Abstract
Parvovirus minute virus of mice (MVMp) is endowed with oncotropic properties so far ascribed only to the dependency of the virus life cycle on cellular factors expressed during S phase and/or modulated by malignant transformation. For other viruses oncotropism relies on their inability to circumvent type I interferon (IFN)-induced innate antiviral mechanisms, the first line of defense triggered by normal cells against viral infections. These agents propagate, therefore, preferentially in transformed/tumor cells, which often lack functional antiviral mechanisms. The present study aimed at investigating whether antiviral processes also contribute to MVMp oncotropism. Our results demonstrate that in contrast to MVMp-permissive transformed mouse A9 fibroblasts, freshly isolated normal counterparts (mouse embryonic fibroblasts [MEFs]) mount, through production and release of type I IFNs upon their infection, an antiviral response against MVMp lytic multiplication. Pretreatment of MEFs with a type I IFN-β-neutralizing antibody, prior to MVMp infection, inhibits the virus-triggered antiviral response and improves the fulfillment of the MVMp life cycle. Our results also show that part of the A9 permissiveness to MVMp relies on the inability to produce type I IFNs upon parvovirus infection, a feature related either to an A9 intrinsic deficiency of this process or to an MVMp-triggered inhibitory mechanism, since stimulation of these cells by exogenous IFN-β strongly inhibits the parvovirus life cycle. Taken together, our results demonstrate for the first time that parvovirus infection triggers an innate antiviral response in normal cells and suggest that the MVMp oncotropism depends at least in part on the failure of infected transformed cells to mount such a response.
The mouse autonomously replicating parvovirus Minute virus of mice (prototype strain, MVMp) is a small icosahedral nonenveloped lytic virus containing a single-stranded DNA genome of about 5.1 kb (8). While infection of adult or neonatal mice with MVMp is asymptomatic, virus injected in utero into developing embryos mounts an aggressive infection which eventually kills the host (26). The MVMp life cycle is best supported in vivo as well as in vitro by fibroblastic cells, especially transformed derivatives like the mouse A9 line. The MVMp genome consists of two overlapping transcription units encoding two nonstructural (NS) and two structural (VP) proteins (7) whose expression is driven by the P4 and P38 promoters, respectively. Among the parvoviral products, the NS1 polypeptide is the major cytotoxic factor. For 2 decades, MVMp has attracted significant attention because of its oncotropic and oncolytic properties, displayed in both rodent and human cells (42). The parvoviral oncotropism has been so far ascribed to the dependency of the virus life cycle on host cell factors present during the S phase of the cell cycle and/or modulated by oncogenic transformation, thereby favoring virus multiplication in proliferating neoplastic tissues (6, 12). However, the nature and function of some of these characterized elements are so far not sufficient to fully explain the parvovirus oncotropism, indicating that still-unknown additional cellular elements must contribute to some extent to this virus property.
The first line of defense developed by cells against a viral invasion consists of the activation of an innate antiviral immune response via the production and release of type I interferons (IFN-α and -β). These antiviral cytokines are produced by invaded cells upon detection of pathogen-associated molecular patterns (PAMPs) consisting of nucleic acids derived from viruses, including double-stranded RNA (dsRNA), single-stranded RNA (ssRNA), or DNA, by cellular pathogen recognition receptors (PRRs) that are either membrane bound (Toll-like receptors [TLRs] 3, 7, 8, or 9) or present in the cytoplasm (protein kinase R [PKR], RIG-I, MDA5, and DAI) (30, 49). Upon activation, PRRs stimulate several downstream latent transcription factors, including NF-κB, ATF2-c-jun, and interferon regulatory factor 3 (IRF-3), which then cooperate to induce the expression of IFN-β molecules (19, 22, 23, 46). This step defines the early phase of the antiviral response. Subsequently, the cytokine is released from infected hosts and interacts in an autocrine and paracrine fashion with specific membrane-bound receptors, thereby stimulating the downstream JAK/STAT pathway. The latter activation is characterized, in particular, by the phosphorylation of the transcription factors STAT1 and STAT2, their heterodimerization, and further association with IRF-9 (40). This heterotrimer translocates to the nucleus, binds to the IFN-stimulated response element present in the promoters of IFN-stimulated genes (ISGs), and enhances their transcription (40). Among the ISGs targeted, several code for polypeptides which exert antiviral activities, like PKR or 2′-5′-oligoadenylate synthetase (2′-5′-OAS) (25). Other ISGs encode proteins that further enhance the antiviral response, such as STAT1 and STAT2 (34), IRF-9, or the transcription factor IRF-7. The latter factor is of major importance for the development of the defense mechanism, since it sets in motion a positive feedback regulation of the JAK/STAT pathway by inducing the transcription of a second wave of type I antiviral cytokines belonging both to the β- as well as to the α-subtype (more then 13 different IFN-α genes in humans and 10 in mice) (21, 24). Since IFN-αs and IFN-β bind to the same receptors, they further activate the JAK/STAT pathway and thereby the antiviral response (19, 21). Thus, release of type I IFNs by invaded hosts is crucial to block viral replication, limit infection, and facilitate virus clearance.
In response to these immune pressures, many viruses developed strategies to inhibit the antiviral innate immune machinery (40). These viral countermeasures block components of the pathways involved in IFN-β production (e.g., IRF-3) and/or JAK/STAT signaling (e.g., PKR, STATs, and 2′-5′-OAS) (40), thereby contributing to the pathogenesis and virulence of these agents. In contrast, some natural viruses (Newcastle disease virus and Sendai virus) or engineered viruses (vesicular stomatitis virus, herpes simplex virus, and influenza virus) are unable to trigger such evasion mechanisms in human cells (16). Their replication, multiplication, and pathogenesis are therefore restricted to cells that are intrinsically deficient in antiviral mechanisms. Interestingly, many human tumor/transformed cells accumulate in the course of the malignant transformation process, mutations hampering the expression/function of key factors of the antiviral response (39, 48). As a consequence, lytic viruses that are unable to counteract antiviral defense mechanisms in human cells are endowed with oncotropic properties and represent potential weapons to fight against cancers (16, 35).
It is presently unclear whether parvoviruses represent triggers and/or are targets of the innate antiviral machinery. While inoculation of mice with MVMp was shown to induce a weak production of type I IFNs (20), no trans-activation of the IFN-β promoter was detected in a mouse fibroblast line after infection with this virus (44). Furthermore, IFN expression was reported to be induced in vivo at a low level after treatment with Kilham rat virus (KRV), another rodent parvovirus (33), while it could not be detected in other studies using this virus (11) or the mink parvoviruses, Aleutian disease virus and mink enteritis virus. On the other hand, Aleutian disease virus and mink enteritis virus were found to be insensitive to the antiviral effects of IFNs (54, 55), whereas MVMp and the porcine parvovirus were shown to be highly (20) and moderately (15) susceptible to these cytokines, respectively. These controversial data, together with the unique oncotropic property of MVMp and the contribution of antiviral innate immune mechanisms to the oncotropic behavior of other lytic viruses, prompted us to further investigate the interplay between MVMp and the IFN-dependent antiviral response. To this end, freshly isolated normal mouse embryonic fibroblasts (MEFs) were compared to the transformed mouse fibroblast line A9 for the induction, release, and antiviral activity of type I IFNs after MVMp infection. Our results show for the first time that the IFN production pathway is mobilized by and active against MVMp in infected primary cells such as MEFs but silent in transformed fibroblasts due either to its intrinsic deficiency or to its inhibition by a virus-mediated evasion mechanism.
MATERIALS AND METHODS
Materials.
The rabbit antiserum anti-SP8 and the monoclonal antibody 3D9, both raised against the parvoviral NS1 protein, as well as the rabbit polyclonal antibody SP6 raised against the parvoviral NS2 protein were described previously (4, 5). The polyclonal rabbit antiserum TATT3 raised against the capsid VP1 and VP2/VP3 proteins of MVMp was a generous gift of P. Tattersall (Yale University, New Haven, CT). Goat polyclonal anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and rabbit anti-STAT1 and -STAT2 antibodies, as well as mouse monoclonal anti-PKR antibody were from Santa Cruz Biotechnology (Heidelberg, Germany). The polyclonal rabbit antibody directed against the phospho(Tyr701)-α and -β isoforms of STAT1 was obtained from Cell Signaling (Frankfurt, Germany). The polyclonal rabbit antibody specific for phospho(Tyr689)-STAT2 was from Millipore (Schwalbach/Ts, Germany). The mouse monoclonal antibodies directed against TLR3 and actin were from eBioscience (Frankfurt, Germany) and MP Biomedicals (Heidelberg, Germany), respectively. The synthetic dsRNA poly(I:C) was from GE Healthcare Europe (Freiburg, Germany). For transfection, Lipofectamine 2000 from Invitrogen (Karlsruhe, Germany) was used. Recombinant mouse beta interferon (rmIFN-β) and the enzyme-linked immunosorbent assay (ELISA) kit for detection of mouse IFN-β were both obtained from R&D Systems (Wiesbaden, Germany). Neutralizing antibodies against mouse IFN-β (7FD3) and IFN-α (4EA1) were produced as previously described (28) and purified through ammonium sulfate precipitation at 45% saturation using standard conditions. Neutralizing titers for 7FD3 and 4EA1 were 1:240,000 and 1:8,000 against 12 IU of recombinant mouse IFN-β and IFN-α4, respectively.
Cell culture and virus production.
Mouse A9 and L929 fibroblasts as well as human fibroblastic NB324K cells were maintained in minimum essential medium (MEM) supplemented with 5% heat-inactivated fetal bovine serum (FBS), 2 mM l-glutamine, 100 μg/ml penicillin, and 10 units/ml streptomycin. 293T cells were grown in Dulbecco's modified Eagle's medium (DMEM) containing 10% FBS and appropriate antibiotics. Low-passage (<5) primary MEFs freshly isolated from 12.5- to 13.5-day postconception embryos of C57BL/6 or CD1 mice were cultured in DMEM containing 10% heat-inactivated FBS with antibiotics. The multiplicity of infection (MOI) is expressed as PFU per cell. Primary stocks of wild-type MVMp were produced at the Virus Production Unit of the DKFZ, by calcium phosphate transfection of 293T cells with the pdBMVp infectious molecular clone of MVM as previously described (10). Cells were harvested 3 days posttransfection, and viruses were collected by repeated cycles of freezing and thawing in vTE (50 mM Tris-HCl [pH 8.3], 0.5 mM EDTA). Crude cell extracts were then used to reinfect human NB324K cells for a single further amplification of the stock. After subjecting infected NB324K cells to another series of free-thaw cycles in vTE buffer, virus stocks were purified by nonionic iodixanol gradient centrifugation (62). Viral stocks titers were determined by plaque assays (32) on human NB324K cell monolayers infected with serial dilutions of virus and are expressed as PFU/ml.
Cell transfection with the synthetic dsRNA poly(I:C).
Transfections of mock- or virus-infected A9 and MEF cells were carried out using Lipofectamine 2000 according to the manufacturer's instructions. Cells were transiently transfected with synthetic dsRNA poly(I:C) at a final concentration of 50 μg/ml for the times indicated, before being processed for further analysis.
Viral DNA extraction and Southern blot analysis.
Viral DNA intermediates were isolated using a modified Hirt extraction method, as previously described (10). Briefly, medium from mock-treated or MVMp-infected cultures was discarded at the time points indicated in the figure legends, and cells were scraped in phosphate-buffered saline (PBS) and pelleted by centrifugation at 500 × g for 5 min at room temperature. Cell pellets were resuspended in a 1:1 (vol/vol) mixture of vTE buffer and 2× Hirt buffer (20 mM Tris [pH 7.4], 20 mM EDTA, 1.2% sodium dodecyl sulfate [SDS]), followed by proteinase K digestion (400 μg/ml) for 18 h at 46°C. Cellular genomic DNA was sheared by five passages through 0.5- and then 0.4-mm needles. DNA samples (2 μg) were fractionated by electrophoresis on a 0.8% agarose gel. After denaturation, the DNA was immobilized onto a nylon Hybond N+ membrane (Amersham Biosciences). Viral DNA intermediates were detected, after denaturation and neutralization, by hybridization with a 32P-labeled DNA probe corresponding to the EcoRV (nucleotide 385)-EcoRI (nucleotide 1084) fragment of the MVMp NS genes.
SDS-PAGE and Western blotting.
At the indicated time points, mock-treated or infected cells were scraped in PBS and centrifuged at 500 × g for 5 min at room temperature. Cell pellets were resuspended in a modified radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.25% Na-deoxycholate, protease inhibitor cocktail [Roche Diagnostics], and phosphatase inhibitors [20 mM NaF, 5 mM β-glycerophosphate, 5 mM p-nitrophenyl phosphate, 5 mM sodium molybdate, 1 mM sodium orthovanadate, 5 mM sodium phosphate]) and stored on ice for 30 min. Samples were centrifuged at 20,000 × g for 15 min at 4°C, and the protein concentration in the supernatants was determined using the BCA protein assay kit according to the manufacturer's instructions (Pierce Biotechnology, Rockford, IL). Samples were then boiled for 5 min in Laemmli buffer, fractionated by 8 or 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE), and blotted onto nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany). The membranes were then blocked with 1× PBS containing 5% low-fat dry milk and 0.1% Tween 20 for 1 h. For detection of phosphorylated proteins, 1× Tris-buffered saline solution (TBS; 20 mM Tris-HCl [pH 7.6], 137 mM NaCl) containing 0.1% Tween 20 and 2% casein was used as a blocking solution. Incubations with primary antibodies were carried out at 4°C overnight either in 1× PBS containing 5% low-fat dry milk and 0.1% Tween 20 or in 1× TBS supplemented with 0.1% Tween 20 and 5% bovine serum albumin. Individual proteins were identified by means of specific antibodies used at a 1:2,000 (anti-SP8-NS1 and TATT3 VPs) or 1:1,000 (others) dilution. Protein-antibody complexes were then visualized with horseradish peroxidase-conjugated anti-rabbit (1:10,000 dilution) or anti-mouse (1:5,000 dilution) immunoglobulin Gs (IgGs; Promega). The immunoreactive total and phosphorylated proteins were detected by enhanced chemiluminescence (Perkin-Elmer Life Sciences, Amersham Biosciences, Freiburg, Germany).
Indirect immunofluorescence microscopy.
Cells were seeded on spot slides (3,000 cells/spot) in 50 μl of complete medium. After 24 h, the medium was removed and cells were mock treated or MVMp infected for 1 h at 37°C at the indicated MOI (50 μl inoculum in serum-free MEM). The inoculum was then removed and replaced with 100 μl of fresh MEM supplemented with 5% FBS. At the indicated time points, cells were fixed in PBS containing 4% paraformaldehyde for 30 min and subsequently permeabilized in PBS containing 0.5% Triton X-100 for 10 min. Before staining, cells were incubated for 1 h at 37°C in PBS containing 5% FBS as blocking solution. After extensive washing with PBS, cells were further incubated for 2 h at 37°C in a PBS solution containing a 1:50 dilution of the anti-NS1 antibody 3D9 (10). After being extensively washed in PBS, the preparations were incubated for 1 h at 37°C with PBS containing a 1:600 dilution of secondary donkey anti-mouse IgGs conjugated to Alexa Fluor 594 (Molecular Probes, Eugene, OR). Before mounting with Elvanol, the stained cells were incubated for 2 min with Hoechst solution to visualize the cell nucleus through DNA labeling and then extensively washed with PBS. Stained cells were then analyzed by conventional epifluorescence microscopy (Leica DMRBE; 40× objective, with immersion oil). Images were captured using a Hamamatsu Orca digital camera and processed using Openlab 2 (Improvision).
LDH assay.
The lytic activity of MVMp was determined by quantifying the amount of lactate dehydrogenase (LDH) released into the culture medium from infected cultures. LDH activity was measured according to the CytoTox96 colorimetric test (Promega, Mannheim, Germany) following the manufacturer's instructions. Briefly, cells (3,000 cells/well) were plated in a 96-well plastic culture dish in a volume of 50 μl of MEM supplemented with 5% FBS. After 24 h, the cells were infected or mock treated by the addition of 50 μl of full medium containing or not the wild-type MVMp (at the indicated MOI). Cells were then kept for 72 h in a CO2 incubator at 37°C. LDH activity was measured in 50 μl of culture medium by using an ELISA reader at the recommended 492 nm. After subtraction of the background value found with nonconditioned complete medium, the fraction of lysed cells in individual infected or noninfected cultures was calculated from the ratio of the LDH activity in the conditioned medium to the total LDH activity of the corresponding culture. The total LDH activity was determined in triplicate cultures after cell lysis by the addition of 10× buffer containing 9% (vol/vol) Triton X-100.
MTT activity assay.
For the determination of cell viability, the metabolic activity of mitochondrial dehydrogenases was measured through the ability of these enzymes to produce a formazan dye through reduction of methylthiazolyldiphenyl-tetrazoliumbromide (MTT). The same cultures were used to determine both MTT and LDH activities. After the removal of 50 μl of medium for LDH activity determination (see above), 10 μl of sterile 5-mg/ml MTT (Sigma) dissolved in PBS was added to the cultures, and incubation was continued for 3 h at 37°C in a CO2 incubator. After centrifugation of the plate, the supernatant (60 μl) was removed and the cells were dried for 4 h at 37°C before being lysed by the addition of 100 μl isopropanol. The absorbance of the formazan dye was measured at 595 nm, using an ELISA plate reader.
The viability of infected cells was expressed as the ratio of the corresponding absorbance to that of noninfected cells taken arbitrarily as 100%.
Detection of IFN production.
Secretion of type I IFNs by parvovirus-infected and/or poly(I:C)-transfected mouse cells was determined by bioassay. Briefly, culture supernatants of stimulated A9 or MEF cells were collected at the indicated time points and cleared of cell debris by a brief centrifugation (500 × g for 5 min). These supernatants were then UV irradiated to inactivate infectious MVMp particles. Biological activity of IFN was measured in a standard cytopathic effect inhibition assay using the murine fibroblast cell line L-929 (ATCC CCL1) cultured in DMEM containing 5% FCS.
Monolayers of 15,000 cells/100 μl/well in flat-bottom microtiter plates were incubated overnight with twofold dilutions of the test samples and then infected with mouse encephalomyocarditis virus (EMCV) at a multiplicity of 0.1. After 20 h, MTT (catalog no. M2128; Sigma Chemicals, Munich, Germany) was added for 4 h at a 1-mg/ml final concentration. In live cells, MTT is metabolized by mitochondria to form an insoluble deposit of dark brown MTT-formazan in the cells. The incorporated MTT-formazan was solubilized in 100 μl of lysis buffer (5% SDS, 10 mM HCl, 50% 2-propanol). Virus-induced cytopathic effect was then quantified in an ELISA reader based on the absorbance at 570 nm. A twofold dilution of an internal laboratory standard preparation of mouse IFN based on the NIH mouse reference IFN-α/β preparation (catalog no. Gu02-901-511) was included in each test. One laboratory unit corresponded to one international unit (IU), defined as the concentration of IFN resulting in 50% protection against viral lysis. A 50% protective effect was assumed at an optical density at 570 (OD570) that was half that obtained for cells fully protected by IFN. Titers of the antiviral bioassay are given in IU. Alternatively, in some experiments, IFN-β was also quantified in supernatants by using an ELISA kit from R&D Systems (Wiesbaden, Germany).
RT-PCR.
Total RNAs of mock-treated, MVMp-infected, and/or poly(I:C)-transfected mouse fibroblasts were isolated using an RNeasy mini kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Isolated RNAs (1 μg total RNA) were then digested with 1 unit of DNase I (Promega, Mannheim, Germany) at 37°C for 20 min to remove genomic DNA contamination before being processed for reverse transcription (RT) using oligo(dT) primers and reverse transcriptase from Moloney murine leukemia virus (Promega, Mannheim, Germany). For each cDNA sample generated in this way, a control was produced using an RT mixture in which no reverse transcriptase was added in order to detect a potential contamination of the cDNA sample with residual genomic DNA. Ten percent portions of the resulting cDNA samples were then used as a template for PCR using Taq DNA polymerase (Invitrogen, Karlsruhe, Germany) and the following specific sets of primers (MWG Biotech, Ebersberg, Germany): for GAPDH, forward primer 5′-ACCACAGTCCATGCCATCAC-3′ and reverse primer 5′-TCCACCACCCTGTTGCTGTA-3′; for IFN-β, forward primer 5′-CCCTATGGAGATGACGGAGA-3′ and reverse primer 5′-CTGTCTGCTGGTGGAGTTCA-3′; for IFN-non-α4, forward primer 5′-A(AG)(GC)(CT)TGT(GC)TGATGCA(AG)CAGGT-3′ and reverse primer 5′-GG(AT)ACACAGTGATCCTGTGG-3′ (13); for 2′-5′-OAS, forward primer 5′-GTGCTCCTCCGCTGTAAGAC-3′ and reverse primer 5′-ACAGAACCTCCCAACAGGTG-3′; for TLR3, forward primer 5′-GAGGGCTGGAGGATCTCTTT-3′ and reverse primer 5′-TGCCTCAATAGCTTGCTGAA-3′; for MVMp, forward primer 5′-ACGCTCACCATTCACGACACCGAAA-3′ and reverse primer 5′-ATCATAGGCCTCGTCGTGCTCTTTG-3′. PCR products were then analyzed by electrophoresis through 2% agarose gels.
RESULTS
Completion of the MVMp life cycle is restricted in infected MEFs.
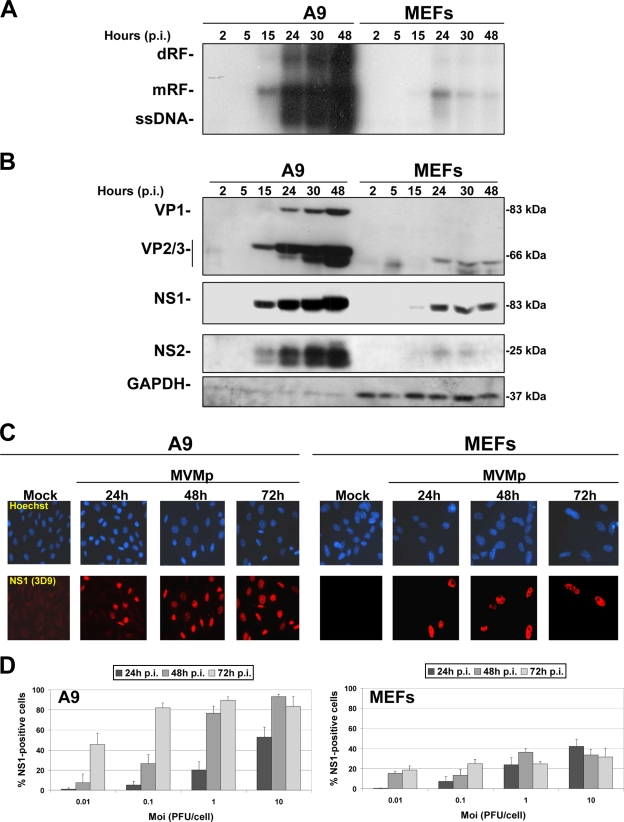
In order to verify the oncotropic feature of MVMp, we first tested whether the viral life cycle is indeed restricted in infected (MOI, 10 PFU/cell) normal MEFs, freshly isolated from C57BL/6 mice, in comparison with transformed A9 fibroblasts known to be permissive to the parvovirus. We first carried out Southern blot experiments, measuring the kinetics of MVMp DNA replication in both cell types. As shown in Fig. 1A, MVMp DNA replication was efficient in A9 cell cultures, as apparent from the time-dependent accumulation of monomeric (mRF) and dimeric (dRF) replicative forms and progeny ssDNA genomes. In contrast, MEF cultures only sustained a low level of MVM DNA replication, which peaked at 24 h postinfection (p.i.) and declined thereafter. Similarly, viral capsid (VP) and NS proteins accumulated at much-reduced levels and only during the first 24 h p.i. in MVMp-infected MEF versus A9 cultures (Fig. 1B). As illustrated in Fig. 1C, both types of cells accumulated nonstructural NS1 proteins in their nucleus upon MVMp infection, a feature which occurred in almost all A9 cells 48 h p.i. (MOI, 10 PFU/cell), whereas only a minor fraction of the MEF population showed such a phenotype over the time frame investigated. Dose- and time-dependent analyses of the latter feature indeed revealed that over 80% of A9 cells showed positive NS1 staining 2 days after infection at an MOI as low as 1 PFU/cell, whereas an MOI of 10 PFU/cell was necessary for NS1 to be detected in a maximum of 40% of MEF cells at 24 h p.i., with no further increase at later times (Fig. 1D). Altogether, these results indicated that MEF cells are poorly permissive for MVMp, which failed to spread in infected cultures.
FIG. 1.
MVMp life cycle in primary (MEF) and transformed (A9) mouse fibroblasts. Kinetics of MVMp replication and viral protein expression were assessed in transformed (A9) and normal mouse embryonic fibroblasts (C57BL/6 MEFs). Cells were infected at an MOI of 10 PFU/cell in 9.5-cm dishes and incubated for the indicated times postinfection. They were then processed for Southern and Western blotting as described in Materials and Methods. (A) Analysis of virus replication by Southern blotting. Two micrograms of total DNA per sample was extracted and run through a 0.8% agarose gel as described in Materials and Methods. Expression of the viral DNA intermediates was investigated using a radiolabeled DNA probe corresponding to the EcoRI-EcoRV fragment of the viral NS genes. The blot shown is representative of three experiments, all of which gave similar results. (B) Western blot analysis of viral structural (VP) and nonstructural (NS) protein expression was performed on 50 μg/sample of total protein extracted in complete RIPA buffer and subjected to 10% SDS-PAGE. Following blotting, membranes were probed with antibodies specific for the respective viral proteins. GAPDH was used as an internal loading control. (C) Analysis of the kinetics of NS1 expression in infected and mock-treated A9 or MEF cells was performed by indirect immunofluorescence as described in Materials and Methods. Cells grown on spot slides (3,000 cells/spot) were infected or not with MVMp at an MOI of 10 PFU/cell and fixed with paraformaldehyde at the indicated time postinfection, and NS1 expression was determined using an anti-NS1 (3D9) primary antibody and a secondary Alexa Fluor 594 donkey anti-mouse IgG antibody (red). Nuclei were visualized with Hoechst solution (blue). (D) MOI-dependent and kinetic analyses of the previous experiment were performed as described for panel C. For each time point and each MOI, five randomly chosen fields within a spot of infected or mock-treated cells stained for NS1 and Hoechst were counted. The values shown are means with standard deviation bars from three independent experiments analyzed.
MVMp is much less toxic for MEFs than for A9 cells, although the extent of its uptake by both cell types seems to be similar.
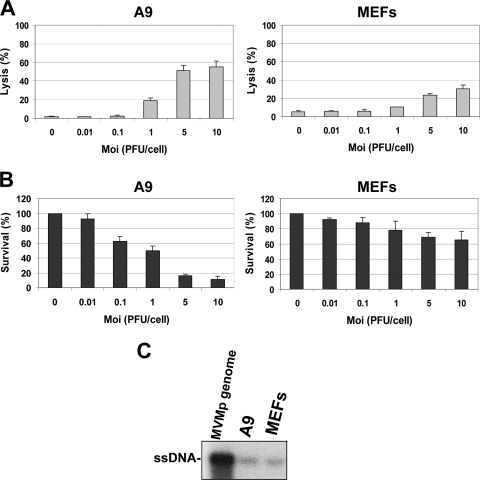
Further analysis of the parvovirus life cycle in both cell types was performed, focusing especially on the cytotoxic activity exerted by MVMp in A9 and MEF cells. The parvovirus was found to be much more toxic for A9 than for MEF cells. While clearly developing in A9 cultures infected at a low multiplicity, cytopathic effects became significant in MEF cells only at the highest virus doses tested (Fig. 2A and B). It should also be stated that similar amounts of inoculated virions were taken up by A9 and MEF cells (Fig. 2C), suggesting that the barrier to MVMp multiplication in the latter cultures occurred intracellularly at a step(s) following entry and limiting viral DNA amplification and expression. These observations raised the question of whether MVMp infection elicited an antiviral response in normal cells which negatively interfered with the completion of the parvoviral life cycle.
FIG. 2.
Cytotoxic effects displayed by MVMp in A9 and MEF cultures. MVMp was tested at different multiplicities of infection for its ability to exert, 72 h p.i., cytotoxic effects in MEF and A9 cultures grown in 96-well plates (3,000 cells per well). (A) Virus lytic activity was measured through quantification of the cytoplasmic release of LDH into the culture medium. Values are expressed as a percentage of total LDH present in the infected or noninfected cultures. (B) The cell-killing activity of the virus was assessed by determining the reduction in the number of living cells able to reduce MTT in a formazan dye in the infected population. Values are expressed as percentages of the value obtained for mock-treated cultures. All LDH and MTT values are means with standard deviation bars from four independent experiments, each carried out in triplicate. (C) Uptake of viral particles by each cell type was assessed by Southern blotting 5 h p.i., using an MOI of 30 PFU/cell after extensive washing of the infected samples with PBS.
MVMp infection of MEFs leads to production and release of type I IFNs.
As a first step in testing this hypothesis, we determined whether type I IFNs, which are known for their antiviral activity, were released into the medium of MVMp-infected A9 and MEF cultures. The presence of IFN-β was first tested by ELISA, since this cytokine mediates the immediate response of cells to pathogen invasion and is known to be the major antiviral cytokine factor produced by infected fibroblasts (40). MVMp infection (10 PFU/cell) was found to induce MEFs to release IFN-β molecules into their culture medium (Fig. 3A). In contrast, no IFN-β secretion could be detected in cell-free supernatant from infected A9 cultures.
FIG. 3.
Production of type I IFNs by MVMp-infected MEF and A9 cultures. Production and release of type I IFNs was assessed in the culture supernatants of MVMp-infected A9 and MEF cultures after an initial centrifugation in order to discard contaminating floating material. Cells grown in 9.5-cm dishes were infected at an MOI of 10 PFU/cell and, at 24 h (A) or at different times p.i. (B), medium was collected, cleared of debris and cells, and subjected to UV irradiation. (A) Concentration of IFN-β in the medium was determined by using a commercially available ELISA kit. (B) The presence of type I IFNs in culture supernatants was determined by bioassays through the protective effect exerted by type I IFNs against EMCV lytic infection in L929 cultures. IFN levels were calculated based on the standard curves generated with recombinant IFN-α. Data reflect averages with standard deviation bars of three experiments performed in triplicate. nd, not detectable.
In a second approach, we analyzed the kinetics of type I IFN release in culture media from MVMp-infected (10 PFU/cell) or mock-treated A9 and MEF cultures, using a bioassay revealing these cytokines through their ability to protect mouse L929 reporter cells from EMCV infection. These experiments were also undertaken to test a possible release of α subtypes of type I IFNs by MVMp-infected A9 cells, since both α and β IFNs are measured by the bioassay, while only the latter one is detected by the above ELISA. As shown in Fig. 3B, this approach confirmed the presence of antiviral cytokines in cell-free supernatants of MVMp-infected (but not mock-infected) MEF cultures, in amounts increasing steadily with time up to 205 ± 45 IU/ml (mean ± standard deviation) at the latest point tested (72 h p.i.). No antiviral activity was detected in medium collected from MVMp-infected A9 cultures, pointing to the failure of these cells to release type I IFNs upon parvovirus infection.
Taken together, these results demonstrate for the first time that normal MEFs release type I IFN upon infection with MVMp, suggesting that these cytokines may play a role in the inhibition of the parvovirus life cycle in these cells.
MVMp infection of MEFs leads to activation of both IFN production and IFN signaling pathways.
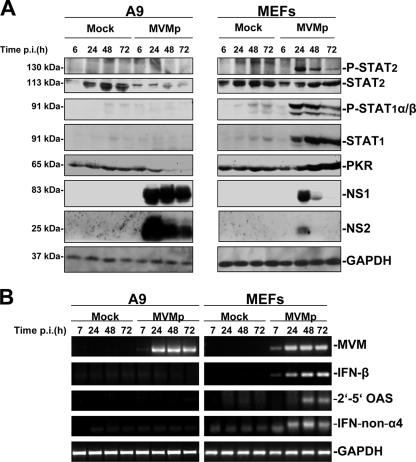
Release of type I IFNs and binding to their membrane-bound receptors activates the cellular JAK/STAT pathway, also termed the IFN signaling pathway. This process is characterized by the phosphorylation of STAT1 and STAT2 transcription factors and the downstream transcriptional upregulation of ISGs, including those encoding PKR, STAT1, STAT2, and 2′-5′-OAS (34, 40). Based on these considerations, we carried out Western blot experiments to determine whether the JAK/STAT pathway was activated in MVMp-infected MEF and A9 cells, using specific antibodies that recognize PKR, total STAT1, total STAT2, or activated STAT1 and STAT2 (phosphorylated on Tyr701 or Tyr689, respectively). As shown in Fig. 4A, STAT1- and STAT2-activating phosphorylations were detected upon virus infection (10 PFU/cell) of MEFs, a feature which peaked about 24 h p.i. and declined afterwards. In addition, a time-dependent increase in the expression of the ISG products STAT1 and PKR was observed in infected MEFs. In contrast, none of these indicators of JAK/STAT pathway mobilization was turned on in MVMp-infected A9 cells. On the contrary, MVM infection of A9 cells was associated with a time-dependent decrease in the steady-state level of PKR, which was already apparent at 24 h p.i. (i.e., long before the occurrence of cytopathic effects) and further progressed until 72 h p.i. (Fig. 4A), suggesting that the virus may be able to downregulate the expression of the antiviral kinase specifically in transformed A9 fibroblasts. This capacity may conceivably contribute to the above-mentioned accumulation of MVMp proteins to a much higher level in A9, compared with MEF, cultures (Fig. 1B).
FIG. 4.
Activation and/or upregulation of expression of antiviral innate immune factors in MVMp-infected MEF and A9 cells. The stimulation of different elements of the antiviral machinery was investigated over time in MVMp-infected A9 or C57BL/6 MEF cultures grown in 9.5-cm dishes. Cells were mock treated or infected at an MOI of 10 PFU/cell and incubated for the times indicated before being subjected to Western blotting and RT-PCR analysis. (A) Western blot analysis of the activation and induction of expression of innate antiviral factors as well as levels of expression of viral proteins were performed on total protein extracts (100 μg/sample) obtained in complete RIPA buffer from infected or mock-treated cells. Extracts were subjected to 10% SDS-PAGE and blotted, and membranes were probed with antibodies specific for phosphorylated and total isoforms of STAT1 and STAT2 as well as for PKR and the viral polypeptides NS1 and NS2. GAPDH was used as a loading control. Each blot shown is representative of three additional blots which all gave similar results. (B) Assessment of IFN-β, IFN-non-α4, 2′-5′-OAS, and viral NS mRNA levels by RT-PCR in MVMp-infected or mock-treated cells. Total RNA was isolated at the indicated times by using an RNeasy kit, and 1 μg was reverse transcribed into cDNA. Ten percent of this product was then subjected to PCR with sets of primers specific to each mRNA. GAPDH was used as a housekeeping gene control. No signal was detected when reverse transcriptase was omitted. The presented data are representative of four independent experiments which gave, under the same experimental conditions, identical results.
These results prompted us to further characterize the temporal activation of both IFNs and IFN-induced genes in infected MEFs. Since the quantitative regulation of these processes is known to occur at the transcriptional level, total RNAs were extracted from MVMp-infected (MOI, 10 PFU/cell) or mock-treated cells, and the transcripts encoding either the viral NS proteins or the cellular factors IFN-β, IFN-non-α4, and 2′-5′OAS were measured by RT-PCR using specific primer sets. As illustrated in Fig. 4B, MVMp infection of MEFs, but not A9 fibroblasts, led to an upregulation of the transcription of all above-mentioned cellular transcripts. Interestingly, the induction of IFN-β gene transcription was apparent already at 7 h p.i., while 2′-5′-OAS and IFN-non-α4 mRNAs started to accumulate to detectable levels at a later time (between 24 and 48 h p.i.), in agreement with the general idea that IFN-β expression represents the immediate response of a cell which leads to the subsequent transcriptional induction of the IFN-α genes.
Altogether, our results showed that IFN-β and IFN-α species were both produced by MEFs upon MVMp infection, arguing for the participation of these cytokines in the resistance of normal cells to the parvovirus through activation of the JAK/STAT pathway. In contrast, these features were not triggered in transformed A9 host cells, which appeared unable to mount an antiviral response against MVMp infection.
Induction of a type I IFN-dependent antiviral response is a general feature of MVMp-infected MEFs.
In order to rule out that the IFN response triggered by wild-type MVMp virus in C57BL/6 MEFs was due to a virus stock specificity or was a peculiarity of this mouse strain, we compared the ability of MVMp batches independently prepared in Heidelberg (MVMp a) and Beer Sheva (MVMp b) to induce the release of type I IFNs and to activate the JAK/STAT pathway in MEFs freshly isolated from either C57BL/6 or CD1 mice. In A9 cells, used as a control, no obvious differences were observed between the viruses. In agreement with the above results (Fig. 1 and 4A), infection of A9 cultures with either virus stock (MOI, 5 PFU/cell) resulted in an amplification of viral DNA (Fig. 5A), an accumulation of both NS1 and NS2 polypeptides, a lack of detectable phosphorylation or increased expression of STATs, and a time-dependent decrease of PKR expression (Fig. 5B). The responses of C57BL/6 and CD1 MEFs to MVMp infection were similar. Indeed, cells of both origins sustained only little viral DNA replication (Fig. 5A) and expression of proteins (Fig. 5B), as previously mentioned (Fig. 1 and 4A). It is noteworthy that CD1 cells seemed to sustain slightly more parvoviral mRF production and ssDNA synthesis at 24 and 48 h, respectively, than C57BL/6 MEFs (Fig. 5A). Nevertheless, this poor permissiveness correlated with a time-dependent induction of ISG (STAT1 and PKR) expression and STAT1/2 phosphorylation in both types of infected MEFs (Fig. 5B), in agreement with previous data (Fig. 4A). As in A9 cells, no significant differences between the MVMp stocks a and b were noticed in MEFs. It is worth noting that compared with their C57BL/6 counterparts, CD1 MEFs revealed a significantly greater ISG induction (STATs and PKR) and activation (P-STATs) upon MVMp infection (Fig. 5B). This enhanced response could be correlated with the release of higher amounts of type I IFNs from infected CD1 (MVM a, 662 ± 100 IU/ml; MVM b, 673 ± 133 IU/ml) versus C57BL/6 MEFs (MVM a, 52 ± 4 IU/ml; MVM b, 46 ± 18 IU/ml) (Fig. 5C). It was concluded from these results that induction of a type I IFN-dependent antiviral response is a general feature of MVMp-infected normal mouse embryonic fibroblasts, although the intensity of this response varies depending on the mouse strain considered.
FIG. 5.
Activation of the JAK/STAT pathway by two unrelated MVMp stocks in MEFs originating from different mouse strains. Cells grown in 9.5-cm dishes were infected at an MOI of 5 PFU/cell for the indicated time points with MVMp produced either in Germany (MVM a) or in Israel (MVM b). (A) Southern blot analysis of MVMp replication. Total DNA was extracted from cells at the indicated times, and the expression levels of viral DNA intermediates dRF, mRF, and ssDNA were assessed as described for Fig. 1 by using a radiolabeled DNA probe corresponding to the EcoRI-EcoRV fragment of the viral NS genes. Results of one representative experiment of three are shown. (B) Western blot analysis of the JAK/STAT pathway activation and assessment of expression of viral proteins, performed as described for Fig. 4. The presented results are representative of three experiments, all of which gave similar results. (C) Quantification of type I IFN production in cell-free culture medium from MVMp a- or MVMp b-infected A9, C57BL/6, or CD1 cultures was performed by bioassays, at 48 h p.i., as described for Fig. 3 and in Materials and Methods. IFN levels were calculated based on the standard curves generated with recombinant IFN-α. Data reflect averages with standard deviation bars of three experiments performed in triplicate.
A9 cells develop an antiviral response upon poly(I:C) transfection.
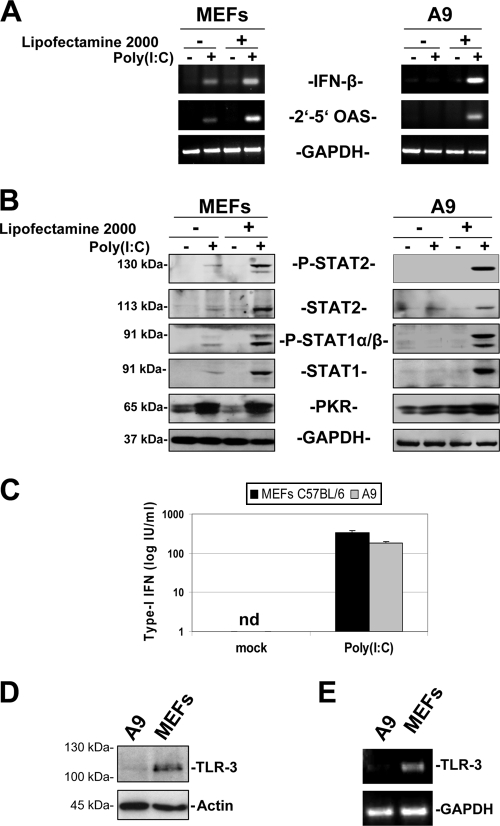
Since type I IFNs were not detected in MVMp-infected A9 supernatants, we decided to assess whether the production and release of type I IFNs could be activated at all in these fibroblasts, using a standard inducer thereof. To this end, A9 cultures as well as MEFs, used as positive controls, were treated with the dsRNA poly(I:C), which is known to trigger the IFN production pathway, either through its recognition by membrane-bound TLR3 when added into the culture medium or through its detection by the cytosolic PRRs RIG-I and MDA5 when transfected into cells (18). The ability of poly(I:C), administered through either route, to stimulate IFN production and JAK/STAT-mediated signaling was determined by RT-PCR quantification of the mRNAs coding for IFN-β and 2′-5′OAS, respectively. As illustrated in Fig. 6A, both the incubation (addition in the medium) or the transfection with poly(I:C) resulted in the upregulation of both transcripts in MEFs, while A9 cells only showed such effects when poly(I:C) was administered through transfection. These results were confirmed by Western blot analysis of components of the JAK/STAT pathway in protein extracts from cells treated, or not, with poly(I:C). As shown in Fig. 6B, a potent stimulation of this pathway was detected upon transfection of MEFs and A9 cells with the dsRNA, as shown by the phosphorylation of STAT1 and STAT2 transcription factors and the enhanced expression of the ISG products PKR, STAT1, and STAT2. As reported for the induction of IFN-β and 2′-5′OAS mRNAs (see above), these protein changes were also achieved in MEFs when poly(I:C) was added to the culture medium, although to a lesser extent than upon transfection, whereas such treatment was ineffective in A9 cells. Finally, the presence of type I IFNs was demonstrated by bioassays in cell-free culture media from poly(I:C)-transfected MEFs and, to a slightly lower extent, A9 fibroblasts (Fig. 6C).
FIG. 6.
Activation of the JAK/STAT pathway in MEFs and A9 cells by poly(I:C) and expression of TLR3 by both cell types. A9 and MEF cells grown in 9.5-cm dishes were stimulated or not with poly(I:C) (50 μg/ml) either through application of the synthetic dsRNA in the culture medium or through its transfection with Lipofectamine 2000. Cells were harvested 24 h later. (A) Assessment by RT-PCR of IFN-β and 2′-5′-OAS mRNA levels in unstimulated or poly(I:C)-treated cells. Total RNA was isolated from cells using an RNeasy kit. One microgram of total RNA was reverse transcribed, and 10% of the resulting cDNA was subjected to PCRs using sets of primers specific for each transcript. GAPDH was used as a housekeeping gene control. No signal was detected when the reverse transcriptase was omitted. Each data set is representative of three experiments, all of which gave similar results. (B) Western blot analysis of JAK/STAT pathway activation in poly(I:C)-treated or untreated cells. Whole-cell extracts obtained in complete RIPA buffer (100 μg/sample) were subjected to 10% SDS-PAGE, blotted, and probed with antibodies specific to phosphorylated and total isoforms of STAT2 and STAT1 as well as to PKR. GAPDH detection was also determined to ensure similar protein loading. The presented blots are representative of three additional experiments, all of which gave similar results. (C) Quantification by bioassays of the type I IFN content of cell-free culture supernatants from poly(I:C)-transfected or unstimulated cultures, performed as described for Fig. 3 and in Materials and Methods. Data are representative of three experiments performed in duplicate. nd, not detectable. (D) Western blot analysis of TLR3 expression by A9 and MEF cells. Total proteins were extracted from cells by using complete RIPA buffer. Protein samples (100 μg/lane) were examined for TLR3 expression by SDS-PAGE, using a TLR3-specific antibody. Equal protein loading was confirmed by using an antibody recognizing α-actin. The presented blots are representative of two experiments that gave similar results. (E) Assessment by RT-PCR of the TLR3 mRNA level in unstimulated A9 or MEF cells. PCRs were performed with sets of primers specific to TLR3 or GAPDH mRNAs and using 10% of the total cDNA produced upon reverse transcription of 1 μg of isolated total RNA. When reverse transcriptase was omitted, no signal could be detected. Data are representative of two experiments that produced similar results.
Altogether, our data indicate that A9 cells, like MEFs, have functional IFN production and signaling pathways, as shown by their induction by the synthetic dsRNA poly(I:C). While supplementing the culture medium with poly(I:C) was sufficient to trigger these effects in MEFs, activation of the IFN response in A9 cells required transfection of the dsRNA. This result suggested to us that TLR3, which is the PRR-sensing poly(I:C) present in the extracellular milieu, is not expressed or expressed only at low levels in A9 cells compared to normal fibroblasts. Indeed, Western blot and RT-PCR experiments failed to reveal TLR3 polypeptides and transcripts in A9 cells, while these products were clearly detected in MEFs (Fig. 6D and E). These results therefore suggest that TLR3 could represent the cellular PRR which senses MVMp infection in MEF cells and that its absence in A9 cells accounts for the failure of the transformed fibroblasts to induce an IFN response upon parvovirus infection.
MVMp is sensitive to the antiviral action of type I IFNs in A9 cells.
The ability of A9 cells to exhibit a number of hallmarks of type I IFN-induced antiviral response activation upon poly(I:C) transfection prompted us to investigate whether the MVMp life cycle is indeed sensitive to this defense mechanism. This is an important issue, given that many human transformed cells have proved to be much less responsive to type I IFNs than their normal counterparts (48), and conflicting data have been reported regarding the sensitivities of autonomous parvoviruses to the antiviral activities of these cytokines (20, 54). In a first step, exogenously applied rmIFN-β was tested for its ability to stimulate the JAK/STAT pathway in transformed A9 fibroblasts, as measured by Western blotting and RT-PCR. We observed (data not shown) that these cells indeed exhibited the hallmarks of IFN-induced signaling, in particular, a dose-dependent phosphorylation of both STAT1 and STAT2 transcription factors, an enhanced expression of STAT1, and a striking accumulation of 2′-5′OAS mRNAs. We next conducted Southern blot experiments to assess the effect of rmIFN-β, applied concomitantly with the virus (10 PFU/cell), on MVMp DNA replication in A9 and MEF cultures. As shown in Fig. 7A, MVMp DNA amplification was severely inhibited by rmIFN-β in a dose-dependent fashion in both cell types. However, while a complete inhibition of the MVMp replication seemed to be achieved in MEFs by the application of rmIFN-β already at the lowest dose used (10 IU/ml) (Fig. 7A), viral DNA replication could not be fully suppressed by the cytokine in A9 cultures and continued to be detected at a residual but significant level even in cells treated with up to 100 IU/ml of rmIFN-β. Nevertheless, a phosphorimager analysis revealed that in these transformed mouse fibroblasts the amount of each viral DNA intermediate was reduced by more than 50% upon treatment with already the lowest IFN-β dose (10 IU/ml) compared to amounts produced by infected cells not treated with the cytokine (data not shown). Similarly, NS1 expression determined by Western blot analysis of MVMp-infected A9 and MEF cells was found to decline upon application of increasing concentrations of rmIFN-β, which correlated with a striking induction of the ISG product PKR (Fig. 7B). Like DNA replication intermediates, residual NS1 production remained detectable at the highest IFN-β dose tested in MVMp-infected A9 cells, while the nonstructural protein became almost undetectable already at the lowest dose of IFN-β tested in infected MEFs. In keeping with the MVMp replication and expression inhibition induced by exogenously applied IFN-β, the cytotoxic and lytic activities of the parvovirus were strongly reduced in cytokine-treated A9 cell cultures, as measured by MTT and LDH assays (Fig. 7C). Taken together, these experiments indicate that MVMp is highly sensitive to the antiviral action of type I IFNs and furthermore that both A9 and MEFs cells are endowed with a functional IFN signaling pathway able to trigger an antiviral response against the parvovirus upon exogenous stimulation with rmIFN-β. They also suggest that the residual MVMp replication and NS1 expression observed in A9 cultures exposed to quite high IFN-β doses were either cell-specific phenomena or, more likely, that the substantially lower levels of basal replication and NS1 expression achieved by MVMp in MEFs compared to A9 cells facilitated the extent of antiviral action exerted by exogenously added rmIFN-β in the former cells.
FIG. 7.
Sensitivity of the MVMp life cycle to the antiviral action of recombinant mouse IFN-β in A9 and MEF cells. Cells grown in 9.5-cm dishes were treated or not with rmIFN-β at the indicated concentration (in IU/ml) concomitantly with MVMp infection (10 PFU/cell). (A and B) Cells were harvested 48 h later and processed for Southern or Western blot analysis. (A) Southern blot analysis of MVMp replication in the presence or absence of increasing doses of rmIFN-β was performed on 2 μg/sample of total extracted DNA as described for Fig. 1 and in Materials and Methods. One blot representative of three performed is shown. (B) Western blot analysis of NS1 and PKR expression in total protein extracts obtained from rmIFN-β-treated or untreated A9 or MEF cells infected with MVMp. Samples (50 μg protein) obtained in complete RIPA buffer were subjected to 10% SDS-PAGE and blotted, and membranes were probed with antibodies specific for NS1 or PKR. GAPDH detection was included as a loading control. Each blot is representative of three experiments, which gave similar results. (C) Analysis of the cytotoxic effects triggered by MVMp in A9 cells in the presence or absence of rmIFN-β were assessed in LDH and MTT experiments. Cells grown in 96-well plates were infected with MVMp (10 PFU/cell) and concomitantly treated, or not, with the indicated doses of rmIFN-β. After an incubation of 72 h, they were then assessed for lysis (LDH assay) and survival (MTT assay) as described in Materials and Methods. For MTT assays, values obtained for mock-treated cells in the presence or absence of the respective amounts of rIFN-β were each time arbitrarily taken as 100% survival. The data shown are means with standard deviation bars from four independent experiments carried out in triplicate.
A9 cells are fully permissive to MVMp, which is routinely propagated in this line. Since we observed that these cells mount an efficient antiviral response against MVMp when stimulated with exogenously applied IFN-β (Fig. 7), and moreover, given that these cells are intrinsically able to produce and release type I IFNs upon stimulation with poly(I:C) (Fig. 6), these findings suggest that the capacity of A9 cultures for sustaining MVMp multiplication can then, at least in part, be assigned to their inability to produce type I IFNs upon MVMp infection. Such features might be caused either by an intrinsic failure in the PRR pathway that senses the parvovirus infection in these cells or by the ability of MVMp to trigger an evasion mechanism which inhibits the latter mechanism specifically in A9 cells.
Treatment with a type I IFN-β-neutralizing antibody prevents IFN-mediated signaling by MVMp and stimulates the parvovirus life cycle in MEFs.
In order to confirm the role of type I IFNs in the stimulation of an antiviral response in MVMp-infected MEFs and to identify the IFN species involved, MEFs were treated with a neutralizing antibody directed specifically either against the β (7FD3) or the α (4EA1) subtype of mouse type I IFNs, starting from 24 h prior to MVMp infection (5 PFU/cell) or mock treatment, or cells were left untreated. Cells were harvested at 40 h p.i. (an interval previously shown to allow full activation of the JAK/STAT pathway in MEFs [Fig. 4A]) and processed for Western blot analysis of STAT phosphorylation and expression, as well as PKR and NS1 accumulation. As shown in Fig. 8A, the antibody that neutralized IFN-β (7FD3), but not the IFN-α-specific one (4EA1), fully inhibited both the MVMp-dependent phosphorylation of STATs as well as the virus-induced upregulation of mediators (STAT1 and STAT2) and effector (PKR) of the IFN response in MEFs. The 7FD3 antibody indeed prevented MVMp from activating an antiviral mechanism in MEFs, as revealed by an increase in (i) the production of MVMp nonstructural protein NS1 (Fig. 8A), (ii) the accumulation of viral DNA replicative forms (Fig. 8B), and (iii) the fraction of MEFs able to express the NS1 polypeptide (Fig. 8C). In keeping with this 7FD3-dependent stimulation of the MVMp life cycle, the capacity of the virus for lysing MEFs was enhanced in the presence of the IFN-β-neutralizing antibody (Fig. 8D). These results demonstrated that the 7FD3 treatment did not interfere with the uptake of MVMp and counteracted the antiviral response downstream of the parvovirus-induced IFN-β production and release. It should be stated that MEFs grew at similar rates, irrespective of whether they were exposed to the 7FD3 antibody (data not shown), ruling out that the enhanced permissiveness of antibody-treated cells for MVMp was due to a stimulation of their proliferation. It is worth noting that IFN-α and -β species were both induced in MVMp-infected MEF cultures (Fig. 4B). The delayed appearance of IFN-αs (Fig. 4B) and the lack of effect elicited by the IFN-α-specific antibody 4EA1 on IFN signaling within 40 h p.i. (Fig. 8A) further confirmed that IFN-β was first induced as a result of MVMp infection of MEFs and subsequently led to the stimulation of IFN-α expression. Importantly, although the 7FD3 antibody treatment fully suppressed the antiviral response induced by MVMp in MEFs (Fig. 8A), thereby improving substantially the MVMp lytic life cycle, we did not detect, as observed in MVMp-infected A9 cells, under these conditions a downregulation in PKR expression compared to mock-treated MEFs. This result demonstrated that the parvovirus is unable to trigger a downregulation in PKR expression in MEFs, a feature which could have been masked by the IFN-β-induced increase in the PKR level.
FIG. 8.
Effects of inhibition of type I IFN activity on the MVMp life cycle in MEFs and A9 cells. Cells grown in 9.5-cm dishes were treated or not with an IFN-β-neutralizing (7FD3) or an IFN-α-neutralizing (4EA1) antibody at a 1:30 dilution starting 24 h before their mock treatment or infection with MVMp (MOI, 5 PFU/cell). They were left to grow further in the presence or absence of antibody for the indicated times before being processed for Western blotting, Southern blotting, indirect immunofluorescence, or LDH and MTT assays. (A) Western blot analysis of JAK/STAT pathway activation and NS1 expression in RIPA buffer extracts from MEF and A9 cells infected or not for 40 h with MVMp and pretreated or not with 7FD3 or 4EA1. A 100-μg aliquot of total protein extract/sample was subjected to 10% SDS-PAGE and blotted, and membranes were probed with antibodies specific to the phosphorylated and total isoforms of STAT1 and STAT2 as well as to PKR and NS1. GAPDH detection was included as a loading control. The data presented are representative of three experiments performed, all of which showed similar results. (B) Southern blot analysis of the kinetics and extent of MVMp replication in infected MEF or A9 cultures, pretreated or not with 7FD3, was performed at the times indicated as described for Fig. 1 and in Materials and Methods. One representative experiment of three is shown. (C) Assessment by immunofluorescence of the effect of IFN-β neutralization on the amount of cells showing NS1 expression in infected A9 or MEF cultures. Cells grown on spot slides (3,000 cells/spot) were treated or not with 7FD3 starting 24 h before their further infection with MVMp for 24 h. They were then fixed with paraformaldehyde and labeled with a primary antibody directed against NS1 (3D9) and a secondary Alexa Fluor 594 donkey anti-mouse IgG. Nuclei were visualized with Hoechst solution. The fraction of NS1-expressing cells was then determined by microscopy. Total and NS1-positive cells were scored for at least five frames per spot under a 20× objective. Values shown are means with standard deviation bars from five independent experiments that were analyzed. In the case of MEF cells, an unpaired t test was performed to determine statistical significance (*, P < 0.05). (D) Analysis by LDH assays of the effects of IFN-β neutralization on the lytic activity of MVMp in A9 or MEF cultures. Cells grown in 96-well plates were treated or not with 7FD3 at a 1:30 dilution starting 24 h before being infected, or not, with MVMp (5 PFU/cell) for a further 72 h. They were then analyzed for virus-induced lysis through quantification of the amount of LDH released into the culture medium as described in Materials and Methods. LDH levels are expressed as a percentage of total LDH present in the respective cultures. The data shown are means with standard deviation bars from three independent experiments, each carried out in triplicate. In the case of MEF cells, an unpaired t test was performed to determine statistical significance (**, P < 0.01). (E) Proliferation of A9 and MEF cultures was investigated by MTT assay (see Materials and Methods) in 96-well plates over a period of 48 h. The data shown are means with standard deviation bars from five independent experiments, each carried out in triplicate.
For the sake of comparison, both IFN-β-neutralizing (7FD3) and IFN-α-neutralizing (4EA1) antibodies were also tested for their effects on the MVMp life cycle in A9 cells. In agreement with the above-mentioned absence of detectable type I IFN production in A9 cultures infected with MVMp (Fig. 3 and 5C), treatment of these cells with 7FD3 or 4EA1 had no effect on the NS1 expression or on the downregulation of PKR expression triggered by MVMp (Fig. 8A). Since 4EA1 showed no effects in either cell type and given that 7FD3 was the only antibody effective against the IFN response triggered by MVMp in infected MEFs (Fig. 8A), we decided to analyze further only the effect displayed by the latter antibody on the parvovirus life cycle in A9 cells. In these transformed fibroblasts, 7FD3 treatment failed to improve the viral DNA replication (Fig. 8B), was unable to increase the fraction of cells expressing NS1 (Fig. 8C), and had no effect on the viral lytic effects (Fig. 8D). It was noted that the capacity of A9 cells for MVMp DNA amplification was much higher than that of 7FD3-treated MEFs (Fig. 8B), i.e., interruption of the antiviral response in the latter cells brought their MVMp permissiveness up to a level which still remained significantly inferior to the A9 one. These observations indicated that the antiviral response displayed by infected MEFs was not the only reason for their lower permissiveness to MVMp compared to A9. Another limitation to the progression of the MVMp life cycle in MEF cultures is likely to lie in the fact that they proliferate at a much lower rate than the transformed A9 cell line (Fig. 8E). Since the onset of MVMp replication is strictly dependent on cellular factors expressed during the S phase of the cell cycle (12), the slow growth of MEF cultures can be expected to restrict the fraction of cells able to initiate the replicative phase of the MVMp life cycle within the time frame analyzed in our experiments.
In conclusion, the above results show that upon infection of normal MEFs, MVMp triggers a type I IFN-mediated antiviral response for which the parvovirus is a target and whose experimental interruption is sufficient to restore a significant extent of MVMp replication in these cells. This response appears to be impaired in a transformed fibroblast line, suggesting that innate antiviral mechanisms may contribute to the oncotropism of autonomous parvoviruses.
DISCUSSION
The oncotropic feature of MVMp has been ascribed so far to the capacity of neoplastic cells to offer a cellular milieu (including cell cycle distribution) suitable for replication and expression of the viral genome and completion of the viral lytic life cycle (12, 42). The present findings indicate that the oncotropism of this parvovirus is also likely to depend on antiviral defense mechanisms triggered by virus infection. Indeed, we showed that normal MEFs can be distinguished from their transformed counterparts (the A9 cell line) by the ability of the former and failure of the latter to mount a robust antiviral response mediated by type I IFNs which very efficiently impairs lytic multiplication of the virus. This work provides the first evidence to suggest that parvovirus infection is sensed by host PRRs, the cellular sentinels triggering type I IFN production upon detection of invading viruses in cells (30). This implies also that the parvoviral genome, DNA replication intermediates, and/or transcription products display pathogen-associated molecular patterns, since these molecules are known to be responsible for the stimulation of PRRs (40). It thus appears that induction of type I IFN expression and the ensuing activation of an innate antiviral response are crucial cellular mechanisms dictating MVMp infectivity in host cells. Our investigations point to IFN-β as the molecule triggering the antiviral state in MVMp-infected MEFs. Indeed, the functional neutralization of this cytokine by means of a specific antibody is sufficient to fully inhibit the host defense response, thereby improving substantially viral lytic replication in these cells. The release of type I IFNs and the establishment of an antiviral state are general reactions of normal mouse fibroblasts to MVMp infection, although the extent of these effects varies between MEFs from different mouse strains. Indeed, MEFs originating from CD1 mice were found to release significantly more antiviral cytokines and undergo a much stronger JAK/STAT pathway activation upon MVMp infection, compared with C57BL/6 MEFs. Given that CD1 cells supported slightly more viral NS protein expression and DNA replication than C57BL/6 cells, it could be that a correlation exists between the extent of MVMp amplification in normal mouse fibroblasts and the type I IFN production. Altogether, our observations are in agreement with an earlier report showing that MVMp-inoculated mice produce low levels of type I IFNs (20) and with the general view that synthesis of IFN-β represents the primary response of fibroblasts to viral infections (19, 40).
It was ruled out that the incapacity of established A9 cells to mount an anti-MVMp response is due to the general lack of sensitivity of these cells to the antiviral action of type I IFNs, as described for many human tumor cells (9, 47). Indeed, exogenous recombinant IFN-β was very efficient in triggering, even at a low dose, a potent antiviral response against MVMp when administered concomitantly with the virus to A9 cells. On the other hand, we failed to detect any induction of either IFN-α or IFN-β mRNAs and proteins in infected A9 cells, which strongly suggests that the permissiveness of these cells for MVMp can be traced back, at least in part, to their incapacity to produce type I IFNs upon parvovirus infection. These results are in line with a previous report showing that MVMp infection did not result in detectable trans-activation of the IFN-β promoter in Moloney sarcoma virus-transformed mouse fibroblasts (44). Similarly, innate antiviral signal transduction pathways leading to IFN-α or -β gene transcription were activated upon myxoma virus infection of normal MEFs but not immortalized mouse embryonic fibroblasts (52). The A9 cell deficiency in IFN production could be either intrinsically acquired, for instance, along with transformation, or caused by MVMp as part of a virus-triggered evasion mechanism operating in transformed mouse cells but not in their normal counterparts (MEFs).
We obtained no evidence to suggest that A9 cells are intrinsically deficient in the PRR-mediated sensing of parvovirus infection. Indeed, poly(I:C)-transfected A9 cells were found to develop a sustained production of IFN-β, indicating that the IFN-producing pathways dependent on the poly(I:C)-responsive cytoplasmic PRRs RIG-I and MDA5 (18, 56, 57) are most likely functional in these cells. On the other hand, A9 cells could be distinguished from MEFs by the lack of detectable expression of TLR3, a well-known membrane-bound PRR (29, 51), in the former line. This difference is, however, unlikely to account for the impairment of type I IFN production in MVMp-infected A9 cells. Indeed, TLR3 receptors are predominantly localized in endosomes (38) and are primarily stimulated by endocytosed extracellular dsRNAs that are either released by RNA virus-infected dying cells or are part of the genome of RNA viruses (1, 31, 45). Although not completely excluded, this feature argues against a major role of TLR3 in the recognition of ssDNA-containing parvoviruses entering cells from the extracellular milieu. However, several parvoviruses, including Kilham rat virus (60) and adeno-associated virus 1 (AAV-1), -2, and -9 (59), were shown to stimulate TLR9 through their ssDNA genomes. Activation of TLR9, a DNA sensor, is known to occur through recognition of CpG DNA motifs, a feature which leads to type I IFN production through engagement of the adaptor MyD88 (30). Thus, it could be envisaged that in A9 cells, but not in MEFs, an absence of TLR9 expression or a defect in its downstream signaling pathway may account for the inability of the former cells to trigger IFN-β production upon MVMp infection. This hypothesis should now be investigated, although the rat parvovirus H-1, a close homologue of MVMp, was found to very weakly stimulate TLR9 (41, 61). The possibility still remains that there could be something wrong with the sensing of MVMp by other DNA sensors in A9 cells. For instance, DAI/ZBP1/DLM1 (a cytoplasmic PRR mediating a potent production of IFN-β upon detection of dsDNA in mouse cells) (50, 53) or its downstream signaling pathway may be specifically altered in A9 cells but not in MEFs.
Alternatively, A9 cells may differ from normal fibroblasts by allowing MVMp to develop an evasion mechanism which inhibits specifically the IFN production pathway that senses the presence of the parvovirus. Although it remains to be demonstrated, this scenario is supported by our observation that the expression of the cytoplasmic, IFN-inducible, dsRNA-dependent protein kinase PKR is time-dependently downregulated in MVMp-infected A9 cells, whereas it is clearly upregulated in infected MEFs through the virus-induced release of type I IFNs. In addition, our study also demonstrates that MVMp is obviously unable to downregulate PKR expression in MEFs, a process which in these cells could have been masked by the IFN-dependent induction of PKR expression. Indeed, the complete inhibition of the latter process by a neutralizing IFN-β antibody does not lead in MVMp-infected MEFs to a reduction of PKR expression below levels detected in noninfected cells, although this treatment significantly improved the parvovirus life cycle. Apart from its classical antiviral role consisting of the downregulation of cellular and viral translation in invaded hosts (25, 43), PKR was also reported to behave as a PRR, thereby contributing to the production of IFN-β upon infection of cells by some viruses (3, 14, 17, 27, 36). This leads us to speculate that MVMp infection may be sensed by PKR, as recently reported for AAV-2 and AAV-5 (two members of the Dependovirus genus of the Parvoviridae family) in human cells (37). This PKR-mediated recognition of MVMp would induce MEFs to produce type I IFNs, whereas this production would not occur in transformed fibroblasts due to the ability of the parvovirus to actively downregulate the expression of this kinase in the latter type of cells. It is worth noting in this context that AAV-2 and -5 require the assistance of helper viruses to inhibit the PKR antiviral activity (37). The proposed participation of PKR in MVMp sensing does not rule out, however, that the virus blocks IFN-β production in A9 cells by targeting other cytoplasmic PRR-dependent pathways besides PKR.
Our data showing that normal mouse fibroblasts release type I IFNs upon MVMp infection may also provide some clues regarding the lethal effect triggered by the parvovirus in embryos after in utero inoculation (26). Indeed, type I IFNs are known to act in both paracrine and autocrine fashions and have pleiotropic effects including, besides the induction of an antiviral response, the inhibition of cell growth, the modulation of apoptosis, and the stimulation of cells belonging to the innate and adaptive immune systems (2, 58). Thus, it may well be that quickly proliferating embryonic cells respond to MVMp infection in utero by producing and releasing substantial amounts of type I IFNs which may interfere with embryonic development through their ability to stimulate apoptosis and/or activate immune cells. This bystander effect may be added to the direct lytic activity of the parvovirus to aggravate the induction of embryonic death.
In summary, our study demonstrates for the first time that MVMp, the parvovirus type species, is both a trigger of and a target for the type I IFN-mediated antiviral response in normal fibroblasts but fails to mobilize this defense pathway in at least some transformed cell derivatives. The dependence of this absence of innate “antiparvoviral” defense on malignant transformation argues for a contribution of the antiviral response to the oncotropism of certain parvoviruses. Our data further show that the inability of MVMp to activate an antiviral state in transformed cells can be traced back to the lack of type I IFN production, most likely through the impairment of cytoplasmic PRR-mediated sensing of virus (products). Hints of an active role of the parvovirus in the suppression of IFN induction in transformed cells were obtained, and intensive work is now being conducted in order to unravel the molecular mechanisms underlying these processes.
Acknowledgments
S.G. was supported by a position from the French Institut National de la Santé et de la Recherche Médicale. This work was partially supported by the German Israel Foundation (no. I-762-210.2/2002; 2004-2007).
We have no commercial affiliations or consultancies, stock or equity interests, or patent-licensing arrangements that could be considered to pose a conflict of interest regarding the manuscript.
We thank Annabel Grewenig for excellent technical assistance. We are indebted to S. Cotmore and P. Tattersall (Yale University, New Haven, CT) for providing us with the infectious MVMp DNA clone and to D. J. Pintel (University of Missouri, Columbia, MO) for his generous gift of the monoclonal mouse anti-NS1 antibody 3D9. We are also deeply thankful to N. Salomé and M. Klein-Vogel (Deutsches Krebsforschungszentrum, Heidelberg, Germany) for providing us with polyclonal anti-NS antibodies. We are also indebted to N. Giese for helpful discussions and constructive suggestions and to B. Leuchs for providing MVMp stocks.
Footnotes
Published ahead of print on 28 October 2009.
REFERENCES
- 1.Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413:732-738. [DOI] [PubMed] [Google Scholar]
- 2.Alsharifi, M., A. Mullbacher, and M. Regner. 2008. Interferon type I responses in primary and secondary infections. Immunol. Cell Biol. 86:239-245. [DOI] [PubMed] [Google Scholar]
- 3.Balachandran, S., P. C. Roberts, L. E. Brown, H. Truong, A. K. Pattnaik, D. R. Archer, and G. N. Barber. 2000. Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity 13:129-141. [DOI] [PubMed] [Google Scholar]
- 4.Bodendorf, U., C. Cziepluch, J. C. Jauniaux, J. Rommelaere, and N. Salome. 1999. Nuclear export factor CRM1 interacts with nonstructural proteins NS2 from parvovirus minute virus of mice. J. Virol. 73:7769-7779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brockhaus, K., S. Plaza, D. J. Pintel, J. Rommelaere, and N. Salome. 1996. Nonstructural proteins NS2 of minute virus of mice associate in vivo with 14-3-3 protein family members. J. Virol. 70:7527-7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornelis, J. J., Y. Q. Chen, N. Spruyt, N. Duponchel, S. F. Cotmore, P. Tattersall, and J. Rommelaere. 1990. Susceptibility of human cells to killing by the parvoviruses H-1 and minute virus of mice correlates with viral transcription. J. Virol. 64:2537-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cotmore, S. F., L. J. Sturzenbecker, and P. Tattersall. 1983. The autonomous parvovirus MVM encodes two nonstructural proteins in addition to its capsid polypeptides. Virology 129:333-343. [DOI] [PubMed] [Google Scholar]
- 8.Cotmore, S. F., and P. Tattersall. 1987. The autonomously replicating parvoviruses of vertebrates. Adv. Virus Res. 33:91-174. [DOI] [PubMed] [Google Scholar]
- 9.Critchley-Thorne, R. J., D. L. Simons, N. Yan, A. K. Miyahira, F. M. Dirbas, D. L. Johnson, S. M. Swetter, R. W. Carlson, G. A. Fisher, A. Koong, S. Holmes, and P. P. Lee. 2009. Impaired interferon signaling is a common immune defect in human cancer. Proc. Natl. Acad. Sci. U. S. A. 106:9010-9105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daeffler, L., R. Horlein, J. Rommelaere, and J. P. Nuesch. 2003. Modulation of minute virus of mice cytotoxic activities through site-directed mutagenesis within the NS coding region. J. Virol. 77:12466-12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darrigrand, A. A., S. B. Singh, and C. M. Lang. 1984. Effects of Kilham rat virus on natural killer cell-mediated cytotoxicity in brown Norway and Wistar Furth rats. Am. J. Vet. Res. 45:200-202. [PubMed] [Google Scholar]
- 12.Deleu, L., A. Pujol, S. Faisst, and J. Rommelaere. 1999. Activation of promoter P4 of the autonomous parvovirus minute virus of mice at early S phase is required for productive infection. J. Virol. 73:3877-3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deonarain, R., A. Alcami, M. Alexiou, M. J. Dallman, D. R. Gewert, and A. C. Porter. 2000. Impaired antiviral response and alpha/beta interferon induction in mice lacking beta interferon. J. Virol. 74:3404-3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Der, S. D., and A. S. Lau. 1995. Involvement of the double-stranded-RNA-dependent kinase PKR in interferon expression and interferon-mediated antiviral activity. Proc. Natl. Acad. Sci. U. S. A. 92:8841-8845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Derbyshire, J. B. 1989. The interferon sensitivity of selected porcine viruses. Can. J. Vet. Res. 53:52-55. [PMC free article] [PubMed] [Google Scholar]
- 16.Everts, B., and H. G. van der Poel. 2005. Replication-selective oncolytic viruses in the treatment of cancer. Cancer Gene Ther. 12:141-161. [DOI] [PubMed] [Google Scholar]
- 17.Gilfoy, F. D., and P. W. Mason. 2007. West Nile virus-induced interferon production is mediated by the double-stranded RNA-dependent protein kinase PKR. J. Virol. 81:11148-11458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gitlin, L., W. Barchet, S. Gilfillan, M. Cella, B. Beutler, R. A. Flavell, M. S. Diamond, and M. Colonna. 2006. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc. Natl. Acad. Sci. U. S. A. 103:8459-8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haller, O., G. Kochs, and F. Weber. 2006. The interferon response circuit: induction and suppression by pathogenic viruses. Virology 344:119-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris, R. E., P. H. Coleman, and P. S. Morahan. 1974. Erythrocyte association and interferon production by minute virus of mice. Proc. Soc Exp. Biol. Med. 145:1288-1292. [DOI] [PubMed] [Google Scholar]
- 21.Hiscott, J. 2007. Convergence of the NF-κB and IRF pathways in the regulation of the innate antiviral response. Cytokine Growth Factor Rev. 18:483-490. [DOI] [PubMed] [Google Scholar]
- 22.Hiscott, J. 2007. Triggering the innate antiviral response through IRF-3 activation. J. Biol. Chem. 282:15325-15329. [DOI] [PubMed] [Google Scholar]
- 23.Honda, K., and T. Taniguchi. 2006. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat. Rev. Immunol. 6:644-658. [DOI] [PubMed] [Google Scholar]
- 24.Honda, K., H. Yanai, H. Negishi, M. Asagiri, M. Sato, T. Mizutani, N. Shimada, Y. Ohba, A. Takaoka, N. Yoshida, and T. Taniguchi. 2005. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 434:772-777. [DOI] [PubMed] [Google Scholar]
- 25.Hovanessian, A. G. 2007. On the discovery of interferon-inducible, double-stranded RNA activated enzymes: the 2′-5′oligoadenylate synthetases and the protein kinase PKR. Cytokine Growth Factor Rev. 18:351-361. [DOI] [PubMed] [Google Scholar]
- 26.Itah, R., J. Tal, and C. Davis. 2004. Host cell specificity of minute virus of mice in the developing mouse embryo. J. Virol. 78:9474-9486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnston, J. B., S. H. Nazarian, R. Natale, and G. McFadden. 2005. Myxoma virus infection of primary human fibroblasts varies with cellular age and is regulated by host interferon responses. Virology 332:235-248. [DOI] [PubMed] [Google Scholar]
- 28.Kawade, Y., and Y. Watanabe. 1987. Proc. Third Int. TNO Meet. Biol. Interferon Syst. Nijhoff, The Hague, The Netherlands.
- 29.Kawai, T., and S. Akira. 2006. Innate immune recognition of viral infection. Nat. Immunol. 7:131-137. [DOI] [PubMed] [Google Scholar]
- 30.Kawai, T., and S. Akira. 2009. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int. Immunol. 21:317-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawai, T., and S. Akira. 2008. Toll-like receptor and RIG-I-like receptor signaling. Ann. N. Y. Acad. Sci. 1143:1-20. [DOI] [PubMed] [Google Scholar]
- 32.Kestler, J., B. Neeb, S. Struyf, J. Van Damme, S. F. Cotmore, A. D'Abramo, P. Tattersall, J. Rommelaere, C. Dinsart, and J. J. Cornelis. 1999. cis requirements for the efficient production of recombinant DNA vectors based on autonomous parvoviruses. Hum. Gene Ther. 10:1619-1632. [DOI] [PubMed] [Google Scholar]
- 33.Kilham, L., C. E. Buckler, V. H. Ferm, and S. Baron. 1968. Production of interferon during rat virus infection. Proc. Soc Exp. Biol. Med. 129:274-278. [DOI] [PubMed] [Google Scholar]
- 34.Lehtonen, A., S. Matikainen, and I. Julkunen. 1997. Interferons up-regulate STAT1, STAT2, and IRF family transcription factor gene expression in human peripheral blood mononuclear cells and macrophages. J. Immunol. 159:794-803. [PubMed] [Google Scholar]
- 35.Liu, T. C., and D. Kirn. 2007. Systemic efficacy with oncolytic virus therapeutics: clinical proof-of-concept and future directions. Cancer Res. 67:429-432. [DOI] [PubMed] [Google Scholar]
- 36.Loving, C. L., S. L. Brockmeier, W. Ma, J. A. Richt, and R. E. Sacco. 2006. Innate cytokine responses in porcine macrophage populations: evidence for differential recognition of double-stranded RNA. J. Immunol. 177:8432-8439. [DOI] [PubMed] [Google Scholar]
- 37.Nayak, R., and D. J. Pintel. 2007. Adeno-associated viruses can induce phosphorylation of eIF2α via PKR activation, which can be overcome by helper adenovirus type 5 virus-associated RNA. J. Virol. 81:11908-11916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishiya, T., E. Kajita, S. Miwa, and A. L. Defranco. 2005. TLR3 and TLR7 are targeted to the same intracellular compartments by distinct regulatory elements. J. Biol. Chem. 280:37107-37117. [DOI] [PubMed] [Google Scholar]
- 39.Pitha, P. M. 2000. Introduction: interferon's connection to cancer. Semin. Cancer Biol. 10:69-72. [DOI] [PubMed] [Google Scholar]
- 40.Randall, R. E., and S. Goodbourn. 2008. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J. Gen. Virol. 89:1-47. [DOI] [PubMed] [Google Scholar]
- 41.Raykov, Z., S. Grekova, B. Leuchs, M. Aprahamian, and J. Rommelaere. 2008. Arming parvoviruses with CpG motifs to improve their oncosuppressive capacity. Int. J. Cancer 122:2880-2884. [DOI] [PubMed] [Google Scholar]
- 42.Rommelaere, J., and J. J. Cornelis. 1991. Antineoplastic activity of parvoviruses. J. Virol. Methods 33:233-251. [DOI] [PubMed] [Google Scholar]
- 43.Saunders, L. R., and G. N. Barber. 2003. The dsRNA binding protein family: critical roles, diverse cellular functions. FASEB J. 17:961-983. [DOI] [PubMed] [Google Scholar]
- 44.Schlehofer, J. R., M. Rentrop, and D. N. Mannel. 1992. Parvoviruses are inefficient in inducing interferon-beta, tumor necrosis factor-alpha, or interleukin-6 in mammalian cells. Med. Microbiol. Immunol. 181:153-164. [DOI] [PubMed] [Google Scholar]
- 45.Schulz, O., S. S. Diebold, M. Chen, T. I. Naslund, M. A. Nolte, L. Alexopoulou, Y. T. Azuma, R. A. Flavell, P. Liljestrom, and C. Reis e Sousa. 2005. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature 433:887-892. [DOI] [PubMed] [Google Scholar]
- 46.Stetson, D. B., and R. Medzhitov. 2006. Type I interferons in host defense. Immunity 25:373-381. [DOI] [PubMed] [Google Scholar]
- 47.Stojdl, D. F., B. Lichty, S. Knowles, R. Marius, H. Atkins, N. Sonenberg, and J. C. Bell. 2000. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat. Med. 6:821-825. [DOI] [PubMed] [Google Scholar]
- 48.Stojdl, D. F., B. D. Lichty, B. R. tenOever, J. M. Paterson, A. T. Power, S. Knowles, R. Marius, J. Reynard, L. Poliquin, H. Atkins, E. G. Brown, R. K. Durbin, J. E. Durbin, J. Hiscott, and J. C. Bell. 2003. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell 4:263-275. [DOI] [PubMed] [Google Scholar]
- 49.Takaoka, A., and S. Shinohara. 2008. DNA sensors in innate immune system. Uirusu 58:37-46. [DOI] [PubMed] [Google Scholar]
- 50.Takaoka, A., Z. Wang, M. K. Choi, H. Yanai, H. Negishi, T. Ban, Y. Lu, M. Miyagishi, T. Kodama, K. Honda, Y. Ohba, and T. Taniguchi. 2007. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 448:501-505. [DOI] [PubMed] [Google Scholar]
- 51.Vercammen, E., J. Staal, and R. Beyaert. 2008. Sensing of viral infection and activation of innate immunity by toll-like receptor 3. Clin. Microbiol. Rev. 21:13-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang, F., J. W. Barrett, Y. Ma, G. A. Dekaban, and G. McFadden. 2009. Induction of alpha/beta interferon by myxoma virus is selectively abrogated when primary mouse embryo fibroblasts become immortalized. J. Virol. 83:5928-5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang, Z., M. K. Choi, T. Ban, H. Yanai, H. Negishi, Y. Lu, T. Tamura, A. Takaoka, K. Nishikura, and T. Taniguchi. 2008. Regulation of innate immune responses by DAI (DLM-1/ZBP1) and other DNA-sensing molecules. Proc. Natl. Acad. Sci. U. S. A. 105:5477-5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wiedbrauk, D. L., M. E. Bloom, and D. L. Lodmell. 1986. Mink parvoviruses and interferons: in vitro studies. J. Virol. 60:1179-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wiedbrauk, D. L., W. J. Hadlow, L. C. Ewalt, and D. L. Lodmell. 1986. Interferon response in normal and Aleutian disease virus-infected mink. J. Virol. 59:514-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoneyama, M., and T. Fujita. 2008. Structural mechanism of RNA recognition by the RIG-I-like receptors. Immunity 29:178-181. [DOI] [PubMed] [Google Scholar]
- 57.Yoneyama, M., M. Kikuchi, T. Natsukawa, N. Shinobu, T. Imaizumi, M. Miyagishi, K. Taira, S. Akira, and T. Fujita. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5:730-737. [DOI] [PubMed] [Google Scholar]
- 58.Yoshida, H., Y. Okabe, K. Kawane, H. Fukuyama, and S. Nagata. 2005. Lethal anemia caused by interferon-beta produced in mouse embryos carrying undigested DNA. Nat. Immunol. 6:49-56. [DOI] [PubMed] [Google Scholar]
- 59.Zhu, J., X. Huang, and Y. Yang. 2009. The TLR9-MyD88 pathway is critical for adaptive immune responses to adeno-associated virus gene therapy vectors in mice. J. Clin. Invest. 119:2388-2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zipris, D., E. Lien, A. Nair, J. X. Xie, D. L. Greiner, J. P. Mordes, and A. A. Rossini. 2007. TLR9-signaling pathways are involved in Kilham rat virus-induced autoimmune diabetes in the biobreeding diabetes-resistant rat. J. Immunol. 178:693-701. [DOI] [PubMed] [Google Scholar]
- 61.Zipris, D., E. Lien, J. X. Xie, D. L. Greiner, J. P. Mordes, and A. A. Rossini. 2005. TLR activation synergizes with Kilham rat virus infection to induce diabetes in BBDR rats. J. Immunol. 174:131-142. [DOI] [PubMed] [Google Scholar]
- 62.Zolotukhin, S., B. J. Byrne, E. Mason, I. Zolotukhin, M. Potter, K. Chesnut, C. Summerford, R. J. Samulski, and N. Muzyczka. 1999. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 6:973-985. [DOI] [PubMed] [Google Scholar]