Abstract
During chronic viral infections, T cells are exhausted due to constant antigen exposure and are associated with enhanced programmed death 1 (PD-1) expression. Deficiencies in the PD-1/programmed death-ligand 1 (PD-L1) pathway are associated with autoimmune diseases, including those of the central nervous system (CNS). To understand the role of PD-1 expression in regulating T-cell immunity in the CNS during chronic infection, we characterized PD-1 expression in cerebrospinal fluid (CSF) and blood of individuals with chronic human immunodeficiency virus type 1 (HIV-1) infection. PD-1 expression was higher on HIV-specific CD8+ T cells than on total CD8+ T cells in both CSF and blood. PD-1 expression on CSF T cells correlated positively with CSF HIV-1 RNA and inversely with blood CD4+ T-cell counts, suggesting that HIV-1 infection drives higher PD-1 expression on CSF T cells. However, in every HIV-positive individual, PD-1 expression was higher on T cells in CSF than on those in blood, despite HIV-1 RNA levels being lower. Among healthy HIV-negative controls, PD-1 expression was higher in CSF than in blood. Furthermore, frequencies of the senescence marker CD57 were lower on CSF T cells than on blood T cells, consistent with our prior observation of enhanced ex vivo functional capacity of CSF T cells. The higher PD-1 expression level on CSF T cells therefore does not reflect cellular exhaustion but may be a mechanism to downregulate immune-mediated tissue damage in the CNS. As inhibition of the PD-1/PD-L1 pathway is pursued as a therapeutic option for viral infections, potential effects of such a blockade on development of autoimmune responses in the CNS should be considered.
Programmed death 1 (PD-1; also called CD279) and its ligands, PD-L1 (also called B7-H1 or CD274) and PD-L2 (also known as B7-DC or CD-273), regulate T-cell activation, peripheral tolerance, and autoimmunity (22, 43). PD-1 can be expressed on CD8+ and CD4+ T cells, B cells, natural killer T cells, and activated monocytes. PD-L1 is expressed on various cells, including T and B cells, dendritic cells, macrophages, mast cells, nonhematopoietic cell types (including vascular endothelial cells, pancreatic islet cells, astrocytes, keratinocytes, and microglial cells), and cells in immune privileged sites, including the placenta and the eye (22). PD-L2 expression is inducible and is restricted to dendritic cells, monocytes, macrophages, and mast cells (22). During chronic infections, the PD-1/PD-L1 pathway inhibits antigen-specific T-cell responses (7, 8, 35, 46). In human immunodeficiency virus type 1 (HIV-1)-infected individuals, PD-1 expression on HIV-specific T cells in peripheral blood is upregulated and correlates positively with plasma viremia and inversely with CD4+ T-cell counts (7, 46). PD-1 expression on HIV-specific T cells is also associated with T-cell exhaustion, as defined by a reduced ability to proliferate and produce cytokines (7, 46). Inhibition of the PD-1/PD-L1 pathway augments HIV-specific CD8+ and CD4+ T-cell function, and antiretroviral therapy is associated with a significant reduction of PD-1 expression on HIV-specific T cells in peripheral blood (8).
The PD-1/PD-L1 pathway also limits immune-mediated tissue damage that may be caused by overreactive peripheral T cells, especially in immune privileged sites such as the central nervous system (CNS). In 1999, the importance of PD-1 for peripheral tolerance was first suggested by studies which showed that PD1−/− mice develop lupus-like autoimmune diseases (32). In humans, polymorphisms in the PDCD1 gene, which encodes PD-1, have been associated with autoimmune diseases, including lupus, diabetes, rheumatoid arthritis, and multiple sclerosis (20, 21, 25). Upregulation of PD-L1 in multiple sclerosis lesions from human brain tissue suggests a role for the PD-1/PD-L1 pathway in regulating T-cell activation and controlling immunopathological damage (33).
The CNS is involved by HIV-1 early during primary infection (6, 13), and approximately 40% of patients who develop advanced AIDS without receiving antiretroviral therapy develop cognitive impairment (6, 13, 38). While HIV-1 proteins gp120 (3, 16) and Tat (30) are directly neurotoxic and may contribute to HIV-associated dementia, detrimental neuropathogenic effects have also been postulated for inflammatory and innate immune cells, especially monocytes/macrophages and T cells (11, 19, 49, 50). Immune responses cause neuropathogenesis during other viral infections, and cytotoxic T lymphocytes can worsen the disease through direct cytotoxicity or release of inflammatory cytokines such as gamma interferon (IFN-γ) (14). However, we recently described higher frequencies of functional HIV-specific CD8+ T cells in cerebrospinal fluid (CSF) than in blood among asymptomatic HIV-positive individuals with little or no HIV-1 RNA in CSF, suggesting that HIV-1-specific CD8+ T cells help to control intrathecal viral replication (40).
To understand the role of the PD-1/PD-L1 pathway in regulating T-cell responses during viral infection of the CNS, we characterized PD-1 expression on T cells in CSF and peripheral blood among asymptomatic HIV-positive individuals. We hypothesized that T-cell PD1 expression would be lower in CSF than in blood, since HIV-1 RNA concentrations are lower in CSF than in plasma and the magnitude and breadth of IFN-γ-secreting HIV-specific T cells are greater in CSF than in blood (40). We show that, in CSF, HIV-1 RNA correlates directly with PD-1 expression on CD4+, CD8+, and HIV-specific CD8+ T cells. Unexpectedly, PD-1 expression on all T cells is higher in CSF than in blood in HIV-positive patients and healthy HIV-negative controls. In contrast, expression of the senescence marker CD57 is lower in CSF than in blood. These data suggest that higher PD-1 expression on T cells in CSF may be a mechanism to regulate T-cell immunity in the CNS, rather than indicating T-cell exhaustion, and that this regulation is increased by HIV-1 replication.
MATERIALS AND METHODS
Study participants.
Individuals with chronic HIV-1 infection were recruited for study participation from the Vanderbilt University-affiliated Comprehensive Care Center (Nashville, TN). Of the 15 participants in the present study, 4 were included in a previous report (40). Participants were antiretroviral naïve. Additional eligibility criteria included at least 100,000 platelets/mm3 within 1 week prior to lumbar puncture, no history of significant CNS abnormality, such as trauma, congenital malformation, or genetic disorder, and no physical examination or magnetic resonance imaging (MRI) evidence of a CNS mass lesion. Typing of HLA class I alleles was performed by sequence-specific primer PCR (DCI, Nashville, TN). HIV-1 RNA was quantified in plasma and cell-free CSF by Cobas Amplicor Monitor HIV-1, version 1.5 (Roche Diagnostic Systems, Branchburg, NJ), with a lower limit of quantitation of 50 copy/ml. The Vanderbilt Institutional Review Board approved this study, and all participants provided written informed consent.
Collection and processing of CSF and peripheral blood.
With the participant in the lateral decubitus position, lumbar CSF was obtained through a 22-gauge spinal needle by gravity drainage. A Whitacre point needle was used if possible to minimize postdural headache. During each procedure, approximately 40 ml of CSF was collected directly into a 50-ml plastic conical tube on ice. Mononuclear cells from CSF were pelleted by centrifugation at 350 × g for 15 min at 4°C. Approximately 100 ml of peripheral blood was obtained immediately before lumbar puncture and processed in parallel. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque density gradient centrifugation.
Flow cytometry.
Mononuclear cells from CSF and blood were labeled ex vivo with a panel of fluorochrome-labeled antibodies. Freshly isolated cells were incubated sequentially with HIV-specific HLA class I tetramers (labeled with phycoerythrin or allophycocyanin; Beckman Coulter or NIH Tetramer Facility) at room temperature for 10 min, anti-PD-1 (Biolegend) at room temperature for 20 min, and goat anti-mouse immunoglobulin G (Pacific Blue; Invitrogen) (secondary antibody for PD-1 antibody) for 30 min on ice, followed by incubation with a cocktail of antibodies to CD3, CD8, CD4, CD14, CD19, CD56, CD57, and CD45RO on ice for 30 min. The anti-PD-1 antibody was initially a kind gift from Gordon Freeman (Harvard Medical School) and subsequently was purchased from Biolegend. Stained samples were acquired on a FACSAria or LSRII flow cytometer (BD Biosciences) and analyzed with FACS Diva (BD Biosciences) or FlowJo (Treestar) software. The choice of tetramers for CSF staining was informed by initial analyses of tetramer responses in blood from each participant.
PBMC culture.
Frozen PBMCs were thawed and cultured at 106/ml in autologous CSF alone, in medium (RPMI 1640 plus 1 mM HEPES, 200 mM glutamine, 5,000 U/ml penicillin, 5,000 μg/ml streptomycin, and 10% heat-inactivated human AB serum) alone, or in medium with 100 U of interleukin-2 (IL-2) at 37οC and 5% CO2. Cells were harvested after 7 days and stained for PD-1, CD3, CD8, CD4, CD14, CD19, CD56, and 7-aminoactinomycin D as described above.
Cytokine assays.
A cytokine bead array assay (BD Biosciences) was used to quantify IL-2 and IL-7 in CSF following the manufacturer's instructions. The theoretical lower limit of detection for the assay was 11.2 pg/ml for IL-2 and 0.5 pg/ml for IL-7.
Neurological examinations and neuroimaging.
Prior to lumbar puncture, each participant underwent a complete neurological examination, as well as standard structural MRI on a 3.0 T Achieva MR scanner (Philips Medical Systems) with an eight-channel Sense (sensitivity-encoding) head coil to rule out significant structural brain abnormalities. Structural MRIs consisted of standard T1 weighted spin echo (TR/TE = 550/14 ms), PD/T2 weighted fast spin echo (TR/TE1/TE2 = 4,000/20/17 ms), FLAIR-TSE (TI/TR/TE = 860/2,000/17 ms), and T1 weighted (inversion prepared) 3D FFE (TI/TR/TE = 900/9.8/4.6 ms) (where TR is repetition time, TE is echo time, FLAIR is fluid-attenuated inversion recovery, TSE is turbo spin echo, TI is inversion time, and FFE is fast field echo).
Statistical analysis.
All comparisons between cells from CSF and peripheral blood, including numbers of epitopes recognized, were performed using a two-tailed Wilcoxon signed rank t test with GraphPad Prism 5. P values of <0.05 were considered significant.
RESULTS
Comparisons of viral loads and T-cell numbers in peripheral blood and CSF.
We compared CSF and blood of 15 HIV-infected adults. The duration of known HIV positivity ranged from 1 to 17 years (median = 8 years). All participants were asymptomatic, had normal neurological examinations, and had no structural abnormalities on neuroimaging. Basic parameters from CSF and peripheral blood are listed in Table 1. Concentrations of HIV-1 RNA in CSF correlated with those in blood (r = 0.8634; P < 0.0001) but were lower in CSF (median = 3.4 log10 copies/ml) than in blood (median = 4.0 log10 copies/ml). Ratios of CD4+ to CD8+ T cells were similar in CSF (median = 0.6) and blood (median = 0.9) (P > 0.05). All participants had CSF total protein levels of <40 mg/dl. Differential counting of CSF cells identified neither neutrophils nor evidence of erythrocyte contamination for any individual, indicating that CSF lymphocytes were not contaminated by peripheral blood lymphocytes introduced during lumbar puncture. The median number of mononuclear cells isolated from CSF was 35,000 (range, 20,000 to 300,000).
TABLE 1.
Characteristics of study participants
| Subject ID | HIV-1 RNA level (log10 copies/ml)a |
CD4+ T-cell count in blood (cells/mm3) | |
|---|---|---|---|
| Plasma | CSF | ||
| 10071 | <1.7 | <1.7 | 896 |
| 10024 | 2.5 | <1.7 | 841 |
| 10067 | 2.6 | <1.7 | 704 |
| 20018 | 2.6 | 1.4 | 961 |
| 10132 | 2.9 | 2.0 | 598 |
| 10068 | 3.3 | 2.3 | 750 |
| 10138 | 3.9 | 2.6 | 232 |
| 10027 | 4.0 | 3.4 | 378 |
| 10097 | 5.6 | 3.5 | 84 |
| 10015 | 5.0 | 3.7 | 680 |
| 10039 | 4.6 | 3.9 | 348 |
| 2098D | 5.2 | 4.0 | 476 |
| 10054 | 4.7 | 4.5 | 290 |
| 20023 | 4.6 | 4.6 | 421 |
| 6847B | 5.4 | 4.6 | 542 |
The value <1.7 indicates that the level was below the assay limit of quantification (50 HIV-1 RNA copies/ml).
Enhanced expression of PD-1 on T cells in CSF compared to that in blood.
At least 5,000 freshly isolated CSF cells from each HIV-positive participant were evaluated, in parallel with PBMCs, for PD-1 expression on CD4+, CD8+, and HIV-specific or cytomegalovirus (CMV)-specific CD8+ T cells (Fig. 1A to C). HIV-specific or CMV-specific CD8+ T cells were detected with HLA class I tetramers chosen based on earlier screening with peripheral blood. Depending on the total yield of CSF cells, we assayed for as many of these tetramer responses as was feasible, using paired CSF and blood samples. Blood and CSF were analyzed with 10 HIV-specific and 4 CMV-specific tetramers, based on the HLA types of the participants, with a total of 24 individual HIV-specific and 6 individual CMV-specific tetramer responses (see Table S1 in the supplemental material). Frequencies of HIV-specific CD8+ T cells in CSF (median = 2.6%) were higher than those in blood (median = 1.2%) (P = 0.0002), as observed previously in a cohort of HIV controllers (40). In contrast, frequencies of CMV-specific CD8+ T cells tended to be lower in CSF (median = 0.7%) than in blood (median = 2.4%) (P = 0.06) (see Fig. S1 in the supplemental material).
FIG. 1.
Representative assay of PD-1 expression on T cells in CSF and blood from an HIV-positive patient. Representative flow cytometric plots show PD-1 expression on HIV-specific and CMV-specific CD8+ T cells in blood (A) and CSF (B) of an HIV-positive patient. (C) Representative histograms of PD-1 expression on CD4+, CD8+, HIV-specific CD8+, and CMV-specific CD8+ T cells in blood (shaded) and CSF (unshaded) of HIV-positive patients. The gate representing PD-1hi cells was defined by gating on CD8+ T cells in blood. The numbers in the plot represent MFIs of PD-1 expression (top numbers) and frequencies of PD-1hi cells, expressed as percentages of the T-cell population (bottom numbers), on T cells in blood (left numbers) and in CSF (right numbers). The tetramers represented in the plot are A*03-QK10 for HIV and A*02-NV9 for CMV.
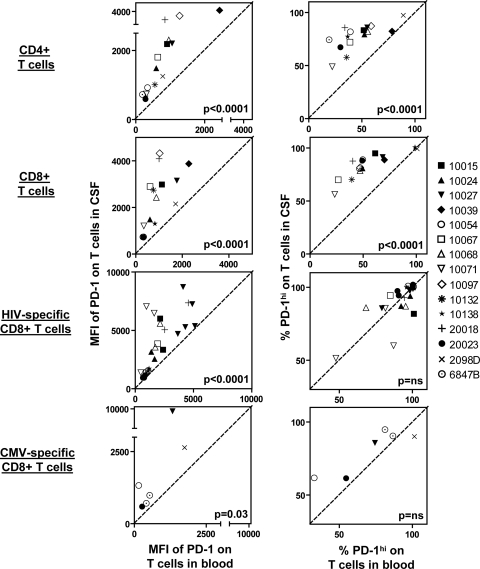
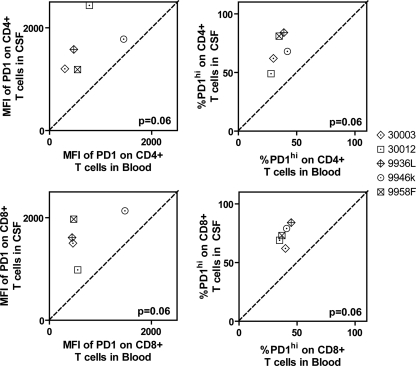
The mean fluorescence intensities (MFIs) of PD-1 expression on CD4+, CD8+, and HIV- and CMV-specific CD8+ T cells were higher in CSF than in blood (Fig. 1C and 2). PD-1hi T cells were gated on CD8+ T cells, and that gate was applied to CD4+ and HIV- and CMV-specific CD8+ T cells (Fig. 1C). Frequencies of PD-1hi T cells were also greater among CD4+, CD8+, and CMV-specific CD8+ T cells (Fig. 2). Among HIV-specific CD8+ T cells in CSF and blood, there was no significant difference in frequency for the PD-1hi subset, apparently because HIV-specific CD8+ T cells in blood were already enriched (>80%) for the PD-1hi subset. To determine whether enhanced PD-1 expression on T cells in CSF was a consequence of HIV infection, we studied five healthy, HIV-negative controls. Among these controls, MFIs of PD-1 expression and frequencies of PD-1hi subsets on total CD4+ and CD8+ cells were also higher in CSF than in blood (Fig. 3), indicating that enhanced PD-1 expression on T cells in CSF is an aspect of normal cellular physiology, rather than solely a consequence of HIV infection.
FIG. 2.
Increased PD-1 expression on T cells in CSF compared to those in blood from HIV-positive patients. (Left) Relationships between MFIs of PD-1 in blood and in CSF. (Right) Relationships between frequencies of PD-1hi T cells in blood and in CSF. Data from 15 HIV-positive patients are shown. On plots for CD4+ and CD8+ T cells, each data point represents an individual participant. On plots for HIV-specific and CMV-specific CD8+ T cells, each data point represents an individual tetramer response for each participant studied. The diagonal dashed line represents the line of unity. P values were derived from a two-tailed Wilcoxon signed-rank t test. Individual subject identifiers are listed to the right.
FIG. 3.
Increased expression of PD-1 on T cells in CSF compared to those in blood from healthy HIV-negative controls. (Left) Relationships between MFIs of PD-1 in blood and in CSF. (Right) Relationships between frequencies of PD-1hi T cells in blood and in CSF. Data from five healthy, HIV-negative volunteers are shown. Each data point represents an individual participant studied. The diagonal dashed line represents the line of unity. P values were derived from a two-tailed Wilcoxon signed-rank t test. Individual subject identifiers are listed to the right.
Higher proportions of memory CD8+ and CD4+ T cells in the CNS have been observed under both normal and pathological conditions (31, 48). To ensure that the apparent enhanced PD-1 expression on T cells in CSF was not simply due to an absence of naïve T cells, CSF and blood cells from five HIV-positive individuals were also stained for CD62L and CD45RO in an expanded panel and analyzed on an LSRII flow cytometer. With naïve T cells (CD45RO− CD62L+) excluded from the analysis, PD-1 expression on the remaining memory T cells was still higher in CSF (CD8+ T-cell PD-1 MFI median = 833; CD4+ PD-1 MFI median = 813) than in blood (CD8+ T-cell PD-1 MFI median = 460 [P = 0.06]; CD4+ T-cell PD-1 MFI median = 424 [P = 0.06]). Failure to achieve statistical significance likely reflects the small sample size for this subanalysis. Among all 15 HIV-positive patients, PD-1 expression on CD45RO+ memory T cells was also significantly higher in CSF (CD4+ T-cell MFI median = 1,838; CD8+ T-cell MFI median = 2,584) than in blood (CD4+ T-cell MFI median = 1,038 [P < 0.0001]; CD8+ T-cell MFI median = 1,517 [P < 0.0001]). Among five healthy controls, PD-1 expression on CD45RO+ memory T cells tended to be higher in CSF (CD4+ T-cell MFI median = 1,268; CD8+ T-cell MFI median = 2,680) than in blood (CD4+ T-cell MFI median = 935 [P = 0.06]; CD8+ T-cell MFI median = 1,290 [P = 0.06]).
Increased PD-1 expression on HIV-specific T cells in CSF and blood.
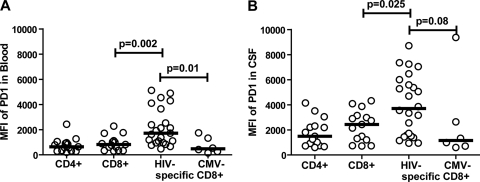
In peripheral blood, PD-1 expression has been shown to be higher on HIV-specific T cells than on total CD8+ and CMV-specific CD8+ T cells (7, 35, 46). To test whether HIV-specific T cells in CSF also have higher expression levels of PD-1, we compared PD-1 expression on different T-cell subsets in CSF and blood from HIV-positive individuals (Fig. 4). Consistent with previous reports, PD-1 expression on T cells in peripheral blood was significantly higher on HIV-specific CD8+ T cells (MFI median = 1,693) than on total CD8+ T cells (MFI median = 822; P = 0.002) or CMV-specific CD8+ T cells (MFI median = 476; P = 0.01) (Fig. 4A). Similar to the case of blood, PD-1 expression on HIV-specific CD8+ T cells in CSF (MFI median = 3,703) was significantly higher than that on total CD8+ T cells in CSF (MFI median = 2,429; P = 0.025) and somewhat higher than that on CMV-specific CD8+ T cells in CSF (MFI median = 1,156; P = 0.08) (Fig. 4B).
FIG. 4.
Comparison of PD-1 expression levels on different T-cell populations in blood and CSF. Comparison of MFIs of PD-1 on CD4+, CD8+, HIV-specific CD8+, and CMV-specific CD8+ T cells in blood (A) and CSF (B) from HIV-positive patients. Data from 15 HIV-positive patients are shown. Markers represent individual patients (PD-1 expression on CD4+ and CD8+ T cells) or individual HIV-specific or CMV-specific CD8+ tetramer populations (may be multiple markers per patient). Horizontal lines represent medians. P values are from Mann-Whitney t tests.
PD-1 expression in CSF correlates with status of HIV disease.
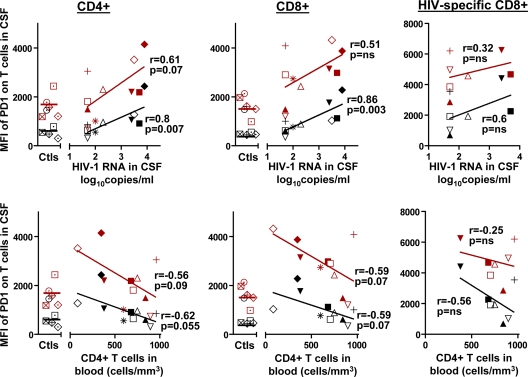
PD-1 expression on CD4+, CD8+, and HIV-specific CD8+ T cells in peripheral blood from HIV patients has been shown to correlate with disease progression (i.e., with higher plasma HIV-1 RNA concentrations and lower CD4+ T-cell counts) (7, 35, 46). We similarly found a direct correlation between PD-1 MFIs on CD4+, CD8+, and HIV-specific CD8+ T cells in blood and the HIV-1 RNA concentration in plasma (r = 0.83 for CD4+ T cells, r = 0.84 for CD8+ T cells, and r = 0.74 for HIV-specific CD8+ T cells) and an inverse correlation with peripheral blood CD4+ T-cell counts (r = −0.68 for CD4+ T cells, r = −0.6 for CD8+ T cells, and r = −0.4 for HIV-specific CD8+ T cells). To evaluate determinants of PD-1 expression on T cells in the CNS, we characterized relationships between the MFI of PD-1 expression on T cells in CSF, HIV-1 RNA in CSF, and peripheral blood CD4+ T-cell counts (Fig. 5). As in blood, MFIs of PD-1 expression on CD4+, CD8+, and HIV-specific CD8+ T cells in CSF correlated positively with HIV-1 RNA in CSF (r = 0.61 for CD4+ T cells, r = 0.51 for CD8+ cells, and r = 0.32 for HIV-specific CD8+ T cells) and inversely with blood CD4+ T-cell counts (r = −0.56 for CD4+ T cells and r = −0.59 for CD8+ T cells).
FIG. 5.
MFI of PD-1 expression on T cells in CSF correlates with status of HIV disease. (Top) Correlations between log10 HIV-1 RNA copies/ml in CSF and MFIs of PD-1 on CD4+, CD8+, and HIV-specific CD8+ T cells in CSF and in blood. (Bottom) Correlations between peripheral blood CD4+ T-cell counts and MFIs of PD-1 on CD4+, CD8+, and HIV-specific CD8+ T cells in CSF and in blood. Spearman's rho (r) and P values are indicated. Data for healthy HIV-negative controls (Ctls) are also shown. Markers for CSF are red, and those for blood are black.
Lower frequencies of CD57hi T cells in CSF than in blood.
In addition to PD-1, we evaluated expression of CD57, a marker for T-cell senescence (4), on T cells in CSF. CD57+ T cells have low proliferation and expansion potential, although they can secrete the cytokines IFN-γ and tumor necrosis factor alpha (TNF-α) in response to cognate antigen (4, 26). We have previously shown that HIV-specific T cells in CSF have the ability to expand readily ex vivo and that among CSF T cells expanded with phytohemagglutinin, HIV-specific IFN-γ responses are broader and of higher magnitude among cells from CSF than among those from blood (40). With anti-CD57 staining, we identified CD57hi, CD57int, and CD57lo populations (Fig. 6A). Gates drawn on CD8+ T cells were applied to CD4+ and HIV- and CMV-specific CD8+ T-cell populations. Frequencies of CD57hi subsets among CD4+, CD8+, and HIV- and CMV-specific CD8+ T cells were lower in CSF (CD4+ median, 1.5%; CD8+ median, 13.7%; HIV-specific CD8+ median, 20%; CMV-specific CD8+ median, 11.2%) than in blood (CD4+ median, 3.2%; CD8+ median, 27.5%; HIV-specific CD8+ median, 24.8%; CMV-specific CD8+ median, 53.8%) (Fig. 6B to E). When CD57 expression levels were analyzed based on any level of positivity (i.e., combining the CD57hi and CD57int subsets), there was no significant difference between frequencies of these subsets in CSF and blood among CD4+, CD8+, and HIV-specific CD8+ T cells. T cells in CSF had increased levels of CD57int populations, while T cells in blood had increased levels of CD57hi populations. To ensure that differences in CD57 expression levels on CSF and blood T cells were not due to differences in frequencies of memory T cells, we analyzed CD57 expression levels on memory T cells in CSF and blood. Frequencies of CD57hi subsets were lower in CSF (CD4+ median, 1.5%; CD8+ median, 16.7%) than in blood (CD4+ median, 4.3% [P = 0.0007]; CD8+ median, 21.8% [P = 0.02]) among CD45RO+ CD4+ and CD8+ T cells. For five HIV-positive patients analyzed additionally for CD62L, frequencies of CD57hi subsets tended to be lower in CSF (CD4+ median, 1%; CD8+ median, 7.1%) than in blood (CD4+ median, 2.6% [P = 0.06]; CD8+ median, 22.2% [P = 0.06]) among memory (based on exclusion of CD45RO− CD62L+ naïve T cells) CD4+ and CD8+ T cells. Thus, T cells in CSF, although expressing higher levels of PD-1, may retain substantial proliferative potential, as indicated by lower frequencies of CD57hi T cells. This is consistent with our prior study in which we observed good expansion potential of HIV-specific T cells from CSF as well as better functional T-cell responses in terms of breadth and magnitude of IFN-γ responses to HIV-specific peptide stimulation than those in cells from blood (40).
FIG. 6.
Lower frequencies of CD57hi T cells in CSF than in blood of HIV-positive patients. (A) Representative histograms of CD57 expression on CD4+, CD8+, HIV-specific CD8+, and CMV-specific CD8+ T cells in blood and CSF of HIV-positive patients. Gates representing CD57hi, CD57int, and CD57lo T cells were defined by gating on CD8+ T cells in blood. (B to E) Relationships between frequencies of CD57hi T cells in CSF and blood of HIV-positive patients. Data from 15 HIV-positive patients are shown. On plots for CD4+ and CD8+ T cells, each data point represents an individual participant. On plots for HIV-specific CD8+ and CMV-specific CD8+ T cells, each data point represents an individual tetramer response for each participant studied. The diagonal dashed line represents the line of unity. P values were derived from a two-tailed Wilcoxon signed-rank t test.
Frequencies of CD57hi T-cell subsets on CD8+ and HIV-specific CD8+ T cells in CSF did not correlate with the MFI of PD-1 expression (see Fig. S2 in the supplemental material). For CD4+ and CMV-specific CD8+ T cells, there was a trend between frequencies of CD57hi T-cell subsets and the MFI of PD-1 expression (see Fig. S2 in the supplemental material). Among CD45RO+ memory T cells, frequencies of CD57hi T-cell subsets on CD8+ T cells did not correlate with the MFI of PD-1 expression (r = 0.18; P was not significant), but there was a correlation among CD4+ CD45RO+ memory T cells (r = 0.55; P = 0.03). Lower CD57 expression levels on CD8+ T cells in CSF and the lack of correlation with PD-1 expression may explain the maintenance of proliferative potential of CSF T cells, despite relatively high levels of PD-1 expression.
Incubating PBMCs in CSF does not induce PD-1 expression.
The common γ-chain cytokines IL-2, IL-7, IL-15, and IL-21 play an important role in peripheral T-cell expansion and survival and upregulate PD-1 expression on T cells in vitro in an antigen-independent manner. To determine whether enhanced PD-1 expression on CSF T cells is a consequence of CSF exposure, perhaps due to cytokines/chemokines in CSF, PBMCs from six HIV-positive individuals and two HIV-negative healthy controls were cultured for 7 days in autologous CSF alone, R-10 culture medium alone, or R-10 with 100 U of IL-2 (see Fig. S3 in the supplemental material). Culture in CSF or R-10 alone did not enhance the MFI of PD-1 expression or the frequency of PD-1hi CD8+ or CD4+ T cells compared to direct staining for PD-1 on PBMCs without ex vivo culture. In contrast, culture with IL-2 significantly enhanced the MFI of PD-1 expression on CD8+ and CD4+ T cells (P = 0.02) (see Fig. S3 in the supplemental material). Neither IL-2 nor IL-7 was detectable in CSF of the 15 HIV-positive individuals in this study (the assay's lower limit of detection was 11.2 pg/ml for IL-2 and 0.5 pg/ml for IL-7). These results indicate that enhanced PD-1 expression is not caused by γ-chain cytokines in CSF. It is possible, however, that cytokines/chemokines in CNS tissue enhance PD-1 expression levels before T cells enter the CSF.
DISCUSSION
Immune control of viral infections in the CNS requires suppression or elimination of virus while minimizing immune-mediated injury to vital cells. T-cell responses in the CNS must therefore be highly regulated. PD-1 is a negative immunoregulatory factor that has also been implicated in exhaustion of T cells during chronic infection. In the present study, we examine the role of PD-1 expression on CSF T cells during chronic asymptomatic HIV-1 infection. We show that PD-1 expression on T cells is upregulated in CSF compared to that in blood. Data from lymphocytic choriomeningitis virus models as well as previous studies of HIV-positive patients suggest that high levels of PD-1 expression on virus-specific T cells are indicative of T-cell exhaustion due to persistent antigen exposure (2, 7, 10, 35, 46). Our study of PD-1 expression on T cells in CSF and blood provides a unique opportunity to relate HIV-1 replication and immune responses within two distinct compartments in the same individual. We show that PD-1 expression on T cells in CSF is enhanced above that on blood T cells, despite lower HIV-1 RNA concentrations in CSF than in plasma. We also show that compared to blood T cells, CSF T cells downregulate the senescence marker CD57. We also previously reported that T cells have broader and higher magnitudes of HIV-specific IFN-γ responses in CSF than those in blood (40). Furthermore, among healthy, HIV-negative controls, we also found higher PD-1 expression on T cells in CSF than on those in blood. These findings indicate that the higher PD-1 expression on CSF T cells may not necessarily reflect exhaustion of T cells, but rather may be a mechanism to control T-cell activation and prevent immune-mediated damage in the CNS.
The senescence marker CD57 was present at a lower frequency on T cells in CSF than on those in blood among these asymptomatic HIV-positive patients. CD57+ T cells have undergone more cell divisions, have shorter telomeres, have limited ability to proliferate, and are more prone to activation-induced cell death (4). CD8+ CD57+ T cells are antigen-driven effector cells still capable of producing IFN-γ and TNF-α and having very high effector cytotoxic capabilities (26). Smaller amounts of HIV-1 antigens in CSF than in blood may, at least in part, explain the relatively lower frequencies of CD57+ T cells in CSF. CD8+ CD57+ T cells were found to have higher expression levels of CX3CR1 than CD8+ CD57− T cells (26), and CX3CL1, a ligand for CX3CR1, is upregulated in astrocytes of patients with HIV-associated dementia (34, 45). It will be important to determine whether patients with HIV-associated dementia have higher expression levels of CD57 on T cells in CSF and whether these are also CX3CR1+.
In HIV-positive patients, the infection appears to drive higher expression of PD-1 on T cells in CSF. PD-1 expression on HIV-specific CD8+ T cells is higher than that on total CD8+, CD4+, and CMV-specific CD8+ T cells, and PD-1 expression on CD8+ and CD4+ T cells in CSF correlates directly with HIV-1 RNA in CSF and inversely with blood CD4+ T-cell counts. Several studies have shown that PD-1 is upregulated on HIV-specific CD8+ T cells in blood and that PD-1 expression correlates with higher plasma viral loads and lower CD4 counts, predictors of disease progression (7, 35, 46). To our knowledge, this is the first human study to show enhanced PD-1 expression on HIV-specific CD8+ T cells in CSF and to correlate PD-1 expression with HIV-1 RNA in another anatomic compartment. In another chronic viral disease, hepatitis C virus infection, enhanced PD-1 expression on T cells from blood and liver is associated with immune dysfunction, with decreased proliferation and IFN-γ and IL-2 secretion (12, 37). In vitro blockade of PD-1 by anti-PD-L1 and anti-PD-L2 antibodies restores functional competence of these cells. In contrast to intrathecal HIV-specific PD-1hi T cells, intrahepatic hepatitis C virus-specific PD-1hi T cells express high levels of CD57 (12). It is possible that although expansion potential and IFN-γ production were not impaired for CSF T cells (40), there may be defects in secretion of other cytokines, such as TNF-α and/or IL-2. The limited number of lymphocytes obtained from CSF limited our ability to assess the polyfunctionality of HIV-specific CD8+ T cells.
This study has potential implications for understanding immune-mediated neuropathogenesis. Normal uninfected brain has multiple intrinsic mechanisms to maintain immune quiescence. These include tight junctions and low levels of adhesion molecule expression by capillary endothelial cells, neuronal expression of CD200, which reduces microglial cell activation, local production of neurotrophins, and transforming growth factor beta secretion by astrocytes and meningeal cells (14). The PD-1/PD-L1 pathway also plays an important role in controlling inflammation in the CNS. Human and mouse microglial cells from the CNS constitutively express PD-L1, and PD-L1 expression is upregulated on microglia under both in vitro (external cytokine stimulation) and in vivo (experimental autoimmune encephalitis and multiple sclerosis) inflammatory conditions (27, 33, 41). In mice, expression levels of PD-L1 were substantially higher on microglial cells than on splenocytes, upon stimulation (27). Thus, PD-L1 expression helps to minimize inflammatory damage by activated immune cells in the CNS. Our finding that PD-1 levels are higher on T cells in CSF than on those in blood, not only in subjects with HIV-1 infection but also in healthy HIV-negative individuals without inflammation, suggests that upregulation of PD-1 on CSF T cells may be a general mechanism which, in combination with upregulation of PD-L1 during inflammation, limits T-cell-mediated damage in the CNS. The present study focused on antiretroviral-naïve HIV-infected individuals without neurological symptoms. In patients with neuro-AIDS, exhaustion of HIV-specific CD8+ T cells in the CNS under conditions of chronic antigen exposure may lead to eventual loss of viral control in the CNS and to the development of neurocognitive symptoms. Characterization of T cells in HIV-positive individuals with advanced AIDS, particularly those with neuropsychological impairment, will further our understanding of the role of PD-1 expression on CNS T cells in the control of HIV replication and immune-mediated neuropathogenesis.
Higher PD-1 expression on T cells in CSF could be explained by either preferential migration of PD-1hi T cells into the CNS, selective retention of PD-1hi T cells in the CNS, or upregulation of PD-1 after T cells enter the CNS. Recent studies indicated that PD-1 expression may also be enhanced during activation and differentiation of T cells (42). HIV-, Epstein-Barr virus- and influenza virus-specific CD8 T cells are mostly PD-1hi, whereas CMV-specific T cells are less frequently PD-1hi (7, 15, 35). In macaques, PD-1 expression increases following DNA vaccination and during persistent lentiviral infection (18). Earlier induction of PD-1 in simian immunodeficiency virus (SIV)-infected sooty mangabeys, with subsequent reduction in immune activation, distinguishes nonpathogenic infection in sooty mangabeys from pathogenic infection in macaques (9). Thus, not all PD-1hi T cells may be exhausted but may indicate a balanced reaction to immune-activating stimuli. In macaques, activated T cells preferentially enter the CNS and increase in frequency early after acute SIV infection (23, 29). In humans, studies showing activated T cells in CSF among both HIV-infected and HIV-uninfected individuals (29, 31, 39) support homing of activated T cells to the CNS. Other groups have noted selective recruitment of memory T cells to the brain and higher proportions of CD8+ and CD4+ memory T cells in the brains of both HIV-infected and uninfected individuals under both normal and neuropathologic conditions (31, 44, 48). Although enhanced PD-1 expression on CSF T cells could be due to an increase frequency of activated memory T cells in CSF, we found PD-1 expression to be higher in CSF even in analyses restricted to memory T-cell subpopulations.
The common gamma-chain (γc) cytokines IL-2, IL-7, IL-15, and IL-21 play an important role in peripheral T-cell expansion and survival. These cytokines upregulate PD-1, and all but IL-21 upregulate PD-L1 on T cells (24). γc cytokine-induced PD-1 does not interfere with cytokine-driven peripheral T-cell expansion/survival but may suppress certain effector functions upon T-cell receptor engagement, thereby minimizing immune-mediated damage to the host. Various cytokines, including IL-2, IL-12, IL-23, IFN-γ, and TNF-α, are produced in the CNS by different neural cells under pathological as well as physiological conditions (5). SIV infection in rhesus macaques leads to enhanced brain levels of IL-15, which has been implicated in enrichment and persistence of SIV-specific T cells in the CNS (28). In the HIV-positive individuals in the present study, IL-2 and IL-7 were undetectable in CSF, and culturing PBMCs ex vivo in autologous CSF did not enhance PD-1 expression on these cells. It is possible that there are cytokines/chemokines produced in the CNS, but undetectable in CSF, that could upregulate PD-1 expression on T cells in the CNS even under normal conditions.
In animal models of virus infection (lymphocytic choriomeningitis virus-infected mice and SIV-infected macaques), in vivo inhibition of the PD-1/PD-L1 pathway by use of monoclonal antibodies has been shown to enhance expansion, cytokine production, and cytotoxicity of virus-specific T cells and to reduce viral replication (2, 47). Humanized anti-PD-1 antibody has been approved for testing in clinical trials for cancer and infectious diseases. Our study suggests that as PD-1 inhibition is pursued as a therapeutic strategy, consideration should be given to potential effects on PD-1/PD-L1-induced T-cell tolerance in the CNS. In this regard, inhibiting PD-L1 during experimental autoimmune encephalitis or diabetes exacerbates disease (1, 41). Therapeutic options for autoimmune diseases currently being tested in animal models include enhancement of PD-L1 expression, leading to enhanced PD-1/PD-L1 signaling (17, 36).
In summary, among asymptomatic HIV-infected individuals, expression of PD-1 is enhanced on CD8+, CD4+, and HIV-specific CD8+ T cells in CSF. This enhanced PD-1 expression in CSF, however, is not associated with T-cell senescence. Enhanced PD-1 expression in the CNS may be a physiologic mechanism to prevent immune-mediated neuronal damage.
Supplementary Material
Acknowledgments
This study was supported by NIH grants MH071205 (D.W.H.) and AI39966 (S.A.K.), by the Vanderbilt-Meharry Center for AIDS Research (AI54999), by Vanderbilt CTSA grant RR024975, and by a Dana Foundation Neuroimmunology Award. Flow cytometry acquisition and analysis were performed at the NIH-funded Vanderbilt-Meharry CFAR Immunopathogenesis Core Facility (grant P30-AI-54999, to S.A.K.) and the Vanderbilt Medical Center Flow Cytometry Shared Resource, supported by the Vanderbilt Ingram Cancer Center (P30 CA68485) and the Vanderbilt Digestive Disease Research Center (DK058404).
We gratefully acknowledge all the study volunteers.
Footnotes
Published ahead of print on 14 October 2009.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Ansari, M. J., A. D. Salama, T. Chitnis, R. N. Smith, H. Yagita, H. Akiba, T. Yamazaki, M. Azuma, H. Iwai, S. J. Khoury, H. Auchincloss, Jr., and M. H. Sayegh. 2003. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J. Exp. Med. 198:63-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barber, D. L., E. J. Wherry, D. Masopust, B. Zhu, J. P. Allison, A. H. Sharpe, G. J. Freeman, and R. Ahmed. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439:682-687. [DOI] [PubMed] [Google Scholar]
- 3.Barks, J. D., X. H. Liu, R. Sun, and F. S. Silverstein. 1997. gp120, a human immunodeficiency virus-1 coat protein, augments excitotoxic hippocampal injury in perinatal rats. Neuroscience 76:397-409. [DOI] [PubMed] [Google Scholar]
- 4.Brenchley, J. M., N. J. Karandikar, M. R. Betts, D. R. Ambrozak, B. J. Hill, L. E. Crotty, J. P. Casazza, J. Kuruppu, S. A. Migueles, M. Connors, M. Roederer, D. C. Douek, and R. A. Koup. 2003. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood 101:2711-2720. [DOI] [PubMed] [Google Scholar]
- 5.Camacho-Arroyo, I., L. Lopez-Griego, and J. Morales-Montor. 2009. The role of cytokines in the regulation of neurotransmission. Neuroimmunomodulation 16:1-12. [DOI] [PubMed] [Google Scholar]
- 6.Davis, L. E., B. L. Hjelle, V. E. Miller, D. L. Palmer, A. L. Llewellyn, T. L. Merlin, S. A. Young, R. G. Mills, W. Wachsman, and C. A. Wiley. 1992. Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology 42:1736-1739. [DOI] [PubMed] [Google Scholar]
- 7.Day, C. L., D. E. Kaufmann, P. Kiepiela, J. A. Brown, E. S. Moodley, S. Reddy, E. W. Mackey, J. D. Miller, A. J. Leslie, C. DePierres, Z. Mncube, J. Duraiswamy, B. Zhu, Q. Eichbaum, M. Altfeld, E. J. Wherry, H. M. Coovadia, P. J. Goulder, P. Klenerman, R. Ahmed, G. J. Freeman, and B. D. Walker. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443:350-354. [DOI] [PubMed] [Google Scholar]
- 8.D'Souza, M., A. P. Fontenot, D. G. Mack, C. Lozupone, S. Dillon, A. Meditz, C. C. Wilson, E. Connick, and B. E. Palmer. 2007. Programmed death 1 expression on HIV-specific CD4+ T cells is driven by viral replication and associated with T cell dysfunction. J. Immunol. 179:1979-1987. [DOI] [PubMed] [Google Scholar]
- 9.Estes, J. D., S. N. Gordon, M. Zeng, A. M. Chahroudi, R. M. Dunham, S. I. Staprans, C. S. Reilly, G. Silvestri, and A. T. Haase. 2008. Early resolution of acute immune activation and induction of PD-1 in SIV-infected sooty mangabeys distinguishes nonpathogenic from pathogenic infection in rhesus macaques. J. Immunol. 180:6798-6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freeman, G. J., E. J. Wherry, R. Ahmed, and A. H. Sharpe. 2006. Reinvigorating exhausted HIV-specific T cells via PD-1-PD-1 ligand blockade. J. Exp. Med. 203:2223-2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gartner, S. 2000. HIV infection and dementia. Science 287:602-604. [DOI] [PubMed] [Google Scholar]
- 12.Golden-Mason, L., B. Palmer, J. Klarquist, J. A. Mengshol, N. Castelblanco, and H. R. Rosen. 2007. Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J. Virol. 81:9249-9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goudsmit, J., F. de Wolf, D. A. Paul, L. G. Epstein, J. M. Lange, W. J. Krone, H. Speelman, E. C. Wolters, J. Van der Noordaa, J. M. Oleske, et al. 1986. Expression of human immunodeficiency virus antigen (HIV-Ag) in serum and cerebrospinal fluid during acute and chronic infection. Lancet ii:177-180. [DOI] [PubMed] [Google Scholar]
- 14.Griffin, D. E. 2003. Immune responses to RNA-virus infections of the CNS. Nat. Rev. Immunol. 3:493-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He, X. H., Q. T. Jia, F. Y. Li, M. Saltis, Y. Liu, L. H. Xu, and Q. B. Zha. 2008. CD8(+) T cells specific for both persistent and non-persistent viruses display distinct differentiation phenotypes but have similar level of PD-1 expression in healthy Chinese individuals. Clin. Immunol. 126:222-234. [DOI] [PubMed] [Google Scholar]
- 16.Hesselgesser, J., D. Taub, P. Baskar, M. Greenberg, J. Hoxie, D. L. Kolson, and R. Horuk. 1998. Neuronal apoptosis induced by HIV-1 gp120 and the chemokine SDF-1 alpha is mediated by the chemokine receptor CXCR4. Curr. Biol. 8:595-598. [DOI] [PubMed] [Google Scholar]
- 17.Hirata, S., S. Senju, H. Matsuyoshi, D. Fukuma, Y. Uemura, and Y. Nishimura. 2005. Prevention of experimental autoimmune encephalomyelitis by transfer of embryonic stem cell-derived dendritic cells expressing myelin oligodendrocyte glycoprotein peptide along with TRAIL or programmed death-1 ligand. J. Immunol. 174:1888-1897. [DOI] [PubMed] [Google Scholar]
- 18.Hokey, D. A., F. B. Johnson, J. Smith, J. L. Weber, J. Yan, L. Hirao, J. D. Boyer, M. G. Lewis, G. Makedonas, M. R. Betts, and D. B. Weiner. 2008. Activation drives PD-1 expression during vaccine-specific proliferation and following lentiviral infection in macaques. Eur. J. Immunol. 38:1435-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jassoy, C., R. P. Johnson, B. A. Navia, J. Worth, and B. D. Walker. 1992. Detection of a vigorous HIV-1-specific cytotoxic T lymphocyte response in cerebrospinal fluid from infected persons with AIDS dementia complex. J. Immunol. 149:3113-3119. [PubMed] [Google Scholar]
- 20.Johansson, M., L. Arlestig, B. Moller, and S. Rantapaa-Dahlqvist. 2005. Association of a PDCD1 polymorphism with renal manifestations in systemic lupus erythematosus. Arthritis Rheum. 52:1665-1669. [DOI] [PubMed] [Google Scholar]
- 21.Keir, M. E., M. J. Butte, G. J. Freeman, and A. H. Sharpe. 2008. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 26:677-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keir, M. E., L. M. Francisco, and A. H. Sharpe. 2007. PD-1 and its ligands in T-cell immunity. Curr. Opin. Immunol. 19:309-314. [DOI] [PubMed] [Google Scholar]
- 23.Kim, W. K., S. Corey, G. Chesney, H. Knight, S. Klumpp, C. Wuthrich, N. Letvin, I. Koralnik, A. Lackner, R. Veasey, and K. Williams. 2004. Identification of T lymphocytes in simian immunodeficiency virus encephalitis: distribution of CD8+ T cells in association with central nervous system vessels and virus. J. Neurovirol. 10:315-325. [DOI] [PubMed] [Google Scholar]
- 24.Kinter, A. L., E. J. Godbout, J. P. McNally, I. Sereti, G. A. Roby, M. A. O'Shea, and A. S. Fauci. 2008. The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. J. Immunol. 181:6738-6746. [DOI] [PubMed] [Google Scholar]
- 25.Kroner, A., M. Mehling, B. Hemmer, P. Rieckmann, K. V. Toyka, M. Maurer, and H. Wiendl. 2005. A PD-1 polymorphism is associated with disease progression in multiple sclerosis. Ann. Neurol. 58:50-57. [DOI] [PubMed] [Google Scholar]
- 26.Le Priol, Y., D. Puthier, C. Lecureuil, C. Combadiere, P. Debre, C. Nguyen, and B. Combadiere. 2006. High cytotoxic and specific migratory potencies of senescent CD8+ CD57+ cells in HIV-infected and uninfected individuals. J. Immunol. 177:5145-5154. [DOI] [PubMed] [Google Scholar]
- 27.Magnus, T., B. Schreiner, T. Korn, C. Jack, H. Guo, J. Antel, I. Ifergan, L. Chen, F. Bischof, A. Bar-Or, and H. Wiendl. 2005. Microglial expression of the B7 family member B7 homolog 1 confers strong immune inhibition: implications for immune responses and autoimmunity in the CNS. J. Neurosci. 25:2537-2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marcondes, M. C., T. H. Burdo, S. Sopper, S. Huitron-Resendiz, C. Lanigan, D. Watry, C. Flynn, M. Zandonatti, and H. S. Fox. 2007. Enrichment and persistence of virus-specific CTL in the brain of simian immunodeficiency virus-infected monkeys is associated with a unique cytokine environment. J. Immunol. 178:5812-5819. [DOI] [PubMed] [Google Scholar]
- 29.Marcondes, M. C., E. M. Burudi, S. Huitron-Resendiz, M. Sanchez-Alavez, D. Watry, M. Zandonatti, S. J. Henriksen, and H. S. Fox. 2001. Highly activated CD8(+) T cells in the brain correlate with early central nervous system dysfunction in simian immunodeficiency virus infection. J. Immunol. 167:5429-5438. [DOI] [PubMed] [Google Scholar]
- 30.Nath, A., K. Psooy, C. Martin, B. Knudsen, D. S. Magnuson, N. Haughey, and J. D. Geiger. 1996. Identification of a human immunodeficiency virus type 1 Tat epitope that is neuroexcitatory and neurotoxic. J. Virol. 70:1475-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neuenburg, J. K., T. A. Cho, A. Nilsson, B. M. Bredt, S. J. Hebert, R. M. Grant, and R. W. Price. 2005. T-cell activation and memory phenotypes in cerebrospinal fluid during HIV infection. J. Acquir. Immune Defic. Syndr. 39:16-22. [DOI] [PubMed] [Google Scholar]
- 32.Nishimura, H., M. Nose, H. Hiai, N. Minato, and T. Honjo. 1999. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 11:141-151. [DOI] [PubMed] [Google Scholar]
- 33.Ortler, S., C. Leder, M. Mittelbronn, A. L. Zozulya, P. A. Knolle, L. Chen, A. Kroner, and H. Wiendl. 2008. B7-H1 restricts neuroantigen-specific T cell responses and confines inflammatory CNS damage: implications for the lesion pathogenesis of multiple sclerosis. Eur. J. Immunol. 38:1734-1744. [DOI] [PubMed] [Google Scholar]
- 34.Pereira, C. F., J. Middel, G. Jansen, J. Verhoef, and H. S. Nottet. 2001. Enhanced expression of fractalkine in HIV-1 associated dementia. J. Neuroimmunol. 115:168-175. [DOI] [PubMed] [Google Scholar]
- 35.Petrovas, C., J. P. Casazza, J. M. Brenchley, D. A. Price, E. Gostick, W. C. Adams, M. L. Precopio, T. Schacker, M. Roederer, D. C. Douek, and R. A. Koup. 2006. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J. Exp. Med. 203:2281-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Probst, H. C., K. McCoy, T. Okazaki, T. Honjo, and M. van den Broek. 2005. Resting dendritic cells induce peripheral CD8+ T cell tolerance through PD-1 and CTLA-4. Nat. Immunol. 6:280-286. [DOI] [PubMed] [Google Scholar]
- 37.Radziewicz, H., C. C. Ibegbu, M. L. Fernandez, K. A. Workowski, K. Obideen, M. Wehbi, H. L. Hanson, J. P. Steinberg, D. Masopust, E. J. Wherry, J. D. Altman, B. T. Rouse, G. J. Freeman, R. Ahmed, and A. Grakoui. 2007. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J. Virol. 81:2545-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Resnick, L., J. R. Berger, P. Shapshak, and W. W. Tourtellotte. 1988. Early penetration of the blood-brain-barrier by HIV. Neurology 38:9-14. [DOI] [PubMed] [Google Scholar]
- 39.Roberts, E. S., S. Huitron-Resendiz, M. A. Taffe, M. C. Marcondes, C. T. Flynn, C. M. Lanigan, J. A. Hammond, S. R. Head, S. J. Henriksen, and H. S. Fox. 2006. Host response and dysfunction in the CNS during chronic simian immunodeficiency virus infection. J. Neurosci. 26:4577-4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sadagopal, S., S. L. Lorey, L. Barnett, R. Basham, L. Lebo, H. Erdem, K. Haman, M. Avison, K. Waddell, D. W. Haas, and S. A. Kalams. 2008. Enhancement of human immunodeficiency virus (HIV)-specific CD8+ T cells in cerebrospinal fluid compared to those in blood among antiretroviral therapy-naive HIV-positive subjects. J. Virol. 82:10418-10428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salama, A. D., T. Chitnis, J. Imitola, M. J. Ansari, H. Akiba, F. Tushima, M. Azuma, H. Yagita, M. H. Sayegh, and S. J. Khoury. 2003. Critical role of the programmed death-1 (PD-1) pathway in regulation of experimental autoimmune encephalomyelitis. J. Exp. Med. 198:71-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sauce, D., J. R. Almeida, M. Larsen, L. Haro, B. Autran, G. J. Freeman, and V. Appay. 2007. PD-1 expression on human CD8 T cells depends on both state of differentiation and activation status. AIDS 21:2005-2013. [DOI] [PubMed] [Google Scholar]
- 43.Sharpe, A. H., E. J. Wherry, R. Ahmed, and G. J. Freeman. 2007. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat. Immunol. 8:239-245. [DOI] [PubMed] [Google Scholar]
- 44.Svenningsson, A., G. K. Hansson, O. Andersen, R. Andersson, M. Patarroyo, and S. Stemme. 1993. Adhesion molecule expression on cerebrospinal fluid T lymphocytes: evidence for common recruitment mechanisms in multiple sclerosis, aseptic meningitis, and normal controls. Ann. Neurol. 34:155-161. [DOI] [PubMed] [Google Scholar]
- 45.Tong, N., S. W. Perry, Q. Zhang, H. J. James, H. Guo, A. Brooks, H. Bal, S. A. Kinnear, S. Fine, L. G. Epstein, D. Dairaghi, T. J. Schall, H. E. Gendelman, S. Dewhurst, L. R. Sharer, and H. A. Gelbard. 2000. Neuronal fractalkine expression in HIV-1 encephalitis: roles for macrophage recruitment and neuroprotection in the central nervous system. J. Immunol. 164:1333-1339. [DOI] [PubMed] [Google Scholar]
- 46.Trautmann, L., L. Janbazian, N. Chomont, E. A. Said, S. Gimmig, B. Bessette, M. R. Boulassel, E. Delwart, H. Sepulveda, R. S. Balderas, J. P. Routy, E. K. Haddad, and R. P. Sekaly. 2006. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 12:1198-1202. [DOI] [PubMed] [Google Scholar]
- 47.Velu, V., K. Titanji, B. Zhu, S. Husain, A. Pladevega, L. Lai, T. H. Vanderford, L. Chennareddi, G. Silvestri, G. J. Freeman, R. Ahmed, and R. R. Amara. 2009. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature 458:206-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.von Geldern, G., S. Cepok, T. Nolting, Y. Du, V. Grummel, O. Adams, H. P. Hartung, G. Arendt, and B. Hemmer. 2007. CD8 T-cell subsets and viral load in the cerebrospinal fluid of therapy-naive HIV-infected individuals. AIDS 21:250-253. [DOI] [PubMed] [Google Scholar]
- 49.Williams, K., S. Westmoreland, J. Greco, E. Ratai, M. Lentz, W. K. Kim, R. A. Fuller, J. P. Kim, P. Autissier, P. K. Sehgal, R. F. Schinazi, N. Bischofberger, M. Piatak, J. D. Lifson, E. Masliah, and R. G. Gonzalez. 2005. Magnetic resonance spectroscopy reveals that activated monocytes contribute to neuronal injury in SIV neuroAIDS. J. Clin. Investig. 115:2534-2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Williams, K. C., and W. F. Hickey. 2002. Central nervous system damage, monocytes and macrophages, and neurological disorders in AIDS. Annu. Rev. Neurosci. 25:537-562. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.