Abstract
It was shown previously that the highly conserved vaccinia virus A35 gene is an important virulence factor in respiratory infection of mice. We show here that A35 is also required for full virulence by the intraperitoneal route of infection. A virus mutant in which the A35 gene has been removed replicated normally and elicited improved antibody, gamma interferon-secreting cell, and cytotoxic T-lymphocyte responses compared to wild-type virus, suggesting that A35 increases poxvirus virulence by immunomodulation. The enhanced immune response correlated with an improved control of viral titers in target organs after the development of the specific immune response. Finally, the A35 deletion mutant virus also provided protection from lethal challenge (1,000 50% lethal doses) equal to that of the wild-type virus. Together, these data suggest that A35 deletion viruses will make safer and more efficacious vaccines for poxviruses. In addition, the A35 deletion viruses will serve as improved platform vectors for other infectious diseases and cancer and will be superior vaccine choices for postexposure poxvirus vaccination, as they also provide improved kinetics of the immune response.
Poxviruses are large, complex viruses with a broad host range and worldwide distribution (30). Members of the family Poxviridae include variola virus, the causative agent of smallpox, which induced a fatality rate of approximately 30% and killed hundreds of millions of people before its eradication in 1980 (28). Currently, the most dangerous extant human-infecting poxvirus is monkeypox virus, which is commonly found in African rodents. Monkeypox virus causes a smallpox-like illness with a 10% fatality rate. A recent study showed that 1.7% of people in the Likouala region in Africa had monkeypox-specific immunoglobulin M (IgM), indicating a significant ongoing infection rate (25). An outbreak of a low-virulence strain of monkeypox virus occurred in the United States in 2003, causing more than 80 human infections and several hospitalizations (6). This outbreak raises concern that monkeypox virus could establish itself in wild-rodent populations in North America (34), thus creating a local zoonotic reservoir for this emerging pathogen. Of further concern are the facts that monkeypox is spreading more efficiently in humans (18, 24, 31) and that the current poxvirus vaccine is not universally protective against monkeypox infection (27). Both variola and monkeypox viruses are considered bioterrorism and biowarfare concerns and are category A select-agent pathogens. There are also other poxvirus infections that sporadically cause human outbreaks, including Cantagalo virus in South America (10, 41) and buffalopox virus in India (23), and the incidence of tanapox virus appears to be increasing (12, 43). Molluscum contagiosum poxvirus accounts for approximately 300,000 doctor visits each year in the United States alone (29). Thus, the study of virulence mechanisms in this group of viruses is important.
The eradication of smallpox was accomplished through the use of the related vaccinia virus (VV) as a live-virus vaccine. Despite its phenomenal success, the public vaccination program was discontinued because of the high incidence of complications due to the virulence of wild-type VV. It is estimated that approximately 25% of the population should not receive this vaccine because of immunodeficiency, eczema, pregnancy, or heart disease (14, 21, 45). Safer vaccines are necessary to protect against emerging or released poxviruses. In addition, poxviruses are being used as platform vaccines for other diseases such as human immunodeficiency virus, malaria, and cancer because they induce a robust immune response and accommodate the insertion of large pieces of foreign DNA. It is therefore of great importance to identify poxvirus virulence genes in order to develop safer and more effective poxvirus vaccines. Replication-defective strains, such as Modified Vaccinia Ankara, have been used in an effort to reduce the risks associated with vaccination (8, 47), but the production of these viruses can be challenging, they require higher doses of vaccine, and their protective efficacy against poxvirus infections in humans is unknown. As poxviruses inhibit the activation of antigen-presenting cells and antigen presentation (26, 38), another way to construct a safer vaccine is to develop replication-competent vaccine strains that exclude immunosuppressive genes (3) while retaining protective antigenic epitopes (17, 32). We show herein that the A35R gene is an excellent candidate for removal from vaccine strains.
The VV A35 gene is highly conserved in mammalian-tropic poxviruses, and a sequence identity search has revealed that the protein has little similarity to any other poxvirus protein or any nonpoxvirus protein, suggesting that this gene has an important and novel function (39). We have shown that the A35 gene is not required for viral replication in vitro but is required for full virulence in the mouse model (39).
We therefore tested the effects of A35R on immune responses during infection in the mouse model and tested its protective efficacy against virulent challenge.
MATERIALS AND METHODS
Cells and virus.
VV strain Western Reserve (WR) and A35Δ mutant virus stocks were propagated by using BS-C-1 cells in minimal essential medium containing 10% fetal bovine serum as previously described (39). P815 cells (ATCC) were grown in Dulbecco's modified Eagle's medium.
Mouse infection and sample collection.
Four 5-week-old BALB/c mice were anesthetized using isoflurane and infected intranasally (i.n.), as previously described (39), with purified virus in 18 μl (100 μl for intraperitoneal [i.p.] infection) or were mock infected with phosphate-buffered saline (PBS). Titers were confirmed for each experiment by using the dilution used to infect mice that day. Mice were weighed and monitored daily for signs of illness and were euthanized if 20% weight loss occurred. For experiments assessing immune responses, five mice from each group were sacrificed, and the spleens were collected in ice-cold RPMI medium. Splenocytes were obtained as previously described and incubated in RPMI medium (7). Blood was collected using cardiac puncture, followed by centrifugation to separate plasma from red blood cells. All experimental protocols were approved by the Animal Care and Use Committee of East Carolina University.
ELISA.
To determine antibody responses, 96-well enzyme-linked immunosorbent assay (ELISA) plates (Immulon H2B; Thermo Electron) were coated overnight with 0.1 μl/well (100 μl) crude WR virus in ELISA coating buffer (10.3 g H2BO4, 7.31 g NaCl, 1 liter double-distilled water [pH 8.5]) at 4°C. Plates were blocked with 1% fetal bovine serum-PBS at room temperature for 30 min. Plates were washed with ELISA wash buffer (1× PBS, 0.02% Tween 20, 0.1% NaN3), and a titration of mouse serum was added. Plates were incubated at room temperature for 2 h and washed. Goat anti-mouse IgG-alkaline phosphatase (Southern Biotech) was added and incubated at room temperature for 1 h. Plates were washed three times and developed (alkaline phosphate substrate kit; Bio-Rad), and the absorbance was read at 405 nm. Anti-B5R ELISAs were similarly performed, by coating plates with purified baculovirus-expressed B5R protein prepared by C-PERL (the kind gift of Stuart Isaacs).
Measurement of neutralizing antibody.
VV-specific neutralizing antibody was measured by incubating 25 μl of sera from PBS-, WR-, or A35Δ-infected mice (n = 5) with WR virus (200 PFU) in 100 μl of medium containing 10% heat-inactivated fetal bovine serum for 1 h on ice. The virus-serum mixture was then added to confluent monolayers of BS-C-1 cells, and plaques were counted 40 h later.
IFN-γ enzyme-linked immunospot (ELISPOT) assay.
Numbers of gamma interferon (IFN-γ)-secreting spleen cells were enumerated similarly to methods described previously (9, 16, 40). Ninety-six-well plates (Immulon H2B; Thermo Electron) were coated overnight with 0.2 μl anti-mouse IFN-γ (1 mg/ml; Pharmingen) at 4°C. Plates were washed with blocking buffer before adding a titration of murine splenocytes in RPMI 1640 medium. The stimulation of splenocytes was achieved either by the addition of WR virus only (multiplicity of infection [MOI] of 2) or with the use of 50,000 WR-infected (MOI of 3) P815 stimulator cells, followed by incubation for 40 h at 37°C. Plates were then washed and incubated with 0.4 μl biotinylated rat anti-mouse IFN-γ (0.5 mg/ml; Pharmingen) for 2 h at 37°C. Plates were washed again and incubated with streptavidin-AP for 1 h at 37°C. Plates were developed with an agarose-BCIP (5-bromo-4-chloro-3-indolylphosphate)-AMP mixture, and spots were counted by using a dissection microscope.
CTL assay.
Cytotoxic T-lymphocyte (CTL) activity was measured as the release of lactate dehydrogenase (LDH) into supernatants from WR virus-infected P815 cells by using a Promega Cytotox kit. P815 target cells were infected (MOI of 5) for 3 h and added to a titration of splenocytes from the vaccinated mice in round-bottom 96-well plates at 37°C. After 6 h, 50 μl of supernatant was collected and assayed for LDH by adding an equal volume of substrate and reading the absorbance at 492 nm. Incubation of splenocytes with uninfected P815 cells was used to measure nonspecific lysis, which was subtracted from each experimental group to yield specific lysis.
Cytokine measurement.
Sera were analyzed by using the LincoPlex Mouse Cytokine/Chemokine Luminex bead immunoassay kit according to the manufacturer's instructions (Linco Research). Sera were incubated with a panel of anticytokine antibodies immobilized on Luminex beads (murine 23-plex; Bio-Rad Laboratories), run according to the manufacturer's instructions, and analyzed on the BioPlex protein array reader (Bio-Rad) at the Duke University Human Vaccine Institute Immune Reconstitution Core Facility (Durham, NC).
Virus titrations.
Groups of mice (n = 5) were sacrificed at various days postinfection (p.i.) (dpi), and the organs were placed into 1 ml of ice-cold RPMI medium. The organs were then freeze-thawed three times, homogenized, and sonicated. Viral replication was evaluated by the titration of the organ on BS-C-1 monolayers and staining with 0.1% crystal violet in 20% ethanol 40 h later.
Statistical analysis.
Experiments were repeated at least three times, and representative data are shown. A two-tailed Student's t test was used to compare groups. P values of <0.05 were considered significant.
RESULTS
Virulence studies.
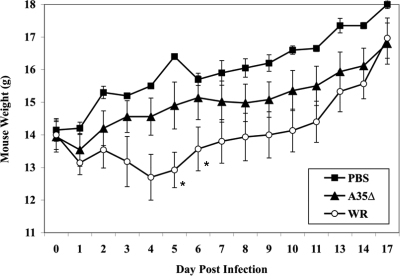
We had shown previously that the VV A35 gene is not required for replication and is a major virulence factor in the mouse i.n. challenge model, increasing virulence almost 100-fold (39). It was unknown whether A35 would also play a role in infection via other challenge routes or if perhaps A35 was required for replication in certain tissues such as nasal or lung tissue. We therefore challenged groups of mice (n = 5) i.p., bypassing the respiratory route, with the wild-type parental VV WR strain, the A35 deletion mutant virus (A35Δ), or PBS as a control. We found that VV (WR and A35Δ) injected i.p. did not cause any weight loss at 105 and 106 PFU/mouse but that at 107 PFU/mouse, the WR virus caused significant weight loss and death for two of five mice, while the A35Δ virus was attenuated, causing only mild weight loss and no mortality (Fig. 1). These data indicate that the A35 gene is required for virulence in mice infected by either the i.n. or i.p. route.
FIG. 1.
i.p. infection of mice. Groups (n = 5) of female BALB/c mice were infected i.p. with 107 PFU/mouse of WR or A35Δ virus or mock-vaccinated with PBS and weighed (grams plus or minus standard errors of the means [SEM]) at various time points p.i. Asterisks indicate that two mice died in the WR-infected group.
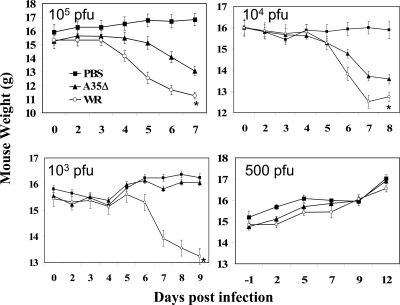
Since A35 is a virulence factor independent of the route of infection, and VV can block major histocompatibility complex (MHC) class II antigen presentation in vitro (26, 38), we wanted to measure the effect of A35 on immune responses in vivo. We anesthetized and i.n. infected groups of mice (n ≥ 5) with the VV WR strain, the A35Δ virus, or PBS as a control. Since we wanted to evaluate the immune responses in the presence or absence of the A35R gene without any confounding effects of overall systemic illness in the animals, we titrated the infectious dose to identify a dose of virus such that the more virulent WR strain did not cause significant weight loss in animals (Fig. 2). We found that 105 to 104 PFU/mouse caused significant weight loss and mortality in >80% of WR-infected mice (approximate 50% lethal dose [LD50] of 5 × 103 PFU [37]) by day 8, while all A35Δ virus-infected mice survived (LD50 of 5 × 105 PFU). At 103 PFU/mouse, WR caused significant weight loss, while the A35Δ virus did not, and 11/12 WR-infected mice and all A35Δ virus-infected mice survived. At 500 PFU/mouse, no weight loss was noted at any time point for either virus (Fig. 2); therefore, we chose 500 PFU/mouse as our infection dose to study immune responses to these viruses.
FIG. 2.
i.n. infection of mice. Groups of mice were infected i.n. with 500 to 105 PFU/mouse of WR or A35Δ virus or mock vaccinated with PBS and weighed (grams plus or minus SEM) for 4 weeks. Weights increased after day 9. Asterisks indicate that all mice (5/5) died in the group infected with 105 PFU WR, 6/7 mice died in the group infected with 104 PFU, and 1/11 mice died in the group infected with 103 PFU WR. No A35Δ virus-infected mice died.
Spleen enlargement.
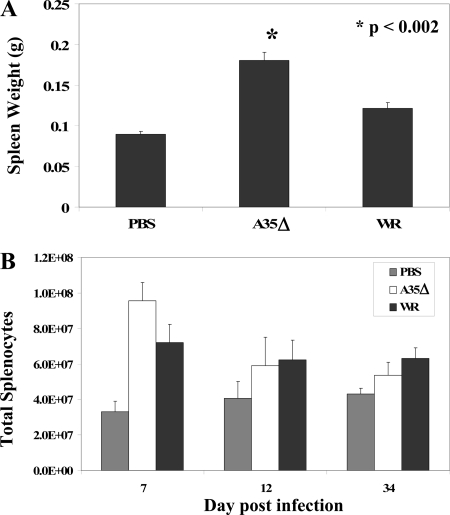
Visual observation during necropsy suggested that spleens of infected/vaccinated mice were larger than spleens of uninfected mice and that spleens of A35Δ virus-infected mice were larger than spleens of WR-infected mice. These observations suggested that A35Δ virus might generate a superior immune response with robust lymphocyte proliferation. B and T lymphocytes proliferate in response to antigen, and a spike of T-cell proliferation in the spleens and lymph nodes of mice at days 5 to 7 after VV infection was reported previously (48). To begin to understand the effects of A35 on the immune response, mice were infected i.n. with 500 PFU of WR or the A35Δ virus. In all experiments, on at least one day (days 6 to 8 p.i.), the spleens from A35Δ virus-infected mice were significantly (P < 0.05) larger than those from WR-infected mice (Fig. 3A). Enlarged spleens correlated with increased numbers of splenocytes isolated from spleens. This difference was reproducible but not significant. A35Δ virus-infected mice had higher numbers of splenocytes than WR-infected mice; however, the difference was lost by 9 dpi (Fig. 3B).
FIG. 3.
Spleen weights and counts. Mice were infected with WR or A35Δ virus i.n. on day 0 with 500 PFU/mouse. Five mice per infection group were sacrificed, their spleens were weighed on day 7 (A), and total splenocytes were counted (B). Data show the averages (±SEM).
Antibody response.
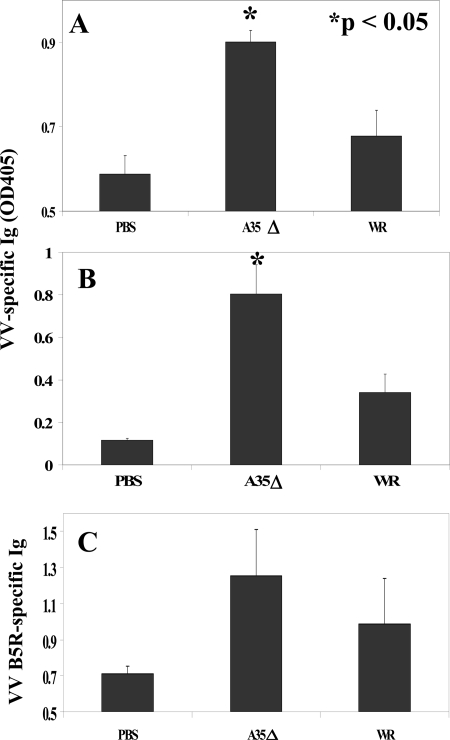
The effects of A35 on VV-specific Ig production in mice were assessed. The generation of anti-VV-specific antibody was previously shown to be crucial in protecting mice from poxvirus infections (2, 5, 33). VV-specific Ig was not detected above background levels on days 5 and 6 p.i. (i.n.). By day 7, there was measurable anti-VV Ig in the serum, and mice vaccinated with the A35Δ virus produced significantly more VV-specific Ig than did mice infected with WR (Fig. 4A), and this difference was maintained to 34 dpi (Fig. 4B), the latest time point tested. These data reveal that A35 inhibits the production of Ig during a specific immune response and that even a highly attenuated virus can induce an equal, or even superior, antibody response compared to that of the wild-type vaccine strain. The B5R antigen is the major target of virus-neutralizing Ig in anti-VV serum (1); therefore, we also measured the Ig-recognizing B5R protein in the sera of VV-infected mice. A35Δ virus-infected mice also produced high titers of B5R-specific Ig, but the difference was not significant (Fig. 4C).
FIG. 4.
VV- and B5R-specific antibody (Ab) response. Mice (n = 5) were infected i.n. with WR or A35Δ virus, and blood was collected on various days p.i. Serum reactivity was measured by ELISA of VV-coated plates for day 7 (A) or day 34 (B) sera or purified B5R-coated plates (C) for day 7 p.i. sera. Data show the average absorbances (±SEM). OD405, optical density at 405 nm.
Since A35Δ virus-infected mice produced higher VV-specific antibody titers, we next compared the levels of neutralizing antibody present in the sera from WR- and A35Δ virus-infected mice on day 34 p.i. Sera from WR-infected mice reduced plaque formation by an average of twofold compared to sera from mock-infected mice (data not shown). Impressively, sera from the A35Δ virus-infected mice were able to reduce plaque formation nearly sixfold compared to sera from mock-infected mice and threefold compared to sera from WR-infected mice. Together, these data indicate that infection with the A35Δ virus not only generated larger amounts of total VV-specific Ig but also led to the production of larger amounts of neutralizing Ig.
IFN-γ-secreting splenocytes.
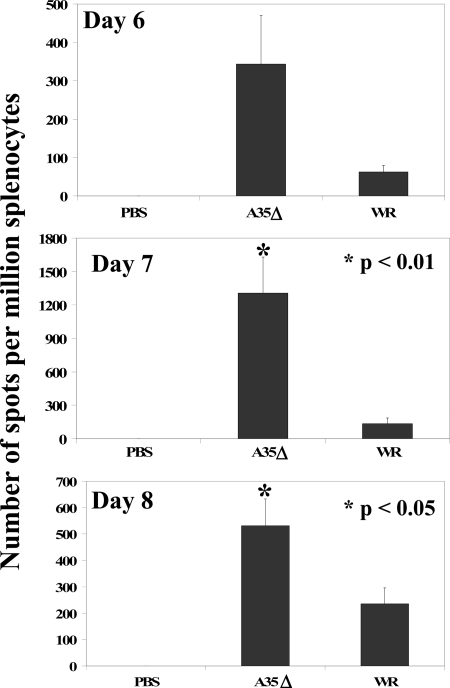
The ELISPOT assay allows the enumeration of cells secreting IFN-γ, a cytokine known to be protective against VV, in response to stimulation by VV antigen presented in the context of MHC (9, 16, 40). We tested how the presence of the A35 gene in the virus infecting mice i.n. affects the induction of IFN-γ-secreting cells in the spleens by using two methods for the antigenic stimulation of IFN-γ: the addition of VV-infected P815 cells (16) or the addition of infectious virus directly to the splenocytes (9). As shown in Fig. 5, splenocytes were stimulated to produce IFN-γ by the addition of infectious virus directly to the cells, and the presence of the A35 gene in the WR virus significantly reduced the number of VV-specific IFN-γ-secreting splenocytes developed by the mouse, in some cases by 10-fold. Similar results were obtained when virus-infected P815 cells were used to stimulate the splenocytes (data not shown). A35-dependent differences were seen at early times p.i. (days 5 to 8), were significant on days 7 to 8, and were not consistently significantly higher at later times as the immune response waned (no significant differences were seen at day 9, 14, or 34). No IFN-γ production was detected in PBS mock-infected mice or in any group without the addition of virus or infected P815 cells as stimulators.
FIG. 5.
VV-specific IFN-γ-producing cells. On days 6, 7, and 8 p.i. (i.n.), the spleens from five mice/group were harvested, and splenocytes were analyzed by ELISPOT for virus-specific IFN-γ production 48 h after stimulation with VV WR virus. Data show averages (±SEM).
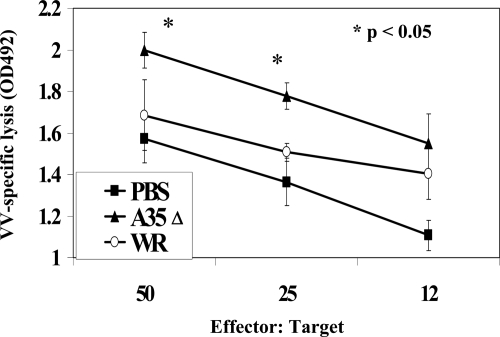
CTL activity.
Since CTL are important for killing virally infected target cells and have been shown to be important for the defense against poxviruses (5, 19, 49), we measured the effect of A35 on CTL generated in spleens of i.n. infected mice. Splenocytes from PBS-, WR-, or A35Δ virus-infected mice were incubated with VV-infected P815 target cells at various ratios, and the specific lysis of the P815 cells was measured through the release of LDH into the supernatants. As shown in Fig. 6, A35Δ virus-infected mice developed significantly stronger VV-specific cytolytic activities than WR-infected mice. Differences were significant only on days 6 and 7 p.i. Together, the immune data suggest that an A35Δ virus induces a significantly stronger B- and T-cell immune response especially early after infection/vaccination than wild-type virus.
FIG. 6.
CTL. Mice were infected i.n. on day 0 with 500 PFU/mouse of WR or A35Δ virus. On day 7, the spleens from five mice/group were harvested, and the splenocytes were incubated with WR-infected (MOI of 5 for 3 h) P815 cells as targets. After 6 h, supernatants were collected and analyzed for the presence of LDH. Data show average lysis (± standard deviations). OD492, optical density at 492 nm.
Cytokine production.
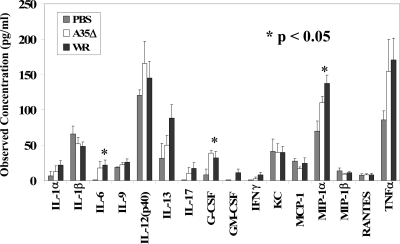
In order to determine whether a cytokine signature could be identified for effective immune responses against poxvirus infection and which cytokines might be mediating immune response differences in WR- and A35Δ virus-infected mice early after infection, we infected mice and harvested sera at days 2 and 3 p.i. and measured 23 cytokines. As shown in Fig. 7, VV infection altered the serum cytokine composition on day 2 p.i. compared to that of PBS mock-infected mice, increasing levels of interleukin-6 (IL-6), granulocyte colony-stimulating factor, and macrophage inflammatory protein 1α significantly. However, there was no significant difference between WR- and A35Δ virus-infected mouse sera that might explain the later differences in immune responses to these virus infections. Serum levels of IL-2, IL-3, IL-4, IL-5, IL-10, IL-12 p70, and eotaxin were below the standard-curve values and are not shown. Day 3 data (not shown) were similar to day 2 data, with smaller differences between groups. Thus, it was not possible to predict the effectiveness of an immune response based on early p.i. serum cytokine levels. We also assessed cytokine levels on days 6 and 7 p.i. On day 6 p.i., differences were small; however, on day 7 p.i., serum levels of monocyte chemoattractant protein 1, IFN-γ, and granulocyte colony-stimulating factor were elevated at least twofold in WR- compared to A35Δ virus-infected mice; however, as discussed below, viral titers were much higher in WR-infected mice at this time point, suggesting that the levels of these cytokines might be elevated in response to viral infection rather than the immune response per se.
FIG. 7.
Cytokines in blood. Mice were infected i.n. on day 0 with 500 PFU/mouse of WR or A35Δ virus or with PBS, and sera were collected on day 2 p.i. and analyzed for cytokine quantities (±SEM) by using a Luminex BioPlex assay. Tumor necrosis factor alpha (TNFα) values were divided by 2. G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; KC, keratinocyte chemoattractant, or CXCL1 (chemokine [C-X-C motif] ligand 1); MCP-1, monocyte chemoattractant protein 1; MIP-1α, macrophage inflammatory protein 1α.
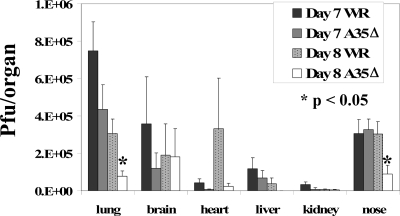
Viral titers in organs.
We showed previously that the A35 gene was not required for replication in numerous tissue culture cell lines (39), but it was possible that A35 increased virulence by allowing VV to replicate in certain animal tissues. We therefore wanted to compare virus replication levels in tissues at both early and late times after infection i.n. with WR and A35Δ viruses. Furthermore, since A35 inhibits the generation of an anti-VV immune response in terms of VV-specific antibody production, IFN-γ secretion, and CTL activity, we assessed viral titers in the organs at time points after the development of the specific immune response (later than day 6) to assess whether the immune response was more effective in controlling virus in A35Δ virus-infected mice. On days 1, 2, and 3 p.i., there were no reproducible significant differences in WR and A35Δ virus titers in the tissues infected early after infection: lung, brain, nose, blood, and spleen (data not shown). However, on days 6 to 9 p.i., WR-infected mice usually had approximately 2- to 10-fold-higher viral titers in most organs (sometimes >100-fold) than A35Δ virus (Fig. 8), suggesting that the improved immune response in A35Δ virus-infected animals was better controlling viral replication. Peak titers were reached on day 7 for most organs, and after day 9 in these sublethally infected animals, titers dropped precipitously in the organs from both WR- and A35Δ virus-infected mice.
FIG. 8.
Viral titers in organs. Mice were infected i.n. with 500 PFU/mouse of WR or A35Δ virus, and organs were harvested from five mice per group per day into 1 ml cold RPMI medium. To quantify viral replication, organs were freeze-thawed three times, homogenized, and sonicated before titration on BS-C-1 cells. Monolayers were stained ∼40 h later with crystal violet, and the plaques were counted. Data show averages (±SEM). Lung and nose titers were divided by 2, and heart, liver, and kidney titers were multiplied by 20, 50, and 100, respectively, in order to make data more visible.
Protection from lethal challenge.
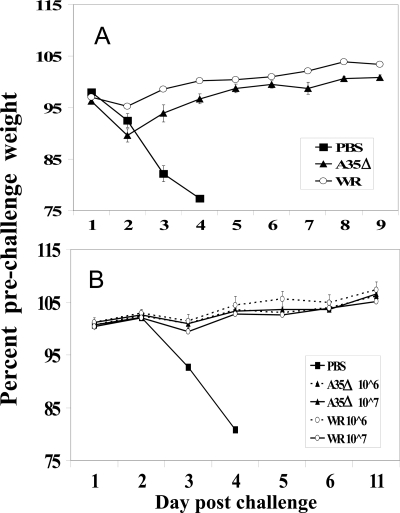
We wanted to determine whether the A35Δ virus would act as a protective vaccine. Since immune recognition of A35 epitopes has not been found in the analysis of human or mouse B (antibody) or T cells (19, 35), we hypothesized that its deletion would not harm vaccine efficacy. Furthermore, the improved immune responses (Fig. 3 to 6) suggest that, in addition to being safer (Fig. 1 and 2), the A35Δ virus might be a more efficacious vaccine. Mice were infected/vaccinated i.n. with a low dose of WR or A35Δ virus (103 PFU/mouse) or mock vaccinated with PBS. Four weeks later, the mice were challenged with a high dose (450 LD50) of virulent WR virus. Mock-vaccinated mice rapidly lost weight, and all died by 4 days postchallenge (Fig. 9A). However, all WR- or A35Δ virus-vaccinated mice survived to 3 weeks, with only slight weight loss immediately after challenge. The A35Δ mutant virus delivered i.p. also protected mice from virulent WR challenge (600 LD50) as well as the WR virus vaccination (Fig. 9B). We tested vaccination over a range from 104 to 107 PFU i.p. and 103 to 105 PFU i.n., with challenge of up to 1,000 LD50, and found that in every case, the A35Δ virus-vaccinated mice were fully protected from weight loss and death and were protected as well as the surviving WR-vaccinated mice. We therefore predict that A35Δ mutant viruses will be superior vaccines.
FIG. 9.
Protection from lethal challenge. (A) Mice were vaccinated i.n. on day 0 with 1,000 PFU of WR or A35Δ virus or mock vaccinated with PBS. Four weeks later, the mice were challenged i.n. with 450 LD50 of virulent WR virus. (B) Mice were vaccinated i.p. with 106 or 107 PFU and challenged 4 weeks postvaccination with 600 LD50 of WR.
DISCUSSION
We have previously shown that the A35 gene is required for full virulence in the mouse respiratory infection model and that the protein is not required for viral replication (39). Here we demonstrate that the A35 protein is also important for virulence when introduced i.p. In the i.n. mouse model, our data here, together with our previous data (39), show that 106 PFU of A35Δ mutant virus is required to reach the same level of weight loss caused by 103 PFU of WR virus, a 1,000-fold difference. It is difficult to compare the virulences of various gene deletion mutants because many have not been tested in the i.n. model measuring weight loss; intracranial inoculations or measures of viral titers in organs have often been performed instead. Available data indicate that the A35Δ virus is similar in virulence to the IL-18 binding protein knockout virus (36), more attenuated than the A46R deletion mutant (42), and less attenuated than the E3L deletion, which causes a 1,000-fold attenuation (46).
The A35 gene affects the development of the host adaptive immune response. Compared to WR-infected mice, mice infected with the A35Δ virus had larger spleens, generated higher titers of VV-specific Ig and neutralizing antibody, and developed higher numbers of VV-specific IFN-γ-secreting cells and improved CTL responses. The faster and greater-magnitude immune response that developed in the A35Δ-infected/vaccinated mice correlated with reduced viral replication in tissues. It is important that viral titers 1 to 4 dpi (before the development of a specific immune response) were not significantly different. Thus, the control of virus replication correlates with the enhanced immunogenicity of the A35Δ virus, suggesting that the immune response is responsible for decreases in virus titers. The relatively uniform reduction in viral load in multiple organs further implicates the immune control of virus rather than a tissue-specific replication defect of the A35Δ mutant.
Interestingly, the improved immune response seen in the A35Δ virus-infected/vaccinated mice was most evident on early days (days 6 to 8) p.i., suggesting that A35 delays the generation of virus-specific immunity, allowing the virus an advantage in establishing a generalized infection. We have shown (Fig. 2) that differences in weight loss between WR- and A35Δ virus-infected mice occur at the time when the specific adaptive immune response is developing in A35Δ virus-infected mice and delayed in WR-infected mice. This A35-mediated delay may allow enough time for the virus to replicate throughout the host organs and establish an infection in the mouse, resulting in weight loss and death. In contrast, the superior immune response in the A35Δ-infected mice results in a reduction in viral replication, clearance of the infection, and survival. While A35 seemed to delay T-cell responses early, and the differences waned later during infection, the antibody response remained elevated in the A35Δ virus-infected mice to day 34 p.i. (the latest time point tested), indicating that the immune response is not only delayed by A35 in WR but also persistently diminished even a month after infection. Impressively, this enhanced antibody response in A35Δ virus-infected mice occurs despite the increased antigen load and persistence of antigen in WR-infected mice (demonstrated by viral titer data for days 6 to 8 p.i.).
Sublethal viral infection in a host may be simplistically considered in several overlapping steps: (i) viral replication in tissues initially infected, (ii) viral stimulation of and interaction with the innate immune system, (iii) development of the specific immune response, (iv) viral replication in target organs, and (v) control of virus by immune mechanisms. Our data suggest that A35 interferes with the development of the specific immune response, because early viral replication up to day 4 is not diminished in the A35Δ mutant, and the difference seen between the A35Δ strain and WR occur with the same kinetics as the development of specific immunity. Since both B- and T-lymphocyte immunities are impeded by A35, it seems likely that A35 acts at an early stage in the initiation of a specific immune response or potentially at the interface between innate immunity and specific immunity. VV is known to block MHC class II antigen presentation to CD4+ T lymphocytes (26). This would be predicted to result in a smaller spleen size, which is indicative of decreased lymphocyte proliferation in this organ, and may also be expected to decrease Ig and CD8+ T-lymphocyte responses that are dependent on CD4+ T-helper cells. CD4+, CD8+, and B lymphocytes are known to be important for protection from poxvirus infections (2, 5, 15, 33, 49).
The fact that the A35Δ mutant virus is highly attenuated and more immunogenic suggests that the deletion of A35 will create superior vaccine strains. Indeed, in a lethal i.n. challenge model, the A35Δ virus vaccine, even at low doses, was as efficacious as WR in multiple challenge experiments with up to 1,000 LD50. It is important not to delete viral genes that contain protective epitopes from vaccine strains. Several groups have identified the CD4+ (4) and CD8+ (13, 19, 22, 32, 44) T-cell epitopes that confer protection to poxviruses. None of these studies has identified A35 as a T-cell determinant. Other groups have analyzed human vaccinia immune globulin in an effort to determine the VV antigens that it recognizes (11, 20). Again, A35 was not among the reactive VV antigens. Thus, the removal of the A35 gene is anticipated to improve vaccine safety while having no detrimental effect on the generation of a protective immune response in mammals. Indeed, the immune response is enhanced in A35Δ virus-infected animals. This is important for the development of poxvirus vaccines, especially for postexposure vaccination, which requires a rapid immune response for protection. In addition, poxvirus platform vaccines for other infectious diseases and cancer will likely benefit from the removal of the A35 gene.
Acknowledgments
We acknowledge the financial support of the North Carolina Biotechnology Center and NIH grant U54 AI057157 from the Southeastern Regional Center of Excellence for Emerging Infections and Biodefense.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or the NCBC.
We thank Tiffany Graham and Parteek Singla for experimental help.
Footnotes
Published ahead of print on 14 October 2009.
REFERENCES
- 1.Bell, E., M. Shamim, J. C. Whitbeck, G. Sfyroera, J. D. Lambris, and S. N. Isaacs. 2004. Antibodies against the extracellular enveloped virus B5R protein are mainly responsible for the EEV neutralizing capacity of vaccinia immune globulin. Virology 325:425-431. [DOI] [PubMed] [Google Scholar]
- 2.Belyakov, I. M., P. Earl, A. Dzutsev, V. A. Kuznetsov, M. Lemon, L. S. Wyatt, J. T. Snyder, J. D. Ahlers, G. Franchini, B. Moss, and J. A. Berzofsky. 2003. Shared modes of protection against poxvirus infection by attenuated and conventional smallpox vaccine viruses. Proc. Natl. Acad. Sci. U. S. A. 100:9458-9463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandt, T. A., and B. L. Jacobs. 2001. Both carboxy- and amino-terminal domains of the vaccinia virus interferon resistance gene, E3L, are required for pathogenesis in a mouse model. J. Virol. 75:850-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calvo-Calle, J. M., I. Strug, M. D. Nastke, S. P. Baker, and L. J. Stern. 2007. Human CD4+ T cell epitopes from vaccinia virus induced by vaccination or infection. PLoS Pathog. 3:1511-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaudhri, G., V. Panchanathan, H. Bluethmann, and G. Karupiah. 2006. Obligatory requirement for antibody in recovery from a primary poxvirus infection. J. Virol. 80:6339-6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, N., G. Li, M. K. Liszewski, J. P. Atkinson, P. B. Jahrling, Z. Feng, J. Schriewer, C. Buck, C. Wang, E. J. Lefkowitz, J. J. Esposito, T. Harms, I. K. Damon, R. L. Roper, C. Upton, and R. M. Buller. 2005. Virulence differences between monkeypox virus isolates from West Africa and the Congo basin. Virology 340:46-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark, R. H., J. C. Kenyon, N. W. Bartlett, D. C. Tscharke, and G. L. Smith. 2006. Deletion of gene A41L enhances vaccinia virus immunogenicity and vaccine efficacy. J. Gen. Virol. 87:29-38. [DOI] [PubMed] [Google Scholar]
- 8.Cosma, A., R. Nagaraj, C. Staib, C. Diemer, F. Wopfner, H. Schatzl, D. H. Busch, G. Sutter, F. D. Goebel, and V. Erfle. 2007. Evaluation of modified vaccinia virus Ankara as an alternative vaccine against smallpox in chronically HIV type 1-infected individuals undergoing HAART. AIDS Res. Hum. Retroviruses 23:782-793. [DOI] [PubMed] [Google Scholar]
- 9.Coulibaly, S., P. Bruhl, J. Mayrhofer, K. Schmid, M. Gerencer, and F. G. Falkner. 2005. The nonreplicating smallpox candidate vaccines defective vaccinia Lister (dVV-L) and modified vaccinia Ankara (MVA) elicit robust long-term protection. Virology 341:91-101. [DOI] [PubMed] [Google Scholar]
- 10.Damaso, C. R., J. J. Esposito, R. C. Condit, and N. Moussatche. 2000. An emergent poxvirus from humans and cattle in Rio de Janeiro State: cantagalo virus may derive from Brazilian smallpox vaccine. Virology 277:439-449. [DOI] [PubMed] [Google Scholar]
- 11.Davies, D. H., X. Liang, J. E. Hernandez, A. Randall, S. Hirst, Y. Mu, K. M. Romero, T. T. Nguyen, M. Kalantari-Dehaghi, S. Crotty, P. Baldi, L. P. Villarreal, and P. L. Felgner. 2005. Profiling the humoral immune response to infection by using proteome microarrays: high-throughput vaccine and diagnostic antigen discovery. Proc. Natl. Acad. Sci. U. S. A. 102:547-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhar, A. D., A. E. Werchniak, Y. Li, J. B. Brennick, C. S. Goldsmith, R. Kline, I. Damon, and S. N. Klaus. 2004. Tanapox infection in a college student. N. Engl. J. Med. 350:361-366. [DOI] [PubMed] [Google Scholar]
- 13.Drexler, I., C. Staib, W. Kastenmuller, S. Stevanovic, B. Schmidt, F. A. Lemonnier, H. G. Rammensee, D. H. Busch, H. Bernhard, V. Erfle, and G. Sutter. 2003. Identification of vaccinia virus epitope-specific HLA-A*0201-restricted T cells and comparative analysis of smallpox vaccines. Proc. Natl. Acad. Sci. U. S. A. 100:217-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eckart, R. E., S. S. Love, J. E. Atwood, M. K. Arness, D. C. Cassimatis, C. L. Campbell, S. Y. Boyd, J. G. Murphy, D. L. Swerdlow, L. C. Collins, J. R. Riddle, D. N. Tornberg, J. D. Grabenstein, and R. J. Engler. 2004. Incidence and follow-up of inflammatory cardiac complications after smallpox vaccination. J. Am. Coll. Cardiol. 44:201-205. [DOI] [PubMed] [Google Scholar]
- 15.Frey, S. E., R. B. Couch, C. O. Tacket, J. J. Treanor, M. Wolff, F. K. Newman, R. L. Atmar, R. Edelman, C. M. Nolan, and R. B. Belshe. 2002. Clinical responses to undiluted and diluted smallpox vaccine. N. Engl. J. Med. 346:1265-1274. [DOI] [PubMed] [Google Scholar]
- 16.Gherardi, M. M., J. C. Ramirez, and M. Esteban. 2003. IL-12 and IL-18 act in synergy to clear vaccinia virus infection: involvement of innate and adaptive components of the immune system. J. Gen. Virol. 84:1961-1972. [DOI] [PubMed] [Google Scholar]
- 17.Hooper, J. W., E. Thompson, C. Wilhelmsen, M. Zimmerman, M. A. Ichou, S. E. Steffen, C. S. Schmaljohn, A. L. Schmaljohn, and P. B. Jahrling. 2004. Smallpox DNA vaccine protects nonhuman primates against lethal monkeypox. J. Virol. 78:4433-4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hutin, Y. J., R. J. Williams, P. Malfait, R. Pebody, V. N. Loparev, S. L. Ropp, M. Rodriguez, J. C. Knight, F. K. Tshioko, A. S. Khan, M. V. Szczeniowski, and J. J. Esposito. 2001. Outbreak of human monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg. Infect. Dis. 7:434-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jing, L., T. M. Chong, C. L. McClurkan, J. Huang, B. T. Story, and D. M. Koelle. 2005. Diversity in the acute CD8 T cell response to vaccinia virus in humans. J. Immunol. 175:7550-7559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones-Trower, A., A. Garcia, C. A. Meseda, Y. He, C. Weiss, A. Kumar, J. P. Weir, and M. Merchlinsky. 2005. Identification and preliminary characterization of vaccinia virus (Dryvax) antigens recognized by vaccinia immune globulin. Virology 343:128-140. [DOI] [PubMed] [Google Scholar]
- 21.Kemper, A., M. Davis, and G. Freed. 2002. Expected adverse events in a mass smallpox vaccination campaign. Eff. Clin. Pract. 5(2):84-90. [PubMed] [Google Scholar]
- 22.Kennedy, R., and G. A. Poland. 2007. T-cell epitope discovery for variola and vaccinia viruses. Rev. Med. Virol. 17:93-113. [DOI] [PubMed] [Google Scholar]
- 23.Kolhapure, R. M., R. P. Deolankar, C. D. Tupe, C. G. Raut, A. Basu, B. M. Dama, S. D. Pawar, M. V. Joshi, V. S. Padbidri, M. K. Goverdhan, and K. Banerjee. 1997. Investigation of buffalopox outbreaks in Maharashtra State during 1992-1996. Indian J. Med. Res. 106:441-446. [PubMed] [Google Scholar]
- 24.Learned, L. A., M. G. Reynolds, D. W. Wassa, Y. Li, V. A. Olson, K. Karem, L. L. Stempora, Z. H. Braden, R. Kline, A. Likos, F. Libama, H. Moudzeo, J. D. Bolanda, P. Tarangonia, P. Boumandoki, P. Formenty, J. M. Harvey, and I. K. Damon. 2005. Extended interhuman transmission of monkeypox in a hospital community in the Republic of the Congo, 2003. Am. J. Trop. Med. Hyg. 73:428-434. [PubMed] [Google Scholar]
- 25.Lederman, E. R., M. G. Reynolds, K. Karem, Z. Braden, L. A. Learned-Orozco, D. Wassa-Wassa, O. Moundeli, C. Hughes, J. Harvey, R. Regnery, J. V. Mombouli, and I. K. Damon. 2007. Prevalence of antibodies against orthopoxviruses among residents of Likouala region, Republic of Congo: evidence for monkeypox virus exposure. Am. J. Trop. Med. Hyg. 77:1150-1156. [PubMed] [Google Scholar]
- 26.Li, P., N. Wang, D. Zhou, C. S. Yee, C. H. Chang, R. R. Brutkiewicz, and J. S. Blum. 2005. Disruption of MHC class II-restricted antigen presentation by vaccinia virus. J. Immunol. 175:6481-6488. [DOI] [PubMed] [Google Scholar]
- 27.Likos, A. M., S. A. Sammons, V. A. Olson, A. M. Frace, Y. Li, M. Olsen-Rasmussen, W. Davidson, R. Galloway, M. L. Khristova, M. G. Reynolds, H. Zhao, D. S. Carroll, A. Curns, P. Formenty, J. J. Esposito, R. L. Regnery, and I. K. Damon. 2005. A tale of two clades: monkeypox viruses. J. Gen. Virol. 86:2661-2672. [DOI] [PubMed] [Google Scholar]
- 28.Mahalingam, S., I. K. Damon, and B. A. Lidbury. 2004. 25 years since the eradication of smallpox: why poxvirus research is still relevant. Trends Immunol. 25:636-639. [DOI] [PubMed] [Google Scholar]
- 29.Molino, A. C., A. B. Fleischer, Jr., and S. R. Feldman. 2004. Patient demographics and utilization of health care services for molluscum contagiosum. Pediatr. Dermatol. 21:628-632. [DOI] [PubMed] [Google Scholar]
- 30.Moss, B. 2001. Poxviruses, p. 2849-2884. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 31.Mukinda, V. B., G. Mwema, M. Kilundu, D. L. Heymann, A. S. Khan, and J. J. Esposito. 1997. Re-emergence of human monkeypox in Zaire in 1996. Monkeypox Epidemiologic Working Group. Lancet 349:1449-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Norbury, C. C., S. Basta, K. B. Donohue, D. C. Tscharke, M. F. Princiotta, P. Berglund, J. Gibbs, J. R. Bennink, and J. W. Yewdell. 2004. CD8+ T cell cross-priming via transfer of proteasome substrates. Science 304:1318-1321. [DOI] [PubMed] [Google Scholar]
- 33.Panchanathan, V., G. Chaudhri, and G. Karupiah. 2006. Protective immunity against secondary poxvirus infection is dependent on antibody but not on CD4 or CD8 T-cell function. J. Virol. 80:6333-6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parker, S., A. Nuara, R. M. Buller, and D. A. Schultz. 2007. Human monkeypox: an emerging zoonotic disease. Future Microbiol. 2:17-34. [DOI] [PubMed] [Google Scholar]
- 35.Pasquetto, V., H. H. Bui, R. Giannino, F. Mirza, J. Sidney, C. Oseroff, D. C. Tscharke, K. Irvine, J. R. Bennink, B. Peters, S. Southwood, V. Cerundolo, H. Grey, J. W. Yewdell, and A. Sette. 2005. HLA-A*0201, HLA-A*1101, and HLA-B*0702 transgenic mice recognize numerous poxvirus determinants from a wide variety of viral gene products. J. Immunol. 175:5504-5515. [DOI] [PubMed] [Google Scholar]
- 36.Reading, P. C., and G. L. Smith. 2003. Vaccinia virus interleukin-18-binding protein promotes virulence by reducing gamma interferon production and natural killer and T-cell activity. J. Virol. 77:9960-9968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reed, L. J., and H. A. Muench. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 38.Rehm, K. E., R. F. Connor, G. J. B. Jones, K. Yimbu, M. D. Mannie, and R. L. Roper. 2009. Vaccinia virus decreases MHC class II antigen presentation, T cell priming, and peptide association with MHC class II. Immunology 128:381-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roper, R. L. 2006. Characterization of the vaccinia virus A35R protein and its role in virulence. J. Virol. 80:306-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.See, R. H., A. N. Zakhartchouk, M. Petric, D. J. Lawrence, C. P. Mok, R. J. Hogan, T. Rowe, L. A. Zitzow, K. P. Karunakaran, M. M. Hitt, F. L. Graham, L. Prevec, J. B. Mahony, C. Sharon, T. C. Auperin, J. M. Rini, A. J. Tingle, D. W. Scheifele, D. M. Skowronski, D. M. Patrick, T. G. Voss, L. A. Babiuk, J. Gauldie, R. L. Roper, R. C. Brunham, and B. B. Finlay. 2006. Comparative evaluation of two severe acute respiratory syndrome (SARS) vaccine candidates in mice challenged with SARS coronavirus. J. Gen. Virol. 87:641-650. [DOI] [PubMed] [Google Scholar]
- 41.Silva-Fernandes, A. T., C. E. Travassos, J. M. Ferreira, J. S. Abrahao, E. S. Rocha, F. Viana-Ferreira, J. R. dos Santos, C. A. Bonjardim, P. C. Ferreira, and E. G. Kroon. 2009. Natural human infections with vaccinia virus during bovine vaccinia outbreaks. J. Clin. Virol. 44:308-313. [DOI] [PubMed] [Google Scholar]
- 42.Stack, J., I. R. Haga, M. Schroder, N. W. Bartlett, G. Maloney, P. C. Reading, K. A. Fitzgerald, G. L. Smith, and A. G. Bowie. 2005. Vaccinia virus protein A46R targets multiple Toll-like-interleukin-1 receptor adaptors and contributes to virulence. J. Exp. Med. 201:1007-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stich, A., H. Meyer, B. Kohler, and K. Fleischer. 2002. Tanapox: first report in a European traveller and identification by PCR. Trans. R. Soc. Trop. Med. Hyg. 96:178-179. [DOI] [PubMed] [Google Scholar]
- 44.Terajima, M., J. Cruz, G. Raines, E. D. Kilpatrick, J. S. Kennedy, A. L. Rothman, and F. A. Ennis. 2003. Quantitation of CD8+ T cell responses to newly identified HLA-A*0201-restricted T cell epitopes conserved among vaccinia and variola (smallpox) viruses. J. Exp. Med. 197:927-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Upfal, M. J., and S. Cinti. 2004. Smallpox vaccination and adverse cardiac events. Emerg. Infect. Dis. 10:961-962. [DOI] [PubMed] [Google Scholar]
- 46.Vijaysri, S., G. Jentarra, M. C. Heck, A. A. Mercer, C. J. McInnes, and B. L. Jacobs. 2008. Vaccinia viruses with mutations in the E3L gene as potential replication-competent, attenuated vaccines: intra-nasal vaccination. Vaccine 26:664-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wyatt, L. S., P. L. Earl, L. A. Eller, and B. Moss. 2004. Highly attenuated smallpox vaccine protects mice with and without immune deficiencies against pathogenic vaccinia virus challenge. Proc. Natl. Acad. Sci. USA 101:4590-4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiao, Z., J. M. Curtsinger, M. Prlic, S. C. Jameson, and M. F. Mescher. 2007. The CD8 T cell response to vaccinia virus exhibits site-dependent heterogeneity of functional responses. Int. Immunol. 19:733-743. [DOI] [PubMed] [Google Scholar]
- 49.Xu, R., A. J. Johnson, D. Liggitt, and M. J. Bevan. 2004. Cellular and humoral immunity against vaccinia virus infection of mice. J. Immunol. 172:6265-6271. [DOI] [PubMed] [Google Scholar]