Abstract
Muscle atrophy is a debilitating process associated with many chronic wasting diseases, like cancer, diabetes, sepsis, and renal failure. Rapid loss of muscle mass occurs mainly through the activation of protein breakdown by the ubiquitin proteasome pathway. Foxo3a transcription factor is critical for muscle atrophy, since it activates the expression of ubiquitin ligase Atrogin-1. In several models of atrophy, inhibition of the phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway induces nuclear import of Foxo3a through an Akt-dependent process. This study aimed to identify signaling pathways involved in the control of Foxo3a nuclear translocation in muscle cells. We observed that after nuclear import of Foxo3a by PI3K/Akt pathway inhibition, activation of stress-activated protein kinase (SAPK) pathways induced nuclear export of Foxo3a through CRM1. This mechanism involved the c-Jun NH2-terminal kinase (JNK) signaling pathway and was independent of Akt. Likewise, we showed that inhibition of p38 induced a massive nuclear relocalization of Foxo3a. Our results thus suggest that SAPKs are involved in the control of Foxo3a nucleocytoplasmic translocation in C2C12 cells. Moreover, activation of SAPKs decreases the expression of Atrogin-1, and stable C2C12 myotubes, in which the p38 pathway is constitutively activated, present partial protection against atrophy.
Muscle atrophy occurs in many pathological states, including cancer, diabetes mellitus, AIDS, and sepsis. It results from a concomitant increase in protein catabolism and decrease in protein synthesis, and this combination induces a massive loss of muscle mass and function. Major insights toward understanding cellular events implicated in the atrophy process have been achieved in recent years. The ubiquitin proteasome pathway (UPS) is considered to play a dominant role (13, 27, 40). A component of the UPS, ubiquitin ligase Atrogin-1, is specifically and dramatically induced during atrophy, and knockout animals lacking Atrogin-1 show a reduced rate of muscle atrophy after denervation (3). In 2004, Sandri et al. demonstrated that, under atrophying conditions, phosphatidylinositol 3-kinase (PI3K)/Akt activity decreases, leading to nuclear translocation of Foxo3a transcription factor and induction of Atrogin-1 (44). When Foxo3a activation is blocked, Atrogin-1 induction during starvation and atrophy of myotubes induced by glucocorticoids are prevented. Therefore, it is now widely accepted that Foxo3a has a critical role in the development of atrophy.
Mammalian Foxo transcription factors are characterized by a DNA binding domain termed the “Forkhead box.” This family is composed of 4 members: Foxo1 (FKHR), Foxo3a (FKHRL1), Foxo4 (AFX), and Foxo6. Foxo factors have a wide range of cellular functions, including regulation of the cell cycle, apoptosis, atrophy, DNA repair, energy metabolism, and defense against oxidative stress (43, 48). They also promote tumor suppression and extend the life span in invertebrates (1, 5, 12). Foxos are regulated by a variety of external stimuli, such as insulin, insulin-like growth factor (IGF-1), nutrients, cytokines, and oxidative stress. Their activity is tightly controlled by signaling pathways through posttranslational modifications, namely, phosphorylation, acetylation, ubiquitination, and protein interactions (48). In particular, Foxo transcription factors are important downstream targets of the PI3K/Akt signaling pathway. Phosphorylation by PI3K/Akt controls a shuttling system that modulates Foxo cellular localization and, thus, its activity (5).
In skeletal muscle, Foxos contribute to several cellular processes, such as myocyte fusion and metabolism regulation (4, 22). Foxo3a is notably involved in both atrophy and autophagy (34, 51), and expression of a constitutively active Foxo3a induces atrophy of muscle cells through activation of Atrogin-1 (44). Upon growth factor or insulin stimulation, PI3K activation induces Akt-mediated phosphorylation of Foxos to promote the association of Foxos with 14-3-3 chaperone proteins. This sequestration of Foxo proteins in the cytoplasm prevents Foxo-dependent gene regulation. Under catabolic conditions, inhibition of PI3K/Akt allows dephosphorylation and nuclear translocation of Foxo3a, which promotes the expression of Atrogin-1 (44).
The mitogen-activated protein kinase (MAPK) family includes stress-activated protein kinases (SAPKs) p38 and c-Jun NH2-terminal kinase (JNK), which mediate a wide variety of cellular processes in response to extracellular stimuli, such as UV radiation, tumor necrosis factor alpha (TNF-α), and oxidative stress (18, 24, 42, 46). Once SAPKs are activated, they phosphorylate target molecules in the cytoplasm and nucleus, resulting in regulation of gene expression. Recently, it has been shown that JNK antagonizes the PI3K/Akt pathway and promotes nuclear translocation of dFoxo-DAF16 to regulate life span in invertebrates (49). In mammals, JNK-dependent phosphorylation is involved in the nuclear translocation and transcriptional activation of Foxo4 after H2O2 treatment (20). In addition, 15 consensus phosphorylation sites for MAPKs have been identified on the Foxo1 sequence, and it has been shown that Foxo1 could be phosphorylated in vitro by ERK and p38 (2).
Progressive loss of skeletal muscle mass is observed during aging. We previously showed that this decline in muscle mass is accompanied by an increase in Atrogin-1 mRNA and oxidative stress (16). Moreover, oxidative stress has been linked to skeletal muscle atrophy in numerous models of muscle wasting, and it also activates Foxo3a in various cell types (7, 11, 20, 38). Although progress in our understanding of the role of SAPK in the regulation of Foxos has been made, the role of these signaling pathways in muscle cells remains unclear. As Foxo3a is a major regulator of muscle atrophy process, the aim of this study was to determine the role of SAPK on Foxo3a regulation in muscle cells. To this end, we first studied the effect of SAPK on Foxo3a cellular localization. We then characterized these signaling pathways by using both molecular and pharmaceutical inhibitors, and this was followed by an evaluation of the effects of SAPK on Foxo3a transcriptional activity and on muscle atrophy.
Our findings indicate that in muscle cells, SAPKs are involved in a Foxo3a nuclear export mechanism. SAPK nuclear export of Foxo3a induces a decrease of Atrogin-1 expression and thereby reduces muscle atrophy.
MATERIALS AND METHODS
Vectors and constructions.
Human Foxo3a was expressed either from the peceFoxo3a-HA wild-type (WT) plasmid producing a hemagglutinin (HA)-tagged form of Foxo3a (hFoxo3a-HA) (5) or from the Foxo3a-green fluorescent protein (GFP) fusion vector (14). A Flag-tagged version of hFoxo3a was also constructed by PCR using a Flag 5′ primer (5′-AGAGAAGCTTGCCGCCACCATGGACTATAAGGACGATGATGACAAAGCAGAGGCACCGGCTTCCCC-3′), a 3′ primer (5′-GGCCACGTACAGGATCGCGG-3′), and the peceFoxo3a-HA backbone.
Vectors expressing different dominant-negative (DN) HA-tagged Akt mutants, KA and ST (8) as well as KM (9), were described previously.
The pGL3-Atrogin-1-Luciferase vector was constructed according to the description of Sandri et al. (44). The Foxo-responsive element (FHRE)-luciferase reporter containing multiple copies of the FHREs was described previously (5). A pCMV-β-galactosidase plasmid was used to normalize luciferase assays (10).
Retroviral vectors (pPrig) expressing a Flag-tagged constitutively active version of human MKK3 (CA MKK3) or MKK4 (CA MKK4) (19) were constructed based on the pPrig vector backbone (35). Expression of CA MKK3 or CA MKK4 proteins was indirectly monitored through GFP expression controlled by an internal ribosome entry site (IRES) sequence included in the pPrig vector.
Wild-type and DN p38 forms used for p38/Foxo3a coimmunoprecipitation experiments (not shown) were expressed from pCDNA3 vectors derived from pCMV5-p38 WT and DN vectors (19).
pCOMBI-Myogenin-CA MKK3 was derived from pCOMBI-CMV (kindly provided by U. Certa, Hoffman la Roche, Basel, Switzerland) (45) in which the cytomegalovirus (CMV) promoter was replaced by the myogenin promoter and constitutively active MKK3 (19) was cloned in the polylinker.
Cell culture, transfection, and transduction.
Murine C2C12 muscle cells (ECACC, reference number 91031101) were grown in a humid atmosphere at 37°C with 5% CO2 in proliferation media (Dulbecco's modified Eagle's medium [DMEM], 4.5 g/liter glucose, 10% fetal bovine serum, 100 U/ml penicillin, 100 g/ml streptomycin). Myoblast differentiation was initiated by replacing the growth medium with differentiation medium (DMEM, 4.5 g/liter glucose, 2% horse serum, 100 U/ml penicillin, 100 g/ml streptomycin). Myotube starvation was induced by Hanks balanced salt solution (HBSS; Invitrogen) incubation. C2C12 cells were transfected with cationic polymer (Lipofectamine 2000; Invitrogen) according to the manufacturer's instructions.
Stable cell lines were obtained by transfecting pCOMBI-Myogenin-CA MKK3 into C2C12 cells. Three independent clones were tested for constitutively active MKK3 expression.
The pPrig-CA-MKK3 and -MKK4 retroviral constructs were transduced in C2C12 cells. To generate retroviruses, 293T cells were transfected with 2.5 μg of an empty pPrig-GFP plasmid or the pPrig-CA-MKK3 and -MKK4 constructs and with 1.25 μg of pCMV-VSVG and 1.25 μg of pCMV-gag-pol plasmids by using the classic calcium phosphate transfection technique. Six hours posttransfection, cells were washed and fresh medium was added. Replication-defective retroviruses were recovered in the culture medium between 24 h and 72 h posttransfection, filtered through sterile 0.45-μm filters, and added directly to C2C12 cells in the presence of 4 μg/ml Polybrene to enhance retroviral transduction efficiency. Mixed populations of control (empty vector) or pPrig-CA-MKK3/MKK4-expressing C2C12 cells were obtained via independent integrations of the retroviruses into the host C2C12 cell genome. GFP-positive transduced cells were observed as early as 24 h posttransduction. Over 95% of the cells were efficiently transduced.
Treatments.
Anisomycin (Tocris, United Kingdom) was used at 10 μg/ml except for when used in luciferase assays and Atrogin-1 mRNA measurements, for which it was used at 50 and 25 ng/ml, respectively, to avoid protein synthesis inhibition. Hydrogen peroxide (H2O2) (Sigma-Aldrich Corporation, MO) was used at 250 μM. Inhibitors were added to culture media 30 min before the start of treatment except for the nuclear export inhibitor, leptomycin B (LC Laboratories, MA), for which pretreatment time was increased (2 h). SC-514 (Calbiochem), an inhibitor of IκB kinase-2 (IKK-2) kinase, was used at 100 μM. SP600125 (Tocris, United Kingdom) was used at 20 μM and 60 μM and LY294002 at 20 μM (Tocris, United Kingdom). MG132 proteasome inhibitor (10 μM) came from Sigma-Aldrich Corporation, MO.
Western blotting.
C2C12 cells were rinsed with phosphate-buffered saline (PBS) and harvested in ice-cold lysis buffer (20 mM Tris, pH 7.5, 150 mM NaCl, 2 mM EDTA, pH 7.5, 25 mM β-glycerophosphate, 1 mM sodium orthovanadate, 2 mM Na-PPi, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride [PMSF]) containing 10 μg/ml of protease inhibitor cocktail (Sigma-Aldrich Corporation, MO). Homogenates were centrifuged at 13,000 × g for 1 h at 4°C, supernatants were saved, and protein concentrations were determined by Lowry's method, with bovine serum albumin (BSA) as a reference (DC protein assay; Bio-Rad, CA).
Equal amounts of protein (20 μg) were run on a 12% SDS-PAGE gel and transferred to a polyvinylidene difluoride (PVDF) membrane (Immobilon-P; Millipore, MA). Membranes were blocked in Tris-buffered saline (TBS)-0.1% Tween 20 containing 5% dry milk and incubated with primary antibody, with the exception of the Cell Signaling antibodies, for which the manufacturer's protocol was strictly followed. The following primary antibodies (obtained from the indicated sources) were used: anti-phospho-Akt (Ser473), anti-total Akt, anti-phospho-JNK, and anti-total JNK (Cell Signaling, United Kingdom); anti-phospho-p38 MAPK and anti-total p38 MAPK (Sigma-Aldrich Corporation, MO); anti-phospho-Foxo3a (Thr32) and anti-total Foxo3a (Millipore, MA); antimyogenin and antitubulin (Developmental Studies Hybridoma Bank-NICHD, University of Iowa, IA); and anti-Flag (Euromedex). Blots were incubated with the appropriate horseradish peroxidase (HRP)-conjugated secondary antibody for 1 h at room temperature. Immunocomplexes were visualized using the Western Lightning Plus chemiluminescence reagent (Millipore, MA) and exposed to a digital camera (LAS-3000 charge-coupled-device [CCD] camera system; Fuji).
Luciferase assays.
Two days after transfection, cells were lysed in 200 μl lysis buffer (100 mM potassium phosphate, pH 7.8, 0.2% Triton X-100, 0.5 mM dithiothreitol [DTT]). Cell lysates were collected and centrifuged at 4°C for 5 min at 12,000 × g. Luciferase activities were measured from supernatants in a luminometer (Centro LB 960; Berthold) by using 25 μl cell extract added extemporaneously to 90 μl of luciferase buffer (25 mM glycylglycine, pH 7.8, 15 mM potassium phosphate, 15 mM MgSO4, 4 mM EGTA, 1 mM DTT, 2 mM ATP) and 50 μl of a 200 μM solution of luciferin. Meanwhile, β-galactosidase activity was determined using Galacto-Light reagent (Applied Biosystems). Measurements were done in triplicate for each sample.
Indirect immunofluorescence.
C2C12 myoblasts were seeded on 6-well plates containing glass coverslips and transfected. The plates were rinsed with cold PBS 48 h posttransfection. The cells were then fixed with a PBS solution containing 3.7% paraformaldehyde (10 min at room temperature [RT]) and permeabilized with a cold PBS-0.2% Triton X-100 solution for 5 min. Saturation was achieved after 30 min at RT in PBS containing 2% of bovine serum albumin. The coverslips were then incubated for 1 h in a saturation solution containing the primary antibody (1/500) directed against the HA epitope (HA11 clone 16B12; Covance, NJ). After 3 washes, the coverslips were incubated for 1 h in the dark with a 1/100-diluted mouse fluorescein isothiocyanate (FITC) antibody (FO261; Dako) in the saturation solution and then mounted on a glass slide with Mowiol solution containing Hoechst stain (0.5 μg/ml). Glass slides were observed with a fluorescence microscope coupled to a CCD camera (Zeiss Axioplan 2).
Myotube diameter analysis.
Myotubes were photographed using a digital camera coupled to a microscope (Axio Scope; Zeiss). The diameters of the 10 biggest myotubes were measured for 10 independent fields (ImageJ software, NIH). Myotube diameters were reported as averages from three independent measurements per myotube.
Quantitative real-time reverse transcription-PCR.
RNA from C2C12 cells was extracted and subjected to DNase digestion by using a Nucleo-Spin RNA II kit (Macherey-Nagel). First-strand cDNA synthesis was performed with random primers by use of Superscript II reverse transcriptase (Invitrogen). Real-time PCR was carried out on a LightCycler (Roche Applied Science) for 45 cycles with an annealing temperature of 65°C. The following primers were used: 5′-GCAGAGAGTCGGCAAGTC-3′ and 5′-CAGGTCGGTGATCGTGAG-3′ (142 bp) for Atrogin-1 and 5′-TGCTGGTGCTGAGTATGTCG-3′ and 5′-CAAGCAGTTGGTGGTACAGG-3′ (177 bp) for 18S rRNA. Relative mRNA abundance was calculated using a standard curve method, and Atrogin-1 mRNA levels were normalized against 18S rRNA gene expression (Relquant 1.01; Roche Diagnostics).
Statistical analysis.
All experiments were performed 3 times, and results are presented as means ± standard errors of the means (SEM). Data were analyzed by Student's t test (Statview). Differences between treatments were considered significant when P was <0.05.
RESULTS
Oxidative stress promotes a delayed nuclear translocation of Foxo3a independent of Akt in C2C12 cells.
To determine the effects of oxidative stress on Foxo3a localization, we transfected C2C12 cells with a plasmid expressing a human wild-type Foxo3a protein (hFoxo3a) (Fig. 1a, upper panel) (5). Two days after transfection, cells were treated with 250 μM hydrogen peroxide (H2O2) (Fig. 1a, lower panel). After 3 h of treatment, unlike other cell types (7), Foxo3a remained mainly in the cytoplasm of C2C12 cells, and only a negligible percentage of cells displayed Foxo3a in the nucleus (less than 5%). However, after 6 h of H2O2 treatment, Foxo3a was detected in the nucleus in more than 60% of cells. Since the Akt signaling pathway can induce nuclear translocation of Foxo3a, we determined the activity of Akt and phosphorylation of Foxo3a by Akt (Thr32) (Fig. 1b). Western blot analysis showed a peak in Akt phosphorylation after 15 min of treatment. This effect was transitory, and phosphorylation returned to the basal level after 1 h. At 6 h of treatment, the level of Akt phosphorylation was slightly higher than that in the control. The level of Foxo3a phosphorylation by H2O2 peaked at 15 min, decreased after 1 h, and was enhanced at 3 h and 6 h, twice as high as that of untreated cells (Fig. 1b). In the canonical Akt-Foxo regulation pathway, basal Akt activity maintains Foxo3a in a phosphorylated state that favors cytoplasmic retention. A decrease in Akt activity reduces Foxo3a phosphorylation (Thr32) and promotes translocation of Foxo3a to the nucleus and subsequent transcriptional activation of its target genes. In our experiments, despite relatively weak levels of Akt and Foxo3a phosphorylation after 1 h of H2O2 treatment, Foxo3a remained in the cytoplasm. However, after 6 h of treatment, although phosphorylation of Foxo3a was elevated, Foxo3a was localized mostly in the nucleus, suggesting that nuclear relocalization of Foxo3a by H2O2 is independent of the classical PI3K/Akt signaling pathway.
FIG. 1.
Oxidative stress promotes delayed nuclear translocation of Foxo3a independent of Akt in C2C12 cells. (a) C2C12 cells were transiently transfected with a peceFoxo3a-WT plasmid expressing the human HA-tagged form of Foxo3a (hFoxo3a-HA). The subcellular localization of hFoxo3a-HA was monitored by immunofluorescence microscopy using an anti-HA antibody. Nuclei were stained with Hoechst stain (upper panel). Forty-eight hours after transfection, an H2O2 (250 μM) time course experiment was performed and localization of hFoxo3a was determined for each time point. On the graph (lower panel), the percentage of cells corresponds to the number of cells distributed among the three types of hFoxo3a-HA cellular localization (150 to 500 counted cells). (b) Akt (Ser473), Foxo3a (Thr32), JNK (Thr183-Tyr185), and p38 phosphorylations in response to H2O2 (250 μM) were assessed by Western blotting using 20 μg of protein loaded on an SDS-PAGE gel. For all panels, results shown are representative of 3 independent experiments.
Previously, it had been shown that oxidative stress can trigger Foxo4 or dFoxo relocalization within the nucleus by a JNK-dependent pathway (20) and that p38 phosphorylates Foxo1 both in vivo and in vitro (2). Therefore, we monitored JNK and p38 activities in C2C12 cells incubated in the presence of H2O2 (Fig. 1b). Whereas H2O2 induced a linear time-dependent increase in p38 phosphorylation, it induced a fast and biphasic (at least 15-min and 1-h) increase in JNK phosphorylation. However, after 6 h of treatment, the level of JNK phosphorylation was lower than the phosphorylation level in the untreated cells. This dropoff in JNK phosphorylation coincided with Foxo3a nuclear translocation, which led us to hypothesize that JNK could be involved in the regulation of nucleocytoplasmic Foxo3a localization.
JNK activation induces nuclear export of Foxo3a in C2C12 cells.
To evaluate the role of JNK signaling on the Foxo3a nucleocytoplasmic transport, C2C12 cells transfected with hFoxo3a were incubated with or without anisomycin, a potent activator of the JNK pathway (25). Anisomycin treatment (10 μg/ml) did not significantly modify the cytoplasmic localization of Foxo3a (Fig. 2a). However, when Foxo3a translocated to the nucleus by inhibition of the Akt pathway with LY294002 (20 μM) or starvation (HBSS), anisomycin abrogated this effect and Foxo3a relocalized to the cytoplasm. Indeed, in response to LY294002 treatment for 1 h, Foxo3a was found in the nucleus of the vast majority of cells (80%), whereas in the presence of anisomycin, less than 10% of the cells displayed a nuclear localization of Foxo3a (Fig. 2a). This effect was not due to phosphorylation of Foxo3a by Akt, since the level of phosphorylation (Thr32) remained constant with and without anisomycin (Fig. 2b). Similarly, by using an SGK inhibitor (H89), we showed that Foxo3a cytoplasmic relocalization induced by anisomycin was independent of SGK (6, 17, 31).
FIG. 2.
JNK activation induces CRM1-dependent nuclear export of Foxo3a in C2C12 cells. (a) Foxo3a localization was monitored by immunofluorescence experiments on C2C12 cells transiently expressing hFoxo3a-HA. On the graph, the percentage of cells corresponds to the number of cells distributed among the three types of hFoxo3a-HA cellular localization (150 to 500 counted cells). Inhibitors SP600125 (60 μM), H89 (10 μM), and SC-514 (100 μM) were added to cells 30 min before the start of treatment (1 h) with LY294002 (20 μM) and LY294002 plus anisomycin (10 μg/ml). SP600125, H89, and SC-514 are inhibitors of JNK, SGK, and IKK, respectively. Inhibition of CRM1-dependent nuclear export was achieved by 1 h of leptomycin B treatment (5 ng/ml). Anisomycin (10 μg/ml) and HBSS treatments were carried out for 1 h. (b) Twenty micrograms of total proteins was separated by SDS-PAGE. The proteins were detected by Western blotting with anti-phospho-Akt (Ser473), anti-phospho-JNK (Thr183-Tyr185), anti-phospho-Foxo3a (Thr32), and antibodies against total proteins. For all panels, results shown are representative of 3 independent experiments.
To estimate the involvement of the JNK signaling pathway, we treated C2C12 cells with LY294002, anisomycin, and SP600125 (60 μM), an inhibitor of JNK (Fig. 2a). The JNK inhibitor suppressed the effect of the anisomycin and Foxo3a was mainly detected within the nucleus (55%). This finding raises the question as to whether activation of the JNK pathway induces nuclear exclusion or cytoplasmic sequestration of Foxo3a. In order to address this issue, we used leptomycin B, a specific inhibitor of nuclear export factor CRM1 (30). Upon incubation of C2C12 cells with LY294002 and leptomycin B (5 ng/ml), Foxo3a was observed in the nucleus of 100% of the cells, and anisomycin treatment was no longer able to counteract the effect of the Akt inhibitor (Fig. 2a). This observation clearly indicates that anisomycin induces nuclear export of Foxo3a through CRM1 rather than cytoplasmic retention.
Previous studies showed that IKK phosphorylates Foxo3a and induces its nuclear exclusion independently of Akt (26). Since anisomycin could under some conditions activate the NF-κB pathway, we treated cells with SC-514 (100 μM), an inhibitor of IKK (29). As shown in Fig. 2a, the effect of SC-514 was limited, excluding a potential role of IKK on the nuclear export of Foxo3a induced by anisomycin. Therefore, it appears that in C2C12 cells, activation of the JNK pathway by anisomycin promotes nuclear export of Foxo3a via CRM1.
Nucleocytoplasmic translocation of Foxo3a by the JNK pathway is independent of Akt in C2C12 cells.
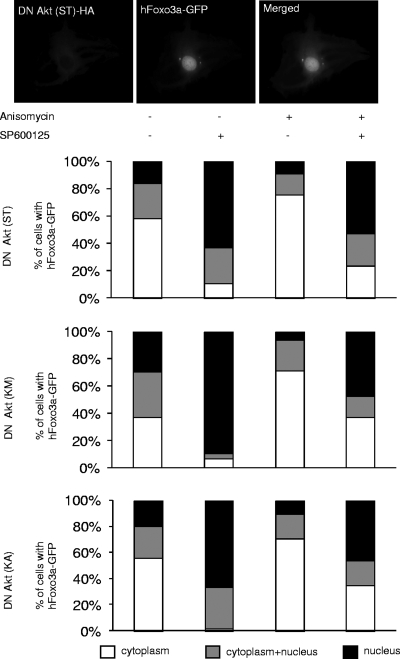
In order to definitely exclude a role for Akt in the nuclear export mechanism of Foxo3a, we cotransfected C2C12 cells with a human Foxo3a protein fused to GFP (Foxo3a-GFP) and three different dominant-negative forms of Akt (Fig. 3) (37). These mutants decreased the activation of the Akt pathway and thereby promoted translocation of Foxo3a to the nucleus. We observed that anisomycin treatment slightly increased the percentage of cells that displayed Foxo3a localization within the cytoplasm compared to the control and, as expected, SP600125 reversed the effect of anisomycin and promoted nuclear relocalization of Foxo3a (Fig. 3). Furthermore, the addition of SP600125 alone, without anisomycin treatment, was sufficient to induce considerable nuclear relocalization of Foxo3a (Fig. 3). Hence, when the basal Akt pathway was diminished by dominant-negative Akt mutants, pharmaceutical blockade of the JNK signaling pathway by SP600125 triggered a massive transfer of Foxo3a to the nucleus.
FIG. 3.
Nucleocytoplasmic translocation of Foxo3a by the JNK pathway is independent of Akt in C2C12 cells. hFoxo3a localization was monitored by fluorescence microscopy on C2C12 cells cotransfected with vectors expressing different Akt dominant-negative (DN) HA-tagged mutants (KA, ST, and KM) and a human Foxo3a-GFP fusion expression vector (upper panel). Forty-eight hours after transfection, the cells were serum deprived for 12 h and then treated with SP600125 (20 μM) and/or anisomycin (10 μg/ml) for 1 h. Subcellular localization of Foxo3a-GFP (150 to 500 counted cells) in cells expressing Akt DN mutants is schematically represented on the graph (lower panel).
The p38 signaling pathway prevents nuclear translocation of Foxo3a and limits atrophy.
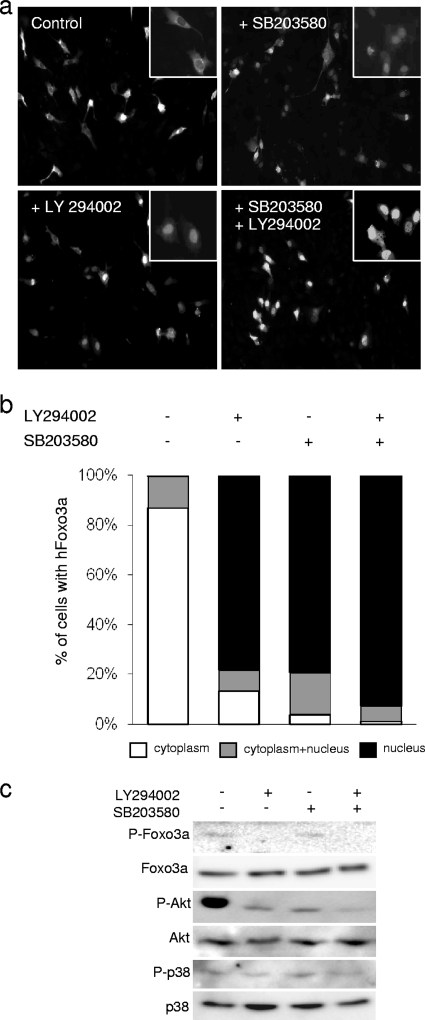
We then wondered whether the export of Foxo3a via activation of the JNK pathway was specific to this kinase or if the other SAPK, p38, was able to induce a similar process. After transient transfection with hFoxo3a, C2C12 cells were treated with SB203580 (25 μM), a p38 inhibitor (Fig. 4a). Surprisingly, we observed that inhibition of p38 in myoblasts induced a rapid and massive nuclear import of Foxo3a (Fig. 4a and b). Hence, after 1 h of treatment, Foxo3a was localized in the nucleus in more than 95% of the cells. We previously showed that inhibition of p38 kinase activity by SB203580 affects the Akt phosphorylation level; however, this effect did not significantly modify Foxo3a Thr32 phosphorylation (Fig. 4c) (9). These results argue in favor of two distinct mechanisms of Foxo3a translocation, one that acts via Akt and another that acts via p38. Therefore, under basal growth (unstressed) conditions, both p38 and Akt signaling pathways regulate Foxo3a and prevent its nuclear import and subsequent activation of target genes. Blockade of the p38 pathway is sufficient to allow activation of Foxo3a.
FIG. 4.
The p38 signaling pathway prevents nuclear translocation of Foxo3a. Cells transfected with hFoxo3a-HA were treated 48 h later (for 1 h) with SB203580 (25 μM) with or without LY294002 (20 μM). (a) hFoxo3a localization was monitored by fluorescence microscopy. (b) The percentage of cells on the graph corresponds to the number of cells distributed among the three types of Foxo3a cellular localization (150 to 500 counted cells) detected by immunofluorescence. (c) Twenty micrograms of total proteins was separated by SDS-PAGE. The proteins were detected by Western blotting with anti-phospho-Akt (Ser473), anti-phospho-p38, anti-phospho-Foxo3a (Thr32), and antibodies against total proteins.
We also performed coimmunoprecipitation experiments to verify whether p38 acts directly or indirectly on Foxo3a (data not shown). For this, we transfected C2C12 cells with constructs expressing human wild-type p38 or a human dominant-negative (DN) p38 mutant in order to optimize any interaction with a partner protein. In neither case did we detect any interaction between p38 and Foxo3a proteins. Even if we could not exclude a direct role for p38, this result prompted us to propose that p38 acts indirectly on Foxo3a.
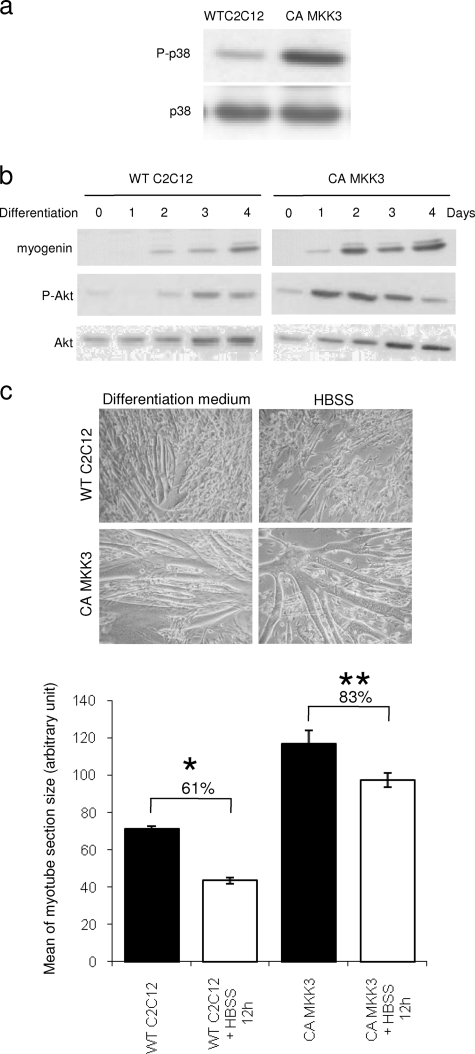
In addition, we used a stable C2C12 cell line overexpressing a constitutively active (CA) form of MKK3 to evaluate the resistance of C2C12 myotubes against starvation-induced atrophy (Fig. 5a). We previously showed, as confirmed by others, that the p38 pathway, in association with PI3K/Akt, is essential for expression of skeletal muscle-specific genes and for fusion of myoblasts into myotubes (9, 28, 33). The expression of myogenin showed that the CA MKK3 mutant cells displayed an accelerated differentiation process (Fig. 5b). In these cells, expression of myogenin was detected at an early differentiation stage and reached a plateau at day 2, whereas a comparable myogenin level was detected only after 4 days of differentiation in wild-type C2C12 cells. Similarly, the Akt activation profile of the mutant was accelerated during differentiation (Fig. 5b). Hence, at day 4, MKK3 CA myotubes displayed an advanced differentiation phenotype relative to the wild type (Fig. 5c). To test atrophy resistance, CA MKK3 myotubes induced to differentiate for 4 days were incubated in HBSS. After 12 h of treatment, the diameter of wild-type C2C12 cells dramatically decreased (≃40%), whereas the size reduction for CA MKK3 myotubes was much less pronounced (≃15%) (Fig. 5c). Therefore, constitutive activation of p38 by the CA MKK3 mutant confers partial protection against atrophy.
FIG. 5.
Constitutive activation of the p38 pathway confers partial protection of myotubes against atrophy. Lysates were prepared from wild-type C2C12 cells and constitutively active MKK3 (CA MKK3)-expressing myoblasts that were grown for 4 days in differentiation media. (a) Total proteins were separated by SDS-PAGE and then analyzed by Western-blotting using an anti-phospho-p38 antibody. (b) Myoblasts from these 2 cell lines were allowed to grow in differentiation media for 4 days and harvested at the different time points. Lysates were probed with antimyogenin, anti-phospho-Akt, and total Akt antibodies. (c) Wild-type C2C12 (WT) cells and constitutively active MKK3 (CA MKK3)-expressing myoblasts were grown for 4 days in differentiation media and then starved in HBSS for 12 h. Cells were observed by transmitted-light microscopy (upper panel). Myotube diameters were determined as described in Materials and Methods. Results are expressed as the mean ± SEM and summarized on a histogram (lower panel). * indicates a significant difference (P < 0.001).
The MKK3 and MKK4 kinases are involved in nucleocytoplasmic translocation of Foxo3a by SAPK in C2C12 cells.
To characterize more precisely the SAPK pathways involved in the regulation of Foxo3a nucleocytoplasmic localization, mutated forms of p38 and JNK upstream activators were used. Stable C2C12 cell lines expressing CA forms of MKK3 and MKK4, kinases that activate p38 and JNK, respectively, were established by retroviral infections. CA MKK3/MKK4 mutants mimic a constitutive activation of p38 and JNK. These stable cell lines were transiently transfected with the human wild-type form of Foxo3a and analyzed by immunofluorescence to determine the localization of Foxo3a (Fig. 6a). In cells expressing the constitutively active form of MKK3 or MKK4, we observed that after treatment with LY294002, the number of cells showing Foxo3a in the cytoplasm was 4 times higher than that in controls (Fig. 6a). Similar observations could be made when HBSS treatment was used instead of LY294002. As shown in Fig. 6b, this process is independent of phosphorylation of Foxo3a by Akt, since the level of Foxo3a phosphorylation (Thr32) was not altered in the stable cell line overexpressing CA MKK3/MKK4. Therefore, it appears that overexpression of the constitutively active MKK3/MKK4 kinases induced nuclear export of Foxo3a. Control of the export mechanism of Foxo3a involved at least partly MKK3 and MKK4.
FIG. 6.
MKK3 and MKK4 are involved in the regulation of Foxo3a nucleocytoplasmic localization by SAPK in C2C12 cells. (a) C2C12 cells transduced with retrovirus vectors (pPrig) expressing a Flag-tagged constitutively active version of human MKK3 (CA MKK3) or MKK4 (CA MKK4) were transiently transfected with the peceFoxo3a WT vector. The presence of either CA MKK3 or CA MKK4 proteins was indirectly observed through GFP expression controlled by an internal ribosome entry site (IRES) sequence included in the pPrig vector. The hFoxo3a localization was observed 48 h posttransfection by immunofluorescence using an anti-HA antibody (upper panel). The percentage of cells in the graph (lower panel) corresponds to the number of CA MKK3- or CA MKK4-expressing cells distributed among the three types of hFoxo3a cellular localization (150 to 500 counted cells). Treatments with LY294002 (20 μM) or HBSS were carried out for 1 h. (b) Proteins extracted from C2C12 cells stably expressing Flag-tagged CA MKK3 and CA MKK4 and treated for 1 h with LY294002 (20 μM) were separated by SDS-PAGE and detected by Western blotting. Results shown are representative of 3 independent experiments. Control, pPrig empty vector.
Nuclear export of Foxo3a by SAPK is not associated with its cytoplasmic degradation by the proteasome pathway.
In previous work, it was demonstrated that Foxo3a is exported by IκB kinase (IKK) from the nucleus toward the cytoplasm, where it is degraded by the ubiquitin proteasome system (26). In our study, we demonstrated that the SAPK pathways are implicated in a Foxo3a nuclear export mechanism. Consequently, we felt it was important to check if Foxo3a was degraded after being exported to the cytoplasm. In order to estimate the degradation of Foxo3a, we treated C2C12 cells with LY294002 and anisomycin with or without MG132 (10 μM), a proteasome inhibitor (data not shown). Anisomycin at a concentration of 10 μg/ml blocks protein synthesis, thereby inducing a decrease in the quantity of Foxo3a. After 1 h, half of the protein was degraded. However, the reductions with and without MG132 were similar. Therefore, this experiment suggests that Foxo3a is not degraded by the proteasome after nuclear export by SAPK.
Activation of SAPK decreases the transcriptional activity of Foxo3a and confers partial protection against atrophy.
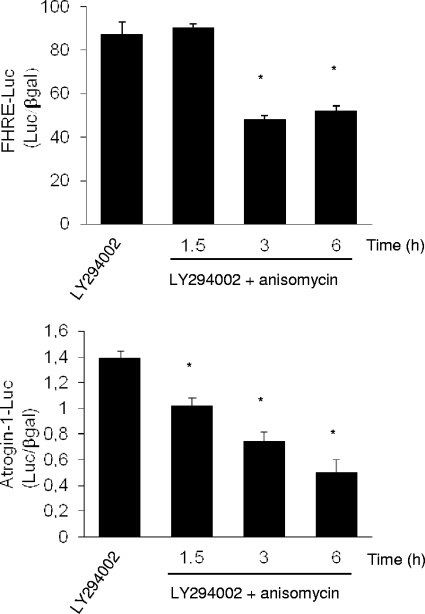
The Atrogin-1 gene has been identified as a crucial gene controlling the atrophy process by the ubiquitin proteasome system in skeletal muscle (3). It has been previously demonstrated that the expression of this gene is regulated by Foxo3a through the PI3K/Akt pathway. Under atrophy conditions, such as starvation, PI3K/Akt activity diminishes, the level of Foxo3a phosphorylation decreases, and Foxo3a translocates to the nucleus and promotes the expression of Atrogin-1. We therefore tested whether JNK activation by anisomycin could decrease the expression of Atrogin-1 after inhibition of the Akt pathway by LY294002. Using a reporter gene strategy, we monitored the activity of both the Atrogin-1 promoter (Atrogin-1-Luc) and a promoter containing multiple copies of the Foxo-responsive element (FHRE-Luc) (Fig. 7). We observed that after treatment with LY294002, the addition of anisomycin at a dose that does not block protein synthesis (50 ng/ml) significantly reduced the activity of FHRE-Luc. Similarly, anisomycin decreased progressively the activity of the Atrogin-1 promoter in a time-dependent manner (Fig. 7). Hence, after 6 h, the activity of the Atrogin-1 promoter was approximately three times lower than that in the control without anisomycin. These results indicate that nuclear export of Foxo3a by stress kinases logically induces a decrease in its transcriptional activity.
FIG. 7.
Activation of SAPK decreases the transcriptional activity of Foxo3a. Wild-type C2C12 cells were cotransfected with either pGL3-Atrogin-1-Luciferase (Atrogin-1-Luc) or FHRE-luciferase (FHRE-Luc) and pCMV-β-galactosidase (βgal) plasmids. Two days later, C2C12 cells were treated with anisomycin (50 ng/ml) for the indicated times in the presence of LY294002 (20 μM). Emitted luminescence was measured on cell extracts, and Atrogin-1 or FHRE promoter activity corresponds to the ratio of Atrogin-1-Luc or FHRE-Luc activity to βgal activity (Luc/βgal). Results are represented as means ± standard deviations. *, significantly different from the control (LY294002; P < 0.001 [Student's t test]).
In order to validate these observations at a physiological level, we starved muscle cells by using HBSS for 4 h with or without anisomycin. Starvation induced a major and rapid Atrogin-1 mRNA increase, which was strongly diminished (2-fold) in the presence of a low concentration of anisomycin (25 ng/ml) (Fig. 8a). Furthermore, we observed that anisomycin notably limits myotube atrophy (Fig. 8b).
FIG. 8.
Activation of SAPK by anisomycin reduces Atrogin-1 expression and limits myotube atrophy. (a) Wild-type C2C12 myotubes were starved (HBSS medium) for 1 to 4 h with or without anisomycin (25 ng/ml). Relative levels of Atrogin-1 mRNA were evaluated by quantitative real-time PCR as described in Materials and Methods. Results are the means ± SEM of 3 independent experiments and are expressed as fold increases relative to the values for the control (nonstarved). *, significant difference (P < 0.05) between HBSS medium and HBSS medium plus anisomycin (25 ng/ml); NS, not significant. (b) Nine-day-differentiated wild-type C2C12 myotubes (left) were incubated for 18 h in HBSS medium (atrophying condition) without (middle) or with (right) anisomycin (50 ng/ml). Cells were photographed, and representative microscope fields are shown (upper panel). Myotube diameters were determined as described in Materials and Methods. Results are expressed as means ± SEM and summarized on a histogram (lower panel). *, significant difference (P < 0.001) from control condition (differentiation medium); †, significant difference (P < 0.001) between HBSS medium and HBSS medium plus anisomycin (50 ng/ml).
DISCUSSION
In this study, we provide evidence that stress kinases are involved in the regulation of Foxo3a nucleocytoplasmic localization in muscle cells. It appears that these signaling pathways trigger Foxo3a nuclear export through the CRM1 nuclear export protein. Following cytoplasmic return of Foxo3a by stress kinases, the transcription factor is not degraded by the proteasome. Moreover, we show that activation of these signaling pathways decreases the expression of Atrogin-1 and confers partial protection against muscle atrophy.
In numerous studies, stress has been linked to skeletal muscle wasting (32, 36, 38). It has also been demonstrated that oxidative stress induces nuclear translocation of Foxo transcription factors and activation of their target genes (7, 20). Since Foxo3a is the only well-described transcription factor controlling the expression of Atrogin-1, an ubiquitin ligase that mediates muscle atrophy (3), we first investigated the effect of oxidative stress on C2C12 muscle cells. In contrast to the case for other cell types (7), nuclear translocation of Foxo3a in C2C12 cells is not progressive throughout time (Fig. 1a). Our experiments suggested that, even at a relatively elevated dose of H2O2, almost all Foxo3a proteins remained in the cytoplasm during at least the first 3 h of treatment. After 6 h, the majority of Foxo3a had translocated to the nucleus, and this event coincided with a drop in JNK activation, whereas Akt activation by H2O2 was maintained (Fig. 1a and b). This observation led us to test the effect of a potent JNK activator (anisomycin) on Foxo3a localization. After nuclear import of Foxo3a consequent to PI3K/Akt blockade, we observed that JNK stimulation induced Foxo3a nuclear export through CRM1 (Fig. 2a). This mechanism appeared to be independent of the Akt/SGK pathway (Fig. 2). In a previous study by M. C. Hu et al. (26), it was demonstrated that direct phosphorylation by IκB induces an Akt-independent nuclear exclusion of Foxo3a. In order to rule out the possibility that anisomycin may possibly activate IκB, an IκB inhibitor was used (Fig. 2a). In addition, contrary to IκB-dependent nuclear export, JNK-induced cytoplasmic relocalization of Foxo3a did not appear to be followed by its proteasome degradation (data not shown).
Furthermore, when the Akt pathway was slightly inhibited by use of inactive forms of this kinase, inhibition of JNK induced a major nuclear translocation of Foxo3a (Fig. 3). To avoid the pleiotropic effects of pharmaceutical activators/inhibitors, involvement of the JNK pathway in Foxo3a nuclear export was confirmed by experiments performed with a constitutively active form of MKK4. Hence, stimulation of JNK by the CA MKK4 mutant drastically reduced the effect of Akt inhibition on Foxo3a nuclear import (Fig. 6a).
It was previously demonstrated that, in invertebrate organisms, JNK modulates nuclear translocation of the Foxo orthologs Daf-16 and dFoxo (41, 50). In mammals, low levels of oxidative stress induce activation of Foxo4 by JNK-dependent phosphorylation on Thr447 and Thr451 (20). These phosphorylation sites are involved in the nuclear translocation and transcriptional activation of Foxo4. However, even if some potential JNK phosphorylation sites located in the C-terminal parts of the proteins have been identified, Thr447 and Thr451 are not conserved in Foxo1 or Foxo3a (2, 20). In a recent study, it was shown by two-dimensional phosphopeptide mapping that Foxo1 contains 9 serine residues specifically phosphorylated in vivo by Erk and p38 (2). To our knowledge, no JNK phosphorylation site on Foxo3a has been identified yet. Thus, a direct role for JNK on Foxo3a seems unlikely. It appears that, in eukaryotic cells, JNK could exert a role on Foxo3a through phosphorylation of 14-3-3 protein and subsequent release of Foxo3a (47) or by phosphorylation of p66Shc, which in turn stimulates Foxo phosphorylation by Akt (23, 39). Thus, our results suggest that in C2C12 cells, the JNK pathway induces nuclear export of Foxo3a independently of Akt/SGK, probably by an indirect mechanism (Fig. 9).
FIG. 9.
Schematic model of SAPK-induced Foxo3a nuclear export in muscle cells. Foxo3a transcription factor is a direct substrate of the Akt protein kinase. In the absence of growth factors or nutrients, PI3K/Akt pathway activation decreases and Foxo3a localizes within the nucleus, where it stimulates Atrogin-1 expression, thereby inducing muscle atrophy. When cells are exposed to growth factors/nutrients, the PI3K/Akt cascade is activated and triggers the export of Foxo3a to the cytoplasm. When the PI3K/Akt pathway is inhibited, stimulation of SAPKs induces Foxo3a nuclear export via the CRM1 nuclear export protein and partly prevents muscle atrophy by decreasing Atrogin-1 promoter (Atrogin-1 prom) activity. Alternatively, activated IKK can directly phosphorylate Foxo3a and promote Akt-independent Foxo3a nuclear export. Data for dominant-negative forms of Akt (DN mutants), the constitutively active forms of MKK3 and MKK4 (CA MKK3 and CA MKK4), LY294002 (inhibitor of PI3K), SC-514 (inhibitor of IKK), SB203580 (inhibitor of p38), SP600125 (inhibitor of JNK), leptomycin B (inhibitor of CRM1 nuclear export protein), and Hanks balanced salt solution (HBSS) (nutrient deprivation condition) are shown.
We and others have demonstrated that the p38 stress kinase plays a fundamental role in muscle development and maintenance (10, 33). It has also been reported by Y. P. Li et al. that H2O2 stimulates Atrogin-1 gene expression in C2C12 myotubes and that this effect could be blunted by p38 inhibitors (32). In addition, some p38 phosphorylation sites on the Foxo1 protein have been recently identified (2). We therefore wanted to elucidate the role of this stress kinase on the regulation of Foxo3a in muscle cells. As widely reported, we noted that p38 is activated by H2O2 (Fig. 1). However, in accordance with recently published data obtained in colorectal cancer cells (15), we observed that, under conditions without stimulation, p38 blockade triggers potent nuclear import of Foxo3a in C2C12 cells (Fig. 4). In order to confirm this role for p38, we established a stable C2C12 cell line expressing a constitutively active form of MKK3 (CA MKK3) to selectively stimulate the p38 pathway. This constitutive activation of p38 significantly reduced Akt-dependent nuclear import of Foxo3a (Fig. 6) and conferred a partial protection against myotube atrophy (Fig. 5c).
We also tried to identify a physical interaction between Foxo3a and p38 by coimmunoprecipitation experiments (data not shown). We could not detect any direct interaction of p38 with Foxo3a in vivo. Nevertheless, direct phosphorylation of Foxo3a by p38 in C2C12 cells cannot be ruled out, and further experiments should be performed to clarify this point. It has otherwise been proposed that AMPK may phosphorylate serine residues on Foxo3a, thereby promoting its transcriptional activity (21). In response to prolonged pharmaceutical inhibition of p38 by SB202190 (up to several days), AMPK was activated by a decrease in ATP level and induced nuclear import of Foxo3a (15). Considering the treatment duration (1 h) in our experiments, such an effect seems unlikely. Collectively, our data suggest that in C2C12 cells, the p38 signaling pathway contributes, along with Akt, to cytoplasmic sequestration of Foxo3a to prevent activation of target genes.
In order to confirm that stress kinases contribute to Foxo3a regulation, we investigated the expression of the Atrogin-1 gene, a major Foxo3a target gene whose expression dramatically increases during muscle atrophy (3, 44). When an atrophying condition was simulated by PI3K/Akt inhibition, activation of stress kinases reduced the expression of a Foxo reporter gene (FHRE-Luc gene) by half and the activity of Atrogin-1 promoter by almost 75% (Fig. 7). Furthermore, during atrophy, stress kinase activation seriously impaired the increase of endogenous Atrogin-1 expression and partially abrogated myotube size reduction (Fig. 8). These results clearly demonstrate that activation of stress kinases could counteract inhibition of the PI3K/Akt pathway and decrease transcriptional activity of Foxo3a, in particular, on Atrogin-1 (Fig. 9).
Taken together, our findings demonstrate that, in C2C12 cells, stress kinase signaling pathways are involved in an Akt-independent nuclear export mechanism of Foxo3a and thereby can reduce muscle atrophy.
Acknowledgments
This work was supported by the National Research Agency (Project Lip-Age, no. ANR-07-PNRA-021) and the French Association against Myopathies (grant no. 6073).
We thank B. Mograbi (EA 4319/INSERM ERI-21, Nice, France) for kindly providing us some Akt mutants, M. Deckert (U576, l'Archet Hospital, Nice, France) for the Foxo3a-GFP and FHRE-Luc plasmids, and P. Martin and P. Pognonec for retroviral constructs (FRE 3094, Faculty of Sciences, Nice, France). We are also grateful to B. Voiland, C. Mondini, and F. Courtin for their technical assistance and to E. Kurkdjian for her engineering expertise and support.
Footnotes
Published ahead of print on 16 November 2009.
REFERENCES
- 1.Arden, K. C. 2008. FOXO animal models reveal a variety of diverse roles for FOXO transcription factors. Oncogene 27:2345-2350. [DOI] [PubMed] [Google Scholar]
- 2.Asada, S., H. Daitoku, H. Matsuzaki, T. Saito, T. Sudo, H. Mukai, S. Iwashita, K. Kako, T. Kishi, Y. Kasuya, and A. Fukamizu. 2007. Mitogen-activated protein kinases, Erk and p38, phosphorylate and regulate Foxo1. Cell. Signal. 19:519-527. [DOI] [PubMed] [Google Scholar]
- 3.Bodine, S. C., E. Latres, S. Baumhueter, V. K. Lai, L. Nunez, B. A. Clarke, W. T. Poueymirou, F. J. Panaro, E. Na, K. Dharmarajan, Z. Q. Pan, D. M. Valenzuela, T. M. DeChiara, T. N. Stitt, G. D. Yancopoulos, and D. J. Glass. 2001. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294:1704-1708. [DOI] [PubMed] [Google Scholar]
- 4.Bois, P. R., V. F. Brochard, A. V. Salin-Cantegrel, J. L. Cleveland, and G. C. Grosveld. 2005. FoxO1a-cyclic GMP-dependent kinase I interactions orchestrate myoblast fusion. Mol. Cell. Biol. 25:7645-7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunet, A., A. Bonni, M. J. Zigmond, M. Z. Lin, P. Juo, L. S. Hu, M. J. Anderson, K. C. Arden, J. Blenis, and M. E. Greenberg. 1999. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96:857-868. [DOI] [PubMed] [Google Scholar]
- 6.Brunet, A., J. Park, H. Tran, L. S. Hu, B. A. Hemmings, and M. E. Greenberg. 2001. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a). Mol. Cell. Biol. 21:952-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunet, A., L. B. Sweeney, J. F. Sturgill, K. F. Chua, P. L. Greer, Y. Lin, H. Tran, S. E. Ross, R. Mostoslavsky, H. Y. Cohen, L. S. Hu, H. L. Cheng, M. P. Jedrychowski, S. P. Gygi, D. A. Sinclair, F. W. Alt, and M. E. Greenberg. 2004. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303:2011-2015. [DOI] [PubMed] [Google Scholar]
- 8.Burgering, B. M., and P. J. Coffer. 1995. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature 376:599-602. [DOI] [PubMed] [Google Scholar]
- 9.Cabane, C., A. S. Coldefy, K. Yeow, and B. Derijard. 2004. The p38 pathway regulates Akt both at the protein and transcriptional activation levels during myogenesis. Cell. Signal. 16:1405-1415. [DOI] [PubMed] [Google Scholar]
- 10.Cabane, C., W. Englaro, K. Yeow, M. Ragno, and B. Derijard. 2003. Regulation of C2C12 myogenic terminal differentiation by MKK3/p38alpha pathway. Am. J. Physiol. Cell Physiol. 284:C658-C666. [DOI] [PubMed] [Google Scholar]
- 11.Cai, D., J. D. Frantz, N. E. Tawa, Jr., P. A. Melendez, B. C. Oh, H. G. Lidov, P. O. Hasselgren, W. R. Frontera, J. Lee, D. J. Glass, and S. E. Shoelson. 2004. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell 119:285-298. [DOI] [PubMed] [Google Scholar]
- 12.Calnan, D. R., and A. Brunet. 2008. The FoxO code. Oncogene 27:2276-2288. [DOI] [PubMed] [Google Scholar]
- 13.Cao, P. R., H. J. Kim, and S. H. Lecker. 2005. Ubiquitin-protein ligases in muscle wasting. Int. J. Biochem. Cell Biol. 37:2088-2097. [DOI] [PubMed] [Google Scholar]
- 14.Charvet, C., I. Alberti, F. Luciano, A. Jacquel, A. Bernard, P. Auberger, and M. Deckert. 2003. Proteolytic regulation of Forkhead transcription factor FOXO3a by caspase-3-like proteases. Oncogene 22:4557-4568. [DOI] [PubMed] [Google Scholar]
- 15.Chiacchiera, F., A. Matrone, E. Ferrari, G. Ingravallo, G. Lo Sasso, S. Murzilli, M. Petruzzelli, L. Salvatore, A. Moschetta, and C. Simone. 2009. p38alpha blockade inhibits colorectal cancer growth in vivo by inducing a switch from HIF1alpha- to FoxO-dependent transcription. Cell Death Differ. 16:1203-1214. [DOI] [PubMed] [Google Scholar]
- 16.Clavel, S., A. S. Coldefy, E. Kurkdjian, J. Salles, I. Margaritis, and B. Derijard. 2006. Atrophy-related ubiquitin ligases, atrogin-1 and MuRF1 are up-regulated in aged rat tibialis anterior muscle. Mech. Ageing Dev. 127:794-801. [DOI] [PubMed] [Google Scholar]
- 17.Davies, S. P., H. Reddy, M. Caivano, and P. Cohen. 2000. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 351:95-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dérijard, B., M. Hibi, I. H. Wu, T. Barrett, B. Su, T. Deng, M. Karin, and R. J. Davis. 1994. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell 76:1025-1037. [DOI] [PubMed] [Google Scholar]
- 19.Dérijard, B., J. Raingeaud, T. Barrett, I. H. Wu, J. Han, R. J. Ulevitch, and R. J. Davis. 1995. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science 267:682-685. [DOI] [PubMed] [Google Scholar]
- 20.Essers, M. A., S. Weijzen, A. M. de Vries-Smits, I. Saarloos, N. D. de Ruiter, J. L. Bos, and B. M. Burgering. 2004. FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J. 23:4802-4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greer, E. L., P. R. Oskoui, M. R. Banko, J. M. Maniar, M. P. Gygi, S. P. Gygi, and A. Brunet. 2007. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J. Biol. Chem. 282:30107-30119. [DOI] [PubMed] [Google Scholar]
- 22.Gross, D. N., A. P. van den Heuvel, and M. J. Birnbaum. 2008. The role of FoxO in the regulation of metabolism. Oncogene 27:2320-2336. [DOI] [PubMed] [Google Scholar]
- 23.Guo, J., Z. Gertsberg, N. Ozgen, and S. F. Steinberg. 2009. p66Shc links alpha1-adrenergic receptors to a reactive oxygen species-dependent AKT-FOXO3A phosphorylation pathway in cardiomyocytes. Circ. Res. 104:660-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han, J., J. D. Lee, L. Bibbs, and R. J. Ulevitch. 1994. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 265:808-811. [DOI] [PubMed] [Google Scholar]
- 25.Hazzalin, C. A., R. Le Panse, E. Cano, and L. C. Mahadevan. 1998. Anisomycin selectively desensitizes signalling components involved in stress kinase activation and fos and jun induction. Mol. Cell. Biol. 18:1844-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu, M. C., D. F. Lee, W. Xia, L. S. Golfman, F. Ou-Yang, J. Y. Yang, Y. Zou, S. Bao, N. Hanada, H. Saso, R. Kobayashi, and M. C. Hung. 2004. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell 117:225-237. [DOI] [PubMed] [Google Scholar]
- 27.Jagoe, R. T., and A. L. Goldberg. 2001. What do we really know about the ubiquitin-proteasome pathway in muscle atrophy? Curr. Opin. Clin. Nutr. Metab. Care 4:183-190. [DOI] [PubMed] [Google Scholar]
- 28.Keren, A., Y. Tamir, and E. Bengal. 2006. The p38 MAPK signaling pathway: a major regulator of skeletal muscle development. Mol. Cell. Endocrinol. 252:224-230. [DOI] [PubMed] [Google Scholar]
- 29.Kishore, N., C. Sommers, S. Mathialagan, J. Guzova, M. Yao, S. Hauser, K. Huynh, S. Bonar, C. Mielke, L. Albee, R. Weier, M. Graneto, C. Hanau, T. Perry, and C. S. Tripp. 2003. A selective IKK-2 inhibitor blocks NF-kappa B-dependent gene expression in interleukin-1 beta-stimulated synovial fibroblasts. J. Biol. Chem. 278:32861-32871. [DOI] [PubMed] [Google Scholar]
- 30.Kudo, N., N. Matsumori, H. Taoka, D. Fujiwara, E. P. Schreiner, B. Wolff, M. Yoshida, and S. Horinouchi. 1999. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc. Natl. Acad. Sci. U. S. A. 96:9112-9117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leong, M. L., A. C. Maiyar, B. Kim, B. A. O'Keeffe, and G. L. Firestone. 2003. Expression of the serum- and glucocorticoid-inducible protein kinase, Sgk, is a cell survival response to multiple types of environmental stress stimuli in mammary epithelial cells. J. Biol. Chem. 278:5871-5882. [DOI] [PubMed] [Google Scholar]
- 32.Li, Y. P., Y. Chen, J. John, J. Moylan, B. Jin, D. L. Mann, and M. B. Reid. 2005. TNF-alpha acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin1/MAFbx in skeletal muscle. FASEB J. 19:362-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lluís, F., E. Perdiguero, A. R. Nebreda, and P. Munoz-Canoves. 2006. Regulation of skeletal muscle gene expression by p38 MAP kinases. Trends Cell Biol. 16:36-44. [DOI] [PubMed] [Google Scholar]
- 34.Mammucari, C., S. Schiaffino, and M. Sandri. 2008. Downstream of Akt: FoxO3 and mTOR in the regulation of autophagy in skeletal muscle. Autophagy 4:524-526. [DOI] [PubMed] [Google Scholar]
- 35.Martin, P., O. Albagli, M. C. Poggi, K. E. Boulukos, and P. Pognonec. 2006. Development of a new bicistronic retroviral vector with strong IRES activity. BMC Biotechnol. 6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McClung, J. M., A. R. Judge, E. E. Talbert, and S. K. Powers. 2009. Calpain-1 is required for hydrogen peroxide-induced myotube atrophy. Am. J. Physiol. Cell Physiol. 296:C363-C371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mograbi, B., R. Bocciardi, I. Bourget, R. Busca, N. Rochet, D. Farahi-Far, T. Juhel, and B. Rossi. 2001. Glial cell line-derived neurotrophic factor-stimulated phosphatidylinositol 3-kinase and Akt activities exert opposing effects on the ERK pathway: importance for the rescue of neuroectodermic cells. J. Biol. Chem. 276:45307-45319. [DOI] [PubMed] [Google Scholar]
- 38.Moylan, J. S., and M. B. Reid. 2007. Oxidative stress, chronic disease, and muscle wasting. Muscle Nerve 35:411-429. [DOI] [PubMed] [Google Scholar]
- 39.Nemoto, S., and T. Finkel. 2002. Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science 295:2450-2452. [DOI] [PubMed] [Google Scholar]
- 40.Nury, D., C. Doucet, and O. Coux. 2007. Roles and potential therapeutic targets of the ubiquitin proteasome system in muscle wasting. BMC Biochem. 8(Suppl. 1):S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oh, S. W., A. Mukhopadhyay, N. Svrzikapa, F. Jiang, R. J. Davis, and H. A. Tissenbaum. 2005. JNK regulates lifespan in Caenorhabditis elegans by modulating nuclear translocation of forkhead transcription factor/DAF-16. Proc. Natl. Acad. Sci. U. S. A. 102:4494-4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raman, M., W. Chen, and M. H. Cobb. 2007. Differential regulation and properties of MAPKs. Oncogene 26:3100-3112. [DOI] [PubMed] [Google Scholar]
- 43.Salih, D. A., and A. Brunet. 2008. FoxO transcription factors in the maintenance of cellular homeostasis during aging. Curr. Opin. Cell Biol. 20:126-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sandri, M., C. Sandri, A. Gilbert, C. Skurk, E. Calabria, A. Picard, K. Walsh, S. Schiaffino, S. H. Lecker, and A. L. Goldberg. 2004. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117:399-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schultze, N., Y. Burki, Y. Lang, U. Certa, and H. Bluethmann. 1996. Efficient control of gene expression by single step integration of the tetracycline system in transgenic mice. Nat. Biotechnol. 14:499-503. [DOI] [PubMed] [Google Scholar]
- 46.Sluss, H. K., T. Barrett, B. Derijard, and R. J. Davis. 1994. Signal transduction by tumor necrosis factor mediated by JNK protein kinases. Mol. Cell. Biol. 14:8376-8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sunayama, J., F. Tsuruta, N. Masuyama, and Y. Gotoh. 2005. JNK antagonizes Akt-mediated survival signals by phosphorylating 14-3-3. J. Cell Biol. 170:295-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van der Horst, A., and B. M. Burgering. 2007. Stressing the role of FoxO proteins in lifespan and disease. Nat. Rev. Mol. Cell Biol. 8:440-450. [DOI] [PubMed] [Google Scholar]
- 49.Wang, M. C., D. Bohmann, and H. Jasper. 2005. JNK extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling. Cell 121:115-125. [DOI] [PubMed] [Google Scholar]
- 50.Wang, M. C., D. Bohmann, and H. Jasper. 2003. JNK signaling confers tolerance to oxidative stress and extends lifespan in Drosophila. Dev. Cell 5:811-816. [DOI] [PubMed] [Google Scholar]
- 51.Zhao, J., J. J. Brault, A. Schild, P. Cao, M. Sandri, S. Schiaffino, S. H. Lecker, and A. L. Goldberg. 2007. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab. 6:472-483. [DOI] [PubMed] [Google Scholar]