Abstract
Varicella-zoster virus (VZV) infection is usually mild in healthy individuals but can cause severe disease in immunocompromised patients. Prophylaxis with varicella-zoster immunoglobulin can reduce the severity of VZV if given shortly after exposure. Glycoprotein H (gH) is a highly conserved herpesvirus protein with functions in virus entry and cell-cell spread and is a target of neutralizing antibodies. The anti-gH monoclonal antibody (MAb) 206 neutralizes VZV in vitro. To determine the requirement for gH in VZV pathogenesis in vivo, MAb 206 was administered to SCID mice with human skin xenografts inoculated with VZV. Anti-gH antibody given at 6 h postinfection significantly reduced the frequency of skin xenograft infection by 42%. Virus titers, genome copies, and lesion size were decreased in xenografts that became infected. In contrast, administering anti-gH antibody at 4 days postinfection suppressed VZV replication but did not reduce the frequency of infection. The neutralizing anti-gH MAb 206 blocked virus entry, cell fusion, or both in skin in vivo. In vitro, MAb 206 bound to plasma membranes and to surface virus particles. Antibody was internalized into vacuoles within infected cells, associated with intracellular virus particles, and colocalized with markers for early endosomes and multivesicular bodies but not the trans-Golgi network. MAb 206 blocked spread, altered intracellular trafficking of gH, and bound to surface VZV particles, which might facilitate their uptake and targeting for degradation. As a consequence, antibody interference with gH function would likely prevent or significantly reduce VZV replication in skin during primary or recurrent infection.
Varicella-zoster virus (VZV) causes chicken pox (varicella) upon primary infection. Lifelong latency is established in neurons of the sensory ganglia, and reactivation leads to shingles (herpes zoster) (1). Disease is usually inconsequential in immunocompetent people but can be severe in immunocompromised patients. The current prophylaxis for these high-risk individuals exposed to VZV is high-titer immunoglobulin to VZV administered within 96 h of exposure. This prophylaxis does not always prevent disease, but the severity of symptoms and mortality rates are usually reduced (32).
Glycoprotein H (gH) is a type 1 transmembrane protein that is required for virus-cell and cell-cell spread in all herpesviruses studied (12, 15, 24, 26). gH is an important target of the host immune system. Individuals who have had primary infection with VZV or herpes simplex virus (HSV), the most closely related human alphaherpesvirus, have humoral and cellular immunity against gH (1, 56). Immunization of mice with a recombinant vaccinia virus expressing VZV gH and its chaperone, glycoprotein L (gL), induced specific antibodies capable of neutralizing VZV in vitro (28, 37). Immunization of mice with purified HSV gH/gL protein resulted in the production of neutralizing antibodies and protected mice from HSV challenge (5, 44), and administration of an anti-HSV gH monoclonal antibody (MAb) protected mice from HSV challenge (16). Antibodies to HSV and Epstein-Barr virus gH effectively neutralize during virus penetration but not during adsorption in vitro, indicating an essential role for gH in the fusion of viral and cellular membranes but not in initial attachment of the virus to the cell (18, 33).
Anti-gH MAb 206, an immunoglobulin G1 (IgG1) antibody which recognizes a conformation-dependent epitope on the mature glycosylated form of gH, neutralizes VZV infection in vitro in the absence of complement (35). MAb 206 inhibits cell-cell fusion in vitro, based on reductions in the number of infected cells and the number of infected nuclei within syncytia, and appears to inhibit the ability of virus particles to pass from the surface of an infected epithelial cell to a neighboring cell via cell extensions (8, 35, 43). When infected cells were treated with MAb 206 for 48 h postinfection (hpi), virus egress and syncytium formation were not apparent, but they were evident within 48 h after removal of the antibody, suggesting that the effect of the antibody was reversible and that there was a requirement for new gH synthesis and trafficking to produce cell-cell fusion. Conversely, nonneutralizing antibodies to glycoproteins E (gE) and I (gI), as well as an antibody to immediate-early protein 62 (IE62), had no effect on VZV spread (46).
Like that of other herpesviruses, VZV entry into cells is presumed to require fusion of the virion envelope with the cell membrane or endocytosis followed by fusion. One of the hallmarks of VZV infection is cell fusion and formation of syncytia (8). Cell fusion can be detected as early as 9 hpi in vitro, although VZV spread from infected to uninfected cells is evident within 60 min (45). In vivo, VZV forms syncytia through its capacity to cause fusion of epidermal cells. Syncytia are evident in biopsies of varicella and herpes zoster skin lesions during natural infection and in SCIDhu skin xenografts (34). VZV gH is produced, processed in the Golgi apparatus, and trafficked to the cell membrane, where it might be involved in cell-cell fusion (11, 29, 35). gH then undergoes endocytosis and is trafficked back to the trans-Golgi network (TGN) for incorporation into the virion envelope (20, 31, 42). Since VZV is highly cell associated in vitro, little is known about the glycoproteins required for entry, but VZV gH is present in abundance in the skin vesicles during human chickenpox and zoster (55).
Investigating the functions of gH in the pathogenesis of VZV infection in vivo is challenging because it is an essential protein and VZV is species specific for the human host. The objective of this study was to investigate the role of gH in VZV pathogenesis by establishing whether antibody-mediated interference with gH function could prevent or modulate VZV infection of differentiated human tissue in vivo, using the SCIDhu mouse model. The effects of antibody administration at early and later times after infection were determined by comparing infectious virus titers, VZV genome copies, and lesion formation in anti-gH antibody-treated xenografts. In vitro experiments were performed to determine the potential mechanism(s) of MAb 206 interference with gH during VZV replication, virion assembly, and cell-cell spread. The present study has implications for understanding the contributions of gH to VZV replication in vitro and in vivo, the mechanisms by which production of antibodies to gH by the host might restrict VZV infection, and the use of passive antibody prophylaxis in patients at high risk of serious illness caused by VZV.
MATERIALS AND METHODS
Cells and virus.
Human melanoma cells and human embryonic lung fibroblasts (HELFs) were grown at 37°C in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (Gemini Bio-Products, Woodland, CA), nonessential amino acids (melanoma cells only) (100 μM; Omega Scientific, Inc., Tarzana, CA), and antibiotics (penicillin at 100 U/ml and streptomycin at 100 μg/ml; Invitrogen, Carlsbad, CA). Wild-type recombinant VZV pOka was generated in melanoma cells by use of a cosmid system (39) and then propagated in HELF cultures.
Preparation, inoculation, and harvest of skin xenografts in SCID mice.
Skin xenografts were prepared in homozygous CB-17scid/scid mice, using human fetal skin tissue obtained according to federal and state regulations (34). Animal protocols complied with the Animal Welfare Act and were approved by the Stanford University Administrative Panel on Laboratory Animal Care. Human tissues were obtained, in accordance with state and federal regulations, from Advanced Bioscience Resources (Alameda, CA). VZV pOka-infected HELF cultures were used to inoculate the xenografts. Infectious virus titer was determined at the time of inoculation by 10-fold serial dilution on melanoma cells. Skin xenografts were harvested at 7, 14, 21, 28, 35, and 42 days postinfection (dpi). Half of each xenograft was stored in 4% paraformaldehyde for histology, and half was homogenized and resuspended in 1 ml phosphate-buffered saline (PBS) for virus titration and DNA extraction. Serum was prepared from blood collected from mice at the time of tissue harvest, allowed to coagulate at room temperature (RT), and then centrifuged at 6,000 × g for 20 min.
Treatment of SCIDhu mice with anti-gH antibody.
Anti-gH MAb 206 is an IgG1 complement-independent neutralizing antibody that recognizes a conformational epitope on mature glycosylated gH (35). Either 100 μl PBS containing 25 μg MAb 206 or 100 μl PBS alone was administered to mice intraperitoneally every 4 days starting at 6 hpi. Mice were treated with antibody beginning at 6 hpi or 4 dpi, and repeated doses were given every 4 days through 12 dpi. The two antibody-treated groups consisted of mice treated with antibody at 6 hpi, 4 dpi, 8 dpi, and 12 dpi (Ab-0-12 group) and mice treated at 4 dpi, 8 dpi, and 12 dpi (Ab-4-12 group). PBS was administered at time points when antibody was not given out to 42 dpi. A control group (PBS group) was given PBS at all comparable time points. The number of xenografts evaluated at each time point was as follows: 7 to 21 dpi, n = 11 or 12; 28 dpi, Ab-0-12 group, n = 5; 28 dpi, Ab-4-12 and PBS groups, n = 11 or 12; and 35 to 42 dpi, n = 5 or 6.
Infectious plaque assay.
Melanoma cells were seeded in a 24-well plate and inoculated in triplicate with 0.1 ml of a 10-fold serial dilution of xenograft homogenate or the inoculum virus to be titrated. For the titration of virus from homogenates, the medium was changed 24 h after inoculation. Cells were cultured for 5 days, and plaques were stained with anti-VZV polyclonal serum. Titers were analyzed using Student's t test to determine if a statistically significant difference (P ≤ 0.05) in titer existed. The number of xenografts positive for virus was analyzed using Fisher's exact test to determine if a statistically significant difference (P ≤ 0.05) existed.
Plaque neutralization assay.
Melanoma cells were seeded in a 24-well plate and inoculated in triplicate with 0.1 ml of 10-PFU/ml pOka in the absence or presence of 0.1 ml of a 10-fold dilution of xenograft homogenate. The medium was changed after 24 h, and plates were incubated for 5 days. Plates were stained as described above, and titers were analyzed using Student's t test to determine if anti-gH antibody within the homogenate neutralized the 10-PFU/ml inoculum.
Enzyme-linked immunosorbent assay.
The IgG1 MAb 206 in mouse serum was measured using a mouse IgG1 enzyme-linked immunosorbent assay quantitation kit from Bethyl Laboratories, Inc. (Montgomery, TX), following the manufacturer's recommended protocol. Briefly, plates were coated with capture antibody and blocked with postcoat solution. Serum samples were diluted 1:100 and 1:1,000 in duplicate and incubated for 60 min at RT. Horseradish peroxidase conjugate and tetramethylbenzidine (TMB) with an acid stop were used to detect the presence of IgG1 antibody. Plates were read in a SpectraMax 190 instrument (Molecular Devices, Sunnyvale, CA). The IgG1 concentration was determined from a standard curve with a range of 250 to 3.9 ng/ml, analyzed using a four-parameter logistic curve fit, as recommended by the manufacturer.
Immunohistochemistry of skin xenograft sections.
Mouse anti-gE antibody (MAb 8612; Millipore, Temecula, CA) was used at 1:2,000 to detect VZV lesions in sectioned xenografts. Slides were developed using an alkaline phosphatase-based enzyme detection method (Millipore, Temecula, CA) with PermaRed substrate (VWR, West Chester, PA). Slides were counterstained with hematoxylin. Xenografts were examined using an Axiovert 200 microscope (Zeiss).
Quantitative PCR.
DNA was isolated from xenograft homogenates by use of DNAzol (Gibco-BRL, Grand Island, NY) following the manufacturer's protocol. VZV genome copy number was assessed using primers/probes to detect ORF31 (encoding gB), ORF62 (encoding IE62), and ORF63 (encoding IE63), as previously reported (58). Each gene target was measured in duplicate, and the mean of each was used to determine the number of genome copies.
VZV DNA in situ hybridization.
A VZV-specific DNA probe was prepared as previously described (45). Paraffin-embedded sections were deparaffinized, incubated with proteinase K (Roche, Indianapolis, IN) in proteinase K buffer (0.1 M Tris-HCl, pH 7.5, 150 mM NaCl, 12.5 mM EDTA) for 10 min at 37°C, and then dried completely. Hybridization mix (15 μl) was added to each section, covered with a glass coverslip, denatured for 10 min at 95°C, and then hybridized overnight at 60°C. Sections were washed twice in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and once in 0.2× SSC for 10 min at 50°C and then blocked for 30 min in digoxigenin (DIG) blocking solution (Roche, Indianapolis, IN). Sections were incubated with anti-DIG MAb (Roche, Indianapolis, IN) (1:50 in blocking solution) for 1 h at 37°C and then with secondary anti-mouse-DIG antibody (1:100 in blocking solution) for 1 h at 37°C to amplify the DIG signal. Finally, sections were incubated with anti-DIG-alkaline phosphatase (1:200 in blocking solution) for 1 h at 37°C, followed by nitroblue tetrazolium-5-bromo-4-chloro-3-indolylphosphate (NBT-BCIP) staining to detect the VZV DNA-specific signal. Sections were counterstained in methyl green and imaged using an Axiovert 200 microscope (Zeiss).
Antibody treatment of pOka-infected HELF cultures in vitro.
Following a protocol adapted from the work of Rodriguez et al. (46), 1 × 106 HELFs/well were seeded in six-well plates 24 h prior to inoculation. Monolayers were inoculated with 3 log10 PFU pOka for 90 min at 37°C. The medium was changed, 25 μg of anti-gH MAb 206 was added, and this was repeated at 24-h intervals. Mock cells received only medium. HELF cultures were harvested for titration and DNA extraction at 24-h intervals.
EM.
HELF monolayers were seeded in 10-cm dishes containing glass coverslips, infected with pOka, and treated with antibody, as detailed above. After 48 h, the cells on the coverslips were washed in PBS, fixed, and processed for transmission electron microscopy (EM) as previously described (7), although without the use of propylene oxide in the dehydration steps. The remaining cells were washed in PBS and collected for cryo-EM. For cryo-EM, treated and untreated samples were fixed in 4% paraformaldehyde with 0.1% glutaraldehyde in phosphate buffer (0.1 M, pH 7.2), washed in PBS, and infiltrated with 2.3 M sucrose overnight at 4°C. The samples were then mounted on pins for cryo-ultramicrotomy and frozen in liquid nitrogen. Ultrathin cryosections (80 nm) were prepared with a diamond knife (Diatome, Hatfield, PA) at −130°C, using an ultramicrotome (Ultracut; Leica) equipped with a cryosectioning chamber. Thawed cryosections were transferred to Formvar- and carbon-coated EM grids (nickel) within a drop of 2.3 M sucrose, washed in PBS, labeled with immunogold, and counterstained with 0.5% uranyl acetate in 2% methylcellulose for 10 min on ice. For immunogold labeling, thawed cryosections were blocked with 1× DIG blocking solution. The mouse monoclonal antibody MAb 206, used for treating the samples, was detected by incubation with 1:100-diluted polyclonal rabbit anti-mouse antibody (Cappel Laboratories) for 1 h at RT, followed by protein A-gold (15 nm) incubation for 30 min at RT.
Confocal microscopy.
HELF cultures were infected with pOka and treated with antibody or mock treated as detailed above. At 48 hpi, cells were fixed in 4% paraformaldehyde and blocked with PBS containing 10% donkey serum and 0.1% Triton X-100. Cellular localization of VZV proteins was performed using primary antibodies to VZV proteins gH (SG3 monoclonal mouse anti-gH; Biodesign, Saco, ME), ORF23 (rabbit polyclonal) (7), and gE (rabbit polyclonal) (25) and to cellular proteins TGN46 (AHP500 polyclonal sheep anti-TGN46; AbD Serotec, Oxford, United Kingdom), early endosome antigen 1 (EEA1) (NB300-502 rabbit anti-EEA1; Novus Biologicals, Littleton, CO), and vacuolar protein sorting 4 (Vps4) (sc-32922 rabbit anti-Vps4; Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Secondary antibodies used were fluorescein isothiocyanate-coupled donkey anti-sheep (Jackson ImmunoResearch, West Grove, PA), Alexa Fluor 555-coupled donkey anti-mouse (Molecular Probes, Carlsbad, CA), Alexa Fluor 647-coupled donkey anti-rabbit (Molecular Probes, Carlsbad, CA), and Hoechst 33342 (Molecular Probes, Carlsbad, CA). Confocal microscopy was performed using a Zeiss LSM510 confocal microscope equipped with two-photon excitation.
RESULTS
Treatment with MAb 206 at 6 hpi significantly reduced the number of skin xenografts infected with VZV.
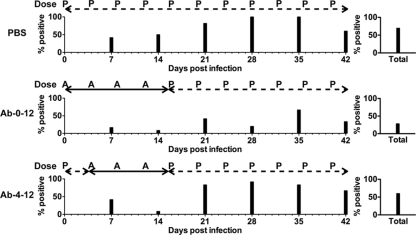
To investigate the effect of anti-gH neutralizing antibody on VZV pathogenesis in vivo, skin xenografts were inoculated with pOka at a titer of 5.3 log10 PFU/ml, and mice were administered intraperitoneally either MAb 206 or PBS. Of 56 xenografts from mock-treated mice (PBS group), 70% were positive for VZV (Fig. 1). Only 28% of the xenografts from the mice treated with the anti-gH MAb 206 at 6 hpi (Ab-0-12 group) were positive for virus, significantly less than the positive number in the PBS group (P ≤ 0.01; Fisher's exact test). In contrast, anti-gH MAb 206 treatment at 4 dpi (Ab-4-12 group) did not significantly reduce the number of infected skin xenografts compared to the PBS group (P = 0.33; Fisher's exact test), as 60% of the xenografts were positive for infectious virus. The number of virus-positive xenografts in the Ab-0-12 group was also significantly reduced compared to that in the Ab-4-12 group (P ≤ 0.01; Fisher's exact test), indicating that early (6 hpi) treatment with MAb 206 was capable of preventing VZV infection in 42% of skin xenografts but that delaying treatment until 4 dpi failed to prevent infection in any skin xenografts.
FIG. 1.
VZV infection of human skin xenografts in SCIDhu mice treated with anti-gH MAb 206 for 0 to 12 days or 4 to 12 days postinoculation. The timeline of treatment is shown above the percentage of xenografts from which infectious virus was recovered. Skin xenografts were inoculated with pOka-infected HELFs at a titer of 5.3 log10 PFU/ml, and mice were administered anti-gH MAb 206 (solid line; administration time points indicated with “A”) or PBS (dashed line; administration time points indicated with “P”). PBS, control group treated with PBS; Ab-0-12, group treated with MAb 206 at 6 hpi, 4 dpi, 8 dpi, and 12 dpi; Ab-4-12, group treated with MAb 206 at 4 dpi, 8 dpi, and 12 dpi. Xenografts were collected at 7-day intervals up to 42 dpi. The total percentage of infected xenografts over the 42-day interval is shown on the right.
VZV titer was significantly reduced following treatment with the anti-gH MAb 206.
For the PBS group, the mean virus titer peaked by 28 dpi, with a 77-fold increase in titer compared to that at day 7 (Fig. 2A). The mean virus titer was unchanged by 35 dpi but fell by 42 dpi. The 30-fold decrease from 28 to 42 dpi was consistent with the depletion of cells permissive for infection in the skin xenograft as a consequence of VZV replication.
FIG. 2.
Replication of VZV in human skin xenografts in SCIDhu mice treated with anti-gH MAb 206 for 0 to 12 days or 4 to 12 days postinoculation. (A) Mean virus titers recovered from VZV-positive xenografts. (B) Neutralization capacity of MAb 206 within xenograft homogenates against a standard inoculum of 10 PFU/ml of VZV. (C) Mean VZV genome copy number per μg human DNA from VZV-positive xenografts, measured by quantitative PCR for ORFs 31 (gB), 62 (IE62), and 63 (IE63). Each gene target was measured in duplicate, and the mean of each was used to determine the genome copy number. (D) Concentrations of IgG1 in sera of MAb 206- or PBS-treated mice. Standard errors of the means are shown on all graphs.
Administration of MAb 206 starting at 6 hpi (Ab-0-12 group) significantly reduced the titers of recoverable virus at 7 to 35 dpi compared to those for the PBS group (P ≤ 0.01; Student's t test), with 7- to 70-fold differences between the two groups. By 35 dpi, 67% of the Ab-0-12 xenografts were positive for virus, the maximum percentage for this group. This was the only time point at which >50% of the xenografts were positive, compared with >50% of xenografts being positive for virus at four and five of the time points for the Ab-4-12 and PBS groups, respectively (Fig. 1). By 42 dpi, the maximum titer reached in the Ab-0-12 group was 88-fold higher than the titer at day 7. The Ab-0-12 titer at 42 dpi was equivalent to the mean titer in the PBS group at 21 dpi, showing a considerable delay in reaching this titer in the Ab-0-12 group. The peak titer in the PBS group occurred at 28 dpi, whereas the maximal titer in the Ab-0-12 group was delayed by at least 14 days, and peak titer in this group may have occurred at an even later time point. Thus, antibody treatment at 6 hpi not only reduced the number of skin xenografts that became infected with pOka but was associated with lower virus titers in those that became infected, both during and after antibody treatment, until 35 dpi, 23 days after the final antibody dose was administered.
Virus titers peaked in the Ab-4-12 group by 35 dpi, with a 33-fold increase from those at 7 dpi. Mean virus titers were significantly reduced at 7 to 28 dpi compared to those of the PBS group (P ≤ 0.02; Student's t test), and virus was recovered from the majority of skin xenografts in the Ab-4-12 group from day 21 onward. The time to the highest virus titer was delayed 1 week compared to that for the PBS group and occurred at least 1 week earlier than that for the Ab-0-12 group. Treatment with MAb 206 was associated with lower virus titers during the administration period, as seen in the Ab-0-12 group, and until 28 dpi, 16 days after the last antibody dose was administered. Virus titers increased with kinetics similar to those for the PBS group titers when antibody treatment was suspended. These data suggested that passive treatment with anti-gH MAb 206 has limitations for control of an established VZV infection.
It was conceivable that the homogenates from skin xenografts might have contained neutralizing antibody that would interfere with plaque formation in the virus titer assay when the xenograft homogenates were tested for infectious virus. A plaque reduction assay was performed in which melanoma cells were inoculated with 10 PFU/ml pOka in the presence or absence of xenograft homogenate. Under these conditions, detection of less than 10 PFU/ml would indicate neutralization of plaque formation by antibody within the homogenate, while a minimum titer of 10 PFU/ml would indicate that antibody within the homogenate did not prevent plaque formation. As expected, virus titers for xenograft homogenates from the PBS group were ≥10 PFU/ml at each time point, with the increased titer being attributable to virus present in the homogenate (Fig. 2B). Evidence of neutralizing activity was not seen for xenograft homogenates from either of the MAb 206-treated groups, as virus titers at early time points remained at approximately 10 PFU/ml but increased at later time points. The lack of plaque neutralization of the 10-PFU/ml inoculum demonstrated that any residual MAb 206 present within the homogenate did not disrupt plaque formation within the virus titer assay.
VZV genome copy numbers in skin xenografts were reduced following treatment with the anti-gH MAb 206.
As expected from the increase in viral titers for the PBS group, viral genome copies increased 794-fold from 7 dpi to 42 dpi in the xenografts from which infectious virus was recovered (Fig. 2C). Xenografts that did not yield infectious virus had a mean genome copy number of 5 to 6 log10 copies per μg of human DNA throughout the experiment, with no increase over time. Xenografts that did not become infected from either of the anti-gH MAb 206-treated groups had similar levels of DNA. This was likely to be residual VZV DNA from the inoculum, and these data were not included in the average copy number for each group.
The mean genome copy number in the infected xenografts from mice treated with MAb 206 at 6 hpi (Ab-0-12 group) followed the same trend as virus titer. The number of genome copies increased 40-fold from 7 dpi to 42 dpi (Fig. 2C). Mean genome copy numbers were significantly lower at 14 to 42 dpi than those in infected xenografts from the PBS group (P ≤ 0.05; Student's t test). Similar to the Ab-0-12 group, treatment of mice with MAb 206 starting at 4 dpi (Ab-4-12 group) significantly reduced the mean genome copy number at each time point, except at 14 dpi, compared to that for the PBS group (P ≤ 0.01; Student's t test). There was a 501-fold increase from 7 dpi to 42 dpi (Fig. 2C). The significantly lower numbers of genome copies in both MAb 206 treatment groups than in the PBS group were highly likely to be caused by the reduced virus spread, as determined by virus titer.
Kinetics of anti-gH MAb 206 accumulation and clearance.
As expected, the PBS group had extremely low levels of serum IgG1 throughout the duration of the 42-day experiment. SCID mice are known to spontaneously develop partial immune reactivity as they age, resulting in low levels of serum IgG in approximately 87% of 2- to 3-month-old mice (40). The highest concentration of IgG1 in the PBS group at 14 dpi was not significantly higher than those at other times points (P ≥ 0.3; Student's t test) (Fig. 2D). The low levels of IgG1 were considered to be background.
The serum levels of IgG1 in mice administered MAb 206 starting at 6 hpi (Ab-0-12 group) were significantly higher than levels in mock-treated mice (P ≤ 0.02; Student's t test), and therefore above background, at all time points (Fig. 2D). IgG1 levels in both MAb 206-treated groups peaked by 14 dpi and then decreased over time to 42 dpi. The Ab-0-12 group had significantly higher IgG1 levels than the Ab-4-12 group at 7, 14, and 42 dpi (P ≤ 0.02; Student's t test). IgG1 levels in the Ab-4-12 group were significantly higher than those in the PBS group at 7 to 35 dpi (P ≤ 0.01; Student's t test). The decrease in serum antibody level correlated with the increases in viral titer and genome copy number for both antibody-treated groups. The additional dose of MAb 206 for the Ab-0-12 group at 6 hpi resulted in higher levels of serum IgG1, likely leading to greater reductions of viral titers and genome copy numbers.
VZV lesion spread in skin xenografts was reduced following treatment with anti-gH MAb 206.
Lesions were identified in almost all xenografts from which infectious virus was recovered. Syncytia were found in all lesions. Xenografts from mock-treated mice had small epidermal lesions at 7 dpi, with only slight disruption of the basement membrane and penetration of virus into the dermis (Fig. 3A). At 14 dpi, lesions extended into the dermis, and by 21 dpi, lesions extended throughout most of the xenograft (Fig. 3B and C). In the majority of xenografts examined at 35 and 42 dpi, the entire xenograft had become infected and had extensive necrosis, as nuclei were not seen with the hematoxylin counterstain (Fig. 3E and F). This correlates with the decrease in titer in the PBS group at these time points.
FIG. 3.
Formation of lesions in VZV-infected human skin xenografts treated with anti-gH MAb 206 for 0 to 12 days or 4 to 12 days postinoculation. Lesions in skin xenografts inoculated with pOka-infected HELFs were identified by VZV gE expression (red) and were counterstained with hematoxylin (blue). Representative lesions are shown at each time point, as follows: 7 dpi, panels A, G, and M; 14 dpi, panels B, H, and N; 21 dpi, panels C, I, and O; 28 dpi, panels D, J, and P; 35 dpi, panels E, K, and Q; and 42 dpi, panels F, L, and R. The total number of xenografts with lesions is shown in the lower left of each panel. Lesions were identified in xenografts from the PBS (A to F) and Ab-4-12 (M to R) groups at all time points. Lesions were observed in xenografts from the Ab-0-12 group (G to L) only at 7, 21, 35, and 42 dpi. Representative uninfected xenografts are shown for the Ab-0-12 group at 14 and 28 dpi. Syncytia were seen in all lesions. Magnification, ×50.
A single lesion was identified among 12 xenografts from the Ab-0-12 group at 7 dpi (Fig. 3G). This lesion was contained within the epidermis and was smaller than the lesions in PBS xenografts at 7 dpi (Fig. 3A). Lesions were not seen at 14 dpi, but at 21 dpi, several lesions, smaller than those seen in the PBS group at 7 dpi, were identified (Fig. 3H and I). These lesions were contained within the epidermis or hair follicles, and only small syncytia were seen. Lesions were not seen at 28 dpi, but by 35 and 42 dpi, lesions extended into the dermis and were approximately the size of those seen in PBS xenografts at 14 dpi (Fig. 3J to L).
Small lesions were identified in the epidermis or hair follicles of the xenografts from the Ab-4-12 group at 7 and 14 dpi (Fig. 3M and N). At 21 dpi, lesions extended into the dermis (Fig. 3O). Lesion size continued to increase until the majority of the tissue was infected, by 35 and 42 dpi, (Fig. 3P to R). Genome-positive cells, identified by in situ hybridization to detect VZV genomic DNA, correlated with protein-positive cells in both Ab-4-12 and PBS xenografts at 14 dpi (data not shown). Overall, lesion size increased in infected xenografts in all groups over time, but the increase was delayed in both groups administered anti-gH MAb 206, with a more pronounced delay in the Ab-0-12 group.
Spread and replication of VZV were decreased in vitro after treatment with anti-gH MAb 206.
MAb 206 was previously shown to neutralize cell-cell spread (8, 35, 43, 46), but its effects on VZV titer and genome copy number have not been determined. To confirm the neutralizing activity of this MAb, HELFs were inoculated with pOka and cultured in the presence or absence of antibody. Small plaques were seen in HELFs infected with pOka in mock-treated cultures at 24 hpi and increased in size until 96 hpi, when nearly all cells were infected (Fig. 4A to D). Treatment of HELFs with MAb 206 (Ab-96 cells) reduced infection to single cells in the monolayer at 24 hpi, with only a few small plaques visible at 48 hpi. A small increase was seen in plaque size at 72 hpi, but no further increase was observed at 96 hpi (Fig. 4E to H). The plaques seen at 72 and 96 hpi were not much larger than the plaques at 24 hpi in the mock-treated cells, and the overall increase between 24 hpi and 72 to 96 hpi was much less than that seen from 24 to 48 hpi in the mock-treated cells, indicating extremely inefficient spread of the virus in the presence of MAb 206.
FIG. 4.
Cell-cell spread of VZV in vitro in the presence of anti-gH MAb 206. HELFs were mock treated (A to D) or treated with antibody (E to H) and then fixed and stained with polyclonal sera against VZV at 24-h intervals. (A and E) 24 hpi; (B and F) 48 hpi; (C and G) 72 hpi; (D and H) 96 hpi. Magnification, ×50.
In the absence of MAb 206 (mock-treated cells), the mean virus titer peaked at 72 hpi, and the titer increased 73-fold from that at 24 hpi (Fig. 5A). The number of genome copies increased 251-fold during the 96-hour period (Fig. 5B). Virus titer and genome copy number did not increase significantly over the first 48 h in HELFs continuously treated with MAb 206 (Ab-96 cells) but increased significantly (P ≤ 0.01; Student's t test) between 48 and 72 hpi (Fig. 4A). No further increase was seen between 72 and 96 hpi (Fig. 5A and B). The peak titer in the Ab-96 wells at 72 to 96 hpi was 12-fold higher than that at 24 hpi. At all time points, titers in Ab-96 wells were significantly lower than the titers in mock-treated wells (P ≤ 0.01; Student's t test), and genome copy numbers remained statistically equivalent to those in the mock-treated cells at 24 hpi. The slight increases in titer and genome copy number occurred at the same time as the small increases in plaque size.
FIG. 5.
VZV replication in vitro in the presence of anti-gH MAb 206. HELFs were inoculated with pOka and grown in medium without antibody (mock) or medium supplemented with MAb 206 for 24, 48, or 96 h (Ab-24, Ab-48, or Ab-96, respectively). Cells were harvested at 24-h intervals for 96 h. (A) Mean titer in HELF cultures. (B) Mean genome copy number based on quantitative PCR for ORFs 31 (gB), 62 (IE62), and 63 (IE63) per ng human DNA. Each gene target was measured in duplicate, and the mean of each was used to determine the number of genome copies. Standard errors of the means are shown for both graphs.
Virus titers were significantly lower than those in mock-treated HELFs at all time points (P ≤ 0.02; Student's t test) for cells treated with MAb 206 for only 24 h (Ab-24) or 48 h (Ab-48) following inoculation (Fig. 5A). The genome copy number in Ab-24 wells was significantly lower than that for mock-treated cells at 48 hpi (P = 0.01; Student's t test) (Fig. 5B). The virus titer increased 440-fold and genome copy number increased 122-fold over the 96 h in HELFs treated for 24 h. At 48 and 72 hpi, the titer was statistically equivalent to that in mock-treated cells at 24 hpi and 48 hpi (P ≥ 0.13; Student's t test), but by 96 hpi, it was significantly lower than that in mock-treated cells at 72 hpi (P ≤ 0.04; Student's t test). A similar trend was seen for HELFs treated with MAb 206 for 48 hpi. Virus titers increased 199-fold and genome copy number increased 89-fold between 24 and 96 hpi. The virus titer at 96 hpi was statistically equivalent to that in mock-treated cells at 48 hpi, and the numbers of genome copies were statistically equivalent at 48 to 96 hpi to the mock values 24 h prior. The in vitro data reflected what was seen in vivo, with neutralizing activity against pOka causing decreased virus spread, titers, and genome copies, but this effect persisted only while antibody was present.
Anti-gH MAb 206 localized to intracellular vacuoles, virus particles on the cell surface, and virus particles within infected cells.
Normal nucleocapsids and enveloped virus particles were found on mock-treated HELFs infected with pOka (Fig. 6A). Some virus particles were observed in aggregates on the surfaces of cells. Nucleocapsids and enveloped virus particles were also identified in HELFs infected with pOka and treated with MAb 206 and were identical to those from mock-treated cells (Fig. 6B). Thus, MAb 206 did not appear to disrupt virion assembly.
FIG. 6.
Virus particle formation and localization of MAb 206 in infected cells in vitro. HELFs inoculated with pOka were mock treated (A) or treated with antibody (B to G) and were fixed at 48 hpi. (A and B) Transmission EM of infected and untreated (A) or treated (B) cells. Arrowheads and right insets show virion particles on the cell surface. Arrows and left insets show nucleocapsids. (C to F) Immunogold labeling of MAb 206 in treated cells. Arrows indicate MAb 206 on the cell surface or within vacuoles. Arrowheads indicate MAb 206 on virus particles. (D) Infected cell (top) with MAb 206 labeling next to uninfected cell (bottom) lacking dense MAb 206 labeling. Magnification, ×10,000 (A and B). Bars, 0.2 μm.
Based on immunogold EM analysis, the anti-gH MAb was enriched in numerous endosome-like structures within treated cells (Fig. 6C and D). Gold particles within endosomes were identified more frequently within infected cells than in neighboring uninfected cells, where only one or two gold particles per cell were found, compared to much more dense labeling in infected cells (Fig. 6D). The limited detection of MAb 206 in uninfected but treated HELFs might have been due to passive uptake of the anti-gH antibody from the medium or to minimal background labeling with unbound gold particles. The increased uptake within infected cells suggested that MAb 206 bound to cell surface gH and was internalized. MAb 206 localized to the surfaces of infected cells (Fig. 6E) and to virus particles (Fig. 6E to G). These antibody-virus complexes were observed on the cell surface and within the cell near the plasma membrane, indicative of endocytosis.
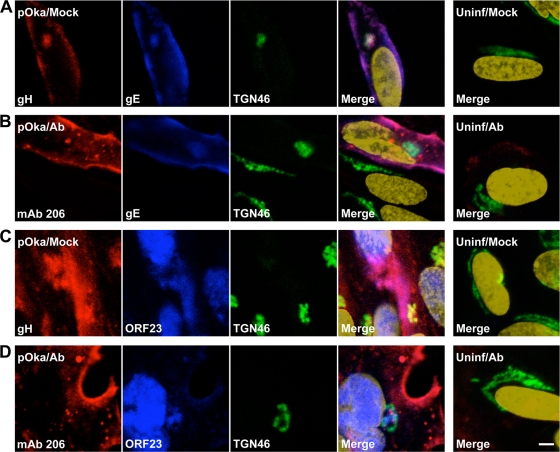
MAb 206 was not trafficked to the TGN but colocalized with the endocytic pathway.
gH localized to the cell surfaces of infected HELFs and to the TGN at 48 hpi, as determined by confocal microscopy (Fig. 7A and C). The TGN is thought to be the site of virus particle secondary envelopment (20). gH often colocalized with gE both in the TGN and on the surfaces of infected HELFs (Fig. 7A). gH also colocalized with a major capsid protein, ORF23 (Fig. 7C). It has previously been demonstrated that ORF23 is found predominantly in the nuclei of infected cells (7), but ORF23 staining on the surfaces of cells or near nuclear membranes has been associated with incoming virions clustered on the plasma membrane or capsids along the nuclear envelope (45). Therefore, in this study, ORF23 staining that did not colocalize with the nuclear marker but did colocalize with gH was identified as representing enveloped virions clustered on the surfaces of infected cells, in agreement with the small aggregates of virion particles or individual virions lining the surfaces of infected cells, as seen by electron microscopy.
FIG. 7.
Localization of gH and MAb 206 relative to VZV gE and ORF23 proteins in fibroblasts in vitro. HELFs inoculated with pOka were mock treated (A and C) or antibody treated (B and D) for 48 h, fixed, permeabilized, stained, and examined by confocal microscopy. Uninfected HELFs were also mock treated (A and C) or antibody treated (B and D) for 48 h, fixed, permeabilized, stained, and examined. (A) gH, red; gE, blue; TGN46, green; nuclei, gold. (B) MAb 206, red; gE, blue; TGN46, green; nuclei, gold. (C) gH, red; ORF23, blue; TGN46, green; nuclei, gold. (D) MAb 206, red; ORF23, blue; TGN46, green; nuclei, gold. Bar, 5 μm.
Similar to the gH localization by confocal microscopy, MAb 206 localized to the cell surface and colocalized with gE and ORF23 on infected cell surfaces but did not localize to the TGN (Fig. 7B and D). Colocalization between ORF23 and gH or MAb 206 on the cell surface was confirmed in nonpermeabilized cells (data not shown). The similarities between gH and MAb 206 localization demonstrated that MAb 206 bound to gH on the surfaces of infected cells and on virions, but the lack of colocalization between MAb 206 and the TGN was markedly different from the localization of gH within the TGN. In treated uninfected cells, minimal MAb 206 was seen, mostly localizing to the surfaces of cells (Fig. 7B and D).
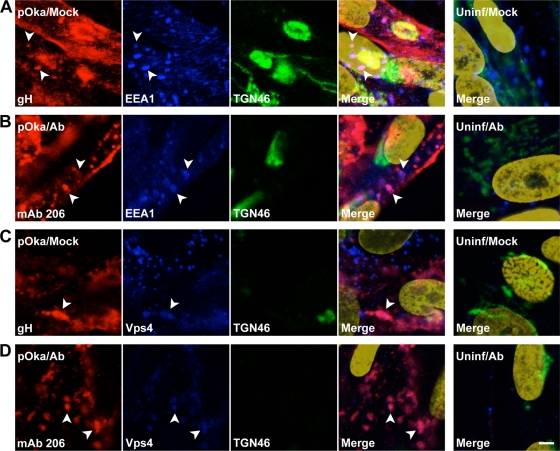
gH and MAb 206 often had a punctate distribution pattern within the cytoplasm of infected cells. Some, but not all, gH and MAb 206 colocalized with the early endosome marker EEA1 (Fig. 8A and B, arrowheads). This indicated that vesicles labeled with MAb 206 by immunogold EM were likely to be endosomes. gH and MAb 206 also colocalized with Vps4, a marker for the multivesicular body (MVB) pathway, in somewhat diffuse patches or punctae within cells (Fig. 8C and D, arrowheads). The colocalization with EEA1 and Vps4 indicated that the endocytic and MVB pathways were involved in trafficking of both gH and MAb 206-bound gH, albeit to different destinations, as the MAb 206-gH complex did not reach the TGN, while gH did.
FIG. 8.
Localization of gH and MAb 206 relative to EEA1 and Vps4 in fibroblasts in vitro. HELFs inoculated with pOka were mock treated (A to D, K to N) or antibody treated (F to I, P to S) for 48 h, fixed, permeabilized, stained, and examined by confocal microscopy. Uninfected HELFs were mock treated (E and O) or antibody treated (J and T) for 48 h, fixed, permeabilized, stained, and examined. Arrowheads highlight colocalization. (A) gH, red; EEA1, blue; TGN46, green; nuclei, gold. (B) MAb 206, red; EEA1, blue; TGN46, green; nuclei, gold. (C) gH, red; Vps4, blue; TGN46, green; nuclei, gold. (D) MAb 206, red; Vps4, blue; TGN46, green; nuclei, gold. Bar, 5 μm.
DISCUSSION
VZV gH is predicted to play an essential role in VZV virulence and is a known target of the humoral immune system during infection (1). The present study of the SCIDhu mouse model showed for the first time that gH contributes to VZV infection and cell-cell spread of virus in skin xenografts in vivo and confirmed this contribution in vitro. The low levels of persistent VZV replication and spread observed in some VZV-infected xenografts in vivo and in HELF cells in vitro in the presence of anti-gH antibody indicate that passive antibody interference with gH functions has some limitations. This residual spread might have resulted from incomplete binding of antibody to all functional gH. Alternatively, other VZV glycoproteins might be capable of mediating some virion-cell or cell-cell fusion in the absence of functional gH. It has been suggested that VZV gH and gL or gB and gE might induce fusion (12, 30), although if so, VZV would be the only herpesvirus investigated to date that does not require both gB and gH for this event.
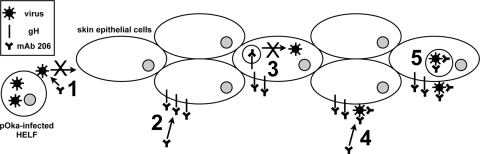
This report also demonstrated for the first time that the administration of anti-gH antibody was effective at preventing or reducing VZV pathogenicity in human skin. Since SCIDhu animals are immunodeficient (4), this inhibitory effect could be assessed in the absence of a polyclonal B-cell response to multiple viral proteins and without VZV-specific cell-mediated immunity. The prevention of infection might be attributed to a block of virus attachment and entry into cells. The reduced pathogenicity in xenografts that became infected might be attributed to the block in spread of virus from cell to cell, inhibition of canonical gH trafficking, and potential virus particle degradation. MAb 206 binds to a conformation-dependent epitope on mature glycosylated gH, and neutralization of VZV is complement independent (35). Two potential mechanisms for MAb 206 neutralization of VZV are inhibition of receptor binding and attachment and inhibition of fusion. The antibody might also disrupt postentry events, such as interactions between virus proteins and trafficking of proteins or virion particles (Fig. 9).
FIG. 9.
Schematic of mechanisms for antibody disruption of gH function and trafficking and potential effects on the pathogenesis of VZV skin infection in vivo. (1) Anti-gH antibody present soon after inoculation can bind and neutralize virus particles on the inoculum cells, preventing VZV attachment or entry into skin epidermal cells. (2) Antibody present after infection has been established can bind gH expressed on the infected cell surface, preventing cell-cell fusion. (3) These antibody-gH complexes are internalized, and antibody-bound gH is not targeted for secondary envelopment and incorporation into virions, but instead is targeted for degradation. (4) Antibody binds to gH on the envelopes of surface virus particles, blocking envelope fusion with neighboring cell membranes and inhibiting cell-cell spread of the virus. (5) Antibody-virion complexes are internalized and targeted for degradation.
Neutralizing antibodies to herpesviruses block attachment by physically blocking interaction and preventing binding of virus proteins to cellular receptors (38, 41, 57). A VZV gH receptor has not been identified, and the cell-associated nature of VZV prevents study of VZV attachment steps. If gH interaction with a cellular protein is required for attachment, then a neutralizing antibody could disrupt binding and prevent infection (Fig. 9, step 1).
Epstein-Barr virus, human cytomegalovirus, and human herpesvirus 6 and 7 gH/gL form a complex with additional glycoproteins. Some of these complexes appear to determine cell-specific infectivity, receptor specificity, or the route of virus entry into the cell (36, 48, 53, 54). No interactions with cellular proteins have been identified for VZV gH, but antibody binding to gH could disrupt or prevent formation of a protein complex required for receptor binding, thereby preventing infection (Fig. 9, step 1).
Neutralizing anti-gH MAb might mask functional domains of VZV gH, preventing fusion and entry. VZV gH and gL can mediate fusion between cell membranes when expressed in vitro in a vaccinia virus vector (12). Many anti-HSV gH neutralizing antibodies block virus penetration and prevent entry (18). HSV gH is required for hemifusion, and one MAb specifically blocks this fusion step (49). Deletion or mutation of predicted HSV gH heptad repeats and α-helical coiled coils disrupts virus infectivity and cell-cell fusion (21, 23). Mimetic peptides of the α-helices interact with lipid membranes (19, 21). When the HSV α-helix 1 was replaced with the positionally conserved α-helix from VZV, the resulting chimeric gH was capable of promoting cell-cell fusion and rescuing infectivity of an HSV gH-negative virus, indicating that VZV gH contains a functional α-helix capable of mediating fusion (22). Thus, the reduced number of infected skin xenografts and cell-cell spread during MAb 206 treatment could potentially have resulted from inhibition of virus entry into cells (Fig. 9, steps 1 and 2).
Glycoprotein trafficking from the plasma membrane to the Golgi apparatus can occur via clathrin-coated vesicles (3). VZV gH endocytosis is antibody independent but clathrin dependent (42), and this report has demonstrated that anti-gH MAb 206 binds gH and is internalized with gH (Fig. 9, step 2). MAb 206 and gH colocalized with EEA1, a marker for early endosomes, and with Vps4, a marker for MVB, indicating that MAb 206 and gH underwent endocytosis and sorting via the MVB pathway. Vps4 is required for transport of endocytosed proteins between prevacuolar endosomes and vacuoles (2). Vps4 is also required for autophagy, presumably for autophagosome-endolysosome fusion (47). Endocytosed proteins can be sorted to the Golgi apparatus via the endocytic recycling pathway or late endosomes. The colocalization between gH and Vps4 might occur during one of the sorting steps, as gH is targeted to the TGN. Alternatively, the association of gH and Vps4 might indicate that VZV uses the MVB pathway for assembly and egress of virus particles, as has been suggested following studies of HSV gB colocalization with Vps4 during envelopment and egress (6). The MAb 206-gH complexes were not targeted to the TGN. The lack of these complexes in the TGN may have resulted from the experimental system, but they may also demonstrate that the antibody disrupts gH trafficking, although the complexes did travel through endosomes and the MVB pathway. VZV gH interacts with gE on the plasma membrane and in virions, and it has been suggested that this interaction results in the targeting of gH to the TGN for secondary envelopment via the TGN-targeting motif of gE (31, 43). Binding of MAb 206 to gH particles expressed on the surfaces of infected cells does not disrupt endocytosis of gH, but the resulting MAb-gH complex might not interact with other virion proteins, resulting in the altered trafficking of gH to sites other than the TGN (Fig. 9, step 3). These complexes colocalize with Vps4, similar to gH, but rather than sorting to the TGN, they could be sorted through late endosomes to lysosomes or autophagosomes, which are induced during VZV infection (50).
MAb 206 might also direct gH complexes to be degraded by interacting with Fc receptors on the surfaces of infected cells. Antigen-antibody complexes bound to Fc receptors on human fibroblasts undergo endocytosis and are targeted for degradation, and these fibroblast Fc receptors mainly interact with monomeric immunoglobulins, especially IgG1 (9, 17). Skin keratinocytes have also been shown to express functional Fc receptors that interact with IgG (51). Antibodies against pseudorabies virus gD and gB expressed on monocytes induce antibody-dependent, clathrin-dependent endocytosis of the glycoproteins, mediated by tyrosine-based endocytosis motifs (13, 14, 52). VZV gH contains a functional tyrosine-based endocytosis motif (42). Thus, MAb 206 would not have to disrupt gH protein interactions, but instead could interact with cellular Fc receptors in order to alter trafficking of gH and target it for degradation via the Vps4/MVB pathway and sorting to either lysosomes or autophagosomes (Fig. 9, step 3).
Immunogold EM analysis of MAb 206 localization within treated infected cells demonstrated that MAb 206 bound to gH on virus particles on the cell surface, which resulted in neutralization of these particles and prevention of virus spread from cell to cell (Fig. 9, step 4). The MAb 206-labeled virions were found within cells, suggesting that labeled particles on the surfaces of infected cells were internalized. A MAb 206 interaction with Fc receptors could direct the internalization and degradation not only of gH but also of virions containing gH in their envelope (Fig. 9, step 5).
Interferon (IFN) production is activated in human epidermal cells in VZV-infected skin xenografts and modulates the progression of lesion formation (27). The experiments reported here with anti-gH antibody showed that infection was suppressed during treatment but progressed to complete destruction of the skin tissue when anti-gH antibody was cleared from the circulation. Together, these findings indicate that the combination of the innate IFN response of epidermal cells and passively administered antibody is not sufficient to eliminate VZV when replication has been initiated in skin. This suggests that an effective cell-mediated immune response to VZV is likely to be necessary to resolve primary VZV infection. Herpes zoster as a result of VZV reactivation has been suggested to result in part from a decreased cellular immune response as patients age, even though the humoral immune response remains high (1). The observations presented here suggest that preexisting anti-gH antibodies might reinforce the innate IFN response and slow the progression of cell-cell spread for an interval that allows for clonal expansion of memory VZV-specific T cells that make IFN-γ and stimulate B cells and cytotoxic T lymphocytes to respond to VZV reactivation.
Anti-gH antibody administration to SCIDhu mice with pOka-infected skin xenografts prevented infection or reduced pathogenicity if infection had been established. This mirrors the prophylaxis of immunocompromised patients, who are administered varicella-zoster immunoglobulin (VZIG) as soon as possible after exposure, up to 96 h postexposure to the virus (32). Neutralization assays demonstrated that humanized MAb 206 had a biological activity that was 2,400-fold that of the standard VZIG preparation (10). Administration of VZIG does not consistently prevent varicella following exposure but can ameliorate the severity of the disease, although severe disease and death can still occur. The data presented here demonstrated that administration of anti-gH antibody immediately after inoculation prevented infection, but delaying treatment by 4 days resulted only in suppression of infection, emphasizing the need to give prophylaxis to exposed patients as soon after exposure as possible. This has potential clinical relevance because maintaining the supply of VZIG has been challenging, and an anti-gH MAb might be developed as an alternative for prophylaxis of patients at risk of severe primary VZV infection.
Acknowledgments
This work was supported by training grants from the National Institutes of Health (R01 AI 020459 and P01CA49605). S. Vleck received support from grants T32 GM007279 and T32 AI07328.
We thank Nafisa Ghori, Department for Microbiology & Immunology, Stanford University, for assistance with transmission electron microscopy and Barbara Berarducci for technical help and scientific discussion.
Footnotes
Published ahead of print on 14 October 2009.
REFERENCES
- 1.Arvin, A. M. 2001. Varicella-zoster virus, p. 2731-2768. In D. M. Knipe et al. (ed.), Fields virology. Lippincott-Williams & Wilkins, Philadelphia, PA.
- 2.Babst, M., T. K. Sato, L. M. Banta, and S. D. Emr. 1997. Endosomal transport function in yeast requires a novel AAA-type ATPase, Vps4p. EMBO J. 16:1820-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bos, C. R., S. L. Shank, and M. D. Snider. 1995. Role of clathrin-coated vesicles in glycoprotein transport from the cell surface to the Golgi complex. J. Biol. Chem. 270:665-671. [DOI] [PubMed] [Google Scholar]
- 4.Bosma, G. C., R. P. Custer, and M. J. Bosma. 1983. A severe combined immunodeficiency mutation in the mouse. Nature 301:527-530. [DOI] [PubMed] [Google Scholar]
- 5.Browne, H., V. Baxter, and T. Minson. 1993. Analysis of protective immune responses to the glycoprotein H-glycoprotein L complex of herpes simplex virus type 1. J. Gen. Virol. 74:2813-2817. [DOI] [PubMed] [Google Scholar]
- 6.Calistri, A., P. Sette, C. Salata, E. Cancellotti, C. Forghieri, A. Comin, H. Gottlinger, G. Campadelli-Fiume, G. Palu, and C. Parolin. 2007. Intracellular trafficking and maturation of herpes simplex virus type 1 gB and virus egress require functional biogenesis of multivesicular bodies. J. Virol. 81:11468-11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaudhuri, V., M. Sommer, J. Rajamani, L. Zerboni, and A. M. Arvin. 2008. Functions of varicella-zoster virus ORF23 capsid protein in viral replication and the pathogenesis of skin infection. J. Virol. 82:10231-10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole, N. L., and C. Grose. 2003. Membrane fusion mediated by herpesvirus glycoproteins: the paradigm of varicella-zoster virus. Rev. Med. Virol. 13:207-222. [DOI] [PubMed] [Google Scholar]
- 9.Danilova, T. A., L. M. Bartova, R. L. Panurina, and I. M. Lyampert. 1981. Studies of Fc receptors of heart valve and joint fibroblasts. Clin. Exp. Immunol. 46:575-580. [PMC free article] [PubMed] [Google Scholar]
- 10.Drew, P. D., M. T. Moss, T. J. Pasieka, C. Grose, W. J. Harris, and A. J. Porter. 2001. Multimeric humanized varicella-zoster virus antibody fragments to gH neutralize virus while monomeric fragments do not. J. Gen. Virol. 82:1959-1963. [DOI] [PubMed] [Google Scholar]
- 11.Duus, K. M., and C. Grose. 1996. Multiple regulatory effects of varicella-zoster virus (VZV) gL on trafficking patterns and fusogenic properties of VZV gH. J. Virol. 70:8961-8971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duus, K. M., C. Hatfield, and C. Grose. 1995. Cell surface expression and fusion by the varicella-zoster virus gH:gL glycoprotein complex: analysis by laser scanning confocal microscopy. Virology 210:429-440. [DOI] [PubMed] [Google Scholar]
- 13.Favoreel, H. W., G. Van Minnebruggen, H. J. Nauwynck, L. W. Enquist, and M. B. Pensaert. 2002. A tyrosine-based motif in the cytoplasmic tail of pseudorabies virus glycoprotein B is important for both antibody-induced internalization of viral glycoproteins and efficient cell-to-cell spread. J. Virol. 76:6845-6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ficinska, J., G. Van Minnebruggen, H. J. Nauwynck, K. Bienkowska-Szewczyk, and H. W. Favoreel. 2005. Pseudorabies virus glycoprotein gD contains a functional endocytosis motif that acts in concert with an endocytosis motif in gB to drive internalization of antibody-antigen complexes from the surface of infected monocytes. J. Virol. 79:7248-7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forrester, A., H. Farrell, G. Wilkinson, J. Kaye, N. Davis-Poynter, and T. Minson. 1992. Construction and properties of a mutant of herpes simplex virus type 1 with glycoprotein H coding sequences deleted. J. Virol. 66:341-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forrester, A. J., V. Sullivan, A. Simmons, B. A. Blacklaws, G. L. Smith, A. A. Nash, and A. C. Minson. 1991. Induction of protective immunity with antibody to herpes simplex virus type 1 glycoprotein H (gH) and analysis of the immune response to gH expressed in recombinant vaccinia virus. J. Gen. Virol. 72:369-375. [DOI] [PubMed] [Google Scholar]
- 17.Frey, J., M. Janes, W. Engelhardt, E. G. Afting, C. Geerds, and B. Moller. 1986. Fc gamma-receptor-mediated changes in the plasma membrane potential induce prostaglandin release from human fibroblasts. Eur. J. Biochem. 158:85-89. [DOI] [PubMed] [Google Scholar]
- 18.Fuller, A. O., R. E. Santos, and P. G. Spear. 1989. Neutralizing antibodies specific for glycoprotein H of herpes simplex virus permit viral attachment to cells but prevent penetration. J. Virol. 63:3435-3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galdiero, S., A. Falanga, M. Vitiello, H. Browne, C. Pedone, and M. Galdiero. 2005. Fusogenic domains in herpes simplex virus type 1 glycoprotein H. J. Biol. Chem. 280:28632-28643. [DOI] [PubMed] [Google Scholar]
- 20.Gershon, A. A., D. L. Sherman, Z. Zhu, C. A. Gabel, R. T. Ambron, and M. D. Gershon. 1994. Intracellular transport of newly synthesized varicella-zoster virus: final envelopment in the trans-Golgi network. J. Virol. 68:6372-6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gianni, T., R. Fato, C. Bergamini, G. Lenaz, and G. Campadelli-Fiume. 2006. Hydrophobic α-helices 1 and 2 of herpes simplex virus gH interact with lipids, and their mimetic peptides enhance virus infection and fusion. J. Virol. 80:8190-8198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gianni, T., P. L. Martelli, R. Casadio, and G. Campadelli-Fiume. 2005. The ectodomain of herpes simplex virus glycoprotein H contains a membrane alpha-helix with attributes of an internal fusion peptide, positionally conserved in the Herpesviridae family. J. Virol. 79:2931-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gianni, T., A. Piccoli, C. Bertucci, and G. Campadelli-Fiume. 2006. Heptad repeat 2 in herpes simplex virus 1 gH interacts with heptad repeat 1 and is critical for virus entry and fusion. J. Virol. 80:2216-2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haddad, R. S., and L. M. Hutt-Fletcher. 1989. Depletion of glycoprotein gp85 from virosomes made with Epstein-Barr virus proteins abolishes their ability to fuse with virus receptor-bearing cells. J. Virol. 63:4998-5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ito, H., M. H. Sommer, L. Zerboni, A. Baiker, B. Sato, R. Liang, J. Hay, W. Ruyechan, and A. M. Arvin. 2005. Role of the varicella-zoster virus gene product encoded by open reading frame 35 in viral replication in vitro and in differentiated human skin and T cells in vivo. J. Virol. 79:4819-4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klupp, B. G., R. Nixdorf, and T. C. Mettenleiter. 2000. Pseudorabies virus glycoprotein M inhibits membrane fusion. J. Virol. 74:6760-6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ku, C. C., L. Zerboni, H. Ito, B. S. Graham, M. Wallace, and A. M. Arvin. 2004. Varicella-zoster virus transfer to skin by T cells and modulation of viral replication by epidermal cell interferon-alpha. J. Exp. Med. 200:917-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kutinova, L., P. Hainz, V. Ludvikova, L. Maresova, and S. Nemeckova. 2001. Immune response to vaccinia virus recombinants expressing glycoproteins gE, gB, gH, and gL of varicella-zoster virus. Virology 280:211-220. [DOI] [PubMed] [Google Scholar]
- 29.Maresova, L., L. Kutinova, V. Ludvikova, R. Zak, M. Mares, and S. Nemeckova. 2000. Characterization of interaction of gH and gL glycoproteins of varicella-zoster virus: their processing and trafficking. J. Gen. Virol. 81:1545-1552. [DOI] [PubMed] [Google Scholar]
- 30.Maresova, L., T. J. Pasieka, and C. Grose. 2001. Varicella-zoster virus gB and gE coexpression, but not gB or gE alone, leads to abundant fusion and syncytium formation equivalent to those from gH and gL coexpression. J. Virol. 75:9483-9492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maresova, L., T. J. Pasieka, E. Homan, E. Gerday, and C. Grose. 2005. Incorporation of three endocytosed varicella-zoster virus glycoproteins, gE, gH, and gB, into the virion envelope. J. Virol. 79:997-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marin, M., D. Guris, S. S. Chaves, S. Schmid, and J. F. Seward. 2007. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 56:1-40. [PubMed] [Google Scholar]
- 33.Miller, N., and L. M. Hutt-Fletcher. 1988. A monoclonal antibody to glycoprotein gp85 inhibits fusion but not attachment of Epstein-Barr virus. J. Virol. 62:2366-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moffat, J. F., M. D. Stein, H. Kaneshima, and A. M. Arvin. 1995. Tropism of varicella-zoster virus for human CD4+ and CD8+ T lymphocytes and epidermal cells in SCID-hu mice. J. Virol. 69:5236-5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montalvo, E. A., and C. Grose. 1986. Neutralization epitope of varicella zoster virus on native viral glycoprotein gp118 (VZV glycoprotein gpIII). Virology 149:230-241. [DOI] [PubMed] [Google Scholar]
- 36.Mori, Y., P. Akkapaiboon, S. Yonemoto, M. Koike, M. Takemoto, T. Sadaoka, Y. Sasamoto, S. Konishi, Y. Uchiyama, and K. Yamanishi. 2004. Discovery of a second form of tripartite complex containing gH-gL of human herpesvirus 6 and observations on CD46. J. Virol. 78:4609-4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nemeckova, S., V. Ludvikova, L. Maresova, J. Krystofova, P. Hainz, and L. Kutinova. 1996. Induction of varicella-zoster virus-neutralizing antibodies in mice by co-infection with recombinant vaccinia viruses expressing the gH or gL gene. J. Gen. Virol. 77:211-215. [DOI] [PubMed] [Google Scholar]
- 38.Nicola, A. V., M. Ponce de Leon, R. Xu, W. Hou, J. C. Whitbeck, C. Krummenacher, R. I. Montgomery, P. G. Spear, R. J. Eisenberg, and G. H. Cohen. 1998. Monoclonal antibodies to distinct sites on herpes simplex virus (HSV) glycoprotein D block HSV binding to HVEM. J. Virol. 72:3595-3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niizuma, T., L. Zerboni, M. H. Sommer, H. Ito, S. Hinchliffe, and A. M. Arvin. 2003. Construction of varicella-zoster virus recombinants from parent Oka cosmids and demonstration that ORF65 protein is dispensable for infection of human skin and T cells in the SCID-hu mouse model. J. Virol. 77:6062-6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nonoyama, S., F. O. Smith, I. D. Bernstein, and H. D. Ochs. 1993. Strain-dependent leakiness of mice with severe combined immune deficiency. J. Immunol. 150:3817-3824. [PubMed] [Google Scholar]
- 41.Ober, B. T., B. Teufel, K. H. Wiesmuller, G. Jung, E. Pfaff, A. Saalmuller, and H. J. Rziha. 2000. The porcine humoral immune response against pseudorabies virus specifically targets attachment sites on glycoprotein gC. J. Virol. 74:1752-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pasieka, T. J., L. Maresova, and C. Grose. 2003. A functional YNKI motif in the short cytoplasmic tail of varicella-zoster virus glycoprotein gH mediates clathrin-dependent and antibody-independent endocytosis. J. Virol. 77:4191-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pasieka, T. J., L. Maresova, K. Shiraki, and C. Grose. 2004. Regulation of varicella-zoster virus-induced cell-to-cell fusion by the endocytosis-competent glycoproteins gH and gE. J. Virol. 78:2884-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peng, T., M. Ponce-de-Leon, H. Jiang, G. Dubin, J. M. Lubinski, R. J. Eisenberg, and G. H. Cohen. 1998. The gH-gL complex of herpes simplex virus (HSV) stimulates neutralizing antibody and protects mice against HSV type 1 challenge. J. Virol. 72:65-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reichelt, M., J. Brady, and A. M. Arvin. 2009. The replication cycle of varicella-zoster virus: analysis of the kinetics of viral protein expression, genome synthesis, and virion assembly at the single-cell level. J. Virol. 83:3904-3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodriguez, J. E., T. Moninger, and C. Grose. 1993. Entry and egress of varicella virus blocked by same anti-gH monoclonal antibody. Virology 196:840-844. [DOI] [PubMed] [Google Scholar]
- 47.Rusten, T. E., T. Vaccari, K. Lindmo, L. M. Rodahl, I. P. Nezis, C. Sem-Jacobsen, F. Wendler, J. P. Vincent, A. Brech, D. Bilder, and H. Stenmark. 2007. ESCRTs and Fab1 regulate distinct steps of autophagy. Curr. Biol. 17:1817-1825. [DOI] [PubMed] [Google Scholar]
- 48.Sadaoka, T., K. Yamanishi, and Y. Mori. 2006. Human herpesvirus 7 U47 gene products are glycoproteins expressed in virions and associate with glycoprotein H. J. Gen. Virol. 87:501-508. [DOI] [PubMed] [Google Scholar]
- 49.Subramanian, R. P., and R. J. Geraghty. 2007. Herpes simplex virus type 1 mediates fusion through a hemifusion intermediate by sequential activity of glycoproteins D, H, L, and B. Proc. Natl. Acad. Sci. USA 104:2903-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takahashi, M. N., W. Jackson, D. T. Laird, T. D. Culp, C. Grose, J. I. Haynes II, and L. Benetti. 2009. Varicella-zoster virus infection induces autophagy in both cultured cells and human skin vesicles. J. Virol. 83:5466-5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tigalonowa, M., J. R. Bjerke, and R. Matre. 1991. Fc gamma-receptors on Langerhans' cells and keratinocytes in suspension from normal skin characterized using soluble immune complexes and monoclonal antibodies. Acta Dermatol. Venereol. 71:99-103. [PubMed] [Google Scholar]
- 52.Van de Walle, G. R., H. W. Favoreel, H. J. Nauwynck, P. Van Oostveldt, and M. B. Pensaert. 2001. Involvement of cellular cytoskeleton components in antibody-induced internalization of viral glycoproteins in pseudorabies virus-infected monocytes. Virology 288:129-138. [DOI] [PubMed] [Google Scholar]
- 53.Wang, D., and T. Shenk. 2005. Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism. Proc. Natl. Acad. Sci. USA 102:18153-18158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang, X., W. J. Kenyon, Q. Li, J. Mullberg, and L. M. Hutt-Fletcher. 1998. Epstein-Barr virus uses different complexes of glycoproteins gH and gL to infect B lymphocytes and epithelial cells. J. Virol. 72:5552-5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weigle, K. A., and C. Grose. 1983. Common expression of varicella-zoster viral glycoprotein antigens in vitro and in chickenpox and zoster vesicles. J. Infect. Dis. 148:630-638. [DOI] [PubMed] [Google Scholar]
- 56.Westra, D. F., G. M. Verjans, A. D. Osterhaus, A. van Kooij, G. W. Welling, A. J. Scheffer, T. H. The, and S. Welling-Wester. 2000. Natural infection with herpes simplex virus type 1 (HSV-1) induces humoral and T cell responses to the HSV-1 glycoprotein H:L complex. J. Gen. Virol. 81:2011-2015. [DOI] [PubMed] [Google Scholar]
- 57.Whitbeck, J. C., M. I. Muggeridge, A. H. Rux, W. Hou, C. Krummenacher, H. Lou, A. van Geelen, R. J. Eisenberg, and G. H. Cohen. 1999. The major neutralizing antigenic site on herpes simplex virus glycoprotein D overlaps a receptor-binding domain. J. Virol. 73:9879-9890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zerboni, L., C. C. Ku, C. D. Jones, J. L. Zehnder, and A. M. Arvin. 2005. Varicella-zoster virus infection of human dorsal root ganglia in vivo. Proc. Natl. Acad. Sci. USA 102:6490-6495. [DOI] [PMC free article] [PubMed] [Google Scholar]