Abstract
Interleukin-7 (IL-7) plays a central role in controlling the homeostasis of both naive and long-term-memory CD4+ T cells. To better understand how human immunodeficiency virus (HIV) perturbs CD4+ T-cell homeostasis, we performed a detailed analysis of IL-7R expression, IL-7 binding, and IL-7-dependent early and late signaling events in CD4+ T-cell subsets from viremic and efficiently treated patients. HIV infection differentially affected the expression of IL-7 receptor (IL-7R) chains, with decreases in IL-7Rα/CD127 expression in the memory subset and increases in γc/CD132 expression in all CD4+ T cells. This resulted in preserved IL-7 binding in the naive compartment and decreased IL-7 binding in the memory compartment of viremic patients. Accordingly, the percentages of cells signaling in response to IL-7, as measured by pSTAT5 induction, were decreased in memory subsets, including conventional CD4+ T cells and regulatory T cells. However, the levels of pSTAT5 induction per responding cell, as measured by pSTAT5 fluorescence intensity, were increased within all naive and memory CD4+ T-cell subsets of viremic patients. The basal level of pSTAT5 was also increased, indicating a constitutive activation of the JAK/STAT5 pathway. IL-7 functional responses, as measured by Bcl-2, CD25, and Foxp3 induction, were impaired in viremic patient CD4+ T cells, suggesting that chronic activation led to downstream defects in the STAT5 signaling pathway. Thus, HIV infection perturbs IL-7 responses at both receptor binding and signaling steps, which likely compromises the regenerative capacity of the CD4+ T-cell pool and may contribute to CD4+ T-cell depletion.
Interleukin 7 (IL-7) plays a central role in controlling CD4+ T-cell homeostasis (2, 30, 52). This cytokine of the gamma-c (γc) family is essential for thymopoiesis and homeostatic proliferation of naive T cells, as exemplified by the profound lymphopenia in IL-7- or IL-7 receptor (IL-7R)-deficient hosts (48, 59, 64, 66). IL-7 is also required for the long-term survival of memory T cells after the clonal expansion phase, especially within the CD4+ T-cell subset (16, 17, 26, 42). CD4+ T-cell lymphopenia triggers a compensatory regulatory loop leading to increased levels of circulating IL-7, which drives homeostatic T-cell proliferation and leads to normalization of T-cell numbers (56, 88). IL-7 promotes T-cell proliferation in lymphopenic hosts by lowering the threshold for T-cell receptor (TCR) activation by foreign and self-antigens (4, 17, 31). IL-7 also supports T-cell growth through inactivation of cell cycle inhibitors and increased metabolism (6, 46, 67, 79, 85). In addition, IL-7 prolongs T-cell survival through induction of antiapoptotic factors, such as Bcl-2 and Bcl-xL (37, 63), and inhibition of proapoptotic factors, such as Bad and Bax (52).
The IL-7/CD4 regulatory loop is activated in human immunodeficiency virus (HIV) infection, as indicated by increased plasma IL-7 levels that inversely correlate with CD4+ T-cell counts of patients (1, 13, 14, 28, 29, 39, 50, 51, 56, 68, 70). Increased IL-7 secretion by bone marrow stromal cells from infected patients suggests a regulation at the level of cytokine production (34, 35). However, IL-7-dependent homeostatic regulation fails in HIV infection, as indicated by decreasing CD4+ T-cell counts in untreated patients in spite of persistently elevated IL-7 levels. A similar phenomenon is observed in pathogenic simian immunodeficiency virus infection of rhesus macaques, where elevated IL-7 levels do not lead to a restoration of the CD4+ T-cell pool (55). The lack of CD4+ T-cell increase in HIV and simian immunodeficiency virus infections may result from continuing CD4+ T-cell destruction by direct viral cytopathic effect and from the generalized immune activation and ensuing activation-induced cell death, which are thought to drive AIDS pathogenesis (25, 32, 76). In addition, several studies point to defective IL-7 responses in T cells from viremic patients. Both CD4+ and CD8+ T cells from patients have reduced sensitivities to the antiapoptotic effects of IL-7 compared to the level for cells from healthy controls (84). We have shown that both Bcl-2 and CD25 inductions are impaired in patient CD4+ and CD8+ T cells cultivated in the presence of IL-7 (21, 23). IL-7 retains the capacities to enhance patient T-cell proliferation and to reduce apoptosis to some extent but does not fully restore these parameters (10, 83). Given the key role of IL-7 in controlling CD4+ T-cell homeostasis, alterations in IL-7 responses are bound to contribute significantly to the pathogenic process that leads to AIDS. IL-7 responses rapidly improve in patients receiving efficient antiretroviral therapy, even though CD4+ T-cell numbers show a slower-paced recovery (23). Importantly, this opens the possibility of IL-7 immunotherapy for restoring CD4+ T-cell counts in patients with efficiently suppressed viral loads (12, 44, 74, 77).
The mechanism underlying defective IL-7 responses in HIV-infected patients remains incompletely understood. One factor may lie in the altered expression of IL-7R components in patient T cells. The IL-7R is composed of the private IL-7Rα chain (CD127) and of the common gamma chain (CD132 or γc), which is shared with receptors for other cytokines of the γc family, namely, IL-2, IL-4, IL-9, IL-15, and IL-21 (16, 36). The two IL-7R chains are differentially regulated: CD127 is downregulated upon T-cell activation through the TCR or through γc family cytokines, including IL-7 itself (3, 52, 62); in contrast, γc is rapidly upregulated upon T-cell activation, through mobilization of intracellular γc stores and transcriptional induction (5). This differential regulation is thought to optimize utilization of γc family cytokines other than IL-7 by activated cells, while sparing limiting IL-7 resources for resting cells (62). CD127 expression is decreased and γc expression is increased in T cells from HIV-infected patients, consistent with changes associated with chronic immune activation (22, 27, 41, 49, 54, 60, 70, 84). CD127 decrease is particularly marked in the memory CD8+ T-cell subset of viremic patients with high levels of chronic immune activation, which may account for the perturbed IL-7 responses observed in this subset (11, 22, 41, 49, 54, 60). However, it should be noted that decreased CD127 expression may be compensated for by increased γc expression and that the net effect of these changes on IL-7 binding capacity has not been investigated so far.
The CD4+ regulatory T-cell (Treg) population plays a key role in controlling the magnitude and duration of adaptive immune responses through suppression of T-cell activation (69). The impairment of Treg function may contribute to the abnormal immune activation characteristic of HIV infection and thus play a driving role in the pathogenic process (45). Since Treg homeostatic regulation differs from that of conventional CD4+ T cells, it is important to distinguish this population in analyzing IL-7 responses. Indeed, Tregs are characterized by a constitutively high level of CD25/IL-2Rα expression and a conversely low expression of CD127/IL-7Rα (47, 72). Accordingly, IL-2 was shown to be the key cytokine in maintaining the homeostasis of the Treg pool, while IL-7 was thought to play a limited role. More-recent studies, however, found that IL-7 could still induce signals in Tregs and contribute to their differentiation and survival in vivo (8, 38, 53, 82). One objective of the present study was to compare IL-7 responses in Tregs and conventional CD4+ T cells to determine whether HIV infection could differentially affect the homeostasis of these two populations.
We have previously shown that CD4+ T cells from viremic patients show detectable but limited decrease of CD127 expression but nevertheless respond as poorly to IL-7 as CD8+ T cells in terms of survival and proliferation (21, 23). Thus, signal transduction defects downstream of IL-7R may also contribute to impaired IL-7 responses, especially within the CD4+ T-cell population. To investigate this issue, we set out to identify the step at which IL-7-dependent signals are perturbed in CD4+ T-cell subsets from HIV-infected patients through a detailed analysis of IL-7R expression, IL-7 binding, and IL-7-dependent signaling events. We provide evidence that HIV infection perturbs IL-7 responses at two levels, by decreasing the IL-7 binding capacity of memory CD4+ T cells and by inducing an abnormal activation of the JAK/STAT5 signaling pathway in all CD4+ T-cell subsets, including Tregs. These changes result in defective STAT5-dependent responses, which may contribute to the progressive CD4+ T-cell loss characteristic of progression to AIDS.
MATERIALS AND METHODS
Study design.
Two groups of patients chronically infected with HIV were included in the study: viremic patients who did not receive antiretroviral therapy (VIR group [n = 12]) and efficiently treated patients with undetectable viral load (HAART group [n = 13]). The criteria for inclusion in the VIR group consisted of a viral load of >10,000 HIV RNA copies/ml plasma, a CD4+ T-cell count of >100/mm3, an absence of antiretroviral treatment or an interruption of treatment for at least 6 months, and no evidence for primary HIV infection. The criteria for inclusion in the HAART group consisted of a viral load of <40 HIV RNA copies/ml plasma for at least 6 months, with treatment initiation dating from at least 1 year and CD4+ T-cell counts of >300/mm3.
Study participants were recruited among the patients followed at the Centre Hospitalo-Universitaire of Bicêtre (CHU Bicêtre, France) and of Necker (CHU Necker, France). The control group consisted of noninfected healthy individuals who donated blood at the Etablissement Français du Sang (Paris, France). The study, designated EP33, was promoted by the Agence Nationale de Recherches sur le SIDA et les Hépatites Virales (ANRS). The study was approved by the Comité de Protection des Personnes de l'Ile de France VII under number 05-15. All participants gave written informed consent prior to blood sampling. A summary of the virological and immunological characteristics of patients is reported in Table 1.
TABLE 1.
Immunological and virological characteristics of study subjects
| Characteristica | Valueb for indicated group |
Pc |
||||
|---|---|---|---|---|---|---|
| HD (n = 22) | VIR (n = 12) | HAART (n = 13) | HD vs VIR | HD vs HAART | VIR vs HAART | |
| Age (yr) | NA | 42 (29-64) | 48 (31-60) | NS | ||
| CD4+ T-cell count | NA | 441 (150-646) | 648 (411-1,116) | <0.005 | ||
| Viral load | <40 | 65,254 (11,260-181,804) | <40 | |||
| CD4+ T-cell nadir | NA | 372 (117-492) | 193 (4-606) | <0.05 | ||
| % HLA-DR+ in: | ||||||
| NTreg | 3.2 (0.4-9.0) | 6.83 (2-26.9) | 9.3 (2.5-21.2) | <0.05 | <0.001 | NS |
| MTreg | 34.6 (17.1-50.7) | 45.3 (19.9-79.1) | 43.3 (19.3-59.6) | NS | NS | NS |
| Nv | 2.0 (0.7-4.6) | 6.3 (1.6-9.8) | 3.2 (1.6-8.5) | <0.0001 | <0.005 | <0.05 |
| Mem | 7.2 (3.6-19.7) | 23.3 (5.3-36) | 8.4 (3.8-20) | <0.0005 | NS | <0.005 |
CD4+ T-cell count, number of CD4+ T cells/mm3 blood; viral load, number of HIV RNA copies/ml plasma; HLA-DR+, HLA-DR positive.
Median values and ranges (in parentheses) are reported. NA, not available.
P values of <0.05 are reported. Statistical analyses were done with the nonparametric Mann-Whitney U test. NS, not significant.
PBMC collection and IL-7 stimulation.
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood through a density gradient on Ficoll-Hypaque (Axis-Schield, Oslo, Norway). PBMC were resuspended at 106 cells/ml in RPMI 1640 medium supplemented with 2 mM glutamine, antibiotics, and 0.5% human AB serum (complete medium). Freshly isolated PBMC (2 ×106) were stimulated with 5 pM or 500 pM of recombinant human glycosylated IL-7 (a gift from M. Morre, Cytheris) for 15 min at 37°C. IL-7 stimulation was stopped by fixation with paraformaldehyde (PFA) at a final concentration of 1.5% for 15 min at 37°C. After centrifugation and PFA removal by aspiration, cells were permeabilized by adding 90% ice-cold methanol drop by drop and incubating cells for 15 min on ice. Fixed cells were stored in methanol at −20°C until processing for intracellular labeling.
Intracellular phosphospecific labeling.
Methanol-permeabilized cells were washed in phosphate-buffered saline (PBS) twice and resuspended in staining buffer (PBS, 4% fetal bovine serum). Cells were stained simultaneously with antibodies for cell surface markers and intracellular markers, including the form of STAT5 phosphorylated at Y694 and the Treg-specific factor Foxp3. Since methanol fixation differentially affected the recognition of cell surface markers, each antibody was initially tested for optimal concentration and fluorophore choice. Through these preliminary experiments, we defined a panel of antibodies that allowed efficient measurement of pSTAT5 within four CD4+ T-cell subpopulations, comprising naive Tregs and memory Tregs (81) as well as naive and memory conventional CD4+ T cells. Cells were stained with an antibody combination consisting of CD4-peridinin chlorophyll protein (CD4-PerCP) and pSTAT5-Alexa Fluor 647 (pSTAT5-AF647) from BD Biosciences; Foxp3-AF488, CD45RA-Pacific Blue (CD45RA-PB), HLA-DR-phycoerythrin-cyanin 7 (HLA-DR-PE-Cy7), and CD3-allophycocyanin-AF750 (CD3-APC-AF750) from eBioscience; and CD25-PE from Dako. Each experiment included a control sample labeled with the same combination, but for the pSTAT5-specific antibody, which was replaced by isotypic control immunoglobulin G1-AF647 (BD Biosciences). Cells were stained for 40 min at 4°C and washed once in staining buffer. Fluorescence was acquired on a Cyan flow cytometer (Beckman Coulter) with Summit version 4.1 software (Beckman Coulter) and analyzed with FlowJo version 8.3.3 software (Tree Star). A minimum of 2 × 106 PBMC were used for each sample, so that sufficient events (at least 200) could be acquired in minor CD4+ T-cell subsets such as naive Tregs.
Measurement of IL-7R receptor expression.
Since antibodies to the IL-7Rα chain (CD127) did not give detectable binding to methanol-permeabilized cells, a separate set of experiments was carried out to evaluate expression of IL-7R receptor chains on CD4+ T cells. Freshly isolated PBMC (1.5 × 106) were labeled for 30 min at 4°C with an antibody combination consisting of CD25-APC, CD127-APC-AF750, CD45RA-PB, and HLA-DR-PE-Cy7 from eBioscience and CD132-PE and CD4-PerCP from BD Biosciences. Cells were fixed and permeabilized with a fixation/permeabilization buffer adapted to Foxp3 detection (eBioscience), labeled for 30 min at 4°C with the Foxp3-AF488 antibody, washed, and resuspended in PBS-1% PFA. Fluorescence was acquired on a Cyan flow cytometer as described above.
Measurement of circulating IL-7.
The IL-7 concentration in plasma collected on EDTA was measured using an immunoassay in accordance with the manufacturer's instructions (Quantikine HS human IL-7; R&D Systems).
IL-7 binding assay.
The binding of recombinant biotinylated IL-7 to primary CD4+ T cells was evaluated by flow cytometry, using a Fluorokine assay kit (R&D Systems). Briefly, 0.04, 0.4, 1.2, 4, 12, and 40 ng of biotinylated IL-7 were added to 105 PBMC resuspended in 25 μl of serum-free PBS for 60 min at 4°C. Cells were then stained with avidin-fluorescein isothiocyanate (avidin-FITC) and an antibody combination consisting of CD4-PerCP, CD45RA-PB, and CD3-APC-AF750 for 30 min at 4°C. PBMC were washed once and resuspended in a 1:1 mixture of wash buffer and fixing solution (PBS, 4% PFA) before immediate fluorescence acquisition on a Cyan flow cytometer. Binding values were determined by the mean fluorescence intensities (MFIs) of the IL-7-biotin-avidin-FITC complex in naive (CD3+ CD4+ CD45RA+) and memory (CD3+ CD4+ CD45RA−) CD4+ T-cell populations. The negative controls included measurement of binding of an unrelated biotinylated protein (soybean trypsin inhibitor) and competition of specific IL-7 binding by a polyclonal anti-IL-7 blocking antibody.
Evaluation of IL-7 functional responses.
Bcl-2, CD25, and Foxp3 inductions were assayed by flow cytometry on PBMC cultivated for 3 days in the presence of IL-7. For these experiments, 2 × 106 PBMC were cultured in complete medium in the presence or absence of 500 pM recombinant human IL-7. After 3 days, cells were fixed by adding PFA to give a final concentration of 1.5%, permeabilized with methanol, washed in PBS, and resuspended in staining buffer. Cells were labeled by using an antibody combination consisting of Bcl-2-FITC (Dako), CD25-PE, Foxp3-AF647, CD3-APC-AF750, CD4-PerCP, and CD45RA-PB. Fluorescence was acquired on a Cyan flow cytometer as described above. Apoptosis at day 3 was measured as the percentage of low-forward-scatter, high-side-scatter cells within the CD3+ CD4+ gate. Experiments with a viability dye (live/dead fixable aqua dead-cell stain; Invitrogen) confirmed that the apoptotic population consisted predominantly of cells that were still viable. Induction of target proteins was measured by the difference in Bcl-2, CD25, or Foxp3 MFI between stimulated and unstimulated cells at day 3.
Statistical analyses.
Analyses were performed with GraphPad Prism 4.0 software, using nonparametric statistical tests in all cases. Differences in variables between subject groups were analyzed with the Mann-Whitney U Test. Horizontal bars on data plots indicate median values. Correlations were analyzed with Spearman's coefficient R. All significant differences between groups (P < 0.05) are reported on data plots.
RESULTS
Differential activation of STAT5 in CD4+ T-cell subpopulations from healthy donors.
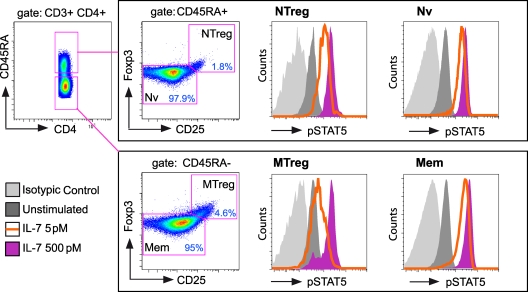
IL-7 signaling responses in CD4+ T cells from healthy donors (HD group; n = 13) were initially analyzed to determine the degrees of responsiveness in different CD4+ T-cell subpopulations. Activation of the JAK/STAT pathway was measured by labeling PBMC with a phospho-specific antibody that recognizes STAT5 phosphorylated at Y694, combined with immunophenotyping and polychromatic flow cytometry analysis. Using a gating strategy based on CD3, CD4, CD45RA, Foxp3, and CD25 expression (Fig. 1), we could measure STAT5 activation in four distinct CD4+ T-cell populations: naive Tregs (NTreg), conventional naive CD4+ T cells (Nv), memory Tregs (MTreg), and conventional memory CD4+ T cells (Mem). All CD4+ T-cell subsets showed efficient signaling responses to a 15-min treatment with IL-7 at a high dose (500 pM), as indicated by the increase in pSTAT5 fluorescence intensity (Fig. 1). However, low-dose IL-7 stimulation (5 pM) induced an intermediate response in NTreg and MTreg cells, while Nv and Mem CD4+ T cells responded almost as efficiently as they did to high-dose IL-7 stimulation (Fig. 1).
FIG. 1.
Gating strategy for the analysis of STAT5 phosphorylation within CD4+ T-cell subsets. PBMC from a healthy blood donor were stimulated or not stimulated with IL-7 for 15 min, permeabilized by methanol treatment, and labeled intracellularly with antibodies to CD3, CD4, CD45RA, CD25, Foxp3, and pSTAT5 (Y694). The gating strategy depicted allowed the characterization of pSTAT5 induction in four CD4+ T-cell subsets: conventional naive CD4+ T cells (Nv), naive Tregs (NTreg), conventional memory CD4+ T cells (Mem), and memory Tregs (MTreg). pSTAT5 inductions in response to high-dose IL-7 stimulation (purple histograms) did not differ markedly between subsets. In contrast, responses to low-dose IL-7 stimulation (orange lines) were more efficient in the Mem and Nv subsets than in the two Treg subsets. Basal pSTAT5 values are represented by dark shaded histograms (unstimulated samples). Negative isotypic control values are represented by light shaded histograms.
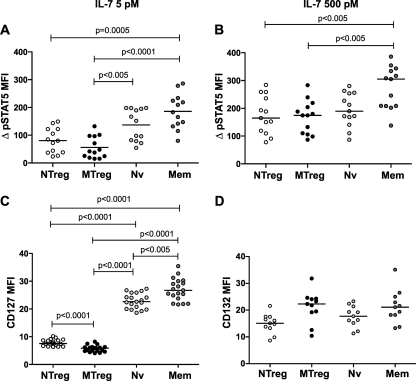
Quantitation of STAT5 activation, as measured by the increase in pSTAT5 MFI in IL-7-treated samples in comparison to the level for unstimulated samples (ΔpSTAT5 MFI), showed that NTreg and MTreg had detectable but significantly lower-level responses than the Nv and Mem subsets to low-dose IL-7 stimulation (Fig. 2A). The level of pSTAT5 responses paralleled that of CD127 expression in the different CD4+ T-cell subsets (Fig. 2C), suggesting that expression of the alpha chain of the receptor was the major parameter controlling STAT5 activation after low-dose IL-7 stimulation. In contrast, expression of the γc chain did not show major variations between CD4+ T-cell subsets, except for slightly lower levels in naive subsets (NTreg and Nv) than in memory subsets (MTreg and Mem) of CD4+ T cells (Fig. 2D). Of note, differences in STAT5 activation were less marked after high-dose IL-7 stimulation, as responses in the two Treg subsets did not differ significantly from those of naive CD4+ T cells (Fig. 2B). The 500 pM dose used is 1 order of magnitude above the estimated Kd of IL-7 for its receptor (close to 30 pM) (57), ensuring full occupancy of the receptor. Therefore, parameters other than CD127 expression may control the level of IL-7 responses once the receptor is saturated.
FIG. 2.
Comparison of STAT5 phosphorylation and CD127 expression between CD4+ T-cell subsets in healthy donors. PBMC from healthy donors (n = 13) were stimulated with 5 pM IL-7 (A) or 500 pM IL-7 (B) for 15 min and evaluated for pSTAT5 induction by flow cytometry within the four CD4+ T-cell subsets (NTreg, MTreg, Nv, and Mem). “ΔpSTAT5 MFI” represents the difference in pSTAT5 MFI between IL-7-stimulated and unstimulated samples. (C) PBMC from healthy donors (n = 19) were evaluated for CD127 expression within the four CD4+ T-cell subsets. (D) PBMC from healthy donors (n = 11) were evaluated for CD132 expression within the four CD4+ T-cell subsets. Statistical analyses were performed with the nonparametric Mann-Whitney U test. A Bonferroni correction was applied because six comparisons were made between four populations. Therefore, the significance threshold was lowered to a P value of <0.0084 (0.05/6). All P values of <0.0084 are reported. Horizontal bars indicate median values.
To better evaluate the efficiency of IL-7 signaling independent of CD127 expression, we computed the ratio of ΔpSTAT5 MFI to CD127 MFI ([Re ratio] Table 2). This ratio was approximately three times lower in conventional CD4+ T cells than in Treg subsets after high-dose IL-7 stimulation, likely pointing to a limiting signaling component (such as the pool of unphosphorylated STAT5) in conventional CD4+ T cells at maximal IL-7R activation. A higher signaling efficiency in Tregs may also contribute to the observed differences in Re ratios (65, 87). Of interest, the Re ratio was higher in MTreg than in NTreg, though these subsets did not differ markedly in CD127 expression. Similarly, the Re ratio was higher in Mem than in Nv CD4+ T cells. Both observations suggested that memory CD4+ T-cell subsets may signal more efficiently via IL-7R than the corresponding naive subsets. Taken together, the analysis of IL-7 responses in healthy donor cells revealed a differential activation of STAT5 in distinct CD4+ T-cell subsets, which differed in receptor expression but also possibly in intrinsic signaling efficiency.
TABLE 2.
Efficiency of IL-7-dependent signaling in different CD4+ T-cell subsetsa
| Subset | Re ratio |
P |
|||
|---|---|---|---|---|---|
| HD | VIR | HAART | HD vs VIR | VIR vs HAART | |
| NTreg | 23.38 | 34.33 | 23.23 | <0.05 | <0.05 |
| MTreg | 30.02 | 32.97 | 32.55 | NS | NS |
| Nv | 8.32 | 13.52 | 9.08 | <0.001 | <0.05 |
| Mem | 10.45 | 15.67 | 11.96 | <0.005 | <0.01 |
The Re ratios of ΔpSTAT5 MFI to CD127 MFI were computed for four CD4+ T cell subsets after high-dose IL-7 stimulation and compared between healthy donors (HD), viremic patients (VIR), and treated patients (HAART). Median values for Re ratio are indicated. Differences between groups were evaluated by the Mann-Whitney U test. P values of <0.05 are reported. NS, not significant. All P values for HD versus HAART were not significant.
HIV infection differentially alters the expression of the two IL-7R chains in CD4+ T-cell subsets.
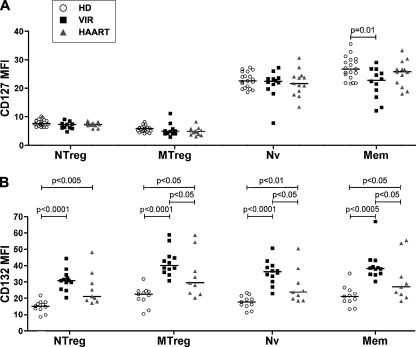
We next examined the effect of HIV infection on IL-7R expression in the different CD4+ T-cell subsets. The studied groups included untreated viremic patients with viral loads of >10,000 HIV RNA copies/ml (VIR group; n = 12) and efficiently treated patients with viral loads of <40 HIV RNA copies/ml (HAART group; n = 13). The immunologic and virologic characteristics of the patients are recapitulated in Table 1. Expression of the activation marker HLA-DR was significantly increased in the NTreg, Nv, and Mem CD4+ T-cell subsets of viremic patients in comparison to the level for healthy donors (Table 1), supporting the notion of chronic immune activation in the viremic group. HLA-DR expression was high in the MTreg population for the three groups (median values between 34 and 45%), consistent with known properties of Tregs (81). Activation levels were not entirely normalized in efficiently treated patients, who maintained increased HLA-DR expression in the Nv and NTreg subsets.
The expression levels of both chains of the receptor, CD127 and CD132, in CD4+ T-cell subsets of patients and control individuals were evaluated. CD127 expression was decreased in the Mem subset of viremic patients (Fig. 3A), in agreement with published studies (22, 27, 41, 54, 70) and consistent with the notion of chronic immune activation in untreated HIV infection. CD127 expression was preserved in the Nv subset, except in one viremic patient, who showed a marked loss for this marker. We cannot rule out that expansion of a CD45RA+ CD127− effector CD4+ T-cell population occurred in this patient, as can happen in advanced HIV infection. Interestingly, CD132 expression was markedly increased in CD4+ T cells from viremic patients, with a near doubling of the fluorescence intensity in all CD4+ T-cell subsets (Fig. 3B). However, no conclusions regarding the expression of functional heterodimeric CD127/CD132 complexes could be drawn, due to the concomitant decrease in CD127 expression. Of note, CD132 expression remained significantly increased in CD4+ T cells from treated patients, indicating that efficient control of viral replication was not sufficient to normalize all signs of abnormal activation.
FIG. 3.
Expression of the two IL-7R chains in CD4+ T cells from HIV-infected patients and healthy donors. PBMC from healthy donors (HD), efficiently treated patients (HAART), and viremic patients (VIR) were evaluated for IL-7Rα/CD127 (A) and γc/CD132 (B) expression by flow cytometry within the four CD4+ T-cell subsets: NTreg, MTreg, Nv, and Mem. Horizontal bars indicate median MFI values.
Circulating-IL-7 concentrations remained below 3.0 pg/ml in both the VIR and the HAART groups, except for one viremic patient, with 6.3 pg/ml IL-7 in plasma (not shown). These low levels of circulating IL-7 were consistent with the relatively preserved CD4+ T-cell counts in our cohort of patients (Table 1). Indeed, IL-7 is known to increase primarily in patients with CD4+ T-cell counts below 200/mm3 (56). Given the low levels of circulating IL-7 detected in the present study, it was unlikely that chronic signaling by this cytokine alone could account for the decreased expression of CD127 in viremic patients.
Decreased binding of IL-7 to memory CD4 T cells of viremic patients.
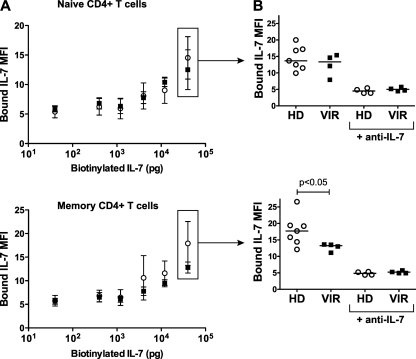
Given the opposite changes in CD127 and CD132 expression, it was difficult to predict whether IL-7 binding to patient CD4+ T cells would increase or decrease. Therefore, we set out to directly assess IL-7 binding, using flow cytometry detection of biotinylated IL-7 bound at the surfaces of PBMC (Fluorokine assay; R&D Systems). Because this method requires intact live cells, we could not perform intracellular detection of Foxp3 and therefore determined binding for the total naive and total memory CD4+ T-cell populations. CD4+ T cells from viremic patients and healthy controls showed dose-dependent increases in biotinylated IL-7 binding in the naive and memory subsets (Fig. 4A, top and bottom panels, respectively). At the highest dose tested, naive CD4+ T cells from viremic patients and healthy controls bound IL-7 at similar levels (Fig. 4B, top panel). In contrast, memory CD4+ T cells from viremic patients bound significantly less IL-7 than those from healthy controls (P < 0.05) (Fig. 4B, bottom panel). Competition with a blocking anti-human IL-7 antibody reduced labeling to the background level, confirming the specificity of the assay (Fig. 4B, +anti-IL-7 samples). Thus, viremic patients showed preserved IL-7 binding to naive CD4+ T cells, which maintained normal CD127 expression, and decreased binding to memory CD4+ T cells, which had decreased CD127 expression. These findings supported the notion that CD127 expression, rather than CD132 expression, was the limiting parameter in determining IL-7 binding.
FIG. 4.
Decreased binding of IL-7 to memory CD4+ T cells from viremic patients. (A) Binding of biotinylated IL-7 to nonpermeabilized PBMC was evaluated by flow cytometry within the naive (CD3+ CD4+ CD45RA+; top panel) and the memory (CD3+ CD4+ CD45RA−; bottom panel) CD4+ T-cell compartments. Bound IL-7 was revealed by incubation with avidin-FITC (Fluorokine assay; R&D Systems). Binding levels were compared between PBMC from healthy donors (n = 7; clear circles) and those from viremic patients (n = 4; solid squares). Error bars represent standard deviations. (B) IL-7 binding values obtained at the highest IL-7 dose (40 ng) were compared between healthy donors and viremic patients, within naive CD4+ T cells (top panel) and memory CD4+ T cells (bottom panel). The negative controls consisting of samples incubated with a blocking IL-7 antibody (+anti-IL-7) and biotinylated IL-7 are represented on the right of the graphs.
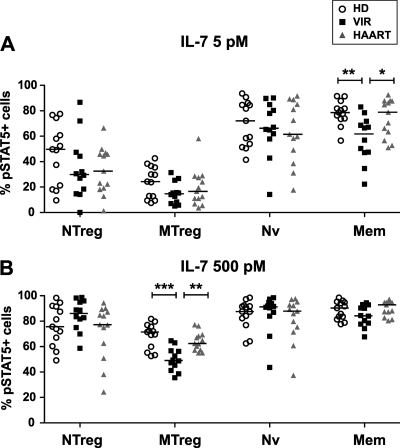
Decreased frequency of memory CD4+ T cells responding to IL-7 in viremic patients.
We then assessed whether the decrease in IL-7 binding capacity seen in memory CD4+ T cells of viremic patients led to a decrease in downstream signaling events. The frequencies of pSTAT5-positive (pSTAT5+) cells at baseline, determined by comparison to the results for isotypic control staining, were low in all groups, with median values below 2% for all CD4+ T-cell subsets (not shown). The frequency of responding cells, corresponding to the percentage of pSTAT5+ cells observed after IL-7 treatment minus the percentage of pSTAT5+ cells observed at baseline, was determined for two IL-7 doses (Fig. 5). Upon low-dose IL-7 stimulation (5 pM) (Fig. 5A), the frequency of responding cells was indeed decreased in the Mem CD4+ T-cell subset of viremic patients in comparison to the levels for healthy donors (P < 0.005) and efficiently treated patients (P < 0.05). The MTreg subset showed low pSTAT5 responses, as expected, with a trend toward lower values in patient groups that did not reach statistical significance. The responses in naive subsets (Nv and NTreg) were more heterogenous and did not differ significantly between groups. High-dose IL-7 stimulation (500 pM) (Fig. 5B) led to high levels of response in conventional Nv and Mem subsets (with median frequencies of responding cells above 85% in all groups), suggesting a saturation of the population of responding cells under these conditions. However, it was interesting that, in the VIR group, the percentage of responding cells within the Mem subset correlated negatively with the viral load (P < 0.05; Spearman correlation coefficient R = −0.65; data not shown). This finding suggested that HIV replication could impair responses to high-dose IL-7 stimulation in the memory CD4+ T cells of the most-advanced patients. IL-7 responses in MTregs remained intermediate and were decreased in viremic patients in comparison to the levels for healthy donors (P < 0.001) and treated patients (P < 0.005). The defect in pSTAT5 response was not detected within the NTreg subset of viremic patients. Taken together, these data were consistent with decreased frequencies of signaling response in memory but not naive CD4+ T-cell subsets of viremic patients, a finding compatible with decreased IL-7 binding capacity.
FIG. 5.
Decreased frequencies of IL-7 response in memory CD4+ T-cell subsets of viremic patients. PBMC from healthy donors (HD), viremic patients (VIR), and efficiently treated patients (HAART) were stimulated with 5 pM IL-7 (A) or 500 pM IL-7 (B) for 15 min and evaluated for STAT5 phosphorylation by flow cytometry within the four CD4+ T-cell subsets: NTreg, MTreg, Nv, and Mem. The percentage of pSTAT5+ cells observed after IL-7 stimulation is reported, with the percentage of pSTAT5+ cells at the baseline subtracted. Horizontal bars indicate median values. Statistically significant differences, as evaluated by the Mann-Whitney U test, are indicated. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
As a control, we measured the frequency of IL-7-responding cells in memory CD8+ T cells defined as the CD3+ CD4− CD45RA− population. Consistent with the literature (11, 84), we found a marked decrease of IL-7 response in memory CD8+ T cells of viremic patients, with the percentage of pSTAT5+ cells decreased about twofold (at 5 pM IL-7, the median percentage pSTAT5+ cells was 10.46 in the VIR group, versus 23.90 in the HD group [P < 0.05]; at 500 pM IL-7, the median percentage of pSTAT5+ cells was 31.80 in the VIR group, versus 62.09 in the HD group [P < 0.05]). The impairment of IL-7 responses could be explained by a drastic decrease of CD127 expression in memory CD8+ T cells, with an MFI also decreased twofold (the median CD127 MFI was 7.2 in the VIR group, versus 13.5 in the HD group [P < 0.0001]). Taken together, these findings supported the notion that CD127 downregulation could account for the loss of IL-7 responder cells in memory T-cell populations.
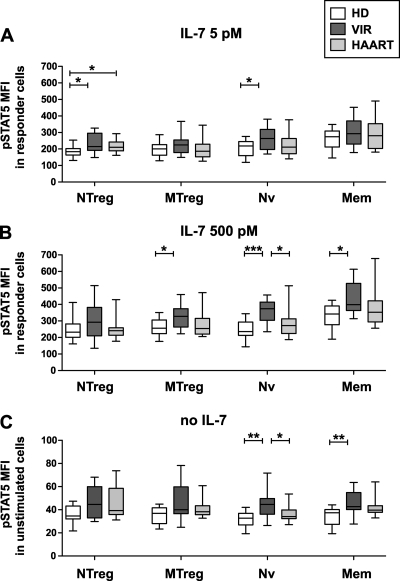
Hyperresponsiveness of the STAT5 pathway in IL-7-responding cells from viremic patients.
We noted that the level of pSTAT5 induction, as measured by the increase in fluorescence intensity following IL-7 treatment (ΔpSTAT5 MFI), did not decrease in viremic patient CD4+ T cells, in spite of a decrease in the frequency of responding cells. On the contrary, there was a trend toward higher-level induction of pSTAT5 in the viremic group, which was statistically significant in the Nv CD4+ T-cell subset (after treatment with 500 pM IL-7, the median ΔpSTAT5 MFIs were 195 in the HD group and 286 in the VIR group; P = 0.005). To clarify this point, we analyzed the level of pSTAT5 induction per responding cell by measuring the pSTAT5 MFI within the pSTAT5+ population (Fig. 6). This analysis confirmed the increased pSTAT5 induction per CD4+ T cell in the viremic group, with significant differences observed in the naive subsets after low-dose IL-7 stimulation (Nv and NTreg) (Fig. 6A) and in both naive and memory subsets after high-dose IL-7 stimulation (Nv, MTreg, and Mem) (Fig. 6B). Thus, HIV infection appeared to perturb IL-7 responses by causing hyperactivation of the JAK/STAT pathway in all CD4+ T-cell subsets. Of note, therapy normalized pSTAT5 induction levels in all CD4+ T-cell subsets except NTreg, suggesting that control of HIV replication could revert to a large extent the abnormal activation of the JAK/STAT pathway.
FIG. 6.
HIV infection induces STAT5 hyperactivation. PBMC from healthy donors (HD; n = 13), viremic patients (VIR; n = 12), and efficiently treated patients (HAART; n = 13) were stimulated with 5 pM IL-7 (A) or 500 pM IL-7 (B). The level of STAT5 phosphorylation in IL-7 responder cells was measured by the MFI of pSTAT5 within the population of pSTAT5+ cells for each of the four CD4+ T-cell subsets analyzed: NTreg, MTreg, Nv, and Mem. (C) Basal STAT5 phosphorylation levels are represented by the pSTAT5 MFI values observed in each CD4+ T-cell subset prior to cytokine stimulation. Boxes indicate median values and interquartile ranges; whiskers extend between minimum and maximum values. Statistically significant differences evaluated by the Mann-Whitney U test are indicated. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Increased basal STAT5 phosphorylation levels in HIV infection.
To determine whether abnormal STAT5 activation occurred chronically, we evaluated the degree of basal STAT5 phosphorylation in CD4+ T cells prior to stimulation (Fig. 6C). The basal pSTAT5 MFI was 5 to 10 times lower than that measured after IL-7 stimulation (median MFI = 40) but significantly higher than the background levels measured with an isotypic control antibody (Fig. 1). Interestingly, CD4+ T cells from viremic patients showed increases in basal pSTAT5 MFI, which were significant in the Nv and Mem subsets (P < 0.01). The basal pSTAT5 MFI in the Nv subset correlated positively with the viral load (P < 0.05; Spearman correlation coefficient R = 0.71; data not shown), supporting the notion that viral replication played a role in driving STAT5 activation. Treated patients showed intermediate levels of basal STAT5 activation that did not differ significantly from those seen in healthy controls. Taken together, these findings confirmed the notion of an abnormal activation of the STAT5 signaling pathway, which occurred chronically in viremic patients and was further amplified upon IL-7 stimulation.
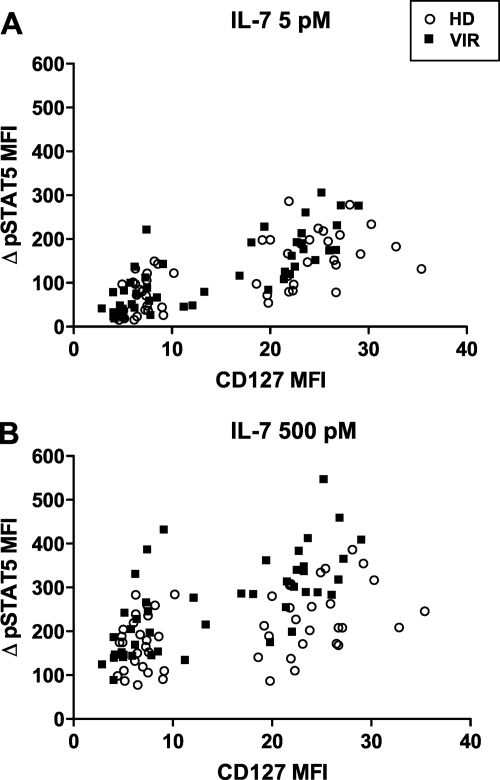
Dependency of STAT5 activation on CD127 expression.
To determine the extent to which phosphorylation of STAT5 depended on receptor expression, we plotted the ΔpSTAT5 MFI response as a function of the CD127 MFI (Fig. 7). Four data points were plotted per study subject, corresponding to the ΔpSTAT5 MFI responses in the four CD4+ T-cell subsets (NTreg, MTreg, Nv, and Mem). STAT5 phosphorylation appeared to increase as a function of CD127 expression, after both low-dose (Fig. 7A) and high-dose (Fig. 7B) IL-7 stimulations, in the HD group as well as the VIR group. Thus, the abnormal activation of the JAK/STAT pathway did not render pSTAT5 production entirely independent of CD127 expression. Interestingly, the VIR group showed a trend toward a higher pSTAT5 increase for a given CD127 expression level after high-dose stimulation (Fig. 7B). We could not quantify these relations by correlation analysis, because of the bimodal distribution of the data set (the presence of distinct low-level-CD127 and high-level-CD127 populations, corresponding to Tregs and conventional CD4+ T-cell subsets, respectively). Therefore, we performed an intrasubset analysis by computing the Re ratios of ΔpSTAT5 MFI to CD127 MFI after high-dose IL-7 stimulation (Table 2). This parameter, which reflects the efficiency of STAT5 phosphorylation, proved significantly higher in the VIR group than in the HD group within the NTreg, Nv, and Mem CD4+ T-cell subsets. This analysis confirmed that for a given CD127 expression level, STAT5 phosphorylation was greater in CD4+ T cells of viremic patients than in those of healthy individuals. Since the analysis was done on the high-dose data set, when all the receptors were saturated by IL-7 and could contribute to signaling, the differences seen between groups must have depended on postreceptor events. Thus, it was possible to conclude that hyperresponsiveness to IL-7 in viremic patients resulted from a perturbation of the signaling machinery downstream of IL-7R.
FIG. 7.
Dependency of STAT5 phosphorylation on CD127 expression. STAT5 phosphorylation (ΔpSTAT5 MFI) in response to IL-7 stimulation was plotted as a function of CD127 expression (CD127 MFI) for each CD4+ T-cell subset. Since four CD4+ T-cell subsets were analyzed per sample (NTreg, Nv, MTreg, and Mem), four data points were plotted per patient. Cells were stimulated with low doses of IL-7 (A) and high doses of IL-7 (B) in the group of healthy donors (HD) and viremic patients (VIR).
Analysis of the Re ratio in the group of treated patients yielded values that did not differ significantly from those found in healthy donors and remained lower than those found in viremic patients (Table 2). Thus, efficient antiretroviral therapy appeared to normalize the abnormal activation of the STAT5 pathway to a large extent.
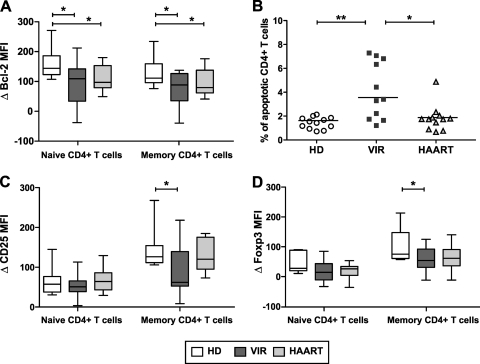
Impairment of IL-7 functional responses in HIV infection.
We next evaluated the functional responses of patient CD4+ T cells cultivated in the presence of IL-7, as measured by the inductions of the survival factor Bcl-2 and of the differentiation factors CD25 and Foxp3. Expression of these three proteins is dependent on STAT5 function, either indirectly in the case of Bcl-2 (74) or directly through the presence of STAT5 binding sequences in the promoters of CD25 and Foxp3 (19, 40, 86). For these experiments, PBMC were cultivated in the presence of a high dose of IL-7 (500 pM) for 3 days and analyzed by flow cytometry using a gating strategy based on CD3, CD4, and CD45RA staining. Treg subsets were not specifically analyzed, since the induction of Foxp3 and CD25 by IL-7 blurred the distinction between Tregs and recently activated cells.
CD4+ T cells from viremic patients showed impaired Bcl-2 induction in both the naive and the memory subsets (Fig. 8A) (P < 0.05). Culture in the presence of IL-7 did not abrogate the propensity of viremic patient CD4+ T cells to apoptose (Fig. 8B), consistent with a defect in the prosurvival function of IL-7. CD4+ T cells from treated patients also showed impaired Bcl-2 induction (P < 0.05), though this did not translate into increased apoptosis, suggesting that Bcl-2 was not the only parameter controlling survival. Both CD25 and Foxp3 inductions were defective in the memory CD4+ T-cell population from viremic patients, pointing to an impairment of STAT5 function (Fig. 8C and D) (P < 0.05 in both cases). Taken together, these findings showed impairment of IL-7 functional responses in CD4+ T cells from viremic patients. While defective IL-7 responses in the memory compartment could result from decreased receptor expression and IL-7 binding capacity, the defect in the naive compartment indicated a postreceptor block in signaling responses.
FIG. 8.
Impaired IL-7 functional responses in CD4+ T cells from viremic patients. PBMC from healthy donors (HD; n = 12), viremic patients (VIR; n = 11), and efficiently treated patients (HAART; n = 12) were cultured for 3 days in complete medium in the presence or absence of 500 pM IL-7. (A) Induction of the survival factor Bcl-2 in the naive (CD3+ CD4+ CD45RA+; left) and the memory (CD3+ CD4+ CD45RA−; right) CD4+ T-cell subsets. Differences in Bcl-2 MFI (ΔBcl-2 MFI) between IL-7-stimulated and unstimulated samples are reported. (B) Percentages of apoptotic cells in the CD3+ CD4+ population after 3 days of culture in the presence of a high dose of IL-7. (C) Induction of the activation marker CD25 in IL-7-treated naive and memory CD4+ T cells. Differences in CD25 MFI between IL-7-stimulated and unstimulated samples are reported. (D) Induction of the differentiation marker Foxp3 in IL-7-treated naive and memory CD4+ T cells. Differences in Foxp3 MFI between IL-7-stimulated and unstimulated samples are reported. Boxes indicate median values and interquartile ranges; whiskers extend between minimum and maximum values. Statistically significant differences, as evaluated by the Mann-Whitney U test, are indicated. *, P < 0.05; **, P < 0.01.
DISCUSSION
IL-7 is the key cytokine that regulates the homeostasis of peripheral CD4+ T cells, through signals that promote both long-term survival and homeostatic proliferation of IL-7R-expressing cells (2, 30, 52). The present work identifies two steps in the impairment of IL-7-dependent responses in patients with active HIV replication. First, IL-7 binding is altered, due to changes in the expression of functional IL-7R heterodimers. We show for the first time that the combination of an increase in γc expression and a decrease of CD127 results in decreased IL-7 binding within the memory CD4+ T-cell population. The reduced binding capacity for IL-7 can in turn explain the reduced frequency of memory CD4+ T cells that respond to IL-7 and contribute to IL-7-dependent functional defects within this population. A second block in IL-7 response at the postreceptor binding stage is needed to account for functional defects within the naive CD4+ T-cell population. These cells have normal IL-7 binding capacity but show reduced IL-7-dependent prosurvival effects, as measured by Bcl-2 induction, pointing to a signaling defect downstream of the receptor. Since early signaling events, as measured by STAT5 phosphorylation at Y694, are increased, rather than decreased, in naive CD4+ T cells of viremic patients, the defect likely occurs at a more distal step of the signaling pathway.
Progressive HIV infection exerted two types of effects on STAT5 phosphorylation responses. On the one hand, the percentage of CD4+ T cells expressing pSTAT5 decreased in conventional and Treg memory CD4+ T-cell subsets, a finding compatible with a recent study reporting defective IL-7R signaling in memory CD4+ T cells of patients with active HIV replication (9). On the other hand, we observed signs of STAT5 hyperactivation, as indicated by (i) an increased level of pSTAT5 induction per cell within the population of CD4+ T cells that still responded to IL-7 stimulation, (ii) a higher level of pSTAT5 induction for a given level of CD127 expression in CD4+ T cells of viremic patients than in those of healthy donors, and (iii) increased STAT5 phosphorylation levels at the baseline in both the naive and the memory CD4+ T-cell compartments of viremic patients. These findings can be reconciled if one considers that HIV perturbs pSTAT5 responses at both receptor binding and postreceptor binding stages. The loss of a population of IL-7 responder cells can be explained by decreased CD127 expression, which limits IL-7 binding capacity and may abrogate pSTAT5 responses under a certain threshold of expression. Since CD127 decrease occurs only within memory CD4+ T cells, the loss of responder cell remains restricted to the same population. Abnormal activation of the signaling machinery downstream of the IL-7R receptor can in turn explain the increased sensitivity of STAT5 phosphorylation responses. The notion of a chronic activation of the JAK/STAT pathway is in agreement with reports of increased basal phosphorylation levels in several signaling pathways, including those of STAT5 and those downstream of the TCR, in progressive HIV infection (20, 71). The dysregulation of multiple signaling pathways likely reflects the generalized immune activation that is thought to drive HIV pathogenesis and lead to the progressive exhaustion of the immune system (25, 32, 76).
A specific reason for the basal activation of STAT5 may lie in the increased levels of circulating IL-7 seen in the plasma of HIV-infected patients with low CD4+ T-cell counts (1, 13, 14, 28, 29, 39, 50, 51, 56, 68, 70). However, we did not detect a correlation between circulating IL-7 and basal or induced pSTAT5 levels in our study group, which comprised viremic patients with moderate CD4+ T-cell depletion (not shown). Also, it is unlikely that the low IL-7 concentrations detected in patients with intermediate CD4 counts can account for the chronic activation of the memory CD4+ T-cell subsets, which have low IL-7 binding capacity. Rather, other cytokines signaling through STAT5 may be responsible for this chronic activation. In this respect, the increase in γc expression detected in viremic patients was of significant magnitude, with a doubling of the MFI, and persisted to some extent in efficiently treated patients. A possibility is that increased expression of the γc chain renders CD4+ T cells more susceptible to stimulation by other γc family cytokines that are induced upon chronic immune activation. IL-15 is an intriguing candidate because IL-15 expression is induced in a variety of inflammatory conditions (18), IL-15 levels are significantly increased in acute and progressive HIV infection (7, 78), and IL-15 can drive the activation and expansion of CCR5+ CD4+ T cells in simian models (58). In addition, we have found that the two molecules that comprise the minimal IL-15 receptor, namely, γc and CD122/IL-2Rβ, are both increased in CD4+ T cells of viremic patients (Fig. 3B and data not shown). Thus, the IL-15 system may contribute to persistent STAT5 activation, since both the ligand and the receptor are upregulated in progressive HIV infection.
Importantly, chronic STAT5 activation did not result in more-efficient functional responses to IL-7, as measured by CD25, FoxP3, and Bcl-2 induction. Chronic stimulation may activate negative regulatory mechanisms that tightly control the activity of STAT5, a potentially oncogenic protein (33). Negative regulators include SLIM proteins, which lead to ubiquitin-dependent degradation of activated STAT (80), and transcriptional regulators, such as PIAS, which prevent STATs from binding to DNA through sumoylation (75). Another possible mechanism for the desensitization of IL-7 responses may be linked to the accumulation of truncated forms of STAT5, which appear to be increased in HIV infection (15), and which were shown to act as dominant-negative mutants of full-length STAT5 (24). Further studies will be needed to pinpoint the distal step at which STAT5 function is impaired.
STAT5 plays a central role in pathways that control CD4+ T-cell survival, through the direct induction of antiapoptotic factors, such as Bcl-XL, and through its effects on the phosphatidylinositol 3-kinase/Akt pathway (37, 52, 63). Phosphatidylinositol 3-kinase activation is required for the increased survival, metabolic rate, and proliferation of IL-7-treated T cells (6, 43, 61, 79). In primary T cells, the IL-7-dependent Akt response is dependent on prior STAT5 activation (85). Thus, STAT5 dysfunction may result in poor Akt activation, which would contribute to the inefficient Bcl-2 induction and the propensity to apoptosis detected in CD4+ T cells from viremic patients. The fact that defective Bcl-2 induction extended to the naive CD4+ T-cell compartment is bound to have profound consequences on CD4+ T-cell homeostasis by compromising the regenerative capacity of the CD4+ T-cell pool.
In designing the study, we chose to analyze conventional and regulatory CD4+ T-cell subsets separately, given their markedly different responses to IL-7 stimulation. Our findings confirm that IL-7 can signal in Tregs, in spite of the low CD127 expression levels characteristic of these cells (8, 38, 53, 82). Tregs were able to respond to an IL-7 concentration in the picomolar range, even though STAT5 activation levels remained lower than in conventional CD4+ T cells. Analyses of IL-2 responses in mouse models have shown that Tregs appear to have a low IL-2R signaling threshold (87), a finding that may be explained in part by a low concentration of the negative regulator of cytokine signaling SOCS3 (65). Given that IL-2R and IL-7R share many signaling pathways, it may prove of interest to determine whether IL-7R-dependent signaling is similarly regulated in Tregs. Our analysis of Re ratios suggests that there may be differences in the efficiency of IL-7R-dependent signaling between Tregs and conventional CD4+ T cells, which warrants further investigations.
Analyses of IL-7 responses were carried out with two Treg subsets that differ in expression of CD45RA but also in activation markers, such as HLA-DR (81). The naive subset of Tregs, defined by coexpression of CD45RA, CD25, and FoxP3, predominates in infants and persists in adults, where it represents about one-third to one-fourth of the total Treg pool (73). Naive Tregs are thought not to have encountered their cognate antigen in the periphery and have low HLA-DR expression levels but can exert bona fide suppressive activity in vitro (81). We found that within Tregs, the naive subset was clearly the more responsive to IL-7. Indeed, the percentage of NTreg cells that responded to low-dose IL-7 stimulation was doubled compared to the level for MTreg (46% versus 24% pSTAT5+ cells in the HD group; P = 0.01), a result likely explained by the higher expression level of CD127 in NTreg. These findings suggest that IL-7 may play a significant role in the homeostasis of the naive subset of Tregs.
HIV infection perturbed Treg responses to IL-7 by inducing a decrease in the frequency of responder pSTAT5+ cells in the MTreg subset. Signs of abnormal activation of STAT5 were also present within both the NTreg and the MTreg subsets, as indicated by increased pSTAT5 expression levels within the responding population. Thus, the pattern of STAT5 dysfunction appeared similar to that seen in conventional CD4+ T cells. A particularly deleterious effect of STAT5 dysfunction for Tregs may be the impairment of Foxp3 induction, given the central role of this protein in Treg differentiation and suppressive capacity (38). Thus, dysfunction of the JAK/STAT pathway may impair the capacity of Tregs to negatively regulate immune responses and thereby contribute to the abnormal immune activation characteristic of progressive HIV infection.
In conclusion, HIV infection perturbs both receptor expression and signal transduction in the IL-7/IL-7R axis, which is bound to have deleterious consequences on CD4+ T-cell homeostatic regulation and may contribute to the progressive CD4+ T-cell loss characteristic of HIV disease. The association between the activation and dysfunction of the JAK/STAT pathway emphasizes the need to devise strategies aimed at controlling abnormal immune activation in HIV infection. In particular, it will be important to ensure efficient control of HIV-induced activation in patients who will receive IL-7 immunotherapy as a means to restore CD4+ T-cell counts.
Acknowledgments
We thank Marie-Thérèse Rannou and the participating nurses from Bicêtre and Necker Hospital for their cooperation, Michel Morre for the gift of recombinant human IL-7, Sandra Pellegrini and Josiane Ragimbeau for advice on STAT5 studies, and Marie-Christine Wagner and Hinde Benjelloun for help with flow cytometry data acquisition. We are especially grateful to the patients who participated in the study.
O.J. is the recipient of a fellowship from the ANRS. This work was supported by the ANRS (study EP33) and the Pasteur Institute, Paris, France.
We declare no competing financial interests.
Footnotes
Published ahead of print on 28 October 2009.
REFERENCES
- 1.Albuquerque, A. S., C. S. Cortesao, R. B. Foxall, R. S. Soares, R. M. Victorino, and A. E. Sousa. 2007. Rate of increase in circulating IL-7 and loss of IL-7Ralpha expression differ in HIV-1 and HIV-2 infections: two lymphopenic diseases with similar hyperimmune activation but distinct outcomes. J. Immunol. 178:3252-3259. [DOI] [PubMed] [Google Scholar]
- 2.Alpdogan, O., and M. R. van den Brink. 2005. IL-7 and IL-15: therapeutic cytokines for immunodeficiency. Trends Immunol. 26:56-64. [DOI] [PubMed] [Google Scholar]
- 3.Alves, N. L., E. M. van Leeuwen, I. A. Derks, and R. A. van Lier. 2008. Differential regulation of human IL-7 receptor alpha expression by IL-7 and TCR signaling. J. Immunol. 180:5201-5210. [DOI] [PubMed] [Google Scholar]
- 4.Baccala, R., and A. N. Theofilopoulos. 2005. The new paradigm of T-cell homeostatic proliferation-induced autoimmunity. Trends Immunol. 26:5-8. [DOI] [PubMed] [Google Scholar]
- 5.Bani, L., V. Pasquier, M. Kryworuchko, J. Salamero, and J. Theze. 2001. Unstimulated human CD4 lymphocytes express a cytoplasmic immature form of the common cytokine receptor gamma-chain. J. Immunol. 167:344-349. [DOI] [PubMed] [Google Scholar]
- 6.Barata, J. T., A. Silva, J. G. Brandao, L. M. Nadler, A. A. Cardoso, and V. A. Boussiotis. 2004. Activation of PI3K is indispensable for interleukin 7-mediated viability, proliferation, glucose use, and growth of T cell acute lymphoblastic leukemia cells. J. Exp. Med. 200:659-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barqasho, B., P. Nowak, A. Tjernlund, S. Kinloch, L. E. Goh, F. Lampe, M. Fisher, J. Andersson, and A. Sonnerborg. 2009. Kinetics of plasma cytokines and chemokines during primary HIV-1 infection and after analytical treatment interruption. HIV Med. 10:94-102. [DOI] [PubMed] [Google Scholar]
- 8.Bayer, A. L., J. Y. Lee, A. de la Barrera, C. D. Surh, and T. R. Malek. 2008. A function for IL-7R for CD4+CD25+Foxp3+ T regulatory cells. J. Immunol. 181:225-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bazdar, D. A., M. Kalinowska, and S. F. Sieg. 2009. Interleukin-7 receptor signaling is deficient in CD4+ T cells from HIV-infected persons and is inversely associated with aging. J. Infect. Dis. 199:1019-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bazdar, D. A., and S. F. Sieg. 2007. Interleukin-7 enhances proliferation responses to T-cell receptor stimulation in naive CD4+ T cells from human immunodeficiency virus-infected persons. J. Virol. 81:12670-12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benoit, A., K. Abdkader, D. Sirskyj, A. Alhetheel, N. Sant, F. Diaz-Mitoma, A. Kumar, and M. Kryworuchko. 2009. Inverse association of repressor growth factor independent-1 with CD8 T cell interleukin (IL)-7 receptor [alpha] expression and limited signal transducers and activators of transcription signaling in response to IL-7 among [gamma]-chain cytokines in HIV patients. AIDS 23:1341-1347. [DOI] [PubMed] [Google Scholar]
- 12.Beq, S., M. T. Nugeyre, R. Ho Tsong Fang, D. Gautier, R. Legrand, N. Schmitt, J. Estaquier, F. Barre-Sinoussi, B. Hurtrel, R. Cheynier, and N. Israel. 2006. IL-7 induces immunological improvement in SIV-infected rhesus macaques under antiviral therapy. J. Immunol. 176:914-922. [DOI] [PubMed] [Google Scholar]
- 13.Beq, S., M. T. Rannou, A. Fontanet, J. F. Delfraissy, J. Theze, and J. H. Colle. 2004. HIV infection: pre-highly active antiretroviral therapy IL-7 plasma levels correlate with long-term CD4 cell count increase after treatment. AIDS 18:563-565. [DOI] [PubMed] [Google Scholar]
- 14.Boulassel, M. R., G. H. Smith, M. D. Edwardes, M. Young, M. Klein, N. Gilmore, J. Macleod, R. Leblanc, P. Rene, J. Allan, R. G. Lalonde, and J. P. Routy. 2005. Influence of RANTES, SDF-1 and TGF-beta levels on the value of interleukin-7 as a predictor of virological response in HIV-1-infected patients receiving double boosted protease inhibitor-based therapy. HIV Med. 6:268-277. [DOI] [PubMed] [Google Scholar]
- 15.Bovolenta, C., L. Camorali, A. L. Lorini, S. Ghezzi, E. Vicenzi, A. Lazzarin, and G. Poli. 1999. Constitutive activation of STATs upon in vivo human immunodeficiency virus infection. Blood 94:4202-4209. [PubMed] [Google Scholar]
- 16.Boyman, O., J. F. Purton, C. D. Surh, and J. Sprent. 2007. Cytokines and T-cell homeostasis. Curr. Opin. Immunol. 19:320-326. [DOI] [PubMed] [Google Scholar]
- 17.Bradley, L. M., L. Haynes, and S. L. Swain. 2005. IL-7: maintaining T-cell memory and achieving homeostasis. Trends Immunol. 26:172-176. [DOI] [PubMed] [Google Scholar]
- 18.Budagian, V., E. Bulanova, R. Paus, and S. Bulfone-Paus. 2006. IL-15/IL-15 receptor biology: a guided tour through an expanding universe. Cytokine Growth Factor Rev. 17:259-280. [DOI] [PubMed] [Google Scholar]
- 19.Burchill, M. A., J. Yang, K. B. Vang, J. J. Moon, H. H. Chu, C. W. Lio, A. L. Vegoe, C. S. Hsieh, M. K. Jenkins, and M. A. Farrar. 2008. Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity 28:112-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Catalfamo, M., M. Di Mascio, Z. Hu, S. Srinivasula, V. Thaker, J. Adelsberger, A. Rupert, M. Baseler, Y. Tagaya, G. Roby, C. Rehm, D. Follmann, and H. C. Lane. 2008. HIV infection-associated immune activation occurs by two distinct pathways that differentially affect CD4 and CD8 T cells. Proc. Natl. Acad. Sci. U. S. A. 105:19851-19856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colle, J. H., J. L. Moreau, A. Fontanet, O. Lambotte, J. F. Delfraissy, and J. Theze. 2007. The correlation between levels of IL-7Ralpha expression and responsiveness to IL-7 is lost in CD4 lymphocytes from HIV-infected patients. AIDS 21:101-103. [DOI] [PubMed] [Google Scholar]
- 22.Colle, J. H., J. L. Moreau, A. Fontanet, O. Lambotte, M. Joussemet, J. F. Delfraissy, and J. Theze. 2006. CD127 expression and regulation are altered in the memory CD8 T cells of HIV-infected patients—reversal by highly active anti-retroviral therapy (HAART). Clin. Exp. Immunol. 143:398-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colle, J. H., J. L. Moreau, A. Fontanet, O. Lambotte, M. Joussemet, S. Jacod, J. F. Delfraissy, and J. Theze. 2006. Regulatory dysfunction of the interleukin-7 receptor in CD4 and CD8 lymphocytes from HIV-infected patients—effects of antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 42:277-285. [DOI] [PubMed] [Google Scholar]
- 24.Crotti, A., M. Lusic, R. Lupo, P. M. Lievens, E. Liboi, G. D. Chiara, M. Tinelli, A. Lazzarin, B. K. Patterson, M. Giacca, C. Bovolenta, and G. Poli. 2007. Naturally occurring C-terminally truncated STAT5 is a negative regulator of HIV-1 expression. Blood 109:5380-5389. [DOI] [PubMed] [Google Scholar]
- 25.Deeks, S. G., C. M. Kitchen, L. Liu, H. Guo, R. Gascon, A. B. Narvaez, P. Hunt, J. N. Martin, J. O. Kahn, J. Levy, M. S. McGrath, and F. M. Hecht. 2004. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood 104:942-947. [DOI] [PubMed] [Google Scholar]
- 26.Dooms, H., K. Wolslegel, P. Lin, and A. K. Abbas. 2007. Interleukin-2 enhances CD4+ T cell memory by promoting the generation of IL-7R alpha-expressing cells. J. Exp. Med. 204:547-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunham, R. M., B. Cervasi, J. M. Brenchley, H. Albrecht, A. Weintrob, B. Sumpter, J. Engram, S. Gordon, N. R. Klatt, I. Frank, D. L. Sodora, D. C. Douek, M. Paiardini, and G. Silvestri. 2008. CD127 and CD25 expression defines CD4+ T cell subsets that are differentially depleted during HIV infection. J. Immunol. 180:5582-5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fluur, C., B. Rethi, P. H. Thang, N. Vivar, F. Mowafi, L. Lopalco, C. U. Foppa, A. Karlsson, G. Tambussi, and F. Chiodi. 2007. Relationship between serum IL-7 concentrations and lymphopenia upon different levels of HIV immune control. AIDS 21:1048-1050. [DOI] [PubMed] [Google Scholar]
- 29.Fry, T. J., E. Connick, J. Falloon, M. M. Lederman, D. J. Liewehr, J. Spritzler, S. M. Steinberg, L. V. Wood, R. Yarchoan, J. Zuckerman, A. Landay, and C. L. Mackall. 2001. A potential role for interleukin-7 in T-cell homeostasis. Blood 97:2983-2990. [DOI] [PubMed] [Google Scholar]
- 30.Fry, T. J., and C. L. Mackall. 2002. Interleukin-7 and immunorestoration in HIV: beyond the thymus. J. Hematother. Stem Cell Res. 11:803-807. [DOI] [PubMed] [Google Scholar]
- 31.Fry, T. J., and C. L. Mackall. 2005. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J. Immunol. 174:6571-6576. [DOI] [PubMed] [Google Scholar]
- 32.Grossman, Z., M. Meier-Schellersheim, W. E. Paul, and L. J. Picker. 2006. Pathogenesis of HIV infection: what the virus spares is as important as what it destroys. Nat. Med. 12:289-295. [DOI] [PubMed] [Google Scholar]
- 33.Hennighausen, L., and G. W. Robinson. 2008. Interpretation of cytokine signaling through the transcription factors STAT5A and STAT5B. Genes Dev. 22:711-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Isgro, A., F. Aiuti, I. Mezzaroma, F. Franchi, A. M. Mazzone, F. Lebba, and A. Aiuti. 2002. Interleukin 7 production by bone marrow-derived stromal cells in HIV-1-infected patients during highly active antiretroviral therapy. AIDS 16:2231-2232. [DOI] [PubMed] [Google Scholar]
- 35.Isgro, A., W. Leti, W. De Santis, M. Marziali, A. Esposito, C. Fimiani, G. Luzi, M. Pinti, A. Cossarizza, F. Aiuti, and I. Mezzaroma. 2008. Altered clonogenic capability and stromal cell function characterize bone marrow of HIV-infected subjects with low CD4+ T cell counts despite viral suppression during HAART. Clin. Infect. Dis. 46:1902-1910. [DOI] [PubMed] [Google Scholar]
- 36.Jiang, Q., W. Q. Li, F. B. Aiello, R. Mazzucchelli, B. Asefa, A. R. Khaled, and S. K. Durum. 2005. Cell biology of IL-7, a key lymphotrophin. Cytokine Growth Factor Rev. 16:513-533. [DOI] [PubMed] [Google Scholar]
- 37.Jiang, Q., W. Q. Li, R. R. Hofmeister, H. A. Young, D. R. Hodge, J. R. Keller, A. R. Khaled, and S. K. Durum. 2004. Distinct regions of the interleukin-7 receptor regulate different Bcl2 family members. Mol. Cell. Biol. 24:6501-6513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Josefowicz, S. Z., and A. Rudensky. 2009. Control of regulatory T cell lineage commitment and maintenance. Immunity 30:616-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalayjian, R. C., J. Spritzler, M. Pu, A. Landay, R. B. Pollard, V. Stocker, L. A. Harthi, B. H. Gross, I. R. Francis, S. A. Fiscus, P. Tebas, R. J. Bosch, V. Valcour, and M. M. Lederman. 2005. Distinct mechanisms of T cell reconstitution can be identified by estimating thymic volume in adult HIV-1 disease. J. Infect. Dis. 192:1577-1587. [DOI] [PubMed] [Google Scholar]
- 40.Kim, H. P., J. Imbert, and W. J. Leonard. 2006. Both integrated and differential regulation of components of the IL-2/IL-2 receptor system. Cytokine Growth Factor Rev. 17:349-366. [DOI] [PubMed] [Google Scholar]
- 41.Koesters, S. A., J. B. Alimonti, C. Wachihi, L. Matu, O. Anzala, J. Kimani, J. E. Embree, F. A. Plummer, and K. R. Fowke. 2006. IL-7Ralpha expression on CD4+ T lymphocytes decreases with HIV disease progression and inversely correlates with immune activation. Eur. J. Immunol. 36:336-344. [DOI] [PubMed] [Google Scholar]
- 42.Kondrack, R. M., J. Harbertson, J. T. Tan, M. E. McBreen, C. D. Surh, and L. M. Bradley. 2003. Interleukin 7 regulates the survival and generation of memory CD4 cells. J. Exp. Med. 198:1797-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lali, F. V., J. Crawley, D. A. McCulloch, and B. M. Foxwell. 2004. A late, prolonged activation of the phosphatidylinositol 3-kinase pathway is required for T cell proliferation. J. Immunol. 172:3527-3534. [DOI] [PubMed] [Google Scholar]
- 44.Levy, Y., C. Lacabaratz, L. Weiss, J. P. Viard, C. Goujard, J. D. Lelievre, F. Boue, J. M. Molina, C. Rouzioux, V. Avettand-Fenoel, T. Croughs, S. Beq, R. Thiebaut, G. Chene, M. Morre, and J. F. Delfraissy. 2009. Enhanced T cell recovery in HIV-1-infected adults through IL-7 treatment. J. Clin. Invest. 119:997-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li, S., E. J. Gowans, C. Chougnet, M. Plebanski, and U. Dittmer. 2008. Natural regulatory T cells and persistent viral infection. J. Virol. 82:21-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li, W. Q., Q. Jiang, E. Aleem, P. Kaldis, A. R. Khaled, and S. K. Durum. 2006. IL-7 promotes T cell proliferation through destabilization of p27Kip1. J. Exp. Med. 203:573-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu, W., A. L. Putnam, Z. Xu-Yu, G. L. Szot, M. R. Lee, S. Zhu, P. A. Gottlieb, P. Kapranov, T. R. Gingeras, B. Fazekas de St. Groth, C. Clayberger, D. M. Soper, S. F. Ziegler, and J. A. Bluestone. 2006. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J. Exp. Med. 203:1701-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma, A., R. Koka, and P. Burkett. 2006. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu. Rev. Immunol. 24:657-679. [DOI] [PubMed] [Google Scholar]
- 49.MacPherson, P. A., C. Fex, J. Sanchez-Dardon, N. Hawley-Foss, and J. B. Angel. 2001. Interleukin-7 receptor expression on CD8(+) T cells is reduced in HIV infection and partially restored with effective antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 28:454-457. [DOI] [PubMed] [Google Scholar]
- 50.Malaspina, A., S. Moir, J. Ho, W. Wang, M. L. Howell, M. A. O'Shea, G. A. Roby, C. A. Rehm, J. M. Mican, T. W. Chun, and A. S. Fauci. 2006. Appearance of immature/transitional B cells in HIV-infected individuals with advanced disease: correlation with increased IL-7. Proc. Natl. Acad. Sci. U. S. A. 103:2262-2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marziali, M., W. De Santis, R. Carello, W. Leti, A. Esposito, A. Isgro, C. Fimiani, M. C. Sirianni, I. Mezzaroma, and F. Aiuti. 2006. T-cell homeostasis alteration in HIV-1 infected subjects with low CD4 T-cell count despite undetectable virus load during HAART. AIDS 20:2033-2041. [DOI] [PubMed] [Google Scholar]
- 52.Mazzucchelli, R., and S. K. Durum. 2007. Interleukin-7 receptor expression: intelligent design. Nat. Rev. Immunol. 7:144-154. [DOI] [PubMed] [Google Scholar]
- 53.Mazzucchelli, R., J. A. Hixon, R. Spolski, X. Chen, W. Q. Li, V. L. Hall, J. Willette-Brown, A. A. Hurwitz, W. J. Leonard, and S. K. Durum. 2008. Development of regulatory T cells requires IL-7R-alpha stimulation by IL-7 or TSLP. Blood 112:3283-3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mercier, F., M. R. Boulassel, B. Yassine-Diab, C. Tremblay, N. F. Bernard, R. P. Sekaly, and J. P. Routy. 2008. Persistent human immunodeficiency virus-1 antigenaemia affects the expression of interleukin-7Ralpha on central and effector memory CD4+ and CD8+ T cell subsets. Clin. Exp. Immunol. 152:72-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muthukumar, A., A. Wozniakowski, M. C. Gauduin, M. Paiardini, H. M. McClure, R. P. Johnson, G. Silvestri, and D. L. Sodora. 2004. Elevated interleukin-7 levels not sufficient to maintain T-cell homeostasis during simian immunodeficiency virus-induced disease progression. Blood 103:973-979. [DOI] [PubMed] [Google Scholar]
- 56.Napolitano, L. A., R. M. Grant, S. G. Deeks, D. Schmidt, S. C. De Rosa, L. A. Herzenberg, B. G. Herndier, J. Andersson, and J. M. McCune. 2001. Increased production of IL-7 accompanies HIV-1-mediated T-cell depletion: implications for T-cell homeostasis. Nat. Med. 7:73-79. [DOI] [PubMed] [Google Scholar]
- 57.Noguchi, M., Y. Nakamura, S. M. Russell, S. F. Ziegler, M. Tsang, X. Cao, and W. J. Leonard. 1993. Interleukin-2 receptor gamma chain: a functional component of the interleukin-7 receptor. Science 262:1877-1880. [DOI] [PubMed] [Google Scholar]
- 58.Okoye, A., H. Park, M. Rohankhedkar, L. Coyne-Johnson, R. Lum, J. M. Walker, S. L. Planer, A. W. Legasse, A. W. Sylwester, M. Piatak, Jr., J. D. Lifson, D. L. Sodora, F. Villinger, M. K. Axthelm, J. E. Schmitz, and L. J. Picker. 2009. Profound CD4+/CCR5+ T cell expansion is induced by CD8+ lymphocyte depletion but does not account for accelerated SIV pathogenesis. J. Exp. Med. 206:1575-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Osborne, L. C., S. Dhanji, J. W. Snow, J. J. Priatel, M. C. Ma, M. J. Miners, H. S. Teh, M. A. Goldsmith, and N. Abraham. 2007. Impaired CD8 T cell memory and CD4 T cell primary responses in IL-7R alpha mutant mice. J. Exp. Med. 204:619-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paiardini, M., B. Cervasi, H. Albrecht, A. Muthukumar, R. Dunham, S. Gordon, H. Radziewicz, G. Piedimonte, M. Magnani, M. Montroni, S. M. Kaech, A. Weintrob, J. D. Altman, D. L. Sodora, M. B. Feinberg, and G. Silvestri. 2005. Loss of CD127 expression defines an expansion of effector CD8+ T cells in HIV-infected individuals. J. Immunol. 174:2900-2909. [DOI] [PubMed] [Google Scholar]
- 61.Pallard, C., A. P. Stegmann, T. van Kleffens, F. Smart, A. Venkitaraman, and H. Spits. 1999. Distinct roles of the phosphatidylinositol 3-kinase and STAT5 pathways in IL-7-mediated development of human thymocyte precursors. Immunity 10:525-535. [DOI] [PubMed] [Google Scholar]
- 62.Park, J. H., Q. Yu, B. Erman, J. S. Appelbaum, D. Montoya-Durango, H. L. Grimes, and A. Singer. 2004. Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity 21:289-302. [DOI] [PubMed] [Google Scholar]
- 63.Paukku, K., and O. Silvennoinen. 2004. STATs as critical mediators of signal transduction and transcription: lessons learned from STAT5. Cytokine Growth Factor Rev. 15:435-455. [DOI] [PubMed] [Google Scholar]
- 64.Peschon, J. J., P. J. Morrissey, K. H. Grabstein, F. J. Ramsdell, E. Maraskovsky, B. C. Gliniak, L. S. Park, S. F. Ziegler, D. E. Williams, C. B. Ware, J. D. Meyer, and B. L. Davison. 1994. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J. Exp. Med. 180:1955-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pillemer, B. B., H. Xu, T. B. Oriss, Z. Qi, and A. Ray. 2007. Deficient SOCS3 expression in CD4+CD25+FoxP3+ regulatory T cells and SOCS3-mediated suppression of Treg function. Eur. J. Immunol. 37:2082-2089. [DOI] [PubMed] [Google Scholar]
- 66.Puel, A., S. F. Ziegler, R. H. Buckley, and W. J. Leonard. 1998. Defective IL7R expression in T(-)B(+)NK(+) severe combined immunodeficiency. Nat. Genet. 20:394-397. [DOI] [PubMed] [Google Scholar]
- 67.Rathmell, J. C., E. A. Farkash, W. Gao, and C. B. Thompson. 2001. IL-7 enhances the survival and maintains the size of naive T cells. J. Immunol. 167:6869-6876. [DOI] [PubMed] [Google Scholar]
- 68.Resino, S., A. Perez, J. A. Leon, M. D. Gurbindo, and M. A. Munoz-Fernandez. 2006. Interleukin-7 levels before highly active antiretroviral therapy may predict CD4+ T-cell recovery and virological failure in HIV-infected children. J. Antimicrob. Chemother. 57:798-800. [DOI] [PubMed] [Google Scholar]
- 69.Sakaguchi, S., T. Yamaguchi, T. Nomura, and M. Ono. 2008. Regulatory T cells and immune tolerance. Cell 133:775-787. [DOI] [PubMed] [Google Scholar]
- 70.Sasson, S. C., J. J. Zaunders, G. Zanetti, E. M. King, K. M. Merlin, D. E. Smith, K. K. Stanley, D. A. Cooper, and A. D. Kelleher. 2006. Increased plasma interleukin-7 level correlates with decreased CD127 and increased CD132 extracellular expression on T cell subsets in patients with HIV-1 infection. J. Infect. Dis. 193:505-514. [DOI] [PubMed] [Google Scholar]
- 71.Schweneker, M., D. Favre, J. N. Martin, S. G. Deeks, and J. M. McCune. 2008. HIV-induced changes in T cell signaling pathways. J. Immunol. 180:6490-6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Seddiki, N., B. Santner-Nanan, J. Martinson, J. Zaunders, S. Sasson, A. Landay, M. Solomon, W. Selby, S. I. Alexander, R. Nanan, A. Kelleher, and B. Fazekas de St. Groth. 2006. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J. Exp. Med. 203:1693-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seddiki, N., B. Santner-Nanan, S. G. Tangye, S. I. Alexander, M. Solomon, S. Lee, R. Nanan, and B. Fazekas de Saint Groth. 2006. Persistence of naive CD45RA+ regulatory T cells in adult life. Blood 107:2830-2838. [DOI] [PubMed] [Google Scholar]
- 74.Sereti, I., R. M. Dunham, J. Spritzler, E. Aga, M. A. Proschan, K. Medvik, C. A. Battaglia, A. L. Landay, S. Pahwa, M. A. Fischl, D. M. Asmuth, A. R. Tenorio, J. D. Altman, L. Fox, S. Moir, A. Malaspina, M. Morre, R. Buffet, G. Silvestri, and M. M. Lederman. 2009. IL-7 administration drives T cell-cycle entry and expansion in HIV-1 infection. Blood 113:6304-6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shuai, K., and B. Liu. 2005. Regulation of gene-activation pathways by PIAS proteins in the immune system. Nat. Rev. 5:593-605. [DOI] [PubMed] [Google Scholar]
- 76.Sodora, D. L., and G. Silvestri. 2008. Immune activation and AIDS pathogenesis. AIDS 22:439-446. [DOI] [PubMed] [Google Scholar]
- 77.Sportes, C., F. T. Hakim, S. A. Memon, H. Zhang, K. S. Chua, M. R. Brown, T. A. Fleisher, M. C. Krumlauf, R. R. Babb, C. K. Chow, T. J. Fry, J. Engels, R. Buffet, M. Morre, R. J. Amato, D. J. Venzon, R. Korngold, A. Pecora, R. E. Gress, and C. L. Mackall. 2008. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J. Exp. Med. 205:1701-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stacey, A. R., P. J. Norris, L. Qin, E. A. Haygreen, E. Taylor, J. Heitman, M. Lebedeva, A. DeCamp, D. Li, D. Grove, S. G. Self, and P. Borrow. 2009. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J. Virol. 83:3719-3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Swainson, L., S. Kinet, C. Mongellaz, M. Sourisseau, T. Henriques, and N. Taylor. 2007. IL-7-induced proliferation of recent thymic emigrants requires activation of the PI3K pathway. Blood 109:1034-1042. [DOI] [PubMed] [Google Scholar]
- 80.Ungureanu, D., and O. Silvennoinen. 2005. SLIM trims STATs: ubiquitin E3 ligases provide insights for specificity in the regulation of cytokine signaling. Sci. STKE 2005:pe49. [DOI] [PubMed] [Google Scholar]
- 81.Valmori, D., A. Merlo, N. E. Souleimanian, C. S. Hesdorffer, and M. Ayyoub. 2005. A peripheral circulating compartment of natural naive CD4 Tregs. J. Clin. Invest. 115:1953-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vang, K. B., J. Yang, S. A. Mahmud, M. A. Burchill, A. L. Vegoe, and M. A. Farrar. 2008. IL-2, -7, and -15, but not thymic stromal lymphopoeitin, redundantly govern CD4+Foxp3+ regulatory T cell development. J. Immunol. 181:3285-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vassena, L., M. Proschan, A. S. Fauci, and P. Lusso. 2007. Interleukin 7 reduces the levels of spontaneous apoptosis in CD4+ and CD8+ T cells from HIV-1-infected individuals. Proc. Natl. Acad. Sci. U. S. A. 104:2355-2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vingerhoets, J., E. Bisalinkumi, G. Penne, R. Colebunders, E. Bosmans, L. Kestens, and G. Vanham. 1998. Altered receptor expression and decreased sensitivity of T-cells to the stimulatory cytokines IL-2, IL-7 and IL-12 in HIV infection. Immunol. Lett. 61:53-61. [DOI] [PubMed] [Google Scholar]
- 85.Wofford, J. A., H. L. Wieman, S. R. Jacobs, Y. Zhao, and J. C. Rathmell. 2008. IL-7 promotes Glut1 trafficking and glucose uptake via STAT5-mediated activation of Akt to support T-cell survival. Blood 111:2101-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yao, Z., Y. Kanno, M. Kerenyi, G. Stephens, L. Durant, W. T. Watford, A. Laurence, G. W. Robinson, E. M. Shevach, R. Moriggl, L. Hennighausen, C. Wu, and J. J. O'Shea. 2007. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood 109:4368-4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yu, A., L. Zhu, N. H. Altman, and T. R. Malek. 2009. A low interleukin-2 receptor signaling threshold supports the development and homeostasis of T regulatory cells. Immunity 30:204-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zubkova, I., H. Mostowski, and M. Zaitseva. 2005. Up-regulation of IL-7, stromal-derived factor-1 alpha, thymus-expressed chemokine, and secondary lymphoid tissue chemokine gene expression in the stromal cells in response to thymocyte depletion: implication for thymus reconstitution. J. Immunol. 175:2321-2330. [DOI] [PubMed] [Google Scholar]