Abstract
While the molecular basis of fusion (F) protein refolding during membrane fusion has been studied extensively in vitro, little is known about the biological significance of membrane fusion activity in parainfluenza virus replication and pathogenesis in vivo. Two recombinant Sendai viruses, F-L179V and F-K180Q, were generated that contain F protein mutations in the heptad repeat A region of the ectodomain, a region of the protein known to regulate F protein activation. In vitro, the F-L179V virus caused increased syncytium formation (cell-cell membrane fusion) yet had a rate of replication and levels of F protein expression and cleavage similar to wild-type virus. The F-K180Q virus had a reduced replication rate along with reduced levels of F protein expression, cleavage, and fusogenicity. In DBA/2 mice, the hyperfusogenic F-L179V virus induced greater morbidity and mortality than wild-type virus, while the attenuated F-K180Q virus was much less pathogenic. During the first week of infection, virus replication and inflammation in the lungs were similar for wild-type and F-L179V viruses. After approximately 1 week of infection, the clearance of F-L179V virus was delayed, and more extensive interstitial inflammation and necrosis were observed in the lungs, affecting entire lobes of the lungs and having significantly greater numbers of syncytial cell masses in alveolar spaces on day 10. On the other hand, the slower-growing F-K180Q virus caused much less extensive inflammation than wild-type virus, presumably due to its reduced replication rate, and did not cause observable syncytium formation in the lungs. Overall, the results show that residues in the heptad repeat A region of the F protein modulate the virulence of Sendai virus in mice by influencing both the spread and clearance of the virus and the extent and severity of inflammation. An understanding of how the F protein contributes to infection and inflammation in vivo may assist in the development of antiviral therapies against respiratory paramyxoviruses.
Sendai virus (SeV), a murine parainfluenza virus (PIV), belongs to the genus Respirovirus within the family Paramyxoviridae (33). Sendai virus is the murine counterpart of human parainfluenza virus 1 (HPIV1), and these two viruses share high sequence homology and antigenic cross-reactivity (23, 38, 58). Both Sendai virus and HPIV1 cause respiratory diseases in their hosts that range from mild to severe, with the greatest morbidity and mortality occurring in immunocompromised hosts (3, 17). In pediatric medicine, HPIV1 is an important cause of bronchiolitis, pneumonia, and laryngotracheobronchitis, or croup (11). Other members of the genus Respirovirus include human and bovine forms of PIV3 (30).
Like other paramyxoviruses, Sendai virus is an enveloped, nonsegmented, negative-strand RNA virus that invades host cells by fusion (F) protein-mediated membrane fusion at the plasma membrane (33). The receptor binding protein for Sendai virus, as well as the other parainfluenza viruses, is the hemagglutinin-neuraminidase (HN) protein. During viral entry, the HN protein binds sialic acid-containing receptors on the surfaces of host cells and then triggers the F protein to refold and cause membrane fusion (34, 40). Paramyxovirus replication occurs in the cytoplasm of infected cells, where the viral nucleocapsid is formed by the encapsidation of the viral genome with the viral nucleoprotein (N), phosphoprotein (P), and the large RNA-dependent RNA-polymerase (L) protein (33). The assembly and budding of infectious parainfluenza virions from the plasma membrane are mediated largely by the matrix (M) protein, which interacts with the viral nucleocapsid and the cytoplasmic tails of the HN and F proteins (56, 63).
The paramyxovirus F protein mediates both virus-cell fusion and cell-cell fusion. Similar to other class I viral fusion proteins, paramyxovirus F proteins are expressed on the surfaces of infected cells and virions as trimers that are trapped in metastable (high energy) conformations (29, 54, 71, 73). In order to become activated for membrane fusion, uncleaved F0 precursor protein trimers must be cleaved into a fusion-capable complex formed by F1 and F2 subunits (55). Field isolates of Sendai virus that have a monobasic cleavage site are cleavage activated by tryptase Clara secreted from respiratory epithelial cells (32, 69) while the pantropic F1-R laboratory isolate of Sendai virus has a mutated cleavage site and is cleaved by more ubiquitously expressed proteases (41, 67). Paramyxovirus F proteins have several regions involved in F protein conformational changes during membrane fusion: a hydrophobic fusion peptide, two 4-3 heptad repeat regions (designated heptad repeat A [HRA] and HRB), a transmembrane domain, and a cytoplasmic tail. The prefusion form of the PIV5 F0 protein has a mushroom-like shape formed by a large globular head attached to a rod-like stalk formed by the HRB region (76). Upon triggering by the HN protein, the HRB region dissociates, the HRA region springs into a coiled coil, and the fusion peptide is inserted into the target membrane (52). Membrane fusion is catalyzed by the formation of a coiled-coil hairpin structure (2, 7, 75, 78), formed by the HRA and HRB regions, that juxtaposes the membrane-interacting fusion peptide and transmembrane domains (52). We recently performed a mutational analysis on a 10-residue sequence in the HRA region of the Sendai virus F protein (37) that forms a β-strand-turn-α-helix structure in the prefusion conformation and part of a triple-stranded coiled coil in the hairpin conformation (75, 76). The mutated residues were found to play important roles in regulating the activation and membrane fusion activity of the Sendai virus F protein, showing that F protein refolding is regulated by residues that undergo dramatic changes in secondary and tertiary structure between the prefusion and hairpin conformations.
Upon triggering by the HN protein, cell surface-expressed F protein trimers mediate cell-cell fusion (syncytium formation) and extend infection in a local area (55). In nonpolarized epithelial cells, virus-induced syncytium formation has long been considered a hallmark of in vitro cytopathogenesis by respiratory paramyxoviruses. However, many questions remain regarding the extent of envelope glycoprotein expression, parainfluenza virus budding, and syncytium formation at the basolateral surfaces of polarized cells (4, 77). In an in vitro model of human airway epithelium (HAE), HPIV3 has been shown to infect ciliated epithelial cells exclusively, predominantly at the apical surface, causing little virus-mediated cytopathology, no spread of the virus beyond ciliated cells, and no syncytium formation (77). As the HAE model lacks innate and adaptive immune cells, this model would not reveal the formation of syncytia involving all cell types in the respiratory tract that are present during infection, including those that play a role in the host response to infection. In immunocompetent mice, the replication of field isolates of Sendai virus is restricted to the respiratory tract, and progeny virions bud from the apical surfaces of polarized epithelial cells (68). While syncytial cell formation in the bronchiolar epithelia of mice infected with Sendai virus has been reported previously (28), the timing of giant cell formation and its contribution to the spread of the virus and the disease it induces in the respiratory tract remain unknown.
To test the hypothesis that the fusogenicity of the F protein contributes to the pathogenicity of Sendai virus in mice, the natural host of this virus, we generated two recombinant Sendai viruses containing F protein mutations in the heptad repeat A region that were found previously to either increase or decrease its fusogenic activity when the F protein was expressed from plasmid DNA constructs (37). In the present study, the effects of the F protein mutations on virus replication, F protein expression, F protein cleavage, and syncytium formation were characterized in vitro. The hyperfusogenic F-L179V virus was found to induce greater morbidity and mortality in DBA/2 mice than wild-type virus, whereas the hypofusogenic and attenuated F-K180Q virus was found to be much less pathogenic. After 1 week of infection, the F-L179V virus induced more extensive interstitial inflammation and necrosis in the lungs than the wild-type virus, including a greater number of syncytial cell masses. On the other hand, the attenuated F-K180Q virus caused much less extensive inflammation than wild-type virus and did not cause observable syncytium formation in the lungs. A comparison of 50% minimal lethal dose (MLD50) values, lung titers, and histopathologic changes reveals a correlation between the membrane fusion activity of the F protein and the virulence of Sendai virus in mice.
MATERIALS AND METHODS
Cell culture.
Monolayer cultures of Vero cells (ATCC CCL-81) and BHK-21 (ATCC CCL-10) and LLC-MK2 (ATTC CCL-7) cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1% glutamine, and 1% penicillin. BHK-21 cells were also supplemented with 10% tryptose phosphate broth.
Recombinant viruses.
Recombinant Sendai viruses (rSeVs) of the Enders strain were rescued by reverse genetics (62). Briefly, monolayers of Vero cells in 10-cm dishes were infected with UV-inactivated recombinant vaccinia virus vTF7.3 (that expresses T7 RNA polymerase) (20) for 1 h at 37°C at a multiplicity of infection (MOI) of 1 PFU per cell. Cells were then cotransfected with 9 μg of the genomic plasmid pSeV (wild type or mutant) and the supporting plasmids pTF1-SV-NP (5.4 μg), pTF1-SV-P (2.9 μg), and pTF1-SV-L (0.4 μg) that express the nucleoprotein (NP), P, and L genes of Sendai virus, respectively, in the presence of Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Cells were maintained for approximately 40 h on DMEM supplemented with 10% FBS and 1% glutamine. Cells were then suspended in phosphate-buffered saline (PBS) and lysed by freeze-thaw cycles. Lysates (0.1 ml) were inoculated into 10-day-old embryonated eggs, and allantoic fluid was harvested 72 h later. After three passages in eggs, the recovered viruses were plaque purified in LLC-MK2 cells, and a clone of each recombinant Sendai virus was amplified in embryonated eggs and subsequently used as a working stock. Reverse transcription-PCR (RT-PCR) was performed on each virus, and the resulting DNA sequences were confirmed to contain no additional mutations in the entire genomes by the Hartwell Center for Bioinformatics and Biotechnology at St. Jude Children's Research Hospital.
In vitro infection of recombinant Sendai viruses to characterize F protein expression and processing.
Monolayers of LLC-MK2 cells at 100% confluence were infected with recombinant Sendai viruses (wild type or mutant) at an MOI of 5 PFU/cell in PBS containing 0.1% gentamicin. Plates were tilted every 10 min while the virus was allowed to be adsorbed for 1 h at 37°C (in 5% CO2). The supernatant was then aspirated, and DMEM was added. The infected cells were incubated overnight at 33°C (5% CO2) and then treated as described below for each experiment.
Radioimmunoprecipitation.
LLC-MK2 cells infected with recombinant virus (wild type or mutant) were maintained in culture for 30 min in methionine- and cysteine-free (Met−Cys−) medium and then labeled for 15 min with 50 μC of [35S]NEG-772 Easytag Express (PerkinElmer, Boston, MA) in 0.5 ml of DMEM (Met− Cys−; 20 mM HEPES buffer, pH 7.3). The cells were then washed once with PBS+ (PBS containing calcium and magnesium at 0.1 g/liter) and chased with 3 ml of DMEM (2 mM Met, 2 mM Cys, 20 mM HEPES buffer, pH 7.3) for 180 min. Samples were lysed with ice-cold radioimmunoprecipitation assay (RIPA) buffer (44) containing one Complete protease inhibitor tablet (Roche). Lysate was spun at 67,000 × g in an Optima TLX ultracentrifuge (Beckman Coulter). Supernatant was incubated overnight (18 to 22 h) at 4°C with 5 μl of a mouse monoclonal antibody that binds the cytoplasmic tail of the Sendai virus F protein (37) at a dilution of 1:200. Immune complexes were adsorbed to protein G-Sepharose 4 Fast Flow (GE Healthcare) for 1 h at 4°C. Samples were washed three times with RIPA buffer containing 0.3 M NaCl, three times with RIPA buffer containing 0.15 M NaCl, and once with 50 mM Tris buffer (0.25 mM EDTA, 0.15 M NaCl, pH 7.4). The samples were resuspended in 50 μl of sample dye buffer containing 200 mM Tris, 8% SDS, 0.2% bromophenol blue, 40% glycerol, and 12% β-mercaptoethanol. The samples were then boiled for 5 min, centrifuged at high speed for 1 min, and fractionated on 12% NuPAGE Bis-Tris polyacrylamide-SDS gels (Invitrogen). Protein bands were visualized using a Typhoon 9200 PhosphorImager (GE Healthcare) and quantified using ImageQuant, version 5.2, software (Molecular Dynamics).
F protein cleavage.
At 24 h after infection, LLC-MK2 cells were washed twice with PBS+ solution. Cells were then radiolabel pulsed for 15 min and chased for 180 min. During the last hour of the chase, TPCK (tosylsulfonyl phenylalanyl chloromethyl ketone)-treated trypsin (5 μg/μl; Sigma) was added to each sample in 0.5 ml of chase medium (DMEM, 1% penicillin, 1% streptomycin, 2 mM methionine, 2 mM cysteine, and 20 mM HEPES). The samples were radioimmunoprecipitated, and F0 and F1 bands were visualized and quantified as described above.
Biotinylation.
At 24 h postinfection, LLC-MK2 cells were washed twice with PBS+ solution. Cells were then radiolabeled and chased for 180 min. The samples were subsequently biotinylated twice for 15 min at 4°C with 2 mg of EZ-Link Sulfo-NHS-SS-biotin [sulfosuccinimidyl 2-(biotinamido)-ethyl-1,3-dithiopropionate; Pierce] in 1 ml of PBS at pH 8. Excess biotin was washed off with PBS containing 50 mM glycine. Samples were then immunoprecipitated as described above. Following the wash with 50 mM Tris buffer (0.25 mM EDTA, 0.15 M NaCl, pH 7.4), the samples were resuspended in 100 μl of 50 mM Tris buffer (0.5% SDS, pH 7.4), boiled for 5 min, and centrifuged at high speed for 1 min. The supernatants were split into two 50-μl fractions. One fraction was saved for direct loading onto the SDS-polyacrylamide gel. The other fraction was diluted to 1 ml with streptavidin buffer (20 mM Tris, 0.15 mM NaCl, 5 mM EDTA, 1% Triton X-100, 0.2% bovine serum albumin, pH 8) and incubated with streptavidin-agarose overnight at 4°C. The samples were washed as described above for radioimmunoprecipitation, resuspended in SDS-PAGE sample buffer containing 12% β-mercaptoethanol, and fractionated on SDS-12% polyacrylamide gels. The F0 bands were visualized and quantified as described above.
Flow cytometry.
At 24 h postinfection, LLC-MK2 cells were washed five times with PBS+ solution, overlaid with PBS+ solution containing a 1:200 dilution of M16 primary mouse monoclonal antibody (47), and incubated at 4°C for 30 min. The infected cells were subsequently washed five times with PBS+, overlaid with PBS+ solution containing fluorescein isothiocyanate (FITC)-labeled goat anti-mouse secondary antibody (dilution, 1:100), incubated at 4°C for 30 min, and washed five times with PBS+. Cells were removed from six-well dishes with PBS containing 50 mM EDTA and were fixed in suspension by adding methanol-free formaldehyde (final concentration, 0.5%). The cell surface fluorescence of 10,000 cells was measured using a FACSCalibur flow cytometer (Becton Dickinson). Mean fluorescence intensity (MFI) values were normalized to the MFI value of the F protein expressed by the wild-type virus.
Syncytium assay of membrane fusion.
At 24 h postinfection, TPCK-treated trypsin (2.5 μg/μl; Sigma) was added for 1 hour to activate the F protein. A second treatment with trypsin (2.5 μg/μl; Sigma) was performed 12 h later, and the infected cells were incubated in fresh medium for an additional 12 h at 37°C. Cells were then fixed and stained with Hema 3 solution (Fisher) according to the manufacturer's instructions. Representative microscopic fields were captured with a Nikon D70 digital camera attached to a Nikon eclipse TS100 inverted microscope.
Single-step and multiple-step growth curves.
Monolayers of LLC-MK2 cells in 24-well dishes (100% confluence) were washed with PBS+ and then infected with 0.1 ml of recombinant virus (wild type or mutant) at an MOI of 5 PFU/cell (single step) or 0.01 PFU/cell (multiple step) suspended in PBS+. One hour after infection at room temperature, the virus was aspirated, and the cells were washed with PBS+. Subsequently, 1 ml of DMEM supplemented with 10% FBS and 1% glutamine was added to the cells, and the cells were incubated at 33°C (5% CO2). For single-step growth curves, supernatants were collected every 6 h for 36 h. For multiple-step growth curves, supernatants were collected every 12 h for 4 days.
Aliquots of supernatants were stored at −80°C until viral titers were determined by plaque assay (see below).
Plaque assay.
Stocks of recombinant virus (wild type or mutant) or supernatants from growth curves were serially diluted with PBS+ supplemented with 0.1% gentamicin. Monolayers of LLC-MK2 cells in six-well dishes (100% confluence) were washed with PBS+ and then infected with 0.25 ml of diluted virus for 1 h at room temperature. Cell supernatants were aspirated, and the infected cells were overlaid with minimal essential medium (MEM) supplemented with 0.45% bicarbonate solution, 1% MEM amino acids, 1% MEM vitamins, 8 mM l-alanyl-l-glutamine (GlutaMax, Invitrogen), 0.1% gentamicin, 0.9% agar, and trypsin at 0.002 mg/ml final concentration. After incubation at 33°C (5% CO2) for 4 to 7 days, a second overlay was performed using MEM supplemented in the absence of trypsin with 0.45% bicarbonate solution, 1% MEM amino acids, 1% MEM vitamins, 8 mM l-alanyl-l-glutamine (GlutaMax, Invitrogen), 0.1% gentamicin, 20% FBS, 0.0035% neutral red, and 0.9% agar. Cells were incubated at 33°C (5% CO2) for 1 to 2 days.
Morbidity and mortality.
Groups of 10 8- to 10-week-old female DBA/2 mice (Jackson Laboratories, Bar Harbor, ME) were infected intranasally with 50 μl of sterile PBS containing recombinant Sendai virus (wild type or F protein mutant). Control mice received PBS only. All experiments were done under general anesthesia with inhaled 2.5% isoflurane (Baxter Healthcare Corp., Deerfield, IL). Mice were monitored for weight loss and survival for 21 days. The Reed-Muench method was used to calculate MLD50 values (49). Weight changes were calculated for each mouse as a percentage of its weight on day 0 before virus infection. All animal studies were performed in a biosafety level 2+ facility in the Animal Resource Center at St. Jude Children's Research Hospital and were approved by the Animal Care and Use Committee at the same institution.
Virus titers in lung.
Groups of 10 8- to 10-week-old female DBA/2 mice (Jackson Laboratories, Bar Harbor, ME) were infected intranasally with 50 μl of sterile PBS containing 5,000 PFU of virus, a dose at which wild-type virus induces weight loss but little mortality while the F-L179V virus induces greater than 50% mortality. Control mice received PBS only. All experiments were done under general anesthesia with inhaled 2.5% isoflurane (Baxter Healthcare Corp., Deerfield, IL). Three mice from each group were sacrificed at days 1, 3, 5, 7, and 9 postinfection. Lungs were removed under sterile conditions, washed with PBS, homogenized, and suspended in a final volume of 1 ml of PBS. Lung homogenates were clarified by centrifugation at 2,000 × g for 10 min at 4°C. Virus titers were obtained by plating serial dilutions in six-well plates with confluent LLC-MK2 cells and performing plaque assays as described above.
Histopathology.
Female DBA/2 mice 8 to 10 weeks old (Jackson Laboratories, Bar Harbor, ME) were infected intranasally with 50 μl of sterile PBS containing 5,000 PFU of virus, the same dose of virus for which lung titers of virus were measured. Control mice received PBS only. All experiments were done under general anesthesia with inhaled 2.5% isoflurane (Baxter Healthcare Corp., Deerfield, IL). Three mice from each group were sacrificed at days 4, 6, 8, and 10 postinfection. Lungs and trachea were removed and fixed in 10% neutral buffered formalin for 24 h. The organs were then embedded in paraffin, sectioned, stained with hematoxylin and eosin, and examined for histopathological changes under the microscope by an expert animal pathologist who was blinded to the composition of the animal groups. The organs were examined for evidence of syncytium formation, inflammation, obstruction of airways, cell infiltration, tissue damage, and interstitial pneumonia.
RESULTS
Replication kinetics of recombinant Sendai viruses that contain mutations in the HRA region of the F protein.
To study the role of the F protein in virus replication and pathogenesis, we rescued recombinant Sendai viruses that contained L179V and K180Q mutations in the HRA region of the F protein. The F-L179V mutation was previously found to increase the membrane fusion activity of plasmid-expressed F protein in syncytium formation, luciferase reporter gene, and dye transfer assays, and the F-K180Q mutation was previously found to decrease F protein expression and membrane fusion activity (37). Wild-type and mutant viruses (Enders strain) were generated by reverse genetics (62), plaque purified, and confirmed to contain no unintended mutations by RT-PCR and DNA sequencing.
To determine if the in vitro replication kinetics of the recombinant Sendai viruses were altered by the F protein mutations, single-step and multiple-step growth curves were performed using LLC-MK2 cells infected with 5 and 0.01 PFU/cell, respectively. The single-step growth curve showed that wild-type Sendai virus grew to a peak titer greater than 7 log10 PFU/ml by 30 h postinfection (Fig. 1A). At a high MOI, the mutant viruses replicated to titers similar to the titer of the wild-type virus by 36 h although titers of the F-K180Q virus were slightly reduced at the 24 and 30 h time points. In multiple-step growth curves (Fig. 1B), wild-type and F-L179V viruses had statistically similar replication kinetics, with peak titers greater than 7 log10 PFU/ml. The multiple-step growth curve for the F-K180Q virus was significantly lower (P < 0.05) and had a maximum titer reduced by more than 1 log10. Overall, the data show that the F-L179V virus had an in vitro replication rate similar to the wild-type virus while the F-K180Q virus had a reduced replication rate.
FIG. 1.
Replication kinetics of recombinant Sendai viruses in vitro. (A) In single-step growth curves, monolayers of confluent LLC-MK2 cells were infected with viruses at an MOI of 5 PFU/cell. Cell culture supernatants were collected every 6 h until 36 h postinfection. (B) In multiple-step growth curves, LLC-MK2 cells were infected at an MOI of 0.01 PFU/cell, and cell culture supernatants were collected every 12 h until 96 h postinfection. Virus yield was measured by plaque titration in LLC-MK2 cells. Error bars represent two standard deviations. WT, wild type.
Expression and cleavage of Sendai virus F proteins.
To measure how the mutations affected the expression and cleavage of the F protein, LLC-MK2 cells were infected at an MOI of 5 PFU/cell. Cell surface expression was measured by biotinylation and flow cytometry experiments (Fig. 2A). The cell surface expression level of F-L179V was similar to that of the wild-type virus, whereas that of F-K180Q was reduced to ∼80% of the wild-type level. To measure the extent to which the F0 proteins were cleaved into fusion-capable F1-F2 complexes, the F proteins were treated with exogenous trypsin and radioimmunoprecipitated (Fig. 2B). Both wild-type and L179V F proteins were cleaved at a level of 67%, while the cleavage percentage of F-K180Q was reduced to 53%. Thus, the F-K180Q mutation resulted in ∼20% reductions in both cell surface expression and cleavage compared to the wild-type F protein.
FIG. 2.
Cell surface expression and cleavage of recombinant Sendai virus F proteins. (A) Cell surface expression of the F proteins was measured by biotinylation and flow cytometry. For biotinylation, virus-infected LLC-MK2 cells were radiolabeled, biotinylated, immunoprecipitated with a mouse monoclonal antibody that binds the cytoplasmic tail of the Sendai virus F protein (37), and then split into fractions of total F protein and surface protein that were pulled down with streptavidin agarose. Samples were analyzed by SDS-PAGE under reducing conditions. The reported band intensities of the biotinylated F proteins were normalized to 100% intensity for the wild-type (wt) F protein. For flow cytometry, virus-infected LLC-MK2 cells were detected using M16, a conformation-specific monoclonal antibody to the F protein ectodomain (47). Mean fluorescence intensities were normalized to 100% surface expression for wild-type F protein. Error bars represent two standard deviations from triplicate experiments. (B) Cleavage of wild-type and mutant F proteins. Virus-infected LLC-MK2 cells were radiolabeled and chased for 3 h. During the last hour of the chase, TPCK-treated trypsin (5 μg/ml) was added to allow cleavage of cell surface-expressed F protein. Wild-type and mutant F proteins were immunoprecipitated with the mouse anti-F protein cytoplasmic tail monoclonal antibody (37) and then analyzed by SDS-PAGE under reducing conditions. The percentages of cleaved F protein for wild-type, F-L179V, and F-K180Q were 67%, 67%, and 53%, respectively, and were calculated by dividing the F1 band intensity by the total observed F protein band intensity (represented by F0 + F1, given that the F2 protein is not observed in the assay).
F protein fusogenicity in vitro.
To examine how the mutations affected the ability of the F protein to cause cell-to-cell membrane fusion in vitro, LLC-MK2 cells were infected at an MOI of 5 PFU/cell, and photomicrographs of syncytia were taken (Fig. 3). Wild-type Sendai virus promoted extensive syncytium formation in the presence, but not the absence, of treatment with exogenous trypsin that is required for cleavage of the Sendai virus F protein into its active F1-F2 form. Wild-type Sendai virus caused an average of 6 giant cells per microscopic field (Table 1). Syncytium formation was more extensive in cells infected with the F-L179V virus (10 giant cells/field) and less extensive in cells infected with the F-K180Q virus (4 giant cells per field). Thus, in vitro the F-L179V virus had a hyperfusogenic phenotype despite having a replication rate similar to the wild-type virus while the F-K180Q virus had a hypofusogenic phenotype and slower replication kinetics.
FIG. 3.
Representative photomicrographs of syncytia formed after infection of BHK-21F cells with recombinant Sendai viruses. At 24 h postinfection, cells were treated with TPCK-treated trypsin (+T; 2.5 μg/μl) to activate the F protein by cleavage or with medium lacking TPCK-treated trypsin (−T) as a negative control. At 48 h postinfection, the cells were fixed, stained, and photographed.
TABLE 1.
Phenotypes of wild-type and mutant recombinant Sendai viruses
| Virus | MLD50 (PFU)a | Cell-cell membrane fusion in: |
|
|---|---|---|---|
| LLC-MK2 cells (avg no. of syncytia/field)b | Mouse lungc | ||
| rSeV F wild type | 3 × 104 | 6 | ++ |
| rSeV F-L179V | 1 × 103 | 10 | +++ |
| rSeV F-K180Q | >1 × 105 | 4 | − |
MLD50 in female DBA/2 mice as calculated by the method of Reed and Muench (49).
A total of 15 fields were averaged for each value.
Relative amount of cell-cell fusion on day 10 postinfection expressed as the average number of focal areas of extensive syncytial cells in alveoli per lung cross-section. −, 0 focal areas of syncytia; ++, 10 to 20 focal areas of syncytia; +++, greater than 20 focal areas of syncytia.
The virulence of Sendai virus in mice is modulated by HRA residues in the F protein.
To determine the titers of mutant and wild-type Sendai virus required for productive infection, groups of 10 female DBA/2 mice were inoculated intranasally with virus titers ranging from 500 to 100,000 PFU, and the body weights and survival of animals were recorded for 21 days. The MLD50 values for F-L179V, wild-type, and F-K180Q viruses were calculated to be 1 × 103, 3 × 104, and >1 × 105 PFU, respectively (Table 1). At each virus titer, the rank order of average weight loss and mortality was F-L179V > wild type > F-K180Q. For example, at a virus dosage of 5,000 PFU, the hyperfusogenic F-L179V virus caused greater weight loss than wild-type virus starting after 8 days of infection (Fig. 4A) and induced 90% mortality, compared to 10% mortality for the wild-type virus (Fig. 4B). On the other hand, at the same dosage of virus, the hypofusogenic F-K180Q virus did not cause mortality or weight loss compared to uninfected control mice. The data show that the hyperfusogenic F-L179V virus induces greater morbidity and mortality than the wild-type virus while the hypofusogenic and attenuated F-K180Q virus causes much less.
FIG. 4.
Morbidity and mortality curves of mice infected with wild-type or mutant recombinant Sendai viruses. Cohorts of 10 mice (8- to 10-week-old female DBA/2 mice) were infected intranasally with 5,000 PFU of the indicated recombinant Sendai virus or were mock infected with the PBS control. (A) Weight loss was monitored for 21 days. An asterisk indicates a significant difference in weight loss compared to mice infected with wild-type virus at the same time point as measured by a Student's t test (P < 0.05). Starting on day 12, there was only one surviving animal that was infected with rSeV F-L179V, and the reported weight change is for that one animal. (B) Survival curves of mice observed daily for 21 days. All of the mice that were mock infected with PBS or infected with rSeV F-K180Q survived.
To examine if the differences in pathogenicities of the viruses were due to virus dissemination outside of the respiratory tract or due to differences in virus replication rates in the lungs of mice, organs were collected from DBA/2 mice on days 1, 3, 5, 7, and 9 after intranasal infection. For all three viruses, no detectable virus titers were measured outside of the respiratory tract in the brain, liver, pancreas, spleen, kidney, or blood (Table 2). For wild-type virus, titers in the lungs reached a peak at day 5 postinfection before clearance of infectious virus was detected by day 9. The titers of F-L179V virus in the lungs of mice were similar to the titers of the wild type on days 1, 3, and 5 postinfection. However, clearance of F-L179V virus from the lungs of mice was delayed starting on day 7. Just as the F-K180Q virus was attenuated in vitro (Fig. 1), the F-K180Q virus also grew to lower titers in the lungs of mice and was cleared earlier than wild-type virus (Table 2). Decreased replication by the F-K180Q virus in the lungs is most likely a major factor in the large reduction in morbidity and mortality induced by the mutant virus. None of the viruses recovered from the lungs had reversion or other unintended mutations.
TABLE 2.
Recombinant Sendai virus titers in lungs of mice infected intranasallya
| Virus | Day p.i. | Titer in lung (PFU/ml)b |
|---|---|---|
| rSeV F wild-type | 1 | 5.7 × 104 |
| 3 | 2.0 × 105 | |
| 5 | 4.2 × 105 | |
| 7 | 2.8 × 104 | |
| 9 | — | |
| rSeV F-L179V | 1 | 7.1 × 104 |
| 3 | 3.4 × 105 | |
| 5 | 6.7 × 105 | |
| 7 | 2.0 × 105 | |
| 9 | 5.6 × 102 | |
| rSeV F-K180Q | 1 | 1.3 × 102 |
| 3 | 7.8 × 102 | |
| 5 | 1.7 × 104 | |
| 7 | — | |
| 9 | — |
Six-week-old female mice of the DBA/2 strain were infected intranasally with 5,000 PFU of each virus. After the reported day postinfection (p.i.), homogenates of the lungs were prepared.
No detectable virus titers were obtained from tissues outside of the respiratory tract (brain, liver, pancreas, spleen, kidney, or blood). Dash, no detectable virus.
To examine how the F protein mutations affect histopathological changes induced by Sendai virus, DBA/2 mice were infected at a dosage of 5,000 PFU, and organs were harvested on days 4, 6, 8, and 10 postinfection. Spleen, liver, kidney, lymph node, brain, and heart tissue were indistinguishable between uninfected animals and animals infected with the wild-type, F-L179V, or F-K180Q Sendai virus. A lack of pathology in these tissues is consistent with the idea that Sendai virus infection is localized to the respiratory tract does not disseminate to these other tissues (Table 2). All observable pathology in infected mice was restricted to the respiratory tract.
Pathology in the trachea was similar for animals infected with either wild-type or F-L179V virus (data not shown). On days 4 and 6 postinfection, mild hyperplasia, individual cell necrosis, and focal areas of attenuation were observed throughout the respiratory mucosa of the trachea. On day 4 postinfection, there was a large amount of exudate composed of neutrophils, macrophages, and mucous that often plugged the tracheal lumen. On day 6 postinfection, most of the exudate had been cleared. Infection by Sendai virus F-K180Q also caused mild hyperplasia on days 4 and 6 postinfection but induced very little exudate composed of neutrophils, macrophages, and mucous. After 8 and 10 days of infection by the wild-type, F-L179V, and F-K180Q Sendai viruses, the trachea of all infected mice appeared to recover fully.
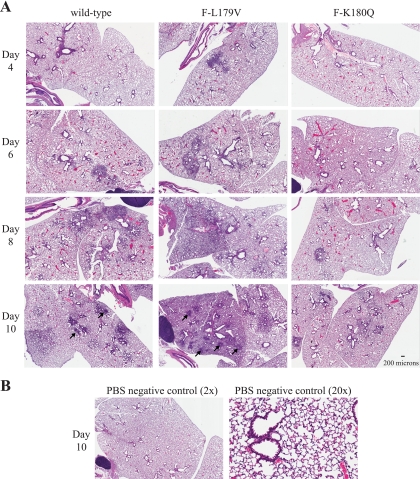
Lung pathology in DBA mice increased progressively between days 4 and 10 after intranasal infection of wild-type Sendai virus (Fig. 5 and 6), as is consistent with previous reports (16, 28). On days 4 to 10 postinfection, peribronchial infiltrates of neutrophils and macrophages were observed around medium and small airways (Fig. 6A). The following pathological traits were first observed on day 6 postinfection: (i) in the medium airways transmigration of inflammatory cells across the mucosa was common; (ii) in terminal airways there was necrosis of the lining epithelium; and (iii) neutrophils, macrophages, mucous, and cell debris often filled the airway lumens. After 8 days of infection, additional symptoms of pulmonary disease included the following: (i) in the medium airways extensive necrosis of the respiratory epithelium was common; (ii) in terminal airways neutrophils (viable and degenerate), macrophages, mucous, and cell debris were observed to adhere to the mucosal surface or were sloughed into the lumen, filling alveolar spaces; (iii) necrosis of alveolar walls was observed in areas of the affected interstitium; and (iv) the mucosa in affected airways were also mildly hyperplastic. Pathology in lung tissue after 10 days of infection with wild-type Sendai virus also included the following: (i) in terminal airways the epithelium was necrotic and sloughed at focal points; (ii) extensive interstitial inflammation was characterized by alveoli filled with lymphocytes, neutrophils, necrotic cellular debris; (iii) occasional necrosis of alveolar walls was present; and (iv) syncytial cells filled many alveolar spaces and were accompanied by lymphocytes, plasma cells, neutrophils, and macrophages.
FIG. 5.
Histopathologic changes in the lungs of mice infected with recombinant Sendai viruses shown at low a magnification. (A) Mice (three per group) were infected intranasally with 5,000 PFU of the indicated recombinant Sendai virus. Animals were sacrificed at the reported day postinfection so that lungs could be harvested, fixed, cut into 5-μm sections, and then stained with hematoxylin and eosin. Low magnification (×2) of the stained section is shown. Black arrows on day 10 postinfection for lungs infected with wild-type and F-L179V virus show examples of large syncytial masses. Interstitial inflammation and necrosis were much more extensive after infection with the F-L179V virus than with wild-type virus on days 8 and 10 postinfection and typically affected an entire lobe in the lungs of mice infected with F-L179V virus. Scale bar, 200 μm. (B) Control mice were intranasally inoculated with PBS. Lungs were harvested on day 10 after inoculation, and sections were prepared as described in panel A. Images from both low magnification (×2) and high magnification (×20) are shown.
FIG. 6.
Higher-magnification images of histopathologic changes in the lungs of mice infected with recombinant Sendai viruses, as described in the legend of Fig. 5. (A) Hematoxylin- and eosin-stained lung sections after 4, 6, 8, or 10 days of infection with wild-type or mutant virus are shown at a magnification of ×20. Pathologies in the lungs of mice infected with wild-type and F-L179V virus were similar in character. There was peribronchiolar cuffing by lymphocytes and plasma cells, airway epithelial necrosis that progressed to hyperplasia, interstitial (alveolar) necrosis, and syncytial cell formation on day 10 postinfection. A major difference between lungs infected with wild-type or F-L179V virus was that pathology due to F-L179V involved more of the total lung tissue than in wild-type virus infection. Thus, the total number of syncytial masses on day 10 postinfection was much greater in the lungs of animals infected with the F-L179V virus. In lungs of mice infected with the F-K180Q virus, there was much less extensive inflammation and necrosis, and no syncytial masses were observed at any time point. Scale bar, 50 μm. (B) Images show ×40 magnification of lung sections on day 10 postinfection. The white arrows show examples of syncytial cells in lungs infected with wild-type or F-L179V virus. No syncytial cells were observed in the lungs of mice infected with the F-K180Q virus. Scale bar, 25 μm.
On days 4 and 6 postinfection, the sizes and number of areas with extensive inflammation and necrosis induced by wild-type and F-L179V virus were similar (Fig. 5A). However, starting on day 8 postinfection, inflammation and necrosis induced by the F-L179V virus were much more extensively disseminated throughout the lungs while inflammation induced by wild-type virus was confined to focal areas. For both the wild-type virus and F-L179V, syncytial cells were focally prominent in alveoli after 10 days of infection but not at day 8 or earlier (Fig. 6B). The number of prominent focal points with masses of syncytia were much greater in the lungs of mice infected with F-L179V virus than in mice infected with wild-type virus (Table 1). Thus, in addition to causing more widespread inflammation in the lungs after 8 days of infection, the hyperfusogenic F-L179V virus also caused an increase in the number of syncytial masses in alveoli after 10 days of infection. In contrast, infection with the hypofusogenic F-K180Q virus resulted in decreased inflammation and pathology (Fig. 5A) and did not induce observable syncytium formation in alveoli at any time point observed (Fig. 6B). The attenuated disease due to the F-K180Q virus is consistent with decreased virus replication in the lungs (Table 2).
DISCUSSION
To investigate how the membrane fusion activity, or fusogenicity, of the F protein influences infection and disease by Sendai virus in mice, two recombinant Sendai viruses were generated that contained point mutations in the HRA region of the F protein. In vitro, an F-K180Q mutation was found to decrease F protein expression, cleavage, and fusogenicity, thereby decreasing Sendai virus replication kinetics. Mice infected with the F-K180Q virus had less severe disease symptoms than mice infected with wild-type virus. Attenuated disease symptoms in F-K180Q-infected mice included lower virus titers in lungs, earlier clearance of infection from the lungs, less extensive inflammation and necrosis in the lungs, no observable syncytial cells in alveolar spaces, less weight loss, and higher MLD50 values. In vitro, an F-L179V mutation was found to significantly increase F-mediated syncytium formation without altering virus replication kinetics, F protein expression, or F protein cleavage. Up until approximately 1 week after infection, mice infected with either wild-type or F-L179V virus had similar lung titers of virus, weight loss, and inflammation. However, after approximately 1 week of infection, disease induced by the F-L179V virus was much more severe. Starting at day 7 postinfection, the rate of clearance of F-L179V virus from the lungs was lower than that of wild-type virus. Moreover, mice infected with wild-type virus started to regain weight after 7 days, while mice infected with F-L179V virus continued to lose weight until approximately day 10, after which they either recovered or succumbed to disease. On days 8 and 10 postinfection, interstitial inflammation and necrosis induced by the F-L179V virus were much more extensive than that induced by the wild type. In fact, inflammation in the lungs of mice infected with F-L179V extended to entire lobes of the lungs, whereas inflammation in the lungs of mice infected with wild-type virus was only focally prominent. Both wild-type and F-L179V virus induced syncytial cell masses in alveolar spaces after 10 days of infection but not at 8 days postinfection or earlier. The number and overall extent of syncytial cell masses in the lungs of mice infected with F-L179V virus were much greater than in animals infected with wild-type virus. The more extensive disease caused by the F-L179V virus ultimately resulted in a significantly lower MLD50 value than for the wild-type virus. The data for the F-L179V virus show that increased fusogenicity by the F protein correlates with greater morbidity and mortality. Overall, the results provide evidence that the fusogenicity of the F protein modulates the virulence of Sendai virus in mice by influencing the spread and clearance of infection and the extent and severity of inflammation.
An L179V mutation in the HRA region of the Sendai virus F protein has been shown previously to increase the membrane fusion activity of plasmid-expressed F protein by decreasing the energy required to initiate F protein refolding (37). During membrane fusion, the spring-loaded HRA region refolds from a crumpled-down, 11-segment structure consisting of α-helices and β-strands and turns into the extended triple-stranded coiled coil that propels the fusion peptide into the target membrane and forms part of the hairpin structure that causes membrane fusion (75, 76). These structural changes associated with the HRA region during refolding from prefusion to postfusion forms have been described in detail previously (37, 76). Point mutations have also been identified that increase the membrane fusion activities of the PIV5, Newcastle disease virus (NDV), HPIV3, and measles virus F proteins. These mutations are located throughout the F protein in the F2 subunit (21, 46), in the fusion peptide (25, 51), in the HRA region (27, 72), between the heptad repeat regions (22, 57), and in the HRB region (15, 45, 53).
While naturally occurring mutations in the F proteins of various paramyxoviruses have been identified that cause increased membrane fusion activity, we are unaware of any previous studies that examine the effects of hyperfusogenic F protein mutations on virus replication and pathogenesis in vivo. Here, we report that an L179V mutation in the Sendai virus F protein results in increased membrane fusion activity and increased virulence in mice. In vitro, the F-L179V mutation was found to increase syncytium formation without altering F protein expression, F protein cleavage, or virus replication. In vitro, the W3A strain of PIV5 has been shown to induce greater cell-cell membrane fusion than the SER strain; however, the rates of virus-cell membrane fusion were found to be similar for both strains of PIV5 that cause different levels of cell-cell membrane fusion (10). Thus, there may be a threshold level of F-protein fusogenicity required for efficient virus entry and replication, and an increase in F-protein fusogenicity above the threshold may provide no growth advantages or disadvantages in vitro. It is unknown whether a selective pressure exists that prevents the Sendai virus F protein from acquiring greater fusogenicity in vivo. Perhaps increased morbidity and mortality induced by a hyperfusogenic virus, such as the F-L179V virus, would ultimately result in decreased transmission between animals. Alternatively, hyperfusogenic mutations that decrease the energy required to initiate irreversible refolding by the F protein could potentially decrease the environmental stability of Sendai virus, thus leading to reduced transmission.
Two other features of the Sendai virus F gene have been previously discovered that prevent the virus from attaining maximum growth and virulence in mice. Unlike other parainfluenza viruses such as PIV5 and HPIV3 (43, 60), the Sendai virus F protein has a monobasic cleavage site at residue R116 that requires the presence of extracellular protease for cleavage of the F0 precursor protein into its fusion-active F1-F2 form (24, 55). Tryptase Clara secreted from respiratory epithelial cells cleaves wild-type Sendai virus F protein in the lungs of mice (32, 64, 69). For the Sendai virus variant F1-R that was isolated from persistently infected tissue culture cells (66), F protein mutations that permit cleavage by more ubiquitously expressed proteases were found to support greater tissue tropism and increased pathogenicity in mice (65). Another naturally occurring mechanism of Sendai virus attenuation is the presence of a nonconsensus gene start signal upstream of the F gene that decreases the reinitiation capacity of the viral polymerase, resulting in decreased expression of the downstream F, HN, and L genes (31). Mutation of the gene start sequence upstream of the F gene (AGGGATAAAG) to the more efficient sequence that is found upstream of the P, M, and HN genes (AGGGTGAAAG; mutated residues are in boldface) has been shown to increase the rate of Sendai virus replication in vitro and result in increased morbidity and mortality in mice (31).
In general, an important role for the F protein in the sustained transmission of paramyxoviruses is implied by the downregulation of F protein expression by numerous paramyxoviruses. The rubulavirus PIV5 and the respiroviruses HPIV1 and HPIV3 have been shown to downregulate F protein expression by readthrough transcription at the M-F gene junction, decreasing F mRNA products by more than 50% (5, 48, 59). Measles virus and canine distemper virus (CDV) have also been shown to downregulate F protein expression by having long untranslated regions (UTR) between the M and F genes (1, 6, 61). For these two morbilliviruses, decreased F protein expression inhibits virus replication and reduces cell-cell fusion. In CDV, replacement of the long M-F UTR with the shorter N-P UTR has been shown to increase F protein expression, virus replication, cell-cell fusion, and F protein incorporation into virions while simultaneously decreasing pathogenicity in ferrets (1). The virulence of CDV in ferrets involves infection in the central nervous system after 14 days of infection, and this occurs only after extensive spread of the virus through lymphatic and epithelial tissues (50, 70). In the case of the mutant CDV with a shorter M-F UTR, increased F protein expression may increase recognition of the virus by the host immune system, thereby promoting earlier virus clearance, less dissemination, and less virulence.
While wild-type Sendai virus infection is an acute infection restricted to the respiratory tract in immunocompetent mice, the virus can persist longer and disseminate to the spleen, pancreas, kidney, liver, brain, and blood in cortisone- and cyclophophasmide-based immunosuppressed mice (39). Persistent and systemic infection by HPIV3 has also been observed in immunosuppressed children (13, 18, 19). It is unknown whether syncytium formation promoted by the F protein contributes to the high morbidity and mortality of parainfluenza virus infection in the immunocompromised (26, 36). Syncytial cell masses have been observed in the bronchiolar and alveolar spaces of immunocompromised children who have succumbed to HPIV3 infection (12, 14). In the immunocompetent mice in the present study, syncytial cell masses in the alveolar spaces were observed after 10 days of infection with wild-type Sendai virus but not at 8 days or earlier. Thus, it is unclear in the mouse model system whether the formation of giant cells directly causes disease in vivo or is a side effect of unknown consequence.
An understanding of how the F protein contributes to respiratory paramyxovirus disease may have important implications for the treatment of infection with antiviral drugs. If F protein-mediated syncytium formation is a direct inducer of enhanced inflammation and morbidity, then F protein inhibitors may provide a double advantage of decreasing both virus replication and syncytium formation. For human respiratory syncytial virus (HRSV), the humanized monoclonal antibody palivizumab, which is directed against the F protein, has been shown to reduce the risk of hospitalization due to lower respiratory tract infection by HRSV (42). Thus, the proof of principle has been established that an F protein inhibitor can be an effective therapeutic. Peptide inhibitors of the F proteins of HRSV and other respiratory paramyxoviruses have also been described (35, 74). These peptides block membrane fusion by binding to the triple-stranded coiled coil formed by the HRA region in a prehairpin conformation of the F protein (52, 53). Small-molecule mimetics of the HRSV peptide inhibit virus replication by binding to a prominent cavity formed by conserved HRA residues (8). The Sendai virus residues that were mutated in the present study, L179 and K180, are located in the corresponding cavity of the Sendai virus F protein. It is unknown whether the widespread use of fusion inhibitors in the clinic could potentially select for resistance mutations that cause increased F protein refolding and increased membrane fusion, resulting in mutant viruses that could perhaps induce greater disease in vivo. Resistance to a small-molecule inhibitor of the HRSV F protein has been mapped to a K394R mutation outside of the drug-binding pocket in the HRA region (9). While this mutation may alter the kinetics of accessibility of the HRA coiled coil in the short-lived prehairpin intermediate conformation of the F protein, it is unknown if the mutation increases the membrane fusion activity of the HRSV F protein. Future studies of the clinical effectiveness of F protein inhibitors and of the medical consequences of potential resistance mutations will be needed to evaluate both benefits and risks of this type of antiviral therapy.
Acknowledgments
We thank Nancy Hutson for help with molecular cloning. We thank Robert Sealy and Julia Hurwitz for generating the mouse monoclonal antibody against the cytoplasmic tail of the Sendai virus F protein. We thank Ruth Ann Scroggs, Irina Alymova, and Vasiliy Mishin for technical advice and Crystal Burke for a critical reading of the manuscript. We thank Bernard Moss for the vTF7.3 vaccinia virus. We thank the Hartwell Center for Bioinformatics and Biotechnology for DNA sequencing. We thank the Animal Resources Center and the Veterinary Pathology Core for supporting animal experiments and histopathology.
The project described was supported by grant R56AI083370 from the National Institute of Allergy and Infectious Diseases and by the American Lebanese Syrian Associated Charities and the Children's Infection Defense Center at St. Jude Children's Research Hospital.
Footnotes
Published ahead of print on 11 November 2009.
REFERENCES
- 1.Anderson, D. E., and V. von Messling. 2008. Region between the canine distemper virus M and F genes modulates virulence by controlling fusion protein expression. J. Virol. 82:10510-10518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker, K. A., R. E. Dutch, R. A. Lamb, and T. S. Jardetzky. 1999. Structural basis for paramyxovirus-mediated membrane fusion. Mol. Cell 3:309-319. [DOI] [PubMed] [Google Scholar]
- 3.Boeckh, M. 2008. The challenge of respiratory virus infections in hematopoietic cell transplant recipients. Br. J. Haematol. 143:455-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bose, S., A. Malur, and A. K. Banerjee. 2001. Polarity of human parainfluenza virus type 3 infection in polarized human lung epithelial A549 cells: role of microfilament and microtubule. J. Virol. 75:1984-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bousse, T., T. Matrosovich, A. Portner, A. Kato, Y. Nagai, and T. Takimoto. 2002. The long noncoding region of the human parainfluenza virus type 1 F gene contributes to the read-through transcription at the M-F gene junction. J. Virol. 76:8244-8251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cattaneo, R., G. Rebmann, K. Baczko, V. ter Meulen, and M. A. Billeter. 1987. Altered ratios of measles virus transcripts in diseased human brains. Virology 160:523-526. [DOI] [PubMed] [Google Scholar]
- 7.Chen, L., J. J. Gorman, J. McKimm-Breschkin, L. J. Lawrence, P. A. Tulloch, B. J. Smith, P. M. Colman, and M. C. Lawrence. 2001. The structure of the fusion glycoprotein of Newcastle disease virus suggests a novel paradigm for the molecular mechanism of membrane fusion. Structure (Camb.) 9:255-266. [DOI] [PubMed] [Google Scholar]
- 8.Cianci, C., E. V. Genovesi, L. Lamb, I. Medina, Z. Yang, L. Zadjura, H. Yang, C. D'Arienzo, N. Sin, K. L. Yu, K. Combrink, Z. Li, R. Colonno, N. Meanwell, J. Clark, and M. Krystal. 2004. Oral efficacy of a respiratory syncytial virus inhibitor in rodent models of infection. Antimicrob. Agents Chemother. 48:2448-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cianci, C., N. Meanwell, and M. Krystal. 2005. Antiviral activity and molecular mechanism of an orally active respiratory syncytial virus fusion inhibitor. J. Antimicrob. Chemother. 55:289-292. [DOI] [PubMed] [Google Scholar]
- 10.Connolly, S. A., and R. A. Lamb. 2006. Paramyxovirus fusion: real-time measurement of parainfluenza virus 5 virus-cell fusion. Virology 355:203-212. [DOI] [PubMed] [Google Scholar]
- 11.Counihan, M. E., D. K. Shay, R. C. Holman, S. A. Lowther, and L. J. Anderson. 2001. Human parainfluenza virus-associated hospitalizations among children less than five years of age in the United States. Pediatr. Infect. Dis. J. 20:646-653. [DOI] [PubMed] [Google Scholar]
- 12.Craighead, J. E. 2000. Parainfluenza Viruses, p. 47-51. In J. E. Craighead (ed.), Pathology and pathogenesis of human viral disease. Academic Press, San Diego, CA.
- 13.Craver, R. D., R. S. Gohd, D. R. Sundin, and J. C. Hierholzer. 1993. Isolation of parainfluenza virus type 3 from cerebrospinal fluid associated with aseptic meningitis. Am. J. Clin. Pathol. 99:705-707. [DOI] [PubMed] [Google Scholar]
- 14.Delage, G., P. Brochu, M. Pelletier, G. Jasmin, and N. Lapointe. 1979. Giant-cell pneumonia caused by parainfluenza virus. J. Pediatr. 94:426-429. [DOI] [PubMed] [Google Scholar]
- 15.Doyle, J., A. Prussia, L. K. White, A. Sun, D. C. Liotta, J. P. Snyder, R. W. Compans, and R. K. Plemper. 2006. Two domains that control prefusion stability and transport competence of the measles virus fusion protein. J. Virol. 80:1524-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faisca, P., D. B. Anh, and D. J. Desmecht. 2005. Sendai virus-induced alterations in lung structure/function correlate with viral loads and reveal a wide resistance/susceptibility spectrum among mouse strains. Am. J. Physiol. Lung Cell. Mol. Physiol. 289:L777-L787. [DOI] [PubMed] [Google Scholar]
- 17.Faisca, P., and D. Desmecht. 2007. Sendai virus, the mouse parainfluenza type 1: a longstanding pathogen that remains up-to-date. Res. Vet. Sci. 82:115-125. [DOI] [PubMed] [Google Scholar]
- 18.Fishaut, M., D. Tubergen, and K. McIntosh. 1980. Cellular response to respiratory viruses with particular reference to children with disorders of cell-mediated immunity. J. Pediatr. 96:179-186. [DOI] [PubMed] [Google Scholar]
- 19.Frank, J. A., Jr., R. W. Warren, J. A. Tucker, J. Zeller, and C. M. Wilfert. 1983. Disseminated parainfluenza infection in a child with severe combined immunodeficiency. Am. J. Dis. Child. 137:1172-1174. [DOI] [PubMed] [Google Scholar]
- 20.Fuerst, T. R., E. G. Niles, F. W. Studier, and B. Moss. 1986. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc. Natl. Acad. Sci. U. S. A. 83:8122-8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gardner, A. E., and R. E. Dutch. 2007. A conserved region in the F2 subunit of paramyxovirus fusion proteins is involved in fusion regulation. J. Virol. 81:8303-8314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gardner, A. E., K. L. Martin, and R. E. Dutch. 2007. A conserved region between the heptad repeats of paramyxovirus fusion proteins is critical for proper F protein folding. Biochemistry 46:5094-5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorman, W. L., D. S. Gill, R. A. Scroggs, and A. Portner. 1990. The hemagglutinin-neuraminidase glycoproteins of human parainfluenza virus type 1 and Sendai virus have high structure-function similarity with limited antigenic cross-reactivity. Virology 175:211-221. [DOI] [PubMed] [Google Scholar]
- 24.Homma, M., and M. Ouchi. 1973. Trypsin action on the growth of Sendai virus in tissue culture cells. 3. Structural difference of Sendai viruses grown in eggs and tissue culture cells. J. Virol. 12:1457-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horvath, C. M., and R. A. Lamb. 1992. Studies on the fusion peptide of a paramyxovirus fusion glycoprotein: roles of conserved residues in cell fusion. J. Virol. 66:2443-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ison, M. G., and F. G. Hayden. 2002. Viral infections in immunocompromised patients: what's new with respiratory viruses? Curr. Opin. Infect. Dis. 15:355-367. [DOI] [PubMed] [Google Scholar]
- 27.Ito, M., M. Nishio, H. Komada, Y. Ito, and M. Tsurudome. 2000. An amino acid in the heptad repeat 1 domain is important for the haemagglutinin-neuraminidase-independent fusing activity of simian virus 5 fusion protein. J. Gen. Virol. 81:719-727. [DOI] [PubMed] [Google Scholar]
- 28.Itoh, T., H. Iwai, and K. Ueda. 1991. Comparative lung pathology of inbred strain of mice resistant and susceptible to Sendai virus infection. J. Vet. Med. Sci. 53:275-279. [DOI] [PubMed] [Google Scholar]
- 29.Jardetzky, T. S., and R. A. Lamb. 2004. Virology: a class act. Nature 427:307-308. [DOI] [PubMed] [Google Scholar]
- 30.Karron, R. A., and P. L. Collins. 2007. Parainfluenza viruses, p. 1497-1526. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 5th ed., vol. 1. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 31.Kato, A., K. Kiyotani, M. K. Hasan, T. Shioda, Y. Sakai, T. Yoshida, and Y. Nagai. 1999. Sendai virus gene start signals are not equivalent in reinitiation capacity: moderation at the fusion protein gene. J. Virol. 73:9237-9246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kido, H., Y. Yokogoshi, K. Sakai, M. Tashiro, Y. Kishino, A. Fukutomi, and N. Katunuma. 1992. Isolation and characterization of a novel trypsin-like protease found in rat bronchiolar epithelial Clara cells. A possible activator of the viral fusion glycoprotein. J. Biol. Chem. 267:13573-13579. [PubMed] [Google Scholar]
- 33.Lamb, R. A., and G. D. Parks. 2007. Paramyxoviridae: the viruses and their replication, p. 1449-1496. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 5th ed., vol. 1. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 34.Lamb, R. A., R. G. Paterson, and T. S. Jardetzky. 2006. Paramyxovirus membrane fusion: lessons from the F and HN atomic structures. Virology 344:30-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lambert, D. M., S. Barney, A. L. Lambert, K. Guthrie, R. Medinas, D. E. Davis, T. Bucy, J. Erickson, G. Merutka, and S. R. Petteway, Jr. 1996. Peptides from conserved regions of paramyxovirus fusion (F) proteins are potent inhibitors of viral fusion. Proc. Natl. Acad. Sci. U. S. A. 93:2186-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lujan-Zilbermann, J., E. Benaim, X. Tong, D. K. Srivastava, C. C. Patrick, and J. P. DeVincenzo. 2001. Respiratory virus infections in pediatric hematopoietic stem cell transplantation. Clin. Infect. Dis. 33:962-968. [DOI] [PubMed] [Google Scholar]
- 37.Luque, L. E., and C. J. Russell. 2007. Spring-loaded heptad repeat residues regulate the expression and activation of paramyxovirus fusion protein. J. Virol. 81:3130-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lyn, D., D. S. Gill, R. A. Scroggs, and A. Portner. 1991. The nucleoproteins of human parainfluenza virus type 1 and Sendai virus share amino acid sequences and antigenic and structural determinants. J. Gen. Virol. 72:983-987. [DOI] [PubMed] [Google Scholar]
- 39.Miyamae, T. 2005. Differential invasion by Sendai virus of abdominal parenchymal organs and brain tissues in cortisone- and cyclophosphamide-based immunosuppressed mice. J. Vet. Med. Sci. 67:369-377. [DOI] [PubMed] [Google Scholar]
- 40.Moscona, A. 2005. Entry of parainfluenza virus into cells as a target for interrupting childhood respiratory disease. J. Clin. Invest. 115:1688-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okada, H., J. T. Seto, N. L. McQueen, H. D. Klenk, R. Rott, and M. Tashiro. 1998. Determinants of pantropism of the F1-R mutant of Sendai virus: specific mutations involved are in the F and M genes. Arch. Virol. 143:2343-2352. [DOI] [PubMed] [Google Scholar]
- 42.Parnes, C., J. Guillermin, R. Habersang, P. Nicholes, V. Chawla, T. Kelly, J. Fishbein, P. McRae, M. Goessler, A. Gatti, J. A. Calcagno, C. Eki, K. A. Harris, J. Joyave, K. McFarland, P. Protter, M. Sullivan, A. Stanford, N. Lovett, M. Ortiz, S. Rojas, S. Cyrus, J. Cyrus, S. Cohen, D. Buchin, L. Riordan, M. Zuniga, R. Shah, C. Minard, A. Quintin, G. Douglas, J. van Houten, S. Freutner, S. Chartrand, P. Nowatzke, J. Romero, T. Rhodes, M. Benoit, E. Walter, L. Walker, L. DeBonnett, M. Cross, T. Free, S. Martin, K. Shank, B. Guedes, L. A. Atkinson, G. J. Halpin, K. Rouse, I. Hand, D. Geiss, J. R. Marshall, L. Burleson, J. Boland, K. Seybold, V. Hunter, S. Unfer, J. Schmucker, M. Gley, M. Marcus, P. Thompson, P. Milla, C. Young, R. Zanni, V. Zinno, A. Fetter-Zarzeka, A. Busey, M. A. Sokunbi, S. Airington, N. Richard, V. Muraligopal, S. Lewis, F. T. Weber, B. P. Giordano, D. Linehan, J. Roach, R. Davis, A. A. Rzepka, T. Booth, D. Smeltzer, J. Walsh, E. Arispe, R. Rowley, C. Bolling, T. Botts, K. Haskett, D. Raby, E. Batiz, A. Gelfand, L. Farrell, S. Butler, L. Colby, P. Schochet, J. Bentler, D. Hirsch, L. Wilkinson, A. Aaronson, E. Bennett, J. Wingate, D. Quinn, et al. 2003. Palivizumab prophylaxis of respiratory syncytial virus disease in 2000-2001: results from The Palivizumab Outcomes Registry. Pediatr. Pulmonol. 35:484-489. [DOI] [PubMed] [Google Scholar]
- 43.Paterson, R. G., T. J. Harris, and R. A. Lamb. 1984. Fusion protein of the paramyxovirus simian virus 5: nucleotide sequence of mRNA predicts a highly hydrophobic glycoprotein. Proc. Natl. Acad. Sci. U. S. A. 81:6706-6710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paterson, R. G., and R. A. Lamb. 1993. The molecular biology of influenza viruses and paramyxoviruses, p. 35-73. In A. Davidson and R. M. Elliott (ed.), Molecular virology: a practical approach. IRL/Oxford University Press, Oxford, United Kingdom.
- 45.Paterson, R. G., C. J. Russell, and R. A. Lamb. 2000. Fusion protein of the paramyxovirus SV5: destabilizing and stabilizing mutants of fusion activation. Virology 270:17-30. [DOI] [PubMed] [Google Scholar]
- 46.Plemper, R. K., and R. W. Compans. 2003. Mutations in the putative HR-C region of the measles virus F2 glycoprotein modulate syncytium formation. J. Virol. 77:4181-4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Portner, A., R. A. Scroggs, and C. W. Naeve. 1987. The fusion glycoprotein of Sendai virus: sequence analysis of an epitope involved in fusion and virus neutralization. Virology 157:556-559. [DOI] [PubMed] [Google Scholar]
- 48.Rassa, J. C., and G. D. Parks. 1998. Molecular basis for naturally occurring elevated readthrough transcription across the M-F junction of the paramyxovirus SV5. Virology 247:274-286. [DOI] [PubMed] [Google Scholar]
- 49.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 50.Rudd, P. A., R. Cattaneo, and V. von Messling. 2006. Canine distemper virus uses both the anterograde and the hematogenous pathway for neuroinvasion. J. Virol. 80:9361-9370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Russell, C. J., T. S. Jardetzky, and R. A. Lamb. 2004. Conserved glycine residues in the fusion peptide of the paramyxovirus fusion protein regulate activation of the native state. J. Virol. 78:13727-13742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Russell, C. J., T. S. Jardetzky, and R. A. Lamb. 2001. Membrane fusion machines of paramyxoviruses: capture of intermediates of fusion. EMBO J. 20:4024-4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Russell, C. J., K. L. Kantor, T. S. Jardetzky, and R. A. Lamb. 2003. A dual-functional paramyxovirus F protein regulatory switch segment: activation and membrane fusion. J. Cell Biol. 163:363-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Russell, C. J., and L. E. Luque. 2006. The structural basis of paramyxovirus invasion. Trends Microbiol. 14:243-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scheid, A., and P. W. Choppin. 1974. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology 57:475-490. [DOI] [PubMed] [Google Scholar]
- 56.Schmitt, A. P., and R. A. Lamb. 2004. Escaping from the cell: assembly and budding of negative-strand RNA viruses. Curr. Top. Microbiol. Immunol. 283:145-196. [DOI] [PubMed] [Google Scholar]
- 57.Sergel, T. A., L. W. McGinnes, and T. G. Morrison. 2000. A single amino acid change in the Newcastle disease virus fusion protein alters the requirement for HN protein in fusion. J. Virol. 74:5101-5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith, F. S., A. Portner, R. J. Leggiadro, E. V. Turner, and J. L. Hurwitz. 1994. Age-related development of human memory T-helper and B-cell responses toward parainfluenza virus type-1. Virology 205:453-461. [DOI] [PubMed] [Google Scholar]
- 59.Spriggs, M. K., and P. L. Collins. 1986. Human parainfluenza virus type 3: messenger RNAs, polypeptide coding assignments, intergenic sequences, and genetic map. J. Virol. 59:646-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spriggs, M. K., R. A. Olmsted, S. Venkatesan, J. E. Coligan, and P. L. Collins. 1986. Fusion glycoprotein of human parainfluenza virus type 3: nucleotide sequence of the gene, direct identification of the cleavage-activation site, and comparison with other paramyxoviruses. Virology 152:241-251. [DOI] [PubMed] [Google Scholar]
- 61.Takeda, M., S. Ohno, F. Seki, Y. Nakatsu, M. Tahara, and Y. Yanagi. 2005. Long untranslated regions of the measles virus M and F genes control virus replication and cytopathogenicity. J. Virol. 79:14346-14354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takimoto, T., J. L. Hurwitz, C. Coleclough, C. Prouser, S. Krishnamurthy, X. Zhan, K. Boyd, R. A. Scroggs, B. Brown, Y. Nagai, A. Portner, and K. S. Slobod. 2004. Recombinant Sendai virus expressing the G glycoprotein of respiratory syncytial virus (RSV) elicits immune protection against RSV. J. Virol. 78:6043-6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takimoto, T., and A. Portner. 2004. Molecular mechanism of paramyxovirus budding. Virus Res. 106:133-145. [DOI] [PubMed] [Google Scholar]
- 64.Tashiro, M., and M. Homma. 1983. Pneumotropism of Sendai virus in relation to protease-mediated activation in mouse lungs. Infect. Immun. 39:879-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tashiro, M., N. L. McQueen, and J. T. Seto. 1999. Determinants of organ tropism of Sendai virus. Front. Biosci. 4:D642-D645. [DOI] [PubMed] [Google Scholar]
- 66.Tashiro, M., E. Pritzer, M. A. Khoshnan, M. Yamakawa, K. Kuroda, H. D. Klenk, R. Rott, and J. T. Seto. 1988. Characterization of a pantropic variant of Sendai virus derived from a host range mutant. Virology 165:577-583. [DOI] [PubMed] [Google Scholar]
- 67.Tashiro, M., M. Yamakawa, K. Tobita, H. D. Klenk, R. Rott, and J. T. Seto. 1990. Organ tropism of Sendai virus in mice: proteolytic activation of the fusion glycoprotein in mouse organs and budding site at the bronchial epithelium. J. Virol. 64:3627-3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tashiro, M., M. Yamakawa, K. Tobita, J. T. Seto, H. D. Klenk, and R. Rott. 1990. Altered budding site of a pantropic mutant of Sendai virus, F1-R, in polarized epithelial cells. J. Virol. 64:4672-4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tashiro, M., Y. Yokogoshi, K. Tobita, J. T. Seto, R. Rott, and H. Kido. 1992. Tryptase Clara, an activating protease for Sendai virus in rat lungs, is involved in pneumopathogenicity. J. Virol. 66:7211-7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.von Messling, V., D. Milosevic, and R. Cattaneo. 2004. Tropism illuminated: lymphocyte-based pathways blazed by lethal morbillivirus through the host immune system. Proc. Natl. Acad. Sci. U. S. A. 101:14216-14221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weissenhorn, W., A. Dessen, L. J. Calder, S. C. Harrison, J. J. Skehel, and D. C. Wiley. 1999. Structural basis for membrane fusion by enveloped viruses. Mol. Membr. Biol. 16:3-9. [DOI] [PubMed] [Google Scholar]
- 72.West, D. S., M. S. Sheehan, P. K. Segeleon, and R. E. Dutch. 2005. Role of the simian virus 5 fusion protein N-terminal coiled-coil domain in folding and promotion of membrane fusion. J. Virol. 79:1543-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.White, J. M., S. E. Delos, M. Brecher, and K. Schornberg. 2008. Structures and mechanisms of viral membrane fusion proteins: multiple variations on a common theme. Crit. Rev. Biochem. Mol. Biol. 43:189-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yao, Q., and R. W. Compans. 1996. Peptides corresponding to the heptad repeat sequence of human parainfluenza virus fusion protein are potent inhibitors of virus infection. Virology 223:103-112. [DOI] [PubMed] [Google Scholar]
- 75.Yin, H. S., R. G. Paterson, X. Wen, R. A. Lamb, and T. S. Jardetzky. 2005. Structure of the uncleaved ectodomain of the paramyxovirus (hPIV3) fusion protein. Proc. Natl. Acad. Sci. U. S. A. 102:9288-9293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yin, H. S., X. Wen, R. G. Paterson, R. A. Lamb, and T. S. Jardetzky. 2006. Structure of the parainfluenza virus 5 F protein in its metastable, prefusion conformation. Nature 439:38-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang, L., A. Bukreyev, C. I. Thompson, B. Watson, M. E. Peeples, P. L. Collins, and R. J. Pickles. 2005. Infection of ciliated cells by human parainfluenza virus type 3 in an in vitro model of human airway epithelium. J. Virol. 79:1113-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhao, X., M. Singh, V. N. Malashkevich, and P. S. Kim. 2000. Structural characterization of the human respiratory syncytial virus fusion protein core. Proc. Natl. Acad. Sci. U. S. A. 97:14172-14177. [DOI] [PMC free article] [PubMed] [Google Scholar]