Abstract
Novel swine-origin influenza viruses of the H1N1 subtype were first detected in humans in April 2009. As of 12 August 2009, 180,000 cases had been reported globally. Despite the fact that they are of the same antigenic subtype as seasonal influenza viruses circulating in humans since 1977, these viruses continue to spread and have caused the first influenza pandemic since 1968. Here we show that a pandemic H1N1 strain replicates in and transmits among guinea pigs with similar efficiency to that of a seasonal H3N2 influenza virus. This transmission was, however, partially disrupted when guinea pigs had preexisting immunity to recent human isolates of either the H1N1 or H3N2 subtype and was fully blocked through daily intranasal administration of interferon to either inoculated or exposed animals. Our results suggest that partial immunity resulting from prior exposure to conventional human strains may blunt the impact of pandemic H1N1 viruses in the human population. In addition, the use of interferon as an antiviral prophylaxis may be an effective way to limit spread in at-risk populations.
A pandemic of novel swine-origin influenza virus (H1N1) is developing rapidly. As of 12 August 2009, nearly 180,000 cases had been reported to the WHO from around the globe (36). Sustained human-to-human transmission has furthermore been observed in multiple countries, prompting the WHO to declare a public health emergency of international concern and to raise the pandemic alert level to phase 6 (7).
Swine are a natural host of influenza viruses, and although sporadic incidences of human infection with swine influenza viruses occur (8, 9, 14, 29, 35), human-to-human transmission is rare. H1N1 influenza viruses have likely circulated in swine since shortly after the 1918 human influenza pandemic (38). From the 1930s, when a swine influenza virus was first isolated, to the late 1990s, this classical swine lineage has remained relatively stable antigenically (34). In the late 1990s, however, genetic reassortment between a human H3N2 virus, a North American avian virus, and a classical swine influenza virus produced a triple reassortant virus, which subsequently spread among North American swine (34). Further reassortment events involving human influenza viruses led to the emergence in pigs of triple reassortants of the H1N1 and H1N2 subtypes (34). None of these swine viruses have demonstrated the potential for sustained human-to-human transmission.
The swine-origin influenza viruses now emerging in the human population possess a previously uncharacterized constellation of eight genes (28). The NA and M segments derive from a Eurasian swine influenza virus lineage, having entered pigs from the avian reservoir around 1979, while the HA, NP, and NS segments are of the classical swine lineage and the PA, PB1, and PB2 segments derive from the North American triple reassortant swine lineage (13). This unique combination of genetic elements (segments from multiple swine influenza virus lineages, some of them derived from avian and human influenza viruses) may account for the improved fitness of pandemic H1N1 viruses, relative to that of previous swine isolates, in humans.
Several uncertainties remain about how this outbreak will develop over time. Although the novel H1N1 virus has spread over a broad geographical area, the number of people known to be infected remains low in many countries, which could be due, at least in part, to the lack of optimal transmission of influenza viruses outside the winter season; thus, it is unclear at this point whether the new virus will become established in the long term. Two major factors will shape the epidemiology of pandemic H1N1 viruses in the coming months and years: the intrinsic transmissibility of the virus and the degree of protection offered by previous exposure to seasonal human strains. Initial estimates of the reproductive number (R0) have been made based on the epidemiology of the virus to date and suggest that its rate of spread is intermediate between that of seasonal flu and that of previous pandemic strains (3, 11). However, more precise estimates of R0 will depend on better surveillance data in the future. The transmission phenotype of pandemic H1N1 viruses in a ferret model was also recently reported and was found to be similar to (16, 27) or less efficient (25) than that of seasonal H1N1 strains. The reason for this discrepancy in the ferret model is unclear.
Importantly, in considering the human population, the impact of immunity against seasonal strains on the transmission potential of pandemic H1N1 viruses is not clear. According to conventional wisdom, an influenza virus must be of a hemagglutinin (HA) subtype which is novel to the human population in order to cause a pandemic (18, 38). Analysis of human sera collected from individuals with diverse influenza virus exposure histories has indicated that in those born in the early part of the 20th century, neutralizing activity against A/California/04/09 (Cal/04/09) virus is often present (16). Conversely, serological analyses of ferret postinfection sera (13) and human pre- and postvaccination sera (4a) revealed that neutralizing antibodies against recently circulating human H1N1 viruses do not react with pandemic H1N1 isolates. These serological findings may explain the relatively small number of cases seen to date in individuals greater than 65 years of age (6). Even in the absence of neutralizing antibodies, however, a measure of immune protection sufficient to dampen transmission may be present in a host who has recently experienced seasonal influenza (10). If, on the other hand, transmission is high and immunity is low, then pandemic H1N1 strains will likely continue to spread rapidly through the population. In this situation, a range of pharmaceutical interventions will be needed to dampen the public health impact of the pandemic.
Herein we used the guinea pig model (4, 21-24, 26, 30) to assess the transmissibility of the pandemic H1N1 strains Cal/04/09 and A/Netherlands/602/09 (NL/602/09) relative to that of previous human and swine influenza viruses. To better mimic the human situation, we then tested whether the efficiency of transmission is decreased by preexisting immunity to recent human H1N1 or H3N2 influenza viruses. Finally, we assessed the efficacy of intranasal treatment with type I interferon (IFN) in limiting the replication and transmission of pandemic H1N1 viruses.
MATERIALS AND METHODS
Viruses.
Human seasonal influenza H1N1 A/Brisbane/59/2007 (Bris/59/07), H3N2 A/Panama/2007/1999 (Pan/99), and pandemic H1N1 Cal/04/09 viruses were obtained from the Centers for Disease Control and Prevention. Influenza H3N2 A/swine/Texas/1998 virus was kindly provided by Richard Webby (St. Jude Children's Hospital, Memphis, TN). Pandemic H1N1 NL/602/09 virus was kindly provided by Ron Fouchier (Erasmus Medical Centre, The Netherlands). Cal/04/09 and NL/602/09 viruses were propagated in MDCK cell culture to avoid the introduction of adaptive changes in HA, while the remaining strains were amplified in 10- to 11-day-old embryonated hens' eggs. The use of differing substrates for the production of virus stocks could result in differences in glycosylation patterns or adaptive changes in viral genes (37). In past experiments, Pan/99 virus stocks grown in eggs or in MDCK cells have been found to exhibit phenotypes which are indistinguishable in the guinea pig model (data not shown).
Animals.
All animal experiments were performed in accordance with the guidelines of the Mount Sinai School of Medicine Institutional Animal Care and Use Committee. Female Hartley strain guinea pigs weighing 300 to 350 g were obtained from Charles River Laboratories (Wilmington, MA). Guinea pigs were anesthetized through intramuscular injection of a ketamine-xylazine mixture (30 mg/kg of body weight and 2 mg/kg of body weight, respectively) prior to all procedures (inoculation, nasal wash collection, collection of blood, and treatment with IFN). During guinea pig transmission experiments, measures were followed to prevent aberrant cross-contamination between cages: exposed animals were handled before inoculated animals, and gloves were disinfected between cages.
Contact transmission.
Four guinea pigs were inoculated intranasally with 104 PFU Cal/04/09 virus in 300 μl phosphate-buffered saline (PBS). At 24 h postinoculation, each infected animal was placed in the same cage with one naïve guinea pig. Nasal washes were collected from all eight guinea pigs on days 2, 4, 6, and 8 postinfection, as previously described (22). Since we have found that environmental conditions have little impact on the efficiency of contact transmission (24), this experiment was performed under ambient conditions, with two animals housed in each enclosed cage.
Aerosol transmission.
Four guinea pigs were inoculated intranasally with 104 PFU of the indicated influenza virus in 300 μl PBS. Exposure of naïve guinea pigs to inoculated guinea pigs was initiated 24 h after infection and continued for 7 days. Nasal washings were collected from animals on days 2, 4, 6, and 8 postinoculation. Aerosol transmission experiments were performed within a Caron environmental test chamber (model 6030) set to 20°C and 20% relative humidity. Cages placed within the chamber were open at the top and at one side, such that air exchange occurred among all eight cages; thus, direct pairings of infected and exposed animals were not made. Herein we define the term aerosol to mean small and large respiratory droplets (that is, with diameters spanning the range of <1 μm to 20 μm). Thus, our experiments do not allow differentiation between exhaled particles with short settling times and those that remain airborne for an extended period.
Transmission following preexposure to a heterologous influenza virus.
For initial exposure, eight guinea pigs were inoculated intranasally with 104 PFU of Bris/59/07 virus (H1N1) or Pan/99 virus (H3N2), as indicated. These animals were subjected to nasal washing on days 2, 4, and 6 postinfection in order to confirm that productive infection had occurred. Challenge with Cal/04/09 virus was carried out at 3 weeks postinoculation by one of two routes, as follows: four previously exposed animals were inoculated intranasally with 104 PFU of virus, while four were simply placed in the same cage with an acutely infected guinea pig. In addition, each intranasally challenged animal was placed in the same cage with one naïve guinea pig at 24 h postinoculation. Thus, two contact transmission experiments were set up with the influenza-experienced guinea pigs, i.e., one in which preexposed animals were the donors in transmission and one in which the preexposed animals were the recipients in transmission. As a control and in parallel with these two experiments, a third experiment was set up using naïve guinea pigs for donors and naïve guinea pigs for recipients.
Transmission of Cal/04/09 virus in the context of IFN treatment.
Recombinant human IFN-αB/D protein (previously described in references 12, 15, and 33) was used for treatment of guinea pigs. The protein was stored lyophilized at 4°C and reconstituted in PBS immediately prior to use. A dose of IFN corresponding to 500,000 U/kg, previously shown to effectively induce an antiviral state among treated animals (32), was given intranasally in a volume of 300 μl, with 150 μl instilled into each nostril. Animals that were not treated with IFN received PBS alone, administered in the same manner. One day prior to infection (day −1), four guinea pigs were treated with IFN and four were treated with PBS. The same eight guinea pigs were then inoculated on day 0 with 104 PFU of Cal/04/09 virus; the inoculum was in a total volume of 300 μl and contained either IFN or PBS alone, as appropriate. Also on day 0, the treatment of 16 guinea pigs due to be exposed to the inoculated animals began: 8 received IFN and 8 received PBS. On day 1 postinfection, exposure of naïve animals to infected animals was initiated by placing one IFN-treated and one PBS-treated guinea pig into the same cage with each infected guinea pig. Nasal washes were collected from all animals on days 2, 4, 6, and 8 postinfection. Treatment with IFN or PBS continued, with daily doses given on days 1, 2, 3, 4, 5, and 6 postinfection, after nasal washing was complete, if applicable. This experiment was performed under ambient conditions, with three animals housed in each enclosed cage.
RESULTS
Transmissibility of pandemic H1N1 influenza viruses in the guinea pig model.
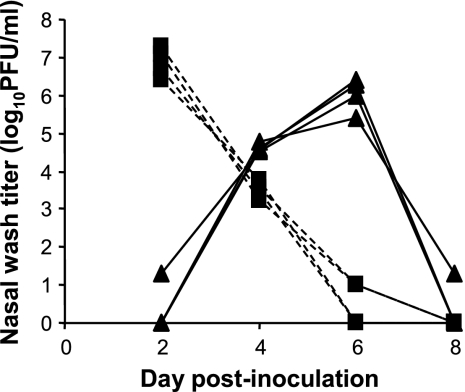
To assess transmission under circumstances where direct and indirect contacts, as well as short-range aerosol transmission, were possible, we exposed naïve guinea pigs to Cal/04/09 virus-infected animals in the same cage. Thus, 24 h after intranasal inoculation, four infected guinea pigs were each placed in the same cage with four naïve guinea pigs. By collecting nasal lavage samples from all animals on alternating days postinoculation, we were able to follow the kinetics of viral growth in the inoculated guinea pigs and the rate of transmission to exposed cagemates. As shown in Fig. 1, the swine-origin virus grew to high titers in all inoculated guinea pigs (peak of approximately 1 × 107 PFU/ml on day 2 postinfection). In addition, Cal/04/09 virus was transmitted efficiently to exposed animals, with all four becoming positive at 3 days postexposure (corresponding to 4 days postinoculation).
FIG. 1.
Efficient transmission of Cal/04/09 between guinea pigs by a contact route. Four guinea pigs were inoculated intranasally with Cal/04/09 (H1N1) virus. At 24 h postinfection, a naïve guinea pig was placed in the same cage with each inoculated guinea pig. On days 2, 4, 6, and 8 postinfection, nasal washings were collected from each guinea pig and the viral load therein quantified by plaque assay. Dashed lines with squares represent nasal wash titers of inoculated animals; solid lines with triangles represent nasal wash titers of exposed animals.
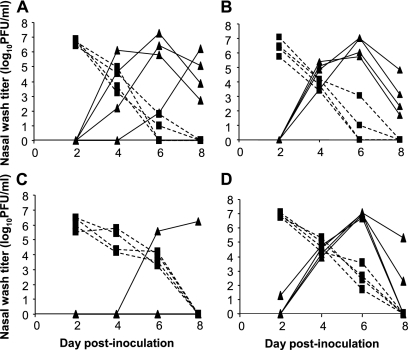
Next, we tested the propensity of Cal/04/09 virus to be transmitted via an aerosol route. Again, four naïve guinea pigs were each exposed to an infected animal at 24 h postinoculation; in this case, however, exposure was achieved by placing the individually caged animals in close proximity (21). Each cage was open to airflow, and cages were placed in such a way that no contact was possible between guinea pigs. Aerosol experiments were performed within an environmentally controlled chamber at 20°C and a relative humidity of 20%. Transmission of Cal/04/09 virus under these conditions also occurred with 100% efficiency: all four exposed animals yielded positive nasal lavage samples by day 5 postexposure (Fig. 2A). To test for strain-specific differences in swine-origin influenza virus transmission, we then evaluated the phenotype of the NL/602/09 isolate under the same conditions. As shown in Fig. 2B, very similar results were obtained: NL/602/09 virus was transmitted to all four exposed guinea pigs, with each yielding positive nasal wash samples by day 3 postexposure. In order to establish a reference for comparison, we also tested the aerosol transmission of a virus isolated from North American swine in 1998 (A/swine/Texas/1998; H3N2) and a recent human isolate of the H3N2 subtype (Pan/99). In contrast to the pandemic H1N1 strains, influenza A/swine/Texas/1998 virus was detected in the nasal washings of only one of four exposed guinea pigs (Fig. 2C). As shown in Fig. 2D and previous publications (21, 22, 30), Pan/99 virus was transmitted with high efficiency, infecting all four exposed guinea pigs. Thus, our results suggest that Cal/04/09 virus possesses a transmission phenotype more similar to that of a seasonal human strain than that of the H3N2 subtype, triple reassortant swine influenza virus tested.
FIG. 2.
Transmission by aerosol route of Cal/04/09, NL/602/09, Pan/99, and A/swine/Texas/1998 influenza viruses. Four guinea pigs were inoculated intranasally with 104 PFU of Cal/04/09 (A), NL/602/09 (B), A/swine/Texas/1998 (C), or Pan/99 (D) virus and isolated for 24 h. At 24 h postinfection, a naïve guinea pig was placed in a cage adjacent to each inoculated guinea pig. Viral loads in nasal washings collected at days 2, 4, 6, and 8 postinfection are presented. Dashed lines with squares represent nasal wash titers of inoculated animals; solid lines with triangles represent nasal wash titers of exposed animals. Experiments shown in panels A and D were performed in parallel; experiments shown in panels B and C were performed separately.
Although the pandemic H1N1 strains were shed at similar peak titers to that of the seasonal Pan/99 virus, we noted that the Cal/04/09 and NL/602/09 strains consistently were cleared more quickly from the guinea pig nasal passages than was Pan/99 virus. Thus, in Cal/04/09- and NL/602/09-infected animals, very low (<100 PFU/ml) virus levels were detected on day 6 postinfection, whereas Pan/99 virus titers usually remained at about 1,000 PFU/ml at this time point (compare Fig. 1 and 2A and B with Fig. 2D and references 21, 22, and 30). This difference in shedding kinetics did not, however, correlate with relative transmission efficiencies.
Impact of preexisting immunity to seasonal H1N1 and H3N2 viruses on pandemic H1N1 virus transmission.
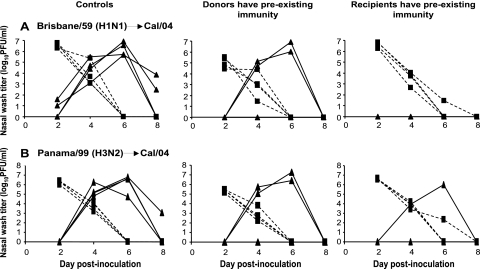
To evaluate the possibility that prior exposure to conventional human H1N1 influenza viruses will decrease the transmissibility of pandemic H1N1 strains, we performed a contact transmission experiment in which either the inoculated or exposed guinea pigs had been preexposed to influenza Bris/59/07 (H1N1) virus 3 weeks previously. Initial exposure comprised intranasal inoculation with 104 PFU of Bris/59/07 virus. Nasal washes were collected to confirm that productive infection with Bris/59/07 virus occurred: peak titers were observed on day 2 postinfection and averaged 1 × 107 PFU/ml (data not shown). Challenge with Cal/04/09 virus was performed in one of three ways. In the first group (Fig. 3A, left column), only guinea pigs with no prior exposure were used as a baseline control. In a like manner to the results shown in Fig. 1, 100% transmission was observed in this group. In the second group (Fig. 3A, middle column), four Bris/59/07-experienced guinea pigs were inoculated intranasally with 104 PFU of Cal/04/09 virus. At 24 h postinoculation, each of these animals was then placed in the same cage with one naïve guinea pig in order to monitor for transmission. Nasal wash titers of the inoculated animals in this group were found to be approximately 40-fold lower at the peak of shedding (on day 2 postinfection) than those of the corresponding naïve control animals. Thus, natural infection with Bris/59/07 virus provides a measure of protection against challenge with Cal/04/09 virus. Furthermore, a reduction in transmission to naïve animals was observed: just two of four contacts contracted infection when the inoculated donor guinea pigs had preexisting immunity. In the third group (Fig. 3A, right column), four Bris/59/07-experienced guinea pigs were challenged through exposure in the same cage to guinea pigs acutely infected with Cal/04/09 virus. Here, complete protection against transmission was seen, with none of the Bris/59/07-experienced animals becoming infected.
FIG. 3.
Previous infection with recent seasonal isolates reduces viral load and limits transmission of Cal/04/09 virus. (A) Transmission of Cal/04/09 virus is reduced from and to guinea pigs with previous exposure to Bris/59/07 (H1N1) virus. (B) Transmission of Cal/04/09 virus is reduced from and to guinea pigs with previous exposure to Pan/99 (H3N2) virus. (Left) Transmission in the absence of preexisting immunity. In each case, naïve animals were productively infected through intranasal inoculation (black squares with dashed lines) and transmitted virus efficiently to contact animals (black triangles with solid lines). (Middle) Transmission from donor guinea pigs with preexisting immunity to a heterologous strain. Four guinea pigs with previous exposure were inoculated intranasally with Cal/04/09 virus. At 24 h postinfection, a naïve guinea pig was placed in the same cage with each inoculated guinea pig. In each case, previously exposed animals were productively infected through inoculation (black squares with dashed lines) but shed lower titers than did previously naïve animals. Transmission to naïve contacts is represented by black triangles with solid lines. (Right) Transmission to recipients possessing preexisting immunity to a heterologous virus. Four naïve guinea pigs were inoculated with Cal/04/09 virus. At 24 h postinfection, a guinea pig previously exposed to the indicated heterologous strain was placed in the same cage with each inoculated guinea pig. In each case, naïve animals were productively infected through inoculation (black squares with dashed lines). Transmission to previously infected contact animals is represented by black triangles with solid lines.
To determine whether the observed protection against Cal/04/09 virus transmission was subtype specific, we then set up a second experiment in which Pan/99 virus (H3N2) was used for the initial exposure, rather than Bris/59/07. As indicated in Fig. 3B, the extent of protection offered by previous Pan/99 infection was very similar to that seen with previous Bris/59/07 infection: full transmission was seen in the naïve control group, while 50% and 25% transmission efficiencies were observed in the groups where donor and recipient guinea pigs, respectively, had preexisting immunity. Thus, the reductions in shedding titers and transmission resulting from prior influenza virus infection were not specific to the H1N1 subtype.
Disruption of viral growth and transmission through intranasal IFN treatment.
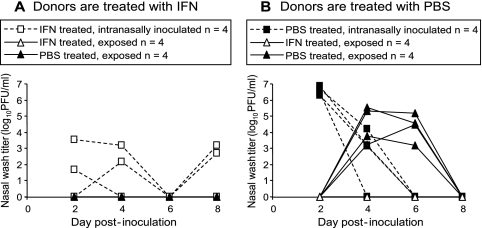
To test the efficacy of locally administered type I IFN in blocking the transmission of Cal/04/09 virus, an experiment was set up in which inoculated donor guinea pigs and/or exposed animals were treated prophylactically. This experiment was performed using a contact transmission setup with three animals in each cage: one was infected intranasally with Cal/04/09 virus and two were exposed. The animals were divided into two groups. In the first group (Fig. 4A), the intranasally infected donor guinea pigs were treated with IFN. This treatment began 1 day prior to inoculation and continued daily until 5 days postinfection (for a total of seven doses). In the second group (Fig. 4B), intranasally infected animals were treated with vehicle (PBS) alone. In both groups, one exposed animal in each cage received IFN treatment, and the second exposed animal in each cage received PBS. Treatment of exposed animals started 1 day prior to exposure and continued until day 5 postexposure (for a total of seven doses). As shown in Fig. 4A, treatment with IFN strongly suppressed viral growth in inoculated guinea pigs. This reduction in shedding also led to the protection of exposed animals in the same cage: neither those guinea pigs treated with IFN nor those treated with PBS contracted infection. The results shown in Fig. 4B indicate that IFN treatment was also sufficient to block transmission from PBS-treated animals to IFN-treated contacts. Control, PBS-treated animals exposed to inoculated guinea pigs receiving PBS treatment did, however, become infected (Fig. 4B). Thus, the results of these experiments show that, at least throughout the period during which animals were monitored, intranasal application of type I IFN effectively limited viral growth in infected animals and prevented transmission both from and to IFN-treated guinea pigs.
FIG. 4.
IFN treatment blocks transmission of Cal/04/09 virus among guinea pigs. (A) Lack of transmission of Cal/04/09 virus from IFN-treated donor guinea pigs. Four guinea pigs treated intranasally with IFN on days −1 to 5 postinfection were infected with 104 PFU of Cal/04/09 virus (open squares with dashed lines). Eight naïve guinea pigs, treated with either IFN (open triangles with solid lines) or PBS (filled triangles with solid lines), were exposed by being placed in the same cage with one infected animal. Transmission was not observed to either IFN- or PBS-treated guinea pigs. (B) Lack of transmission of Cal/04/09 virus to IFN-treated recipient guinea pigs. Four guinea pigs treated intranasally with PBS alone on days −1 to 5 postinfection were inoculated with 104 PFU of Cal/04/09 virus (filled squares with dashed lines). Eight naïve guinea pigs, treated with either IFN (open triangles with solid lines) or PBS (filled triangles with solid lines), were exposed by being placed in the same cage with one infected animal. Transmission was observed to all four PBS-treated animals but to none of the IFN-treated guinea pigs.
DISCUSSION
Herein we have used the guinea pig model to evaluate the transmissibility of pandemic H1N1 influenza viruses. This work extends our previous characterization of the guinea pig model for the study of seasonal and H5N1 influenza viruses (22, 23, 30). We favor the use of the guinea pig as a model host due to its high susceptibility to and ability to transmit low-passage human isolates (in contrast to mice), and based on several practical considerations such as size and cost, which make them considerably more convenient for research purposes than ferrets.
Our data indicate that pandemic H1N1 viruses possess a similar transmission phenotype to that of seasonal strains well adapted to the human host. These findings are in agreement with and therefore reinforce results obtained with the ferret model (16, 27). Furthermore, the transmission efficiency of the pandemic H1N1 strains tested was much higher than that of an H3N2 subtype swine influenza virus isolate of the triple reassortant lineage. Although already apparent from the extensive spread of pandemic H1N1 influenza viruses in humans, this finding highlights the fact that the pandemic H1N1 viruses tested are fundamentally different from a previous swine influenza virus and opens the way for the identification of the viral factors which support efficient human-to-human transmission. Our data from the guinea pig model also suggest that partial immunity against pandemic H1N1 viruses, which will slow their spread, may exist in the human population. The protection against Cal/04/09 virus transmission conferred by previous exposure to a seasonal influenza virus was found to be similar whether the initial infection was with an H1N1 or an H3N2 subtype virus. This result suggests that the basis for protection was not a neutralizing antibody response targeted against the viral surface glycoproteins. Consistent with this, no hemagglutination inhibition activity against Cal/04/09 virus was detected in the prechallenge sera of Bris/59/07- or Pan/99-experienced guinea pigs (data not shown). Due to the relatively short interval between infections (3 weeks), it is possible that elements of a nonspecific, innate response to the primary infection remained active at the time of challenge and could account for the protection seen. Our own unpublished work, in which we performed a very similar experiment using the pairing of A/duck/Ukraine/1963 (H3N8) and Pan/99 viruses, suggests that perhaps this is not the case. When guinea pigs were initially exposed to A/duck/Ukraine/1963 virus and then challenged 3 weeks later with Pan/99 virus, very little protection was seen (75% and 100% transmission efficiencies were observed when the donor and recipient animals, respectively, had preexisting immunity [data not shown]). The differing outcomes could be explained by differences in the innate responses induced by A/duck/Ukraine/1963 virus compared to those resulting from infection of guinea pigs with a seasonal human strain. A/duck/Ukraine/1963 virus grew to a peak titer of about 105 PFU/ml in the inoculated guinea pigs, that is, 100-fold lower than that of Bris/59/07 or Pan/99 virus. Thus, it is possible that some nonspecific effect of Bris/59/07 or Pan/99 virus infection was still active at 3 weeks postinfection and accounts for the protection seen in Fig. 3. Alternatively, the protection seen herein following serial infection may be due to a component of the adaptive response, perhaps mediated by T cells or antibodies recognizing epitopes that are shared among Cal/04/09 and both seasonal influenza virus lineages. These epitopes would most likely be lacking from A/duck/Ukraine/1963 virus, in which all eight gene segments are derived directly from the Eurasian avian influenza virus lineage. Serological assays designed to detect nonneutralizing antibodies were not performed; thus, the role that such antibodies may have played in the protection against reinfection remains unclear.
Precedents for heterosubtypic immunity following influenza virus infection have been reported, both for humans who experienced the 1957 pandemic (10) and for animal models (19, 31). It is nevertheless important that the level of protection against pandemic H1N1 viruses seen in humans with prior exposure to seasonal strains may not be as high as that seen in experimentally infected guinea pigs, since (i) as discussed above, the interval between infections will most likely have an impact; (ii) the profiles of antibody and T-cell specificities produced in a guinea pig may differ from those in a human, especially when one considers humans with a complex exposure history to influenza viruses; and (iii) although we saw partial protection following natural infection with Bris/59/07 and Pan/99 viruses, vaccination may not produce the same effect.
Similar to previous studies assessing the impact of IFN treatment of guinea pigs on the replication of influenza A/Viet Nam/1203/04 (H5N1) virus and the 1918 pandemic strain (33), we have found that daily IFN treatment effectively reduces the growth of Cal/04/09 virus, by at least 1,000-fold. We furthermore showed that this treatment regimen completely blocks transmission both to and from treated guinea pigs, at least up to day 8 postinfection. The high efficacy observed, especially with regard to viral spread, could make intranasal type I IFN an attractive treatment option in the context of the current influenza pandemic. IFN is already in use for the treatment of hepatitis C viral infections (17) and has been shown to be effective against influenza in other animal models (2, 20, 32). Currently circulating pandemic H1N1 viruses are resistant to the adamantanes, leaving oseltamivir and zanamivir as the only FDA-approved antiviral drugs effective against these viruses (5). Should supplies of these drugs run short or, even more likely, should resistance to them become prevalent in the pandemic virus, alternative treatment approaches will be in high demand to protect those at increased risk of developing complications following influenza virus infection.
Acknowledgments
We thank the Centers for Disease Control and Prevention for providing the Cal/04/09, Bris/59/07, and Pan/99 isolates and Ron Fouchier and Richard Webby for providing the NL/602/09 and A/swine/Texas/1998 viruses, respectively. We are grateful to Rafael Medina and Balaji Manicassamy for producing the stock of Cal/04/09 used in this work. We also thank Heinz Hochkeppel (Novartis) for providing recombinant human IFN-αB/D.
This research was supported by the Center for Research on Influenza Pathogenesis (NIAID contract HHSN266200700010C) and the W. M. Keck Foundation (to P.P. and A.G.-S.). A.C.L. is a Parker B. Francis Fellow in Pulmonary Research. S.M. was supported by Sunnybrook Health Sciences Centre, Toronto, Canada, and by a Ruth L. Kirschstein Physician Scientist Research Training in Pathogenesis of Viral Diseases Award (ST32A1007623-07). P.S. was on sabbatical leave from the University of Freiburg, Germany.
Footnotes
Published ahead of print on 14 October 2009.
REFERENCES
- 1.Reference deleted.
- 2.Beilharz, M. W., J. M. Cummins, and A. L. Bennett. 2007. Protection from lethal influenza virus challenge by oral type 1 interferon. Biochem. Biophys. Res. Commun. 355:740-744. [DOI] [PubMed] [Google Scholar]
- 3.Boelle, P. Y., P. Bernillon, and J. C. Desenclos. 2009. A preliminary estimation of the reproduction ratio for new influenza A(H1N1) from the outbreak in Mexico, March-April 2009. Euro. Surveill 14:19205. [DOI] [PubMed] [Google Scholar]
- 4.Bouvier, N. M., A. C. Lowen, and P. Palese. 2008. Oseltamivir-resistant influenza A viruses are transmitted efficiently among guinea pigs by direct contact but not by aerosol. J. Virol. 82:10052-10058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4a.CDC. 2009. Serum cross-reactive antibody response to a novel influenza A (H1N1) virus after vaccination with seasonal influenza vaccine. MMWR Morb. Mortal. Wkly. Rep. 58:521-524. [PubMed] [Google Scholar]
- 5.CDC. 2009. Interim guidance on antiviral recommendations for patients with novel influenza A (H1N1) virus infection and their close contacts. CDC, Atlanta, GA. http://www.cdc.gov/h1n1flu/recommendations.htm.
- 6.CDC. 2009. Novel H1N1 flu: facts and figures. CDC, Atlanta, GA. http://www.cdc.gov/h1n1flu/surveillanceqa.htm.
- 7.Chan, M. 2009. World now at the start of 2009 influenza pandemic. World Health Organization, Geneva, Switzerland. http://www.who.int/mediacentre/news/statements/2009/h1n1_pandemic_phase6_20090611/en/index.html.
- 8.Dacso, C. C., R. B. Couch, H. R. Six, J. F. Young, J. M. Quarles, and J. A. Kasel. 1984. Sporadic occurrence of zoonotic swine influenza virus infections. J. Clin. Microbiol. 20:833-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Jong, J. C., J. M. de Ronde-Verloop, P. J. Bangma, E. van Kregten, J. Kerckhaert, M. F. Paccaud, F. Wicki, and W. Wunderli. 1986. Isolation of swine-influenza-like A(H1N1) viruses from man in Europe, 1986. Lancet ii:1329-1330. [DOI] [PubMed] [Google Scholar]
- 10.Epstein, S. L. 2006. Prior H1N1 influenza infection and susceptibility of Cleveland Family Study participants during the H2N2 pandemic of 1957: an experiment of nature. J. Infect. Dis. 193:49-53. doi: 10.1086/498980. [DOI] [PubMed] [Google Scholar]
- 11.Fraser, C., C. A. Donnelly, S. Cauchemez, W. P. Hanage, M. D. Van Kerkhove, T. D. Hollingsworth, J. Griffin, R. F. Baggaley, H. E. Jenkins, E. J. Lyons, T. Jombart, W. R. Hinsley, N. C. Grassly, F. Balloux, A. C. Ghani, N. M. Ferguson, A. Rambaut, O. G. Pybus, H. Lopez-Gatell, C. M. Apluche-Aranda, I. B. Chapela, E. P. Zavala, D. M. Guevara, F. Checchi, E. Garcia, S. Hugonnet, and C. Roth. 2009. Pandemic potential of a strain of influenza A (H1N1): early findings. Science 324:1557-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gangemi, J. D., J. Lazdins, F. M. Dietrich, A. Matter, B. Poncioni, and H. K. Hochkeppel. 1989. Antiviral activity of a novel recombinant human interferon-alpha B/D hybrid. J. Interferon Res. 9:227-237. [DOI] [PubMed] [Google Scholar]
- 13.Garten, R. J., C. T. Davis, C. A. Russell, B. Shu, S. Lindstrom, A. Balish, W. M. Sessions, X. Xu, E. Skepner, V. Deyde, M. Okomo-Adhiambo, L. Gubareva, J. Barnes, C. B. Smith, S. L. Emery, M. J. Hillman, P. Rivailler, J. Smagala, M. de Graaf, D. F. Burke, R. A. Fouchier, C. Pappas, C. M. Alpuche-Aranda, H. Lopez-Gatell, H. Olivera, I. Lopez, C. A. Myers, D. Faix, P. J. Blair, C. Yu, K. M. Keene, P. D. Dotson, Jr., D. Boxrud, A. R. Sambol, S. H. Abid, K. St. George, T. Bannerman, A. L. Moore, D. J. Stringer, P. Blevins, G. J. Demmler-Harrison, M. Ginsberg, P. Kriner, S. Waterman, S. Smole, H. F. Guevara, E. A. Belongia, P. A. Clark, S. T. Beatrice, R. Donis, J. Katz, L. Finelli, C. B. Bridges, M. Shaw, D. B. Jernigan, T. M. Uyeki, D. J. Smith, A. I. Klimov, and N. J. Cox. 2009. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science 325:197-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gregory, V., M. Bennett, Y. Thomas, L. Kaiser, W. Wunderli, H. Matter, A. Hay, and Y. P. Lin. 2003. Human infection by a swine influenza A (H1N1) virus in Switzerland. Arch. Virol. 148:793-802. [DOI] [PubMed] [Google Scholar]
- 15.Horisberger, M. A., and K. de Staritzky. 1987. A recombinant human interferon-alpha B/D hybrid with a broad host-range. J. Gen. Virol. 68:945-948. [DOI] [PubMed] [Google Scholar]
- 16.Itoh, Y., K. Shinya, M. Kiso, T. Watanabe, Y. Sakoda, M. Hatta, Y. Muramoto, D. Tamura, Y. Sakai-Tagawa, T. Noda, S. Sakabe, M. Imai, Y. Hatta, S. Watanabe, C. Li, S. Yamada, K. Fujii, S. Murakami, H. Imai, S. Kakugawa, M. Ito, R. Takano, K. Iwatsuki-Horimoto, M. Shimojima, T. Horimoto, H. Goto, K. Takahashi, A. Makino, H. Ishigaki, M. Nakayama, M. Okamatsu, K. Takahashi, D. Warshauer, P. A. Shult, R. Saito, H. Suzuki, Y. Furuta, M. Yamashita, K. Mitamura, K. Nakano, M. Nakamura, R. Brockman-Schneider, H. Mitamura, M. Yamazaki, N. Sugaya, M. Suresh, M. Ozawa, G. Neumann, J. Gern, H. Kida, K. Ogasawara, and Y. Kawaoka. 2009. In vitro and in vivo characterization of new swine-origin H1N1 influenza viruses. Nature 460:1021-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keam, S. J., and R. S. Cvetkovic. 2008. Peginterferon-alpha-2a (40 kD) plus ribavirin: a review of its use in the management of chronic hepatitis C mono-infection. Drugs 68:1273-1317. [DOI] [PubMed] [Google Scholar]
- 18.Kilbourne, E. D. 2006. Influenza pandemics of the 20th century. Emerg. Infect. Dis. 12:9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kreijtz, J. H., R. Bodewes, G. van Amerongen, T. Kuiken, R. A. Fouchier, A. D. Osterhaus, and G. F. Rimmelzwaan. 2007. Primary influenza A virus infection induces cross-protective immunity against a lethal infection with a heterosubtypic virus strain in mice. Vaccine 25:612-620. [DOI] [PubMed] [Google Scholar]
- 20.Kugel, D., G. Kochs, K. Obojes, J. Roth, G. P. Kobinger, D. Kobasa, O. Haller, P. Staeheli, and V. von Messling. 2009. Intranasal administration of alpha interferon reduces seasonal influenza A virus morbidity in ferrets. J. Virol. 83:3843-3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lowen, A. C., S. Mubareka, J. Steel, and P. Palese. 2007. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathog. 3:1470-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lowen, A. C., S. Mubareka, T. M. Tumpey, A. García-Sastre, and P. Palese. 2006. The guinea pig as a transmission model for human influenza viruses. Proc. Natl. Acad. Sci. USA 103:9988-9992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowen, A. C., J. Steel, S. Mubareka, E. Carnero, A. Garcia-Sastre, and P. Palese. 2009. Blocking interhost transmission of influenza virus by vaccination in the guinea pig model. J. Virol. 83:2803-2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lowen, A. C., J. Steel, S. Mubareka, and P. Palese. 2008. High temperature (30°C) blocks aerosol but not contact transmission of influenza virus. J. Virol. 82:5650-5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maines, T. R., A. Jayaraman, J. A. Belser, D. A. Wadford, C. Pappas, H. Zeng, K. M. Gustin, M. B. Pearce, K. Viswanathan, Z. H. Shriver, R. Raman, N. J. Cox, R. Sasisekharan, J. M. Katz, and T. M. Tumpey. 2009. Transmission and pathogenesis of swine-origin 2009 A(H1N1) influenza viruses in ferrets and mice. Science 325:484-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mubareka, S., A. C. Lowen, J. Steel, A. L. Coates, A. Garcia-Sastre, and P. Palese. 2009. Transmission of influenza virus via aerosols and fomites in the guinea pig model. J. Infect. Dis. 199:858-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munster, V. J., E. de Wit, J. M. van den Brand, S. Herfst, E. J. Schrauwen, T. M. Bestebroer, D. van de Vijver, C. A. Boucher, M. Koopmans, G. F. Rimmelzwaan, T. Kuiken, A. D. Osterhaus, and R. A. Fouchier. 2009. Pathogenesis and transmission of swine-origin 2009 A(H1N1) influenza virus in ferrets. Science 325:481-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palese, P., and M. L. Shaw. 2006. Orthomyxoviridae: the viruses and their replication, p. 1647-1690. In D. M. Knipe et al. (ed.), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 29.Rota, P. A., E. P. Rocha, M. W. Harmon, V. S. Hinshaw, M. G. Sheerar, Y. Kawaoka, N. J. Cox, and T. F. Smith. 1989. Laboratory characterization of a swine influenza virus isolated from a fatal case of human influenza. J. Clin. Microbiol. 27:1413-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steel, J., A. C. Lowen, S. Mubareka, and P. Palese. 2009. Transmission of influenza virus in a mammalian host is increased by PB2 amino acids 627K or 627E/701N. PLoS Pathog. 5:e1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Straight, T., M. Ottolini, G. Prince, and M. Eichelberger. 2008. Antibody contributes to heterosubtypic protection against influenza A-induced tachypnea in cotton rats. Virol. J. 5:44. doi: 10.1186/1743-422X-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tumpey, T. M., K. J. Szretter, N. Van Hoeven, J. M. Katz, G. Kochs, O. Haller, A. Garcia-Sastre, and P. Staeheli. 2007. The Mx1 gene protects mice against the pandemic 1918 and highly lethal human H5N1 influenza viruses. J. Virol. 81:10818-10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Hoeven, N., J. A. Belser, K. J. Szretter, H. Zeng, P. Staeheli, D. E. Swayne, J. M. Katz, and T. M. Tumpey. 2009. Pathogenesis of the 1918 pandemic and H5N1 influenza virus infection in a guinea pig model: the antiviral potential of exogenous alpha-interferon to reduce virus shedding. J. Virol. 83:2851-2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vincent, A. L., W. Ma, K. M. Lager, B. H. Janke, and J. A. Richt. 2008. Swine influenza viruses: a North American perspective. Adv. Virus Res. 72:127-154. [DOI] [PubMed] [Google Scholar]
- 35.Wells, D. L., D. J. Hopfensperger, N. H. Arden, M. W. Harmon, J. P. Davis, M. A. Tipple, and L. B. Schonberger. 1991. Swine influenza virus infections. Transmission from ill pigs to humans at a Wisconsin agricultural fair and subsequent probable person-to-person transmission. JAMA 265:478-481. [DOI] [PubMed] [Google Scholar]
- 36.WHO. 2009. Influenza A(H1N1)—update 61. WHO, Geneva, Switzerland. http://www.who.int/csr/don/2009_08_12/en/index.html.
- 37.Widjaja, L., N. Ilyushina, R. G. Webster, and R. J. Webby. 2006. Molecular changes associated with adaptation of human influenza A virus in embryonated chicken eggs. Virology 350:137-145. [DOI] [PubMed] [Google Scholar]
- 38.Wright, P. F., G. Neumann, and Y. Kawaoka. 2006. Orthomyxoviruses, p. 1691-1740. In D. M. Knipe et al. (ed.), Fields virology, 5th ed., vol. 1. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]