Abstract
Every year, influenza virus infection causes significant mortality and morbidity in human populations. Although egg-based inactivated viral vaccines are available, their effectiveness depends on the correct prediction of the circulating viral strains and is limited by the time constraint of the manufacturing process. Recombinant subunit vaccines are easier to manufacture with a relatively short lead time but are limited in their efficacy partly because the purified recombinant membrane proteins in the soluble form most likely do not retain their native membrane-bound structure. Nanodisc (ND) particles are soluble, stable, and reproducibly prepared discoid shaped nanoscale structures that contain a discrete lipid bilayer bound by two amphipathic scaffold proteins. Because ND particles permit the functional reconstitution of membrane/envelope proteins, we incorporated recombinant hemagglutinin (HA) from influenza virus strain A/New Caledonia/20/99 (H1N1) into NDs and investigated their potential to elicit an immune response to HA and confer immunity to influenza virus challenge relative to the commercial vaccines Fluzone and FluMist. HA-ND vaccination induced a robust anti-HA antibody response consisting of predominantly the immunoglobulin G1 (IgG1) subclass and a high hemagglutination inhibition titer. Intranasal immunization with HA-ND induced an anti-HA IgA response in nasal passages. HA-ND vaccination conferred protection that was comparable to that of Fluzone and FluMist against challenge with influenza virus strain A/Puerto Rico/8/1934 (H1N1).
The influenza A virus-type viral genome encodes 11 proteins including hemagglutinin (HA) and neuraminidase (NA). HA is important in virus transmission and is also a major determinant of host range (16). NA prevents viral aggregation and helps in the release of new viruses from the infected cell (25). These glycoproteins are the principal antigens against which humoral immune responses of the host are directed. Vaccination has been accepted as the most effective method of preventing influenza virus. Current licensed vaccines against influenza virus include conventional inactivated virus vaccine, live-attenuated vaccine, or inactivated “split-virus” vaccines, all grown in embryonated chicken eggs. Influenza virus vaccines may contain residual egg-derived antigens, which is a risk factor for persons with hypersensitivity to eggs. In the case of live-attenuated vaccines that are delivered by the mucosal route, there are several potential safety concerns including the possibility that the vaccine strain could undergo spontaneous genetic change and in a rare case of simultaneous infection with another influenza virus could undergo antigenic shift. These factors are of special concern for children and the elderly, who are the primary populations at risk for influenza virus infection (9). Therefore, there is a continuing need for developing more efficacious and safer vaccines.
Apart from licensed vaccines, a number of different vaccine formulations including soluble glycoproteins, virus-like particles, and subunit vaccines (6, 9, 14) with various efficacies have been developed. Recombinant glycoprotein vaccines offer many distinct advantages, including cost, the possibility of adapting them to rapidly changing strains within a short time, and independence from egg-based formulations. In experimental setups, recombinant HA (rHA) and recombinant NA have provided protection against lethal challenge to mice (18, 27). The safety, immunogenicity, and efficacy of trivalent rHA vaccines have been established (26), and a potential trivalent HA vaccine (FluBlok; Protein Sciences Corporation) is currently in phase III clinical trials.
Some rHA-based vaccines elicit high titers of anti-HA antibodies. However, these antibodies do not necessarily possess a high capacity for virus neutralization. This apparent discrepancy likely results from the use of soluble HA protein that may not accurately mimic the native structure of the membrane-embedded glycoprotein on the viral envelope for immunization. This could result in a robust antibody response with a limited ability to react with “native epitopes.” This notion is supported by data from previously reported studies that indicated that antigens expressed in their native three-dimensional conformation can elicit a more effective antibody response than proteins in their nonnative forms (19). Therefore, we investigated whether rHA presented in a lipid-bilayer-embedded formulation would elicit a potent neutralizing antibody response.
The Nanodisc (ND) system was developed as a novel method for functionally reconstituting membrane proteins into soluble nanoscale lipid bilayers (3, 4, 12, 22). NDs are robust, reproducible, and monodisperse discoidal particles 5.5 nm high and nominally 10 nm in diameter that are formed via a self-assembly process. ND particles contain two copies of an alpha-helical, amphipathic protein, termed membrane scaffold protein (MSP), which encircles a lipid bilayer in a “belt-like” fashion (Fig. 1a). A mixture of phospholipids and MSP are placed in a nonequilibrium solubilized state, for instance, using detergent or high hydrostatic pressure, and the system is then allowed to approach equilibrium by the gentle removal of the perturbant. This initiates a process of self-assembly, wherein the phospholipids and MSP find each other and generate a discoidal phospholipid bilayer encircled by the MSP. The resulting nanostructures represent a highly stable and homogeneous population with an aqueous solubility in the millimolar range (11).
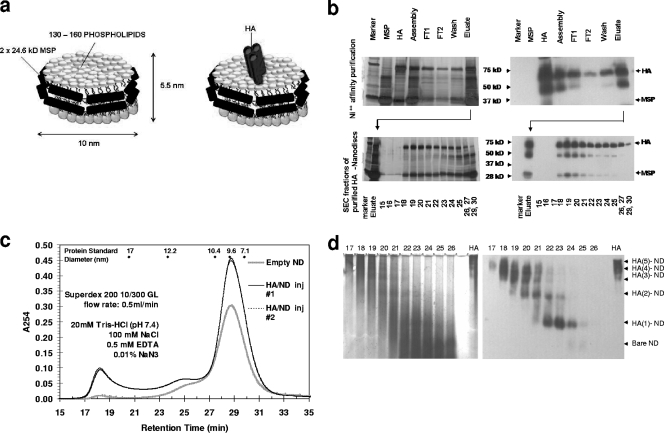
FIG. 1.
Construction of HA-NDs. (a) Schematic showing an ND particle that contains a phospholipid bilayer encircled by membrane scaffold proteins (left) (5) and the same ND particle with an embedded transmembrane protein (right) (17). (b) HA-ND assemblies were first purified by Ni2+ affinity chromatography. (Top left) Silver-stained SDS-PAGE showing flowthrough, wash, and elution of HA-ND assembly mix over a Ni-nitrilotriacetic acid column (FT1 and FT2 are flowthrough, and the eluate contains the eluted protein). Arrows show the positions of the 72-kDa HA band and the 25-kDa MSPs. (Top right) Anti-HA Western blotting of the same SDS-PAGE gel. Depending on the quality of purification, a certain fraction of full-length 72-kDa rHA (HA0) can exist as proteolytically cleaved HA1 (∼50-kDa) and HA2 (∼28-kDa) subunits. (Bottom left) Ni2+ column eluates were further purified by SEC. Silver-stained SDS-PAGE gel shows size-based fractionation of Ni2+ column eluate. The numbers at the bottom correspond to the fractions collected. The MSP amounts are largest at fractions 27 to 30, showing that empty NDs eluted at those fractions. (Bottom right) Anti-HA Western blotting of the same SDS-PAGE gel showing that HA-ND assemblies eluted mainly between fractions 18 and 26. (c) Elution profile of HA-ND following SEC separation. The elution times for protein standards used for calibration are indicated at the top. The control profile for empty NDs is superimposed. HA-ND assemblies have a shorter retention time than empty NDs. inj, injection. (d) HA-ND assemblies from different SEC fractions separate as discrete-sized molecules upon native PAGE separation. Silver staining (left) and anti-HA Western blotting (right) of native PAGE gels from size exclusion fractions show different HA polymers contained in NDs. Earlier fractions are rich in higher-polymeric forms of HA, while later fractions are richer in monomeric HA. Control HA was loaded in the last well to the right in both cases.
The value of the ND self-assembly process is that one can simply and reproducibly incorporate membrane proteins into these structures. This is accomplished by including the membrane protein in the initial mixture of MSP, lipid, and detergent prior to the initiation of the self-assembly process. An incorporated membrane protein then finds itself in a native-like environment with stability and activity normally found in vivo. By using phospholipids with different chemical characteristics (charge, degree of unsaturation, and length of acyl chains), the bilayer environment can be optimized to accommodate functional requirements. Furthermore, larger scaffold proteins, which in turn create a larger-diameter particle, can be employed to incorporate multimers or membrane protein complexes. Numerous membrane proteins from the three major classes-integral, tethered, and embedded (including monomers and multimers)-in the lipid bilayer environment created by NDs have been studied (2-5, 8, 10, 13, 20, 23). Since the ND system creates a stable bilayer environment that mimics that encountered by a membrane protein in the cell membrane, membrane proteins display normal folding, native ligand binding kinetics, and intact signaling activity (1, 3, 5, 8, 10, 13, 17, 23).
In this study, we successfully incorporated recombinant baculovirus-derived HA into NDs (HA-ND) and compared its efficacy to induce a relevant immune response and confer protection against influenza virus challenge with those of existing licensed vaccines by using a mouse model.
MATERIALS AND METHODS
Proteins, vaccines, and viruses.
Insect cell-derived rHA from influenza virus A/New Caledonia/20/99 (H1N1) was purchased from Protein Sciences Corporation (Meriden, CT). MSP1T2 was grown and purified as described previously (11). The MSPs used in the experiments have N-terminal polyhistidine tags. Concentrations were determined by the absorbance at 280 nm by using calculated extinction coefficients (15). Palmitoyloleoyl phosphatidylcholine (POPC) was obtained from Avanti Polar Lipids (Alabaster, AL), dissolved in chloroform, and quantified by phosphate analysis (7).
The Fluzone (Sanofi Pasteur) intramuscular (i.m.) vaccine for the 2007-2008 influenza season used in this study is formulated to contain 45 μg HA per 0.5-ml dose in a ratio of 15 μg HA from the following three prototype strains: A/Solomon Islands/3/2006 (H1N1), A/Wisconsin/67/2005 (H3N2), and B/Malaysia/2506/2004. The FluMist (MedImmune) trivalent intranasal (i.n.) vaccine for the 2007-2008 season used in this study is formulated to contain 106.5 to 107.5 50% tissue culture infective doses (TCID50) of each of the three strains of live-attenuated influenza virus (LAIV) reassortants detailed above for Fluzone.
The influenza viruses A/New Caledonia/20/99, A/Solomon Islands/3/06, and A/Puerto Rico/8/1934 (PR/8/34) were a gift from A. Garcia-Sastre (Mount Sinai School of Medicine, New York, NY).
Mice.
Female BALB/c mice, 6 to 8 weeks of age, were purchased from Charles River Laboratories (Wilmington, MA). Three to five mice per cage were housed in the Biologic Resources Laboratory facilities at the University of Illinois (Chicago, IL) and were provided food and water ad libitum. All mice were cared for in accordance with the guidelines set forth by the University of Illinois Animal Care and Use Committee.
Construction of HA-ND.
rHA was initially solubilized in β-octylglucoside (β-OG) to a final concentration of 100 mM. ND particles were prepared by using the conditions and MSP, cholate, and phospholipid ratios previously described (11). Briefly, dried phospholipid was reconstituted with HA-disc buffer (30 mM Tris-HCl [pH 7.5], 0.3 M NaCl) containing 100 mM sodium cholate. MSP from stock solution (200 to 400 μM) was combined with the POPC-cholate mixture and incubated on ice with gentle agitation. Detergent-solubilized rHA (typically ∼190 μM) or β-OG alone was added to the MSP-POPC-cholate mixture at mole ratios of 2 MSPs to 3 HAs with various amounts of phospholipid to optimize self-assembly. The mixtures were incubated for 1 h on ice, with the final concentration of β-OG maintained at 100 mM. Self-assembly was initiated by the addition of ∼500 mg wet SM-2 Bio-Beads (Bio-Rad, Hercules, CA) per ml of solution. Mixtures were incubated for an additional 1 h on ice with gentle agitation to keep the beads suspended. Supernatant was removed from the Bio-Beads by centrifugation.
IMAC and SEC.
NDs containing rHA were purified by immobilized metal affinity chromatography (IMAC) by using a 3-ml column containing Fast-Flow Sepharose (GE Healthcare, Piscataway, NJ) charged with Ni2+ and separated according to the manufacturer's recommended protocol by using IMAC buffer (40 mM Tris-HCl [pH 7.5], 0.3 M NaCl). Following extensive column washing with IMAC buffer (without and with 50 mM imidazole), NDs containing rHA were eluted in the presence of IMAC buffer containing 0.4 M imidazole. The recovery of HA-ND from the Ni-nitrilotriacetic acid resin is typically in the range of 50 to 70% due mostly to the incomplete elution of the sample. IMAC eluates were concentrated by centrifugal ultrafiltration. ND and HA-ND samples were fractionated by size exclusion chromatography (SEC) as previously described for NDs (11). Briefly, samples were filtered (0.22 μm) and injected onto a Superdex 200 HR 10/30 column (GE Healthcare) equilibrated with disc buffer (20 mM Tris-HCl [pH 7.4], 0.5 mM EDTA, 150 mM NaCl, 0.1% [wt/vol] sodium azide) run at 0.5 ml/min at room temperature. Fractions were collected every 1 min, and peak elution was monitored at 254 nm. Following IMAC and SEC separations, HA-ND and ND samples were analyzed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and Western blotting using anti-HA (goat immunoglobulin G [IgG]; BEI Sources, ATCC) and anti-His (rabbit IgG; Santa Cruz) antibodies.
HA-ND quantification.
HA content and ND content (determined by the concentration of MSP) were measured by enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well ELISA plates were coated with various amounts of (i) rHA, (ii) MSP, and (iii) HA-ND SEC eluates in 0.1 M carbonate bicarbonate buffer (pH 9.6) by overnight incubation at 4°C. Plates were blocked (25°C for 2 h) with phosphate-buffered saline (PBS) containing Tween 20 (0.05%, vol/vol) and bovine serum albumin (3%, wt/vol). Following a thorough washing in PBS-Tween 20 (0.05%, vol/vol), the plates were further incubated (25°C for 2 h) with anti-HA antibodies (1:5,000) (goat IgG; BEI Sources, ATCC) or anti-His antibodies (1:5,000) (rabbit IgG; Santa Cruz). After further washing, samples were incubated (25°C for 1 h) separately with horseradish peroxidase-conjugated polyclonal anti-goat (for rHA) or anti-rabbit (for MSP) IgG (1:5,000; Invitrogen). Samples were then incubated with tetramethylbenzidine substrate solution in the dark for 15 min. The reaction was stopped by adding 1 M HCl to the mixture, and the colorimetric change was measured as the optical density at 450 nm (OD450) by using a microplate reader (model 550; Bio-Rad). Concentration-dependent standard curves were constructed for rHA and MSP from the respective OD450 values. The HA and MSP contents of unknown HA-ND mixtures were then calculated from the corresponding OD450 values by extrapolation using standard curves.
Hemagglutination assay.
The HA activity of rHA, ND, and HA-ND in SEC fractions was tested in untreated 96-well U-bottom plates (Nunc, Naperville, IL). Equal volumes (50 μl each) of 0.6% chicken red blood cells (RBCs) (cRBCs) in PBS and test samples (rHA, NDs, or HA-NDs) twofold serially diluted in PBS were combined at room temperature and incubated for 75 to 120 min. The HA titer was the reciprocal of the greatest sample dilution that did not allow the sedimentation of the cRBCs compared to negative control wells that contained only buffer. The positive control for HA was an influenza A virus (PR/8/34 [H1N1]), grown in eggs, for which the titer had been previously determined.
HI assay.
To inactivate nonspecific inhibitors, sera were treated with receptor-destroying enzyme (RDE; Sigma) prior to testing. Briefly, 4 parts RDE was added to 1 part serum and incubated overnight at 37°C. Five parts 1.5% (wt/vol) sodium citrate was added to the mix. RDE was inactivated by incubation at 56°C for 30 min. RDE-treated serum was twofold serially diluted in 96-well U-bottom tissue culture plates starting with a 10-fold initial dilution. An equal volume of virus (of each of three strains, A/New Caledonia/20/99, A/Solomon Islands/3/06, or PR/8/34), adjusted to approximately 8 HA units/50 μl, was added to each well. Anti-PR/8/34 serum was used as a positive control. Nonimmunized serum was used as a negative control. The plates were covered and incubated at room temperature for 20 min followed by the addition of 0.6% (vol/vol) cRBCs (Lampire Biologicals, Pipersville, PA) in PBS and mixed by agitation. The plates were covered, and the cRBCs were allowed to settle for 30 min at room temperature. The hemagglutination inhibition (HI) titer was determined by the reciprocal dilution of the last well that contained nonagglutinated cRBCs.
Immunization, bleeding, and viral challenge.
For dose-response studies and comparison of HA-ND with commercially available vaccines, two routes of immunization were used, i.m. and i.n. For dose optimization studies, groups of three mice each were immunized i.m. (50 μl in the thigh muscle) or i.n. (20 μl i.n. after sedation with 200 μl of 0.05 mg/ml fentanyl intraperitoneally) with HA-ND having (i) 0.005 μg, (ii) 0.05 μg, (iii) 0.5 μg, or (iv) 5.0 μg of rHA incorporated into NDs per mouse and boosted with an identical dose on day 14. Mice were tail bled on days 0, 7, 10, and 13. The mice were euthanized on day 26, serum was collected for analyses of the humoral response, and spleen was collected for T-cell proliferation studies.
For comparisons between buffer, HA alone, or HA-ND, three groups of 10 mice each were immunized subcutaneously on day 1 and i.m. boosted on days 8 and 15 with 50 μl of (i) Tris-buffered saline (TBS), (ii) HA alone (5 μg/mouse), and (iii) ND-incorporated HA (containing 5 μg of HA/mouse). ND particles in sterile TBS at a physiological pH were provided by Nanodisc Inc. (Champaign, IL) for controls. A 50-μl sample of blood was collected from the tail vein of these mice on days 0, 7, and 13. Three mice from each group were sacrificed on day 20. Sera from the bleeds were used for analyses of the immune response. Spleens were collected from these mice at the time of sacrifice for in vitro studies with cultured lymphocytes. On day 21, thrice-immunized mice from all groups (n = 7 per group) were sedated with 200 μl fentanyl (0.05 mg/ml) per mouse and i.n. challenged with 100 50% lethal doses (LD50) of live influenza virus PR/8/34 in a 20-μl volume. LD50 titers were determined by the method of Reed and Muench (21a) by inoculating groups of four mice i.n. with dilutions of virus. Infected mice were observed for a period of 14 days for weight loss and mortality. Mice were euthanized if they lost 30% of their body weight.
For comparison of the immunogenicity of HA-ND with that of Fluzone, three groups of three mice each were immunized with (i) 5 μg HA-ND and (ii) the inactivated vaccine Fluzone containing 5 μg of HA from H1N1 alone as part of a trivalent vaccine formulation per mouse in a 50-μl volume on day 1 and boosted with an equal amount of the corresponding vaccine on day 14. Mice were tail bled on day 0 and day 13 and euthanized on day 26. Sera were used for analyses of antibody responses.
For comparisons between HA-ND and FluMist, two groups of three mice each were sedated with 200 μl fentanyl per mouse and immunized i.n. with either (i) HA-ND containing 5.0 μg of rHA incorporated into NDs per mouse and (ii) the live-attenuated vaccine FluMist containing 106.5 to 107.5 TCID50 of trivalent LAIV per mouse, each in a 20-μl volume on day 1, and boosted with an equal amount of the same on day 14. Mice were tail bled on day 0, day 7, day 10, day 13, and day 26, at which point they were euthanized. Sera were used for analyses of antibody responses. Nasal wash specimens were collected by pushing 0.5 ml of RPMI medium with 5% (vol/vol) fetal calf serum through each nostril and capturing effluent from the posterior opening.
For viral challenge studies, six groups of 10 mice each were immunized on days 1 and 14 with (i) TBS i.m., (ii) ND alone (∼10 μg ND/mouse) i.m., (iii) HA-ND (with 5 μg HA/mouse) i.m., (iv) Fluzone (15 μg of total HA as a trivalent inactivated virus) i.m., (v) HA-ND (with 5 μg of HA/mouse) i.n., and (vi) FluMist (containing 106.5 to 107.5 TCID50 of trivalent LAIV/mouse) i.n. after fentanyl-induced sedation. Mice were challenged with 100 LD50 of a live H1N1 influenza virus A strain (PR/8/1934) on day 25. The mice were monitored for weight loss or other distress after viral challenge over a period of 14 days and scored for survival.
Serological assays.
A quantitative ELISA was performed to assess anti-HA-specific IgM, IgA, or IgG isotypes in immune serum. Briefly, each well of a 96-well plate was coated with 20 ng of purified rHA from H1N1 strain NC 20/99 (Protein Sciences, Meriden, CT) and 0.1 M carbonate-bicarbonate buffer (pH 9.6) by overnight incubation at 4°C. Plates were blocked (25°C for 2 h) with PBS containing Tween 20 (0.05%, vol/vol) and bovine serum albumin (3%, wt/vol) and then incubated with serial dilutions of sera (25°C for 2 h). Following thorough washings in PBS-Tween 20 (0.05%, vol/vol), samples were incubated (25°C for 1 h) separately with horseradish peroxidase-conjugated anti-mouse IgM, IgG1, IgG3, IgG2a, IgG2b, and IgA (1:5,000; Zymed-Invitrogen) diluted in PBS-Tween 20 (0.05%, vol/vol). Upon thorough washing, samples were incubated with tetramethylbenzidine substrate solution in the dark for 15 min. The reaction was stopped by adding 1 M HCl to the mixture, and the colorimetric change was measured as the OD450 by using a microplate reader (model 550; Bio-Rad). All samples were analyzed in triplicates, and results were recorded as average OD450 units ± standard errors. All calculations were carried out by using Microsoft Excel.
For determination of antibody titers of Fluzone-, FluMist-, or ND-specific antibodies, each well was coated separately with Fluzone containing 60 ng of HA as a trivalent vaccine, FluMist containing 5 × 102.5 to 5 × 103.5 TCID50 of LAIV as a trivalent vaccine, or 30 ng of ND. The subsequent steps of the quantitative ELISA were the same as those described above.
T-cell proliferation assay.
Spleens were collected from euthanized mice, and splenocytes were cultured at 106 cells/ml in 96 wells at a final volume of 100 μl in 96-well tissue culture plates (Becton Dickinson) in RPMI medium with 10% (vol/vol) fetal calf serum in the presence of different concentrations of HA, HA-ND, or TBS. Cells were incubated for 72 to 96 h at 37°C in the presence of [3H]thymidine (10 μCi/well). Cell proliferation was assessed by measuring [3H]thymidine incorporation. Results were plotted as counts per minute after normalization with buffer-treated controls.
Statistical analysis.
Statistical analyses for antibody titers and HI titers or T-cell proliferations were performed by using a one-tailed t test with unequal variance. Samples from HA-ND-vaccinated mice were compared to samples from HA-alone-, Fluzone-, or FluMist-vaccinated mice, and significance was considered at a P value of <0.05.
RESULTS
Incorporation of HA into NDs.
rHA (72 kDa) from influenza virus A (A/New Caledonia/20/99 [H1N1]), solubilized with β-OG, was incubated with POPC and MSP1T2 (24.6 kDa). Following the removal of detergent, HA-ND assemblies (illustrated in Fig. 1a) were formed via self-assembly. Because HA-ND particles are prepared in the presence of an excess of MSP and phospholipid, self-assembly yields a mixture of HA-ND and empty ND particles. Unincorporated HA was removed by negative selection using an IMAC purification step, which utilizes a His6 affinity tag present on the MSP (Fig. 1b, top). The eluate contains a mix of (i) empty NDs and (ii) HA-NDs consisting of HA molecules trapped inside NDs. Because the incorporation of HA into an ND particle is predicted to increase the hydrodynamic radius of HA-ND compared to that of ND alone, HA-ND particles are expected to exhibit a decreased retention time compared to that of ND only. Therefore, the mixture was further fractionated by SEC and analyzed by SDS-PAGE and Western blotting (Fig. 1b, bottom, and c). Fractions 18 to 30 contained various amounts of HA as determined by ELISA and Western blotting using anti-HA antibodies (Fig. 1b, bottom). The HA protein can form a trimer; therefore, HA-ND SEC fractions were tested to determine whether they contained monomers and higher multimers of HA per ND.
The HA and ND contents of SEC fractions were determined by ELISA using anti-HA and anti-MSP antibodies (Table 1). Assuming a monomeric-HA mass of 72 kDa and a single-ND mass of ∼150 kDa, the ratio of the number of HA molecules contained per ND was calculated. Later fractions (fractions 22 and above) had an HA-to-ND ratio of less than 1, suggesting that there was a significant amount of NDs eluting at those fractions without any trapped HA. Fractions 21 and 20 had HA-to-ND ratios of between 1 and 2, suggesting predominant populations of NDs with monomeric and dimeric HA. Fractions 19 and 18 show HA-to-ND ratios above 3, which implies the presence of HA trimers or higher oligomers. These HA-ND SEC fractions, separated by 6% native PAGE and subjected to Western blotting with anti-HA antibodies, also revealed that the earlier fractions were rich in trimers (or higher multimers) of HA, while the later fractions had monomeric and dimeric forms (Fig. 1d, right). rHA alone appeared to exist primarily as trimers and higher oligomers, which is expected in the absence of any detergent to stabilize its monomeric form (Fig. 1d). When the same native PAGE gel was silver stained, the later fractions were revealed to be rich in empty NDs (Fig. 1d, left). Because of their smaller hydrodynamic sizes, empty NDs have a longer retention time than HA-NDs and elute primarily within fractions 27 to 31. These later fractions, enriched in empty NDs, were excluded, and fractions 18 to 24, which contained the bulk of HA, were pooled. HA and ND contents were quantified by ELISA by using anti-HA and anti-MSP antibodies, respectively. The pooled fractions were found to contain 11.3 μg of HA and 17.2 μg of ND per ml. The HA-ND mix was concentrated 10-fold, and buffer was exchanged with TBS and sterile filtered for use as a test vaccine.
TABLE 1.
HA and ND contents of SEC fractions and their hemagglutination activitiesa
| Fraction | HA (μg/ml) | ND (μg/ml) | Mol ratio (HA to ND) | HA units/ml | HA units/μg of HA |
|---|---|---|---|---|---|
| 18 | 11.4 | 6.2 | 3.8 | 3,200.0 | 280.7 |
| 19 | 16.8 | 8.9 | 3.9 | 6,400.0 | 381.0 |
| 20 | 13.8 | 14.3 | 2.0 | 3,200.0 | 231.9 |
| 21 | 10.5 | 15.0 | 1.5 | 1,600.0 | 152.4 |
| 22 | 8.0 | 19.2 | 0.9 | 400.0 | 50.0 |
| 23 | 7.3 | 24.3 | 0.6 | 200.0 | 27.4 |
| 24 | 7.9 | 32.0 | 0.5 | 100.0 | 12.7 |
| HA alone | 20 | 3,200.0 | 160 | ||
| ND alone | 50.0 | 0.0 |
HA and ND contents of SEC fractions were determined by anti-HA and anti-MSP ELISA. The approximate HA-to-ND ratio for each fraction was determined for a monomeric HA mass of 72 kDa and an ND mass of 150 kDa. The extent of hemagglutination for each fraction was calculated from the results of a hemagglutination assay using twofold serial dilutions. The number of hemagglutination units per microgram of HA was determined for each fraction by correcting for the HA content. Hemagglutination values for control HA and empty NDs are also shown.
The native HA structure is necessary for efficient binding to sialic acid residues on RBCs and for subsequent hemagglutination. To determine the effect of ND incorporation on HA structure, we performed in vitro hemagglutination tests with the SEC fractions. Briefly, we diluted each fraction 25-fold and, using those fractions as starting concentrations, set up twofold serial dilutions for each fraction in sterile PBS, and we added equal volumes of 0.6% cRBCs. rHA alone, at 20 μg/ml, was used as a control. Empty NDs at 50 μg/ml were used as a negative control and did not cause any hemagglutination. The number of HA units/ml was calculated for each fraction based on the last dilution showing complete hemagglutination (Table 1). Correcting for the HA content, the number of hemagglutination units/μg of HA was calculated for each fraction. As shown in Table 1, it is apparent that the fractions with monomeric and dimeric HA trapped in NDs show low hemagglutination, while those containing predominantly trimeric HA exhibit a very high capacity for hemagglutination. In all fractions containing significant trimeric HA embedded in NDs, the numbers of hemagglutination units/μg of HA were higher than that observed for rHA alone, indicating that ND incorporation provides a stable membrane-like scaffolding to rHA to help maintain its native structure.
HA-ND immunization can induce IgM and IgG responses.
We tested the efficacy of HA-ND vaccination by both the i.m and i.n routes. First, two equal i.m doses (primary and boosting) were used to establish an optimum HA-ND dose and to evaluate its potential as a vaccine. To determine an optimal i.m. vaccine dose, four groups of three mice each were immunized (referred to as the i.m. set) on day 1 with doses of 0.005 μg, 0.05 μg, 0.5 μg, or 5.0 μg of HA-ND. The CDC recommendation for a single i.m. dose in humans is 15 μg HA equivalents of inactivated virus per strain in a trivalent vaccine formulation. Assuming that the average body weight of a human is 60 kg, this corresponds to 0.005 μg of HA per 20-g mouse. Therefore, this amount was chosen as the minimum dose for the i.m. set. We also evaluated the potential of i.n. administration of HA-ND as a means of generating a significant IgA response. An i.n. dose that maintained parity with the i.m. immunization was chosen: four groups of three mice each were immunized i.n. (referred to as the i.n. set) on day 1 with doses of 0.005 μg, 0.05 μg, 0.5 μg, or 5.0 μg of HA-ND. The anti-HA IgM response was evaluated by ELISA at 1:200 dilutions of sera.
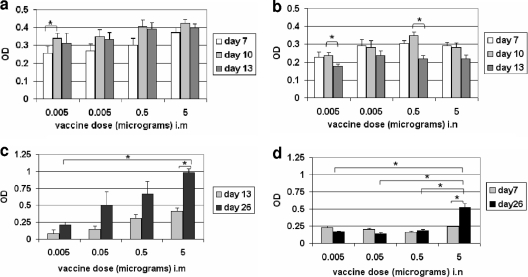
For the i.m. set, the IgM response peaked at around 10 days postimmunization and showed a marginal reduction by day 13 across different doses, although the difference was not statistically significant (Fig. 2a). Similarly, in the i.n. group, the primary IgM response peaked at around day 10 (Fig. 2b) and was reduced by day 13. The reduction of IgM levels from day 10 to day 13 was significant for the 0.005- and 0.5-μg/mouse dosage groups. Irrespective of the route of immunization, the degree of the IgM response (except for the 5-μg i.n. dose) appeared to correlate with the immunizing dose (Fig. 2a and b). Sera from unimmunized BALB/c mice were consistently negative for the presence of any anti-HA IgM or IgG.
FIG. 2.
Optimization of HA-ND doses. (a) Time course of IgM response to i.m. HA-ND vaccination showing a correlation of the HA content of the immunizing dose with serum antibody levels. OD450 values for anti-HA ELISA are shown as bars against immunizing HA content. Standard errors of sample means and statistical significances of the differences between the time points were calculated. Statistically significant differences are shown (*, P ≈ 0.05). (b) Time course of IgM response to i.n. HA-ND vaccination. OD values for anti-HA ELISA are shown as bars against immunizing HA contents. (c) Total IgG response (ELISA) after i.m. primary and boosting HA-ND immunizations. Serum anti-HA IgG levels correlate with immunizing doses. (d) Total IgG response (ELISA) after i.n. primary and boosting HA-ND immunizations.
On day 14, mice were immunized with a second and equal dose of HA-ND. The mice were euthanized on day 26, and the anti-HA serum IgG level was measured by ELISA at a 1:200 dilution. In the i.m. set, the serum IgG levels correlated with increasing doses of HA-ND, and the differences between the highest- and the lowest-dose groups were significantly different. There was a consistent increase in serum IgG levels from day 13 to day 26 across the different dosage groups, indicating a recall response (Fig. 2c). The increase was significant for the 5-μg group. It was not statistically significant in the other groups, possibly due to variation between individual samples, as reflected by the error bars. However, in the i.n. set, except for the 5-μg group, the other groups showed no significant differences either in anti-HA serum IgG levels or in recall responses. The differences in IgG responses between the 5-μg group and the other dosage groups were significant, as was the difference between the primary and secondary IgG responses within this group. This demonstrated that the immunological threshold for i.n. HA-ND vaccination was 5 μg per dose (Fig. 2d). Therefore, in all subsequent studies (both i.m. and i.n.), we used 5 μg/dose of HA in HA-ND.
ND incorporation contributes to the immunogenicity of HA.
Since rHA alone can elicit an immune response, we wanted to see if ND incorporation significantly enhanced its immunogenicity. We immunized three groups of 10 mice each subcutaneously on day 1 and boosted mice i.m. on days 8 and 15 with TBS alone, HA alone, and HA-ND. Three mice per group were tail bled on days 0, 7, and 14 and euthanized on day 21. Sera from these mice were tested for antibody responses. Day 14 sera from HA-ND-immunized mice had significantly higher anti-HA IgG levels than HA-only-immunized mice, although IgM levels were comparable (Fig. 3a), at 1:200 dilutions of sera. There was little or no immune response to ND even upon two immunizations (data not shown).
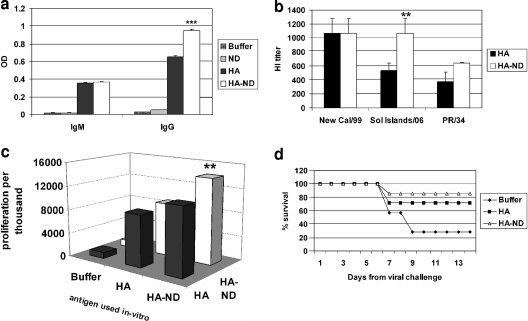
FIG. 3.
HA-ND is more immunogenic than HA alone. (a) Comparison of serum anti-HA IgM and total IgG levels (ELISA) between HA-ND- and HA-only-immunized mice after two immunizations (day 14). Statistically significant differences are marked (**, P = 0.05 to 0.01; ***, P < 0.001). Corresponding values for buffer- and ND-immunized mice are shown as controls. (b) HI titers of sera from mice immunized twice (day 14) with HA or HA-ND were compared. HI values are plotted as reciprocals of the serum dilution that could neutralize 8 HA units of the indicated influenza A (H1N1) virus. New Cal/99, A/New Caledonia/20/99; Sol Islands/06, A/Solomon Islands/3/2006. (c) 3H incorporation is shown as a measure of the extent of T-cell proliferation when T cells from HA-ND- or HA-only-immunized mice were challenged in vitro with either HA-ND or HA only (day 21). Proliferation levels without antigenic challenge were used for normalization. (d) Protective efficacy of HA or HA-ND vaccination of mice after live-influenza-virus PR/8/34 viral challenge. A 14-day survival chart for immunized mice after viral challenge is shown.
Sera were also tested for the presence of virus-neutralizing antibodies through HI assays (Fig. 3b). The HI titers of both HA- and HA-ND-immunized sera were similar and maximum against the A/New Caledonia/20/99 virus, which is the parent virus strain for the HA used in this study. However, sera from HA-ND-immunized mice had higher HI titers against the other two viruses tested. This difference was significant against the A/Solomon Islands/3/2006 strain (and marginally missing significance against the PR/8/34 strain). These results indicated that HA-ND can elicit an HI antibody response that is more broadly reactive than that induced by HA alone.
Splenocytes from HA-ND-immunized mice showed higher proliferation values than did splenocytes from HA-immunized mice in the presence of either antigen as determined by [3H]thymidine incorporation (Fig. 3c). Although the difference was smaller when HA alone was used, it was significant when HA-ND was used as the in vitro challenge antigen.
The remaining seven mice from each group were challenged with a live H1N1 strain of influenza virus (PR/8/34) on day 23 and scored for survival over a period of 14 days. By the end of the 14-day observation period, five mice out of seven died in the control group (Fig. 3d). Two mice died from the HA-immunized group, while only one died from the HA-ND-immunized group. Furthermore, the HA-ND-immunized group showed a lower amount of weight loss than the HA-immunized group (not shown). Collectively, these data indicated that incorporation into ND can confer greater protective efficacy to rHA.
i.m. HA-ND vaccination elicits an immune response that is comparable to that elicited by a commercial “split-virus” vaccine.
Fluzone is one of the commercially available inactivated “split-virus” vaccines administered i.m. For the 2007-2008 influenza virus season, it was formulated to contain 45 μg HA per 0.5-ml dose, in the recommended ratio (for human vaccination) of 15 μg HA from the following three prototype strains: A/Solomon Islands/3/2006 (H1N1), A/Wisconsin/67/2005 (H3N2), and B/Malaysia/2506/2004. To allow direct comparisons between HA-ND and Fluzone, we used similar HA contents between HA-ND and Fluzone. Since we previously determined 5 μg/mouse of HA incorporated into NDs to be the experimental dose, we wanted to compare it to the Fluzone dose that contained 5 μg of HA from the H1N1 strain alone. This dose of Fluzone would have 15 μg (3 × 5 = 15 μg) of total HA combined from all three strains.
Two groups of three mice each were immunized i.m. with either Fluzone containing 15.0 μg of total HA (∼5 μg from an H1N1 strain only) or 5 μg of HA-ND on day 1 and boosted with an identical dose on day 14. Mice were prebled on day 0 for controls and on days 13 and 26, after which they were euthanized.
At this stage, it is pertinent that the rHA contained in the HA-ND formulation was derived from the H1N1 strain A/New Caledonia/20/99. We compared anti-HA (A/New Caledonia/20/99 [H1N1]) antibody levels assuming cross-reactivity between HAs from different H1N1 strains. The anti-HA serum IgM and IgG levels between HA-ND and Fluzone immunizations were comparable at 1:200 dilutions of sera (Fig. 4a). However, the anti-Fluzone total IgG levels were significantly higher in Fluzone (∼2.5-fold)-immunized than in HA-ND-immunized mice (data not shown), indicating that most of the antibody response was most likely directed against either HA from the other two strains. The anti-Fluzone response in the HA-ND-immunized mice is presumably limited to the H1N1-specific HA component of Fluzone only, which constitutes only one-third of the total formulation. The anti-HA subclass-specific responses were determined by ELISA (Fig. 4b). We noted a significant difference in the IgG subclass responses between the groups. For the HA-ND-immunized group, the major contribution was from the IgG1 subclass, while in the Fluzone-immunized group, the dominant subclass was IgG2a. Although both groups showed considerable IgG2b responses, the response was greater in the Fluzone-immunized group.
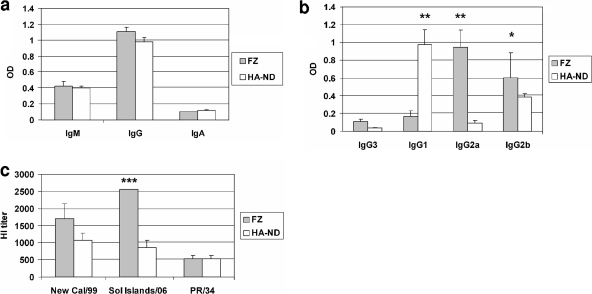
FIG. 4.
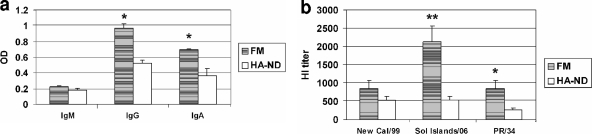
Comparison of immune responses upon i.m. immunization with HA-ND or Fluzone. (a) Anti-HA (A/New Caledonia/20/99 [New Cal/99]) IgM and total IgG and IgA antibody responses were compared (ELISA) between HA-ND and Fluzone immunizations. Statistically significant differences are marked (*, P ≈ 0.05; **, P = 0.05 to 0.01; ***, P < 0.001). (b) Anti-HA IgG subclasses (IgG1, IgG3, IgG2a, and IgG2b) were compared between Fluzone- and HA-ND-immunized mice. (c) HI titers of sera from HA-ND- and Fluzone-immunized mice were compared. Sol Islands/06, A/Solomon Islands/3/2006.
Immune sera were twofold serially diluted and tested for their capacities to neutralize three different H1N1 viruses, namely, A/New Caledonia/20/99, A/Solomon Islands/3/2006, and PR/8/34 (Fig. 4c). As expected, sera from Fluzone-immunized mice had significantly higher HI titers against the A/Solomon Islands/3/2006 virus, which is the parent virus strain for the HA contained in Fluzone. These sera also contained higher HI titers against A/New Caledonia/20/99 (although not statistically significant) than did sera from HA-ND-immunized mice. However, the HI titers in sera from Fluzone- and HA-ND-immunized mice against PR/8/34 were comparable. Interestingly, HA from strain A/Solomon Islands/3/2006 has a higher degree of identity to HA from strain A/New Caledonia/20/99 (97% at the DNA level) than the HA from strain PR/8/34 (88% at the DNA level). These results indicated that the higher the antigenic drift of the HA molecule, the lesser the protective efficacy of the commercial “split-virus” vaccine. However, immunization with HA-ND not only induced comparable HI titers against PR/8/34 but also elicited considerable cross-HI activity against the other two strains. This indicated to us that HA-ND can indeed provide significant cross-protection against the drifted strains.
i.n. HA-ND vaccination leads to secretory IgA production but has lower immunogenicity than commercial LAIV.
To compare the efficacies of HA-ND and FluMist vaccinations, on days 1 and 14, two groups of three mice each were immunized i.n. with either FluMist containing 106.5 to 107.5 TCID50 of total LAIV from three strains combined (which amounts to 0.33 × 106.5 to 0.33 × 107.5 TCID50 virus from the H1N1 strain alone) in a 20-μl volume or 5 μg of HA-ND. Mice were prebled on day 0 and again on days 10 and 26, at which point they were euthanized.
We compared anti-HA (H1N1 strain A/New Caledonia/20/99) as well as anti-FluMist (H1N1 strain A/Solomon Islands/3/2006) antibody levels in these immunized mice. The anti-HA serum IgM levels (at 1:500 dilutions of sera) between HA-ND and FluMist immunizations were comparable (Fig. 5a). The total IgG levels in sera and IgA levels in nasal wash specimens of FluMist-immunized mice were higher than those noted for HA-ND-immunized mice (at 1:200 dilutions of sera). Similarly, the anti-FluMist IgG and IgA levels in the nasal wash specimens of FluMist-immunized mice were significantly higher and were detectable at dilutions of 1:12,500 and 1:2,500, respectively. However, no anti-FluMist antibodies were detected in HA-ND-immunized mice at those dilutions (not shown).
FIG. 5.
Comparison of immune responses upon i.n. immunization with HA-ND or FluMist (FM). (a) Anti-HA (A/New Caledonia/20/99 [New Cal/99]) IgM and total IgG levels in sera and IgA levels in nasal wash specimens (ELISA) were compared. Statistically significant differences are marked (*, P ≈ 0.05; **, P = 0.05 to 0.01). (c) HI titers in the sera from i.n. wash specimens were compared. Sol Islands/06, A/Solomon Islands/3/2006.
Surprisingly, when we tested for HI activity in sera from immunized mice (Fig. 5b), we noted that the higher ELISA IgG antibody titer seen in FluMist-immunized mice did not completely correlate with the HI activity against the A/New Caledonia/20/99 virus. However, as expected, sera from FluMist-immunized mice had significantly higher HI titers than the sera from HA-ND-immunized mice against the A/Solomon Islands/3/2006 and PR/8/34 viruses. We conclude that although i.n. immunization with HA-ND produced both anti-HA IgG and IgA responses, FluMist immunization was more robust in terms of virus neutralization at the compared doses.
Incorporation of HA into ND allows it to react better with sera from vaccinated mice.
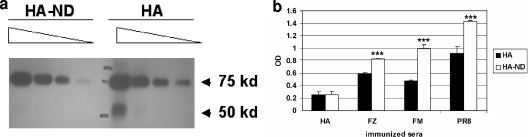
To determine whether HA-ND displayed epitopes that were more akin to virus envelope-associated HA, we measured the capacity of split- or whole-virus-immunized sera to react with either HA alone or HA-ND. For this, we used approximately equal amounts of HA either alone or as ND-incorporated material and compared the HA contents by Western blotting (Fig. 6a) by using a series of dilutions. The blot revealed that a slightly larger amount of HA was present in the HA preparation than in the HA-ND preparation. We then measured the capacities of different immunized sera to recognize either HA or HA-ND by ELISA. Although anti-HA serum showed a higher OD with HA alone than HA-ND, all sera from animals immunized with either split-virus subunits (Fluzone) or whole-virus preparations (FluMist and PR/8/34) showed higher ODs with HA-ND than with HA alone. We corrected for HA content by using values that were obtained with anti-HA sera and plotted the data. The differences between the OD values obtained for HA-ND were found to be significantly higher than those for HA alone (Fig. 6b). This clearly demonstrated that sera from both split- and whole-virus-immunized animals recognized HA-ND more efficiently than HA alone. This could imply that relative to soluble HA, the ND-embedded HA molecules mimic the viral envelope better, perhaps as a consequence of HA assuming a conformation that is closer to that of the HA on the viral envelope when it is embedded in the NDs.
FIG. 6.
Sera from whole-virus-immunized mice recognize HA-ND more efficiently than HA alone. (a) HA-ND from SEC fraction 19 was first diluted to approximately 300 ng/ml and then twofold serially diluted. Control HA was also diluted similarly, with an approximate starting concentration of 300 ng/ml. Twenty-five microliters of each dilution was resolved on an SDS-PAGE gel and Western blotted with anti-HA (PR/8/34 [PR8]) antibody. (b) HA-ND and HA alone (100 μl/well of ∼300 ng/ml) were used to coat Polysorp (Nunc) ELISA plates. Sera from HA-, Fluzone (FZ)-, and FluMist (FM)-immunized (twice) mice and anti-PR/8/34 (H1N1) antisera (BEI Resources, ATCC) were used at different dilutions to bind to coated HA and then probed with anti-mouse (Promega) (for HA-, Fluzone-, and FluMist-immunized sera) or anti-goat (for anti-PR/8/34) antibodies (Zymax; Invitrogen). The data from this assay were plotted after correcting for HA contents in both samples as measured by using anti-HA serum.
HA-ND vaccination provides protection against influenza virus PR/8/34 infection.
Finally, we tested whether our in vitro data correlated with protection against challenge with live influenza virus. We immunized six groups of 10 mice each two times (days 1 and 14) with one of the following antigens or control: (i) TBS, (ii) ND alone (∼10 μg ND/mouse), (iii) HA-ND (with 5 μg HA/mouse) i.m., (iv) Fluzone i.m. (15 μg of total HA as trivalent inactivated virus), (v) HA-ND (with 5 μg HA/mouse) i.n., or (vi) FluMist (containing 106.5 to 107.5 TCID50′of trivalent LAIV/mouse) i.n. On day 25 all immunized mice were challenged with a live H1N1 influenza virus strain (PR/8/34). Mice were sacrificed on day 14 postinfection or when mice showed >30% weight loss from the baseline, showed severe respiratory distress, and were unresponsive to stimuli. Six out of 10 mice died in the TBS control group, while 8 out of 10 died in the ND-immunized control group. We wondered whether the slight increase in mortality in ND-immunized mice was due to increased cytokine production and/or toxic effects of ND. To address these concerns, we measured the levels of proinflammatory cytokines in the sera of immunized mice by ELISA (eBiosciences). Although the positive-control standards were readily detected, the sera from both buffer-and ND-immunized mice contained less than 8 pg/ml interleukin-1β (IL-1β), 15 pg/ml IL-12, 4 pg/ml IL-6, 15 pg/ml gamma interferon, 2 pg/ml IL-2, and 8 pg/ml tumor necrosis factor alpha (data not shown). We also harvested various organs and conducted pathology with hematoxylin and eosin staining. We found no evidence of any toxicity associated with NDs (data not shown). Therefore, we conclude that the additional deaths in the ND-immunized group are the result of experimental variation and not due to a toxicity of NDs.
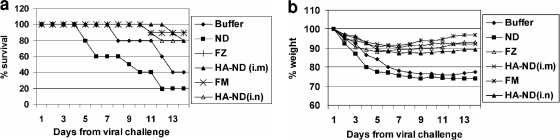
Only one mouse each died in the groups immunized with the commercial vaccines Fluzone and FluMist, while two died in each of the HA-ND-immunized groups (i.e., i.m. or i.n.), clearly demonstrating the protective efficacy of HA-ND immunization against live-virus challenge (Fig. 7a). While mortality figures show a higher efficacy of the commercial vaccines, the per-day weight loss in various groups of immunized mice failed to show any significant difference between the various vaccinated groups (Fig. 7b).
FIG. 7.
HA-ND provides protective immunity comparable to that of live H1N1 challenge with commercial vaccines. (a) Protective efficacies of i.m. and i.n. HA-ND vaccination in mice against H1N1 strain PR/8/34 live viral challenge were compared with those of commercial vaccines. A 14-day survival chart for the different groups of immunized mice after viral challenge with live influenza virus (PR/8/34) is shown. (b) Average cumulative weight loss in each of the immunized groups. FZ, Fluzone; FM, FluMist.
DISCUSSION
We have demonstrated that the rHA protein incorporated into the membrane environment of an ND particle and used to immunize mice results in a stronger immune response and a stronger protective effect than those induced by HA alone. Moreover, the HA-ND-elicited humoral immune response showed significant cross-reactivity against nonhomologous strains relative to the response induced by immunization with HA alone (Fig. 3). This indicated that HA-ND elicited a better response not only against the corresponding vaccine strain but also against drifted strains.
In the course of this study we noticed that while the anti-HA reaction in HA-ND-immunized sera was mainly of the IgG1 subclass, in Fluzone-vaccinated mice it was predominantly of the IgG2a subclass. Additionally, HA-ND induced an antibody response that could be favorably compared with that induced by FDA-approved commercial vaccines. In live-virus challenge experiments, the efficacy of i.m. delivered HA-ND was comparable to that of Fluzone.
The respiratory tract mucosa is the primary site of infection with influenza virus. Secretory IgA antibodies in the mucosa are believed to provide defense against influenza viruses (24). Nasally delivered influenza virus vaccines are expected to induce a secretory IgA response that can be detected in nasal wash specimens (21). However, in one study, when mice were immunized twice with soluble HA alone, no IgA was detected in nasal wash specimens (28). To overcome this, adjuvants like cholera toxin B subunits and Escherichia coli heat-labile toxin are often administered as mucosal adjuvants to induce IgA antibodies. However, we show here that i.n. vaccination with HA-ND induced not only serum anti-HA IgG responses but also secretory IgA in the nasal wash specimens. Interestingly, HA-ND has not been optimized either for the dose or for i.n. delivery. Nevertheless, upon i.n. inoculation, it elicited an effective protective immune response that could be compared favorably with that elicited by FluMist.
There are many advantages to using recombinant vaccines. From a manufacturing perspective, the cloning, expression, and manufacturing of rHAs are less time-consuming than for whole-virus vaccines, allowing more rapid and scalable vaccine production in response to emerging viruses. Because rHA can be highly purified and does not contain egg protein, it should be safer to use, particularly for individuals with egg allergies. Additionally, since no live influenza virus is used in the process, the need for biocontainment during vaccine manufacture is eliminated. Previous studies found recombinant vaccines to have a narrower specificity and potentially less immunogenicity than whole-virus vaccines. One possible explanation, however, may be that rHA is unlikely to retain its native conformation in solution. This speculation is based on the fact that HA is an envelope protein, and in the absence of the stabilizing influence of the membrane environment, the protein is apt to misfold and/or become degraded. Incorporation into the ND therefore not only may allow a naturalistic presentation of HA epitopes but also might help stabilize the HA protein. This notion is supported by previous work involving G-protein coupled receptors (GPCRs), chemotaxis receptors, and P450 enzymes, whose function is retained and whose stability is improved by ND incorporation. While soluble GPCRs had a much reduced hormone binding capacity, the ND-incorporated GPCRs exhibited hormone binding affinities that were nearly identical to that of the receptors expressed on the cell membrane (17). Direct proof for a functional higher-order oligomeric structure was obtained for the E. coli chemotaxis receptor Tar by using NDs after proving to be previously unobtainable by using soluble subunits or other membrane reconstitution systems (5). Finally, refined kinetic data and improved stability have been obtained for ND-incorporated P450 proteins (1).
In this study, we have incorporated rHA into NDs and demonstrated that a distribution of multimeric HA molecules is associated with the HA-ND population. We have shown that all HA-ND preparations with significant fractions of trimeric HA embedded in NDs have a higher hemagglutination activity than rHA alone (Table 1). Since the extent of hemagglutination depends on the capacity of HA molecules to bind to sialic acid residues on RBCs, it may also be a function of its native structure. This is further supported by our observation that sera from whole-virus- or split-virus-immunized animals recognized HA-ND more efficiently than HA alone (Fig. 6). Therefore, we hypothesize that, at least in part, ND-incorporated HA is more likely to have a three-dimensional structure that approximates that of the native HA associated with the viral envelope, although further study will be necessary to confirm whether this is the case.
This work represents a proof of principle in which we show that subunit vaccines in a lipid-bilayer-associated form can elicit a robust immune response and confer protective immunity against viral challenge. Although our results do not indicate that ND-incorporated rHA has a higher immunogenicity than whole-virus commercial vaccines, it provides intriguing results considering that in this study, we used a formulation consisting of a single influenza virus antigen that is not yet fully optimized. Because rHA can be manufactured, purified, and incorporated into NDs on a large scale in a relatively short time, the ND system represents a potentially useful platform for not only influenza virus vaccines but also a range of other subunit vaccines that use envelope proteins.
Acknowledgments
This work was funded by Nanodisc Inc., Champaign, IL.
Footnotes
Published ahead of print on 14 October 2009.
REFERENCES
- 1.Baas, B. J., I. G. Denisov, and S. G. Sligar. 2004. Homotropic cooperativity of monomeric cytochrome P450 3A4 in a nanoscale native bilayer environment. Arch. Biochem. Biophys. 430:218-228. [DOI] [PubMed] [Google Scholar]
- 2.Bayburt, T. H., J. W. Carlson, and S. G. Sligar. 1998. Reconstitution and imaging of a membrane protein in a nanometer-size phospholipid bilayer. J. Struct. Biol. 123:37-44. [DOI] [PubMed] [Google Scholar]
- 3.Bayburt, T. H., and S. G. Sligar. 2003. Self-assembly of single integral membrane proteins into soluble nanoscale phospholipid bilayers. Protein Sci. 12:2476-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bayburt, T. H., and S. G. Sligar. 2002. Single-molecule height measurements on microsomal cytochrome P450 in nanometer-scale phospholipid bilayer disks. Proc. Natl. Acad. Sci. U. S. A. 99:6725-6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boldog, T., S. Grimme, M. Li, S. G. Sligar, and G. L. Hazelbauer. 2006. Nanodiscs separate chemoreceptor oligomeric states and reveal their signaling properties. Proc. Natl. Acad. Sci. U. S. A. 103:11509-11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bright, R. A., D. M. Carter, S. Daniluk, F. R. Toapanta, A. Ahmad, V. Gavrilov, M. Massare, P. Pushko, N. Mytle, T. Rowe, G. Smith, and T. M. Ross. 2007. Influenza virus-like particles elicit broader immune responses than whole virion inactivated influenza virus or recombinant hemagglutinin. Vaccine 25:3871-3878. [DOI] [PubMed] [Google Scholar]
- 7.Chen, P. S., T. Y. Toribara, and H. Warner. 1956. Microdetermination of phosphorus. Anal. Chem. 28:1756-1758. [Google Scholar]
- 8.Civjan, N. R., T. H. Bayburt, M. A. Schuler, and S. G. Sligar. 2003. Direct solubilization of heterologously expressed membrane proteins by incorporation into nanoscale lipid bilayers. Biotechniques 35:556-560, 562-563. [DOI] [PubMed] [Google Scholar]
- 9.Cox, R. J., K. A. Brokstad, and P. Ogra. 2004. Influenza virus: immunity and vaccination strategies. Comparison of the immune response to inactivated and live, attenuated influenza vaccines. Scand. J. Immunol. 59:1-15. [DOI] [PubMed] [Google Scholar]
- 10.Denisov, I. G., B. J. Baas, Y. V. Grinkova, and S. G. Sligar. 2007. Cooperativity in cytochrome P450 3A4: linkages in substrate binding, spin state, uncoupling, and product formation. J. Biol. Chem. 282:7066-7076. [DOI] [PubMed] [Google Scholar]
- 11.Denisov, I. G., Y. V. Grinkova, A. A. Lazarides, and S. G. Sligar. 2004. Directed self-assembly of monodisperse phospholipid bilayer Nanodiscs with controlled size. J. Am. Chem. Soc. 126:3477-3487. [DOI] [PubMed] [Google Scholar]
- 12.Denisov, I. G., M. A. McLean, A. W. Shaw, Y. V. Grinkova, and S. G. Sligar. 2005. Thermotropic phase transition in soluble nanoscale lipid bilayers. J. Phys. Chem. B 109:15580-15588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duan, H., N. R. Civjan, S. G. Sligar, and M. A. Schuler. 2004. Co-incorporation of heterologously expressed Arabidopsis cytochrome P450 and P450 reductase into soluble nanoscale lipid bilayers. Arch. Biochem. Biophys. 424:141-153. [DOI] [PubMed] [Google Scholar]
- 14.Fiers, W., S. Neirynck, T. Deroo, X. Saelens, and W. M. Jou. 2001. Soluble recombinant influenza vaccines. Philos. Trans. R. Soc. Lond. B Biol. Sci. 356:1961-1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gill, S. C., and P. H. von Hippel. 1989. Calculation of protein extinction coefficients from amino acid sequence data. Anal. Biochem. 182:319-326. [DOI] [PubMed] [Google Scholar]
- 16.Hoffmann, E., J. Stech, Y. Guan, R. G. Webster, and D. R. Perez. 2001. Universal primer set for the full-length amplification of all influenza A viruses. Arch. Virol. 146:2275-2289. [DOI] [PubMed] [Google Scholar]
- 17.Leitz, A. J., T. H. Bayburt, A. N. Barnakov, B. A. Springer, and S. G. Sligar. 2006. Functional reconstitution of Beta2-adrenergic receptors utilizing self-assembling Nanodisc technology. Biotechniques 40:601-602, 604, 606. [DOI] [PubMed] [Google Scholar]
- 18.Martinet, W., T. Deroo, X. Saelens, E. Beirnaert, P. Vanlandschoot, R. Contreras, W. Fiers, and W. M. Jou. 1998. Evaluation of recombinant A/Victoria/3/75 (H3N2) influenza neuraminidase mutants as potential broad-spectrum subunit vaccines against influenza A. Arch. Virol. 143:2011-2019. [DOI] [PubMed] [Google Scholar]
- 19.McBurney, S. P., K. R. Young, and T. M. Ross. 2007. Membrane embedded HIV-1 envelope on the surface of a virus-like particle elicits broader immune responses than soluble envelopes. Virology 358:334-346. [DOI] [PubMed] [Google Scholar]
- 20.Nath, A., W. M. Atkins, and S. G. Sligar. 2007. Applications of phospholipid bilayer nanodiscs in the study of membranes and membrane proteins. Biochemistry 46:2059-2069. [DOI] [PubMed] [Google Scholar]
- 21.Plante, M., T. Jones, F. Allard, K. Torossian, J. Gauthier, N. St-Félix, G. L. White, G. H. Lowell, and D. S. Burt. 2001. Nasal immunization with subunit proteosome influenza vaccines induces serum HAI, mucosal IgA and protection against influenza challenge. Vaccine 20:218-225. [DOI] [PubMed] [Google Scholar]
- 21a.Reed, L. H., and H. Muench. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 22.Shaw, A. W., M. A. McLean, and S. G. Sligar. 2004. Phospholipid phase transitions in homogeneous nanometer scale bilayer discs. FEBS Lett. 556:260-264. [DOI] [PubMed] [Google Scholar]
- 23.Shaw, A. W., V. S. Pureza, S. G. Sligar, and J. H. Morrissey. 2007. The local phospholipid environment modulates the activation of blood clotting. J. Biol. Chem. 282:6556-6563. [DOI] [PubMed] [Google Scholar]
- 24.Shvartsman, Y. S., and M. P. Zykov. 1976. Secretory anti-influenza immunity. Adv. Immunol. 22:291-330. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki, Y. 2005. Sialobiology of influenza: molecular mechanism of host range variation of influenza viruses. Biol. Pharm. Bull. 28:399-408. [DOI] [PubMed] [Google Scholar]
- 26.Treanor, J. J., G. M. Schiff, F. G. Hayden, R. C. Brady, C. M. Hay, A. L. Meyer, J. Holden-Wiltse, H. Liang, A. Gilbert, and M. Cox. 2007. Safety and immunogenicity of a baculovirus-expressed hemagglutinin influenza vaccine: a randomized controlled trial. JAMA 297:1577-1582. [DOI] [PubMed] [Google Scholar]
- 27.Vanlandschoot, P., G. Maertens, W. M. Jou, and W. Fiers. 1993. Recombinant secreted haemagglutinin protects mice against a lethal challenge of influenza virus. Vaccine 11:1185-1187. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe, I., T. M. Ross, S. Tamura, T. Ichinohe, S. Ito, H. Takahashi, H. Sawa, J. Chiba, T. Kurata, T. Sata, and H. Hasegawa. 2003. Protection against influenza virus infection by intranasal administration of C3d-fused hemagglutinin. Vaccine 21:4532-4538. [DOI] [PubMed] [Google Scholar]