Abstract
A recent clinical trial of a T-cell-based AIDS vaccine delivered with recombinant adenovirus type 5 (rAd5) vectors showed no efficacy in lowering viral load and was associated with increased risk of human immunodeficiency virus type 1 (HIV-1) infection. Preexisting immunity to Ad5 in humans could therefore affect both immunogenicity and vaccine efficacy. We hypothesized that vaccine-induced immunity is differentially affected, depending on whether subjects were exposed to Ad5 by natural infection or by vaccination. Serum samples from vaccine trial subjects receiving a DNA/rAd5 AIDS vaccine with or without prior immunity to Ad5 were examined for the specificity of their Ad5 neutralizing antibodies and their effect on HIV-1 immune responses. Here, we report that rAd5 neutralizing antibodies were directed to different components of the virion, depending on whether they were elicited by natural infection or vaccination in HIV vaccine trial subjects. Neutralizing antibodies elicited by natural infection were directed largely to the Ad5 fiber, while exposure to rAd5 through vaccination elicited antibodies primarily to capsid proteins other than fiber. Notably, preexisting immunity to Ad5 fiber from natural infection significantly reduced the CD4 and CD8 cell responses to HIV Gag after DNA/rAd5 vaccination. The specificity of Ad5 neutralizing antibodies therefore differs depending on the route of exposure, and natural Ad5 infection compromises Ad5 vaccine-induced immunity to weak immunogens, such as HIV-1 Gag. These results have implications for future AIDS vaccine trials and the design of next-generation gene-based vaccine vectors.
Recombinant adenovirus (rAd)-based vectors are currently under investigation in a variety of gene therapy and T-cell-based vaccine clinical trials. There are more than 370 such ongoing clinical trials for broad applications, including infectious diseases and cancer therapy (http://www.wiley.co.uk/genetherapy/clinical/). Based on supportive data from nonhuman primate studies, rAd-based vectors have been developed and tested in human clinical trials to deliver human immunodeficiency virus (HIV-1) gene products that stimulate HIV-specific immune responses. Preexisting immunity to Ad serotype 5 (Ad5), from which most vectors are derived, is common in humans. Though neutralizing antibodies to Ad5 may reduce the immunogenicity of Ad5-based vectors in animal models (16), their effect on immunity in subjects with previous Ad5 infection is poorly understood. In the STEP trial, which tested a Merck rAd5 vaccine encoding HIV-1 Gag, Pol, and Nef, vaccination failed to show protection, either by lowering viral load or by decreasing acquisition of infection (3, 9, 12, 21). Furthermore, the possibility was raised that subjects with preexisting neutralizing antibodies from natural Ad5 infection may have carried an increased risk of HIV infection after vaccination. Thus, understanding the nature and immune effects of Ad5 seropositivity in humans is important to the development of vaccines against AIDS and other diseases.
Ad5 is a common cause of respiratory disease and an occasional cause of gastroenteritis in humans, and exposure before adolescence is common in human populations (19). Such exposure stimulates both innate and adaptive immune responses that generate neutralizing antibodies and virus-specific T-cell responses (6). These antibodies can also synergize with each other to achieve maximum viral neutralization (7, 22). The capsid protein specificity of Ad5 neutralizing antibodies has been reported for humans following administration of rAd5 gene therapy vectors for advanced liver or lung cancer (7, 10). However, results were presented solely for antibodies induced by administration of rAd5. One report has assessed Ad5 neutralizing antibodies with a healthy human population that was Ad5 seropositive from natural exposure to the virus (18). The median titer of the population was presented, but the frequency of protein-specific neutralizing antibody has not been defined for humans.
Here we describe the first report of the natural frequency and effect on immunization of neutralizing antibodies specific for different Ad capsid proteins in human subjects. We address the fundamental mechanisms of how humans generate neutralizing antibodies to a common cold virus that is in widespread use as a vector for gene therapy and vaccines. Such mechanisms may also be applicable to other nonenveloped viruses, including adeno-associated viruses and other viruses containing multiple envelope surface proteins, like influenza. To analyze the contribution of anti-capsid antibodies to neutralization by different human serum samples, wild-type and chimeric vectors were utilized. For example, a rAd type 5 (rAd5) vector with a fiber derived from Ad35 fiber (rAd5 F35) can be used to analyze the anti-Ad5 capsid response independent of fiber. Conversely, a rAd35 vector with a fiber transposed from Ad5 can determine the specificity of neutralization mediated by the Ad5 fiber. Using these vectors, we have analyzed human serum samples from two HIV vaccine clinical trials, VRC 006 and HVTN 204, in which a single-dose rAd5 vaccine alone and a three-dose DNA prime/single dose rAd5 boost vaccine encoding HIV-1 Env A,B, and C; Gag; and Pol, respectively, were administered. Thus, we sought to characterize the specificity of rAd5 neutralizing antibodies in Ad5-immune subjects and to determine their effect on immune responses elicited by vaccination.
MATERIALS AND METHODS
Ad vector construction, production, and purification.
Replication-deficient rAd5 F35 and rAd35 F5 were generated in 293-ORF6 cells essentially as described previously (2, 8, 11). The region encoding the Ad35 shaft and knob (amino acids 45 to 323) was cloned into an Ad5 shuttle plasmid, pASE3(10)F35. The region encoding the Ad5 shaft and knob (amino acids 46 to 581) was cloned into the Ad35 shuttle plasmid pUC19.Ad35.F(5S.5K)_E4. The region of the fiber protein that interacts with the viral capsid, the tail, was therefore homologous to the capsid; this feature ensured that there was minimal perturbation of the virion structure. The plasmids with the fiber-modified genes were recombined in Rec+ Escherichia coli with Ad5 or Ad35 vector-genome plasmids encoding an expression cassette for luciferase. The fiber-modified genome plasmid clones were isolated through repeated bacterial transformation-colony formation. Fiber-modified viral vectors were generated by transfection of 293-ORF6 cells, amplified, and purified as described previously (11). Replication-deficient rAd5 and rAd35 were generated and produced using similar methods. The quality of the rAd stocks was verified by growth kinetics, comparative analysis of transgene expression, particle/infectious unit ratios, PCR analysis of the genomes, and DNA sequencing of the modified regions.
Clinical trials.
VRC006 is a Vaccine Research Center-sponsored phase I trial described previously (4). A total of 36 healthy volunteers were divided into three groups that were injected with 109, 1010, or 1011 viral particles of Ad5-based multivalent HIV vaccine vectors encoding HIV Envs from clades A, B, and C and Gag and Pol from HIV clade B. Each group also included two persons injected with placebo.
HVTN204 is a phase II trial to test a multivalent HIV DNA vaccine prime and multivalent HIV Ad5 vector boost in human volunteers. The vaccine vectors encode six HIV antigens, including three HIV Envs (Env clade A, Env clade B, and Env clade C) and Gag, Nef, and Pol from HIV clade B. The volunteers were given either four injections of placebo or three dosages of DNA vaccine at month 0, 1, and 2 and then given an Ad5 vector boost at month 6 (week 24).
Neutralization of adenovectors with human serum samples.
The method for analyzing the neutralization of adenovectors with human serum samples was developed based on procedures published previously (17). A total of 127 samples of prevaccinated human serum samples (week 0) from volunteers enrolled in the TRIAD (HVTN204) HIV vaccine trial and 32 available prevaccination (week 0) and postvaccination (week 4) serum samples from the VRC006 HIV vaccine trial were obtained from the VRC immunology core laboratory. The serum samples were inactivated by heating at 56°C for 60 min, and the inactivated serum samples were diluted with 10% fetal bovine serum containing RPMI medium and mixed with the indicated rAd vector encoding luciferase for 30 min at room temperature for neutralization. To prepare A549 cell suspensions for transduction, A549 cells grown in a 75-cm2 flask were harvested by treatment with 4 mM EDTA and suspended in 10% fetal bovine serum containing RPMI medium. The neutralized virus was used to transfect A549 cells at 100 PU/cell, and luciferase expression was analyzed using the luciferase assay kit (Promega, Inc.) at 24 h posttransduction. Serum samples that at the lowest dilution (1:36 due to limited serum availability as noted) reduced viral transduction activity by more than 90% were defined as seropositive (17). The neutralization titer for such seropositive serum samples was also determined and defined as the maximum dilution that can reduce the viral transduction by 90% as calculated using GraphPad Prism 5 software.
Analysis of T-cell responses to vaccination.
At 28 weeks after the first vaccination (4 weeks post-rAd vaccination), the T-cell responses to individual HIV antigen peptide pools were analyzed according to previously published methods (4) by an intracellular cytokine staining (ICS) assay. The percentages of CD4+ and CD8+ T cells producing cytokines were reported with background correction.
rAd5 HIV vaccine responders were identified by comparing ICS data with antigen peptide stimulation and data without antigen peptide stimulation. A composite definition was used to identify T-cell responses based on both statistical criteria and the magnitude of the response. First, the proportion of positive cells in response to the antigen must be statistically significantly different than the proportion of positive cells in the background-stimulated sample at an α of 0.01 by a one-sided Fisher exact test. In addition, the background-subtracted magnitude must be above a predefined antigen-specific threshold. The cutoff frequency for a positive response was set at 0.045% for CD4+ response to all HIV antigens, 0.070% for CD8+ response to Env-C, 0.058% for CD8+ response to Gag, and 0.045% for CD8+ response to all other HIV antigens. Only if both of these criteria are met is a sample considered to have a positive response to that antigen. This definition is consistent with that used by the Vaccine Research Center, NIH, in the analysis of other clinical trial data.
Statistical analysis.
Serum samples were categorized as seropositive or seronegative for each of the four recombinant vectors, with a neutralization titer of more than 36 categorized as positive. The subjects were also categorized into rAd5 HIV vaccine responders or nonresponders as described above. Fisher's exact (two-sided) tests were used to calculate P values between subjects seronegative or seropositive for antibodies to Ad5 and vaccine responder or nonresponder.
The magnitudes of CD4+ or CD8+ T-cell immune responses were compared between seropositive and seronegative subjects using two-sided Wilcoxon rank sum tests. These nonparametric tests were chosen due to the presence of large influential points for some of the groups and to the moderate sample sizes for some of the groups. As multiple comparisons were carried out at the same time, which could lead to false significance, a Bonferroni adjustment was used to define a threshold for statistical significance of 0.001; P values above this threshold but below 0.05 are noted in the text as marginally significant or suggestive of a trend.
RESULTS
Analysis of human sera for neutralization directed to different Ad5 proteins.
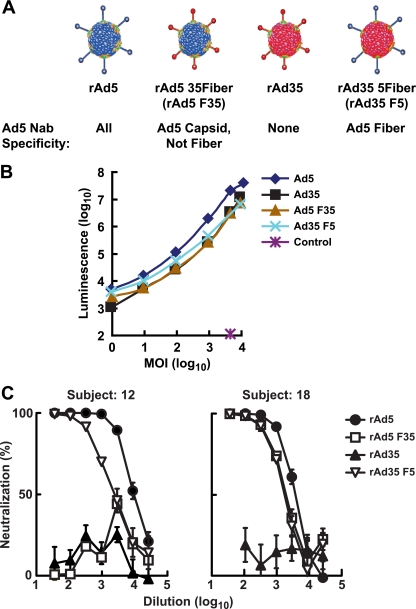
Both Ad5 and Ad35 contain three major surface proteins: fiber, hexon and penton base. These external proteins play an important role in viral infection and are the major targets of neutralizing antibodies in human sera. We adapted a previously described assay (17) to differentiate between neutralization through fiber antibodies and through antibodies against other capsid proteins. In the modified protocol, EDTA rather than trypsin was used to detach A549 cells grown as a monolayer in a flask in order to avoid degradation of cell surface receptors for Ad. Since the primary mechanism by which Ad5 infects human cells is through direct interaction between fiber and its primary receptor, coxsackie B, and Ad receptor (CAR), we focused on the analysis of fiber-related and non-fiber-related neutralization. The panel of rAd vectors included two chimeric vectors and the parental, unmodified vectors (Fig. 1A) that showed similar transduction frequencies on the A549 target cells (Fig. 1B). The rare serotype Ad35 was used as the source for non-Ad5 capsid proteins, since serum samples from North American populations rarely neutralize Ad35. The Ad5 fiber was replaced with Ad35 fiber to generate rAd5 F35, and this vector was used to detect neutralization of Ad5 independent of fiber. In contrast, the complementary chimera, rAd35 F5, rAd35 typed with Ad5 fiber, allowed the detection of Ad5 fiber-directed neutralization. The parental viruses, rAd5 and rAd35, facilitated comparison of the potencies of Ad5 immunity among subjects and excluded the possibility of cross-reactive antibodies that might confound interpretation of neutralization of the chimeras. Different patterns of neutralization were observed. Serum from a representative VRC 006 trial subject neutralized rAd35 F5 at high titers but inhibited rAd5 F35 at low levels, suggesting Ad5 fiber was the main neutralization target (Fig. 1C, left). In contrast, serum from another subject neutralized both chimeras at high titers, consistent with the presence of neutralizing antibodies directed to both fiber and other capsid proteins (Fig. 1C, right).
FIG. 1.
Recombinant and chimeric Ads determine the specificity of neutralizing antibodies in selected subjects. (A) Wild-type and chimeric vectors based on Ad5 and Ad35 were used to analyze the neutralizing antibody activities against different viral capsid proteins. Fiber and other major capsid proteins (including hexon and penton) in wild-type Ad5 contain neutralizing epitopes targeted by human antibodies. rAd5 F35 is an Ad5-based chimeric vector that contains the fiber of Ad35 in place of Ad5 fiber. It contains all the Ad5 capsid proteins except fiber and was used to analyze specific neutralizing antibodies to Ad5 capsid proteins excluding fiber. rAd35 F5 contains only one Ad5-specific neutralizing target, the fiber. It was used to analyze specific neutralizing antibodies to Ad5 fiber. (B) Comparable transduction of A549 cells by different chimeric rAd vectors. A549 cells were transduced with rAd vectors encoding luciferase (Ad5, Ad35, Ad5 F35, and Ad35 F5) or green fluorescent protein (Control) at indicated multiples of infection viral particles (MOI). Luminescence was measured at 24 h after transduction. (C) Two examples of human serum samples from the VRC006 trial that can neutralize Ad vectors. Human serum samples at week 0 (prior to vaccination) were diluted as indicated, and their ability to neutralize adenoviral vectors was tested. Neutralization assays were performed in triplicate, and the average with standard error is shown. Serum VRC006-12 could neutralize more than 90% of the infectivity of rAd5 and rAd35 F5 but not of rAd35 and rAd5 F35, showing primarily Ad5 fiber-specific neutralization. Serum VRC006-18 could neutralize more than 90% of the infectivity of rAd5, rAd5 F35, and rAd35 F5 but not of rAd35, showing that Ad5 fiber and other capsid proteins were all targeted for neutralization.
Analysis of Ad5 neutralization in serum samples from the VRC 006 trial subjects.
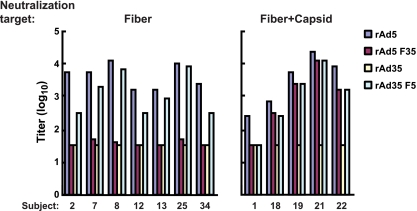
Serum samples from 12 out of the 32 participants in VRC 006 were Ad5 seropositive at a titer of >1:36 prior to vaccination, presumably due to prior natural exposure to Ad5 virus. All but one serum sample from the 12 seropositive subjects inhibited transduction of the rAd35 F5 reporter, indicating the high prevalence of Ad5 fiber-related neutralizing antibodies in these 12 subjects. Of these 12 serum samples, 7 serum samples contained only Ad5 fiber-specific neutralizing activity (58%) and 4 serum samples (33%) contained neutralizing antibodies to both fiber and other capsid proteins (Fig. 2, left and right, respectively). These seven serum samples failed to block Ad35 entry, confirming their specificity for Ad5 (Fig. 2, left). This preliminary analysis revealed two characteristic profiles of response, one in which neutralizing activity is focused mainly on Ad5 fiber and a second in which recognition is directed to both fiber and other capsid proteins. In the latter case, neutralization could also require the concurrent presence of Ad5 fiber and other Ad5 capsid proteins (Fig. 2, subject 1), suggesting either recognition of a more complex conformational epitope or the synergistic effect of low-level fiber and capsid specificities.
FIG. 2.
Ad neutralizing titers in prevaccination serum samples from volunteers involved in the HIV vaccine trial VRC006 at week 0. Serum samples from 32 volunteers were analyzed for seropositivity to Ad5, and 12 serum samples showed titers of greater than 36. These 12 serum samples were used to neutralize Ad vectors, and their neutralizing titers to each vector were determined. Due to the limited amount of serum available, the minimum titer was set at 1:36 and the maximum was 1:26,244. The serum samples can be grouped by their neutralizing activities into two major groups: one group with neutralizing activity solely directed to Ad5 fiber (Fiber) and another group with neutralizing activity to fiber plus other Ad5 capsid proteins (Fiber+Capsid). In only serum sample number 1, neutralization required the concurrent presence of Ad5 fiber and other Ad5 capsid proteins.
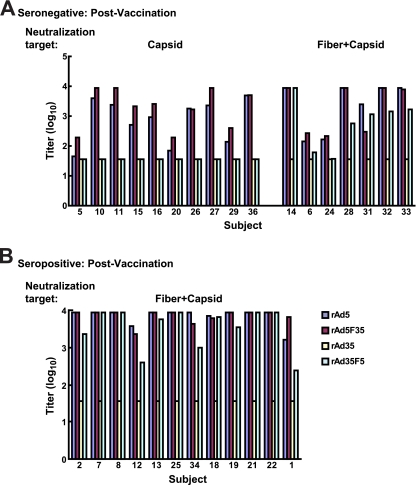
To characterize the nature of antibodies induced by vaccination with rAd vectors in Ad5-naïve individuals, neutralization profiles of these subjects were analyzed after a single vaccination with the Ad5 vaccine. By 4 weeks after vaccination, 17 of the 20 Ad5-naïve subjects developed Ad5 neutralizing antibodies at titers of ≥1:36, while 3 subjects fell below this threshold (Fig. 3A). All serum samples neutralized rAd5 F35, indicating that capsid proteins other than fiber represented the target of neutralization. Indeed, serum samples from 10 subjects did not inhibit entry of the rAd35 F5 reporter, demonstrating an absence of Ad5 fiber neutralizing activity in more than half of the group. Serum samples that contained Ad5 fiber neutralizing antibodies also had neutralizing antibodies to other capsid proteins. Thus, Ad5-naïve subjects immunized with Ad vaccines generated neutralizing antibodies to capsid proteins other than fiber with the highest frequency.
FIG. 3.
Adenoviral neutralizing antibody titers in serum samples from volunteers involved in the HIV vaccine trial VRC006 at 4 weeks postvaccination. The postvaccination neutralizing titers are shown for naïve or seronegative subjects (A) and subjects with preexisting seropositivity (B) (shown in the same order as shown in Fig. 2). For volunteers with preexisting seropositivity, all serum samples showed neutralizing antibodies to both fiber and other capsid proteins, as they could neutralize rAd5, rAd5 F35, and rAd35 F5 but not rAd35 vectors. In naïve volunteers, two neutralization patterns were seen following vaccination. One group could neutralize rAd5 and rAd5 F35 but not rAd35 or rAd35 F5, showing capsid (non-fiber-mediated) neutralization; another group could neutralize rAd5, rAd5 F35, and rAd35 F5 but not rAd35, showing neutralization directed to both fiber and other capsid proteins. Capsid denotes Ad5 capsid proteins other than fiber; Fiber+Capsid indicates Ad5 fiber and other capsid proteins. The minimum titer was set at 1:36, and the maximum was 1:8,748.
Vaccination of individuals who were Ad5 seropositive elicited antibodies that neutralized rAd5, rAd5 F35, and rAd35 F5 in all 12 subjects, thus showing high titer to both Ad5 fiber and other capsid proteins (Fig. 3B). Because anti-fiber antibodies were present in Ad5-seropositive subjects before vaccination, immunization with rAd5 vectors increased anti-fiber titers and expanded reactivity to other capsid proteins (Fig. 4, upper versus lower right). Similarly, all seronegative subjects who generated anti-Ad5 antibodies following vaccination generated neutralizing antibodies to capsid proteins other than fiber, while a subset also produced anti-fiber antibodies (Fig. 4, upper versus lower left).
FIG. 4.
Distribution of adenoviral neutralization targets detectable in serum samples from volunteers in the VRC006 trial. Data shown in Fig. 2 and 3 are summarized based on the percentage of serum samples containing antibodies directed to specific viral capsid proteins. In the naïve volunteers, vaccination generated neutralization targeted mainly to capsid proteins other than fiber alone. In the seropositive volunteers, neutralization directed to the fiber was detected broadly before vaccination, whereas the neutralization extended to fiber plus other capsid proteins after vaccination. Fiber and capsid are defined as described in the legend for Fig. 3.
The impact of neutralization activity on rAd vaccine immunogenicity.
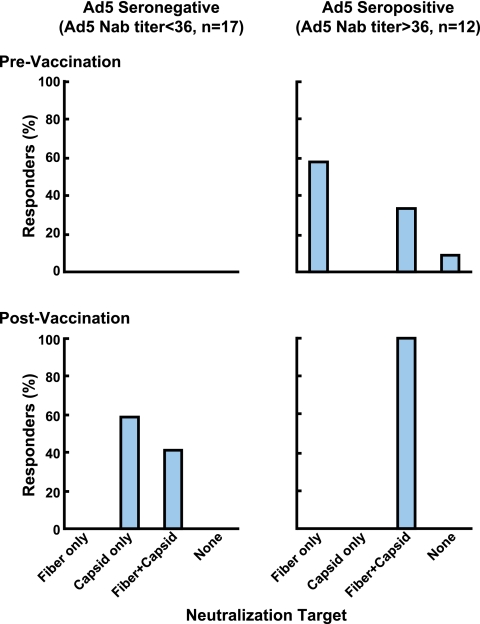
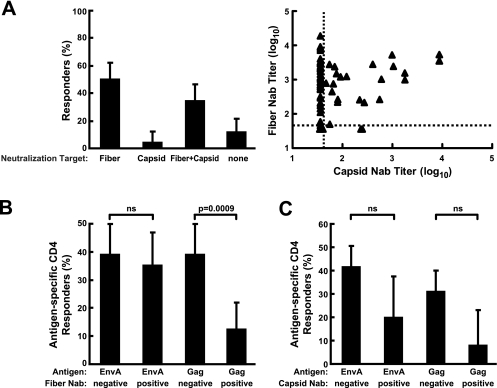
To determine the impact of preexisting Ad5 neutralizing activity on vaccine-induced immune responses, we examined a larger number of subjects from trial HVTN 204, a phase II study of the DNA/rAd Env A, B, and C; Gag; Pol; and Nef vectors conducted with 180 individuals in North America. Prior to vaccination, Ad5 seropositivity in trial participants was 55% (70 out of 127 samples analyzed). Fifty percent of the seropositive subjects had neutralizing antibodies specific for Ad5 fiber (Fig. 5A, left, Fiber). Antibodies to both fiber and other capsid proteins were detected in 34% of subjects (Fig. 5A, left, Fiber+Capsid). Thus, similar to Ad5-seropositive VRC 006 volunteers, nearly every subject generated neutralizing antibodies to fiber, and neutralization targeted solely to nonfiber capsid proteins was rare, ∼4% (Fig. 5A, left, Capsid). Serum samples from 11% of seropositive volunteers did not neutralize either chimera, thus requiring the concurrent presence of capsid and fiber proteins for inhibition (Fig. 5A, left, none versus Capsid and/or Fiber), similar to one subject from VRC 006. Overall, the neutralization patterns were consistent with the pattern of reactivity seen prior to vaccination in trial VRC 006 (Fig. 4 and 5A). The Nab titers to Ad5 capsid and fiber for each individual subject are also shown (Fig. 5A, right).
FIG. 5.
Distribution of adenoviral neutralization targets detected in serum samples from volunteers in the HVTN204 trial at week 0 and its association with the vaccine response rate. (A) Seventy serum samples from 127 volunteers showed preexisting neutralizing antibodies to Ad5 (left). In the 70 positive serum samples, the percentage of sera that contained neutralizing antibodies directed to specific viral capsid proteins is shown with a bar representing the 90% confidence interval for the true percentage of responders in each category. Fiber and capsid are defined as described in the legend for Fig. 3. The distribution of preexisting anti-capsid (Ad5 F35) and anti-fiber (Ad35 F5) neutralizing antibody (Nab) titers for the 121 volunteers who participated in HVTN204 clinical trials are also indicated (right). The cutoff titer (shown as a dashed line) for Nab negatives was set at 36 for both anti-capsid (n = 96) and anti-fiber (n = 64) Nab. (B and C) The responders to the rAd5 HIV vaccine among 121 volunteers for whom valid immune response data are available were categorized according to Ad35 F5 seropositive or seronegative (positive or negative Ad5 fiber neutralizing antibodies, respectively; panel B) and Ad5 F35 seropositive or seronegative (positive or negative Ad5 capsid neutralizing antibodies, respectively; panel C). The percentage of responders with positive CD4+ T-cell response to EnvA or Gag is shown, with a bar representing the 90% confidence interval for the true percentage of responders in each category. ns, not significant, with P > 0.001.
To determine the effect of these neutralizing antibodies on vaccine-induced T-cell immunity, we analyzed ICS data that were available for 121 subjects at week 28, 4 weeks after the rAd HIV vaccine boost. Groups were stratified according to the presence and pattern of rAd5 neutralizing antibodies. HIV antigen-specific responses were first analyzed by magnitude in seropositive and seronegative groups. The median CD4+ and CD8+ Gag responses of Ad5 seronegative subjects (n = 54) were significantly higher than those of Ad5 seropositive subjects (n = 67; P < 0.001 and P < 0.01, respectively) (Table 1). In contrast, the median responses to Env, Pol, and Nef in Ad5 seronegative or seropositive vaccinees remained similar (Table 1).
TABLE 1.
Effect of Ad5 seropositivity on HIV vaccine vector-induced responsesa
| Specific T cell | Median response (% of cells positive [P value compared to Ad5 seronegative]) |
|||
|---|---|---|---|---|
| Ad5 seronegative (n = 54) | Ad5 seropositive (n = 67) | Ad5 fiber-only seropositive (n = 35) | Ad5 fiber + capsid seropositive (n = 22) | |
| Gag CD4 | 0.041 | 0.013 (0.0009) | 0.009 (0.004) | 0.013 (0.002) |
| Gag CD8 | 0.021 | 0.007 (0.007) | 0.007 (0.04) | 0.003 (0.02) |
| EnvA CD4 | 0.033 | 0.021 (0.1) | 0.025 (0.3) | 0.018 (0.2) |
| EnvA CD8 | 0.030 | 0.015 (0.07) | 0.021 (0.3) | 0.013 (0.06) |
| EnvB CD4 | 0.030 | 0.016 (0.08) | 0.024 (0.3) | 0.014 (0.09) |
| EnvB CD8 | 0.013 | 0.007 (0.2) | 0.009 (0.4) | 0.005 (0.2) |
| EnvC CD4 | 0.036 | 0.024 (0.1) | 0.025 (0.4) | 0.024 (0.3) |
| EnvC CD8 | 0.020 | 0.016 (0.2) | 0.016 (0.4) | 0.017 (0.4) |
| Nef CD4 | 0.003 | 0.001 (0.5) | 0.001 (0.8) | 0.001 (0.2) |
| Nef CD8 | 0.000 | 0.004 (0.053) | 0.005 (0.057) | 0.002 (0.6) |
| Pol CD4 | 0.019 | 0.013 (0.3) | 0.013 (0.4) | 0.013 (0.4) |
| Pol CD8 | 0.016 | 0.013 (0.6) | 0.014 (0.9) | 0.010 (0.3) |
The magnitude of CD4+ and CD8+ T-cell responses to the indicated HIV antigens in different serostatus groups.
To analyze the effect of neutralizing antibodies directed to different proteins of Ad5, the Ad5-seropositive group was split into four subsets: Ad5 fiber only (fiber seropositive but capsid seronegative, n = 35), Ad5 fiber and capsid seropositive (n = 22), Ad5 capsid only (n = 3), and neutralizing target undefined (n = 7). The Ad5-capsid-only and the neutralizing-target-undefined subsets were too small for subset analysis. Ad5-fiber-only seropositivity (n = 35) correlated with reduced CD4+ and CD8+ responses to Gag compared to Ad5 seronegativity (CD4+ P = 0.004; CD8+ P = 0.04), and the responses did not differ from those of the Ad5-seropositive group (Table 1; median CD4+ response 0.013% versus 0.009%; median CD8+ response 0.007% versus 0.007%). Similarly, Ad5 fiber-plus-capsid seropositivity (n = 22) correlated with reduced CD4+ and CD8+ responses compared to Ad5 seronegativity (Table 1; CD4+ P = 0.002; CD8+ P = 0.02); and the responses did not differ from those of the Ad5-seropositive group. Additionally, there was no difference in Gag-specific responses between the Ad5 fiber only and fiber-plus-capsid subsets (P > 0.05). Thus, the decline in Gag-specific T-cell response correlated significantly with the presence of anti-fiber neutralizing antibodies, and the presence of anti-capsid antibodies did not further reduce responses.
We then determined whether Ad5 fiber seropositivity affected the response rate to Gag. Vaccine positive responders were defined as showing significant responses after rAd5 vaccination by analysis of ICS data. In subjects with natural neutralizing antibodies to Ad5 fiber, 12% (7 out of 57 rAd35 F5-seropositive subjects) showed positive CD4+ T-cell responses to Gag (Fig. 5B). In individuals without Ad5 fiber antibodies, 39% (25 out of 64 Ad35 F5-seronegative subjects) had a positive CD4 T-cell response to Gag that was significantly higher than the 12% response rate among seropositive subjects (Fig. 5B, Gag, negative versus positive; P < 0.001). On the other hand, seropositivity to Ad5 fiber did not affect the response to other antigens, such as EnvA (Fig. 5B, EnvA, negative versus positive; P > 0.05), for which the magnitude of the response was also not affected. In individuals with anti-capsid neutralizing antibodies, the frequency of positive CD4 responses to HIV Gag also trended lower compared to those of capsid seronegative individuals, but it did not reach the threshold P value of 0.001 required for statistical significance because of the multiple group comparisons (Fig. 5C; P = 0.02). For example, 42% and 31% of the capsid seronegatives responded with positive CD4 responses to EnvA and Gag, respectively, compared to 20% and 8% in seropositives. Similar, but weaker, nonstatistically significant trends were observed for other HIV antigens (data not shown). We also analyzed the correlation between the titers of preexisting neutralizing antibodies and the magnitude of responses generated by the vaccines. There were weak inverse relationships of antibody titer to T-cell responses, but these correlations were not maintained when relationships were restricted to only the people with titers above the neutralization cutoff value. This analysis is consistent with the data shown in the tables demonstrating that the dominant effect was seropositivity and not the magnitude of the neutralization titer. Thus, the data and analyses consistently demonstrated that only seropositivity to Ad5 fiber was significantly associated with a lower HIV Gag-specific T-cell response compared to seronegativity.
DISCUSSION
The specificity and immune effects of the rAd5 neutralizing antibodies of subjects receiving DNA/rAd vectors have been analyzed in this study. We found that the molecular target of neutralization differs depending on whether Ad5 immunity is generated from natural infection or from vaccination with replication-defective viral vectors. Antibodies generated by natural infection are directed primarily to fiber components, while vector exposure elicits responses primarily to capsid proteins other than fiber. Injection of vector into Ad5 seropositive individuals elicits antibodies to both fiber and capsid in nearly all subjects. The presence of antibodies to fiber in naturally infected individuals reduces vaccine-induced immunity to HIV-1 Gag, whereas the response to Env is not substantially affected, although the vaccines generated detectable immune responses to both Gag and Env in Ad5 seronegatives. This observation may be caused by differential trafficking and processing of an aggregated protein in contrast to a transmembrane glycoprotein that could affect antigen presentation. It is also possible that Ad5 immune effects are underestimated in this study because of the DNA immunization.
As shown in this study and documented elsewhere, Ad5 neutralizing antibodies are prevalent in humans. The seroprevalence seems to vary among populations from different continents, and up to 85% of sera can be seropositive for Ad5 (13). The only study prior to the one reported here that had assessed the contribution of individual capsid-specific antibodies to viral neutralization in humans from natural infection with Ad did not describe the frequency of samples positive for specific capsid proteins (18). Instead, a population median titer was presented, which is an assessment of avidity, not frequency. In addition, there was no statistical significance of the apparent titer differences presented, and supporting data were only from immunization of mice with rAd5. Taking the findings together, it is unclear whether the median titers across the populations of human samples could be extrapolated to determine immunodominant targets for neutralization. Thus, based on the frequency assessment in the study presented here, neutralizing antibodies to the fiber protein are more common than antibodies to hexon. However, the frequency of hexon antibodies was still significant and must be taken into account when designing the next generation of rAd vaccine vectors.
Chimeric rAd5-based vectors have been demonstrated to overcome vector neutralization (5, 18). Our results support the use of chimeric Ads with non-Ad5 fibers in combination with other non-Ad5 capsid proteins. Modification of capsid while retaining an Ad5 fiber alone, as is done with mutant hypervariable region Ad5 vectors, is unlikely to fully overcome preexisting neutralizing antibodies from natural Ad infection. The results also emphasize the contribution of anti-fiber antibodies to neutralization generated by natural viral exposure, consistent with previous studies showing the importance of anti-fiber antibody and its synergy with anti-penton antibody for neutralization (7, 22). These antibodies are likely targeted to the fiber knob (5). Since the modification of the hexon protein has been shown to affect the distribution of cellular transduction in vivo (20), it will be important to assess the anti-Ad and anti-transgene immune responses to capsid-modified rAd vaccine vectors.
Vaccination of naïve volunteers with rAd5 rarely generated neutralizing antibodies targeted solely to fiber. This result may reflect differences in the exposure to fiber and hexon in natural infection compared to vaccination. During natural infection, new fiber molecules are transcribed from viral genes in excess of the amount of fiber incorporated into virions, and they may be released from infected cells that undergo cytolysis. The significant late protein synthesis, particularly of fiber, from infected cells not only increases the fiber antigen content, but also exerts biological effects on intercellular adhesion or signaling through cellular receptors that will result in a very different set of immune and inflammatory responses than in E1,E4-deleted vectors. In contrast, vaccination with the replication-incompetent rAd5 vector would result in exposure to smaller amounts of fiber, as the viral proteins introduced in vivo would be restricted to preformed virus, the incoming replication-incompetent virions of the E1,E4 replication defective vector. An Ad virion is composed of 36 fiber protein monomers and 720 hexon protein monomers (15), and vaccine recipients would be expected to respond better to the more abundant hexon protein. Viral replication in vivo may also affect the presentation of epitopes to B cells and stimulation of helper and memory T cells. Vaccination with rAd5 vectors in volunteers previously seropositive to Ad5 generated sera that targeted all capsid components, including fiber, showing the mobilization and recall of more diverse B-cell target repertoires to fend off the vaccine vector.
Many studies have used intramuscular injection of rAd5 vectors to generate Ad5 immunity in an effort to mimic natural exposure to infectious virus in animal models. Our study shows that vaccination with rAd vectors generates a profile of neutralizing antibodies different than natural infection, presumably due to the differences of route, amount of viral exposure, and capability of viral replication. Such differences call for caution in interpreting animal model data from studies of vector preexposure with respect to their effect on vaccine immunogenicity. In fact, these studies better reflect the effect of repeated intramuscular administration of vector rather than the effect of prior natural exposure.
While some data are available regarding the effect of preexisting neutralizing antibodies on the immunogenicity of rAd5-based vectors (4, 14), the specificity of these antibodies and correlation with immune stimulation has not been analyzed. In previous trials, there was a trend toward suppression of immunity in the presence of preexisting Ad5 neutralizing antibodies, especially for HIV-1 Gag-specific enzyme-linked immunospot assay responses. Here, we show that such an effect blunted both CD4+ and CD8+ T-cell Gag responses in seropositive volunteers, but we could not detect a significant effect on Env responses after DNA prime/rAd5 boost vaccination. As the response was not completely abolished in seropositive populations (4, 14), it is likely that the effective dose of vector was decreased but not completely inactivated. It is tempting to suggest that efficient HIV Gag antigen presentation may need a higher level or longer time of antigen expression than other antigens. In support of this hypothesis, it has been shown that the immunogenicity of HIV-1 Gag generated by DNA vaccination can be more dependent on the formation of viral-like particles than other antigens (1). The results shown here suggest that rAd5-based vectors, especially Gag-encoding vectors, are less likely to stimulate HIV-1 immunity in an Ad5 seropositive population; such a suppressive effect may also have contributed to the lack of rAd5 vaccine efficacy in the recent STEP trial. These data also document qualitative differences in immunity between Ad5 infection and vaccination that may affect the responses to subsequent rAd5 vector exposure. Possibly, these responses could also differentially affect the proinflammatory responses to vaccination that might affect susceptibility to infection, a possibility raised in the STEP study. These results also document a biological basis for differences between responses to immunization with rAd5 vaccines in Ad5 naïve and seropositive subjects that are relevant to proposed DNA/rAd5 efficacy studies; such studies should be preferably performed in Ad5 seronegative subjects.
In summary, this study highlights differences in the specificities of neutralizing antibodies generated by natural infection compared to vaccination. Furthermore, we have shown that anti-Gag cellular immune responses are more sensitive to the effects of preexisting neutralizing antibody than those directed against Env when using these vaccine formulations. The identification of the Ad5 vector proteins targeted by human neutralizing antibodies also suggests that next generation chimeric Ad vectors be developed without rAd5 hexon and fiber, which could avoid preexisting immunity and increase the likelihood of eliciting cellular immunity to HIV-1 antigens encoded in the vaccine.
Acknowledgments
We thank Ati Tislerics for manuscript preparation, Brenda Hartman for preparation of figures, and members of the Nabel lab for helpful discussions and comments.
This research was supported by the Intramural Research Program of the NIH, Vaccine Research Center, NIAID.
We declare that we have no material conflicts of interest, although intellectual property applications have been filed through the National Institutes of Health on the background DNA/rAd5 vaccine reported here.
Footnotes
Published ahead of print on 21 October 2009.
REFERENCES
- 1.Arrode, G., R. Hegde, Y. Jin, D. K. Singh, O. Narayan, and Y. Chebloune. 2008. Nef modulates the immunogenicity of Gag encoded in a non-infectious HIV DNA vaccine. Vaccine 26:3795-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brough, D. E., A. Lizonova, C. Hsu, V. A. Kulesa, and I. Kovesdi. 1996. A gene transfer vector-cell line system for complete functional complementation of adenovirus early regions E1 and E4. J. Virol. 70:6497-6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchbinder, S. P., D. V. Mehrotra, A. Duerr, D. W. Fitzgerald, R. Mogg, D. Li, P. B. Gilbert, J. R. Lama, M. Marmor, C. Del Rio, M. J. McElrath, D. R. Casimiro, K. M. Gottesdiener, J. A. Chodakewitz, L. Corey, and M. N. Robertson. 2008. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372:1881-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catanzaro, A. T., R. A. Koup, M. Roederer, R. T. Bailer, M. E. Enama, Z. Moodie, L. Gu, J. E. Martin, L. Novik, B. K. Chakrabarti, B. T. Butman, J. G. Gall, C. R. King, C. A. Andrews, R. Sheets, P. L. Gomez, J. R. Mascola, G. J. Nabel, and B. S. Graham. 2006. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 candidate vaccine delivered by a replication-defective recombinant adenovirus vector. J. Infect. Dis. 194:1638-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng, C., J. G. Gall, W. P. Kong, R. L. Sheets, P. L. Gomez, C. R. King, and G. J. Nabel. 2007. Mechanism of ad5 vaccine immunity and toxicity: fiber shaft targeting of dendritic cells. PLoS Pathog. 3:e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chirmule, N., K. Propert, S. Magosin, Y. Qian, R. Qian, and J. Wilson. 1999. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 6:1574-1583. [DOI] [PubMed] [Google Scholar]
- 7.Gahéry-Ségard, H., F. Farace, D. Godfrin, J. Gaston, R. Lengagne, T. Tursz, P. Boulanger, and J. G. Guillet. 1998. Immune response to recombinant capsid proteins of adenovirus in humans: antifiber and anti-penton base antibodies have a synergistic effect on neutralizing activity. J. Virol. 72:2388-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamilton, M. M., G. A. Byrnes, J. G. Gall, D. E. Brough, C. R. King, and L. L. Wei. 2008. Alternate serotype adenovector provides long-term therapeutic gene expression in the eye. Mol. Vis. 14:2535-2546. [PMC free article] [PubMed] [Google Scholar]
- 9.Hanke, T. 2008. STEP trial and HIV-1 vaccines inducing T-cell responses. Expert Rev. Vaccines 7:303-309. [DOI] [PubMed] [Google Scholar]
- 10.Hong, S. S., N. A. Habib, L. Franqueville, S. Jensen, and P. A. Boulanger. 2003. Identification of adenovirus (ad) penton base neutralizing epitopes by use of sera from patients who had received conditionally replicative ad (addl1520) for treatment of liver tumors. J. Virol. 77:10366-10375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lemiale, F., H. Haddada, G. J. Nabel, D. E. Brough, C. R. King, and J. G. Gall. 2007. Novel adenovirus vaccine vectors based on the enteric-tropic serotype 41. Vaccine 25:2074-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore, J. P., P. J. Klasse, M. J. Dolan, and S. K. Ahuja. 2008. AIDS/HIV. A STEP into darkness or light? Science 320:753-755. [DOI] [PubMed] [Google Scholar]
- 13.Nwanegbo, E., E. Vardas, W. Gao, H. Whittle, H. Sun, D. Rowe, P. D. Robbins, and A. Gambotto. 2004. Prevalence of neutralizing antibodies to adenoviral serotypes 5 and 35 in the adult populations of The Gambia, South Africa, and the United States. Clin. Diagn. Lab. Immunol. 11:351-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Priddy, F. H., D. Brown, J. Kublin, K. Monahan, D. P. Wright, J. Lalezari, S. Santiago, M. Marmor, M. Lally, R. M. Novak, S. J. Brown, P. Kulkarni, S. A. Dubey, L. S. Kierstead, D. R. Casimiro, R. Mogg, M. J. DiNubile, J. W. Shiver, R. Y. Leavitt, M. N. Robertson, D. V. Mehrotra, and E. Quirk. 2008. Safety and immunogenicity of a replication-incompetent adenovirus type 5 HIV-1 clade B gag/pol/nef vaccine in healthy adults. Clin. Infect. Dis. 46:1769-1781. [DOI] [PubMed] [Google Scholar]
- 15.Shenk, T. E. 2001. Adenoviridae: the viruses and their replication, p. 2265-2300. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 16.Shiver, J. W., and E. A. Emini. 2004. Recent advances in the development of HIV-1 vaccines using replication-incompetent adenovirus vectors. Annu. Rev. Med. 55:355-372. [DOI] [PubMed] [Google Scholar]
- 17.Sprangers, M. C., W. Lakhai, W. Koudstaal, M. Verhoeven, B. F. Koel, R. Vogels, J. Goudsmit, M. J. Havenga, and S. Kostense. 2003. Quantifying adenovirus-neutralizing antibodies by luciferase transgene detection: addressing preexisting immunity to vaccine and gene therapy vectors. J. Clin. Microbiol. 41:5046-5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sumida, S. M., D. M. Truitt, A. A. Lemckert, R. Vogels, J. H. Custers, M. M. Addo, S. Lockman, T. Peter, F. W. Peyerl, M. G. Kishko, S. S. Jackson, D. A. Gorgone, M. A. Lifton, M. Essex, B. D. Walker, J. Goudsmit, M. J. Havenga, and D. H. Barouch. 2005. Neutralizing antibodies to adenovirus serotype 5 vaccine vectors are directed primarily against the adenovirus hexon protein. J. Immunol. 174:7179-7185. [DOI] [PubMed] [Google Scholar]
- 19.Thorner, A. R., R. Vogels, J. Kaspers, G. J. Weverling, L. Holterman, A. A. Lemckert, A. Dilraj, L. M. McNally, P. M. Jeena, S. Jepsen, P. Abbink, A. Nanda, P. E. Swanson, A. T. Bates, K. L. O'Brien, M. J. Havenga, J. Goudsmit, and D. H. Barouch. 2006. Age dependence of adenovirus-specific neutralizing antibody titers in individuals from sub-Saharan Africa. J. Clin. Microbiol. 44:3781-3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waddington, S. N., J. H. McVey, D. Bhella, A. L. Parker, K. Barker, H. Atoda, R. Pink, S. M. Buckley, J. A. Greig, L. Denby, J. Custers, T. Morita, I. M. Francischetti, R. Q. Monteiro, D. H. Barouch, N. van Rooijen, C. Napoli, M. J. Havenga, S. A. Nicklin, and A. H. Baker. 2008. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell 132:397-409. [DOI] [PubMed] [Google Scholar]
- 21.Watkins, D. I., D. R. Burton, E. G. Kallas, J. P. Moore, and W. C. Koff. 2008. Nonhuman primate models and the failure of the Merck HIV-1 vaccine in humans. Nat. Med. 14:617-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wohlfart, C. 1988. Neutralization of adenoviruses: kinetics, stoichiometry, and mechanisms. J. Virol. 62:2321-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]