Abstract
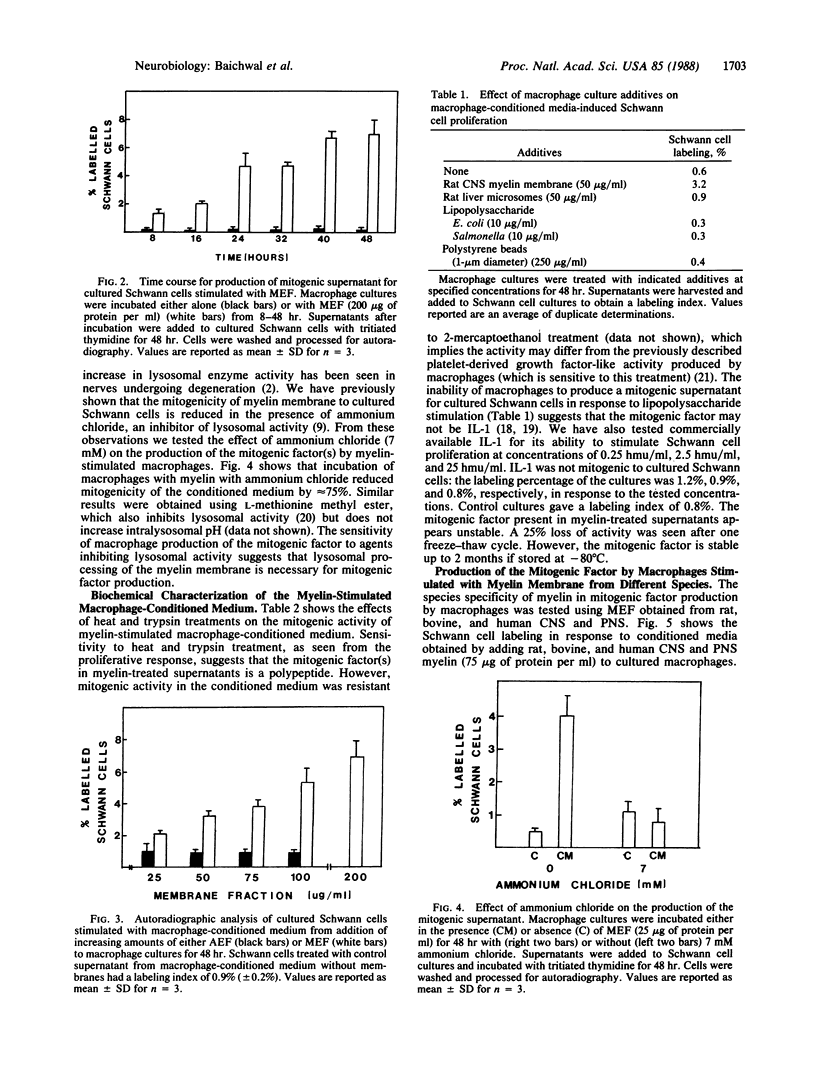
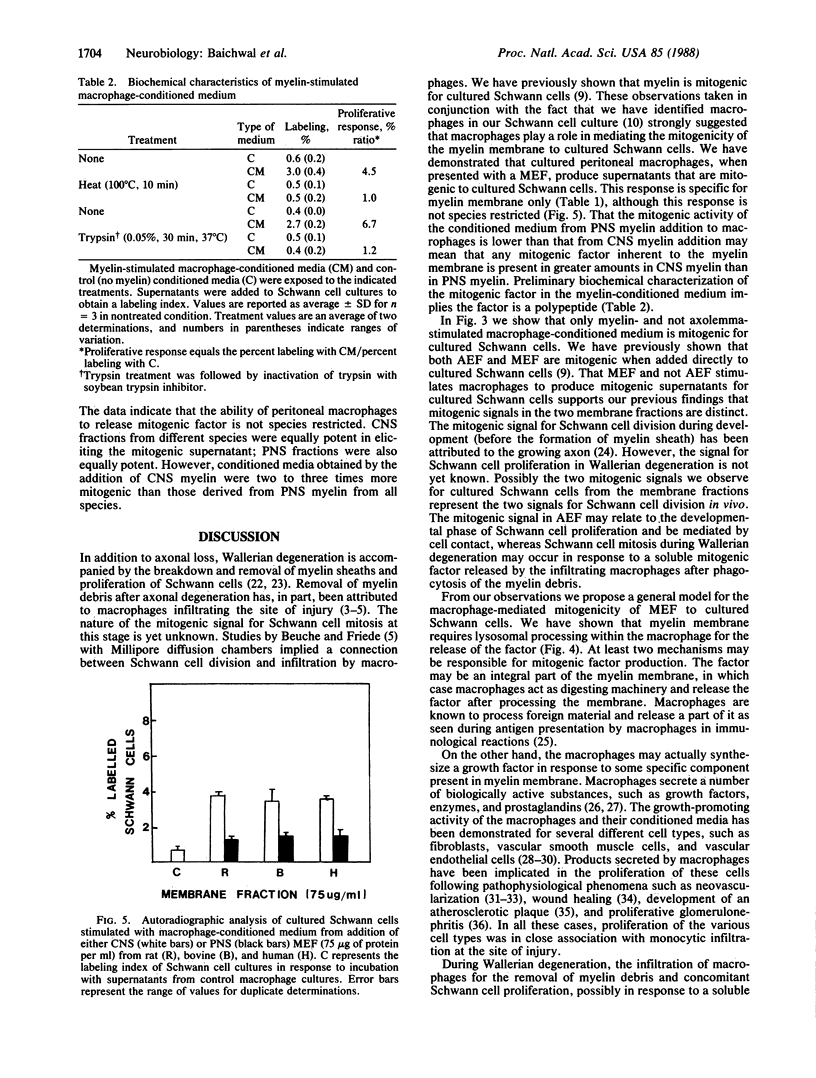
Conditioned medium from cultured peritoneal macrophages that have phagocytosed a myelin membrane fraction is mitogenic for cultured Schwann cells. Production of the mitogenic supernatant was time- and dose-dependent with a maximal Schwann cell-proliferative response from supernatants after 48-hr incubation of cultured macrophages with myelin-enriched fraction (200 micrograms of protein per ml). The response was specific for myelin membrane: supernatants derived from macrophages incubated with axolemma, liver microsomes, polystyrene beads, or lipopolysaccharide were not mitogenic. Lysosomal processing of the myelin membrane was necessary for the production of the mitogenic factor, which was shown to be heat labile and trypsin sensitive. There was no species specificity because myelin membranes isolated from the central and peripheral nervous systems of rat, bovine, and human were equally potent in eliciting mitogenic supernatant. However, supernatants derived from central nervous system myelin membranes were two to three times more mitogenic than those obtained from peripheral nervous system fractions of the same species. Previous observations that myelin is mitogenic for cultured Schwann cells may, in part, involve the intermediate processing of myelin by macrophages that are present in Schwann cell cultures. These results suggest that macrophages play a crucial role in Schwann cell proliferation during Wallerian degeneration.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asbury A. K. Schwann cell proliferation in developing mouse sciatic nerve. A radioautographic study. J Cell Biol. 1967 Sep;34(3):735–743. doi: 10.1083/jcb.34.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baichwal R. R., Yan L., Bosler A., DeVries G. H. An automated method for autoradiographic analysis of cultured Schwann cells. J Neurosci Methods. 1987 Aug;20(4):295–305. doi: 10.1016/0165-0270(87)90062-8. [DOI] [PubMed] [Google Scholar]
- Berner A., Torvik A., Stenwig A. E. Origin of macrophages in traumatic lesions and Wallerian degeneration in peripheral nerves. Acta Neuropathol. 1973;25(3):228–236. doi: 10.1007/BF00685202. [DOI] [PubMed] [Google Scholar]
- Beuche W., Friede R. L. The role of non-resident cells in Wallerian degeneration. J Neurocytol. 1984 Oct;13(5):767–796. doi: 10.1007/BF01148493. [DOI] [PubMed] [Google Scholar]
- Bigbee J. W., Yoshino J. E., DeVries G. H. Morphological and proliferative responses of cultured Schwann cells following rapid phagocytosis of a myelin-enriched fraction. J Neurocytol. 1987 Aug;16(4):487–496. doi: 10.1007/BF01668503. [DOI] [PubMed] [Google Scholar]
- Bradley W. G., Asbury A. K. Duration of synthesis phase in neuilemma cells in mouse sciatic nerve during degeneration. Exp Neurol. 1970 Feb;26(2):275–282. doi: 10.1016/0014-4886(70)90125-1. [DOI] [PubMed] [Google Scholar]
- Brockes J. P., Fields K. L., Raff M. C. Studies on cultured rat Schwann cells. I. Establishment of purified populations from cultures of peripheral nerve. Brain Res. 1979 Apr 6;165(1):105–118. doi: 10.1016/0006-8993(79)90048-9. [DOI] [PubMed] [Google Scholar]
- Brunden K. R., Poduslo J. F. Lysosomal delivery of the major myelin glycoprotein in the absence of myelin assembly: posttranslational regulation of the level of expression by Schwann cells. J Cell Biol. 1987 Mar;104(3):661–669. doi: 10.1083/jcb.104.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Stone R. D., Leung D. Y., Silver I., Hohn D. C., Hunt T. K. Role of macrophages in would healing. Surg Forum. 1976;27(62):16–18. [PubMed] [Google Scholar]
- Cotran R. S. Monocytes, proliferation, and glomerulonephritis. J Lab Clin Med. 1978 Dec;92(6):837–840. [PubMed] [Google Scholar]
- Crang A. J., Blakemore W. F. Observations on Wallerian degeneration in explant cultures of cat sciatic nerve. J Neurocytol. 1986 Aug;15(4):471–482. doi: 10.1007/BF01611730. [DOI] [PubMed] [Google Scholar]
- DeVries G. H., Anderson M. G., Johnson D. Fractionation of isolated rat CNS myelinated axons by sucrose density gradient centrifugation in a zonal rotor. J Neurochem. 1983 Jun;40(6):1709–1717. doi: 10.1111/j.1471-4159.1983.tb08146.x. [DOI] [PubMed] [Google Scholar]
- DeVries G. H., Minier L. N., Lewis B. L. Further studies on the mitogenic response of cultured Schwann cells to rat CNS axolemma-enriched fractions. Brain Res. 1983 Jul;285(1):87–93. doi: 10.1016/0165-3806(83)90112-8. [DOI] [PubMed] [Google Scholar]
- Edelson P. J., Zwiebel R., Cohn Z. A. The pinocytic rate of activated macrophages. J Exp Med. 1975 Nov 1;142(5):1150–1164. doi: 10.1084/jem.142.5.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friede R. L., Johnstone M. A. Responses of thymidine labeling of nuclei in gray matter and nerve following sciatic transection. Acta Neuropathol. 1967 Jan 2;7(3):218–231. doi: 10.1007/BF00686373. [DOI] [PubMed] [Google Scholar]
- Gery I., Davies P., Derr J., Krett N., Barranger J. A. Relationship between production and release of lymphocyte-activating factor (interleukin 1) by murine macrophages. 1. Effects of various agents. Cell Immunol. 1981 Nov 1;64(2):293–303. doi: 10.1016/0008-8749(81)90481-0. [DOI] [PubMed] [Google Scholar]
- Greenburg G. B., Hunt T. K. The proliferative response in vitro of vascular endothelial and smooth muscle cells exposed to wound fluids and macrophages. J Cell Physiol. 1978 Dec;97(3 Pt 1):353–360. doi: 10.1002/jcp.1040970310. [DOI] [PubMed] [Google Scholar]
- Holtzman E., Novikoff A. B. Lysomes in the rat sciatic nerve following crush. J Cell Biol. 1965 Dec;27(3):651–669. doi: 10.1083/jcb.27.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibovich S. J., Ross R. A macrophage-dependent factor that stimulates the proliferation of fibroblasts in vitro. Am J Pathol. 1976 Sep;84(3):501–514. [PMC free article] [PubMed] [Google Scholar]
- Leibovich S. J., Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol. 1975 Jan;78(1):71–100. [PMC free article] [PubMed] [Google Scholar]
- Martin B. M., Gimbrone M. A., Jr, Unanue E. R., Cotran R. S. Stimulation of nonlymphoid mesenchymal cell proliferation by a macrophage-derived growth factor. J Immunol. 1981 Apr;126(4):1510–1515. [PubMed] [Google Scholar]
- Martinet Y., Bitterman P. B., Mornex J. F., Grotendorst G. R., Martin G. R., Crystal R. G. Activated human monocytes express the c-sis proto-oncogene and release a mediator showing PDGF-like activity. Nature. 1986 Jan 9;319(6049):158–160. doi: 10.1038/319158a0. [DOI] [PubMed] [Google Scholar]
- Meador-Woodruff J. H., Yoshino J. E., Bigbee J. W., Lewis B. L., Devries G. H. Differential proliferative responses of cultured Schwann cells to axolemma and myelin-enriched fractions. II. Morphological studies. J Neurocytol. 1985 Aug;14(4):619–635. doi: 10.1007/BF01200801. [DOI] [PubMed] [Google Scholar]
- Page R. C., Davies P., Allison A. C. The macrophage as a secretory cell. Int Rev Cytol. 1978;52:119–157. doi: 10.1016/s0074-7696(08)60755-x. [DOI] [PubMed] [Google Scholar]
- Pellegrino R. G., Politis M. J., Ritchie J. M., Spencer P. S. Events in degenerating cat peripheral nerve: induction of Schwann cell S phase and its relation to nerve fibre degeneration. J Neurocytol. 1986 Feb;15(1):17–28. doi: 10.1007/BF02057901. [DOI] [PubMed] [Google Scholar]
- Polverini P. J., Cotran R. S., Sholley M. M. Endothelial proliferation in the delayed hypersensitivity reaction: an autoradiographic study. J Immunol. 1977 Feb;118(2):529–532. [PubMed] [Google Scholar]
- Rosenthal A. S., Shevach E. M. Function of macrophages in antigen recognition by guinea pig T lymphocytes. I. Requirement for histocompatible macrophages and lymphocytes. J Exp Med. 1973 Nov 1;138(5):1194–1212. doi: 10.1084/jem.138.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer J. L., Bunge R. P. Studies of Schwann cell proliferation. I. An analysis in tissue culture of proliferation during development, Wallerian degeneration, and direct injury. J Cell Biol. 1980 Mar;84(3):739–752. doi: 10.1083/jcb.84.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakral K. K., Goodson W. H., 3rd, Hunt T. K. Stimulation of wound blood vessel growth by wound macrophages. J Surg Res. 1979 Apr;26(4):430–436. doi: 10.1016/0022-4804(79)90031-3. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. Secretory function of mononuclear phagocytes: a review. Am J Pathol. 1976 May;83(2):396–418. [PMC free article] [PubMed] [Google Scholar]
- Wall R. T., Harker L. A., Quadracci L. J., Striker G. E. Factors influencing endothelial cell proliferation in vitro. J Cell Physiol. 1978 Aug;96(2):203–213. doi: 10.1002/jcp.1040960209. [DOI] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]
- Yoshino J. E., Dinneen M. P., Lewis B. L., Meador-Woodruff J. H., Devries G. H. Differential proliferative responses of cultured Schwann cells to axolemma- and myelin-enriched fractions. I. Biochemical studies. J Cell Biol. 1984 Dec;99(6):2309–2313. doi: 10.1083/jcb.99.6.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino J. E., Griffin J. W., DeVries G. H. Identification of an axolemma-enriched fraction from peripheral nerve. J Neurochem. 1983 Oct;41(4):1126–1130. doi: 10.1111/j.1471-4159.1983.tb09061.x. [DOI] [PubMed] [Google Scholar]