Abstract
Coxsackie B viruses (CVB) are enteroviruses that have been associated with a variety of human diseases, including myocarditis. In the present study, we found that MDA5 and its adaptor molecule MAVS are critical for type I interferon responses to CVB, since the absence of either MAVS or MDA5 leads to deficient type I interferon production and early mortality in mice infected with CVB. Pancreatic and hepatic necrosis were observed on histopathological examination of MAVS and MDA5 knockout mice infected with CVB. Inflammatory cytokine production in response to systemic CVB infection was independent of MAVS. Surprisingly, virus titers were not elevated in MAVS-deficient mice, despite significant reductions in type I interferon levels. These data highlight the importance of type I interferon in host defense and provide insight on the mechanisms of innate immune responses following coxsackievirus infection.
Coxsackieviruses are nonenveloped, single-stranded RNA enteroviruses of the Picornavirus family that are common pathogens in humans. They are conventionally divided into subgroups A (serotypes A1 to A24) and B (serotypes B1 to B6). While coxsackie A viruses are most commonly associated with skin exanthema, the coxsackie B viruses (CVB) are associated with serious illnesses such as myocarditis, pericarditis, and meningitis, among other diseases. All six serotypes of CVB bind and enter cells through a common receptor protein, the coxsackie and adenovirus receptor (CAR) (5).
Type I interferons (IFNs), including IFN-β and multiple IFN-α subtypes, are produced early during viral infection and induce antiviral effects within target cells and mediate the development of the innate and adaptive immune responses. Type I IFN is critical in the early control of CVB infection, since mice deficient in the type I IFN receptor (IFN-α/βR−/−) die more rapidly than wild-type mice when infected with CVB (32). Deonarain et al. observed increased susceptibility of IFN-β-deficient mice after CVB infection (6). Rapid recognition of the virus by the innate immune system is essential for the activation of type I IFNs. We and others have reported that enteroviruses, including CVB, can stimulate the production of cytokines including type I IFN through endosomal Toll-like receptors (TLRs), specifically TLR7 and TLR8 (28, 30). TLR3, which recognizes double-stranded RNA (dsRNA) (2), has been shown to be crucial for the survival of mice infected with CVB4 (23). However, cytoplasmic RIG-like helicases, including retinoic acid-inducible gene I (RIG-I) and melanoma differentiation-associated gene 5 (MDA5), are also important mediators of intracellular viral nucleic acid sensing. Each consists of a C-terminal DEXD/H-box RNA-helicase domain and an N-terminal caspase recruitment domain and can induce IFN gene transcription in response to dsRNA. RIG-I has the additional capability of responding to the 5′ triphosphorylated RNA (13), while MDA5 can recognize polyriboinosinic:polyribocytidylic acid (10). RIG-I and MDA5 use the signaling adaptor mitochondrial antiviral signaling (MAVS), also called IPS-1, Cardif, and VISA, to coordinate the activation of the transcription factor interferon regulatory factor 3 (IRF3) to induce the production of type I IFN (16, 21, 26, 33). RIG-I, MDA5, and MAVS knockout mice have been used to establish the importance of these molecules in type I IFN responses to viruses. Notably, induction of type I IFN by certain picornaviruses, including encephalomyocarditis virus, Theiler's virus, and Mengo virus, is mediated by MDA5 and not RIG-I (10, 15). MDA5 has also been proven to sense a murine norovirus (19).
The goal of the present study was to determine whether MAVS and MDA5 participate in the induction of type I IFN in response to CVB infection. This goal was achieved using an in vivo model of infection comparing wild-type and knockout mice. We have found that MAVS, the adaptor molecule for both MDA5 and RIG-I, is essential for type I IFN production, but not inflammatory cytokine production, in response to coxsackievirus B3 (CVB3) infection. Mortality studies performed in MAVS and MDA5 knockout mice proved that both play crucial roles in survival after systemic CVB3 infection. These data suggest that MDA5 is a predominant receptor for protective innate immune responses against CVB.
MATERIALS AND METHODS
Viruses.
CVB3 strain Nancy was maintained and quantified by plaque assay on HeLa cells as previously described (3).
Mice.
C57BL/6 IFN-α/βR−/− were provided by J. Sprent (Scripps Research Institute, La Jolla, CA) (17) and were backcrossed for more than 10 generations. B6.129 MAVS−/− mice were provided by Z. Chen (University of Texas Southwestern Medical Center, Dallas) (26) and were backcrossed with C57BL/6 mice for three generations; MAVS−/−, MAVS+/−, and matched littermate wild-type controls were used for mortality studies. F1 129 SvJ MDA5−/− mice were provided by M. Colonna (Washington University, St. Louis, MO) (10). MyD88−/− and TLR3−/− mice, a gift from S. Akira (Osaka University, Osaka, Japan) (1, 12), were each backcrossed with C57BL/6 mice for more than six generations. Age- and sex-matched control mice were obtained from the Jackson Laboratory (Bar Harbor, ME). All mice were maintained in accordance with the guidelines of the Institutional Animal Care and Use Committee of the University of Massachusetts Medical School.
Histopathological analyses.
Tissues were recovered from mice at necropsy, fixed in Bouin's solution, and embedded in paraffin. Sections 5 μm thick were cut. For routine histology, sections were stained with hematoxylin and eosin. The sections were evaluated by R. T. Bronson without knowledge of the experimental design.
ELISA.
Blood was collected from mice and sera were stored frozen (−80°C) until use. To quantify the amounts of IFN protein, sera were assayed in duplicates using an enzyme-linked immunosorbent assay (ELISA) specific for mouse IFN-α or IFN-β (PBL Biomedical Laboratories, Piscataway, NJ). The ELISA was performed in accordance with the manufacturer's protocol and analyzed at an absorbance of 450 nm. The limits of detection of IFN-α and IFN-β were 12.5 pg/ml and 15.6 pg/ml, respectively. Murine MCP-1 was measured by using the OptEIA ELISA set (BD Biosciences, San Jose, CA).
Statistical analyses.
Survival analysis was performed by using the Kaplan Meier method and log-rank test. Differences in the means of data were compared by using the Student unpaired t test. Significant differences were assumed for P values < 0.05. All statistical analyses were performed by using GraphPad Prism software (version 4.0c; GraphPad, San Diego, CA).
RESULTS
MAVS and MDA5 are critical for survival following CVB3 infection.
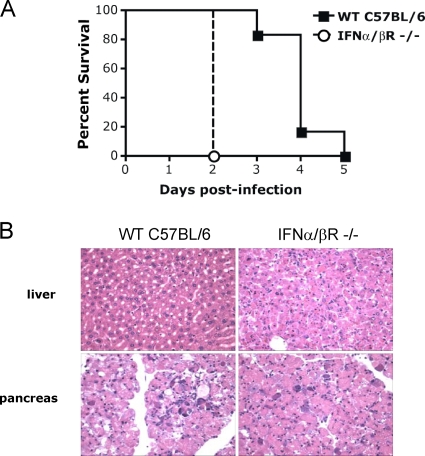
To examine the consequences of absence of type I IFN signaling during CVB3 infection, mortality was assessed in IFN-α/βR−/− mice. We observed 100% mortality at 2 days after infection with 104 PFU of CVB3 given intraperitoneally (i.p.) (Fig. 1A). In contrast, the median survival time was 4 days in control wild-type C57BL/6 mice. This was in agreement with a previous study that examined the importance of the type I IFN signaling pathway during CVB3 infection in vivo (32). Total body necropsy studies revealed severe necrosis of the acinar cells of the pancreas in both wild-type and IFN-α/βR−/− mice at 48 h after infection. Pancreatic islet cells did not appear to be affected, a finding consistent with published reports (20). However, severe hepatic cell necrosis without inflammatory cell infiltration was consistently seen in IFN-α/βR−/− mice but not in wild-type mice (Fig. 1B). Damage to both the pancreas and the liver appeared to be a primary cause of early CVB-induced mortality in these mice, since necropsies did not reveal differences between wild-type and knockout mice in any other organs. Heart and brains did not demonstrate any histopathologic abnormalities.
FIG. 1.
IFN-α/βR-deficient mice show increased susceptibility to lethal infection with CVB3. (A) IFN-α/βR−/− mice (n = 6) and age- and sex-matched wild-type C57BL/6 (n = 6) were injected with 104 PFU of CVB3 and monitored for survival. The median survival was 2 days for IFN-α/βR−/− mice (solid line) versus 4 days for wild-type C57BL/6 mice (dashed line, P = 0.0009). (B) Histopathology of livers and pancreata from a wild-type C57BL/6 mouse and an IFN-α/βR−/− mouse 48 h after infection with CVB3. Both pancreata demonstrate severe necrosis of the acinar cells, but only the IFN-α/βR−/− mouse liver shows severe necrosis without associated inflammatory cellular infiltration. Magnification, ×400.
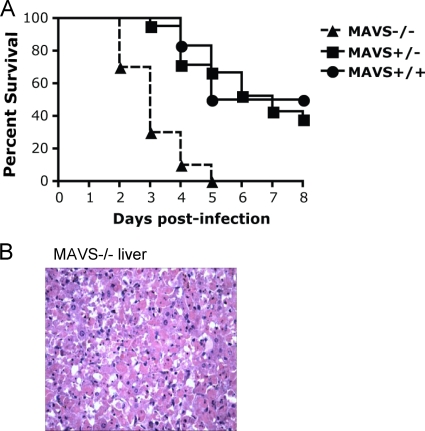
To determine whether the absence of MAVS signaling influences mortality associated with CVB3 infection, we similarly infected MAVS−/− mice. MAVS+/− and MAVS+/+ littermates were used as controls. MAVS−/− mice had a median survival time of 3 days, while MAVS+/− and MAVS+/+ littermates had median survival times of 7 and 6.5 days (Fig. 2A). All moribund MAVS−/− mice had mottled livers and enlarged pancreata on gross examination, and histopathology revealed early severe hepatic necrosis without associated inflammatory cells (Fig. 2B), as well as severe necrosis of the acinar pancreatic tissue.
FIG. 2.
Absence of MAVS is associated with high mortality in CVB3-infected mice. (A) MAVS−/− mice (n = 10) and littermate controls, including MAVS+/+ mice (n = 6) and MAVS+/− mice (n = 21), were injected i.p. with 104 PFU of CVB3 and then monitored for survival. Median survival times were 3 days for MAVS−/− mice versus 7 days for MAVS+/+ mice (P < 0.001) and 6.5 days for MAVS+/− mice (P = 0.021). The graph shows data combined from two independent experiments. (B) Histopathology of a liver from a MAVS−/− mouse 2 days postinfection reveals severe necrosis without inflammatory cells. Magnification, ×400.
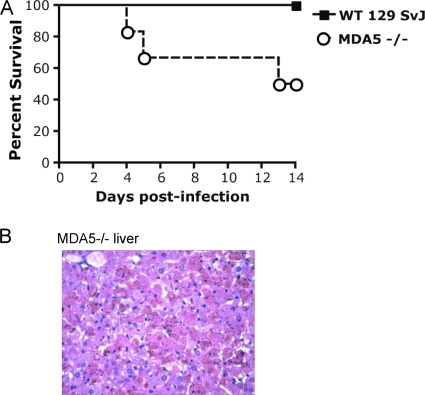
We also found that MDA5 expression plays an important role in survival after challenge with CVB3. We compared MDA5−/− mice to wild-type 129 SvJ mice, which were the appropriate controls for these knockout mice. 129 SvJ mice had a relatively diminished inflammatory response after CVB3 infection, with much lower cytokine levels and virus titers compared to C57BL/6 and B6.129 mice infected with the same dose of virus (see Fig. 4 and 5). No deaths occurred in CVB3-infected wild-type 129 SvJ mice over 14 days of infection. However, a mortality of 50% was observed in MDA5−/− mice on a pure 129 SvJ background (Fig. 3A). Total body necropsy was performed on a moribund MDA5−/− mouse and was notable for severe pancreatic and hepatic necrosis (Fig. 3B). Mice that did not die during the first 14 days after infection recovered with weight gain and full activity. In all three mortality studies, the severity of liver damage correlated with early mortality.
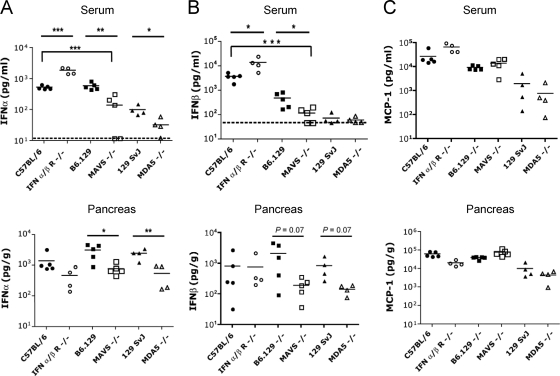
FIG. 4.
Pancreatic IFN-α levels are significantly reduced in MAVS−/− and MDA5−/−mice infected with CVB3. Age- and sex-matched wild-type (C57BL/6 or B6.129 or 129 SvJ), IFN-α/βR−/−, MAVS−/−, or MDA5−/− mice were inoculated with 104 PFU of CVB3 i.p. Organs were harvested 48 h after infection, and the cytokine levels of serum and pancreata (normalized to organ weight) were determined by ELISA. Statistical significance was calculated by using the Student t test (*, P < 0.05; **, P < 0.01; ***, P < 0.001). The data are shown from four to five animals per group. Dashed line indicates limit of detection of the assay.
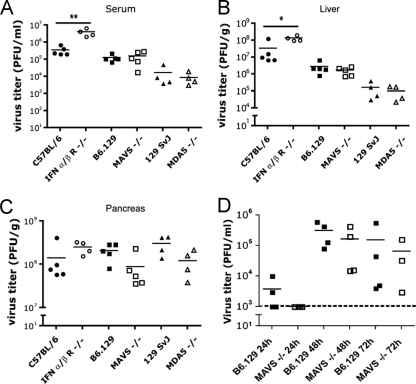
FIG. 5.
Virus titers were significantly elevated in IFN-α/βR−/− but not MAVS−/− or MDA5−/− mouse sera and livers after infection with CVB3. Wild-type (C57BL/6, B6.129, or 129 SvJ), IFN-α/βR−/−, MAVS−/−, or MDA5−/− mice were inoculated with 104 PFU of CVB3 i.p. Organs were harvested 48 h after infection, and the virus titers of serum (A), pancreas (B), and liver (C) were determined by plaque assay on HeLa cells. Statistical significance was calculated by using the Student t test (*, P < 0.05; **, P < 0.01; ***, P < 0.001). The data are shown from four to five animals per group. (D) In a separate experiment, wild-type B6.129 or MAVS−/− mice were similarly inoculated with CVB3 and tail bled daily. Serum virus titers were determined by plaque assay on HeLa cells. The data are shown from four animals per group. The dashed line indicates limit of detection of the assay.
FIG. 3.
MDA5-deficient mice have increased susceptibility to lethal infection with CVB3. (A) MDA5−/− mice (n = 6) and age- and sex-matched wild-type controls on a pure 129 SvJ background (n = 7) were injected i.p. with 104 PFU of CVB3 and then monitored for survival. A total of 50% of MDA5−/− mice died, whereas all of the wild-type mice survived more than 14 days (P = 0.03). (B) Histopathology of a liver from a MDA5−/− mouse at 4 days postinfection reveals severe hepatic necrosis. Necrotic hepatocytes stain bright red. Brown granules overlying necrotic cells represent acid hematin. Magnification, ×400.
Production of type I IFN, but not inflammatory cytokines, depends on MAVS during infection with CVB3.
In screening experiments, we determined that IFN-α and IFN-β reached peak levels in both serum and pancreata of wild-type C57BL/6 mice at 48 h and waned by 4 days after i.p. infection with 104 PFU of CVB3. We subsequently performed a comparative assessment of cytokines from IFN-α/βR-, MAVS-, and MDA5-deficient mice, along with their appropriate wild-type controls, at 48 h postinfection. We limited our cytokine analysis to serum and pancreas and liver, since these particular organs showed significant pathology in our infection model. We observed several profound differences between MAVS−/− and wild-type B6.129 mice. Both serum and pancreatic IFN-α levels were significantly diminished in MAVS−/− mice compared to B6.129 wild-type controls; serum IFN-α levels were also significantly diminished in comparison to C57BL/6 wild-type controls (Fig. 4A). Serum and pancreatic IFN-β levels were also diminished in MAVS−/− mice compared to either B6.129 or C57BL/6 control mice, although differences in the pancreata did not meet statistical significance (Fig. 4B). Similarly, MDA5−/− mice had significantly lower levels of IFN-α than wild-type 129 SvJ mice in both serum and pancreas (Fig. 4A). 129 SvJ and MDA5−/− mice had levels of IFN-β in the serum that were at the lower limit of detection for the assay (Fig. 4B). Mean IFN-β levels in the pancreata of MDA5−/− mice were lower than that of wild-type control mice, but the difference was not statistically significant (Fig. 4B). Although pancreatic type I IFN levels were considerably elevated in wild-type CVB3-infected mice, liver type I IFN levels were low at 48 h postinfection and did not differ between infected mice and uninfected wild-type C57BL/6 mice (data not shown).
As for type I IFN, MCP-1 serum levels peak at 48 h after infection of wild-type C57BL/6 mice with CVB3. However, MCP-1 levels in serum and pancreata did not differ between wild-type mice and MAVS−/− or MDA5−/− mice, highlighting the distinct role of MAVS and MDA5 in type I IFN rather than NF-κB-mediated pathways (Fig. 4C). To identify additional cytokines elevated at 48 h postinfection, we performed a comprehensive survey of cytokines in sera from wild-type C57BL/6 (n = 8), wild-type B6.129 (n = 8), and MAVS−/− (n = 6) mice using a Bio-Plex mouse 23-plex panel on the Luminex-100 station (Bio-Rad, Hercules, CA). The following cytokines were significantly increased in mice infected with CVB3 compared to those treated with saline control at 48 h: MCP-1, IL-6, IL-10, IL-12 p40, KC, and RANTES. However, none of these cytokine levels significantly differed between wild-type mice and MAVS−/− mice (data not shown). Serum type I IFN levels in infected wild-type mice were again significantly elevated, whereas type I IFN levels in MAVS−/− mice were comparable to those of saline-treated mice.
Viral replication did not significantly differ between wild-type and MAVS−/− or MDA5−/− mice 48 h postinfection.
We assessed for differences in viral replication in mice by measuring virus titers in the serum and organ samples used for Fig. 4. Consistent with published reports (32), infected IFN-α/βR−/− mice had significantly higher virus titers in serum and liver than infected wild-type mice (Fig. 5A and B). However, surprisingly, the virus titers in the serum and liver did not differ for either MAVS−/− mice or MDA5−/− mice compared to the appropriate wild-type control mice at 48 h. Pancreatic virus titers were relatively consistent for all mice (Fig. 5C). Histopathology from these mice at 48 h revealed that wild-type C57BL/6 mice had only mild hepatic necrosis, whereas IFN-α/βR−/− mice uniformly had severe liver necrosis. Wild-type B6.129 mice had mild hepatic necrosis, whereas MAVS−/− mice had mild to moderate hepatic necrosis 48 h postinfection. At 48 h, both wild-type 129 SvJ and MDA5−/− mice had relatively normal livers and normal or only mildly necrotic pancreata. This further supported our observation that the 129 SvJ mice are relatively resistant to coxsackievirus disease.
We also serially examined virus titers in serum from wild-type B6.129 mice and MAVS−/− mice after infection with CVB3 (Fig. 5D). Our goal was to determine whether any differences in virus titer occurred beyond 48 h since some MAVS−/− mice survived beyond this time point. At 24 h, serum virus titers were nearly all at the lower limit of detection of the assay. At 48 h, serum titers were elevated but no differences were observed between wild-type and MAVS−/− samples, a finding consistent with previous data. At 72 h, surviving MAVS−/− mice exhibited signs of illness, but again their titers were comparable to those of wild-type control mice. By day four, 75% of the MAVS−/− mice were dead, whereas wild-type mice only began to succumb beginning at day 5 postinfection. This demonstrated that virus titers were not significantly elevated in MAVS−/− mice compared to wild-type control mice at any day over the time course of infection.
DISCUSSION
We have used a murine model of infection to examine the role of specific innate immune sensors of CVB3, namely, the RIG-like helicase MDA5 and its adaptor MAVS. Our model identifies both as key molecules for coxsackievirus innate immune recognition and type I IFN induction during primary systemic infection, as summarized in Fig. 6. The absence of the MDA5-MAVS pathway leads to increased mortality in mice after CVB3 infection. Our data also demonstrate that the genetic background of the mouse impacts the overall response to systemic infection with CVB3. C57BL/6 and B6.129 mice show high virus titers in the serum and liver, whereas 129 SvJ mice have relatively low virus titers and systemic cytokine levels. Unlike the C57BL/6 and B6.129 mice, 129 SvJ mice were able to survive infection with the virus at the given dose. Delineating the pathways involved in the innate sensing of CVB3 by using knockout mice generated on different background strains is challenging and data must be carefully interpreted. However, regardless of the background strain, we found that if the type I IFN response pathway was disrupted, the mice succumbed, and the unique finding on pathological examination was severe necrosis of the liver. Although necrosis of the acinar cells of the pancreas is a consistent pathological finding in CVB3-infected mice that has been confirmed by others (20), hepatic necrosis is early and severe in mice deficient in the type I IFN response. The dual insult to both liver and pancreas certainly could contribute to early mortality.
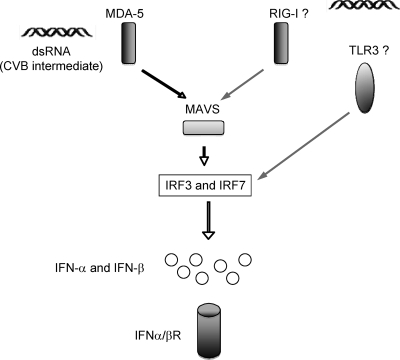
FIG. 6.
Viral RNA recognition pathways for CVB and their impact on type I IFN induction, viral replication, and mortality in mice in vivo. The dsRNA replicative intermediate of CVB interacts with the helicase-binding domain of MDA5 in the cell cytoplasm. Activation of the adaptor MAVS triggers downstream IRFs 3 and 7. Production of IFN-α and IFN-β leads to the engagement of the IFN-α/βR. Absence of the IFN-α/βR leads to decreased survival and increased virus titers after CVB challenge. In contrast, absence of MAVS or MDA5 leads to decreased serum levels of type I IFN and decreased survival but does not lead to increased virus titers in serum after challenge with CVB.
Deficiency of the IFN-α/βR led to a 10-fold increase in virus titer in the liver and serum compared to control wild-type mice. Severe organ damage was consistently observed in the livers of MAVS- and MDA5-deficient mice, even though virus titers were not significantly elevated in the MAVS- and MDA5-deficient mice compared to wild-type mice. This was true for the MAVS−/− mice even during peak morbidity (day 3 postinfection). This suggests that liver necrosis in the absence of appropriate systemic type I IFN signaling may occur independently of viral replication. Interestingly, while pancreatic type I IFN levels are high 48 h postinfection in the CVB3 mouse infection model, liver type I IFN levels are low (i.e., comparable to uninfected mice) despite the presence of high virus titers in the liver. There was a notable paucity of inflammatory cell infiltrate in the CVB-infected livers. This contrasts the murine cytomegalovirus model of viral infection in which high amounts of type I IFN and inflammatory cells, including NK cells, are detected in murine livers within 2 days of infection (25). Future studies are warranted to determine which organs and cells produce type I IFN.
We speculate that type I IFN deficiency might have an effect on mortality in the setting of viral infection independent of its effect on viral replication. Type I IFN could have a protective role against apoptosis or could have protective immunomodulatory effects during systemic CVB3 infection in mice. In a recent study, Richer et al. compared TLR3−/− mice and wild-type nonobese diabetic mice infected with CVB4 but could not establish an association between type I IFN deficiency and increased viral replication (23). Similarly, Hardarson et al. found that encephalomyocarditis virus infection led to increased mortality and increased viral replication in TLR3−/− mice, even though expression of type I IFN was unimpaired (11).
We have previously found that TLR7 and FcR can mediate type I IFN responses to CVB3 in human and murine plasmacytoid dendritic cells (30). However, in preliminary mortality studies, the absence of MyD88, the adaptor molecule for TLR7, does not negatively impact survival during primary CVB3 infection in our studies (data not shown). Also, TLR3 has been shown to play a critical role for survival after CVB4 infection (23), and yet in preliminary studies we have found no differences in mortality when comparing TLR3−/− and wild-type mice after CVB3 infection (data not shown). Our studies differed in that we used a different serotype of CVB and the CVB4/TLR3 studies were performed in nonobese diabetic mice, an autoimmune strain characterized by T-cell dysregulation. We cannot exclude a cooperative role for TLR3 on type I IFN production after CVB3 infection, since a pronounced effect on mortality could be potentially observed in MDA5/TLR3 double-knockout mice. Interestingly, absence of TLR3 has been shown to be beneficial in certain viral models of infection, including West Nile virus and influenza virus, presumably due to decreased proinflammatory signaling (11, 18, 31). Overall, the role of TLR3 in different viral infections in vivo remains controversial and needs further clarification (29).
Of note, others have reported that MDA5 protein is degraded after in vitro infection with the enterovirus poliovirus, possibly as a mechanism to antagonize type I IFN production (4). Our data suggest that, in vivo, MDA5 is required for survival after infection with CVB3 and that if enterovirus-induced degradation of MDA5 does occur in murine infection with coxsackievirus, it is not likely to be relevant to pathogenesis in this model.
CVB is an established cause of cardiac disease, but evidence exists that CVB may play a role in the development of type 1 diabetes. Type 1 diabetes results from the destruction of insulin-producing pancreatic islet β cells in genetically predisposed subjects. Epidemiological data and clinical observations have suggested a link between enteroviral infections and type 1 diabetes (7, 8, 34). Primary human islet cells can be infected by different strains of CVB (24) and possess mRNA for RIG-I, MDA5, and all TLRs (9, 14). An intriguing link was established between viral infection and type 1 diabetes when MDA5 was identified as a strong candidate gene within a type 1 diabetes susceptibility locus (27). Several rare variants of MDA5 were recently found to be protective against type 1 diabetes in genome-wide association studies (22). In separate studies, we infected wild-type C57BL/6 mice with CVB3 and assessed for signs of diabetes. Over the course of 40 days, mice did not develop glycosuria and, as determined by histopathologic analysis, the pancreatic islets did not appear to be affected in any of the mice infected with CVB3 (data not shown). However, our discovery of the mechanistic association between type I IFN production and the MAVS-MDA5 pathway are striking since innate immune recognition of coxsackievirus RNA may important role in the pathogenesis of diabetes in humans. Although our mouse model of infection may not be an appropriate model in which to study the relationship between coxsackievirus, MDA5 expression, cytokine production, and type 1 diabetes, the potential interactions between MDA5 and CVB within human islets need to be further defined. We anticipate that determining how MDA5 drives the innate immune response to CVB may lead to novel approaches to disease prevention.
Acknowledgments
This study was supported by NIH grant K08 AI 053542 (to J.P.W.), NIH grant R01 AI064349 (R.W.F.), and JDRFI grant 24-2008-950 (to J.P.W. and R.W.F.).
We thank Stuart Levitz for constructive comments.
Footnotes
Published ahead of print on 21 October 2009.
REFERENCES
- 1.Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9:143-150. [DOI] [PubMed] [Google Scholar]
- 2.Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413:732-738. [DOI] [PubMed] [Google Scholar]
- 3.Asher, D. R., A. M. Cerny, and R. W. Finberg. 2005. The erythrocyte viral trap: transgenic expression of viral receptor on erythrocytes attenuates coxsackievirus B infection. Proc. Natl. Acad. Sci. USA 102:12897-12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barral, P. M., J. M. Morrison, J. Drahos, P. Gupta, D. Sarkar, P. B. Fisher, and V. R. Racaniello. 2007. MDA-5 is cleaved in poliovirus-infected cells. J. Virol. 81:3677-3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320-1323. [DOI] [PubMed] [Google Scholar]
- 6.Deonarain, R., D. Cerullo, K. Fuse, P. P. Liu, and E. N. Fish. 2004. Protective role for interferon-beta in coxsackievirus B3 infection. Circulation 110:3540-3543. [DOI] [PubMed] [Google Scholar]
- 7.Dotta, F., S. Censini, A. G. van Halteren, L. Marselli, M. Masini, S. Dionisi, F. Mosca, U. Boggi, A. O. Muda, S. D. Prato, J. F. Elliott, A. Covacci, R. Rappuoli, B. O. Roep, and P. Marchetti. 2007. Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc. Natl. Acad. Sci. USA 104:5115-5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elfaitouri, A., A. K. Berg, G. Frisk, H. Yin, T. Tuvemo, and J. Blomberg. 2007. Recent enterovirus infection in type 1 diabetes: evidence with a novel IgM method. J. Med. Virol. 79:1861-1867. [DOI] [PubMed] [Google Scholar]
- 9.Giarratana, N., G. Penna, S. Amuchastegui, R. Mariani, K. C. Daniel, and L. Adorini. 2004. A vitamin D analog downregulates proinflammatory chemokine production by pancreatic islets inhibiting T-cell recruitment and type 1 diabetes development. J. Immunol. 173:2280-2287. [DOI] [PubMed] [Google Scholar]
- 10.Gitlin, L., W. Barchet, S. Gilfillan, M. Cella, B. Beutler, R. A. Flavell, M. S. Diamond, and M. Colonna. 2006. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc. Natl. Acad. Sci. USA 103:8459-8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardarson, H. S., J. S. Baker, Z. Yang, E. Purevjav, C. H. Huang, L. Alexopoulou, N. Li, R. A. Flavell, N. E. Bowles, and J. G. Vallejo. 2007. Toll-like receptor 3 is an essential component of the innate stress response in virus-induced cardiac injury. Am. J. Physiol. Heart Circ. Physiol. 292:H251-H258. [DOI] [PubMed] [Google Scholar]
- 12.Honda, K., S. Sakaguchi, C. Nakajima, A. Watanabe, H. Yanai, M. Matsumoto, T. Ohteki, T. Kaisho, A. Takaoka, S. Akira, T. Seya, and T. Taniguchi. 2003. Selective contribution of IFN-alpha/beta signaling to the maturation of dendritic cells induced by double-stranded RNA or viral infection. Proc. Natl. Acad. Sci. USA 100:10872-10877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hornung, V., J. Ellegast, S. Kim, K. Brzozka, A. Jung, H. Kato, H. Poeck, S. Akira, K. K. Conzelmann, M. Schlee, S. Endres, and G. Hartmann. 2006. 5′-Triphosphate RNA is the ligand for RIG-I. Science 314:994-997. [DOI] [PubMed] [Google Scholar]
- 14.Hultcrantz, M., M. H. Huhn, M. Wolf, A. Olsson, S. Jacobson, B. R. Williams, O. Korsgren, and M. Flodstrom-Tullberg. 2007. Interferons induce an antiviral state in human pancreatic islet cells. Virology 367:92-101. [DOI] [PubMed] [Google Scholar]
- 15.Kato, H., O. Takeuchi, S. Sato, M. Yoneyama, M. Yamamoto, K. Matsui, S. Uematsu, A. Jung, T. Kawai, K. J. Ishii, O. Yamaguchi, K. Otsu, T. Tsujimura, C. S. Koh, C. Reis e Sousa, Y. Matsuura, T. Fujita, and S. Akira. 2006. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441:101-105. [DOI] [PubMed] [Google Scholar]
- 16.Kawai, T., K. Takahashi, S. Sato, C. Coban, H. Kumar, H. Kato, K. J. Ishii, O. Takeuchi, and S. Akira. 2005. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat. Immunol. 6:981-988. [DOI] [PubMed] [Google Scholar]
- 17.Kolumam, G. A., S. Thomas, L. J. Thompson, J. Sprent, and K. Murali-Krishna. 2006. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J. Exp. Med. 202:637-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Goffic, R., V. Balloy, M. Lagranderie, L. Alexopoulou, N. Escriou, R. Flavell, M. Chignard, and M. Si-Tahar. 2006. Detrimental contribution of the Toll-like receptor (TLR)3 to influenza A virus-induced acute pneumonia. PLoS Pathog. 2:e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCartney, S. A., L. B. Thackray, L. Gitlin, S. Gilfillan, H. W. Virgin, and M. Colonna. 2008. MDA-5 recognition of a murine norovirus. PLoS Pathog. 4:e1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mena, I., C. Fischer, J. R. Gebhard, C. M. Perry, S. Harkins, and J. L. Whitton. 2000. Coxsackievirus infection of the pancreas: evaluation of receptor expression, pathogenesis, and immunopathology. Virology 271:276-288. [DOI] [PubMed] [Google Scholar]
- 21.Meylan, E., J. Curran, K. Hofmann, D. Moradpour, M. Binder, R. Bartenschlager, and J. Tschopp. 2005. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 437:1167-1172. [DOI] [PubMed] [Google Scholar]
- 22.Nejentsev, S., N. Walker, D. Riches, M. Egholm, and J. A. Todd. 2009. Rare variants of IFIH1, a gene implicated in antiviral responses, protect against type 1 diabetes. Science 324:387-389. [DOI] [PMC free article] [PubMed]
- 23.Richer, M. J., D. J. Lavallee, I. Shanina, and M. S. Horwitz. 2009. Toll-like receptor 3 signaling on macrophages is required for survival following coxsackievirus B4 infection. PLoS ONE 4:e4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roivainen, M., S. Rasilainen, P. Ylipaasto, R. Nissinen, J. Ustinov, L. Bouwens, D. L. Eizirik, T. Hovi, and T. Otonkoski. 2000. Mechanisms of coxsackievirus-induced damage to human pancreatic beta-cells. J. Clin. Endocrinol. Metab. 85:432-440. [DOI] [PubMed] [Google Scholar]
- 25.Salazar-Mather, T. P., C. A. Lewis, and C. A. Biron. 2002. Type I interferons regulate inflammatory cell trafficking and macrophage inflammatory protein 1α delivery to the liver. J. Clin. Investig. 110:321-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seth, R. B., L. Sun, C. K. Ea, and Z. J. Chen. 2005. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-κB and IRF 3. Cell 122:669-682. [DOI] [PubMed] [Google Scholar]
- 27.Smyth, D. J., J. D. Cooper, R. Bailey, S. Field, O. Burren, L. J. Smink, C. Guja, C. Ionescu-Tirgoviste, B. Widmer, D. B. Dunger, D. A. Savage, N. M. Walker, D. G. Clayton, and J. A. Todd. 2006. A genome-wide association study of nonsynonymous SNPs identifies a type 1 diabetes locus in the interferon-induced helicase (IFIH1) region. Nat. Genet. 38:617-619. [DOI] [PubMed] [Google Scholar]
- 28.Triantafilou, K., G. Orthopoulos, E. Vakakis, M. A. Ahmed, D. T. Golenbock, P. M. Lepper, and M. Triantafilou. 2005. Human cardiac inflammatory responses triggered by coxsackie B viruses are mainly Toll-like receptor (TLR) 8-dependent. Cell Microbiol. 7:1117-1126. [DOI] [PubMed] [Google Scholar]
- 29.Vercammen, E., J. Staal, and R. Beyaert. 2008. Sensing of viral infection and activation of innate immunity by Toll-like receptor 3. Clin. Microbiol. Rev. 21:13-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang, J. P., D. R. Asher, M. Chan, E. A. Kurt-Jones, and R. W. Finberg. 2007. Cutting edge: antibody-mediated TLR7-dependent recognition of viral RNA. J. Immunol. 178:3363-3367. [DOI] [PubMed] [Google Scholar]
- 31.Wang, T., T. Town, L. Alexopoulou, J. F. Anderson, E. Fikrig, and R. A. Flavell. 2004. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat. Med. 10:1366-1373. [DOI] [PubMed] [Google Scholar]
- 32.Wessely, R., K. Klingel, K. U. Knowlton, and R. Kandolf. 2001. Cardioselective infection with coxsackievirus B3 requires intact type I interferon signaling: implications for mortality and early viral replication. Circulation 103:756-761. [DOI] [PubMed] [Google Scholar]
- 33.Xu, L. G., Y. Y. Wang, K. J. Han, L. Y. Li, Z. Zhai, and H. B. Shu. 2005. VISA is an adapter protein required for virus-triggered IFN-beta signaling. Mol. Cell 19:727-740. [DOI] [PubMed] [Google Scholar]
- 34.Yin, H., A. K. Berg, T. Tuvemo, and G. Frisk. 2002. Enterovirus RNA is found in peripheral blood mononuclear cells in a majority of type 1 diabetic children at onset. Diabetes 51:1964-1971. [DOI] [PubMed] [Google Scholar]