Abstract
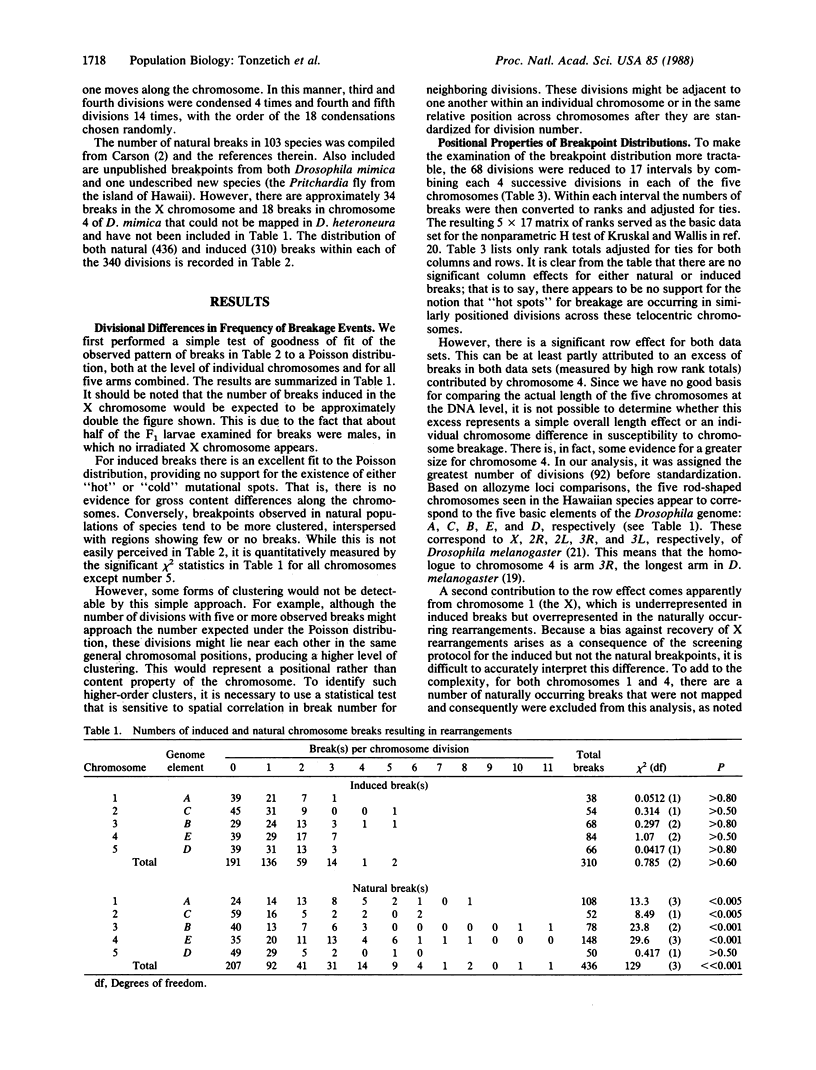
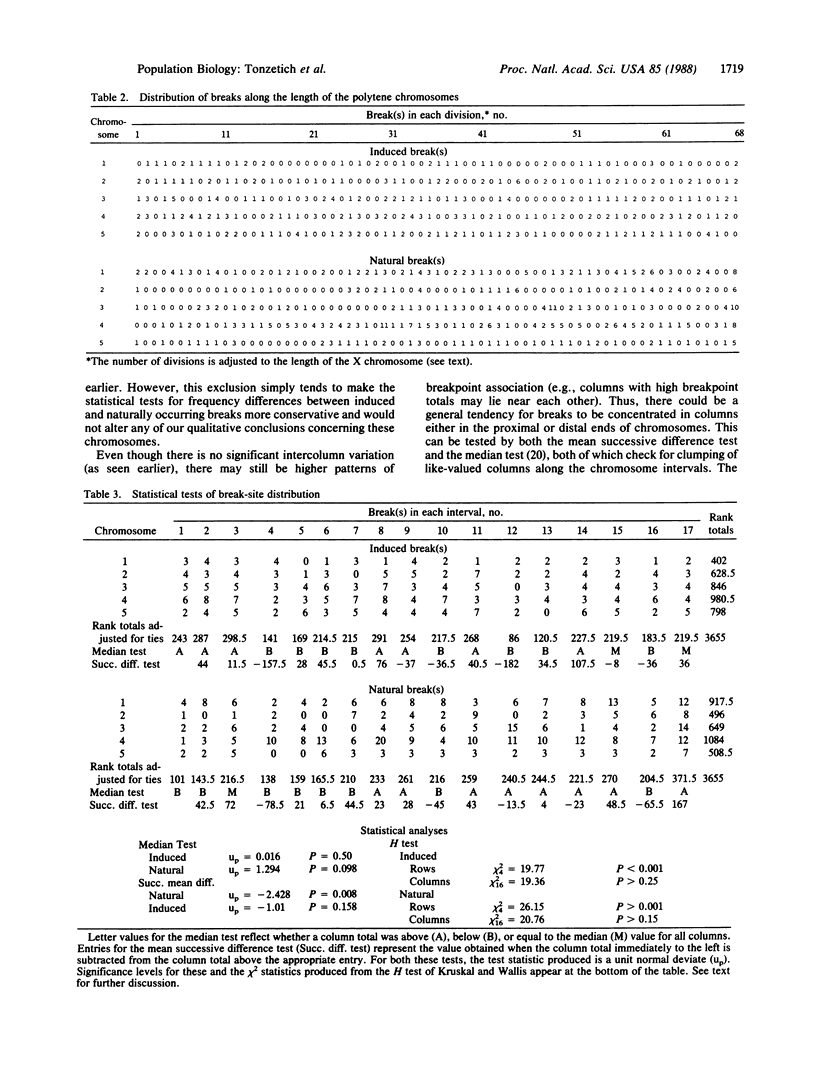
gamma-Irradiation of a laboratory strain of the Hawaiian species Drosophila heteroneura yielded 310 breaks in the five major acrocentric polytene chromosomes. Their map positions conform to the Poisson distribution, unlike most of the 436 natural breaks mapped in 105 closely related species endemic to Hawaii. Genome element E is longer and has more induced breaks than the others. Both in Hawaiian and related species groups, this element shows increased polymorphism and fixation of naturally occurring inversions. The X chromosome (element A) also accumulates many natural breaks; the majority of the resulting aberrations become fixed rather than remain as polymorphisms. Although size may play a small role in initial break distribution, the major effects relative to the establishment of a rearrangement in natural populations are ascribed to the interaction of selection and drift. Nonconformance of the natural breaks to the Poisson distribution appears to be due to the tendency for breaks to accumulate both in the proximal euchromatic portion of each arm and in heterochromatic regions that are not replicated in the polytene chromosomes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baimai V. Heterochromatin and multiple inversions in a Drosophila chromosome. Can J Genet Cytol. 1975 Mar;17(1):15–20. doi: 10.1139/g75-003. [DOI] [PubMed] [Google Scholar]
- Carson H. L. Chromosomal sequences and interisland colonizations in hawaiian Drosophila. Genetics. 1983 Mar;103(3):465–482. doi: 10.1093/genetics/103.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels W. R., Preston C. R. Formation of chromosome rearrangements by P factors in Drosophila. Genetics. 1984 Aug;107(4):657–678. doi: 10.1093/genetics/107.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatti M., Tanzarella C., Olivieri G. Analysis of the chromosome aberrations induced by x-rays in somatic cells of Drosophila melanogaster. Genetics. 1974 Aug;77(4):701–719. doi: 10.1093/genetics/77.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley D. L., Sandler L., Baker B. S., Carpenter A. T., Denell R. E., Hall J. C., Jacobs P. A., Miklos G. L., Davis B. K., Gethmann R. C. Segmental aneuploidy and the genetic gross structure of the Drosophila genome. Genetics. 1972 May;71(1):157–184. doi: 10.1093/genetics/71.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart W. D., Bishop J. G., Carson H. L., Frank M. B. Location of the 18/28S ribosomal RNA genes in two Hawaiian Drosophila species by monoclonal immunological identification of RNA.DNA hybrids in situ. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3751–3754. doi: 10.1073/pnas.78.6.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelker R. A. The genetics and cytology of a mutator factor in Drosophila melanogaster. Mutat Res. 1974 Mar;22(3):265–276. doi: 10.1016/0027-5107(74)90027-x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi O., Cardellino R. A., Mukai T. High Rates of Occurrence of Spontaneous Chromosome Aberrations in DROSOPHILA MELANOGASTER. Genetics. 1976 Jun;83(2):409–422. doi: 10.1093/genetics/83.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yannopoulos G., Stamatis N., Zacharopoulou A., Pelecanos M. Site-specific breaks induced by the male recombination factor 23.5 MRF in Drosophila melanogaster. Mutat Res. 1983 Mar;108(1-3):185–202. doi: 10.1016/0027-5107(83)90120-3. [DOI] [PubMed] [Google Scholar]



