Abstract
Patients with advanced melanoma usually do not benefit from conventional chemotherapy treatment. There is therefore a true need for a new kind of therapy for melanoma. One factor responsible for the poor prognosis of melanoma is the inhibitor of apoptosis protein (IAP) family member Livin. In this study, we applied a novel approach for the treatment of melanoma, using a unique strain of the oncolytic Newcastle disease virus (NDV-HUJ). We found that, unlike chemotherapeutic drugs, NDV-HUJ, a one-cycle replicating virus, overcomes the resistance to apoptosis of melanoma primary cultures that over express the Livin protein. In contrast, melanoma tumor cells that do not express Livin are relatively resistant to NDV-HUJ treatment. Furthermore, we show that NDV-HUJ-induced oncolysis is attributed to the dual function of Livin: although Livin inhibits apoptosis through the inhibition of caspases, under the robust apoptotic stimulation of NDV-HUJ, caspases can cleave Livin to create a truncated protein with a paradoxical proapoptotic activity. Thus, NDV-HUJ is a potent inducer of apoptosis that can overcome the antiapoptotic effect of Livin and allow cleavage of Livin into the proapoptotic tLivin protein. Moreover, the results indicate that the interferon system, which is functional in melanoma, is not involved in NDV-induced oncolysis. Taken together, our data offer the possibility of a new viral oncolytic treatment for chemoresistant melanoma.
Newcastle disease virus (NDV) is an avian paramyxovirus that has a potential selective oncolytic effect on human tumors (5, 7, 13, 21, 25, 26). NDV's natural host is avian, and while mammalian cells bear the sialic acid receptor for NDV and may be infected by the virus, the virus has limited replication capacity in normal mammalian cells (21). We recently reported the development of an attenuated (lentogenic) isolate of NDV (HUJ) that undergoes only one cycle replication in infected mammalian cells (7, 25). NDV-HUJ is a single clone derived from the parental strain NDV Hitchner B1, which contains a mixed viral population. The new virus clone is attenuated due to multiple passages in specific-pathogen-free (SPF) eggs, and its intracerebral pathogenicity index (ICPI) value is low (an ICPI of 0.01 versus an ICPI of 0.93 for the parental NDV Hitchner B1). Sequence analysis of NDV-HUJ indicated 156 changes at the nucleotide sequence level and multiple amino acid changes from the parental B1 virus in all six viral genes (see Fig. S1 in the supplemental material). Although NDV-HUJ is an attenuated virus in chicken, it retains a selective cytotoxic potential for cancer cells, as determined in vitro and in vivo, using murine and human lung carcinomas (25). The oncolytic effect of the virus is apoptosis dependent (25). NDV-HUJ has been applied to treat glioblastoma patients in a phase I/II clinical trials and found to be safe and potentially active (7).
The inhibitors of apoptosis proteins (IAPs) are receiving increased attention as key players in the initiation of tumors, their progression, and resistance to chemotherapy treatment (17). To date, eight human IAPs have been identified, including Livin. IAPs are characterized by one or more repeats of a highly conserved 70-amino-acid domain termed the baculovirus IAP repeat (BIR) that can bind and inhibit caspases, some IAPs also contain a conserved sequence termed the RING finger. RING finger proteins might function as E3 ubiquitin ligases; however, the exact nature of the E3 ligase activity of IAPs is still largely unclear.
IAPs inhibit apoptosis induced by a variety of stimuli, mainly through their ability to bind and inhibit specific caspases (17). Intense study has shown that the role of IAP in apoptosis regulation is highly diverse, with a prominent role in tumorigenesis and resistance to therapy. Among the human IAPs, XIAP is the best characterized and the most potent caspase inhibitor. The most recently discovered member of this family is Livin, found by us and others (3, 9, 12, 24). Livin contains a single BIR domain and a RING finger (3, 12). We previously found that Livin is specifically cleaved by caspases at the Asp52 residue to produce a large C-terminal fragment, containing both the BIR and the RING domains. After cleavage, truncated Livin (tLivin) acts paradoxically as a proapoptotic factor (18, 19).
In the present study we show that chemoresistant melanoma primary cultures that highly express the Livin protein are sensitive to oncolytic NDV-HUJ treatment. This appears to be a result of activation of caspases 8, 3, and 7 that in turn cleave Livin to produce the tLivin. This is a novel regulatory mechanism in which NDV-HUJ can overcome the antiapoptosis function of Livin and expose the “good side” of Livin by inducing the cleavage of Livin to produce the proapoptotic tLivin that subsequently leads to metastatic melanoma cell death.
MATERIALS AND METHODS
Patients.
The present study includes melanoma patients who participated in previous studies for treatment with autologous melanoma vaccine as adjuvant therapy (14, 15). Tumor specimens were obtained by the surgeon in consultation with the oncologist at Hadassah Medical Center. This study was approved by the Institutional Review Board, and informed consent was obtained from all study participants. The study was conducted according to the Declaration of Helsinki, the International Conference on Harmonization, and the Guidelines for Good Clinical Practice.
Melanoma cell culture preparation.
The methods of cell culture preparation for vaccine were described previously (14, 15). In brief, tumor specimens were procured fresh and sterile. Cells were extracted mechanically or by enzymatic dissociation with collagenase and DNase (Sigma, St. Louis, MO). Cell suspensions were put into culture bottles with Dulbecco modified Eagle medium (Gibco-BRL, Gaithersburg, MD), 10% fetal calf serum (Gibco-BRL), HEPES (1:500), penicillin-streptomycin (1:100), and glutamine (1:100). Cells were cultured to at least 1.0 × 107 to 2.5 × 107. Culturing the cells resulted in the preferential selection of melanoma cells.
Virus.
NDV B1 Hitchner strain was obtained from the American Type Culture Collection and was passaged in our lab 54 times, using embryonated eggs, from nonvaccinated chickens, before cloning by limiting dilution in eggs. Five additional passages were carried out in SPF eggs, twice by the limiting dilution method and subsequently by three regular passages. Multiple passages of the original ATTC NDV B1strain in embryonated eggs in our lab resulted in a virus with a high ICPI (0.93 as determined in 24-h-old SPF chicks). The mean time to death (MTD) caused by the virus was 97.6 h, as determined in SPF embryonated eggs (2). The cloning resulted in a single virus, NDV-HUJ, of low virulence (ICPI = 0.01 and MTD = 112.1 h). The titer (expressed as the 50% tissue culture infective dose) of NDV-HUJ after replication in chicken embryo fibroblasts in culture was 101 without trypsin versus 106.6 with trypsin, and for NDV-B1 it was 102.5 without trypsin versus 106 with trypsin. Sequence analysis of NDV-HUJ and comparison with two other NDV strains, Hitchner B1 and LaSota, revealed a unique viral sequence with similarities and differences to both Hitchner B1 and LaSota strains (see Fig. S1 in the supplemental material). Moreover, the cleavage site sequence in the F protein of NDV-HUJ is similar to that of the parental strain NDV B1 (112-GRQGR-116). This sequence is recognized by trypsinlike proteases, and the virus multiple cycles replication in most cultured cells is dependent on added trypsin. To maintain the properties of the cloned virus, only one passage of the working bank was used in all our experiments. Determination of the pathogenicity index (i.e., the ICPI) was repeated twice by an independent institute (The Israel Veterinary Institute, Beit Dagan, Israel). In all of the experiments, infection with NDV-HUJ was performed at an multiplicity of infection (MOI) of 10 without the addition of trypsin.
NDV-MTH is a virulent-mesogenic strain that is trypsin independent. Correspondingly, the cleavage site sequence of the NDV-MTH F protein is (112-RRQRR-116), and the pathogenicities of this strain are high (ICPI = 1.38 and MDT = 75.6 h).
Western blot analysis and antibodies.
Whole-cell lysates were prepared using lysis buffer containing 20 mM Tris-HCl, 2 mM EDTA, 6 mM β2-mercaptoethanol, 1% NP-40, and 0.1% sodium dodecyl sulfate. Protease inhibitors included 1 mM phenylmethylsulfonyl fluoride, protease inhibitor cocktail (Sigma) diluted 1:10 and complete inhibitor cocktail (Roche) diluted 1:25. About 0.25 × 106 to 1 × 106 cells were lysed in a total volume of 100 μl by incubation at 4°C for 20 min with vigorous vortexing. The protein content was assessed by the DC protein assay (Bio-Rad, Hercules, CA). Samples were resolved on 10% Bis-Tris precast gels (Invitrogen, Carlsbad, CA). The antibodies used for Western blot analysis were Livin (IMG-347; Imgenex), caspase-3 (catalog no. 9662; Cell Signaling), caspase-7 (catalog no. 9492; Cell Signaling), caspase-8 (catalog no. 9746; Cell Signaling), XIAP (catalog no. 2045; Cell Signaling), β-actin (catalog no. 4967; Cell Signaling), and α-tubulin (sc-8035; Santa Cruz).
siRNA assay.
Livin expression was silenced in the primary melanoma cultures by infection with pGIPZ lentivirus (Open Biosystems) that express green fluorescent protein (GFP) and small interfering RNA (siRNA) vector construct (22). The lentivector was pseudotyped with VSV-G (11). About 90% of the cells were infected, as determined by fluorescence-activated cell sorting (FACS) analysis for GFP expression.
Transfection.
Transfection of the melanoma cell line LB33 Mel-A1 (a generous gift from P. G. Coulie) with the pIRES2-EGFP plasmid (Qiagen, Hilden, Germany) that encodes for wild-type Livin-β or Livin β-D52A or empty vector control was carried out using FuGENE-6 (Roche). Because pIRES2-EGFP contains an internal ribosome entry site, it permits both the gene of interest and the EGFP gene to be translated from a single bicistronic mRNA.
Analysis of NDV surface proteins in infected cells.
The cells were infected with NDV-HUJ at an MOI of 10, as described previously (13). Cell samples were stained using anti-NDV chicken serum (1:500 dilution) for 1 h and with a goat anti-chicken immunoglobulin G-fluorescein isothiocyanate or Cy5-conjugated 1:500 dilution (catalog no. 703-096-155 and 703-176-155; Jackson Laboratories, West Grove, PA) for 30 min. After incubation, the cells were washed with phosphate-buffered saline (PBS) and stained with 0.5 μg/ml of propidium iodide (PI; Sigma, St. Louis, MO), an indicator for cell mortality. The relative levels of surface NDV antigens were assessed by FACS analysis using FACSort (Becton Dickinson, Franklin Lakes, NJ). The data were analyzed using FCS Express software (De Novo Software, Los Angeles, CA). The fluorescence geometric mean (FGM) value was used to quantify the intensity of antibody fluoresces staining, which correlates with the amount of viral antigen on the surfaces of the infected cells.
Cell cycle analysis.
Cells at 48 h posttreatment were harvested, washed with PBS, and fixed in 70% ethanol. Cells were then washed twice with PBS and incubated in 1 ml of PBS containing 50 μg/ml of PI and 200 μg/ml of RNase A (Sigma) at 37°C for 10 min. The stained cells were analyzed for red fluorescence (FL2-A), and data were analyzed by using FCS Express software.
Semiquantitative RT-PCR.
Total RNA was isolated by using the RNeasy RNA isolation kit (Qiagen). In order to distinguish between the minus and plus strands of NDV RNA, we used a two-step reverse transcription-PCR (RT-PCR). For the synthesis of cDNA, identical amounts of RNA (1 μg) were used as a template for avian myeloblastosis virus reverse transcriptase (Promega, Madison, WI). The RT reaction was carried out to specifically identify the positive-strand genomic NDV RNA, with a reverse (negative-sense) primer to the NDV leader plus-strand RNA sequence (nucleotide [nt] 330)5′-TGCCTGAGTGGTTTGTTGGC-3′(nt 310) from the NDV NP gene. For PCR amplification of the NDV cDNA, we used the forward (positive-sense) primer, Leader Start (nt 1)5′-ACCAAACAGAGAATCGGTGAG-3′(nt 21), and the reverse primer, Leader End (nt 330)5′-TGCCTGAGTGGTTTGTTGGC-3′(nt 310), from the NDV NP gene to produce a double-stranded DNA fragment of 330 bp, containing the Leader and part of the NP gene region. In order to avoid a nonspecific amplification of RNA positive strand of the virus due to secondary RNA structures, a control assay was performed with each sample, without primers, to ensure specific amplification of the positive RNA strand. DNA products were collected after 20, 25, 30, and 35 PCR cycles and resolved on 1% agarose gel. The results presented are from the linear range of the amplification after 25 or 30 cycles. Analysis of amounts of PCR DNA products was done using an ImageMaster VDS-CL scanner (Amersham Pharmacia, Piscataway, NJ) with TINA20 software. Relative NDV RNA values were calculated by dividing the PCR DNA band density by that derived from the 18S total RNA in each sample.
RESULTS
Preferential killing of advanced primary melanoma cells by NDV-HUJ.
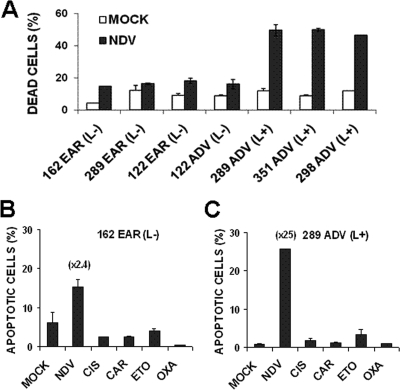
Primary metastatic melanoma cells were obtained from the lymph nodes of melanoma patients at two time points correlating with early and advanced disease progression. These tumor cells were grown in culture and tested for response to the oncolytic activity of NDV-HUJ. Cells were infected with NDV-HUJ for 48 h and stained with anti-NDV specific antibodies to monitor NDV infection and with PI to evaluate cell death (Fig. 1A). The results indicated that whereas most cells, from both advanced (ADV) and early (EAR) melanomas were infected with the virus (∼95%, see Fig. 6 and 7), the level of cell death varied significantly. ADV melanoma cultures underwent significantly more extensive cell death upon infection compared to EAR melanoma cultures. Infected ADV melanoma cell death relative to the respective mock-infected cells was ∼5-fold greater, whereas for the EAR it was only ∼2-fold greater. However, not all ADV melanoma cultures displayed the same level of cell death upon infection. For example, the sensitivity of 122 ADV to the oncolytic effect of the virus was low and was similar to that of 122 EAR melanoma cells obtained from the same patient (Fig. 1A).
FIG. 1.
Preferential killing of advanced primary melanoma cells by NDV-HUJ. (A) Cultures were infected with NDV-HUJ (MOI = 10) for 48 h. The extent of cell death was determined by PI staining using FACS analysis as described in Materials and Methods. The y axis represents the percentage of PI-stained cells (cell death). Open bars represent PI staining of mock-infected cells; black bars represent PI staining of NDV-infected cells. (B and C) Cell killing by NDV-HUJ and chemotherapy in primary melanoma cultures. Melanoma primary cultures were either mock infected or infected with NDV-HUJ (MOI = 10). Parallel cultures were treated with a panel of chemotherapeutic drugs: 0.5 μM cisplatin, 10 μM carboplatin, 1 μM oxaplatin, or 30 μM etoposide. The concentration of chemotherapeutic drugs used was previously applied to test activity of primary melanoma cells (16). Cells were harvested 48 h posttreatment and taken for FACS analysis for DNA content. The y axis represents the fraction of cells in the sub-G1 phase of cell cycle that indicates dead cells. Melanoma samples are designated by patient number and as EAR (early) or ADV (advanced) melanoma. The level of Livin expression is marked in each sample as high (L+) or Livin undetectable (L−), based on Western blot analysis (Fig. 2 to 3). Numbers in parentheses above the bars indicate the fold increase in cell death due to NDV-HUJ infection relative to mock-infected control cells.
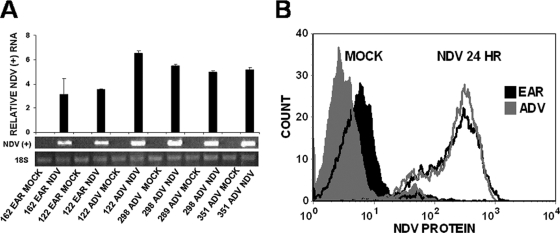
FIG. 6.
NDV-HUJ RNA and protein synthesis in infected primary melanoma cultures. (A) Analysis of NDV plus RNA strand synthesis. RNA (1 μg) prepared from mock-infected and NDV-HUJ-infected (MOI = 10) cultures at 24 h postinfection was subjected to RT-PCR amplification to trace the positive (+) RNA strand of NDV. Analysis of viral (+) RNA in the infected cells was done as described in Materials and Methods and in reference 25. (B) Cells of patient 289 both from EAR (black-shaded areas) and ADV (gray-shaded areas) stage of melanoma were mock infected (filled histograms) or NDV-HUJ infected (MOI = 10) (solid line). Cells were harvested 24 h after infection, stained with anti-NDV antibodies, and analyzed by flow cytometry to quantify viral surface proteins, using the FGM value as described in Materials and Methods.
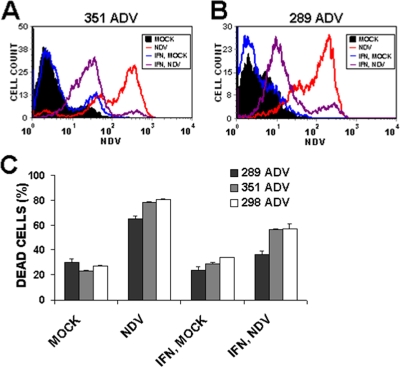
FIG. 7.
The IFN system is functional in primary melanoma cultures. Melanoma cultures 351 ADV (A) and 289 ADV (B) were either mock infected (filled black) or NDV-HUJ infected at an MOI of 10 (red line). Cultures were pretreated with human IFN-β (1,000 U/ml) for 16 h before NDV (purple line) or mock (blue line) infection. Cells were harvested 72 h postinfection and stained with anti-NDV antibodies. Surface NDV protein was quantified using the FGM value, as described in Materials and Methods. (C) Cell death was analyzed by flow cytometry of PI-stained 289 ADV, 351 ADV, and 298 ADV melanoma cultures as described in Materials and Methods.
NDV-HUJ induces cell death in chemoresistant advanced primary melanoma cells.
The oncolytic effect of NDV-HUJ was compared to several chemotherapeutic drugs such as cisplatin, carboplatin, oxaplatin, and etoposide (Fig. 1B and C and see Fig. S1 in the supplemental material). In a variety of different tumor cells the killing mechanism of both NDV-HUJ and chemotherapy drugs is apoptosis dependent. Using FACS analysis to monitor DNA content, we demonstrate that NDV-HUJ infection causes a significant increase in cell death compared to treatment with chemotherapeutic drugs under optimal conditions (Fig. 1B and C). Furthermore, the relative fraction of dead cells in the NDV-HUJ-infected cultures was higher in the advanced melanoma compared to early melanoma cells (Fig. 1B and C and see Fig. S2 in the supplemental material). One of the four advanced melanoma primary cultures tested (122 ADV) showed resistance to death after viral infection (Fig. 1); the explanation for the resistance of the 122 ADV melanoma is discussed further below (Fig. 2).
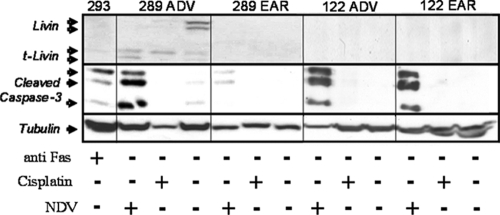
FIG. 2.
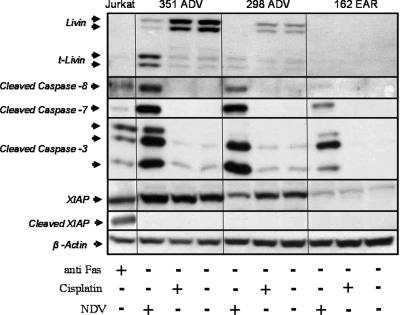
NDV-HUJ activates apoptosis in chemoresistant melanoma tumor cells. Primary cultures from two patients with both EAR and ADV melanoma samples (289 ADV versus EAR and 122 ADV versus EAR) were either mock infected or NDV-HUJ infected (MOI = 10) or treated with 0.5 μM cisplatin for 48 h. 293HEK cells were treated with anti-Fas protein (300 ng/ml for 24 h) to serve as a positive control for caspase-3 activation. Cell lysates were analyzed by Western blotting with monoclonal anti-Livin antibody, which detects both the full-length Livin and the cleavage product tLivin, and anti-caspase 3 and anti-tubulin antibodies (each antibody was used individually in a separate blot). The cleaved active caspase-3 serves as a marker of apoptosis. A plus sign (+) represents either NDV-HUJ infection or cisplatin or anti-FAS addition, while a minus sign (−) indicates no treatment.
Correlation between the oncolytic effect of NDV-HUJ, caspase activation, and cleavage of Livin.
Several melanoma primary cultures from both early and advanced patients were infected for 48 h with NDV-HUJ, and cellular proteins of the apoptotic cascade were analyzed by Western blotting with specific antibodies (Fig. 2 and 3). NDV-HUJ infection triggers the apoptosis process by the activation of caspases 8, 3, and 7 in all tested cells. We found a correlation between expression of the Livin protein and disease progression (I. Lazar et al., unpublished data). Indeed, Western blot analysis of Livin in the melanoma primary cultures described above shows that Livin is present in advanced melanoma cells.
FIG. 3.
Cisplatin, unlike NDV-HUJ, fails to induce the apoptosis cascade in advanced melanoma cells. Melanoma primary cultures were either mock infected or NDV-HUJ infected at an MOI of 10 or treated with 0.5 μM cisplatin for 48 h. Livin, active caspases 3, 7, and 8, XIAP, and actin proteins were determined in three primary melanoma cultures by Western blot analysis. Jurkat cells were treated with anti-Fas protein (300 ng/ml for 24 h) to serve as a positive control for caspases 3, 7, and 8 activation and cleaved XIAP. A plus sign (+) represents either NDV-HUJ infection or cisplatin or anti-FAS addition, while a minus sign (−) indicates no treatment. All cells were resistant to cisplatin treatment, with no cleavage of Livin or caspase proteins.
One advanced melanoma primary culture, 122 ADV, did not express the Livin protein (Fig. 2). This particular cell culture was also less sensitive to the oncolytic effect of the virus and thus similar to the early melanoma cultures (Fig. 1A). Infection with NDV-HUJ results in a specific cleavage of the Livin protein to produce the proapoptotic tLivin (Fig. 2 and 3). In contrast, infection with NDV-HUJ does not lead to the cleavage of XIAP (Fig. 3), another member of the IAP family, that is cleaved as a result of apoptotic stimulation (4, 12), demonstrating the specificity of NDV-HUJ for Livin.
All melanoma samples in the present study were resistant to apoptosis induced by cisplatin, a first-line chemotherapeutic agent used to treat melanoma patients (Fig. 1B and C and see Fig. S1 in the supplemental material). In order to compare directly the effects of cisplatin and NDV-HUJ on the apoptotic cascade proteins, Western blot analysis was conducted on cell extracts of both early and advanced melanoma after a treatment with either the virus or the drug. The results shown in Fig. 2 and 3 indicate minimal cleavage of caspases 8, 3, and 7 after treatment of both early and advanced melanoma cells with cisplatin at high concentrations. In contrast, tumor cells derived from advanced melanoma patients (289 ADV, 351 ADV, and 298 ADV) exhibit extensive caspase 8, 3, and 7 activation upon infection with NDV-HUJ compared to the mock-infected cells. Taken together, the results presented in Fig. 1, 2, and 3 indicate that the oncolytic effect of NDV-HUJ is associated with expression of the Livin protein and its cleavage to the proapoptotic tLivin.
Livin knockdown (using siRNA) decreases the oncolytic effect of NDV-HUJ in advanced melanoma cells.
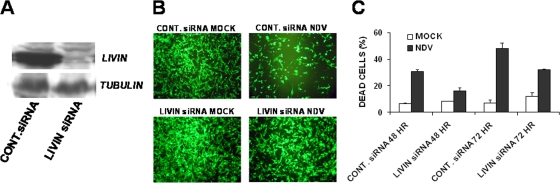
In order to directly examine whether Livin is the key mediator in the apoptosis process induced by NDV-HUJ infection, Livin gene expression was knocked down in advanced melanoma cells using the siRNA methodology. Advanced melanoma cultures were transduced with lentiviral vectors expressing either Livin-specific siRNA or a control nonspecific siRNA (Fig. 4A). The lentiviral vector also expressed GFP to enable the tracking of transduction efficiencies. Stably transduced melanoma cells were then infected with NDV-HUJ for 48 and 72 h. Percentage of GFP-positive cells and cell death after infection were monitored by FACS analysis (see Fig. S3 in the supplemental material). Advanced melanoma cultures that did not express Livin, due to the expression of the Livin-specific siRNA (Fig. 4A), were less sensitive to NDV HUJ mediated killing after 48 and 72 h compared to cells expressing the control siRNA (Fig. 4C). The cell cultures were also photographed at 72 h postinfection under fluorescence microscope to further document the rescue effect of the Livin-specific siRNA from NDV-HUJ induced cell death (Fig. 4B).
FIG. 4.
Livin siRNA rescues ADV melanoma from the oncolytic effect of NDV-HUJ. (A) Melanoma primary culture 351 ADV was transduced with GIPZ lentiviral vector expressing GFP and siRNA, either a control siRNA that does not affect Livin protein expression or Livin siRNA, which abolishes Livin protein expression, as indicated by Western analysis. At 24 h after lentiviral transduction, cultures express a high level of GFP. Next, transduced cultures were either mock infected (□) or NDV-HUJ infected (MOI = 10, ▪). Cultures were harvested at 48 and 72 h postinfection. (B) NDV-HUJ killing activity was documented in situ, using fluorescence microscopy, for residual GFP-expressing cells (72 h postinfection). (C) Cultures were harvested at 48 and 72 h postinfection, and cell death was determined by FACS analysis of PI staining. Cells were first selected for GFP expression (Lenti vector transduced cells represent ca. 90% of total cells), and PI staining was determined on this cell fraction (see Fig. S3 in the supplemental material). The results presented are based on doubly stained cells that are positive for both GFP and PI.
Truncated Livin is a key factor in cell death induced by NDV-HUJ.
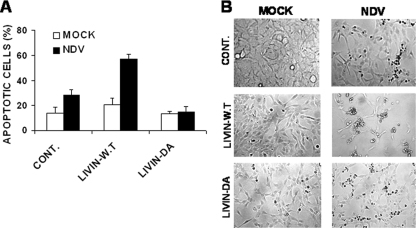
In order to demonstrate that tLivin indeed plays a key role in NDV-induced apoptosis, we used a human melanoma cell line, Mel-A1, that does not express the endogenous Livin. These cells were transiently transfected with plasmid vectors containing wild-type Livin (WT Livin), a Livin mutant that cannot be cleaved to produce tLivin due to introduction of a mutation at the cleavage site (Livin DA), and an empty vector as a control. These expression vectors were previously used to study the dual role of Livin in apoptosis (1, 18). The transfected cells were infected with NDV-HUJ, and the extent of apoptosis was monitored by FACS analysis (Fig. 5A). Mel-A1 cells expressing WT Livin protein were clearly more sensitive to NDV-HUJ-induced apoptosis compared to the control infected Mel-A1 cells. Furthermore, Mel-A1 cells expressing the cleavage mutant Livin-DA were resistant to NDV-HUJ-induced apoptosis. The cultures were photographed 48 h postinfection to document the extent of apoptosis induced by NDV-HUJ in these different lines (Fig. 5B). These results indicate that whereas expression of exogenous WT Livin increases the oncolytic activity of NDV-HUJ, expression of the noncleavable mutant Livin-DA does not stimulate apoptosis after NDV-HUJ infection. We conclude therefore that resistance to apoptosis induction by NDV-HUJ infection most likely results from the antiapoptotic activity of the full-length Livin-DA that is unable to undergo cleavage. Thus, a delicate balance between the anti- and proapoptotic activities of Livin determines cell fate after NDV-HUJ infection.
FIG. 5.
tLivin is a key mediator of cell death induced by NDV-HUJ. Melanoma cell line LB33 Mel-A1, which does not express the endogenous Livin protein (18), was transiently transfected with plasmid vectors expressing WT Livin or a mutant Livin that cannot be cleaved to tLivin (Livin DA) (18) and empty vector as control. Cultures were either mock (□) or NDV-HUJ (MOI = 10, ▪) infected for 48 h. (A) Cell apoptosis was determined by calculating the fraction of sub-G1 cells, in a DNA content staining, using FACS. (B) Cell morphology was documented using light microscopy at ×50 magnification.
NDV-HUJ RNA and protein synthesis in the melanoma cells.
In the previous section, we demonstrated that expression of Livin is critical for the oncolytic effect of NDV-HUJ, and yet it is also possible that the virus preferentially infects and thus kills ADV melanoma cells over EAR melanoma cells. NDV-HUJ is an attenuated strain that readily infects mammalian cells to express viral RNA (+ and - strands) and proteins but fails to produce infectious progeny virus (25). To compare the infection capacity of EAR and ADV melanoma cultures, the level of viral RNA and protein synthesis between the cultures was evaluated (Fig. 6). Total RNA from NDV-HUJ-infected and mock-infected melanoma cells was isolated and the plus (+), antigenomic, RNA strand of the virus was monitored by RT-PCR. In order to avoid nonspecific amplification of RNA positive-strand of the virus due to secondary structures, a control assay was performed for each RNA sample without primers to ensure specific (+) RNA detection. All of the control reactions, without primers, were indeed negative (data not shown). The virus appears to infect both EAR and ADV melanoma cells, as indicated by the presence of the (+) RNA strand (Fig. 6A). In repeated experiments the relative level of the positive-strand virus RNA is somewhat higher (50 to 100%) in the advanced melanoma cells compared to the early melanoma cells. However, viral protein synthesis in the EAR and ADV cell cultures is similar (the fluorescence geometric mean [FGM] of infected 289 EAR cells is 196.6, whereas the FGM of infected 289 ADV cells is 194.8; Fig. 6B). To further investigate the relative contribution of viral infection and Livin expression in the selective oncolytic effect of the virus, Livin expression was knocked down in ADV melanoma cultures by a specific siRNA, as described in Fig. 4, and the cultures were infected with NDV-HUJ. NDV-HUJ protein synthesis in the Livin siRNA knockdown cells appears somewhat higher than in the control siRNA cells (at 24 h postinfection the FGM of Livin siRNA cells is 5,447.8, whereas the FGM of control siRNA cells is 3,063 [see Fig. S4 in the supplemental material]). In contrast, the oncolytic effect of the virus was ∼2-fold higher in the control siRNA cells compared to the Livin knockdown siRNA cells (Fig. 4C). The observation that Livin expression and the level of viral infection in the melanoma cultures are unrelated events is further supported by the finding that viral RNA appears higher in 122 ADV melanoma compared to 122 EAR melanoma (Fig. 6A), whereas both cultures do not express Livin (Fig. 2) and are both not sensitive to the viral oncolytic effect (Fig. 1A). Taken together, all of these results indicate that Livin expression, and not levels of viral RNA and proteins, is the key factor in NDV-HUJ induced oncolysis of the ADV melanomas.
The interferon system is functional in primary melanoma cells.
One hypothesis for the selective oncolysis of tumor cells by viruses is that tumor cells have a deficient interferon (IFN) system and that this deficiency enables the virus to replicate efficiently and selectively in tumor cells compared to normal cells (23). To test whether the IFN system is functional in the advanced melanoma primary cultures, we treated the cells with exogenous human IFN-β 16 h before infection. The amount of viral proteins in the infected 351 ADV cells was clearly reduced due to the IFN pretreatment (FGM reduced from 117.3 to 31.4; Fig. 7A). A similar result was observed in the infected 289ADV melanoma cells, where the level of NDV protein-antigen was reduced in the IFN-treated infected cells (FGM reduced from 69.8 to 14.5; Fig. 7B). Thus, the response to exogenous IFN is functional in the advanced melanomas. However, it is possible that the induction of IFN in the melanoma cells after infection is defective and therefore the antiviral cascade is not initiated. To address this issue, the relative level of IFN-β mRNA transcription was monitored in control and NDV-HUJ-infected cells (see Fig. S5 in the supplemental material). The relative level of IFN mRNA was higher in the infected samples than in mock-infected control cells. Furthermore, the biological activity of IFN production in the infected melanoma cultures was measured as follows. Media from mock-infected and NDV-HUJ-infected 162 EAR and 351 ADV melanoma cultures were collected 48 h postinfection. Clear diluted medium was added to 351 ADV melanoma cultures 16 h prior to infection with the replicative NDV-MTH (6). Pretreatment with medium from NDV-HUJ-infected melanoma cultures resulted in reduced NDV-MTH-induced cell death compared to pretreatment with control medium (mock-infected cells). This reduction was similar whether pretreatment was with medium from EAR or ADV melanoma cultures. In addition, pretreatment with medium of NDV-HUJ-infected melanoma cultures resulted in decreased NDV-MTH replication (see Fig. S6 in the supplemental material). These results demonstrated that in ADV and EAR melanoma cultures IFN production was induced after infection. We therefore conclude that in the primary cultures tested the IFN system is functional in both ADV and EAR melanoma cells; however, a larger analysis of more tumor specimens is required before a general conclusion can be drawn.
DISCUSSION
Melanoma is the most aggressive form of skin cancer and accounts for more than 7,000 deaths annually in the United States (20). Early melanoma is curable if treated at an initial stage by surgical excision, with a 95% of 10-year survival rate. However, the prognosis decreases to a ca. 50% 5-year survival rate when the tumor reaches the lymph nodes (stage III melanoma) and becomes worse in patients with metastatic (stage IV) advanced melanoma, with only 10 to 20% 5-year survival rate (8). Patients with advanced melanoma are usually not affected by conventional chemotherapy treatment, accounting for their poor prognosis. Therefore, there is a true need for a new mode of therapy for melanoma.
One of the unique markers of melanoma is the protein Livin, also called melanoma inhibitor of apoptosis (ML-IAP) (9). Livin is a member of the inhibitor of apoptosis protein (IAP) family, and yet we demonstrated that it is not only an apoptosis inhibitor but has a bifunctional role as an apoptosis regulator. Livin can act as an antiapoptotic protein by its ability to bind and inhibit caspases 3, 7, and 9. However, during robust apoptotic stimulation, caspases 3 and 7 can cleave Livin to create a truncated protein (tLivin) with a paradoxical proapoptotic activity (17, 18).
In the present study we investigated the potential of NDV-HUJ as an oncolytic virus against melanoma. The virus is an attenuated new isolate of NDV with a limited replication capacity of one cycle in mammalian cells, and the progeny virions that bud and release from the cells are not infectious, either due to uncleaved F0 protein and trypsin dependence or due to the formation of defective particles (25). It is not known whether NDV needs to replicate in vivo for its oncolytic effect. We recently discovered that NDV-HUJ induces apoptotic cell death in mouse and human tumor lung cells (25).
Our present results, obtained with seven melanoma primary cultures, indicate that the virus induces apoptosis in advanced melanoma cells, as observed by an increase of the sub-G1 cell fraction and the activation of caspases 8, 3, and 7 after infection (Fig. 2 and 3). Surprisingly, we found that only advanced melanoma cells expressing Livin are highly sensitive to the oncolytic activity of NDV-HUJ (Fig. 1 to 3). We showed that NDV-HUJ activated caspases and subsequently apoptosis (Fig. 1 to 3) (25). We also demonstrated that during apoptosis caspases cleave Livin to produce tLivin (18). Thus, we hypothesize that the sensitivity of advanced melanoma expressing Livin to NDV-HUJ relies on the ability of the virus to overcome the antiapoptotic effect of Livin and to significantly activate caspases 8, 3, and 7. These activated caspases in turn trigger apoptosis and, in parallel, lead to the cleavage of Livin into the proapoptotic tLivin (Fig. 2 and 3), thus accelerating cell death. Support for this explanation comes from several experiments described in the results section, including the knockdown of Livin expression in the ADV melanoma cultures that resulted in a significant reduction in the oncolytic effect of NDV-HUJ (Fig. 4). Moreover, ectopic expression of WT Livin in Mel-A1, a melanoma cell line that does not express endogenous Livin, enhanced the oncolytic effect of the virus on these cells (Fig. 5). However, when Mel-A1 cells were transfected with a noncleavable Livin mutant (Livin-DA), the cells became extremely resistant to NDV-HUJ treatment (Fig. 5). Thus, the proapoptotic tLivin protein is a key factor in NDV-HUJ-induced apoptosis in melanomas.
In contrast to NDV-HUJ, all primary melanoma cells tested were highly resistant to a panel of chemotherapy drugs, including cisplatin, carboplatin, oxaplatin, and etoposide (Fig. 1B and C and see Fig. S1 in the supplemental material). This observation indicates that in melanoma, the antiapoptotic mechanisms acting against cytotoxic drugs are not effective against NDV-HUJ-induced cell death.
To investigate the correlation of viral infection, Livin expression, and cell death, viral RNA and protein synthesis were monitored in the infected melanoma cells, with or without siRNA-mediated knockdown of Livin expression (see Fig. S4 in the supplemental material). Viral protein synthesis was somewhat higher in the Livin siRNA knockdown cells, whereas the oncolytic effect of the virus was stronger in the control siRNA cells, expressing high levels of Livin (Fig. 4A). We interpret these seemingly conflicting data as follows: when Livin protein is knocked down, using a specific siRNA, the proapoptotic activity of tLivin is denied, and the cells survive for a longer period of time (Fig. 4C). Under these conditions, the rescue effect of knockdown Livin and subsequently its product tLivin on cell viability provide the virus more time to synthesize RNA and proteins to a higher extent. We conclude therefore that Livin expression, rather than higher levels of viral RNA and protein synthesis, is responsible for the selective apoptosis of advanced melanoma after NDV infection.
One possible explanation for the selective killing of tumor cells by oncolytic viruses is that tumors frequently have a deficient IFN system, which in normal cells prevents viral replication (10, 23). However, our results indicate that the IFN system is functional in the melanoma cells tested, since pretreatment of the cells with recombinant IFN-β decreases viral protein translation, as well as cell mortality, compared to the control cells (Fig. 7). Moreover, the induction of biologically active IFN in the melanoma cells postinfection is functional (see Fig. S6 in the supplemental material).
In summary, we present here a new mechanism of virus-mediated oncolysis. Although the work was done on a limited number of primary melanoma cultures, the findings point to a potentially new treatment to chemoresistant melanoma using the oncolytic virus NDV-HUJ. Since the oncolytic effect of the virus depends on the Livin protein, pretesting for Livin expression in a biopsy tissue of the tumor should increase the frequency of successful treatments.
Supplementary Material
Acknowledgments
This study was supported by grants from the European Community Program 6, by the Cinigene Network of Excellence, by Philip Morris US and its international external research program, by a grant from the Israel Science Foundation (grant 524/06), by the Public Committee for the Allocation of Estate Funds, by the Israel Ministry of Justice (grant 3130), and by the Caesarea Edmond Benjamin de Rothschild Foundation.
Footnotes
Published ahead of print on 28 October 2009.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Abd-Elrahman, I., K. Hershko, T. Neuman, B. Nachmias, R. Perlman, and D. Ben-Yehuda. 2009. The inhibitor of apoptosis protein Livin (ML-IAP) plays a dual role in tumorigenicity. Cancer Res. 69:5475-5480. [DOI] [PubMed] [Google Scholar]
- 2.Alexander, D. J. 1989. A laboratory manual for the isolation and identification of avian pathogens, 3rd ed., p. 114-120. American Association of Avian Pathologists, Jacksonville, FL.
- 3.Ashhab, Y., A. Alian, A. Polliack, A. Panet, and D. Ben Yehuda. 2001. Two splicing variants of a new inhibitor of apoptosis gene with different biological properties and tissue distribution pattern. FEBS Lett. 495:56-60. [DOI] [PubMed] [Google Scholar]
- 4.Deveraux, Q. L., E. Leo, H. R. Stennicke, K. Welsh, G. S. Salvesen, and J. C. Reed. 1999. Cleavage of human inhibitor of apoptosis protein XIAP results in fragments with distinct specificities for caspases. EMBO J. 18:5242-5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fabian, Z., C. M. Csatary, J. Szeberenyi, and L. K. Csatary. 2007. p53-independent endoplasmic reticulum stress-mediated cytotoxicity of a Newcastle disease virus strain in tumor cell lines. J. Virol. 81:2817-2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fabian, Z., B. Torocsik, K. Kiss, L. K. Csatary, B. Bodey, J. Tigyi, C. Csatary, and J. Szeberenyi. 2001. Induction of apoptosis by a Newcastle disease virus vaccine (MTH-68/H) in PC12 rat phaeochromocytoma cells. Anticancer Res. 21:125-135. [PubMed] [Google Scholar]
- 7.Freeman, A. I., Z. Zakay-Rones, J. M. Gomori, E. Linetsky, L. Rasooly, E. Greenbaum, S. Rozenman-Yair, A. Panet, E. Libson, C. S. Irving, E. Galun, and T. Siegal. 2006. Phase I/II trial of intravenous NDV-HUJ oncolytic virus in recurrent glioblastoma multiforme. Mol. Ther. 13:221-228. [DOI] [PubMed] [Google Scholar]
- 8.Helmbach, H., E. Rossmann, M. A. Kern, and D. Schadendorf. 2001. Drug resistance in human melanoma. Int. J. Cancer 93:617-622. [DOI] [PubMed] [Google Scholar]
- 9.Kasof, G. M., and B. C. Gomes. 2001. Livin, a novel inhibitor of apoptosis protein family member. J. Biol. Chem. 276:3238-3246. [DOI] [PubMed] [Google Scholar]
- 10.Krishnamurthy, S., T. Takimoto, R. A. Scroggs, and A. Portner. 2006. Differentially regulated interferon response determines the outcome of Newcastle disease virus infection in normal and tumor cell lines. J. Virol. 80:5145-5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kunicher, N., H. Falk, B. Yaacov, T. Tzur, and A. Panet. 2008. Tropism of lentiviral vectors in skin tissue. Hum. Gene Ther. 19:255-266. [DOI] [PubMed] [Google Scholar]
- 12.Lin, J. H., G. Deng, Q. Huang, and J. Morser. 2000. KIAP, a novel member of the inhibitor of apoptosis protein family. Biochem. Biophys. Res. Commun. 279:820-831. [DOI] [PubMed] [Google Scholar]
- 13.Lorence, R. M., P. A. Rood, and K. W. Kelley. 1988. Newcastle disease virus as an antineoplastic agent: induction of tumor necrosis factor-alpha and augmentation of its cytotoxicity. J. Natl. Cancer Inst. 80:1305-1312. [DOI] [PubMed] [Google Scholar]
- 14.Lotem, M., T. Peretz, O. Drize, Z. Gimmon, D. Ad El, R. Weitzen, H. Goldberg, I. Ben David, D. Prus, T. Hamburger, and E. Shiloni. 2002. Autologous cell vaccine as a post operative adjuvant treatment for high-risk melanoma patients (AJCC stages III and IV). Br. J. Cancer 86:1534-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lotem, M., E. Shiloni, I. Pappo, O. Drize, T. Hamburger, R. Weitzen, R. Isacson, L. Kaduri, S. Merims, S. Frankenburg, and T. Peretz. 2004. Interleukin-2 improves tumor response to DNP-modified autologous vaccine for the treatment of metastatic malignant melanoma. Br. J. Cancer 90:773-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohammed, M. Q., and S. Retsas. 2000. Oxaliplatin is active in vitro against human melanoma cell lines: comparison with cisplatin and carboplatin. Anticancer Drugs 11:859-863. [DOI] [PubMed] [Google Scholar]
- 17.Nachmias, B., Y. Ashhab, and D. Ben-Yehuda. 2004. The inhibitor of apoptosis protein family (IAPs): an emerging therapeutic target in cancer. Semin. Cancer Biol. 14:231-243. [DOI] [PubMed] [Google Scholar]
- 18.Nachmias, B., Y. Ashhab, V. Bucholtz, O. Drize, L. Kadouri, M. Lotem, T. Peretz, O. Mandelboim, and D. Ben-Yehuda. 2003. Caspase-mediated cleavage converts Livin from an antiapoptotic to a proapoptotic factor: implications for drug-resistant melanoma. Cancer Res. 63:6340-6349. [PubMed] [Google Scholar]
- 19.Nachmias, B., S. Mizrahi, M. Elmalech, I. Lazar, Y. Ashhab, R. Gazit, G. Markel, D. Ben-Yehuda, and O. Mandelboim. 2007. Manipulation of NK cytotoxicity by the IAP family member Livin. Eur. J. Immunol. 37:3467-3476. [DOI] [PubMed] [Google Scholar]
- 20.Parmiani, G., C. Castelli, M. Santinami, and L. Rivoltini. 2007. Melanoma immunology: past, present and future. Curr. Opin. Oncol. 19:121-127. [DOI] [PubMed] [Google Scholar]
- 21.Sinkovics, J. G., and J. C. Horvath. 2000. Newcastle disease virus (NDV): brief history of its oncolytic strains. J. Clin. Virol. 16:1-15. [DOI] [PubMed] [Google Scholar]
- 22.Stegmeier, F., G. Hu, R. J. Rickles, G. J. Hannon, and S. J. Elledge. 2005. A lentiviral microRNA-based system for single-copy polymerase II-regulated RNA interference in mammalian cells. Proc. Natl. Acad. Sci. USA 102:13212-13217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stojdl, D. F., B. Lichty, S. Knowles, R. Marius, H. Atkins, N. Sonenberg, and J. C. Bell. 2000. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat. Med. 6:821-825. [DOI] [PubMed] [Google Scholar]
- 24.Vucic, D., H. R. Stennicke, M. T. Pisabarro, G. S. Salvesen, and V. M. Dixit. 2000. ML-IAP, a novel inhibitor of apoptosis that is preferentially expressed in human melanomas. Curr. Biol. 10:1359-1366. [DOI] [PubMed] [Google Scholar]
- 25.Yaacov, B., E. Eliahoo, I. Lazar, M. Ben-Shlomo, I. Greenbaum, A. Panet, and Z. Zakay-Rones. 2008. Selective oncolytic effect of an attenuated Newcastle disease virus (NDV-HUJ) in lung tumors. Cancer Gene Ther. 15:795-807. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.