling a sample of GP-2ΔTM/Δcyt in 0.2% SDS for 10 min before subjecting it to a corresponding sucrose gradient analysis (lower panel, broken line). An additional gradient analysis was performed using the marker proteins RNase (14 kDa), chymotrypsinogen A (25 kDa), ovalbumin (45 kDa), bovine serum albumin (67 kDa), and aldolase (158 kDa). (B) Cross-linking of the GP-2 ectodomain. Purified GP-2ΔTM/Δcyt was treated with the indicated concentrations of the chemical cross-linker EGS. The cross-linked proteins were then separated by SDS-PAGE, and GP-2 was detected by immunoblotting with an anti-GP-2-N antibody. (C) Evidence for N glycosylation of the GP-2 ectodomain. Purified GP-2ΔTM/Δcyt was incubated with PNGase F as indicated. The samples were analyzed by SDS-PAGE and immunoblotting by using the anti-GP-2-N antibody. GP-2ΔTM/Δcyt* represents the unglycosylated protein form.
Requirement for cholesterol in the viral envelope for Lassa virus infection.
For numerous viruses, it has been shown that cholesterol within the viral envelope is essential for virus infectivity, as was first described for HIV-1 (15). Here, we wanted to address whether LASV also requires cholesterol in its viral envelope for successful infection. Therefore, we performed cholesterol depletion experiments using methyl-β-cyclodextrin (MβCD). Virions were treated with MβCD prior to infection of Vero E6 cells. Virus titers were then analyzed from TCID50 at indicated times postinfection. Further, to examine whether other LASV factors in addition to the glycoprotein are important for virus infectivity influenced by cholesterol, we compared LASV with recombinant VSVΔG/LASV-GP. Following MβCD treatment, both viruses showed impaired infectivity (Fig. 5A). The infectivity of LASV decreases about 10-fold after maximum cholesterol depletion, whereas the infectivity of VSVΔG/LASV-GP is 1,000-fold reduced. The infectivity of the control virus VSV is also 1,000-fold decreased after MβCD treatment (data not shown), which is consistent with results in previous studies (40, 79).
FIG. 5.
Virus infectivity is dependent on cholesterol within the virus envelope. (A) Infectivity of virions containing LASV GP after cholesterol depletion. LASV and VSVΔG/LASV-GP were pretreated with increasing concentrations of methyl-β-cyclodextrin (MβCD), and cells were infected at an MOI of 1. Virus titers were measured from TCID50 after 48 h for LASV and after 10 h for VSVΔG/LASV-GP. (B) Infectivity of MβCD-treated virions after replenishment with exogenous cholesterol. Cholesterol of LASV and VSVΔG/LASV-GP was depleted by incubation with 10 mM MβCD as performed for panel A. Afterwards, cholesterol, dissolved in ethanol, was added to virions in increasing concentrations. Virus titers were measured as explained for panel A. Virions treated only with MβCD were set to 100%. Results are means from three independent experiments with standard deviations. Asterisks indicate significance values by t test as related to the value of the untreated samples (***, P < 0.001; **, P < 0.01; *, P < 0.05). (C) GP oligomerization after cholesterol depletion. VSVΔG/LASV-GP was treated with increasing concentrations of the cross-linker sulfo-GMBS after preincubation with 10 mM MβCD as described for panel A. Cross-linked proteins were separated by SDS-PAGE and subjected to immunoblotting using an anti-GP-1 antibody.
We wanted to further confirm the importance of cholesterol for virus infectivity by restoring infection with MβCD-treated viruses through the addition of exogenous cholesterol. As shown in Fig. 5B, the addition of exogenous cholesterol successfully reversed the inhibitory effect of MβCD on LASV and VSVΔG/LASV-GP infection in a dose-dependent manner, confirming that efficient virus infection requires cholesterol in the viral membrane.
To determine whether decreased infectivity caused by MβCD treatment is based on impaired stability of LASV GP trimers due to disruption or aggregation of glycoprotein spikes, we examined the oligomeric properties of cholesterol-depleted virions by cross-linking analysis. For this purpose, recombinant VSVΔG/LASV-GP virions were treated with MβCD or were left untreated before cross-linker was added in increasing concentrations. However, no differences in the oligomeric patterns were observed between cholesterol-depleted and untreated virions (Fig. 5C).
Taken together, these data show that LASV infectivity is impaired by depletion of cholesterol from the virus envelope. However, a decrease in GP complex stability resulting in decreased infectivity was not shown. GP function might also be affected by altered fluidity of the viral envelope. Therefore, further studies investigating the lipid composition of the virus envelope are needed.
Lassa virus budding from detergent-soluble membrane areas.
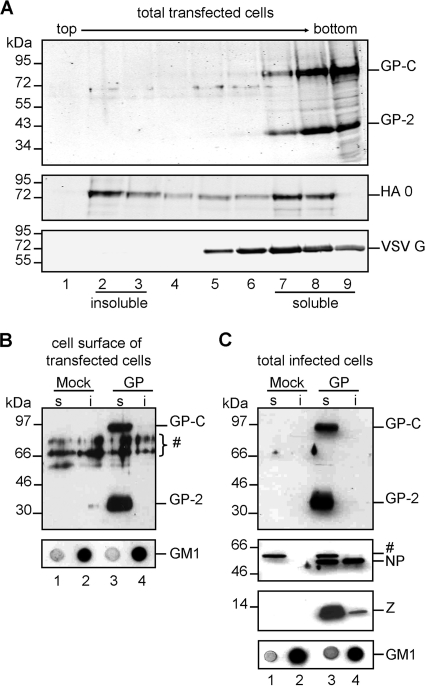
For the New World arenavirus Junin virus, it was recently shown that assembly of GP occurs in detergent-soluble membrane microdomains (1). However, given the biological variations among New World and Old World arenaviruses in terms of receptor usage and mechanisms of cell entry, it is of interest whether these viruses also differ with respect to their assembly platform at the plasma membrane. Moreover, the functional role of cholesterol within the LASV envelope for virus infectivity described in the present study led us to investigate the association of viral proteins with detergent-resistant membrane microdomains (DRMs), also known as lipid rafts. These DRMs are characterized by their high content of cholesterol and sphingolipids and their resistance to extraction with nonionic detergents at low temperatures (72). In order to isolate DRMs, CHO-K1 cells expressing plasmid-encoded LASV GP were treated with TNE buffer containing 0.5% Triton X-100 at 4°C, and detergent-soluble fractions were separated from detergent-resistant fractions by sucrose gradient ultracentrifugation. Fractions were collected and analyzed for the presence of GP by immunoblotting. Influenza virus hemagglutinin (HA), which is known to associate with detergent-resistant membranes, and VSV G protein, which lacks DRM association, were used as markers (38, 68, 69). Western blot analyses revealed that both noncleaved GP-C and cleaved GP-1/GP-2 complexes are present in detergent-soluble membrane fractions (Fig. 6A, fractions 7 to 9). This result was also observed in other mammalian cell types, including Vero E6 and Huh 7 cells, indicating that sensitivity of LASV GP to detergent extraction is cell type independent (data not shown).
FIG. 6.
Localization of LASV proteins in detergent-soluble membrane areas. (A) Flotation analysis of transiently LASV GP-expressing cells. Cells expressing LASV GP, VSV G, or influenza virus HA, respectively, were solubilized with 0.5% Triton X-100 at 4°C, and the lysate was subjected to flotation in a sucrose gradient. Gradient fractions were collected from the top after ultracentrifugation and analyzed using SDS-PAGE and Western blotting with the antiserum anti-GP-2-C, anti-HA, or anti-VSV G. The influenza virus HA, a DRM-located protein, and VSV glycoprotein G, a DRM-excluded protein, served as controls. (B) Cell surface distribution of LASV GP after Triton X-100 treatment. Transiently LASV GP-expressing cells were cell surface labeled with biotin. Then, cells were lysed in TNE buffer containing 0.5% Triton X-100 at 4°C and separated into detergent-soluble (s) and detergent-insoluble (i) fractions by centrifugation. Biotinylated proteins from each fraction were precipitated using streptavidin-coupled beads and subjected to SDS-PAGE and immunoblotting. LASV GP was stained with an anti-GP-2-C antibody. As a marker for detergent-insoluble fractions, GM1 was visualized on a nitrocellulose membrane by incubation with HRP-coupled cholera toxin subunit B. The number symbol denotes unspecific protein bands. (C) Effect of Triton X-100 treatment on LASV GP in infected cells. Cells were infected with LASV at an MOI of 1. Two days postinfection, they were divided into detergent-soluble (s) and detergent-insoluble (i) fractions and analyzed as described for panel B. Immunoblots were stained with an anti-GP-2-C, anti-NP, or anti-Z antibody. GM1 was visualized as described for panel B. The number symbol denotes unspecific protein bands.
Since budding of arenaviruses is presumed to occur at the plasma membrane, we performed cell surface biotinylation experiments with subsequent DRM extraction in order to investigate the surface distribution of GP. For this, LASV GP was expressed in Huh 7 cells, and 24 h posttransfection, cells were labeled with biotin. After biotinylation, cells were treated with 0.5% Triton X-100 at 4°C and the detergent-soluble fraction was separated from the detergent-insoluble fraction by centrifugation. Biotinylated proteins were precipitated from both fractions using streptavidin-coupled Sepharose, and GP was visualized by immunoblotting. As a control for the quality of DRM preparations, fractions were tested for the presence of the DRM resident glycolipid GM1 by dot blot analysis using HRP-conjugated cholera toxin B. Both the precursor GP-C and the cleaved subunit GP-2 were detected on the cell surface only in the detergent-soluble fraction, confirming the data obtained from analysis of total cell lysate (Fig. 6B).
Production of infectious LASV requires the interplay of viral components during assembly and release. Recently an interaction between GP and the matrix protein Z, which is the major driving force for virus budding, was demonstrated (17). It is therefore possible that either GP-Z interaction or involvement of other viral factors may result in altered GP membrane localization during virus infection. To test this, Huh 7 cells were infected with LASV at an MOI of 1. Forty-eight hours postinfection, cells were treated with TNE buffer containing 0.5% Triton X-100 at 4°C and detergent-soluble and detergent-insoluble fractions were separated by centrifugation. Western blot analysis of LASV proteins of these fractions showed that both the precursor GP-C and the cleaved GP-2 subunit were localized in infected cells in membrane areas that were sensitive to Triton X-100 (Fig. 6C). The matrix protein Z was predominantly found in the detergent-soluble fraction, and only a small fraction remained in the detergent-insoluble fraction. In contrast, NP was present in both fractions. However, the detergent-insoluble portion of NP might be explained by the interaction with detergent-insoluble matrices like the cytoskeleton, as shown for the NP of the New World arenavirus Junin virus (16).
Taken together, the major determinants for the production of infectious Lassa virions, GP and matrix protein Z, are present in detergent-soluble membrane areas, indicating that LASV buds from membrane areas distinct from lipid rafts. Thus, despite their differences in several steps in the virus life cycle, Old World and New World arenaviruses share characteristics in respect to their cellular platform for assembly and release.
DISCUSSION
Viral surface glycoproteins mediate entry of enveloped viruses into host cells through receptor binding at the cell surface and subsequent fusion of viral and cellular lipid membranes. Therefore, the characterization of viral glycoproteins is of fundamental interest to understanding the infection mechanism. The oligomeric nature of viral fusion proteins is essential for correct biological function (39). Previous studies regarding the oligomerization of LCMV glycoprotein have revealed conflicting results, indicating formation of tetramers and trimers, respectively (14, 31). In this study, we investigated the oligomeric state of Lassa virus (LASV) glycoprotein spikes using two independent approaches, sucrose gradient ultracentrifugation and chemical cross-linking analysis. We defined the mature LASV glycoprotein complex, as well as the spikes of recombinant VSV and plasmid-expressed mature LASV glycoprotein, as trimeric. The mature LASV GP complex is composed of three heterodimers, each formed by one GP-1 subunit and one GP-2 subunit that are noncovalently linked. Furthermore, we showed that the trimers of heterodimers are also formed by noncovalent linkages. A trimeric status of mature glycoprotein spikes was described for a number of viral fusion proteins requiring maturation cleavage prior to their fusion activation (18, 50, 74, 82, 83, 85).
We further generated a soluble and N-glycosylated construct of the entire GP-2 ectodomain that is secreted from insect cells and assembles as a trimer under native conditions, independently of other parts of the LASV glycoprotein. Our results are in agreement with a structural prediction for the LCMV glycoprotein and with results obtained by Eschli and coworkers for a nonglycosylated, truncated GP-2 ectodomain of LCMV expressed by E. coli (31, 34). These trimers containing only the membrane-anchored ectodomain of class I fusion proteins most likely exist in a postfusion state as six-helix bundles consisting of three molecules, each possessing two α-helices (80). Such a spontaneous formation of six-helix bundles is the result of the preferred low-energy state of the postfusion conformation, which was previously described by structural analyses of the ectodomains of the membrane-anchored subunits HIV gp41, influenza virus hemagglutinin 2, Ebola virus GP-2, parainfluenza virus F2, and the glycoprotein of Moloney murine leukemia virus (Mo-55) (32, 41, 66, 81). Thus, in this study we have demonstrated a trimeric state of LASV GP in both its prefusion and postfusion conformation. These characteristics are then in agreement with the classification of LASV GP as a class I fusion protein (47, 73).
In contrast to the trimeric organization of the mature glycoprotein complexes, we observed a different oligomerization pattern for noncleaved GP-C, which exhibited various oligomeric forms and/or aggregates. Nevertheless, the various oligomers of noncleaved GP-C are transported to the plasma membrane similarly to trimeric GP-1/GP-2 complexes, demonstrating that they are not aggregates accumulating in the ER due to misfolding. However, despite the presence of the cleaved and noncleaved GP on the surface of virus-infected cells, only cleaved GP-1/GP-2 complexes are inserted into nascent LASV virions (25, 52). The underlying mechanism for the discrimination of cleaved and noncleaved GP during virus assembly is currently unknown. It is possible that the matrix protein Z of LASV plays a critical role in the selective incorporation of mature glycoprotein complexes into budding particles. However, this seems rather unlikely, since the selective incorporation of cleaved glycoprotein subunits is also observed in a recombinant VSV expressing the glycoprotein of LASV (55; this study). This indicates that structural rearrangements in GP following proteolytic processing are an essential prerequisite for selective envelope glycoprotein incorporation. We have previously shown that the precursor glycoprotein and the cleaved subunits GP-1 and GP-2 differ in their glycosylation status. While immature GP-C remains completely Endo H sensitive, only the cleaved subunits acquired complex-type N glycosylation (25). Future studies will need to elucidate whether this differential glycosylation also contributes to selective GP incorporation into virions.
The cholesterol content within membranes is known to be crucial for oligomerization of several protein complexes. For example, oligomerization of various cell surface receptors is dependent on lipid rafts, which are cholesterol-rich membrane areas (7, 13, 67, 72, 84). An impact of cholesterol on virus infectivity has been observed for a number of viruses, including VSV, canine distemper virus, influenza virus, HIV-1, Borna disease virus, and hepatitis B and C viruses (4, 9, 15, 20, 40, 76, 79). In this report, we show that depletion of cholesterol from the LASV envelope resulted in impaired virus infectivity. This observation is supported by the finding that cholesterol addition following cholesterol depletion restores infectivity of virions. Further, investigations of several viruses have revealed that cholesterol depletion results in impaired fusion efficiency (6, 37, 76, 78), which might be explained by the absence of a stabilizing effect of cholesterol on the quarternary protein structure of the fusion protein. Such structural effects of cholesterol on protein functions were shown for the receptors CC chemokine receptor 5 and CXCR4 (58, 59). A possible dependence on cholesterol for the quarternary structure of LASV GP was analyzed by cholesterol depletion experiments with subsequent cross-linking analyses of the oligomeric pattern of GP spikes. However, an effect of cholesterol depletion on the stability of GP trimers could not be observed. Nevertheless, an impaired fusion competence of LASV GP is possible, which also might be based on altered fluidity of GP trimers within the lipid layer of the viral envelope membrane.
The level of impairment of virus infectivity by cholesterol depletion is observed to be much lower for LASV than for VSVΔG/LASV-GP and VSV. This is probably linked to different lipid compositions of LASV and VSV. The lipid compositions of viral envelopes are dependent on the membrane areas from which viruses are released. VSV is suggested to bud from detergent-soluble membrane areas. This was demonstrated by the localization of VSV glycoprotein in detergent-soluble membrane areas and the observation that the cholesterol content of VSV is significantly lower than that of influenza virus, which is known to bud from lipid rafts (38, 68). In this report, we have shown that both membrane-associated LASV proteins, the glycoprotein and matrix protein Z, are located within detergent-soluble membranes in LASV-infected cells, strongly indicating that LASV buds from detergent-soluble membrane areas. Thus, LASV, VSV, and VSVΔG/LASV-GP are all suggested to be released from detergent-soluble membrane areas. Nevertheless, differences in the impact of cholesterol on virus infectivity might relate to different lipid compositions independent of detergent solubility. The lower dependence of virus infectivity on cholesterol of LASV than of VSVΔG/LASV-GP might reflect that either matrix protein Z or cooperation of GP and Z is important for the lipid composition of LASV. This interplay was investigated with VSV in previous studies. Here, the lipid compositions of vesicles differed when only VSV glycoprotein or VSV matrix protein or both proteins were expressed (54). Further observations of VSV glycoprotein and VSV matrix protein revealed different locations of the proteins at the plasma membrane, also indicating that the two proteins do not share affinities to certain lipids (77). A similar result was observed using electron microscopy analysis of the plasma membrane of cells transfected with plasmids encoding the Z protein and GP-C of the arenavirus Junin virus (1). Currently, participation of the glycoprotein and matrix protein of arenaviruses in lipid selection of the viral envelope has not been investigated. The mechanism for sorting lipids into the viral envelope and the identification of the responsible viral proteins are important subjects for future investigations.
Acknowledgments
We thank J. K. Rose (Department of Pathology, Yale University School of Medicine, New Haven, CT) and G. Herrler (Institute of Virology, University of Hannover, Hannover, Germany) for generously providing the vesicular stomatitis virus reverse-genetics system and plasmid-encoded VSV glycoprotein G, respectively. We are grateful to M. C. Georges-Courbot (Unit of Biology of Viral Emerging Infections, Institute Pasteur, Lyon, France) for kindly providing monoclonal LASV antibodies. We gratefully acknowledge H. D. Klenk for critical manuscript review and A. Groseth for carefully editing the manuscript. We also thank P. Neubauer-Rädel for expert technical assistance and G. Ludwig and M. Schmidt for technical support with BSL-4 procedures.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (Ga-282/4-1, Ga-282/5-1, SFB 535, and SFB 593).
Footnotes
Published ahead of print on 4 November 2009.
REFERENCES
- 1.Agnihothram, S. S., B. Dancho, K. W. Grant, M. L. Grimes, D. S. Lyles, and J. H. Nunberg. 2009. Assembly of arenavirus envelope glycoprotein GPC in detergent-soluble membrane microdomains. J. Virol. 83:9890-9900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agnihothram, S. S., J. York, and J. H. Nunberg. 2006. Role of the stable signal peptide and cytoplasmic domain of G2 in regulating intracellular transport of the Junin virus envelope glycoprotein complex. J. Virol. 80:5189-5198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agnihothram, S. S., J. York, M. Trahey, and J. H. Nunberg. 2007. Bitopic membrane topology of the stable signal peptide in the tripartite Junin virus GP-C envelope glycoprotein complex. J. Virol. 81:4331-4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aizaki, H., K. Morikawa, M. Fukasawa, H. Hara, Y. Inoue, H. Tani, K. Saito, M. Nishijima, K. Hanada, Y. Matsuura, M. M. Lai, T. Miyamura, T. Wakita, and T. Suzuki. 2008. Critical role of virion-associated cholesterol and sphingolipid in hepatitis C virus infection. J. Virol. 82:5715-5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beyer, W. R., D. Popplau, W. Garten, D. von Laer, and O. Lenz. 2003. Endoproteolytic processing of the lymphocytic choriomeningitis virus glycoprotein by the subtilase SKI-1/S1P. J. Virol. 77:2866-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biswas, S., S. R. Yin, P. S. Blank, and J. Zimmerberg. 2008. Cholesterol promotes hemifusion and pore widening in membrane fusion induced by influenza hemagglutinin. J. Gen. Physiol. 131:503-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bose, S., J. Feix, S. Seetharam, and B. Seetharam. 1996. Dimerization of transcobalamin II receptor. Requirement of a structurally ordered lipid bilayer. J. Biol. Chem. 271:11718-11725. [DOI] [PubMed] [Google Scholar]
- 8.Böttcher, E., T. Matrosovich, M. Beyerle, H. D. Klenk, W. Garten, and M. Matrosovich. 2006. Proteolytic activation of influenza viruses by serine proteases TMPRSS2 and HAT from human airway epithelium. J. Virol. 80:9896-9898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bremer, C. M., C. Bung, N. Kott, M. Hardt, and D. Glebe. 2009. Hepatitis B virus infection is dependent on cholesterol in the viral envelope. Cell Microbiol. 11:249-260. [DOI] [PubMed] [Google Scholar]
- 10.Briese, T., J. T. Paweska, L. K. McMullan, S. K. Hutchison, C. Street, G. Palacios, M. L. Khristova, J. Weyer, R. Swanepoel, M. Egholm, S. T. Nichol, and W. I. Lipkin. 2009. Genetic detection and characterization of Lujo virus, a new hemorrhagic fever-associated arenavirus from southern Africa. PLoS Pathog. 5:e1000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown, D. A., and J. K. Rose. 1992. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 68:533-544. [DOI] [PubMed] [Google Scholar]
- 12.Brown, E. L., and D. S. Lyles. 2003. Organization of the vesicular stomatitis virus glycoprotein into membrane microdomains occurs independently of intracellular viral components. J. Virol. 77:3985-3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burger, K., G. Gimpl, and F. Fahrenholz. 2000. Regulation of receptor function by cholesterol. Cell Mol. Life Sci. 57:1577-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burns, J. W., and M. J. Buchmeier. 1991. Protein-protein interactions in lymphocytic choriomeningitis virus. Virology 183:620-629. [DOI] [PubMed] [Google Scholar]
- 15.Campbell, S. M., S. M. Crowe, and J. Mak. 2002. Virion-associated cholesterol is critical for the maintenance of HIV-1 structure and infectivity. AIDS 16:2253-2261. [DOI] [PubMed] [Google Scholar]
- 16.Candurra, N. A., M. J. Lago, L. Maskin, and E. B. Damonte. 1999. Involvement of the cytoskeleton in Junin virus multiplication. J. Gen. Virol. 80(Pt. 1):147-156. [DOI] [PubMed] [Google Scholar]
- 17.Capul, A. A., M. Perez, E. Burke, S. Kunz, M. J. Buchmeier, and J. C. de la Torre. 2007. Arenavirus Z-glycoprotein association requires Z myristoylation but not functional RING or late domains. J. Virol. 81:9451-9460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Center, R. J., P. Schuck, R. D. Leapman, L. O. Arthur, P. L. Earl, B. Moss, and J. Lebowitz. 2001. Oligomeric structure of virion-associated and soluble forms of the simian immunodeficiency virus envelope protein in the prefusion activated conformation. Proc. Natl. Acad. Sci. U. S. A. 98:14877-14882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charrel, R. N., X. de Lamballerie, and S. Emonet. 2008. Phylogeny of the genus Arenavirus. Curr. Opin. Microbiol. 11:362-368. [DOI] [PubMed] [Google Scholar]
- 20.Clemente, R., A. de Parseval, M. Perez, and J. C. de la Torre. 2009. Borna disease virus requires cholesterol in both cellular membrane and viral envelope for efficient cell entry. J. Virol. 83:2655-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Danthi, P., and M. Chow. 2004. Cholesterol removal by methyl-beta-cyclodextrin inhibits poliovirus entry. J. Virol. 78:33-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delgado, S., B. R. Erickson, R. Agudo, P. J. Blair, E. Vallejo, C. G. Albarino, J. Vargas, J. A. Comer, P. E. Rollin, T. G. Ksiazek, J. G. Olson, and S. T. Nichol. 2008. Chapare virus, a newly discovered arenavirus isolated from a fatal hemorrhagic fever case in Bolivia. PLoS Pathog. 4:e1000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desplanques, A. S., H. J. Nauwynck, D. Vercauteren, T. Geens, and H. W. Favoreel. 2008. Plasma membrane cholesterol is required for efficient pseudorabies virus entry. Virology 376:339-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Simone, C., M. A. Zandonatti, and M. J. Buchmeier. 1994. Acidic pH triggers LCMV membrane fusion activity and conformational change in the glycoprotein spike. Virology 198:455-465. [DOI] [PubMed] [Google Scholar]
- 25.Eichler, R., O. Lenz, W. Garten, and T. Strecker. 2006. The role of single N-glycans in proteolytic processing and cell surface transport of the Lassa virus glycoprotein GP-C. Virol. J. 3:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eichler, R., O. Lenz, T. Strecker, M. Eickmann, H. D. Klenk, and W. Garten. 2003. Identification of Lassa virus glycoprotein signal peptide as a trans-acting maturation factor. EMBO Rep. 4:1084-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eichler, R., O. Lenz, T. Strecker, M. Eickmann, H. D. Klenk, and W. Garten. 2004. Lassa virus glycoprotein signal peptide displays a novel topology with an extended endoplasmic reticulum luminal region. J. Biol. Chem. 279:12293-12299. [DOI] [PubMed] [Google Scholar]
- 28.Eichler, R., O. Lenz, T. Strecker, and W. Garten. 2003. Signal peptide of Lassa virus glycoprotein GP-C exhibits an unusual length. FEBS Lett. 538:203-206. [DOI] [PubMed] [Google Scholar]
- 29.Eichler, R., T. Strecker, L. Kolesnikova, J. ter Meulen, W. Weissenhorn, S. Becker, H. D. Klenk, W. Garten, and O. Lenz. 2004. Characterization of the Lassa virus matrix protein Z: electron microscopic study of virus-like particles and interaction with the nucleoprotein (NP). Virus Res. 100:249-255. [DOI] [PubMed] [Google Scholar]
- 30.Emonet, S., J. J. Lemasson, J. P. Gonzalez, X. de Lamballerie, and R. N. Charrel. 2006. Phylogeny and evolution of old world arenaviruses. Virology 350:251-257. [DOI] [PubMed] [Google Scholar]
- 31.Eschli, B., K. Quirin, A. Wepf, J. Weber, R. Zinkernagel, and H. Hengartner. 2006. Identification of an N-terminal trimeric coiled-coil core within arenavirus glycoprotein 2 permits assignment to class I viral fusion proteins. J. Virol. 80:5897-5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fass, D., and P. S. Kim. 1995. Dissection of a retrovirus envelope protein reveals structural similarity to influenza hemagglutinin. Curr. Biol. 5:1377-1383. [DOI] [PubMed] [Google Scholar]
- 33.Froeschke, M., M. Basler, M. Groettrup, and B. Dobberstein. 2003. Long-lived signal peptide of lymphocytic choriomeningitis virus glycoprotein pGP-C. J. Biol. Chem. 278:41914-41920. [DOI] [PubMed] [Google Scholar]
- 34.Gallaher, W. R., C. DiSimone, and M. J. Buchmeier. 2001. The viral transmembrane superfamily: possible divergence of Arenavirus and Filovirus glycoproteins from a common RNA virus ancestor. BMC Microbiol. 1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garbutt, M., R. Liebscher, V. Wahl-Jensen, S. Jones, P. Möller, R. Wagner, V. Volchkov, H. D. Klenk, H. Feldmann, and U. Ströher. 2004. Properties of replication-competent vesicular stomatitis virus vectors expressing glycoproteins of filoviruses and arenaviruses. J. Virol. 78:5458-5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glushakova, S. E., I. S. Lukashevich, and L. A. Baratova. 1990. Prediction of arenavirus fusion peptides on the basis of computer analysis of envelope protein sequences. FEBS Lett. 269:145-147. [DOI] [PubMed] [Google Scholar]
- 37.Guyader, M., E. Kiyokawa, L. Abrami, P. Turelli, and D. Trono. 2002. Role for human immunodeficiency virus type 1 membrane cholesterol in viral internalization. J. Virol. 76:10356-10364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harder, T., P. Scheiffele, P. Verkade, and K. Simons. 1998. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J. Cell Biol. 141:929-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harrison, S. C. 2008. Viral membrane fusion. Nat. Struct. Mol. Biol. 15:690-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Imhoff, H., V. von Messling, G. Herrler, and L. Haas. 2007. Canine distemper virus infection requires cholesterol in the viral envelope. J. Virol. 81:4158-4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joshi, S. B., R. E. Dutch, and R. A. Lamb. 1998. A core trimer of the paramyxovirus fusion protein: parallels to influenza virus hemagglutinin and HIV-1 gp41. Virology 248:20-34. [DOI] [PubMed] [Google Scholar]
- 42.Katzman, R. B., and R. Longnecker. 2003. Cholesterol-dependent infection of Burkitt's lymphoma cell lines by Epstein-Barr virus. J. Gen. Virol. 84:2987-2992. [DOI] [PubMed] [Google Scholar]
- 43.Kielian, M., and F. A. Rey. 2006. Virus membrane-fusion proteins: more than one way to make a hairpin. Nat. Rev. Microbiol. 4:67-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klewitz, C., H. D. Klenk, and J. ter Meulen. 2007. Amino acids from both N-terminal hydrophobic regions of the Lassa virus envelope glycoprotein GP-2 are critical for pH-dependent membrane fusion and infectivity. J. Gen. Virol. 88:2320-2328. [DOI] [PubMed] [Google Scholar]
- 45.Krautkrämer, E., S. I. Giese, J. E. Gasteier, W. Muranyi, and O. T. Fackler. 2004. Human immunodeficiency virus type 1 Nef activates p21-activated kinase via recruitment into lipid rafts. J. Virol. 78:4085-4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kretzschmar, E., L. Buonocore, M. J. Schnell, and J. K. Rose. 1997. High-efficiency incorporation of functional influenza virus glycoproteins into recombinant vesicular stomatitis viruses. J. Virol. 71:5982-5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lamb, R. A., and T. S. Jardetzky. 2007. Structural basis of viral invasion: lessons from paramyxovirus F. Curr. Opin. Struct. Biol. 17:427-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lawson, N. D., E. A. Stillman, M. A. Whitt, and J. K. Rose. 1995. Recombinant vesicular stomatitis viruses from DNA. Proc. Natl. Acad. Sci. U. S. A. 92:4477-4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lecompte, E., J. ter Meulen, S. Emonet, S. Daffis, and R. N. Charrel. 2007. Genetic identification of Kodoko virus, a novel arenavirus of the African pigmy mouse (Mus Nannomys minutoides) in West Africa. Virology 364:178-183. [DOI] [PubMed] [Google Scholar]
- 50.Lee, J. E., M. L. Fusco, A. J. Hessell, W. B. Oswald, D. R. Burton, and E. O. Saphire. 2008. Structure of the Ebola virus glycoprotein bound to an antibody from a human survivor. Nature 454:177-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lenz, O., J. ter Meulen, H. Feldmann, H. D. Klenk, and W. Garten. 2000. Identification of a novel consensus sequence at the cleavage site of the Lassa virus glycoprotein. J. Virol. 74:11418-11421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lenz, O., J. ter Meulen, H. D. Klenk, N. G. Seidah, and W. Garten. 2001. The Lassa virus glycoprotein precursor GP-C is proteolytically processed by subtilase SKI-1/S1P. Proc. Natl. Acad. Sci. U. S. A. 98:12701-12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liao, Z., L. M. Cimakasky, R. Hampton, D. H. Nguyen, and J. E. Hildreth. 2001. Lipid rafts and HIV pathogenesis: host membrane cholesterol is required for infection by HIV type 1. AIDS Res. Hum. Retroviruses 17:1009-1019. [DOI] [PubMed] [Google Scholar]
- 54.Luan, P., L. Yang, and M. Glaser. 1995. Formation of membrane domains created during the budding of vesicular stomatitis virus. A model for selective lipid and protein sorting in biological membranes. Biochemistry 34:9874-9883. [DOI] [PubMed] [Google Scholar]
- 55.Maisa, A., U. Ströher, H. D. Klenk, W. Garten, and T. Strecker. 2009. Inhibition of lassa virus glycoprotein cleavage and multicycle replication by site 1 protease-adapted alpha(1)-antitrypsin variants. PLoS Negl. Trop. Dis. 3:e446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Medigeshi, G. R., A. J. Hirsch, D. N. Streblow, J. Nikolich-Zugich, and J. A. Nelson. 2008. West Nile virus entry requires cholesterol-rich membrane microdomains and is independent of alphavbeta3 integrin. J. Virol. 82:5212-5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Neuman, B. W., B. D. Adair, J. W. Burns, R. A. Milligan, M. J. Buchmeier, and M. Yeager. 2005. Complementarity in the supramolecular design of arenaviruses and retroviruses revealed by electron cryomicroscopy and image analysis. J. Virol. 79:3822-3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nguyen, D. H., and D. Taub. 2002. Cholesterol is essential for macrophage inflammatory protein 1 beta binding and conformational integrity of CC chemokine receptor 5. Blood 99:4298-4306. [DOI] [PubMed] [Google Scholar]
- 59.Nguyen, D. H., and D. Taub. 2002. CXCR4 function requires membrane cholesterol: implications for HIV infection. J. Immunol. 168:4121-4126. [DOI] [PubMed] [Google Scholar]
- 60.Ono, A., and E. O. Freed. 2005. Role of lipid rafts in virus replication. Adv. Virus Res. 64:311-358. [DOI] [PubMed] [Google Scholar]
- 61.Perez, M., R. C. Craven, and J. C. de la Torre. 2003. The small RING finger protein Z drives arenavirus budding: implications for antiviral strategies. Proc. Natl. Acad. Sci. U. S. A. 100:12978-12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roche, S., A. A. Albertini, J. Lepault, S. Bressanelli, and Y. Gaudin. 2008. Structures of vesicular stomatitis virus glycoprotein: membrane fusion revisited. Cell Mol. Life Sci. 65:1716-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rojek, J. M., A. M. Lee, N. Nguyen, C. F. Spiropoulou, and S. Kunz. 2008. Site 1 protease is required for proteolytic processing of the glycoproteins of the South American hemorrhagic fever viruses Junin, Machupo, and Guanarito. J. Virol. 82:6045-6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rojek, J. M., M. Perez, and S. Kunz. 2008. Cellular entry of lymphocytic choriomeningitis virus. J. Virol. 82:1505-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rose, N. F., A. Roberts, L. Buonocore, and J. K. Rose. 2000. Glycoprotein exchange vectors based on vesicular stomatitis virus allow effective boosting and generation of neutralizing antibodies to a primary isolate of human immunodeficiency virus type 1. J. Virol. 74:10903-10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ruigrok, R. W., A. Aitken, L. J. Calder, S. R. Martin, J. J. Skehel, S. A. Wharton, W. Weis, and D. C. Wiley. 1988. Studies on the structure of the influenza virus haemagglutinin at the pH of membrane fusion. J. Gen. Virol. 69(Pt. 11):2785-2795. [DOI] [PubMed] [Google Scholar]
- 67.Saffarian, S., Y. Li, E. L. Elson, and L. J. Pike. 2007. Oligomerization of the EGF receptor investigated by live cell fluorescence intensity distribution analysis. Biophys. J. 93:1021-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scheiffele, P., A. Rietveld, T. Wilk, and K. Simons. 1999. Influenza viruses select ordered lipid domains during budding from the plasma membrane. J. Biol. Chem. 274:2038-2044. [DOI] [PubMed] [Google Scholar]
- 69.Scheiffele, P., M. G. Roth, and K. Simons. 1997. Interaction of influenza virus haemagglutinin with sphingolipid-cholesterol membrane domains via its transmembrane domain. EMBO J. 16:5501-5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schrempf, S., M. Froeschke, T. Giroglou, D. von Laer, and B. Dobberstein. 2007. Signal peptide requirements for lymphocytic choriomeningitis virus glycoprotein C maturation and virus infectivity. J. Virol. 81:12515-12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shah, W. A., H. Peng, and S. Carbonetto. 2006. Role of non-raft cholesterol in lymphocytic choriomeningitis virus infection via alpha-dystroglycan. J. Gen. Virol. 87:673-678. [DOI] [PubMed] [Google Scholar]
- 72.Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature 387:569-572. [DOI] [PubMed] [Google Scholar]
- 73.Skehel, J. J., and D. C. Wiley. 2000. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu. Rev. Biochem. 69:531-569. [DOI] [PubMed] [Google Scholar]
- 74.Song, H. C., M. Y. Seo, K. Stadler, B. J. Yoo, Q. L. Choo, S. R. Coates, Y. Uematsu, T. Harada, C. E. Greer, J. M. Polo, P. Pileri, M. Eickmann, R. Rappuoli, S. Abrignani, M. Houghton, and J. H. Han. 2004. Synthesis and characterization of a native, oligomeric form of recombinant severe acute respiratory syndrome coronavirus spike glycoprotein. J. Virol. 78:10328-10335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Strecker, T., R. Eichler, J. Meulen, W. Weissenhorn, H. D. Klenk, W. Garten, and O. Lenz. 2003. Lassa virus Z protein is a matrix protein and sufficient for the release of virus-like particles [corrected]. J. Virol. 77:10700-10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sun, X., and G. R. Whittaker. 2003. Role for influenza virus envelope cholesterol in virus entry and infection. J. Virol. 77:12543-12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Swinteck, B. D., and D. S. Lyles. 2008. Plasma membrane microdomains containing vesicular stomatitis virus M protein are separate from microdomains containing G protein and nucleocapsids. J. Virol. 82:5536-5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Teissier, E., and E. I. Pecheur. 2007. Lipids as modulators of membrane fusion mediated by viral fusion proteins. Eur. Biophys. J. 36:887-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang, W., Y. J. Fu, Y. G. Zu, N. Wu, J. Reichling, and T. Efferth. 2009. Lipid rafts play an important role in the vesicular stomatitis virus life cycle. Arch. Virol. 154:595-600. [DOI] [PubMed] [Google Scholar]
- 80.Weissenhorn, W., L. J. Calder, S. A. Wharton, J. J. Skehel, and D. C. Wiley. 1998. The central structural feature of the membrane fusion protein subunit from the Ebola virus glycoprotein is a long triple-stranded coiled coil. Proc. Natl. Acad. Sci. U. S. A. 95:6032-6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weissenhorn, W., S. A. Wharton, L. J. Calder, P. L. Earl, B. Moss, E. Aliprandis, J. J. Skehel, and D. C. Wiley. 1996. The ectodomain of HIV-1 env subunit gp41 forms a soluble, alpha-helical, rod-like oligomer in the absence of gp120 and the N-terminal fusion peptide. EMBO J. 15:1507-1514. [PMC free article] [PubMed] [Google Scholar]
- 82.Wilk, T., F. de Haas, A. Wagner, T. Rutten, S. Fuller, R. M. Flugel, and M. Lochelt. 2000. The intact retroviral Env glycoprotein of human foamy virus is a trimer. J. Virol. 74:2885-2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wilson, I. A., J. J. Skehel, and D. C. Wiley. 1981. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature 289:366-373. [DOI] [PubMed] [Google Scholar]
- 84.Yi, J. S., H. J. Choo, B. R. Cho, H. M. Kim, Y. N. Kim, Y. M. Ham, and Y. G. Ko. 2009. Ginsenoside Rh2 induces ligand-independent Fas activation via lipid raft disruption. Biochem. Biophys. Res. Commun. 385:154-159. [DOI] [PubMed] [Google Scholar]
- 85.Yin, H. S., X. Wen, R. G. Paterson, R. A. Lamb, and T. S. Jardetzky. 2006. Structure of the parainfluenza virus 5 F protein in its metastable, prefusion conformation. Nature 439:38-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.York, J., S. S. Agnihothram, V. Romanowski, and J. H. Nunberg. 2005. Genetic analysis of heptad-repeat regions in the G2 fusion subunit of the Junin arenavirus envelope glycoprotein. Virology 343:267-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.York, J., V. Romanowski, M. Lu, and J. H. Nunberg. 2004. The signal peptide of the Junin arenavirus envelope glycoprotein is myristoylated and forms an essential subunit of the mature G1-G2 complex. J. Virol. 78:10783-10792. [DOI] [PMC free article] [PubMed] [Google Scholar]