Abstract
Measles virus (MV), a member of the family Paramyxoviridae, is a nonsegmented negative-strand RNA virus. The RNA helicases retinoic acid-inducible gene I (RIG-I) and melanoma differentiation-associated gene 5 (MDA5) are differentially involved in the detection of cytoplasmic viral RNAs and induction of alpha/beta interferon (IFN-α/β). RIG-I is generally believed to play a major role in the recognition of paramyxoviruses, whereas many viruses of this family produce V proteins that can inhibit MDA5. To determine the individual roles of MDA5 and RIG-I in IFN induction after MV infection, small interfering RNA-mediated knockdown of MDA5 or RIG-I was performed in the human epithelial cell line H358, which is susceptible to wild-type MV isolates. The production of IFN-β mRNA in response to MV infection was greatly reduced in RIG-I knockdown clones compared to that in H358 cells, confirming the importance of RIG-I in the detection of MV. The IFN-β mRNA levels were also moderately reduced in MDA5 knockdown clones, even though these clones retained fully functional RIG-I. A V protein-deficient recombinant MV (MVΔV) induced higher amounts of IFN-β mRNA at the early stage of infection in H358 cells compared to the parental virus. The reductions in the IFN-β mRNA levels in RIG-I knockdown clones were less pronounced after infection with MVΔV than after infection with the parental virus. Taken together, the present results indicate that RIG-I and MDA5 both contribute to the recognition of MV and that the V protein promotes MV growth at least partly by inhibiting the MDA5-mediated IFN responses.
Alpha/beta interferons (IFN-α/β) play central roles in the host defense against viral infections (42). Pattern recognition receptors are the host molecules that detect pathogen-associated molecular patterns and activate the innate immune responses that operate at the early stage of infections (1). Toll-like receptor 3 (TLR3) and TLR7 recognize viral RNAs in the endosome and induce the production of various cytokines, including IFNs. In the cytoplasm of virus-infected cells, two RNA helicases, melanoma differentiation-associated gene 5 (MDA5) and retinoic acid-inducible gene I (RIG-I), are involved in virus recognition and IFN induction (21, 58, 59). Studies have shown that MDA5 recognizes long double-stranded RNAs (dsRNAs) (22), which are produced in cells infected with picornaviruses and reoviruses (23, 28), while RIG-I detects single-stranded RNAs with 5′-triphosphate (19, 37) and short dsRNAs (22), which are found in cells infected with a variety of RNA viruses. Therefore, it is generally believed that RIG-I plays a major role in the recognition of many RNA viruses, including paramyxoviruses (23, 28), whereas MDA5 only acts as an RNA sensor for certain RNA viruses.
Measles is a febrile acute infectious disease that remains a major cause of child deaths worldwide, especially in developing countries (7). Measles virus (MV) is a member of the genus Morbillivirus in the family Paramyxoviridae. The MV genome possesses six genes, which encode N (nucleocapsid), P (phospho-), M (matrix), F (fusion), H (hemagglutinin), and L (large) proteins, respectively (16). The P gene encodes two additional proteins, the V and C proteins (5, 10). During transcription of the P gene, an additional guanine residue may be inserted at a specific site in the nascent transcript via the recognition of an editing motif, producing the V mRNA (10). Consequently, the V protein shares the N-terminal 231 amino acid residues with the P protein but has a unique C-terminal region with 68 amino acid residues. The C protein is translated from the P and V mRNAs using an alternative reading frame (5). Although the V and C proteins are dispensable for MV growth in some cultured cells (40, 45), they promote viral replication by circumventing the host innate immune responses (11, 31, 53) and are associated with MV virulence in vivo (12, 35, 53, 54). The V protein blocks signal transduction in response to IFN-α/β (8, 13, 14, 32, 34, 41, 51, 57) and inhibits TLR7- and TLR9-mediated IFN-α/β production in human plasmacytoid dendritic cells by binding to IκB kinase α and IFN regulatory factor (IRF) 7 (36). Furthermore, the V proteins of many paramyxoviruses, including MV, bind to MDA5 and inhibit its function (2). Therefore, the role of MDA5 in the recognition of paramyxoviruses may have been underestimated in previous studies.
In the present study, we generated RIG-I and MDA5 knockdown human cells and a V protein-deficient recombinant MV (MVΔV) and examined the roles of RIG-I and MDA5 in the detection of MV and the resulting IFN induction. Our results indicated that these antiviral RNA helicase proteins both contribute to IFN induction in response to MV infection and that the V protein promotes MV growth at least partly by inhibiting the MDA5-mediated response in infected cells.
MATERIALS AND METHODS
Plasmid constructions.
Using the pBAsi-hU6 pur DNA vector (Takara Bio, Inc., Shiga, Japan), we constructed three plasmids—pBAsi-MDA5, pBAsi-RIG-I, and pBAsi-luciferase—that generated stem-loop type small interfering RNAs (siRNAs). The siRNAs from pBAsi-MDA5, pBAsi-RIG-I, and pBAsi-luciferase targeted the mRNAs of human MDA5 and RIG-I and firefly luciferase, respectively. The target sequences for the human MDA5 and RIG-I and firefly luciferase mRNAs were 5′-GGAGAAUAACUCAUCAGAAUC-3′, 5′-GCCAGAAUCUUAGUGAGAAUU-3′, and 5′-GCCCGCGAACGACAUUUAUAA-3′, respectively.
The plasmid p(+)MV323-EGFP was derived from p(+)MV323, which encoded the full-length antigenomic cDNA of the virulent IC-B strain of MV, and contained an additional transcriptional unit for enhanced green fluorescent protein (EGFP) (17, 50). The plasmid p(+)MVΔV-EGFP was generated by introducing four nucleotide substitutions into the region corresponding to the RNA editing motif of the P gene of p(+)MV323-EGFP. All four substitutions were synonymous in the reading frame of the P protein. Recombinant MVs generated from p(+)MV323-EGFP and p(+)MVΔV-EGFP were designated wild-type (wt) MV and MVΔV, respectively, in the present study.
Cells and viruses.
H358 (49), VV5-4 (3), and B95a (24) cells were maintained in RPMI 1640 medium (ICN Biomedicals, Aurora, OH) supplemented with 7.5% fetal bovine serum (FBS). To generate cells constitutively expressing the siRNAs targeting the mRNAs of human MDA5 and RIG-I and firefly luciferase, H358 cells cultured in 3.5-cm dishes were transfected with 5 μg of pBAsi-MDA5, pBAsi-RIG-I, or pBAsi-luciferase using Lipofectamine 2000 (Invitrogen Life Technologies, Carlsbad, CA), according to the manufacturer's instructions. At 36 h posttransfection, the cells were harvested, transferred onto 10-cm dishes, and selected in RPMI 1640 medium supplemented with 7.5% FBS and 1 μg of puromycin/ml. After 3 weeks of culture, the puromycin-resistant clones formed colonies on the 10-cm dishes, and three clones each were isolated from the pBAsi-MDA5-transfected cells (clones M1, M2, and M3) and pBAsi-RIG-I-transfected cells (clones R1, R2, and R3). One clone was also isolated from the pBAsi-luciferase-transfected cells. These clones were propagated for further analyses. Vero cells constitutively expressing human signaling lymphocyte activation molecule (SLAM) (Vero/hSLAM) (33) were maintained in Dulbecco's modified Eagle medium (ICN Biomedicals) supplemented with 7.5% FBS and 500 μg of Geneticin (G418; Nacalai Tesque, Tokyo, Japan)/ml. Recombinant MVs were generated in VV5-4 cells from the cloned cDNAs and propagated as described previously (29, 47).
Reagents and antibodies.
A fusion blocking peptide (FBP), Z-d-Phe-Phe-Gly, was purchased from Peptide Institute (Osaka, Japan) (43). IFN-αA/D was purchased from Sigma-Aldrich (St. Louis, MO). A serum sample from a patient with subacute sclerosing panencephalitis (56) was kindly provided by M. B. A. Oldstone, The Scripps Research Institute, La Jolla, CA, and used to detect the MV N protein. Rabbit polyclonal antibodies against the MV P and V proteins and a mouse monoclonal antibody against the MV C protein (clone 2D10) were described previously (30, 31). Rabbit polyclonal antibodies against MDA5 and RIG-I were purchased from ProSci., Inc. (Poway, CA). A rabbit polyclonal antibody against IRF3 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). A mouse monoclonal antibody against human actin (clone C2) and a polyclonal antibody against IFN-induced protein with tetratricopeptide repeats 1 (ISG56) were purchased from Santa Cruz Biotechnology and Abnova Corp. (Taipei City, Taiwan), respectively. Horseradish peroxidase-conjugated donkey anti-rabbit and sheep anti-mouse immunoglobulin antibodies were purchased from GE Healthcare (Piscataway, NJ). A horseradish peroxidase-conjugated goat anti-human immunoglobulin antibody was purchased from EY Laboratories (San Mateo, CA).
Virus titration.
Monolayers of Vero/hSLAM cells on 12-well plates were incubated with serially diluted virus samples for 1 h at 37°C, washed with phosphate-buffered saline, and overlaid with Dulbecco's modified Eagle medium containing 2% FBS and 1.5% methylcellulose 4000 (Wako Pure Chemical Industries, Osaka, Japan). At 5 days after infection, the numbers of PFU were determined.
Western blot analysis.
After being washed with phosphate-buffered saline, cells were lysed with a buffer (150 mM NaCl, 10 mM Tris-HCl [pH 7.4], 1% Triton X-100, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS]), and the polypeptides in the lysates were reduced by heating in the presence of β-mercaptoethanol (Nacalai Tesque). For the detection of IRF3 dimers, cells were lysed with a different lysis buffer (150 mM NaCl, 50 mM Tris-HCl [pH 8.0], 1% NP-40) (20) supplemented with serine/threonine phosphatase inhibitor (Sigma-Aldrich), tyrosine phosphatase inhibitor (Sigma-Aldrich), and protease inhibitor cocktails (Sigma-Aldrich), and the polypeptides in the lysates were not reduced. The polypeptides were separated by SDS-polyacrylamide gel electrophoresis (PAGE), except for those prepared to detect IRF3 dimers, which were separated by native PAGE (20). The separated polypeptides were electroblotted onto polyvinylidene difluoride (PVDF) membranes (Hybond-P; GE Healthcare). The PVDF membranes were incubated with appropriate primary antibodies for 16 h at 4°C, washed three times with Tris-buffered saline containing 0.05% Tween 20 (TBST), and incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. After three washes with TBST, the PVDF membranes were treated with ECL Plus reagents (GE Healthcare), and chemiluminescent signals were detected and visualized using a VersaDoc 3000 Imager (Bio-Rad, Hercules, CA). The relative amounts of the proteins were determined by densitometry using the Quantity One software (Bio-Rad).
Reverse transcription-quantitative PCR (RT-qPCR).
Total RNAs were extracted and purified from cells using TRIzol reagent (Invitrogen Life Technologies), treated with RQ1 DNase (Promega, Madison, WI), and reverse transcribed into cDNAs by using a PrimeScript RT reagent kit and an oligo(dT) primer (Takara Bio, Inc.), according to the manufacturer's instructions. The amounts of cDNAs for the viral mRNAs and host β-actin and IFN-β mRNAs were quantified by using SYBR Premix Ex Taq II (Takara Bio, Inc.) and a LightCycler (Roche Diagnostics, Indianapolis, IN) as described previously (31, 48).
Transfection with MV leader RNA.
MV leader RNA was synthesized in vitro by using a MEGAshortscript kit (Ambion, Austin, TX) according to the manufacturer's instructions, and the in vitro transcription products were purified by using TRIzol reagent as previously described (31). Monolayers of cells on six-well plates were transfected with 1 μg of MV leader RNA by using Lipofectamine 2000. Cellular RNAs were collected at 6 h posttransfection.
RESULTS
Generation of MDA5 or RIG-I knockdown cells.
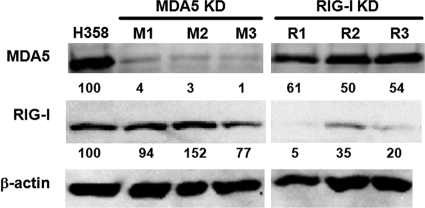
For the present study, we used the human epithelial cell line H358, which is susceptible to clinical MV isolates via an as-yet-unidentified receptor (49). Our preliminary results indicated that the V protein may play an important role in MV growth in H358 cells, since MV lacking the V protein exhibited reduced growth in this cell line, but not in other cell lines (see below). To evaluate the individual contributions of MDA5 and RIG-I to the induction of IFN-α/β after MV infection, we produced H358 cells that constitutively expressed siRNAs targeting the MDA5 and RIG-I mRNAs. Three MDA5 knockdown clones and three RIG-I knockdown clones were selected to minimize the effects of clonal variations. The expression levels of MDA5 and RIG-I were examined by Western blot analysis in these clones at 24 h after treatment with IFN-αA/D (Fig. 1), since MDA5 and RIG-I were only expressed at low levels without IFN treatment. The signal intensities for MDA5 and RIG-I were quantified and normalized by that for β-actin. All three MDA5 knockdown clones (M1, M2, and M3) expressed MDA5 at levels of <5% compared to the parental H358 cells, whereas the RIG-I knockdown clones (R1, R2, and R3) expressed RIG-I at levels of 5 to 35% compared to the parental H358 cells.
FIG. 1.
Expression of MDA5 and RIG-I in H358 cells and knockdown clones. The expression levels of MDA5 and RIG-I in the parental H358 cells, MDA5 knockdown clones (MDA5 KD: M1, M2, and M3), and RIG-I knockdown clones (RIG-I KD: R1, R2, and R3) were examined by Western blot analysis at 24 h after treatment with IFN-αA/D. β-actin was evaluated as an internal control. Indicated blow each band are the signal intensities of MDA5 and RIG-I, which were normalized by that of β-actin. The value of the parental H358 cells was set to 100%.
IFN-β mRNA expression in MDA5 and RIG-I knockdown clones after MV infection.
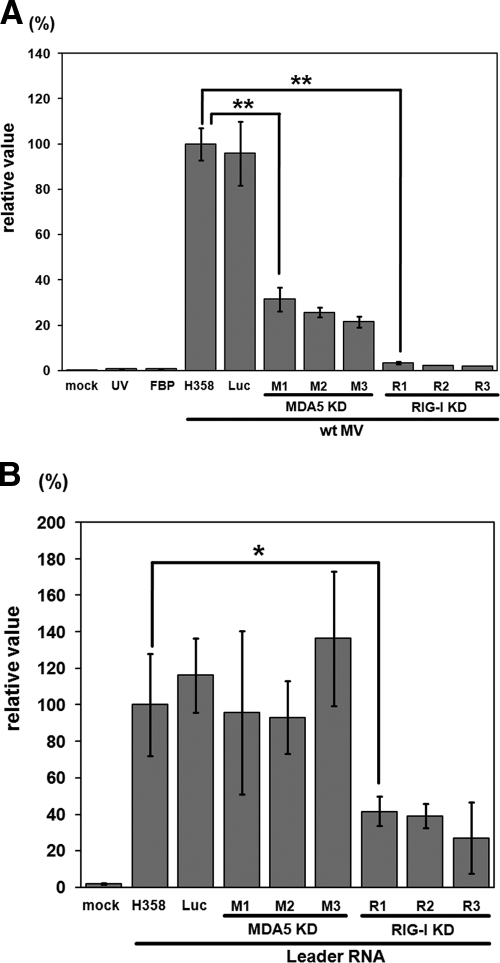
IFN-β mRNA expression was examined by RT-qPCR in the MDA5 and RIG-I knockdown clones, as well as in H358 cells after infection with wt MV. A clone expressing a nontargeting luciferase siRNA was also included as a control for the analysis. The IFN-β mRNA levels were very low in all cells at 24 h after infection (data not shown). At 48 h after infection, all three RIG-I knockdown clones produced IFN-β mRNA at levels of <5% compared to the parental H358 cells (Fig. 2A), in agreement with previous findings that RIG-I is a chief cytoplasmic RNA sensor for the detection of paramyxovirus infections (23, 28). H358 cells inoculated with wt MV in the presence of the FBP or with UV-inactivated wt MV expressed as low levels of IFN-β mRNA as the mock-infected cells, confirming that MV replication in the cytoplasm was required for the induction of IFN-β mRNA expression in H358 cells. Furthermore, the control H358 cells expressing a nontargeting siRNA produced almost the same amount of IFN-β mRNA as the parental H358 cells after infection with wt MV, indicating that the presence of siRNA per se does not affect the expression of IFN-β mRNA.
FIG. 2.
IFN-β mRNA expression in H358 cells and knockdown clones after MV infection or transfection with MV leader RNA. (A) The parental H358 cells, the clone expressing a nontargeting luciferase siRNA (Luc), MDA5 knockdown clones (MDA5 KD: M1, M2 and M3) and RIG-I knockdown clones (RIG-I KD: R1, R2 and R3) were infected with wt MV at a multiplicity of infection (MOI) of 0.5. H358 cells were also infected with UV-inactivated wt MV (UV), treated with an FBP before wt MV infection (FBP), or mock infected (mock). Total RNAs were extracted from the cells at 48 h after infection, and the IFN-β mRNA levels were quantified by RT-qPCR. (B) H358 cells, the clone expressing a luciferase siRNA, and MDA5 and RIG-I knockdown clones were transfected with in vitro-transcribed MV leader RNA. H358 cells were also mock transfected (mock). Total RNAs were extracted from the cells at 6 h posttransfection, and the IFN-β mRNA levels were quantified by RT-qPCR. All data were normalized by the corresponding β-actin mRNA levels in the respective cells. The mean value in the parental H358 cells infected with wt MV (A) or transfected with MV leader RNA (B) was set to 100%. The data represent the means ± the standard deviations of triplicate samples. *, P < 0.05; **, P < 0.01 (significant differences based on a t test).
If RIG-I is largely responsible for detecting MV RNA, knockdown of MDA5 should not affect the expression levels of IFN-β mRNA in H358 cells. However, expression of IFN-β mRNA was reduced by 60 to 80% in the three MDA5 knockdown clones (Fig. 2A). To confirm that RIG-I was functioning properly in these MDA5 knockdown clones, the clones were treated with MV leader RNA transcribed in vitro, which has been shown to induce IFN-α/β via RIG-I (38). All three MDA5 knockdown clones, as well as the control clone expressing a nontargeting siRNA, expressed similar or even higher levels of IFN-β mRNA in response to the MV leader RNA compared to the parental H358 cells (Fig. 2B). As expected, the RIG-I knockdown clones exhibited reduced expression of IFN-β mRNA in response to the MV leader RNA. These results indicate that not only RIG-I but also MDA5 is involved in IFN induction after MV infection.
Generation and properties of a recombinant MV lacking the V protein.
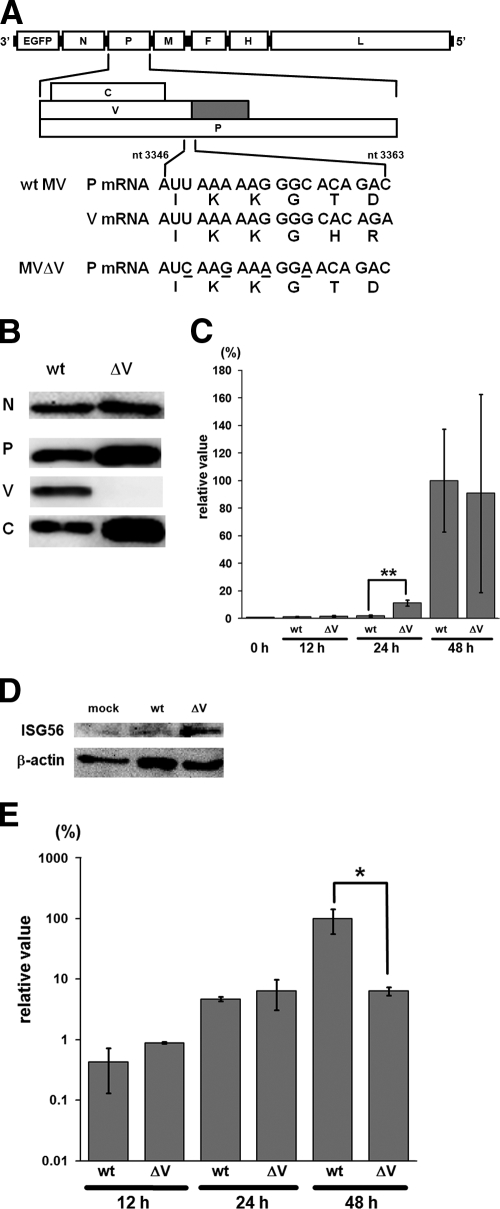
Many previous studies may have failed to demonstrate the contribution of MDA5 to IFN induction because paramyxoviruses produce V proteins that bind to MDA5, thereby inhibiting its function. To examine the activity of MDA5 in the absence of the V protein, we generated a recombinant MV incapable of producing the V protein (MVΔV). To this end, four point mutations were introduced into the P gene editing motif of wt MV, such that they were synonymous in the reading frame of the P protein (Fig. 3A). MVΔV grew efficiently in SLAM-positive B95a cells (data not shown). cDNAs of the P and V mRNAs from MVΔV-infected B95a cells were amplified by PCR, and the PCR products were cloned and sequenced to determine the frequency of the RNA editing. Although 42% (20/48) of the PCR products from wt MV-infected cells had one or more additional guanine residues at the editing site, none (0/45) of the PCR products from MVΔV-infected cells had any additional nucleotides. Western blot analysis further confirmed that the P and C proteins, but not the V protein, were produced in MVΔV-infected B95a cells (Fig. 3B).
FIG. 3.
Generation and properties of the V protein-deficient MV (MVΔV). (A) Diagram of the MV genome indicating the locations of the introduced mutations to generate MVΔV. Underlines indicate the mutated nucleotides. The predicted amino acids are shown below the trinucleotide codons. nt, nucleotide. (B) Protein synthesis in MV-infected cells. B95a cells were infected with wt MV or MVΔV at an MOI of 0.01. At 36 h after infection, viral proteins (N, P, V, and C) in the infected cells were detected by Western blot analysis. (C) IFN-β mRNA levels in H358 cells infected with wt MV or MVΔV at an MOI of 0.5. At 12, 24, and 48 h after infection, total RNA was extracted from the MV-infected cells, and the IFN-β mRNA levels were quantified by RT-qPCR. The data were normalized by the β-actin mRNA levels, and the mean value in wt MV-infected cells at 48 h after infection was set to 100%. The data represent the means ± the standard deviations of triplicate samples. The IFN-β mRNA level in uninfected cells is also shown (0 h). **, P < 0.01 (significant difference based on a t test). (D) Induction of ISG56 in MV-infected cells. H358 cells were mock infected or infected with wt MV or MVΔV at an MOI of 0.5. At 24 h after infection, the ISG56 levels in the infected cells were detected by Western blot analysis. β-Actin was analyzed as an internal control. (E) N mRNA levels in H358 cells infected with wt MV or MVΔV at an MOI of 0.5. At 12, 24, and 48 h after infection, total RNA was extracted from MV-infected cells, and the N mRNA levels were quantified by RT-qPCR. The data were normalized by the β-actin mRNA levels, and the mean value in wt MV-infected cells at 48 h after infection was set to 100%. *, P < 0.05 (significant difference based on a t test).
In H358 cells, IFN-β mRNA production was hardly increased above the background (uninfected) level at 12 and 24 h after infection with wt MV (Fig. 3C). MVΔV induced ∼6 times more IFN-β mRNA in H358 cells at 24 h after infection compared to wt MV. Similarly, MVΔV induced a larger amount of the IRF3-activated protein ISG56 at 24 h after infection compared to wt MV (Fig. 3D). At 12 and 24 h after infection, the amounts of the N mRNA determined by RT-qPCR were comparable between wt MV- and MVΔV-infected H358 cells (Fig. 3E). Therefore, a difference in the amounts of viral RNAs produced was unlikely to be responsible for the difference in the amounts of IFN-β mRNA and ISG56 induced. Taken together, these results suggest that the absence of the V protein led to enhanced activity of MDA5 in MVΔV-infected cells, which in turn caused higher inductions of IFN-β and ISG56. The amount of the N mRNA at 48 h after MVΔV infection was ∼10-fold lower than that after wt MV infection (Fig. 3E), which was probably due to the higher levels of IFN induced at the early stage of infection (24 h after infection) in MVΔV-infected cells, resulting in the inhibition of virus replication. This reduction of MV replication (and therefore viral RNA synthesis) at later times in MVΔV-infected cells may also explain why at 48 h after infection, there is little difference in the amount of IFN produced between wt MV- and MVΔV-infected cells (Fig. 3C).
Infection of MDA5 and RIG-I knockdown cells with MVΔV.
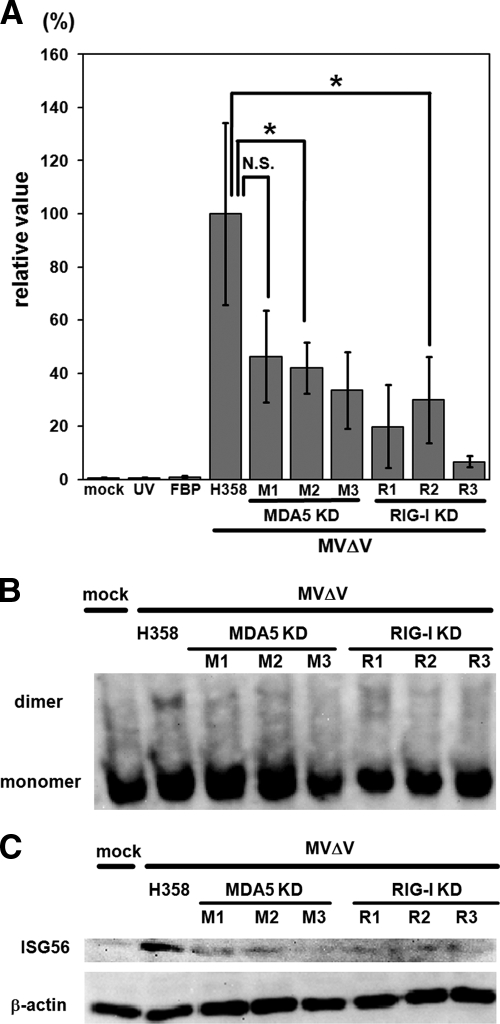
Next, we infected MDA5 and RIG-I knockdown clones, as well as H358 cells, with MVΔV. Detection of MV infection appeared to be suppressed in MVΔV-infected MDA5 knockdown clones, as evidenced by decreases in the production of IFN-β mRNA (Fig. 4A), the dimerization of IRF3 (Fig. 4B), and the production of ISG56 (Fig. 4C). As expected, these three parameters were also suppressed in RIG-I knockdown clones (Fig. 4A to C). In control experiments, all of the knockdown clones were found to exhibit IRF3 dimer formation and express ISG56 after transfection with Cardif/VISA/MAVS/IPS-1, the common adaptor molecule downstream of MDA5 and RIG-I (42; data not shown). These findings indicate that MDA5 does indeed contribute to the detection of MV and resulting induction of IFN-β and that MDA5 and RIG-I are both required for H358 cells to fully produce IRF3-activated IFN-β and ISG56 after MVΔV infection. It was noted that significant levels of IFN-β mRNA were produced in MVΔV-infected RIG-I knockdown clones (Fig. 4A), unlike the case for RIG-I knockdown clones infected with wt MV, in which the production of IFN-β mRNA was negligible compared to wt MV-infected parental H358 cells (Fig. 2A). These observations may further support the role of MDA5 in IFN induction after MV infection, which is only revealed in the absence of the V protein.
FIG. 4.
Inductions of IFN-β mRNA and ISG56 and dimerization of IRF3 in MVΔV-infected cells. (A) The parental H358 cells, MDA5 knockdown clones (MDA5 KD: M1, M2, and M3) and RIG-I knockdown clones (RIG-I KD: R1, R2, and R3) were infected with MVΔV at an MOI of 0.5. H358 cells were also infected with UV-inactivated MVΔV (UV), treated with an FBP before MVΔV infection (FBP), or mock infected (mock). Total RNAs were extracted from the cells at 24 h after infection, and the IFN-β mRNA levels were quantified by RT-qPCR. The data were normalized by the β-actin mRNA levels, and the mean value in MVΔV-infected H358 cells was set to 100%. *, P < 0.05 (significant difference based on a t test). N.S., not significant. (B and C) H358 cells and MDA5 and RIG-I knockdown clones were infected with MVΔV at an MOI of 0.5. H358 cells were also mock infected. At 24 h after infection, the monomeric and dimeric forms of IRF3 in the cells were examined by native PAGE and Western blot analysis (B), and the ISG56 levels in the cells were detected by SDS-PAGE and Western blot analysis (C). β-Actin was examined as an internal control.
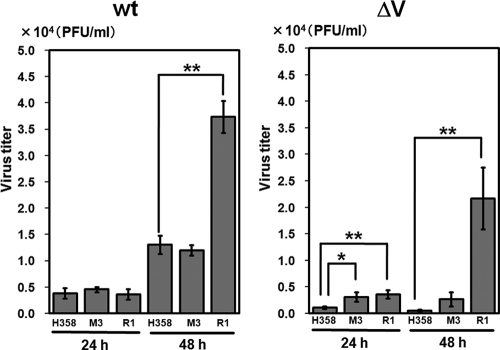
Growth of wt MV and MVΔV in knockdown clones.
The growth kinetics of wt MV and MVΔV were examined in H358 cells, an MDA5 knockdown clone (M3), and a RIG-I knockdown clone (R1) (Fig. 5). At 24 h after infection when the productions of IFN-β mRNA were still very limited (Fig. 3C), there was little difference in the growth of wt MV among H358, M3, and R1 cells. At 48 h after infection, the growth of wt MV was accelerated in R1 cells, but not in M3 cells, compared to H358 cells. Virus titers may be determined by the balance between the speed of virus replication and antiviral action of IFN. Thus, the reduction of IFN-β mRNA found in M3 cells may not have been sufficient to enhance wt MV replication at 48 h after infection, unlike that in R1 cells. On the other hand, the growth of MVΔV was strongly suppressed in H358 cells. Growth retardation of MVΔV may be expected to be compensated for in MDA5 knockdown cells, and MVΔV did indeed grow better in M3 cells than in H358 cells. However, the growth of MVΔV in M3 cells was still less efficient than that of wt MV in H358 cells. Furthermore, MVΔV growth was greatly enhanced in R1 cells compared to H358 and M3 cells at 48 h after infection, reconfirming the primary importance of RIG-I in the detection of MV and subsequent IFN production.
FIG. 5.
Growth kinetics of wt MV and MVΔV in H358 cells and knockdown clones. The parental H358 cells, an MDA5 knockdown clone (M3) and an RIG-I knockdown clone (R1) were infected with wt MV or MVΔV at an MOI of 0.5. At 24 and 48 h after infection, the cells were harvested, together with the culture media, and the virus titers were determined by plaque assays. The data represent the means ± the standard deviations of triplicate samples. *, P < 0.05; **, P < 0.01 (significant differences based on a t test).
DISCUSSION
In the present study, we have shown that RIG-I and MDA5 are both involved in the detection of MV in human cells and that the V protein plays an important role in MV growth by inhibiting the MDA5-mediated induction of the host IFN responses.
Previous studies using embryonic fibroblasts from MDA5−/− or RIG-I−/− knockout mice in vitro, as well as these knockout mice in vivo, have shown that these RNA helicases have differential roles in the recognition of different viruses. Specifically, RIG-I is essential for the detection of many RNA viruses including paramyxoviruses, orthomyxoviruses, rhabdoviruses and flaviviruses, while MDA5 is critical for the detection of picornaviruses (15, 23, 28). Interestingly, dengue virus type 2, a flavivirus, and reoviruses were shown to induce innate immune responses in embryonic fibroblasts obtained from either MDA5−/− or RIG-I−/− mice, indicating that they trigger both RIG-I- and MDA5-dependent responses (28).
Although the above generalization may be made, the data are more complicated for individual viruses. In studies with knockout mice, IFN production was significantly diminished in RIG-I−/− cells, but not in MDA5−/− cells, after infection with paramyxoviruses such as Sendai virus (SeV), including that lacking the V protein, Newcastle disease virus (NDV), and respiratory syncytial virus (23, 28). On the other hand, Yoneyama et al. (58) demonstrated that expression of IFN mRNA after NDV infection was inhibited in mouse fibroblast L929 cells by siRNA-mediated knockdown of either RIG-I or MDA5. Studies with the monoclonal antibody J2, which specifically recognizes dsRNAs of more than 40 bp in length (55), detected dsRNAs in NDV-infected cells (52) but not in SeV-infected cells (52, 55).
In the case of MV, infection was found to trigger IFN responses in human Huh7 cells but not in their derivative Huh7.5 cells with nonfunctional RIG-I, although the MDA5 pathway was intact in both cell lines (38). Another study suggested that MDA5 is involved in MV-induced expression of IFN-β since forced expression of MDA5, but not RIG-I or TLR3, led to enhanced IFN-β promoter activity in MV-infected human A549 cells (6). Shingai et al. (46) reported that vaccine strains of MV contain defective interfering (DI) particles, which are responsible for IFN-β induction. These authors also showed that both RIG-I and MDA5 detect the stem-loop structure of DI RNAs, whereas only RIG-I senses DI RNAs with 5′-triphosphate. Therefore, differences in the cell types (knockout mouse-derived cells versus cultured cells) and assay systems used may have led to various conclusions regarding the relative roles of RIG-I and MDA5 in the detection of different paramyxoviruses. Furthermore, although all paramyxoviruses have a common life cycle including genome replication and transcription (25), host cells may recognize different paramyxoviruses in different manners.
To determine the individual roles of MDA5 and RIG-I in IFN induction after MV infection, we established human epithelial H358 cells constitutively expressing siRNAs targeting the MDA5 and RIG-I mRNAs. Even in the RIG-I knockdown clones that still expressed considerable levels of RIG-I (clones R2 and R3), induction of IFN-β mRNA after MV infection was almost completely blocked, indicating that RIG-I plays a major role in the recognition of MV. The results are consistent with those obtained using Huh7 and Huh7.5 cells (38), as well as with the general notion regarding the importance of RIG-I in the detection of paramyxoviruses. However, our data further showed that MDA5 is also involved in the detection of MV, since IFN-β mRNA production in MDA5 knockdown clones was reduced compared to that in the parental H358 cells. This observation appears to contradict the finding that RIG-I knockdown clones (expressing normal levels of MDA5) hardly produced IFN-β mRNA after MV infection. Without RIG-I, MDA5-mediated signaling probably does not function sufficiently to induce IFN in the presence of the antagonizing V protein.
To evaluate the contribution of MDA5 to IFN induction in the absence of the V protein, a V protein-deficient MV (MVΔV) was generated. IFN-β mRNA and ISG56 were induced at higher levels in MVΔV-infected cells than in wt MV-infected cells at 24 h after infection. Similar enhancement of IFN production has also been reported for the paramyxovirus simian virus 5 with a defective V protein (18). Activation of IRF3 and production of IFN-β mRNA and ISG56 in response to MVΔV infection were reduced in MDA5 knockdown cells compared to the parental H358 cells. Importantly, significant levels of IFN-β mRNA were produced in RIG-I knockdown cells infected with MVΔV, unlike the case for those infected with wt MV. Furthermore, the growth of MVΔV, but not that of wt MV, was enhanced in MDA5 knockdown cells. All of these results indicate that MDA5 is indeed involved in the recognition of MV and that the V protein is required for MV to inhibit the MDA5-mediated IFN induction.
MDA5 and RIG-I appear to recognize different RNA structures (19, 22, 37, 44). Leader RNA, a 5′-triphosphate ended single-stranded RNA, is produced during the transcription of MV and other paramyxoviruses (9, 26), and in vitro-synthesized MV leader RNA was found to induce IFN through RIG-I (38). Therefore, RIG-I probably detects leader RNA in MV-infected cells and induces IFN production. Using the dsRNA-specific antibody J2 (55), we tried to detect intracellular dsRNA, which is the ligand for MDA5 (22). Although dsRNA was not found in wt MV- or MVΔV-infected Vero/hSLAM cells, it was clearly detected in MVΔC-infected cells (data not shown). A recent study also reported that dsRNA exists in cells infected with a C protein-deficient SeV, but not in SeV-infected cells (52). Therefore, the C proteins of both SeV and MV may act to limit the generation of intracellular dsRNA. It is likely that dsRNA is generated at a low level in wt MV- or MVΔV-infected cells, although it cannot be detected by the J2 antibody in the presence of the C protein. Alternatively, unknown structural motifs may exist in MV RNA that can activate MDA5.
The V protein interferes with multiple steps of host antiviral responses such as IFN-α/β induction (36, 39) and IFN-α/β-mediated signal transduction (32, 34, 51, 57). Since the MV P protein can inhibit IFN signaling like the V protein (13, 32), the raison d'être for the MV V protein may largely reside in its blockade of MDA5-mediated IFN induction. However, the significance of this V protein function may vary among different cell types, because MV lacking the V protein grows comparably to the parental virus in B95a cells (present study) but not in H358 cells (present study) and rhesus monkeys (12). In the present study, knockdown of MDA5 weakened IFN induction in H358 cells after infection with wt MV or MVΔV. On the other hand, the growth defect of MVΔV was not completely compensated for in MDA5 knockdown H358 cells, indicating that the V protein may contribute to efficient MV growth partly by inhibiting MDA5-mediated IFN induction and partly by other mechanisms.
Given the importance of RIG-I in the recognition of paramyxoviruses, including MV, inhibition of RIG-I, rather than MDA5, may be a better strategy for MV to achieve efficient growth. Indeed, some paramyxoviruses block RIG-I-mediated IFN-α/β induction (4, 27). Strong suppression of innate immunity through blockade of RIG-I may increase MV growth but possibly kill the host too quickly for the virus to spread to other hosts. Blockade of MDA5 may be more advantageous for MV survival, by allowing both sufficient growth within a host and efficient spread to different hosts.
Acknowledgments
We thank M. B. A. Oldstone for providing the reagent to detect MV proteins.
This study was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology and the Ministry of Health, Labor, and Welfare of Japan.
Footnotes
Published ahead of print on 21 October 2009.
REFERENCES
- 1.Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499-511. [DOI] [PubMed] [Google Scholar]
- 2.Andrejeva, J., K. S. Childs, D. F. Young, T. S. Carlos, N. Stock, S. Goodbourn, and R. E. Randall. 2004. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-beta promoter. Proc. Natl. Acad. Sci. USA 101:17264-17269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bair, C. H., C. S. Chung, I. A. Vasilevskaya, and W. Chang. 1996. Isolation and characterization of a Chinese hamster ovary mutant cell line with altered sensitivity to vaccinia virus killing. J. Virol. 70:4655-4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bao, X., T. Liu, Y. Shan, K. Li, R. P. Garofalo, and A. Casola. 2008. Human metapneumovirus glycoprotein G inhibits innate immune responses. PLoS. Pathog. 4:e1000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellini, W. J., G. Englund, S. Rozenblatt, H. Arnheiter, and C. D. Richardson. 1985. Measles virus P gene codes for two proteins. J. Virol. 53:908-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berghall, H., J. Siren, D. Sarkar, I. Julkunen, P. B. Fisher, R. Vainionpaa, and S. Matikainen. 2006. The interferon-inducible RNA helicase, mda-5, is involved in measles virus-induced expression of antiviral cytokines. Microbes Infect. 8:2138-2144. [DOI] [PubMed] [Google Scholar]
- 7.Bryce, J., C. Boschi-Pinto, K. Shibuya, and R. E. Black. 2005. W.H.O. estimates of the causes of death in children. Lancet 365:1147-1152. [DOI] [PubMed] [Google Scholar]
- 8.Caignard, G., M. Guerbois, J. L. Labernardiere, Y. Jacob, L. M. Jones, F. Wild, F. Tangy, and P. O. Vidalain. 2007. Measles virus V protein blocks Jak1-mediated phosphorylation of STAT1 to escape IFN-alpha/beta signaling. Virology 368:351-362. [DOI] [PubMed] [Google Scholar]
- 9.Castaneda, S. J., and T. C. Wong. 1989. Measles virus synthesizes both leaderless and leader-containing polyadenylated RNAs in vivo. J. Virol. 63:2977-2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cattaneo, R., K. Kaelin, K. Baczko, and M. A. Billeter. 1989. Measles virus editing provides an additional cysteine-rich protein. Cell 56:759-764. [DOI] [PubMed] [Google Scholar]
- 11.Devaux, P., and R. Cattaneo. 2004. Measles virus phosphoprotein gene products: conformational flexibility of the P/V protein amino-terminal domain and C protein infectivity factor function. J. Virol. 78:11632-11640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devaux, P., G. Hodge, M. B. McChesney, and R. Cattaneo. 2008. Attenuation of V- or C-defective measles viruses: infection control by the inflammatory and interferon responses of rhesus monkeys. J. Virol. 82:5359-5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devaux, P., V. von Messling, W. Songsungthong, C. Springfeld, and R. Cattaneo. 2007. Tyrosine 110 in the measles virus phosphoprotein is required to block STAT1 phosphorylation. Virology 360:72-83. [DOI] [PubMed] [Google Scholar]
- 14.Fontana, J. M., B. Bankamp, W. J. Bellini, and P. A. Rota. 2008. Regulation of interferon signaling by the C and V proteins from attenuated and wild-type strains of measles virus. Virology 374:71-81. [DOI] [PubMed] [Google Scholar]
- 15.Gitlin, L., W. Barchet, S. Gilfillan, M. Cella, B. Beutler, R. A. Flavell, M. S. Diamond, and M. Colonna. 2006. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc. Natl. Acad. Sci. USA 103:8459-8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griffin, D. E. 2007. Measles virus, p. 1551-1585. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 5th ed. Lippincott/The Williams & Wilkins Co., Philadelphia, PA.
- 17.Hashimoto, K., N. Ono, H. Tatsuo, H. Minagawa, M. Takeda, K. Takeuchi, and Y. Yanagi. 2002. SLAM (CD150)-independent measles virus entry as revealed by recombinant virus expressing green fluorescent protein. J. Virol. 76:6743-6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He, B., R. G. Paterson, N. Stock, J. E. Durbin, R. K. Durbin, S. Goodbourn, R. E. Randall, and R. A. Lamb. 2002. Recovery of paramyxovirus simian virus 5 with a V protein lacking the conserved cysteine-rich domain: the multifunctional V protein blocks both interferon-beta induction and interferon signaling. Virology 303:15-32. [DOI] [PubMed] [Google Scholar]
- 19.Hornung, V., J. Ellegast, S. Kim, K. Brzozka, A. Jung, H. Kato, H. Poeck, S. Akira, K. K. Conzelmann, M. Schlee, S. Endres, and G. Hartmann. 2006. 5′-Triphosphate RNA is the ligand for RIG-I. Science 314:994-997. [DOI] [PubMed] [Google Scholar]
- 20.Iwamura, T., M. Yoneyama, K. Yamaguchi, W. Suhara, W. Mori, K. Shiota, Y. Okabe, H. Namiki, and T. Fujita. 2001. Induction of IRF-3/-7 kinase and NF-κB in response to double-stranded RNA and virus infection: common and unique pathways. Genes Cells 6:375-388. [DOI] [PubMed] [Google Scholar]
- 21.Kang, D. C., R. V. Gopalkrishnan, Q. Wu, E. Jankowsky, A. M. Pyle, and P. B. Fisher. 2002. mda-5: an interferon-inducible putative RNA helicase with double-stranded RNA-dependent ATPase activity and melanoma growth-suppressive properties. Proc. Natl. Acad. Sci. USA 99:637-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kato, H., O. Takeuchi, E. Mikamo-Satoh, R. Hirai, T. Kawai, K. Matsushita, A. Hiiragi, T. S. Dermody, T. Fujita, and S. Akira. 2008. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J. Exp. Med. 205:1601-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kato, H., O. Takeuchi, S. Sato, M. Yoneyama, M. Yamamoto, K. Matsui, S. Uematsu, A. Jung, T. Kawai, K. J. Ishii, O. Yamaguchi, K. Otsu, T. Tsujimura, C. S. Koh, C. Reis e Sousa, Y. Matsuura, T. Fujita, and S. Akira. 2006. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441:101-105. [DOI] [PubMed] [Google Scholar]
- 24.Kobune, F., H. Sakata, and A. Sugiura. 1990. Marmoset lymphoblastoid cells as a sensitive host for isolation of measles virus. J. Virol. 64:700-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lamb, R. A. 2007. Paramyxoviridae, p. 1449-1496. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 5 ed. Lippincott/The Williams & Wilkins Co., Philadelphia, PA.
- 26.Leppert, M., L. Rittenhouse, J. Perrault, D. F. Summers, and D. Kolakofsky. 1979. Plus and minus strand leader RNAs in negative strand virus-infected cells. Cell 18:735-747. [DOI] [PubMed] [Google Scholar]
- 27.Ling, Z., K. C. Tran, and M. N. Teng. 2009. Human respiratory syncytial virus nonstructural protein NS2 antagonizes the activation of beta interferon transcription by interacting with RIG-I. J. Virol. 83:3734-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loo, Y. M., J. Fornek, N. Crochet, G. Bajwa, O. Perwitasari, L. Martinez-Sobrido, S. Akira, M. A. Gill, A. Garcia-Sastre, M. G. Katze, and M. Gale, Jr. 2008. Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. J. Virol. 82:335-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakatsu, Y., M. Takeda, M. Kidokoro, M. Kohara, and Y. Yanagi. 2006. Rescue system for measles virus from cloned cDNA driven by vaccinia virus Lister vaccine strain. J. Virol. Methods 137:152-155. [DOI] [PubMed] [Google Scholar]
- 30.Nakatsu, Y., M. Takeda, S. Ohno, R. Koga, and Y. Yanagi. 2006. Translational inhibition and increased interferon induction in cells infected with C protein-deficient measles virus. J. Virol. 80:11861-11867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakatsu, Y., M. Takeda, S. Ohno, Y. Shirogane, M. Iwasaki, and Y. Yanagi. 2008. Measles virus circumvents the host interferon response by different actions of the C and V proteins. J. Virol. 82:8296-8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohno, S., N. Ono, M. Takeda, K. Takeuchi, and Y. Yanagi. 2004. Dissection of measles virus V protein in relation to its ability to block alpha/beta interferon signal transduction. J. Gen. Virol. 85:2991-2999. [DOI] [PubMed] [Google Scholar]
- 33.Ono, N., H. Tatsuo, Y. Hidaka, T. Aoki, H. Minagawa, and Y. Yanagi. 2001. Measles viruses on throat swabs from measles patients use signaling lymphocytic activation molecule (CDw150) but not CD46 as a cellular receptor. J. Virol. 75:4399-4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palosaari, H., J. P. Parisien, J. J. Rodriguez, C. M. Ulane, and C. M. Horvath. 2003. STAT protein interference and suppression of cytokine signal transduction by measles virus V protein. J. Virol. 77:7635-7644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patterson, J. B., D. Thomas, H. Lewicki, M. A. Billeter, and M. B. Oldstone. 2000. V and C proteins of measles virus function as virulence factors in vivo. Virology 267:80-89. [DOI] [PubMed] [Google Scholar]
- 36.Pfaller, C. K., and K. K. Conzelmann. 2008. Measles virus V protein is a decoy substrate for IκB kinase alpha and prevents Toll-like receptor 7/9-mediated interferon induction. J. Virol. 82:12365-12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pichlmair, A., O. Schulz, C. P. Tan, T. I. Naslund, P. Liljestrom, F. Weber, and C. Reis e Sousa. 2006. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science 314:997-1001. [DOI] [PubMed] [Google Scholar]
- 38.Plumet, S., F. Herschke, J. M. Bourhis, H. Valentin, S. Longhi, and D. Gerlier. 2007. Cytosolic 5′-triphosphate ended viral leader transcript of measles virus as activator of the RIG I-mediated interferon response. PLoS ONE 2:e279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poole, E., B. He, R. A. Lamb, R. E. Randall, and S. Goodbourn. 2002. The V proteins of simian virus 5 and other paramyxoviruses inhibit induction of interferon-beta. Virology 303:33-46. [DOI] [PubMed] [Google Scholar]
- 40.Radecke, F., and M. A. Billeter. 1996. The nonstructural C protein is not essential for multiplication of Edmonston B strain measles virus in cultured cells. Virology 217:418-421. [DOI] [PubMed] [Google Scholar]
- 41.Ramachandran, A., J. P. Parisien, and C. M. Horvath. 2008. STAT2 is a primary target for measles virus V protein-mediated alpha/beta interferon signaling inhibition. J. Virol. 82:8330-8338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Randall, R. E., and S. Goodbourn. 2008. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J. Gen. Virol. 89:1-47. [DOI] [PubMed] [Google Scholar]
- 43.Richardson, C. D., A. Scheid, and P. W. Choppin. 1980. Specific inhibition of paramyxovirus and myxovirus replication by oligopeptides with amino acid sequences similar to those at the N termini of the F1 or HA2 viral polypeptides. Virology 105:205-222. [DOI] [PubMed] [Google Scholar]
- 44.Saito, T., D. M. Owen, F. Jiang, J. Marcotrigiano, and M. Gale, Jr. 2008. Innate immunity induced by composition-dependent RIG-I recognition of hepatitis C virus RNA. Nature 454:523-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schneider, H., K. Kaelin, and M. A. Billeter. 1997. Recombinant measles viruses defective for RNA editing and V protein synthesis are viable in cultured cells. Virology 227:314-322. [DOI] [PubMed] [Google Scholar]
- 46.Shingai, M., T. Ebihara, N. A. Begum, A. Kato, T. Honma, K. Matsumoto, H. Saito, H. Ogura, M. Matsumoto, and T. Seya. 2007. Differential type I IFN-inducing abilities of wild-type versus vaccine strains of measles virus. J. Immunol. 179:6123-6133. [DOI] [PubMed] [Google Scholar]
- 47.Takeda, M., S. Ohno, F. Seki, K. Hashimoto, N. Miyajima, K. Takeuchi, and Y. Yanagi. 2005. Efficient rescue of measles virus from cloned cDNA using SLAM-expressing Chinese hamster ovary cells. Virus Res. 108:161-165. [DOI] [PubMed] [Google Scholar]
- 48.Takeda, M., S. Ohno, F. Seki, Y. Nakatsu, M. Tahara, and Y. Yanagi. 2005. Long untranslated regions of the measles virus M and F genes control virus replication and cytopathogenicity. J. Virol. 79:14346-14354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takeda, M., M. Tahara, T. Hashiguchi, T. A. Sato, F. Jinnouchi, S. Ueki, S. Ohno, and Y. Yanagi. 2007. A human lung carcinoma cell line supports efficient measles virus growth and syncytium formation via a SLAM- and CD46-independent mechanism. J. Virol. 81:12091-12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takeda, M., K. Takeuchi, N. Miyajima, F. Kobune, Y. Ami, N. Nagata, Y. Suzaki, Y. Nagai, and M. Tashiro. 2000. Recovery of pathogenic measles virus from cloned cDNA. J. Virol. 74:6643-6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takeuchi, K., S. I. Kadota, M. Takeda, N. Miyajima, and K. Nagata. 2003. Measles virus V protein blocks interferon (IFN)-alpha/beta but not IFN-gamma signaling by inhibiting STAT1 and STAT2 phosphorylation. FEBS Lett. 545:177-182. [DOI] [PubMed] [Google Scholar]
- 52.Takeuchi, K., T. Komatsu, Y. Kitagawa, K. Sada, and B. Gotoh. 2008. Sendai virus C protein plays a role in restricting PKR activation by limiting the generation of intracellular double-stranded RNA. J. Virol. 82:10102-10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takeuchi, K., M. Takeda, N. Miyajima, Y. Ami, N. Nagata, Y. Suzaki, J. Shahnewaz, S. Kadota, and K. Nagata. 2005. Stringent requirement for the C protein of wild-type measles virus for growth both in vitro and in macaques. J. Virol. 79:7838-7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Valsamakis, A., H. Schneider, P. G. Auwaerter, H. Kaneshima, M. A. Billeter, and D. E. Griffin. 1998. Recombinant measles viruses with mutations in the C, V, or F gene have altered growth phenotypes in vivo. J. Virol. 72:7754-7761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weber, F., V. Wagner, S. B. Rasmussen, R. Hartmann, and S. R. Paludan. 2006. Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses. J. Virol. 80:5059-5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yanagi, Y., B. A. Cubitt, and M. B. Oldstone. 1992. Measles virus inhibits mitogen-induced T-cell proliferation but does not directly perturb the T-cell activation process inside the cell. Virology 187:280-289. [DOI] [PubMed] [Google Scholar]
- 57.Yokota, S., H. Saito, T. Kubota, N. Yokosawa, K. Amano, and N. Fujii. 2003. Measles virus suppresses interferon-alpha signaling pathway: suppression of Jak1 phosphorylation and association of viral accessory proteins, C and V, with interferon-alpha receptor complex. Virology 306:135-146. [DOI] [PubMed] [Google Scholar]
- 58.Yoneyama, M., M. Kikuchi, K. Matsumoto, T. Imaizumi, M. Miyagishi, K. Taira, E. Foy, Y. M. Loo, M. Gale, Jr., S. Akira, S. Yonehara, A. Kato, and T. Fujita. 2005. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J. Immunol. 175:2851-2858. [DOI] [PubMed] [Google Scholar]
- 59.Yoneyama, M., M. Kikuchi, T. Natsukawa, N. Shinobu, T. Imaizumi, M. Miyagishi, K. Taira, S. Akira, and T. Fujita. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5:730-737. [DOI] [PubMed] [Google Scholar]