Abstract
Crimean-Congo hemorrhagic fever virus (CCHFV) is a tick-borne virus (genus Nairovirus, family Bunyaviridae) associated with high case fatality disease outbreaks in regions of Africa, Europe, and Asia. The CCHFV genome consists of three negative-strand RNA segments, S, M, and L. The unusually large virus L polymerase protein and the need for biosafety level 4 (BSL-4) containment conditions for work with infectious virus have hampered the study of CCHFV replication. The L protein has an ovarian tumor (OTU) protease domain located in the N terminus, which has led to speculation that the protein may be autoproteolytically cleaved to generate the active virus L polymerase and additional functions. We report the successful development of efficient CCHFV helper virus-independent S, M, and L segment minigenome systems for analysis of virus RNA and protein features involved in replication. The virus RNA segment S, M, and L untranslated regions were found to be similar in support of replication of the respective minigenomes. In addition, the OTU domain located in the N terminus of the expressed virus L protein was shown to be a functional protease. However, no evidence of L protein autoproteolytic processing was found, and the OTU protease activity was dispensable for virus RNA replication. Finally, physiologically relevant doses of ribavirin inhibited CCHFV minigenome replication. These results demonstrated the utility of the minigenome system for use in BSL-2 laboratory settings to analyze CCHFV biology and in antiviral drug discovery programs for this important public health and bioterrorism threat.
Crimean-Congo hemorrhagic fever (CCHF) is a tick-borne viral zoonosis. Human infections are associated with a rapidly progressive acute febrile illness with high case fatality (10). CCHF outbreaks occur on a frequent basis in regions of Africa, Asia, and Europe. The broad distribution of the virus reflects the extensive range of the tick hosts, which are predominantly hard ticks of the Hyalomma genus (22). CCHF virus (CCHFV) belongs to the genus Nairovirus, family Bunyaviridae. This family contains more then 350 identified species classified into five genera: Orthobunyavirus, Phlebovirus, Nairovirus, Hantavirus, and Tospovirus. CCHFV is primarily transmitted to humans by tick bite or unprotected contact with blood or tissues of infected livestock. In addition, nosocomial transmission of the virus can occur in the absence of good barrier nursing or infection control practices. In spite of the medical importance and severity of the disease, CCHFV biology and pathogenesis remain poorly characterized, mainly because outbreaks are sporadic and usually restricted to a relatively small number of cases, no adequate animal model is available, and handling of the infectious virus requires the highest level of biosafety measures, biosafety level 4 (BSL-4).
The genome of CCHFV consists of three single-stranded negative-sense RNA segments referred to as the small (S), medium (M), and large (L) segments. Comparison of full-length genomes of multiple geographically diverse CCHFV strains and related nairoviruses demonstrated that all of these viruses possessed unusual encoded protein domains of unknown function relative to viruses of other genera of the family Bunyaviridae (7, 21, 33, 34, 41). Strikingly, the virus L RNA segment is ∼12 kb in length compared to ∼6.5 kb for most viruses of other genera. The segment contains a single open reading frame (ORF) that is predicted to encode a large protein of approximately 448 kDa and includes the L RNA-dependent RNA polymerase (L-RdRp). In addition to the predicted RdRp activity, the L-segment-encoded protein has also been predicted to include an ovarian tumor (OTU) protease domain (21, 27). OTU domains are present in eukaryotic, viral, and bacterial proteins. The CCHFV OTU domain functions as a core element of an authentic cysteine protease capable of deconjugating a broad spectrum of proteins modified with ubiquitin or ubiquitin-like proteins (17). Furthermore, viral OTU protease activity has been suggested to be part of the virus arsenal used to evade innate immunity (17), but it remains unknown whether this activity is required for virus replication. In addition, the CCHFV M segment is ∼5.4 kb in length, in contrast to ∼3.6 to 4.4 kb for most viruses of other genera. The M segment encodes a polyprotein that is rapidly processed by host cell proteases into the surface glycoprotein precursors (41) and the nonstructural NSM protein (2). The S RNA segment is ∼1.6 kb in size and contains a single ORF that encodes the nucleoprotein (N) which is responsible for the encapsidation of the viral RNA (vRNA) genome and cRNA genome into ribonucleoprotein particles (RNPs). The encapsidated vRNA is used as a template for both virus mRNA transcription and replication into its full-length counterpart (cRNA). The N-encapsidated cRNA is, in turn, used as a template to synthesize the genomic vRNA.
Reverse genetics systems are a powerful tool to dissect genetic determinants for transcription, replication, assembly, and pathogenesis of negative-strand RNA viruses (9, 36, 40). Full reverse genetics systems, which rescue infectious virus entirely from cloned cDNAs, have been successfully developed for several members of the family Bunyaviridae, including Bunyamwera virus, La Crosse virus, and Akabane virus (genus Orthobunyavirus), and Rift Valley fever virus (genus Phlebovirus) (6, 18, 19, 23, 37). Such systems have demonstrated that transcription, replication, and packaging of the RNA genome require cis-acting elements in the 5′ and 3′ untranslated regions (UTRs) and the virus L-RdRp and nucleoprotein (N) acting in trans (8, 13, 16, 30). The terminal UTRs are complementary and form a panhandle RNA structure that coordinates the activity of transcription and replication (4, 28).
Reverse genetics systems for negative-strand RNA viruses commonly use the bacteriophage T7 promoter and T7 polymerase or mammalian polymerase I (PolI) promoters to drive transcription of the virus genome RNA (vRNA), virus antigenome, cRNA, or minigenome versions of the vRNA or cRNA where the virus ORFs have been replaced with a reporter gene (enhanced green fluorescent protein [EGFP], luciferase, chloramphenicol transferase, etc.). To generate an accurate 3′ RNA terminus, a PolI terminator or ribozyme/T7 terminator sequence was included after the viral 3′ UTR. Several minigenome systems have been designed for members of the Bunyaviridae family in which minigenome RNA expression together with the L protein and N protein leads to the formation of transcription and replication-competent RNPs and translation of mRNAs containing a reporter protein of choice. These minigenome systems have been useful tools to rapidly assess the transcription and replication activity of the virus RdRp. While full infectious virus rescue systems have yet to be developed for members of the Hantavirus or Nairovirus genus, reconstitution of active RNPs without the use of a helper virus has been reported for Hantaan virus (genus Hantavirus) (13, 44). In the case of CCHFV, the UTRs were shown to contain all the signals required for transcription, replication, and packaging by use of a system involving PolI-driven expression of CCHFV S segment minigenome RNAs followed by infection with a helper CCHFV (15). Nevertheless, little is known about the transcription and replication requirements of CCHFV or other nairoviruses. The requirement of a BSL-4 containment laboratory together with the unusually large size of the virus L (∼11.8-kb ORF and 448-kDa protein) has further hampered advances in this area.
Here, we report the successful development of a reverse genetic minigenome system which facilitates BSL-2 laboratory studies of the CCHFV RNA transcription/replication process and the potential role of the unique nairovirus-specific L protein domains on the L-RdRp activity. We demonstrate that the UTRs of the CCHFV S, M, and L RNA segments are sufficient for virus minigenome RNA transcription, replication, and packaging. We also conclude that transcription and replication of CCHFV minigenomes do not require protease activity of the OTU domain in the L protein. In addition, using ribavirin as an example, we demonstrate the utility of the minigenome reporter system for antiviral drug screening without the need for BSL-4 containment facilities.
MATERIALS AND METHODS
Plasmid constructions.
CCHFV strain IbAr10200 RNA was isolated from infected SW-13 cell supernatants as described previously (1). Reverse transcriptase reactions were conducted with a Thermoscript cDNA kit (Invitrogen) in the presence of segment-specific primers designed to anneal with the 3′ end of the corresponding vRNA segments. Full-length S, L, and M segment cDNAs were amplified by PCR with Phusion high-fidelity polymerase (NEB) with strand-specific terminal primers. All primer sequences are available upon request. Approximately 12-kb L, 5.5-kb M, and 1.5-kb S PCR products were gel purified and cloned in pJAZZ-OC (Lucigen) for S and L and in pCR-Blunt II-TOPO (Invitrogen) for the M segment. Clones were sequenced and selected to match the published CCHFV sequences (S, NC_005302; M, NC_005300; L, AY389508).
The plasmid V0.0, a generous gift from L. Andrew Ball (University of Alabama at Birmingham, AL), was modified in order to generate the V0.0/B used in this study. First, the restriction sites XbaI, HincII, PstI, SphI, and HindIII and two BsmBI sites and the SP6 promoter were removed from V0.0. Finally, we replaced a previous cloning site (BbsI) with a polylinker containing two BsmBI sites and one EcoRI site. Authentic S and M segment clones and an L clone with three mutations (L-mut) were obtained. All full-length segments were cloned by In-Fusion PCR cloning (Clontech) using the BsmBI site between a T7 RNA polymerase promoter and a hepatitis D virus ribozyme in the V0.0/B vector to create V0.0/B-S, V0.0/B-M, and V0.0/B-L-mut. V0.0/B-L-mut point mutations were corrected with QuikChange mutagenesis (Stratagene) and verified by sequencing. Two extra Gs were added following the T7 promoter in order to support efficient T7 transcription of RNA transcripts. This will result in two extra Gs at the 5′ ends of T7-derived S, M, and L transcripts. All clones were verified by sequencing.
The CCHF ORFs in V0.0/B-S, -M, and -L were subsequently replaced with EGFP or Gaussia luciferase (Gluc; NEB) by restriction digestion with KpnI and BglII restriction enzymes to generate T7-cS, T7-cM, and T7-cL-EGFP or -Gluc cRNA minigenomes. PCR primers were designed to clone the cS-Gluc, cM-Gluc, and cL-Gluc in viral sense orientation relative to the T7 promoter using the BsmB1 site to obtain plasmids that will express the vRNA minigenome versions (T7-vS-Gluc, T7-vM-Gluc, and T7-vL-Gluc) containing one extra G at the 5′ end of the transcripts.
Runoff in vitro transcripts were synthesized with a MEGAscript T7 kit (Ambion) using PCR products obtained with a forward primer annealing upstream of the T7 promoter and a reverse primer annealing with the 3′ UTR extremities of the T7-vS-Gluc, T7-vM-Gluc, and T7-vL-Gluc plasmids. The PCR template was removed by Turbo DNase I treatment (Ambion). In vitro RNA minigenome transcripts were subsequently purified with an RNeasy Kit (Qiagen) and analyzed by agarose gel electrophoresis to confirm the size and purity of the product. In addition, the vS-Gluc minigenome was cloned into pRF207 (kind gift from Ramon Flick, formerly from University of Texas Medical Branch, Galveston) with a BsmBI restriction enzyme to obtain a clone that will express vS-Gluc from the murine PolI promoter (PolI-vS-Gluc).
Two plasmids encoding defective versions of the virus L protein were constructed by site-directed mutagenesis of the V0.0/B-L clone. The Cys40 (OTU domain active site) was mutated to Ala to produce the plasmid V0.0/B-L-C40A, and amino acids DD2519 (RdRp conserved motif C) were deleted by QuickChange mutagenesis (Stratagene) to produce plasmid V0.0/B-L-ΔDD. These two plasmids were designed to encode L proteins defective in OTU protease or RdRp polymerase function. All clones were entirely sequenced to confirm the introduction of the desired mutation and verify the absence of any other errors. A V5 epitope tag (Invitrogen) was inserted at the N terminus of the proteins encoded by V0.0/B-L, V0.0/B-L-C40A, and V0.0/B-L-ΔDD by In-Fusion PCR cloning (Clontech) to generate the V0.0/B-V5-L, V0.0/B-V5-L-C40A, and V0.0/B-V5-L-ΔDD plasmids. The V0.0/B-V5-L ORF and its mutant ORFs were then subcloned into the mammalian expression vector pCAGGS (35) digested with AgeI and SmaI by In-Fusion PCR cloning to build the pC-V5-L, pC-V5-L-C40A, and pC-V5-L-ΔDD versions. The nucleoprotein (S segment ORF) was also subcloned by In-Fusion PCR cloning into the pCAGGS digested with KpnI and SmaI to generate the pC-N vector.
CCHFV infection of virus minigenome-expressing cells.
BSR-T7/5 cells constitutively expressing T7 polymerase and HuH-7 cells were generous gifts from Klaus Conzelmann (Max von Pettenkofer Institut, Munich, Germany) and Charles Rice (Rockefeller University, New York, NY), respectively. BSR-T7/5 and Huh-7 cells were grown with Dulbecco's modified Eagle's medium supplemented with 5% fetal bovine serum and 1 mg/ml of G418 (Sigma) or 10% fetal bovine serum, respectively. A total of 500 ng of either T7-cS-Gluc, T7-cM-Gluc, or T7-cL-Gluc plasmid or their EGFP-expressing variants were transfected into 24 wells of subconfluent BSR-T7/5 cells with LT1 transfection reagent (Mirus Bio). Transfection complexes were removed at 16 h posttransfection, and cells were then mock infected or infected with CCHFV strain IbAr10200 for 1 h at 37°C at a multiplicity of infection of 0.1. At 48 h postinfection, cell supernatants were collected and used to inoculate HuH-7 cell monolayers. At 36 h postinoculation, the HuH-7 cell supernatants were collected and kept frozen before virus inactivation by gamma-irradiation (5 × 106 rads). Luciferase activity was measured with a Ready-To-Glow secreted luciferase system (Clontech) and a Synergy 4 microplate reader (Biotek). For cells expressing EGFP-tagged minigenomes, digital pictures of live cells were captured 36 h after the virus passage, using an inverted fluorescence microscope (Nikon) equipped with a charge-coupled-device camera (Q Imaging).
Immunofluorescence assay and Western blotting.
BSR-T7/5 cells were transfected with a 3:1 DNA ratio of pC-V5-L, pC-V5-L-C40A, or pC-V5-L-ΔDD to pC-N. At 24 h posttransfection cells were fixed with phosphate-buffered saline (PBS)-4% formaldehyde, permeabilized with 0.5% Triton X-100 for 5 min at room temperature, and blocked with PBS containing 2% bovine serum albumin. Cells were then incubated with a monoclonal antibody specific for CCHF nucleoprotein (clone 21B2) provided by J. Smith (formerly from United States Army Medical Research Institute for Infectious Diseases, USAMRIID) and a rabbit anti-giantin polyclonal antibody (Covance). Unbound antibodies were washed away with PBS and incubated with Alexa Fluor 546-conjugated anti-mouse IgG1, Alexa Fluor 633 anti-rabbit, and anti-V5-fluorescein isothiocyanate (FITC) (Invitrogen) antibodies. Images were captured with a DM6000B confocal microscope (Leica).
For Western blot analysis, cells were lysed in mammalian protein extraction reagent lysis buffer supplemented with Halt protease inhibitors (Thermo Scientific). Reduced protein samples were separated with NuPAGE Novex 3 to 8% Tris-acetate gradient gels. Proteins were transferred to nitrocellulose membranes with an iBlot Dry Blotting System (Invitrogen). Immunoreactive proteins were revealed with an anti-V5 monoclonal antibody (MAb; Invitrogen) or anti-N MAb (clone 9D5 from USAMRIID) using a One-Step Western Advanced Kit (Genscript). Chemiluminescence was detected following membrane incubation with SuperSignal West Dura substrate (Thermo Scientific), and images were captured with a FluorChem HD2 cooled charge-coupled-device camera (Alpha Innotech). Apparent protein molecular weights were determined with HiMark Prestained Protein Standard (Invitrogen).
Recombinant L protein minigenome assays.
Forty-eight wells of subconfluent BSR-T7/5 cells were transfected with LT1 transfection reagent complexed with a mix of 25 ng of pSEAP2-Control (secreted alkaline phosphatase [AP] constitutive expression control; Clontech), 300 ng of pC-V5-L, and 100 ng of pC-N together with either 100 ng of T7-vS, PolI-vS, or no minigenome. At 24 h postinfection conditioned medium was collected and analyzed for Gluc and AP activity with a Renilla luciferase assay system (Promega) according to the manufacturer's protocol. For RNA transfection, cells were transfected with pC-V5-L or its variants together with pC-N (DNA ratio, 3:1). At 24 h posttransfection medium was changed, and cells were transfected with 250 ng of T7-minigenome in vitro transcripts with a TransIT-mRNA Transfection Kit (Mirus Bio). At 24 h after RNA transfection cell supernatants were collected to perform Gluc (Renilla luciferase assay system; Promega) and AP (Great EscAPe SEAP Chemiluminescence Kit; Clontech) assays. The luminescence for Gluc and AP assays was measured with a Synergy 4 multimode microplate reader (Biotek).
L-OTU in vitro protease activity.
Subconfluent monolayers of BSR-T7/5 cells were transfected with a 3:1 DNA ratio of pC-V5-L or pC-V5-L-C40A and pC-N. At 48 h after transfection cells were washed with PBS and lysed on ice in lysis/reaction buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1% Triton X-100, 5 mM dithiothreitol). Cell lysates were clarified at 4°C by centrifugation (10,000 × g). The total volume was then incubated with anti-V5 MAb (1:500 dilution) with rotation for 2 h at 4°C before addition of protein A-coated magnetic beads (Invitrogen) for 1 h. Immunocomplexes were washed five times with lysis/reaction buffer. Beads were resuspended and vortexed in lysis/reaction buffer. Fifty-microliter aliquots containing the precipitated V5-L and V-L-C40A were then incubated with 1 μM ubiquitin-7-amino-4-methylcoumarin (Ub-AMC) or ISG15-AMC (Boston Biochemicals) at 25°C. Hydrolysis of the substrates was measured with an excitation wavelength of 380 nm and emission of 460 nm every 30 s for 600 s with a Synergy 4 Multi-Mode Microplate Reader (Biotek).
RESULTS
CCHFV virus RNA genome 3′ and 5′ UTRs are sufficient for RNA transcription, replication, and packaging.
As a general rule, the virus genome RNA UTRs contain all of the cis-acting elements necessary for virus transcription, replication, and genome packaging for the members of the family Bunyaviridae. To confirm this observation for CCHFV, we first experimentally determined the 3′ termini of the vRNA and cRNA for the virus S, M, and L segments by 3′ rapid amplification of cDNA ends (RACE) and sequencing of the DNA products. The derived sequences matched those of the published S, M, and L segment terminal sequences with the exception of the S segment, where an A was found at nucleotide position 1661 instead of the published G (data not shown). We next designed luciferase- and EGFP-expressing minigenome versions of the S, M, and L cRNAs, which incorporated the correct terminal sequences that matched our 3′ RACE analysis. The cS, cM, and cL minigenomes (Fig. 1) were flanked by a T7 RNA polymerase promoter and a hepatitis delta virus ribozyme in order to drive minigenome synthesis and obtain the authentic 3′ termini of the full-length complementary sense virus RNAs (cRNAs).
FIG. 1.
CCHFV minigenome expression systems. (A) T7-cRNA minigenome expression vector diagram. The vector V0.0/B was used to produce cRNA minigenome under the control of a T7 RNA polymerase promoter (T7-p) which is expected to synthesize minigenomes containing two nonviral Gs at the 5′ terminus. Hepatitis D virus ribozyme (HDV-Rz) sequence was added to obtain minigenome RNAs with the 3′ termini identical to CCHFV cRNA 3′ termini. T7 terminator (T7-Ter) was also added to stop T7 RNA polymerase transcription. The segment ORF was replaced by either the secreted Gaussia luciferase (Luc) or EGFP (GFP). (B) vRNA minigenome expression vector diagram. This minigenome was cloned in negative orientation relative to the T7 promoter (shown as upside-down Luc), and one nonviral G is incorporated at the 5′ end; ribozyme cleavage would yield the correct 3′ end. (C) PolI-vRNA minigenome expression diagram. vRNA minigenome was cloned into PolI expression vector (pRF207); murine PolI promoter (PolI-p) and human PolI terminator (Pol-ter) are present to ensure the fidelity of the termini of minigenome RNAs. (D) In vitro vRNA minigenome diagram. This vRNA minigenome was generated from the DNA construct shown in panel B.
Initial experiments were performed to determine whether the T7/ribozyme system was a suitable alternative to the rather limited CCHFV PolI S segment minigenome system previously developed (15). Of note, the S minigenome UTRs used in this study differ by one nucleotide in comparison to studies performed by Flick et al. (Fig. 1). Transfection of T7 cS, cM, and cL minigenome plasmids into BSR-T7/5 cells (cells expressing the T7 RNA polymerase) should produce virus RNA transcripts predicted to form a panhandle structure similar to the authentic virus cRNA with the exception that the T7 polymerase will incorporate two nonviral Gs at the 5′ end of the cRNA minigenomes. Infection of these cells with CCHFV is expected to provide the viral proteins necessary for the minigenome replication.
To determine if the nonviral Gs are maintained during replication of the minigenome, total RNA was isolated from BSR-T7/5 cells transfected with the cL-EGFP minigenome plasmid and then infected with CCHFV. Sequence analysis of the 3′ RACE products of viral sense RNAs yielded authentic virus 3′ termini, demonstrating that the nonviral Gs were lost during minigenome replication (data not shown). Therefore, the T7 polymerase-driven minigenome system produced authentic vRNA capable of replication.
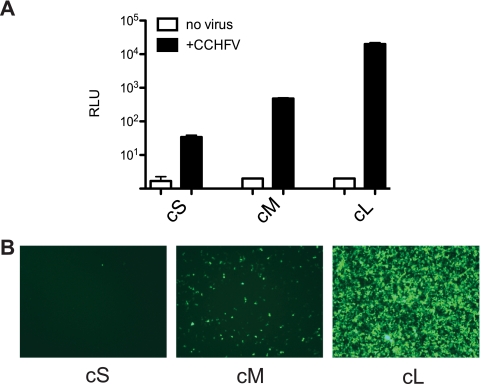
We next asked whether the virus RNA UTRs were sufficient for packaging into CCHFV particles. BSR-T7/5 cells were transfected with T7-cS, T7-cM, or T7-cL minigenomes and subsequently infected with CCHFV. Supernatants were removed and passaged to naïve HuH-7 cells. These cells are susceptible to CCHFV infection and contain no T7 RNA polymerase. Any reporter activity in these cells resulting from supernatant passage would not result from T7-driven minigenome plasmid contaminants in the transfected BSR-T7 cell supernatants. At 36 h postinfection, the cells and supernatants were monitored for evidence of virus replication. Significant levels of luciferase were expressed from each of the passaged cS, cM, and cL minigenomes, indicating that each minigenome RNA contained the minimum signal required for packaging in the UTR (Fig. 2A). A gradient of luciferase signal was observed, with L segment > M segment > S segment. The L minigenome gave >4 logs of activity over background while the S was ∼3,000 times and M ∼40 times less than the activity of L. An equivalent result was observed by monitoring the expression of EGFP after passage of EGFP-expressing minigenomes (Fig. 2B). Numerous green cells were seen for the L UTR construct, less for the M UTR, and only one or two green cells per field for the S UTR. Furthermore, comparable results were obtained when the transfected and infected cell supernatants were passaged onto BSR-T7/5 cells rather than HuH-7 cells (data not shown). In summary, these data clearly show that all the T7-driven minigenomes were replicated and packaged into CCHFV particles although with different efficiencies.
FIG. 2.
Replication and packaging of CCHFV minigenomes. BSR-T7/5 cells were transfected with cRNA minigenomes (cS, cM, and cL) and subsequently infected (black bars) or not (white bars) with CCHFV. Supernatants were passaged to Huh-7 monolayers. Following CCHFV passage, luciferase activity was measured (n = 3) (A) and an EGFP picture was taken (B) at 36 h after the passage.
Expression of CCHFV recombinant L and N proteins.
We next attempted to reconstitute replication of the minigenome without the use of helper virus. As the S, M, and L RNA segment UTRs had each been shown to contain the minimal cis-acting elements required for transcription, replication, and packaging, we next designed plasmid vectors with a strong PolII promoter to express the L protein and N protein, which are the minimal trans-acting proteins required for efficient transcription and replication (8, 16, 30). A V5 epitope was inserted at the N terminus of the L protein ORF to allow immunodetection of the protein as no antibodies reactive with the CCHFV L protein are available. In addition to the wild-type (WT) L protein, a polymerase-defective control was created by deletion of amino acids DD2519 (L-ΔDD), thus removing the critical aspartic acid residues of the SDD polymerase motif (1, 5, 24). In order to investigate the biological relevance for the OTU protease in the L protein, we constructed a variant where the L-OTU protease is inactivated by mutating the active site nucleophile C40 to A (L-C40A) (17).
We first examined the cellular localization of the CCHFV nucleoprotein (N) and the WT and mutant L proteins (Fig. 3). The N was observed mostly in perinuclear structures and often observed in close proximity to the Golgi apparatus. In contrast, the CCHFV WT L protein and L-ΔDD and L-C40A mutant proteins had a cytoplasmic distribution when expressed alone. Interestingly, coexpression of the L protein with N resulted in a redistribution of the L protein into perinuclear structures containing N, indicating that potential N-L protein interaction redirected L protein to cytoplasmic N protein inclusions. Similar findings were obtained with mutant L protein defective in polymerase function (L-ΔDD) or OTU protease function (L-C40A), indicating that neither of these functions is necessary for the colocalization of L with N into these perinuclear structures.
FIG. 3.
WT and mutant L protein colocalize with the nucleoprotein (N). BSR-T7/5 cells were cotransfected with either pC-V5-L (L), pC-V5-L-ΔDD (L-ΔDD), or pC-V5-L-C40A (L-C40A) together with empty plasmid or N-expressing vector, pC-N (N). The V5 fusion L proteins were detected with V5-FITC MAb (green), anti-N MAb (red), and Golgi compartment marker (white).
Western blot analysis with an antibody specific for the V5 epitope-tagged L protein and CCHFV N antibodies verified the relative level of expression of each of the proteins and the molecular weight of the L protein and N. Detection of V5-tagged L protein revealed a band of ∼450 kDa (Fig. 4) and additional faster-migrating proteins. The ∼450-kDa protein closely matched the full-length L protein's predicted molecular mass of 448 kDa. Two other major immunoreactive proteins were detected for the WT L protein, RdRp-inactive L protein (L-ΔDD) or OTU protease-inactive L protein (L-C40A). In addition, coexpression of N (∼50 kDa) with L protein did not grossly alter the expression of the various isoforms of tagged V5 L proteins. All of the protein bands observed were specific to the V5-tagged proteins as no bands were detected from lysates of control cells lacking L expression plasmids (Fig. 4). Some minor bands appeared to be reduced in intensity in the lane containing L-C40A plus N, but the significance of this is unclear. In summary, these data confirm that the various full-length forms of L protein were expressed to similar levels and that the two major faster-migrating forms of L protein were readily detected even when the L protein OTU protease was inactive.
FIG. 4.
WT and mutant L proteins display similar expression levels. BSR-T7/5 cells were cotransfected (as described in the legend of Fig. 3) 24 h posttransfection, and cell lysate proteins were separated by NuPAGE electrophoresis under reducing conditions. Western blot analyses were performed with anti-V5 (upper panel) and anti-CCHFV N (lower panel) MAbs. Apparent molecular masses are indicated in kilodaltons (kDa).
Recombinant L protein RdRp activity.
Since we failed to detect any differences between the WT and OTU mutant L proteins by confocal microscopy and Western blotting, we next compared the activity of these L proteins with the CCHFV luciferase minigenomes. Various approaches to express luciferase-S minigenome were optimized to achieve maximum signal over background readings of the reporter activity. The Gluc gene was inserted into both T7 promoter and PolI promoter vectors with S segment UTRs (Fig. 1). To avoid direct translation of the cRNA minigenome transcripts in the absence of replication, we designed vectors that would synthesize vRNA (negative sense) minigenome transcripts (Fig. 1). The T7-vS minigenome was also used to generate in vitro T7-runoff RNA transcripts. In parallel experiments we measured the amount of secreted luciferase as an indicator of CCHFV L-RdRp activity, together with the amount of secreted AP activity, used as a control of the transfection efficiency. The optimal assay should give high levels of luciferase in the presence of active transcription and minimal or undetectable luciferase activity in the complete absence of L or when cells were transfected with the inactive L polymerase mutant (ΔDD).
As shown in Fig. 5A, high levels of reporter activity were detected using T7-vS as the template producing the negative-sense S minigenome transcripts. However, reporter activity was almost as high even with the inactive L polymerase (L-ΔDD) or empty vector. This obviously was an unacceptable level of signal to noise (ratio of relative light units [RLU] of L to RLU of L-ΔDD, ∼3). This background activity potentially reflects cryptic promoter activity in the plasmid backbone or the virus genome. When the PolI vector was used to express the same minigenome RNA, the signal-to-noise ratio improved markedly, with values of ∼20. This represents a workable, but not ideal, system. We next tested whether transfection of in vitro transcribed vS minigenome RNA could further increase the signal-to-noise ratio. Since RdRp enzymes do not exist in mammals, we anticipated that this approach would eliminate transcripts derived from a possible cryptic promoter synthesized by host cell RNA polymerase(s) (20). Transfection of in vitro transcripts resulted in undetectable luciferase secretion in the absence of L or with the L-ΔDD mutant while high levels of activity were observed with the WT L protein. This approach resulted in an excellent signal-to-noise ratio that was ∼5,000. These data confirmed that expression of recombinant L protein with N was sufficient to reconstitute the transcription and replication activity of the virus RNP and that this activity was abolished by deletion of the DD2519 of the L protein. Furthermore, direct transfection of minigenome RNA in place of plasmid DNA proved to be a sensitive and specific method to assess the L-RdRp activity.
FIG. 5.
Recombinant L-RdRp activity. (A) BSR-T7/5 cells were cotransfected with either the V0.0/B-vS-Gluc (T7), pRF207-vS-Gluc (PolI), or empty plasmid (RNA) together with empty plasmid (−), RdRp-inactive L protein (L-ΔDD) or WT L protein (L) and N, and secreted AP was used as an internal control. At 24 h after transfection, cells that had been transfected with empty plasmid were transfected with vS-Gluc minigenome in vitro transcripts (RNA). Conditioned medium was collected 24 h after DNA (T7 and PolI) or RNA (RNA) transfections for luciferase assays. Average RLU and standard deviation (n = 4) values are shown. (B) Cells were transfected as described in panel A (RNA) except that vM-Gluc and vL-Gluc in vitro transcript minigenomes were tested in parallel with the vS-Gluc minigenome. Average RLU of Gluc (open bars) and AP (black bars) activity with standard deviation were determined (n = 6).
The initial experiments examining CCHF minigenome replication and passage supported by virus infection (Fig. 2) reproducibly found a gradient of activity between the different RNA segment UTRs in the following order: L > M > S. However, the readout of those assays was a result of RNA segment replication and packaging efficiency. Using the L and N expression system described above, we can now directly assess the efficiency of the UTRs to support replication. To do this we compared the signal from minigenome RNAs flanked by either the S, M, or L UTR (Fig. 5B). A high level of activity was observed when the WT L protein was cotransfected with the vS, vM, and vL minigenome RNAs, and yet no specific luciferase was detected with RNA minigenomes in the presence of the L-ΔDD mutant (Fig. 5B). Only minor differences were noted among the S-, M-, and L-containing minigenomes, suggesting that the ability of the three-segment UTRs to support replication was approximately equivalent in contrast to results obtained in the superinfection experiments (Fig. 2). Taken together, these data suggest that the gradient of reporter activity (L > M > S) observed after passage of the minigenomes with live virus reflects the relative efficiency at which the different UTRs contribute to packaging and not replication.
Ribavirin inhibition of the L-RdRp activity.
Ribavirin is the only drug with proven efficacy against CCHFV in cell culture, suckling mice, and possibly in patients when it is administered early during the course of infection (11, 12, 42, 43). This drug is a guanosine analogue with a broad antiviral spectrum whose mechanism of action still remains unclear (39). We wanted to determine if our minigenome system could be used to screen antiviral compounds and provide further insight into the ribavirin anti-CCHFV mechanism of action. Earlier studies had shown that ribavirin doses of 0.5 to 250 μg/ml caused significant reductions in infectious CCHFV yields (43). We tested if CCHF minigenomes could replicate in the presence of nontoxic doses of the drug, ranging from 1 to 50 μg/ml (Fig. 6). A dose-dependent inhibition of reporter production by ribavirin was observed, reaching maximal inhibition at ∼25 μg/ml. Importantly, the control AP levels remained unchanged at all ribavirin doses, ruling out a nonspecific action of the drug. These data clearly indicate that vS minigenome transcription, replication, and/or translation are inhibited by ribavirin and that this platform has excellent potential for use to rapidly test the efficacy of compounds against CCHFV.
FIG. 6.
Inhibition of the L-RdRp activity by anti-CCHFV drug. BSR-T7/5 cells were transfected as described in the legend of Fig. 5, except that complete medium with increasing doses of ribavirin was added 4 h after transfection of the vS-Gluc minigenome in vitro transcripts. Conditioned medium was collected, and average activities (RLU) of Gluc and AP were determined (n = 4).
L-OTU protease activity is not essential for minigenome replication.
One intriguing and unusual feature of the N-terminal domain of the CCHFV L protein is that it contains an authentic protease of the OTU family (17). The L protein amino acids 1 to 169 have been shown to be sufficient for deubiquitination and deISGlyation of target cellular proteins (17); however, the relevance of this protease activity in the context of CCHFV replication remains unexplored. We took advantage of our minigenome system and ability to manipulate the L sequence to ask whether the OTU protease activity was required for L protein polymerase activity. In order to evaluate whether the mutation C40A inactivated the OTU protease activity in the context of the full-length L protein, we compared the peptidase activities of WT L and L-C40A with those of two physiologically relevant fluorogenic substrates (Ub-AMC and ISG15-AMC). WT L and L-C40A were immunoprecipitated with V5 antibody. Purified WT L protein material was found to hydrolyze both Ub-AMC and ISG15-AMC while the L-C40A was devoid of any activity, thus confirming that OTU domain protease function was inactivated by the C40A mutation (Fig. 7A and B). The RdRp activities of the WT L and L-C40A proteins were then compared and shown to be comparable in activity with the minigenome systems (Fig. 7C). Therefore, we concluded that that the L protein OTU protease activity is not required for RdRp activity.
FIG. 7.
Active L-OTU domain is not required for L-RdRp function. (A and B) BSR-T7/5 cells were cotransfected with either WT L or L-C40A mutant together with N. L and L-C40A were immunoprecipitated with V5 MAb. Equal aliquots of immunoprecipitated material were incubated with either Ub-AMC or ISG15-AMC substrates. AMC release was measured every 30 s for 600 s and plotted as relative fluorescence units (RFU). (C) Cells were transfected as described for panels A and B and subsequently transfected with vS-Gluc minigenome in vitro transcripts. Conditioned medium was collected, and average activities (RLU) of Gluc and AP were determined (n = 4).
DISCUSSION
Here, we describe a highly efficient reverse genetics system that does not require infectious CCHFV and allows genetic modification of the virus L and N proteins in addition to the RNA template, permitting precise studies of virus mechanisms such as transcription, replication, and packaging. Although such systems are available for viruses of other genera of the Bunyaviridae family, this is the first such system available for a member of the Nairovirus genus. A previous report describing the generation of CCHFV carrying either S-GFP or S-chloramphenicol acetyltransferase minigenomes, confirmed that the S segment UTRs were sufficient for transcription, replication, and packaging (15). Unfortunately, this system was rather limited by the fact that it required live CCHFV to drive minigenome replication; thus, its use was restricted to a BSL-4 laboratory, and the viral L and N genes could not be manipulated for functional studies.
To assay the functions of the L protein, cells were transfected with S-, M-, and L-segment minigenomes and subsequently infected with CCHFV. Passage of virus particles containing the S, M, or L minigenomes was demonstrated, indicating that the mature T7-derived primary transcripts were replicated, transcribed, and packaged by CCHFV. In addition, nonvirus templated nucleotides (5′ GG), introduced to improve T7 transcription, were found to be removed after replication, further demonstrating the ability of these transcripts to initiate replication after their T7 transcription. The S, M, or L segment UTRs contributed to significant differences in the expression of two different reporters on passage of transfected cell supernatants to new cell monolayers, with the L UTR being the most efficient and S the least (L > M > S). Similar observations in S, M, and L UTR efficiencies were previously reported for Bunyamwera and Uukuniemi virus minigenome systems (14, 29). However, superinfection experiments should be interpreted with caution as the reporter activity measured is the result of the combination of transcription, replication, encapsidation, and packaging. The results of the Bunyamwera and Uukuniemi virus experiments suggested that the packaging signals within their L UTRs were the strongest while the S segment UTRs were the weakest of the three (L > M > S). In contrast, approximately equivalent levels of activity were observed with CCHFV S, M, and L UTRs in our minigenome replication experiments, where replication occurred with coexpressed L and N protein as opposed to infection of a helper virus.
These findings suggest that the differences observed in the virus superinfection and passage experiments may be the result of differences in packaging efficiency rather than replication promoter efficiency, with UTR packaging signal strengths being L > M > S. Analysis of passage efficiency of infectious virus-like particles with the different UTRs could more precisely address this question as the current observation involves comparison of a helper-virus-driven system relative to a plasmid-driven system. It is interesting that the gradation observed in packaging efficiency of RNA segment UTRs (L > M > S) appears to be a broad feature of bunyaviruses, given that this gradation has now been seen with Bunyamwera virus (genus Orthobunyavirus), Uukuniemi virus (genus Phlebovirus), and CCHFV (genus Nairovirus) (14, 29). The packaging strength is inversely proportional to the size of the RNA segment, perhaps compensating for the increased difficulty in packaging larger RNPs into virion particles so that the small RNA does not outcompete the larger RNAs during virus passage. This hypothesis could be tested by examining passage efficiencies of L UTR-containing minigenomes of increasing size, varying from authentic S to L segment size (∼1.6 to 13 kb).
We demonstrated that the expressed recombinant CCHFV nucleoprotein localized to the perinuclear region of transfected cells (Fig. 3) and closely resembles nucleoprotein localization in CCHFV-infected cells, a pattern dependent on the cellular actin network (3). By tagging V5 on the N terminus of the L protein, we show the interaction of L protein with N when the proteins are coexpressed in transfected cells. When expressed independently, L was detected throughout the cytoplasm but colocalized with N in the perinuclear regions in close proximity to the Golgi cisternae, where CCHFV assembly is reported to occur. This recruitment of L protein by N was independent of L polymerase or OTU protease function as their colocalization was not altered when these functions were abolished. How L protein and N interact with actin filaments and whether actin is required for formation of virus replication complexes are areas for future research.
The L proteins encoded by the viruses of the Nairovirus genus are among the largest of the negative-strand RNA viruses and are almost twice the size of those encoded by most viruses of other genera in the Bunyaviridae family. Sequence comparison of the known RdRp motifs suggests that the additional size of these L proteins is due to the presence of extra N-terminal domains in the nairovirus L proteins. One domain includes an OTU protease. These features have led to the speculation that the large L protein is further cleaved and that the N terminus harbors additional functions not present in other viruses of the family Bunyaviridae (21, 27). Recent work from Frías-Stäheli et al. has confirmed that the L-OTU domain is indeed a functional cysteine protease that is capable of deconjugating ubiquitin and ISG15 from cellular proteins (17). The OTU domain of the positive-stranded RNA arterivirus porcine respiratory and reproductive syndrome virus undergoes cleavage which activates the virus polymerase (17, 45). In addition, herpes simplex virus type 1 encodes a ubiquitin-specific cysteine protease within the amino terminus of the large (3,161 amino acids) tegument protein. Interestingly, it has been suggested, based on ubiquitin suicide inhibitor probe studies, that the protein may require posttranslational cleavage to generate the active form of the ubiquitin-specific protease (25, 31). Such observations led to considerable interest in determining whether the CCHFV L protein was proteolytically cleaved and whether this was a result of autocatalytic cleavage activity of the OTU domain.
Western blot analysis of the V5-tagged WT L protein revealed a ∼450-kDa band, which corresponds with the predicted size (448 kDa) of the full-length L protein. Smaller proteins sharing the amino terminus V5 epitope were also detected and could be cleavage products. However, these smaller proteins were also detected in cells expressing the mutant L protein lacking L-OTU activity (L-C40A) and therefore do not appear to be the result of OTU protease activity. Some minor N-tagged L protein bands appeared reduced with the mutant L protein lacking OTU activity, but the significance of these and the question of whether these faster-migrating bands represent authentic posttranslational products or an artifact of L overexpression remain an open question.
In the course of establishing the CCHFV minigenome system, we determined that the transfection of minigenome RNA, as opposed to expression from a DNA plasmid, provided the most robust system, with signal-to-noise ratios approaching 5,000-fold above background. It is unclear why such high background signals were seen when plasmid DNA constructs were transfected, but this finding strongly suggests the presence of mammalian cryptic promoter(s). The same V0.0/B plasmid backbone had been used with Rift Valley fever virus reverse genetics experiments without excessive background issues (data not shown), suggesting that the CCHFV-specific inserts were enhancing the cryptic promoter activity in some way. Transfecting in vitro synthesized CCHFV S minigenome RNA instead of plasmid DNA greatly improved the signal-to-noise ratio, similar to results in the previously reported Lassa virus minigenome system (20).
We show that CCHFV minigenome replication is inhibited by ribavirin, illustrating that this system can be used for antiviral drug screening. The ribavirin mechanism of action appears complex and varies according to the viruses studied (39). The first proposed mode of action of this drug is mediated by inhibition of the inositide-5′-monophosphate dehydrogenase, which depletes intracellular GTP levels and interferes with a subset of virus polymerases. The second is through the stimulation of the virus mutation rate, which results in lethal error catastrophe. Finally, direct interaction with virus polymerase (39) or mimicry of the m7G cap can also contribute to ribavirin activity (26). Here, we found inhibition of minigenome replication at a ribavirin dose of approximately 2 μg/ml, with maximal inhibition of reporter activity at 25 μg/ml. This inhibitory effect is in a similar range to that observed with infectious virus assays (38, 43). For instance, Watts et al. have shown that ribavirin doses as low as 5 μg/ml caused a transient reduction of virus yields, and a rapid and sustained reduction of virus to undetectable levels was seen at doses of 50 μg/ml or greater (43). Here, we showed that ribavirin preferentially inhibited the synthesis of minigenome reporter protein over cellular RNA polymerase activity (as measured by AP activity), demonstrating that the effect of ribavirin is specific for CCHFV. One of the main advantages of this method over the use of live CCHFV (38, 43) is that it does not require a BSL-4 laboratory.
The identification of a putative L-OTU protease domain in the L protein N terminus, together with the unusually large size of the protein, had led to earlier speculation that the L protein may undergo autocatalytic processing (21, 27). However, no evidence of OTU protease domain-specific proteolytic processing of L was detected. The L-RdRp activity of the CCHFV L protein defective in RdRp or OTU protease activity further illustrates the use of this reverse genetics system for CCHFV structure-function studies. In order to address the role of the L-OTU protease activity on the L-RdRp activity, we compared the rate of replication of an L-OTU inactive mutant (L-C40A) to the WT L protein. Interestingly, the inactivation of L-OTU protease yielded comparable RdRp activity to the WT L protein, thus providing direct evidence that the L-OTU activity is dispensable for L-RdRp function. Taken as a whole, our results suggest that the L-segment-encoded protease has a role in another step of the virus life cycle such as immune evasion (17) or at another uncharacterized step. If the L-OTU activity is not required for virus replication, as suggested by the minigenome data presented here, it may well be possible to engineer a CCHFV mutant with no L-OTU activity once a full CCHFV reverse genetics system capable of generating infectious virus has been developed. Such an OTU-deficient CCHFV would help to precisely clarify the L-OTU function in its most relevant biological context.
The current study lays the foundation for building an infectious clone system. We demonstrated that T7 and murine PolI vectors both yield vRNA templates which are replicated by CCHFV viral proteins or recombinant L protein and nucleoprotein in BSR-T7 cells which stably express T7. This cell line has been successfully used in past reverse genetics systems to rescue RNA viruses, and CCHFV infection of these cells does support growth of virus to decent titers. Efficient rescue systems have been developed for bunyaviruses and arenaviruses by reducing the number of plasmids needed to initiate virus rescue to only the T7-expressed full-length antigenome RNA segments and omitting any support plasmids (containing the individual N and L ORFs) (1, 6, 18, 32). Apparently, translation of these uncapped virus segment antigenome RNAs is sufficient to launch virus replication. Given the demonstrated efficiency of the CCHFV minigenome system, such an approach should be promising for the future development of an infectious CCHFV rescue system.
Acknowledgments
We thank Laura McMullan for critical reading of the manuscript.
Eric Bergeron was supported by an Oak Ridge Institute for Science and Education fellowship and ASM/CCID fellowship in Infectious Disease and Public Health Microbiology.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Published ahead of print on 28 October 2009.
REFERENCES
- 1.Albariño, C. G., É. Bergeron, B. R. Erickson, M. L. Khristova, P. E. Rollin, and S. T. Nichol. 2009. Efficient reverse genetics generation of infectious Junin viruses differing in glycoprotein processing. J. Virol. 83:5606-5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altamura, L. A., A. Bertolotti-Ciarlet, J. Teigler, J. Paragas, C. S. Schmaljohn, and R. W. Doms. 2007. Identification of a novel C-terminal cleavage of Crimean-Congo hemorrhagic fever virus PreGN that leads to generation of an NSM protein. J. Virol. 81:6632-6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson, I., M. Simon, A. Lundkvist, M. Nilsson, A. Holmstrom, F. Elgh, and A. Mirazimi. 2004. Role of actin filaments in targeting of Crimean Congo hemorrhagic fever virus nucleocapsid protein to perinuclear regions of mammalian cells. J. Med. Virol. 72:83-93. [DOI] [PubMed] [Google Scholar]
- 4.Barr, J. N., and G. W. Wertz. 2005. Role of the conserved nucleotide mismatch within 3′- and 5′-terminal regions of Bunyamwera virus in signaling transcription. J. Virol. 79:3586-3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biswas, S. K., and D. P. Nayak. 1994. Mutational analysis of the conserved motifs of influenza A virus polymerase basic protein 1. J. Virol. 68:1819-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blakqori, G., and F. Weber. 2005. Efficient cDNA-based rescue of La Crosse bunyaviruses expressing or lacking the nonstructural protein NSs. J. Virol. 79:10420-10428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deyde, V. M., M. L. Khristova, P. E. Rollin, T. G. Ksiazek, and S. T. Nichol. 2006. Crimean-Congo hemorrhagic fever virus genomics and global diversity. J. Virol. 80:8834-8842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunn, E. F., D. C. Pritlove, H. Jin, and R. M. Elliott. 1995. Transcription of a recombinant bunyavirus RNA template by transiently expressed bunyavirus proteins. Virology 211:133-143. [DOI] [PubMed] [Google Scholar]
- 9.Ebihara, H., A. Groseth, G. Neumann, Y. Kawaoka, and H. Feldmann. 2005. The role of reverse genetics systems in studying viral hemorrhagic fevers. Thromb. Haemost. 94:240-253. [DOI] [PubMed] [Google Scholar]
- 10.Ergonul, O. 2006. Crimean-Congo haemorrhagic fever. Lancet Infect. Dis. 6:203-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ergonul, O. 2008. Treatment of Crimean-Congo hemorrhagic fever. Antiviral Res. 78:125-131. [DOI] [PubMed] [Google Scholar]
- 12.Fisher-Hoch, S. P., J. A. Khan, S. Rehman, S. Mirza, M. Khurshid, and J. B. McCormick. 1995. Crimean Congo-haemorrhagic fever treated with oral ribavirin. Lancet 346:472-475. [DOI] [PubMed] [Google Scholar]
- 13.Flick, K., J. W. Hooper, C. S. Schmaljohn, R. F. Pettersson, H. Feldmann, and R. Flick. 2003. Rescue of Hantaan virus minigenomes. Virology 306:219-224. [DOI] [PubMed] [Google Scholar]
- 14.Flick, K., A. Katz, A. Overby, H. Feldmann, R. F. Pettersson, and R. Flick. 2004. Functional analysis of the noncoding regions of the Uukuniemi virus (Bunyaviridae) RNA segments. J. Virol. 78:11726-11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flick, R., K. Flick, H. Feldmann, and F. Elgh. 2003. Reverse genetics for Crimean-Congo hemorrhagic fever virus. J. Virol. 77:5997-6006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flick, R., and R. F. Pettersson. 2001. Reverse genetics system for Uukuniemi virus (Bunyaviridae): RNA polymerase I-catalyzed expression of chimeric viral RNAs. J. Virol. 75:1643-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frías-Stäheli, N., N. V. Giannakopoulos, M. Kikkert, S. L. Taylor, A. Bridgen, J. Paragas, J. A. Richt, R. R. Rowland, C. S. Schmaljohn, D. J. Lenschow, E. J. Snijder, A. Garcia-Sastre, and H. W. Virgin IV. 2007. Ovarian tumor domain-containing viral proteases evade ubiquitin- and ISG15-dependent innate immune responses. Cell Host Microbe 2:404-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerrard, S. R., B. H. Bird, C. G. Albarino, and S. T. Nichol. 2007. The NSm proteins of Rift Valley fever virus are dispensable for maturation, replication and infection. Virology 359:459-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Habjan, M., N. Penski, M. Spiegel, and F. Weber. 2008. T7 RNA polymerase-dependent and -independent systems for cDNA-based rescue of Rift Valley fever virus. J. Gen. Virol. 89:2157-2166. [DOI] [PubMed] [Google Scholar]
- 20.Hass, M., U. Golnitz, S. Muller, B. Becker-Ziaja, and S. Gunther. 2004. Replicon system for Lassa virus. J. Virol. 78:13793-13803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Honig, J. E., J. C. Osborne, and S. T. Nichol. 2004. Crimean-Congo hemorrhagic fever virus genome L RNA segment and encoded protein. Virology 321:29-35. [DOI] [PubMed] [Google Scholar]
- 22.Hoogstraal, H. 1979. The epidemiology of tick-borne Crimean-Congo hemorrhagic fever in Asia, Europe, and Africa. J. Med. Entomol. 15:307-417. [DOI] [PubMed] [Google Scholar]
- 23.Ikegami, T., S. Won, C. J. Peters, and S. Makino. 2006. Rescue of infectious Rift Valley fever virus entirely from cDNA, analysis of virus lacking the NSs gene, and expression of a foreign gene. J. Virol. 80:2933-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin, H., and R. M. Elliott. 1992. Mutagenesis of the L protein encoded by Bunyamwera virus and production of monospecific antibodies. J. Gen. Virol. 73:2235-2244. [DOI] [PubMed] [Google Scholar]
- 25.Kattenhorn, L. M., G. A. Korbel, B. M. Kessler, E. Spooner, and H. L. Ploegh. 2005. A deubiquitinating enzyme encoded by HSV-1 belongs to a family of cysteine proteases that is conserved across the family Herpesviridae. Mol. Cell 19:547-557. [DOI] [PubMed] [Google Scholar]
- 26.Kentsis, A., I. Topisirovic, B. Culjkovic, L. Shao, and K. L. Borden. 2004. Ribavirin suppresses eIF4E-mediated oncogenic transformation by physical mimicry of the 7-methyl guanosine mRNA cap. Proc. Natl. Acad. Sci. U. S. A. 101:18105-18110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kinsella, E., S. G. Martin, A. Grolla, M. Czub, H. Feldmann, and R. Flick. 2004. Sequence determination of the Crimean-Congo hemorrhagic fever virus L segment. Virology 321:23-28. [DOI] [PubMed] [Google Scholar]
- 28.Kohl, A., E. F. Dunn, A. C. Lowen, and R. M. Elliott. 2004. Complementarity, sequence and structural elements within the 3′ and 5′ non-coding regions of the Bunyamwera orthobunyavirus S segment determine promoter strength. J. Gen. Virol. 85:3269-3278. [DOI] [PubMed] [Google Scholar]
- 29.Kohl, A., A. C. Lowen, V. H. Leonard, and R. M. Elliott. 2006. Genetic elements regulating packaging of the Bunyamwera orthobunyavirus genome. J. Gen. Virol. 87:177-187. [DOI] [PubMed] [Google Scholar]
- 30.Lopez, N., R. Muller, C. Prehaud, and M. Bouloy. 1995. The L protein of Rift Valley fever virus can rescue viral ribonucleoproteins and transcribe synthetic genome-like RNA molecules. J. Virol. 69:3972-3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Love, K. R., A. Catic, C. Schlieker, and H. L. Ploegh. 2007. Mechanisms, biology and inhibitors of deubiquitinating enzymes. Nat. Chem. Biol. 3:697-705. [DOI] [PubMed] [Google Scholar]
- 32.Lowen, A. C., C. Noonan, A. McLees, and R. M. Elliott. 2004. Efficient bunyavirus rescue from cloned cDNA. Virology 330:493-500. [DOI] [PubMed] [Google Scholar]
- 33.Marriott, A. C., A. A. el-Ghorr, and P. A. Nuttall. 1992. Dugbe Nairovirus M RNA: nucleotide sequence and coding strategy. Virology 190:606-615. [DOI] [PubMed] [Google Scholar]
- 34.Marriott, A. C., and P. A. Nuttall. 1996. Large RNA segment of Dugbe nairovirus encodes the putative RNA polymerase. J. Gen. Virol. 77:1775-1780. [DOI] [PubMed] [Google Scholar]
- 35.Miyazaki, J., S. Takaki, K. Araki, F. Tashiro, A. Tominaga, K. Takatsu, and K. Yamamura. 1989. Expression vector system based on the chicken beta-actin promoter directs efficient production of interleukin-5. Gene 79:269-277. [DOI] [PubMed] [Google Scholar]
- 36.Neumann, G., and Y. Kawaoka. 2004. Reverse genetics systems for the generation of segmented negative-sense RNA viruses entirely from cloned cDNA. Curr. Top. Microbiol. Immunol. 283:43-60. [DOI] [PubMed] [Google Scholar]
- 37.Ogawa, Y., K. Sugiura, K. Kato, Y. Tohya, and H. Akashi. 2007. Rescue of Akabane virus (family Bunyaviridae) entirely from cloned cDNAs by using RNA polymerase I. J. Gen. Virol. 88:3385-3390. [DOI] [PubMed] [Google Scholar]
- 38.Paragas, J., C. A. Whitehouse, T. P. Endy, and M. Bray. 2004. A simple assay for determining antiviral activity against Crimean-Congo hemorrhagic fever virus. Antiviral Res. 62:21-25. [DOI] [PubMed] [Google Scholar]
- 39.Parker, W. B. 2005. Metabolism and antiviral activity of ribavirin. Virus Res. 107:165-171. [DOI] [PubMed] [Google Scholar]
- 40.Pekosz, A., B. He, and R. A. Lamb. 1999. Reverse genetics of negative-strand RNA viruses: closing the circle. Proc. Natl. Acad. Sci. U. S. A. 96:8804-8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanchez, A. J., M. J. Vincent, and S. T. Nichol. 2002. Characterization of the glycoproteins of Crimean-Congo hemorrhagic fever virus. J. Virol. 76:7263-7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tignor, G. H., and C. A. Hanham. 1993. Ribavirin efficacy in an in vivo model of Crimean-Congo hemorrhagic fever virus (CCHF) infection. Antiviral Res. 22:309-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watts, D. M., M. A. Ussery, D. Nash, and C. J. Peters. 1989. Inhibition of Crimean-Congo hemorrhagic fever viral infectivity yields in vitro by ribavirin. Am. J. Trop. Med. Hyg. 41:581-585. [DOI] [PubMed] [Google Scholar]
- 44.Zhang, Y., X. H. Li, H. Jiang, C. X. Huang, P. Z. Wang, D. L. Mou, L. Sun, Z. Xu, X. Wei, and X. F. Bai. 2008. Expression of L protein of Hantaan virus 84FLi strain and its application for recovery of minigenomes. APMIS 116:1089-1096. [DOI] [PubMed] [Google Scholar]
- 45.Ziebuhr, J., E. J. Snijder, and A. E. Gorbalenya. 2000. Virus-encoded proteinases and proteolytic processing in the Nidovirales. J. Gen. Virol. 81:853-879. [DOI] [PubMed] [Google Scholar]