Abstract
HIV-1 infection is characterized by loss of CD56dim CD16+ NK cells and increased terminal differentiation on various lymphocyte subsets. We identified a decrease of CD57− and CD57dim cells but not of CD57bright cells on CD56dim CD16+ NK cells in chronic HIV infection. Increasing CD57 expression was strongly associated with increasing frequencies of killer immunoglobulin-like receptors (KIRs) and granzyme B-expressing cells but decreasing percentages of cells expressing CD27+, HLA-DR+, Ki-67+, and CD107a. Our data indicate that HIV leads to a decline of less-differentiated cells and suggest that CD57 is a useful marker for terminal differentiation on NK cells.
NK cells are effector cells of innate immunity which are pivotal as first-line defense against viral infections, such as HIV infection (14). Large genotypic studies demonstrated a delayed onset of AIDS in HIV-seropositive individuals carrying the activating receptor KIR3DS1 and/or alleles of the inhibiting receptor KIR3DL1 in conjunction with HLA-Bw4-80I (18, 19). Development of NK cells mainly takes place in the bone marrow, from which mature NK cells move out to reside and circulate in peripheral sites (13). Mature NK cells are characterized by granules which harbor granzymes and perforin. These NK cells are fully armed, “ready-to-go” effector cells (17).
A number of NK cell abnormalities have been reported in HIV infection (9), including high activation status (2, 10), increased turnover (16), differential expression of activating and inhibitory receptors (20), impaired interaction with dendritic cells (12), and loss of CD56dim CD16+ NK cells (23). CD56dim CD16+ NK cells represent the largest NK cell subset in peripheral blood in healthy individuals. The expression of killer immunoglobulin-like receptors (KIRs) and CD57 are predominant features of this subpopulation (8, 15). CD57 expression on NK cells has been previously associated with replicative senescence on T and NK cells (4), raising the question of how HIV-1 infection alters CD57 expression on CD56dim CD16+ NK cells.
To the best of our knowledge, no one has addressed the phenotypic and functional properties of CD56dim CD16+ NK cells that are preferentially lost during HIV infection. Here, we provide evidence that increasing CD57 expression indicates terminal differentiation in healthy individuals, as well in as HIV-infected subjects. We furthermore show that HIV infection is associated with preferential loss of less-differentiated cells, which are characterized by high activation status and turnover.
In this study, blood samples from 37 HIV-seropositive individuals and 15 healthy subjects were analyzed; all HIV-infected patients were either antiretroviral therapy naïve or untreated for more than one year. The HIV-positive study cohort comprised 10 patients with a viral load of less than 2,000 copies/ml, 14 patients with a viral load ranging from 2,000/ml to 20,000 copies/ml, and 13 patients with a viral load above 20,000 copies/ml. CD4 T cell counts ranged from 180/μl to 1,355/μl, the average being 457.3/μl.
The study was approved by the local ethics commission (Ethikkommission der Medizinischen Hochschule Hannover, Votum No. 3150), and all study participants gave informed written consent for their participation.
Flow cytometric analysis was performed on cryopreserved peripheral blood mononuclear cells (PBMCs) as previously described (21, 22). A list of monoclonal antibodies employed in this study is available upon request. For intracellular analysis of granzyme B, perforin, and Ki-67, we used a fixation and permeabilization kit (Invitrogen). At least 1 million events were acquired for each sample, using either a FACSAria or LSR II flow cytometer (BD Biosciences). Data were analyzed with FlowJo (TreeStar). Lymphocytes were defined by forward and side scatter. CD3+, CD14+, CD19+, dead cells, and cell aggregates were removed from analysis based on peridinin chlorophyll protein and Viaprobe staining and gating on a plot of forward-scatter area versus forward-scatter height (Fig. 1A). NK cells and their distinctive subpopulations were defined based on their CD56 and/or CD16 expression. Fluorescence-minus-one (FMO) staining was used to determine threshold values for the expression of specific markers.
FIG. 1.
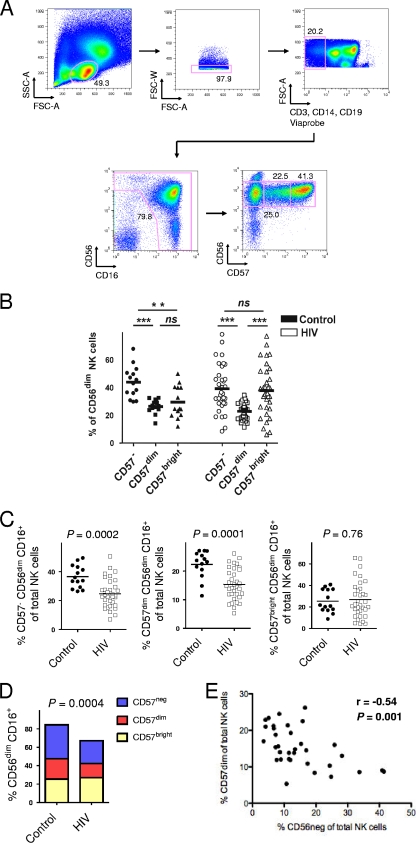
HIV infection is associated with loss of CD57− and CD57dim but not CD57bright CD56dim CD16+ NK cells. (A) Representative gating scheme for identification of NK cells. NK cells were defined as CD3− CD14− CD19− lymphocytes expressing either CD56 or CD16 or both. We divided CD56dim CD16+ NK cells into three subsets based on their level of CD57 expression: CD57−, CD57dim, and CD57bright cells. Numbers on FACS plots indicate frequency of gated population. SSC-A, side scatter area; FSC-A, forward scatter area; FSC-W, forward scatter width. (B) Comparison of percentages of the CD57−, CD57dim, and CD57bright subpopulations in control subjects (n = 14) and HIV-seropositive individuals (n = 34) on CD56dim CD16+ NK cells. ns, not significant (P > 0.05); **, P < 0.01; ***, P < 0.001. (C) Frequencies of CD57−, CD57dim, and CD57bright expressing CD56dim CD16+ NK cells in relation to total NK cells in control subjects (n = 14) and HIV-seropositive individuals (n = 34). (D) Mean frequency of CD56dim CD16+ NK cells in 14 control individuals and in 34 HIV-infected people and the distribution of CD57−, CD57dim, and CD57bright cells within CD56dim CD16+ NK cells is shown. (E) Relationship between percentage of CD57dim CD56dim CD16+NK cells and percentage of CD56neg CD16+ NK cells on total NK cells. Horizontal bars in dot plots show the means.
NK cells as defined above were sorted from cryopreserved PBMCs on a FACSAria (purities ranged from 91% to 99%). An amount of 105 NK cells was plated per well and stimulated with 10 ng/ml interleukin-15 (IL-15), 100 ng/ml IL-12, and 5 × 104 K562 cells. A CD107a degranulation assay was performed as described previously (1, 12). GraphPad Prism (version 5.0) software was used for statistical evaluation of data. Correlation analysis was performed using the Pearson test. The unpaired t test was performed when two groups were compared, and all t tests were two tailed. Comparison of more than two groups was performed using one-way analysis of variance followed by Tukey's post-hoc test. P values of less than 0.05 were considered significant.
We found that CD57 on NK cells was predominantly expressed on the CD56dim CD16+ population (Fig. 1A). The expression patterns of CD57 allowed us to differentiate between three subfractions within CD56dim CD16+ NK cells, namely, CD57−, CD57dim, and CD57bright cells. The frequency of the CD57bright subpopulation on CD56dim CD16+ NK cells was increased compared to the frequency of the CD57dim subpopulation on CD56dim CD16+ NK cells in HIV-seropositive patients but not in HIV-seronegative control subjects (Fig. 1B). This relative increase was associated with substantial reductions of the CD57− CD56dim and the CD57dim CD56dim NK cell subpopulations of total NK cells in our HIV-seropositive cohort compared to these subpopulations in healthy control subjects (means, 36.6% versus 24.8% [P = 0.0002] and 22.4% versus 15.4% [P = 0.0001]), but the frequencies of CD57bright CD56dim NK cells within total NK cells were similar between HIV-infected patients and HIV-seronegative individuals (Fig. 1C). In accordance with previously published data (3, 23), we could confirm that there is a relative loss of CD56dim CD16+ NK cells in HIV infection (mean, 84.3% versus 67.0%, P = 0.0004) (Fig. 1D). Our data indicate that this loss is predominantly due to decreased numbers of CD57− CD56dim and CD57dim CD56dim NK cells, leading to a relative overrepresentation of CD57bright cells within CD56dim CD16+ NK cells in HIV infection (Fig. 1C). There was no significant correlation between the relative loss of CD57− and CD57dim NK cells and absolute numbers of CD56dim CD16+ NK cells, but there was a significant inverse correlation between loss of CD57dim NK cells and increasing percentages of CD56− CD16+ cells (Pearson r = −0.54, P = 0.001) (Fig. 1E).
To determine whether the relative decrease of CD57− and CD57dim NK cells was associated with parameters of HIV disease progression, we performed correlation analysis of the percentages of CD57− or CD57dim cells with viral load and CD4 T cell counts. We found no such correlations (Pearson r < 0.2 and P > 0.05 for all) (data not shown). A recent cross-sectional and longitudinal study demonstrated that changes in the NK cell compartment, as shown by down-modulation of Siglec-7 on CD56dim NK cells, are associated with HIV viremia (5). The longitudinal data in the study indicated that the full restoration of NK cell pathologies required 24 months of antiviral treatment. This suggests that alterations in the NK cell compartment can be driven by HIV viral load but that these changes seem to require a significant amount of time.
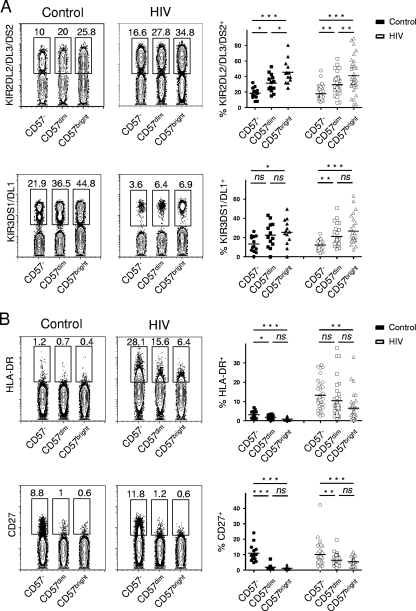
We next investigated the phenotypic and functional properties of the CD57−, CD57dim, and CD57bright subpopulations on CD56dim CD16+ NK cells. For KIR2DL2/DL3/DS2, we detected increasing prevalences of KIR-expressing NK cells with increasing expression of CD57 in both healthy control subjects and HIV-infected blood donors (Fig. 2A). As for KIR3DS1/DL1, we found an increase of KIR+-expressing NK cells between CD57− and CD57bright cells in control individuals and significant differences in percentages of KIR3DS1/DL1-expressing NK cells between CD57− and CD57dim, as well as between CD57− and CD57bright, NK cells in our HIV-positive cohort (Fig. 2A). These results suggest that increasing CD57 expression is associated with higher numbers of KIR-expressing NK cells in control subjects and HIV-infected subjects.
FIG. 2.
Phenotypic characterization of the CD57−, CD57dim, and CD57bright subpopulations of CD56dim CD16+ NK cells. Representative flow cytometry plots for one control and one HIV-infected subject and summary data for all individuals whose PBMCs were analyzed are shown. CD57−, CD57dim, and CD57bright NK cells are concatenated to visualize them in a single dot plot. Numbers in contour plots indicate percentages of gated events of the respective subset. (A) Percentages of KIR2DL2/DL3/DS2 and KIR3DS1/DL1-expressing CD57−, CD57dim, and CD57bright cells were analyzed in control individuals (n = 15) and HIV-infected subjects (n = 37). (B) Numbers of HLA-DR-expressing and CD27-expressing CD57−, CD57dim, and CD57bright cells in control individuals' (n = 15) and HIV-infected subjects' (n = 37) PBMCs were analyzed. Horizontal bars in dot plots show the means. ns, not significant (P > 0.05); *, P < 0.05; **, P < 0.01; ***, P < 0.001.
We next addressed the question of whether increasing CD57 expression is linked to differential phenotypic properties of NK cells and analyzed the HLA-DR and CD27 expression of the CD57−, CD57dim, and CD57bright subpopulations on CD56dim CD16+ NK cells. A significantly higher fraction of NK cells expressed HLA-DR in the CD57− than in the CD57bright subset in both healthy control individuals and HIV-infected subjects (Fig. 2B). A considerably higher portion of NK cells was positive for HLA-DR in HIV-infected individuals than in control subjects (means, 3.2% versus 13.2% [P < 0.0001], 1.8% versus 10.4% [P = 0.001], and 0.9% versus 6.5% [P = 0.005] for CD57−, CD57dim, and CD57bright subpopulations, respectively). We furthermore detected marked differences in frequencies of cells expressing CD27, a member of the tumor necrosis factor (TNF) receptor family (24). CD57− NK cells displayed the highest percentages of CD27+ cells, whereas CD57bright cells were almost all negative for CD27, in both control individuals and HIV-seropositive subjects (Fig. 2B). We thus show that increasing expression of CD57 is associated with differential activation status and differential phenotype.
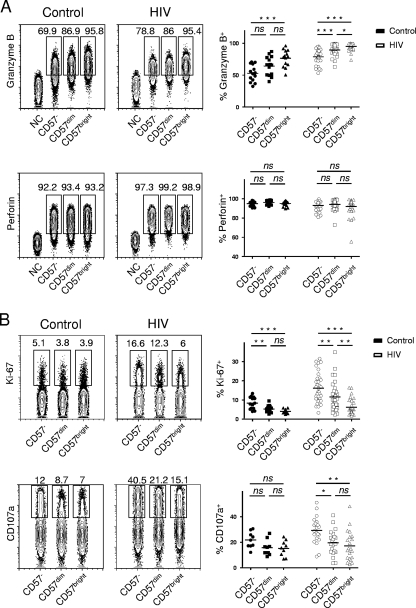
Next, we sought to determine whether CD57 is linked to differential functional phenotypes by assessing the intracellular expression of granzyme B, perforin, and Ki-67. The frequencies of perforin-expressing NK cells did not vary within the different CD57 subsets of CD56dim CD16+ NK cells (Fig. 3A). However, we found that CD57bright cells displayed the highest frequencies of granzyme B+ in both control and HIV-seropositive subjects, whereas CD57− cells exhibited the lowest percentages for granzyme B+ cells (Fig. 3A). Conversely, when we studied the expression of Ki-67, we identified the opposite trend: less than 5% of CD57bright cells in control individuals and less than 10% of CD57bright cells in HIV-infected study subjects expressed Ki-67 (Fig. 3B). The highest numbers of Ki-67+ cells were found in the CD57− population.
FIG. 3.
Functional characterization of CD57−, CD57dim, and CD57bright cells within the CD56dim CD16+ NK cell population. (A) Representative staining results for granzyme B and perforin and summary data for control (n = 14) and HIV-seropositive subjects (n = 36). Numbers in the concatenated contour plots indicate percentages of gated events of the respective subset. B cells were defined as the negative control for granzyme and perforin staining. (B) Percentages of Ki-67+ and CD107a+ cells on CD57−, CD57dim, and CD57bright cells within the CD56dim NK cell population in control (n = 14 and n = 9, respectively) and HIV-seropositive (n = 36 and n = 21, respectively) subjects' PBMCs were analyzed. Horizontal bars in dot plots show the means. NC, negative control; ns, not significant (P > 0.05); *, P < 0.05; **, P < 0.01; ***, P < 0.001.
We also assessed the presence of the degranulation marker CD107a on CD57−, CD57dim, and CD57bright subpopulations of CD56dim CD16+ NK cells after stimulation with IL-12 and IL-15 and exposure to K562 cells. Similarly to what we had observed for Ki-67 expression, CD57− cells were the most efficient at degranulation when compared with CD57dim and CD57bright cells in HIV-infected individuals. Comparison to healthy controls revealed that there was a higher expression of CD107a in HIV-seropositive subjects for each CD57 subset. However, the most effective degranulation occurred in the CD57− and CD57dim subsets, which are preferentially depleted in HIV infection.
We focused our analysis on CD56dim CD16+ NK cells because they constitute the largest NK cell subset in peripheral blood, they are the major NK cell subset expressing CD57 and KIRs, and they are the most prominent subpopulation for cytolytic activity. CD56dim CD16+ cells but not CD56bright CD16− NK cells were reported to be decreased in HIV-infected subjects (23), which we could confirm in our experiments (data not shown). We did not find CD57 on CD56bright CD16− NK cells either in healthy or in HIV-infected individuals. CD57 has been described as a marker for replicative senescence, and its expression has been associated with shorter telomeres and diminished proliferative capacities on T and NK cells (4). The presence of this marker on CD56dim CD16+ but not on CD56bright CD16+ NK cells might explain why the latter subset was shown to proliferate more efficiently upon cytokine stimulation (6). We demonstrated that increasing CD57 expression on NK cells was associated with lower numbers of CD27-expressing cells, a marker which is mainly expressed by CD56bright CD16− NK cells (24). CD56bright CD16− cells were suggested to be early NK cells, which differentiate from CD34dim CD45RA+ hematopoietic precursor cells with high expression of integrin α4β7 (11). These cells can furthermore give rise to CD56dim CD16+ NK cells (7). Our data support this hypothesis, as we show that CD57 can be found on CD56dim CD16+ NK cells but not on CD56bright NK cells, whereas the opposite is observed for CD27.
We demonstrate that differential CD57 expression is associated with distinct functional characteristics. We show for the first time that increasing expression of CD57 on CD56dim CD16+ NK cells is associated with increasing prevalence of KIR+ and granzyme B+ cells. These cells appear to be more mature and differentiated in terms of KIR and granzyme B expression but less functionally active, as shown by decreased expression of Ki-67 and CD107a. We therefore propose that CD57 is not only a marker for replicative senescence but, in addition, a marker for terminal differentiation on NK cells, which is characterized by increased expression of KIR and higher granzyme B content and “counterbalanced” by decreased degranulation (CD107a) and decreased proliferation (Ki-67).
Notably, we observed consistently higher frequencies of granzyme B+ cells in all three subsets within CD56dim CD16+ NK cells from HIV-seropositive individuals than in healthy control subjects (means, 52.9% versus 78.7% [P < 0.0001], 65.3% versus 89.6% [P < 0.0001], and 76.5% versus 95.0% [P < 0.0001]for CD57−, CD57dim, and CD57bright subpopulations, respectively) (Fig. 1C). Furthermore, HIV infection was associated with higher numbers of Ki-67-expressing NK cells (means, 8.4% versus 16.1% [P = 0.0005], 5.3% versus 11.6% [P = 0.0016], and 4.1% versus 6.2% [P = 0.04]) (Fig. 1C). These changes, including the strong increase in HLA-DR-expressing NK cells, probably reflect the systemic immune activation in HIV-infected individuals.
In summary, these findings support a view of a differential regulation of NK function and are in concordance with maturation of NK cells with high expression of CD57 on NK cells with a more terminally differentiated phenotype. Our data indicate that high turnover; activation status; and active degranulation as characterized by the expression of Ki-67, HLA-DR, and CD107a are mainly features of CD57− and much less of CD57dim NK cells. HIV infection is associated with increased activation, proliferation, and cytotoxicity during “early” stages of CD56dim CD16+ NK cell differentiation compared to their occurrence in healthy controls, but those are the very cells that are significantly decreased in chronic HIV infection. A loss of these functionally more active NK cells may be a yet-unappreciated factor in overall NK cell pathology and a further possible explanation for the impairment of NK cells in their contribution to viral control in HIV infection.
Acknowledgments
We thank Christina Reimer for her excellent assistance in flow cytometry and cell sorting and Mathias Rhein for support in cell sorting.
D.M.-O. is funded by grants from the Bundesministerium für Bildung und Forschung (Kompetenznetz HIV/AIDS) and from the Helmholtz-Zentrum für Infektionsforschung (IG-SCID-TwinPro02). H.S.H. is supported by a fellowship of the MD/Ph.D. program of the Hannover Biomedical Research School (HBRS) at Hannover Medical School. R.E.S. is supported by grant IND 06/20 from Bundesministerium für Bildung und Forschung.
Footnotes
Published ahead of print on 11 November 2009.
REFERENCES
- 1.Alter, G., J. M. Malenfant, and M. Altfeld. 2004. CD107a as a functional marker for the identification of natural killer cell activity. J. Immunol. Methods 294:15-22. [DOI] [PubMed] [Google Scholar]
- 2.Alter, G., J. M. Malenfant, R. M. Delabre, N. C. Burgett, X. G. Yu, M. Lichterfeld, J. Zaunders, and M. Altfeld. 2004. Increased natural killer cell activity in viremic HIV-1 infection. J. Immunol. 173:5305-5311. [DOI] [PubMed] [Google Scholar]
- 3.Alter, G., N. Teigen, B. T. Davis, M. M. Addo, T. J. Suscovich, M. T. Waring, H. Streeck, M. N. Johnston, K. D. Staller, M. T. Zaman, X. G. Yu, M. Lichterfeld, N. Basgoz, E. S. Rosenberg, and M. Altfeld. 2005. Sequential deregulation of NK cell subset distribution and function starting in acute HIV-1 infection. Blood 106:3366-3369. [DOI] [PubMed] [Google Scholar]
- 4.Brenchley, J. M., N. J. Karandikar, M. R. Betts, D. R. Ambrozak, B. J. Hill, L. E. Crotty, J. P. Casazza, J. Kuruppu, S. A. Migueles, M. Connors, M. Roederer, D. C. Douek, and R. A. Koup. 2003. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood 101:2711-2720. [DOI] [PubMed] [Google Scholar]
- 5.Brunetta, E., M. Fogli, S. Varchetta, L. Bozzo, K. L. Hudspeth, E. Marcenaro, A. Moretta, and D. Mavilio. 2009. The decreased expression of Siglec-7 represents an early marker of dysfunctional natural killer cell subsets associated with high levels of HIV-1 viremia. Blood 114:3822-3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carson, W. E., J. G. Giri, M. J. Lindemann, M. L. Linett, M. Ahdieh, R. Paxton, D. Anderson, J. Eisenmann, K. Grabstein, and M. A. Caligiuri. 1994. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J. Exp. Med. 180:1395-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan, A., D. L. Hong, A. Atzberger, S. Kollnberger, A. D. Filer, C. D. Buckley, A. McMichael, T. Enver, and P. Bowness. 2007. CD56bright human NK cells differentiate into CD56dim cells: role of contact with peripheral fibroblasts. J. Immunol. 179:89-94. [DOI] [PubMed] [Google Scholar]
- 8.Chattopadhyay, P. K., M. R. Betts, D. A. Price, E. Gostick, H. Horton, M. Roederer, and S. C. De Rosa. 2009. The cytolytic enzymes granyzme A, granzyme B, and perforin: expression patterns, cell distribution, and their relationship to cell maturity and bright CD57 expression. J. Leukoc. Biol. 85:88-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fauci, A. S., D. Mavilio, and S. Kottilil. 2005. NK cells in HIV infection: paradigm for protection or targets for ambush. Nat. Rev. Immunol. 5:835-843. [DOI] [PubMed] [Google Scholar]
- 10.Fogli, M., P. Costa, G. Murdaca, M. Setti, M. C. Mingari, L. Moretta, A. Moretta, and A. De Maria. 2004. Significant NK cell activation associated with decreased cytolytic function in peripheral blood of HIV-1-infected patients. Eur. J. Immunol. 34:2313-2321. [DOI] [PubMed] [Google Scholar]
- 11.Freud, A. G., B. Becknell, S. Roychowdhury, H. C. Mao, A. K. Ferketich, G. J. Nuovo, T. L. Hughes, T. B. Marburger, J. Sung, R. A. Baiocchi, M. Guimond, and M. A. Caligiuri. 2005. A human CD34(+) subset resides in lymph nodes and differentiates into CD56bright natural killer cells. Immunity 22:295-304. [DOI] [PubMed] [Google Scholar]
- 12.Hong, H. S., N. Bhatnagar, M. Ballmaier, U. Schubert, P. Henklein, T. Volgmann, H. Heiken, R. E. Schmidt, and D. Meyer-Olson. 2009. Exogenous HIV-1 Vpr disrupts IFN-alpha response by plasmacytoid dendritic cells (pDCs) and subsequent pDC/NK interplay. Immunol. Lett. 125:100-104. [DOI] [PubMed] [Google Scholar]
- 13.Huntington, N. D., C. A. Vosshenrich, and J. P. Di Santo. 2007. Developmental pathways that generate natural-killer-cell diversity in mice and humans. Nat. Rev. Immunol. 7:703-714. [DOI] [PubMed] [Google Scholar]
- 14.Iannello, A., O. Debbeche, S. Samarani, and A. Ahmad. 2008. Antiviral NK cell responses in HIV infection. I. NK cell receptor genes as determinants of HIV resistance and progression to AIDS. J. Leukoc. Biol. 84:1-26. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs, R., G. Hintzen, A. Kemper, K. Beul, S. Kempf, G. Behrens, K. W. Sykora, and R. E. Schmidt. 2001. CD56bright cells differ in their KIR repertoire and cytotoxic features from CD56dim NK cells. Eur. J. Immunol. 31:3121-3127. [DOI] [PubMed] [Google Scholar]
- 16.Kottilil, S., J. O. Jackson, K. N. Reitano, M. A. O'Shea, G. Roby, M. Lloyd, J. Yang, C. W. Hallahan, C. A. Rehm, J. Arthos, R. Lempicki, and A. S. Fauci. 2007. Innate immunity in HIV infection: enhanced susceptibility to CD95-mediated natural killer cell death and turnover induced by HIV viremia. J. Acquir. Immune Defic. Syndr. 46:151-159. [DOI] [PubMed] [Google Scholar]
- 17.Lanier, L. L. 2005. NK cell recognition. Annu. Rev. Immunol. 23:225-274. [DOI] [PubMed] [Google Scholar]
- 18.Martin, M. P., X. Gao, J. H. Lee, G. W. Nelson, R. Detels, J. J. Goedert, S. Buchbinder, K. Hoots, D. Vlahov, J. Trowsdale, M. Wilson, S. J. O'Brien, and M. Carrington. 2002. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat. Genet. 31:429-434. [DOI] [PubMed] [Google Scholar]
- 19.Martin, M. P., Y. Qi, X. Gao, E. Yamada, J. N. Martin, F. Pereyra, S. Colombo, E. E. Brown, W. L. Shupert, J. Phair, J. J. Goedert, S. Buchbinder, G. D. Kirk, A. Telenti, M. Connors, S. J. O'Brien, B. D. Walker, P. Parham, S. G. Deeks, D. W. McVicar, and M. Carrington. 2007. Innate partnership of HLA-B and KIR3DL1 subtypes against HIV-1. Nat. Genet. 39:733-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mavilio, D., J. Benjamin, M. Daucher, G. Lombardo, S. Kottilil, M. A. Planta, E. Marcenaro, C. Bottino, L. Moretta, A. Moretta, and A. S. Fauci. 2003. Natural killer cells in HIV-1 infection: dichotomous effects of viremia on inhibitory and activating receptors and their functional correlates. Proc. Natl. Acad. Sci. U. S. A. 100:15011-15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer-Olson, D., K. W. Brady, M. T. Bartman, K. M. O'Sullivan, B. C. Simons, J. A. Conrad, C. B. Duncan, S. Lorey, A. Siddique, R. Draenert, M. Addo, M. Altfeld, E. Rosenberg, T. M. Allen, B. D. Walker, and S. A. Kalams. 2006. Fluctuations of functionally distinct CD8+ T-cell clonotypes demonstrate flexibility of the HIV-specific TCR repertoire. Blood 107:2373-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyer-Olson, D., N. H. Shoukry, K. W. Brady, H. Kim, D. P. Olson, K. Hartman, A. K. Shintani, C. M. Walker, and S. A. Kalams. 2004. Limited T cell receptor diversity of HCV-specific T cell responses is associated with CTL escape. J. Exp. Med. 200:307-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tarazona, R., J. G. Casado, O. Delarosa, J. Torre-Cisneros, J. L. Villanueva, B. Sanchez, M. D. Galiani, R. Gonzalez, R. Solana, and J. Pena. 2002. Selective depletion of CD56(dim) NK cell subsets and maintenance of CD56(bright) NK cells in treatment-naive HIV-1-seropositive individuals. J. Clin. Immunol. 22:176-183. [DOI] [PubMed] [Google Scholar]
- 24.Vossen, M. T., M. Matmati, K. M. Hertoghs, P. A. Baars, M. R. Gent, G. Leclercq, J. Hamann, T. W. Kuijpers, and R. A. van Lier. 2008. CD27 defines phenotypically and functionally different human NK cell subsets. J. Immunol. 180:3739-3745. [DOI] [PubMed] [Google Scholar]