Abstract
Enterovirus 71 (EV71) causes childhood hand, foot, and mouth disease and neurological complications, and no vaccines or therapeutic drugs are currently available. Formaldehyde-inactivated whole-virus vaccines derived from EV71 clinical isolates and a mouse-adapted virus (MAV) were tested in a mouse model of EV71 encephalomyelitis. After only two immunizations, given to mice at 1 and 7 days of age, the MAV vaccine protected mice at 14 days of age from disease. Tissues from immunized mice were negative for virus by viral culture, reverse transcriptase PCR, immunohistochemistry analysis, and in situ hybridization. Cross-neutralizing EV71 antibodies to strains with genotypes B3, B4, and C1 to C5 generated in immunized adult mice were able to passively protect 14-day-old mice from disease.
Enterovirus 71 (EV71), like poliovirus, is a nonenveloped, single-stranded RNA virus that belongs to the genus Enterovirus within the Picornaviridae family (22). Outbreaks of EV71-associated hand, foot, and mouth diseases have been reported worldwide, and hundreds of children have died from severe complications of encephalomyelitis (1, 3, 4, 6, 8-10, 12, 13, 14-16, 18-20, 24).
There are currently no effective antiviral drugs available for severe EV71 infection. The success of both formaldehyde-inactivated and live-attenuated poliovirus vaccines in controlling poliovirus outbreaks highlights the potential control of EV71 epidemics by mass vaccination.
Recently, several new formats of EV71 candidate vaccines have been successfully developed. These included a DNA vaccine, an EV71-like particle vaccine, and a recombinant VP1 protein vaccine derived from milk samples from transgenic mice engineered to produce VP1 capsid protein (5, 7, 28). All of these vaccines are immunogenic and show good immune responses in vaccinated mice (5, 7, 28). However, only the VP1 vaccine appears to show some direct evidence of protection in that suckling mice feeding on milk that contained the vaccine did not lose weight after virus challenge (5). However, the efficacy of this vaccine was not evaluated by survival rate studies and pathological and virological analyses of immunized animals.
Challenge studies following vaccination with the DNA and EV71-like particle vaccines have not been reported, perhaps because adult mice are not susceptible to clinical EV71 infection and so far only suckling mice of up to a week old are susceptible to infection (26).
We recently described a new mouse model for EV71 encephalomyelitis (21) that is similar to the human disease (27). In this model, a genotype B3 clinical isolate (herein designated 13903; GenBank accession number AY207648) was serially passaged five times to create a mouse-adapted virus (MAV) that was able to infect mice up to 14 days old. In the present study, this mouse model was used to test the protective efficacy of formaldehyde-inactivated whole-virus vaccines. Following immunization of mice at birth and administration of a booster dose at day 7, we found complete protection from disease in mice at day 14.
Vaccine preparation.
Human EV71 strains 13903 (21), A10/4 (genotype B4; GenBank accession number AF376067) (2), and MAV (21), purified by limiting dilution, were grown in Vero cells at a multiplicity of infection of 5 at 36°C in Dulbecco's modified Eagle's medium without fetal bovine serum. The infected culture was kept until all cells showed a cytopathic effect. The culture was subjected to three freeze-thaw cycles and clarified by centrifugation at 4,500 rpm for 10 min at 4°C. The supernatant was filtered through a 0.2-μm-pore-size filter (Sartorius, Germany). The virus titer was determined using the 50% cell culture infective dose (CCID50) assay with Vero cells as described previously (21). Prior to formaldehyde inactivation, the virus suspension was again filtered as described above to remove any viral aggregates in order to facilitate formaldehyde penetration into all virus particles. Inactivation was carried out by adding 37% formaldehyde (Merck, Denmark) to the virus suspension to give a final formaldehyde dilution of 1/4,000 and incubating the suspension for 14 days at 37°C (17). The inactivated virus suspension was filtered again as described above and stored at −80°C. In this way, three inactivated-virus vaccines, the 13903, A10/4, and MAV vaccines, were prepared. No live viruses were detected after repeated Vero cell cultures for a period of up to 3 weeks. The protein concentrations in inactivated virus preparations were determined using the Coomassie Plus (Bradford) assay kit according to the procedures of the manufacturer (Pierce).
Immunogenicities of inactivated-virus vaccines in adult mice.
Three groups (n = 6 per group) of 4- to 6-week-old ICR mice were each immunized via the intraperitoneal (i.p.) route with one of the three inactivated whole-virus vaccines in a dose of 0.25 ml (20 μg) containing 0.5 mg/ml aluminum hydroxide (Sigma). The immunization schedule consisted of two inoculations given 1 week apart. The mice were sacrificed at day 14 postimmunization, and sera were tested for neutralizing antibodies against several EV71 clinical isolates (isolates 1 to 5) (Table 1) by the microneutralization test as described previously (28). Briefly, serial twofold dilutions of serum samples were mixed with 100 CCID50 of virus, and the neutralization antibody titer was the highest dilution of serum that protected 50% of the cultures. Our preliminary data indicated that the MAV vaccine induced the highest neutralizing antibody titers and, hence, was likely to have greater potential to be effective against genotype B viruses than the other two vaccines (Table 2). Hence, further testing with the MAV-induced antibodies was performed with clinical isolates of genotypes C1 to C5 (isolates 6 to 10) (Table 1). Antisera generated by immunization with the MAV vaccine not only neutralized genotype B3 EV71 strains, but also neutralized strains of genotypes B4 and C1 to C5, with the highest neutralizing activity against genotypes B3 and B4 and the lowest activity against genotype C4 (Table 2).
TABLE 1.
Clinical isolates of EV71 strains used in this study
| Isolate no. | Virus strain | Genotype | Origin | Year | Reference |
|---|---|---|---|---|---|
| 1 | 13903 | B3 | Malaysia | 1997 | 21 |
| 2 | MF | B3 | Malaysia | 1997 | 11 |
| 3 | MY104-9 | B3 | Malaysia | 1998 | 2 |
| 4 | CS | B4 | Malaysia | 1997 | 11 |
| 5 | A10/4 | B4 | Malaysia | 2000 | 2 |
| 6 | 9522 | C1 | Malaysia | 2003 | 23 |
| 7 | 8 M/6/99 | C2 | Australia | 1999 | 2 |
| 8 | 001-KOR-00 | C3 | Korea | 2000 | 2 |
| 9 | VN5559 | C4 | Vietnam | 2005 | 25 |
| 10 | VN5784 | C5 | Vietnam | 2005 | 25 |
TABLE 2.
Titers of cross-neutralizing antibodies against clinical isolates of EV71 after immunization with formaldehyde-inactivated virus vaccines
| Vaccine strain or material | Titer of antibodiesa against: |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 13903 | MF | MY104-9 | CS | A10/4 | 9522 | 8 M/6/99 | 001-KOR-00 | VN5559 | VN5784 | |
| MAV | ≥1/512 | ≥1/512 | ≥1/512 | ≥1/512 | ≥1/512 | 1/256 | 1/64 | 1/256 | 1/32 | 1/64 |
| A10/4 | 1/32 | 1/32 | 1/32 | 1/32 | 1/32 | ND | ND | ND | ND | ND |
| 13903 | 1/64 | 1/32 | 1/32 | 1/32 | 1/32 | ND | ND | ND | ND | ND |
| Control | <1/8 | <1/8 | <1/8 | <1/8 | <1/8 | <1/8 | <1/8 | <1/8 | <1/8 | <1/8 |
Virus strains were tested in microneutralizing assays. ND, not determined.
Active immunization of and challenge experiments with suckling mice. (i) Survival rates.
Since the MAV vaccine showed the highest neutralizing antibody titers, it was used for further vaccination experiments; the other two vaccines were not studied further. All animal experiments were performed after obtaining ethical clearance and in accordance with institutional (University of Malaya) guidelines on animal welfare and the use of laboratory animals.
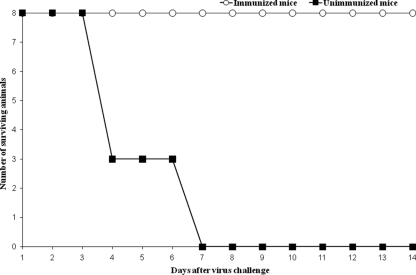
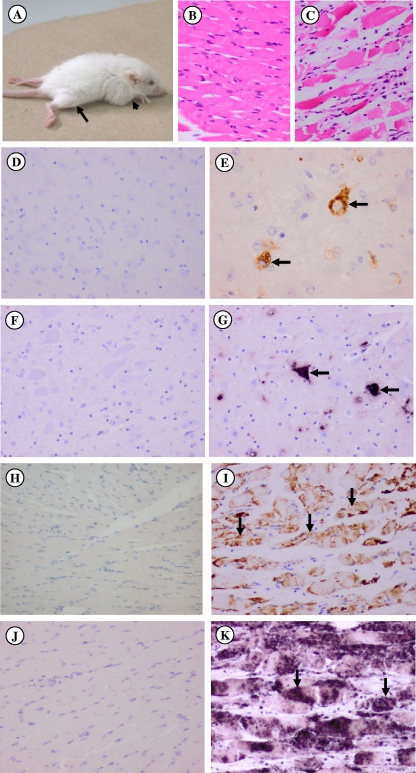
The MAV vaccine was tested with 1-day-old ICR mice (n = 8) by the subcutaneous route using 0.05 ml (4 μg) of vaccine containing 0.5 mg/ml aluminum hydroxide. Eight additional mice served as negative controls and received only normal saline injections. Mice were boosted at day 7 and challenged by the i.p. route with 50 50% lethal doses (LD50) of live MAV at day 14. The mice were then monitored for paralysis or death for the next 14 days. The survival graph for immunized and unimmunized animals after live-virus challenge is shown in Fig. 1. The MAV vaccine protected 100% of immunized mice from weight loss, paralysis, and death. The protection level was only 50% when no booster was given (results not shown). All unimmunized animals developed signs of acute disease characterized by progressive hind or front limb paralysis (Fig. 2A) and weight loss at 3 days postinfection (dpi) and death at 4 to 7 dpi.
FIG. 1.
Survival graph for mice (immunized and unimmunized) after i.p. challenge with lethal doses of mouse-adapted EV71.
FIG. 2.
Pathological findings for immunized and unimmunized mice after i.p. challenge with lethal doses of mouse-adapted EV71. (A to C) An unimmunized mouse exhibited hind limb (arrow) and front limb (arrowhead) paralysis at 3 dpi (A). No significant lesions in the skeletal muscle from an immunized mouse were observed (B); in contrast, severe necrosis with mild inflammatory cell infiltration was observed in muscle from the unimmunized mouse (C). (D to K) In the brain stem of the immunized mouse, no viral antigens (D) or RNA (F) were detected. Similarly, no viral antigens (H) and RNA (J) were detected in skeletal muscle fibers. In the unimmunized mouse, numerous brain stem neurons were positive for viral antigens (E, arrows) and RNA (G, arrows). Skeletal muscle fibers were also positive for viral antigens (I, arrows) and RNA (K, arrows). Samples in panels B and C were stained with hematoxylin and eosin stain; samples in panels D, E, H, and I were subjected to IHC analysis with DAB (3,3′-diaminobenzidine) chromogen and hematoxylin counterstain; samples in panels F, G, J, and K were analyzed by ISH with Nitro Blue Tetrazolium-BCIP (5-bromo-4-chloro-3-indolylphosphate) substrate and hematoxylin counterstain. Original magnifications, ×20 (B to K).
(ii) Histopathology, virus titer determination, and virus detection.
Histopathological changes, virus titers, and viral antigens/RNA in tissues from two further sets of nine immunized and nine unimmunized suckling mice after virus challenge were studied. However, all mice were sacrificed at 4 dpi. Three mice from each group were used for (i) histopathology analysis, (ii) virus titer determination, and (iii) reverse transcriptase PCR (RT-PCR). For histopathology analysis, the whole mouse was fixed in 10% buffered formalin for 5 days. Seven standard whole-body, cross-sectional, paraffinized tissue blocks were prepared. Tissue sections were stained with hematoxylin and eosin, and immunohistochemistry (IHC) and in situ hybridization (ISH) analyses were performed as described previously (21). In unimmunized mice, limb skeletal muscles revealed extensive, severe necrotizing myositis (Fig. 2C). Viral antigens (Fig. 2E) and viral RNA (Fig. 2G) were detected in neurons of the central nervous system (CNS). Viral antigens (Fig. 2I) and RNA (Fig. 2K) were diffusely observed within the skeletal muscle fibers. In contrast, no significant pathology was observed in the muscles (Fig. 2B, H, and J) and CNS tissues (Fig. 2D and F) of immunized mice.
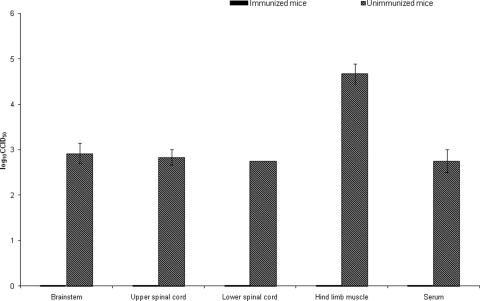
For virus titer determination, hind limb muscles, lower and upper spinal cords, and brain stems were weighed and homogenized in phosphate-buffered saline to 10% (wt/vol). Virus titers were determined as described above. Virus titers in tissues were expressed as the mean log10 CCID50 ± the standard error of the mean for pooled 10% tissue homogenates from groups of three mice at 4 dpi. Virus titers in sera were expressed as the log 10 CCID50 per milliliter. In general, all unimmunized mice showed evidence of virus in sera, hind limb muscles, and CNS tissues, whereas no virus was ever isolated from brain stems, upper and lower spinal cords, hind limbs, or sera from immunized mice (Fig. 3).
FIG. 3.
Virus titers in brain stems, upper and lower spinal cords, hind limb muscles, and sera from immunized and unimmunized mice challenged with lethal doses of mouse-adapted EV71 by the i.p. route.
Total RNA was extracted from homogenized hind limb muscles, upper and lower spinal cords, brain stems, and sera by using Trizol reagent according to the procedure of the manufacturer (Invitrogen). RT-PCR with gene-specific primers (27) and beta-actin primers as an internal control was then performed using the Titan One-Tube RT-PCR system according to the procedures of the manufacturer (Roche, Germany). Similar to virus titer, IHC, and ISH analyses, the RT-PCR was negative for tissues from all the immunized mice but positive for the unimmunized group.
Passive immunization of and challenge experiments with suckling mice.
To confirm the importance of the humoral immune response in protection, undiluted, heat-inactivated, pooled sera from previously immunized adult mice were given to a group of 14-day-old ICR mice (n = 8) while a control group (n = 8) received normal sera from naïve mice. Mice were given 0.2 ml of sera with neutralizing antibody titers of 1/512 or normal sera by the i.p. route. One hour later, the animals were challenged with 10 LD50 of live MAV, and they received a second injection of 0.2 ml of sera 24 h later. The mice were observed for signs of disease for 7 days. All mice that received the hyperimmune sera were completely protected, with no signs of disease whatsoever. In contrast, all mice that received the naïve sera developed paralysis or died within 4 to 5 dpi.
In summary, suckling mice immunized with the adjuvant-carrying formaldehyde-inactivated MAV vaccine at 1 and 7 days of age were effectively protected from lethal virus challenge and disease at 14 days. The results of this study further suggest that protection against disease as indicated by the absence of weight loss, paralysis, death, and virus in sera and CNS and skeletal muscle tissues correlates with the presence of neutralizing antibodies. In this mouse model, viral replication in skeletal muscles appears to precede viral spread to the CNS, and indeed, retrograde peripheral motor nerve transmission was thought to be responsible (21). We believe that the inhibition of viremia by neutralizing antibodies induced by vaccines prevented skeletal muscle and CNS infection in the mouse model. However, further studies are needed to determine the possible roles of cellular immunity in protection.
Genomic comparison between EV71 strains 13903 and MAV revealed several nucleotide differences, including two each in the VP2 and VP1 structural genes (unpublished data). However, analysis of deduced amino acid sequences showed that MAV had only one amino acid substitution each in the VP2 and VP1 regions that is different from the sequence in the 13903 strain. Whether or not these differences acting alone or in combination with other unknown factors cause increased immunogenicity of the MAV vaccine needs to be further investigated.
This formaldehyde-inactivated MAV vaccine could serve as a useful prototype vaccine, as it appears to be highly effective against EV71 infection in our mouse model. Similar approaches may be applied for the development of vaccines against EV71 infection in humans.
Acknowledgments
We are deeply indebted to Hiroyuki Shimizu for providing us with the EV71 antiserum.
This work was supported by grant no. 36-02-03-6010 from the Malaysian government (Intensified Research Priority Area).
Footnotes
Published ahead of print on 28 October 2009.
REFERENCES
- 1.Alexander, J. P., L. Baden, M. A. Pallansch, and G. Anderson. 1994. Enterovirus 71 infections and neurologic disease—United States, 1977-1991. J. Infect. Dis. 169:905-908. [DOI] [PubMed] [Google Scholar]
- 2.Cardosa, M. J., D. Perera, B. A. Brown, D. Cheon, H. M. Chan, K. P. Chan, H. Cho, and P. McMinn. 2003. Molecular epidemiology of human enterovirus 71 strains and recent outbreaks in the Asia-Pacific region: comparative analysis of the VP1 and VP4 genes. Emerg. Infect. Dis. 9:461-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan, K. P., K. T. Goh, C. Y. Chong, E. S. Teo, G. Lau, and A. E. Ling. 2003. Epidemic hand, foot, and mouth disease caused by human enterovirus 71, Singapore. Emerg. Infect. Dis. 9:78-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan, L. G., U. D. Parashar, M. S. Lye, F. G. L. Ong, S. R. Zaki, J. P. Alexander, K. K. Ho, L. L. Han, M. A. Pallansch, A. B. Suleiman, M. Jegathesan, and L. J. Anderson. 2000. Deaths of children during an outbreak of hand, foot, and mouth disease in Sarawak, Malaysia: clinical and pathological characteristic of the disease. Clin. Infect. Dis. 31:678-683. [DOI] [PubMed] [Google Scholar]
- 5.Chen, H. L., J. Y. Huang, T. W. Chu, T. C. Tsai, C. M. Hung, C. C. Lin, F. C. Liu, L. F. Wang, Y. J. Chen, M. F. Lin, and C. M. Chen. 2008. Expression of VP1 protein in the milk of transgenic mice: a potential oral vaccine protects against enterovirus 71 infection. Vaccine 26:2882-2889. [DOI] [PubMed] [Google Scholar]
- 6.Chong, C. Y., K. P. Chan, V. A. Shah, W. Y. M. Ng, G. Lau, T. S. Teo, S. H. Lai, and A. E. Ling. 2003. Hand, foot and mouth disease in Singapore: a comparison of fatal and non-fatal cases. Acta Paediatr. 92:1163-1169. [PubMed] [Google Scholar]
- 7.Chung, Y. C., M. S. Ho, J. C. Wu, W. J. Chen, J. H. Huang, S. T. Chou, and Y. C. Hu. 2008. Immunization with virus-like particles of enterovirus 71 elicits potent immune responses and protects mice against lethal challenge. Vaccine 26:1855-1862. [DOI] [PubMed] [Google Scholar]
- 8.Fujimoto, T., M. Chikahira, S. Yoshida, H. Ebira, A. Hasegawa, A. Totsuka, and O. Nishio. 2002. Outbreak of central nervous system disease associated with hand, foot, and mouth disease in Japan during the summer of 2000: detection and molecular epidemiology of enterovirus 71. Microbiol. Immunol. 46:621-627. [DOI] [PubMed] [Google Scholar]
- 9.Hagiwara, A., I. Tagaya, and T. Yoneyama. 1978. Epidemic of hand, foot and mouth disease associated with enterovirus 71 infection. Intervirology 9:60-63. [DOI] [PubMed] [Google Scholar]
- 10.Hanson, R. R. 1988. Case of paralytic illness associated with enterovirus 71 infection. JAMA 259:1621-1622. [PubMed] [Google Scholar]
- 11.Herrero, L. J., C. S. Lee, R. J. Hurrelbrink, B. H. Chua, K. B. Chua, and P. C. McMinn. 2003. Molecular epidemiology of enterovirus 71 in peninsular Malaysia, 1997-2000. Arch. Virol. 148:1369-1385. [DOI] [PubMed] [Google Scholar]
- 12.Huang, C. C., C. C. Liu, Y. C. Chang, C. Y. Chen, S. T. Wang, and T. F. Yeh. 1999. Neurologic complication in children with enterovirus 71 infection. N. Engl. J. Med. 341:929-935. [DOI] [PubMed] [Google Scholar]
- 13.Ishimaru, Y., S. Nakano, K. Yamaoka, and S. Takami. 1980. Outbreaks of hand, foot, and mouth disease by enterovirus 71. High incidence of complication disorders of central nervous system. Arch. Dis. Child. 55:583-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kennett, M. L., C. J. Birch, F. A. Lewis, A. P. Yung, S. A. Locarnini, and L. D. Gust. 1974. Enterovirus type 71 infection in Melbourne. Bull. World Health Organ. 51:609-614. [PMC free article] [PubMed] [Google Scholar]
- 15.Komatsu, K., Y. Shimizu, Y. Takeuchi, H. Ishiko, and H. Takada. 1999. Outbreak of severe neurologic involvement associated with enterovirus 71 infection. Pediatr. Neurol. 20:17-23. [DOI] [PubMed] [Google Scholar]
- 16.Lum, L. C. S., K. T. Wong, S. K. Lam, K. B. Chua, A. Y. T. Goh, W. L. Lim, B. B. Ong, G. Paul, S. AbuBakar, and M. Lamber. 1998. Fatal enterovirus 71 encephalomyelitis. J. Pediatr. 133:795-798. [DOI] [PubMed] [Google Scholar]
- 17.Martín, J., G. Crossland, D. J. Wood, and P. D. Minor. 2003. Characterization of formaldehyde-inactivated poliovirus preparations made from live-attenuated strains. J. Gen. Virol. 84:1781-1788. [DOI] [PubMed] [Google Scholar]
- 18.Melnick, J. L. 1984. Enterovirus 71 infections: a varied clinical pattern sometimes mimicking poliomyelitis. Rev. Infect. Dis. 6(Suppl. 2):S387-S390. [DOI] [PubMed] [Google Scholar]
- 19.Nagy, G., S. Takátsy, E. Kukán, L. Mihaly, and I. Dömök. 1982. Virological diagnosis of enterovirus type 71 infections: experiences gained during epidemic of acute CNS diseases in Hungary in 1978. Arch. Virol. 71:217-227. [DOI] [PubMed] [Google Scholar]
- 20.Ng, D. K. K., A. K. W. Law, S. W. W. Cherk, and L. L. Mak. 2001. First fatal case of enterovirus 71 infection in Hong Kong. Hong Kong Med. J. 7:193-196. [PubMed] [Google Scholar]
- 21.Ong, K. C., B. Munisamy, S. Devi, M. J. Cardosa, and K. T. Wong. 2008. Pathologic characterization of a murine model of human enterovirus 71 encephalomyelitis. J. Neuropathol. Exp. Neurol. 67:532-542. [DOI] [PubMed] [Google Scholar]
- 22.Pallansch, M. A., and R. P. Roos. 2001. Enteroviruses: poliovirus, coxsackieviruses, echoviruses, and newer enteroviruses, p. 723-775. In D. M. Knipe, P. M. Howley, D. E. Griffin, M. A. Martin, R. A. Lamb, and B. Roizman (ed.), Fields virology, 4th ed., vol. 1. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 23.Podin, Y., E. L. Gias, F. Ong, Y. W. Leong, S. F. Yee, M. A. Yusof, D. Perera, B. Teo, T. Y. Wee, S. C. Yao, S. K. Tao, A. Kiyu, M. T. Arif, and M. J. Cardosa. 2006. Sentinel surveillance for human enterovirus 71 in Sarawak, Malaysia: lessons from the first 7 years. BMC Public Health 6:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shindarov, L. M., M. P. Chumakov, M. K. Voroshilova, S. Bojinov, S. M. Vasilenko, I. Iordanov, I. D. Kirov, E. Kamenov, E. V. Leshchinskaya, G. Mitov, I. A. Robinson, S. Sivchev, and S. Staikov. 1979. Epidemiological, clinical, and pathomorphological characteristics of epidemic poliomyelitis-like disease caused by enterovirus 71. J. Hyg. Epidemiol. Microbiol. Immunol. 23:284-295. [PubMed] [Google Scholar]
- 25.Tu, P. V., N. T. T. Thao, D. Perera, T. H. Huu, N. T. K. Tien, T. C. Thuong, O. M. How, M. J. Cardosa, and P. C. McMinn. 2007. Epidemiological and virologic investigation of hand, foot, and mouth disease, Southern Vietnam, 2005. Emerg. Infect. Dis. 13:1733-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang, Y. F., C. T. Chou, H. Y. Lei, C. C. Liu, S. M. Wang, J. J. Yan, I. J. Su, J. R. Wang, T. M. Yeh, S. H. Chen, and C. K. Yu. 2004. A mouse-adapted enterovirus 71 strain causes neurological disease in mice after oral infection. J. Virol. 78:7916-7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong, K. T., M. Badmanathan, K. C. Ong, H. Kojima, N. Nagata, K. B. Chua, B. B. Ong, and K. Nagashima. 2008. The distribution of inflammation and virus in human enterovirus 71 encephalomyelitis suggests possible viral spread by neural pathways. J. Neuropathol. Exp. Neurol. 67:162-169. [DOI] [PubMed] [Google Scholar]
- 28.Wu, C. N., Y. C. Lin, C. Fann, N. S. Liao, S. R. Shih, and M. S. Ho. 2002. Protection against lethal enterovirus 71 infection in newborn mice by passive immunization with subunit VP1 vaccines and inactivated virus. Vaccine 20:895-904. [DOI] [PubMed] [Google Scholar]