Abstract
The target of rapamycin (TOR) complex 1 (TORC1) signaling pathway is a critical regulator of translation and cell growth. To identify novel components of this pathway, we performed a kinome-wide RNA interference (RNAi) screen in Drosophila melanogaster S2 cells. RNAi targeting components of the p38 stress-activated kinase cascade prevented the cell size increase elicited by depletion of the TOR negative regulator TSC2. In mammalian and Drosophila tissue culture, as well as in Drosophila ovaries ex vivo, p38-activating stresses, such as H2O2 and anisomycin, were able to activate TORC1. This stress-induced TORC1 activation could be blocked by RNAi against mitogen-activated protein kinase kinase 3 and 6 (MKK3/6) or by the overexpression of dominant negative Rags. Interestingly, p38 was also required for the activation of TORC1 in response to amino acids and growth factors. Genetic ablation either of p38b or licorne, its upstream kinase, resulted in small flies consisting of small cells. Mutants with mutations in licorne or p38b are nutrition sensitive; low-nutrient food accentuates the small-organism phenotypes, as well as the partial lethality of the p38b null allele. These data suggest that p38 is an important positive regulator of TORC1 in both mammalian and Drosophila systems in response to certain stresses and growth factors.
The target of rapamycin, TOR, is a highly conserved serine/threonine kinase that is a critical regulator of cell growth. It is a core component of two signaling complexes, TORC1 and TORC2 (60, 74). TORC1 is defined by the presence of Raptor in the complex, while TORC2 contains Rictor. Rictor and Raptor are mutually exclusive. Activation of the TORC1 pathway leads to increased protein translation, increased cell size, and increased proliferation, making this pathway an important target for emerging cancer therapies. Rapamycin is an inhibitor of TORC1 that is commonly used as an immunosuppressant following kidney transplantation (51). At least three analogs of rapamycin are currently being tested in solid and hematological tumors and have shown some promising results (21).
The TORC1 pathway responds to numerous inputs, sensing both the desirability of and the capacity for growth. Many of these pathways control TORC1 signaling through phosphorylation of the tuberous sclerosis protein TSC2. TSC2 associates with TSC1 to form a heterodimeric GTPase-activating protein complex (GAP) that inactivates the small GTPase Rheb (24, 29, 67). While the exact molecular mechanism remains a topic of debate, activation of Rheb promotes the kinase activity of TORC1 (24, 29, 67). Rheb is required for the activation of TORC1 in response to both amino acids and growth factors (55, 62). In Drosophila melanogaster, mutation of either TOR or Rheb inhibits growth, leading to reduced body size and reduced cell size in mutant clones (42, 64). Mutation of either TSC1 or TSC2 has the predicted opposite effect, as tissue deficient for either of these proteins overgrows and contains large cells (49, 66).
TORC1 is activated via the phosphatidylinositol 3′ kinase (PI3′K) pathway by growth-promoting mitogens, such as insulin and growth factors. Drosophila mutants with mutations of PI3′K pathway components have size phenotypes similar to those of the TOR and Rheb mutants (71). In mammalian cells, the PI3′K-mediated activation of TORC1 occurs at least in part through the phosphorylation of TSC2 by the PI3′K target AKT (30, 50). Interestingly, mutation of these residues in Drosophila has no impact on TSC2 function in vivo, suggesting that there may be other mechanisms through which PI3′K can activate Drosophila TOR (20). Recent work has suggested that the proline-rich AKT substrate PRAS40 may provide part of this link (23, 59, 69, 70). In addition, signaling through RAS activates extracellular signal-regulated kinase (ERK) and ribosomal S6 kinase (RSK), which can phosphorylate TSC2 and Raptor to activate TORC1 (13, 40, 56). There are also likely to be additional mechanisms through which growth factors activate Drosophila TOR that have not yet been identified.
TORC1 activity is also controlled by the intracellular building blocks necessary to support cellular growth. The energy-sensing AMP-activated protein kinase (AMPK) pathway relays information about the energy status of the cell to TORC1 by phosphorylating TSC2. Unlike the inactivating phosphorylation of TSC2 by Akt, phosphorylation of TSC2 by AMPK promotes the GAP activity of the TSC complex (31). AMPK also phosphorylates Raptor, leading to decreased TORC1 activity (28). Thus, when energy levels are low, active AMPK inhibits TORC1.
Amino acids also activate the TORC1 pathway, through a mechanism that requires Rheb, as well as the type III PI3′K VPS34 and the serine/threonine kinase mitogen-activated protein kinase kinase kinase kinase 3 (MAP4K3) (11, 22, 43). TORC1 thereby integrates information about the availability of amino acids and the amount of energy available for growth with growth factor signaling. Given its ancient function in adapting growth rates to environmental conditions, it is likely that TOR responds to a variety of stimuli, suggesting that many TOR control mechanisms remain to be uncovered. The Rag family of Ras-related small GTPases has recently been identified as a key component of the amino acid-sensing pathway, acting in parallel to Rheb (34, 58). Rag GTPases form heterodimers; RagA or RagB interacts with RagC or RagD. RagA and RagB are active when GTP bound, while RagC and RagD are active when bound to GDP (34, 58). Activation of the Rags by amino acids results in TOR relocalization to Rab7-containing vesicles (58). While the function of these vesicles in TORC1 signaling remains unclear, this relocalization is associated with increased TORC1 activity.
TORC1 controls cell growth and translation through the phosphorylation and activation of components of the translational machinery, such as S6 kinase (S6K) and 4EBP1, an inhibitor of eukaryotic translation initiation factor 4E (eIF4E) activity (reviewed in reference 27). S6K phosphorylates the S6 ribosomal subunit, thereby increasing translation. Mice deficient for S6K1 are small and have small pancreatic beta cells and a correspondingly low level of circulating insulin (45). Mutation of the phosphorylation sites on S6 results in a similar phenotype, with small beta cells and fibroblasts (57). In Drosophila, mutation of S6K again reduces both cell and organism size (42), as does the overexpression of 4EBP (41).
Interestingly, while mutation of the TORC1 pathway in mammalian cells reduces cell size by 10 to 15%, ablation of core TORC1 pathway components in Drosophila cells can affect cell size by up to 40% (73). In an attempt to identify novel components of the TORC1 pathway, we undertook an RNA interference (RNAi)-based screen of Drosophila S2 cells. We reasoned that the extreme size phenotypes observed in Drosophila cells upon TORC1 manipulations would facilitate the identification of modulators. In order to increase the likelihood of isolating novel regulators of TOR, we uncoupled TOR activity from many of its known nutritional controls by depleting TSC2 and screened for double-stranded RNAs (dsRNAs) that could reverse the cell size increase elicited by loss of TSC2. Depletion of multiple components of the p38 pathway was found to revert the TSC2 RNAi-induced cell size increase. Furthermore, activation of p38 is necessary and sufficient for the activation of TOR. Strikingly, mutation of components of the stress-activated p38 pathway in Drosophila has a similar phenotype to mutations in the TOR and insulin signaling pathway: a cell-autonomous cell size decrease, reduced body size, and a sensitization to the effects of nutritional stress.
MATERIALS AND METHODS
Chemicals.
Anisomycin, rapamycin, SB202190, insulinlike growth factor (IGF), epidermal growth factor (EGF), and 4-hydroxytamoxifen (4-OHT) were from Calbiochem. H2O2 was from BDH Laboratories, and bovine insulin was from Sigma-Aldrich.
RNAi screen.
An RNAi library targeting 335 kinases and phosphatases was generated. Primer information can be found at http://flight.licr.org. S2 cells were plated in 96-well plates, and the library RNAi was transfected along with Tsc2 RNAi using Fugene 6. After five days, cells were harvested, diluted in phosphate-buffered saline (PBS), and analyzed using a Z2 Coulter counter (Multisizer II; Beckman-Coulter).
Primer sequences for dsRNA.
Primer sequences for dsRNAs are as follows: for TOR, TAATACGACTCACTATAGGCTTTTGAGGTGCTCAGAGGC and TAATACGACTCACTATAGGGTAGCCGCGGCACTAGAGTAT; for S6K, TAATACGACTCACTATAGGTCCCAGTTGACGTGTTTGAA and TAATACGACTCACTATAGGGGCGTGAGGGCATCTTCTTAG; for TSC1, TAATACGACTCACTATAGGGGTCAGGTCTTCAGTTTGGGAAC and TAATACGACTCACTATAGGGTATGTCAGTTCTGTCCGTGTCCfor TSC2, TAATACGACTCACTATAGGAATGTGCTGACAGCCTTCCT and TAATACGACTCACTATAGGGGCACACTCGACTCCAGATGA; for 4EBP, TAATACGACTCACTATAGGGAATCAGCTAAGATGTCCGCTTC and TAATACGACTCACTATAGGGACAAGGTAACGGGGTCAATATG; for Lic, TAATACGACTCACTATAGGGTAAGCAAACCGATACGGTCC and TAATACGACTCACTATAGGGTACCAGCCAGAGGTAGGCAC for set 1 and TAATACGACTCACTATAGGGGAGTATAGGCAAGGCCCAA and TAATACGACTCACTATAGGGTGACGCTTATTGCTTATTGCTGATTGC for set 2; for Mekk1, TAATACGACTCACTATAGGGCGCTTCGAAAAGGTACTTG and TAATACGACTCACTATAGGGTGTCAACGATGAGAGCAAGC for set 1 and TAATACGACTCACTATAGGGTACCCAATGGCTCGACACT and TAATACGACTCACTATAGGGTGCAGAGGCACTCTACCCTT for set 2; for MK2, TAATACGACTCACTATAGGGGGATTGCCCAGTACAATGCT and TAATACGACTCACTATAGGGATCCTACGCCCATCTCTCCT; for Gcn2, TAATACGACTCACTATAGGACGAGTGCGTACTGTGCATC and TAATACGACTCACTATAGGGGCTGGACGGTGTTAGGATGT; for MAST205, TAATACGACTCACTATAGGGCAACTCAAACTCAGGCGACA and TAATACGACTCACTATAGGGTGATGCTTCACACTGCTTCC; for Trc, TAATACGACTCACTATAGGGGTAGCAGCTTGAAGGTTGCC and TAATACGACTCACTATAGGGAGCCAATGGAAGGACATTTG; for IKKb, TAATACGACTCACTATAGGGTGCAGCTGTATGTGGAGGAG and TAATACGACTCACTATAGGGTCTCGCAAACTTCTTTCCGT; for Psk, TAATACGACTCACTATAGGGCCTGTCGGACTCTTTTGAGC and TAATACGACTCACTATAGGGAAGCGGTGTATCTGGTTTGG; for Pdp1, TAATACGACTCACTATAGGGTGCGAAAAACCCCTTCATAC and TAATACGACTCACTATAGGGTGGTTTTGCACATTTTTCCA; for Puc, TAATACGACTCACTATAGGGAGTGTGCGTGCTACAAGTGG and TAATACGACTCACTATAGGGCGCTTTATCCGCATTTTCAT; for p38a, TAATACGACTCACTATAGGGCCCTTCCTCAAGCAATACCA and TAATACGACTCACTATAGGGTCAGATCTGCGTCCATCAAG; and for p38b, TAATACGACTCACTATAGGGGCACCTGAGCGTACAGAACA and TAATACGACTCACTATAGGGGCACCTGAGCGTACAGAACA.
Semiquantitative reverse transcription-PCR (RT-PCR).
RNA was prepared from S2 cells using a Qiagen RNeasy mini kit. One microgram of total RNA was used for cDNA synthesis using an Advantage RT-for-PCR kit (Clontech). The same gene-specific primers listed above were used for amplification from total cDNA, except for Licorne, for which the primers GAACAGCACCGCCTTGTAAT and GTGACGCTTATTGCTGATTGC were used. GGACGATATGGAGAAGATCTGG and CATGATCTGGGTCATCTTCTCA were used to amplify actin.
Transfection of RNAi oligonucleotides into Drosophila S2 cells.
RNAi molecules were generated as described previously (73). S2 cells were plated in serum-free medium and incubated with dsRNA. Serum-containing medium was added after 1 h. Cells were harvested after five days. For primer information, see materials and methods in the supplemental material.
Transfection of RNAi oligonucleotides into human cell lines.
A pool of four (SMART pools; Dharmacon) predesigned small interfering RNA (siRNA) molecules were used for all transfections except for MKK3/6, where Ambion siRNA against MKK3 and MKK6 were pooled together. Cells were transfected using Dharmafect 1 and assayed after three days.
Western blotting.
Cells were harvested directly into protein sample buffer (Invitrogen). Western blots were blocked in TBS-T (Tris-buffered saline containing 0.1% Tween-20) with 5% milk, probed with primary antibody, washed thrice with TBS-T, incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies, and washed again thrice in TBS-T. Antibodies against phospho-S6, phospho-4EBP1, S6, 4EBP1, TSC2, p38, mammalian TOR (mTOR), phospho-S6K, MKK3, MKK6 (Cell Signaling Technology), actin, mTOR (Santa Cruz), phospho-p38 (Biosource), Raptor (Novus), Drosophila S6K (gift from D. Alessi), polyglutamate (EE), hemagglutinin (HA) (CRUK monoclonal antibody production facility), phospho-Raptor S863 (Abgent), tubulin, and armadillo (Developmental Studies Hybridoma Bank) were used.
Constructs.
A cDNA library generated from S2 cells (Advantage RT-PCR; Clontech) was used to amplify Drosophila S6K cDNA, using the primers AAGCTTGGTGGTGGTAACACACACACGGCAATGGCGGAC and ACTAGTAGCAATCGCTCCAGCCTTTAGACC, and cloned into pcDNA3.1Flag using the HindIII and SpeI restriction cut sites in the primers. The resulting N-terminally Flag-tagged version of S6K was excised and cloned into pMK33. Mutagenesis of T238 and T398 was performed using a QuikChange site-directed mutagenesis kit (Stratagene). Rag constructs were a gift from D. Sabatini (Addgene plasmids 19302, 19303, 19305, and 19306). To generate the Raptor S863G mutant, HA-Raptor (Addgene plasmid 8513) was mutagenized on S863 using a QuikChange XL site-directed mutagenesis kit and primers CGGCCCCCGCCGGCCCCACCAAC and GTTGGTGGGGCCGGCGGGGGCCG.
Ex vivo stimulation of ovaries.
Ovaries from adult females were dissected in PBS and placed into glass dishes containing Schneider's medium (Invitrogen). Ovaries were stimulated as indicated in the legend to Fig. 3, with gentle agitation for 1 h, harvested by centrifugation, lysed in protein sample buffer, and sonicated.
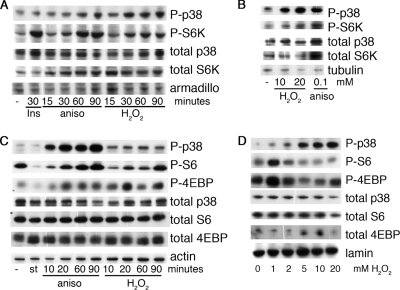
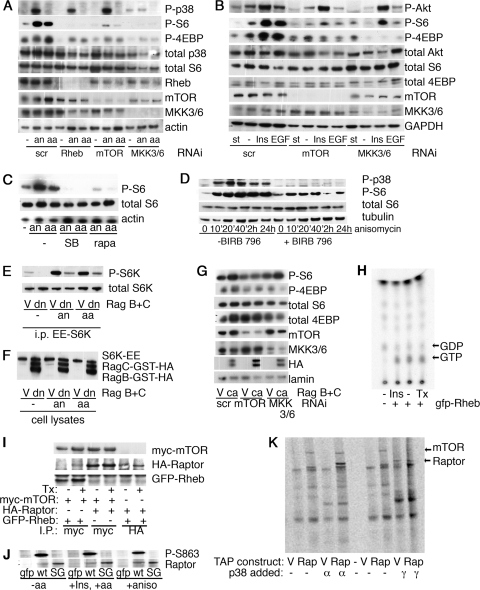
FIG. 3.
Stimulus and dose-specific activation of p38 induces phosphorylation of Tor targets. (A) S2 cells were treated with 1 μM insulin (Ins), 10 μg/ml anisomycin (aniso), or 1 mM H2O2 as indicated. Cell extracts were collected and analyzed by immunoblotting. (B) Ovaries from wiso females were dissected into PBS and stimulated for 1 h at room temperature as indicated. The ovaries were lysed in protein sample buffer, sonicated, and analyzed by immunoblotting. (C) A549 cells were starved of serum overnight and then starved of amino acids for 90 min (st), treated with 10 μg/ml anisomycin (aniso) or 1 mM H2O2 for the indicated times, and analyzed by immunoblotting. −, cells were grown in 10% serum. (D) Induction of S6 phosphorylation by H2O2 is dose dependent. A549 cells were treated for 30 min with the indicated concentrations of H2O2 and analyzed by immunoblotting.
Stress assays.
To assay high-salt stress, L1 larvae from homozygous matings were placed on medium containing 0.2 M NaCl2 at a concentration of 25 larvae per vial. Adults were counted 12 days later. For the stress assay with 1% H2O2, adult flies (2 to 5 days old) were placed into vials containing 1% H2O2. Surviving adults were counted twice per day. For the dry starvation stress assay, adult flies (2 to 5 days old) were placed into empty vials. Surviving adults were counted twice per day. To assay heat shock stress, adult flies (2 to 5 days old) were placed into vials containing moist paper towel. Surviving adults were counted every hour.
Rheb-GTP loading.
Transfected green fluorescent protein (GFP)-Rheb was immunoprecipitated from 293 cells overexpressing MEK kinase 3 (MEKK3)-estrogen receptor (ER) using 1 mg of anti-GFP antibody (3E1). Lysis, immunoprecipitation, and thin-layer chromatography were performed as previously described (39).
TAP.
pMSCV-TAP Raptor (Addgene) was transfected into 293 cells, and stable clones were isolated by puromycin selection. Tandem affinity purification (TAP) was performed as described previously (www.embl-heidelberg.de/ExternalInfo/seraphin/TAP.html) except that 0.3% CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate} was used instead of 0.1% NP-40 in all of the buffers.
Immunoprecipitation.
293 cells transfected with myc-mTOR, HA-Raptor (Addgene plasmids 1861 and 8513), or GFP-Rheb (9) were lysed 72 h after transfection in CHAPS buffer (9). Immunoprecipitations were performed from 6-well dishes with 1 mg of 12CA5 (HA) or 9E10 (myc) monoclonal antibodies for 2 h and then with immunoglobulin G (IgG) for an additional hour before washing three times in CHAPS buffer.
Larval protein concentration measurements.
Ten larvae per genotype were collected 72 h after egg lay and frozen in liquid nitrogen. Larvae were thawed into 0.3% CHAPS buffer, homogenized, and sonicated. Protein concentration was determined by Bradford assay (Pierce) in triplicate.
Measuring wing area and adult mass.
L1 larvae were placed onto low-nutrient medium (see below) at a concentration of 50 larvae per vial. The resulting adults were weighed in groups of 20. These adults were then dehydrated in 95% ethanol, and wings were mounted for determination of area as described previously (44).
Drosophila media.
Each liter of high-nutrient medium contained 7.2 g agar, 36 g maize, 7.3 g yeast, 4.4 g soya, 72 g malt, 24 ml molasses, 5.2 ml propionic acid, and 74 μl of orthophosphoric acid. Each liter of low-nutrient medium contained 10 g agar, 83 g maize, 17 g yeast, and 60 g sucrose.
Survival of lic null larvae.
lic/FM7,Kr>GFP females were mated with FM7,Kr>GFP/Y; dTor2L19/CyO,actin>GFP or FM7,Kr>GFP/Y; dRheb2D1 males to generate larvae null for both lic and either dTor or dRheb, respectively. lic/FM7,Kr>GFP; da>Gal4/da>Gal4 females were mated with FM7,Kr>GFP/Y; UAS-dRheb47-49/UAS-dRheb47-49 males to generate larvae null for lic and overexpressing dRheb.
Flow cytometry analysis of wing discs.
Flies of genotype FRT19A,lic/FRT19A,Ub>GFP; hs>FLP or hs>FLP; FRP40A,p38b/FRT40A,Ub>GFP; p38a/p38a were heat shocked at 37°C for 60 min 48 h after egg lay. Wing discs were processed as previously described (66).
RT-PCR for lic, p38b, and actin.
Ten larvae of the indicated genotypes were collected 72 h after egg lay and frozen in liquid nitrogen. RNA was prepared using an RNeasy minikit (Qiagen), and cDNA was prepared using an Advantage RT-for-PCR kit (Clontech). The following primers were used for the PCR: for lic, GAACAGCACCGCCTTGTAAT and GTGACGCTTATTGCTGATTGC; for p38b, GCTGGAGAAGATGCTGGAAC and GCAAATCGAAGGTTCGAAAA; and for actin, GGACGATATGGAGAAGATCTGG and CATGATCTGGGTCATCTTCTCA.
RESULTS
An RNAi screen for modulation of TSC2-induced cell size increase.
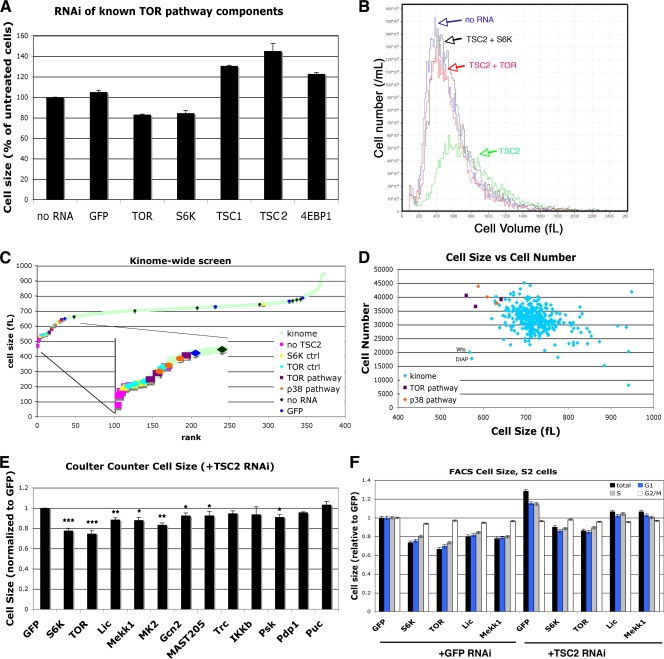
Activation of the insulin/TOR pathway in Drosophila S2 cells has dramatic effects on cell size (73). Treatment of these cells with dsRNAs directed against the tuberous sclerosis protein TSC1 or TSC2 increases cell size by up to 40%, while RNAi against TOR or S6K decreases cell size by approximately 20% (Fig. 1A). Once activated, TOR phosphorylates S6K and 4EBP1 (27). The phosphorylation of S6K is activating, while the phosphorylation of 4EBP1 prevents it from binding to eIF4E. Since the interaction between 4EBP1 and eIF4E is inhibitory, the phosphorylation of both S6K and 4EBP1 activates translation. Accordingly, RNAi against S6K itself results in a 20% decrease in cell size, while RNAi against 4EBP1 increases cell size (Fig. 1A). Thus, the changes in Drosophila S2 cell size in response to RNAi against known TOR pathway components are consistent with previously published data establishing the roles of S6K and 4EBP1 in the TOR-mediated control of cell size.
FIG. 1.
A genetic screen for regulators of TSC2-mediated cell size identifies members of the p38 pathway. (A) RNAi targeting known members of the Tor pathway in Drosophila S2 cells. S2 cells were treated with the indicated RNAi for 5 days, and cell size was measured by Coulter counter. Error bars represent standard errors of the means (SEMs) across 3 independent experiments. (B) RNAi targeting either S6K or TOR can rescue the large-cell phenotype caused by RNAi targeting TSC2. Cells were treated with both RNAis together for 5 days. Cell size was measured using a Coulter counter. (C, D) S2 cells were treated with TSC2 RNAi together with RNAi molecules targeting each Drosophila kinase or phosphatase. “TOR ctrl,” “S6K ctrl,” and “GFP” indicate RNAi molecules that were synthesized independently from the rest of the RNAi collection. “TOR pathway” and “p38 pathway” indicate RNAi molecules targeting components of these two pathways within the screened RNAi collection. (C) Each RNAi in the screen is presented in order of increasing cell size. (D) Components of the p38 pathway affect both cell size and cell number. RNAi molecules targeting components of either the TOR or the p38 pathway result in increased cell number and decreased cell size. Data points in panels C and D are averages of two independent repeats. (E) A second, nonoverlapping RNAi was used to validate 10 RNAi molecules identified in the screen. As in the initial screen, S2 cells were treated with the indicated RNAi for 5 days, either alone or with TSC2 RNAi. Error bars represent SEMs across 3 independent experiments. (F) Cells were treated with RNAi as indicated, together with either TSC2 or GFP RNAi. Cells were harvested, stained with propidium iodide, and analyzed by flow cytometry. All populations were normalized to the results for GFP RNAi, set to 1, to allow for comparisons between experiments. The forward scatter of the total population, as well as of the G1-, S-, and G2/M-gated populations, are shown. Error bars represent the SEMs within one representative experiment. ***, P < 0.005; **, P < 0.01; *, P < 0.05.
In mammalian cells, TSC1 and TSC2 have been implicated in pathways other than TOR (4, 72). In S2 cells, however, RNAi against either TOR or S6K completely reversed the large-cell phenotype induced by TSC2 RNAi (Fig. 1B). TSC2 depletion may therefore provide a useful background to identify novel components of the TOR signaling pathway, as RNAi directed against these components should reverse the large-cell phenotype induced by TSC2 RNAi. An RNAi library targeting 335 known Drosophila kinases and phosphatases was used to search for novel TOR pathway components. In this screen, cells were treated with RNAi against TSC2 in combination with individual dsRNAs from the library, and cell size and cell number were measured using a Coulter counter (Fig. 1C and D).
Of the 5% of dsRNAs that reduced cell size the most dramatically, three were known components of the TOR signaling pathway (Fig. 1C and D): TOR itself, S6K, and phosphoinositide-dependent kinase 1 (PDK1), a kinase known to be required for S6K activation (5, 36, 54). RNAi against any of these three kinases was able to reverse the TSC2-mediated increase in cell size (Fig. 1C; also see Table S1 in the supplemental material). Other RNAi molecules within this top 5% of “hits” include IκB kinase β (IKKβ), which has recently been shown to activate TOR (37); two regulators of apoptosis (DIAP1 and Wts); and other kinases, such as CG14163, MYT1, and MAST205 (see Table S1 in the supplemental material).
Interestingly, among the 5% of dsRNAs with the strongest effect in reversing the large-cell phenotype were three core components of the stress-activated p38 signaling pathway: Licorne, Mekk1, and MAPK-activated protein kinase 2 (MK2) (Fig. 1C and D). Mekk1 phosphorylates and activates Licorne, which in turn phosphorylates and activates p38. p38 has numerous downstream targets, including MK2. In mammals, MK2 may also be involved in a positive feedback loop, as RNAi against MK2 destabilizes p38 (35). Neither of the Drosophila p38 homologues p38a and p38b were included in the library, and they were therefore were not recovered in our screen.
In addition to altering cell size, RNAi against TSC2 also affects cell proliferation (6) (Fig. 1D). Rapamycin, a TOR inhibitor, inhibits progression from G1 to S phase and induces a G1 arrest in many mammalian cells. However, rapamycin treatment and TOR inhibition also accelerate the progression from G2 into M (47, 73). In S2 cells, the latter mechanism predominates, and inhibition of the TOR pathway with either low levels of rapamycin or RNAi against the insulin/TOR pathway accelerates progression through G2/M and increases cell number (73). Consistent with these observations, treatment of S2 cells with TSC2 RNAi decreases cell number, and the TSC2 RNAi-mediated decrease in cell number can be reversed by RNAi against S6K, TOR, Mekk1, Lic, or MK2 (Fig. 1D). In contrast, RNAi against Wts or DIAP1, while reducing cell size, also reduced cell number (Fig. 1D). Given their known roles in inhibiting apoptosis, Wts and DIAP1 were excluded from further analysis.
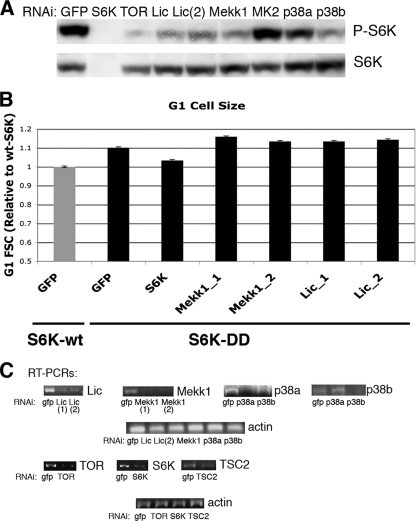
To confirm some of the results of this screen, second, nonoverlapping RNAis targeting eight genes identified as putative negative regulators, as well as two genes (Puc and Pdp1) identified as putative positive regulators, were generated. S2 cells were treated with these RNAis with TSC2 RNAi, and cell size was measured by Coulter counter. Targeting of any of the three identified p38 pathway components with this second RNAi also decreased the size of cells treated with TSC2 RNAi. The levels of mRNA remaining after selected RNAi treatments in S2 cells are shown in Fig. 2C.
FIG. 2.
Licorne RNAi prevents the cell size increase mediated by Tsc2 RNAi but does not affect the size of cells overexpressing S6K-DD. (A) S2 cells were treated with Tsc2 RNAi plus the indicated RNAi. Total cell extracts were immunoblotted with the indicated antibodies. (B) S2 cells were stably transfected with either wild-type S6K (S6K-wt) or with a mutated form of S6K in which both T238 and T398 were mutated to aspartate (S6K-DD). Pools of stably transfected cells were treated with RNAi as indicated, stained with propidium iodide, and analyzed by flow cytometry. The forward scatter (FSC) for the G1-gated population is shown. Error bars represent the SEMs from a representative experiment. (C) Semiquantitative RT-PCR was used to measure the indicated mRNA species in S2 cells treated with RNAi for 5 days.
As cells progress through the cell cycle, they grow before they divide. Thus, RNAis that block cells in G2/M would be predicted to increase the average cell size of a population due to an accumulation of the larger G2 cells. Conversely, RNAis that block cells in G1/S should decrease the average size. Indeed, many of the RNAi molecules that increased cell size in our screen are known cell cycle regulators (see Table S1 in the supplemental material). p38β has been identified as a regulator of cell cycle (and therefore cell size) in a genome-wide RNAi screen (6). To distinguish bona fide regulation of growth from changes in cell cycle progression, cells were treated with RNAi and subjected to fluorescence-activated cell sorting (FACS) analysis. By analyzing forward scatter and, hence, cell size, we could demonstrate that both TOR and p38 pathway components reduced cell size in all phases of the cell cycle (Fig. 1F). Interestingly, treatment of cells with RNAi targeting p38 pathway components alone also had a small effect on cell size (Fig. 1F). Thus, both TOR and p38 pathway components affect both cell size and cell cycle, but the cell size effect is likely due to altered cell growth rather than the consequence of a cell cycle phasing defect.
Inhibition of p38 signaling decreases phosphorylation of S6K.
We next sought to investigate the molecular interaction between p38 and TORC1 signaling. First, we examined whether inhibition of p38 signaling had any effect on the phosphorylation of the TOR target S6K. As S6K phosphorylated at T398 (P-S6K) is not detectable in unstimulated S2 cells, TSC2 RNAi was used to activate TOR and induce S6K phosphorylation. RNAi against Lic, Mekk1, and p38b dramatically reduces the TSC2 RNAi-mediated phosphorylation of S6K (Fig. 2A). In contrast, p38a and MK2 RNAi had little effect. This may be due to inefficient RNAi, or MK2 may affect cell size through another mechanism.
To investigate this possibility further, we generated S2 cells stably expressing S6K in which two activating phosphorylation sites, T238 and T398, were mutated to phosphomimetic sites (S6K-DD) (10, 52). Mutation of these two sites constitutively activates S6K and increases cell size (Fig. 2B). In these cells, RNAi against p38 pathway components does not affect cell size (Fig. 2B). Thus, S6K activation is dominant to p38, and activation of p38 results in phosphorylation of S6K. Taken together, these results suggest that p38 acts upstream of S6K in the control of cell growth.
Activation of p38 results in the phosphorylation of TOR targets.
p38 becomes activated in response to numerous stresses (1, 16, 76). This occurs through the phosphorylation of both the Thr and Tyr within the primary sequence TGY of p38 by the upstream kinase Lic (Drosophila) (MKK3 and MKK6 in mammals). Whereas previous work has suggested that TOR becomes inhibited upon the induction of stresses such as hypoxia (3, 17, 53, 63), our data demonstrate that RNAi against p38 prevents phosphorylation of S6K, suggesting that in some situations stress-induced activation of p38 may play a positive role in the activation of TOR targets (Fig. 2A). S2 cells were therefore treated with one of the stress-inducing reagents H2O2 and anisomycin, and levels of phosphorylated S6K were measured (Fig. 3A). Treatment of cells with anisomycin for 1 h increased both phosphorylation of the TGY motif of p38 (P-p38) and phosphorylation of S6K.
Drosophila ovaries are sensitive to manipulation of the insulin/TOR pathway (44). Furthermore, the p38 pathway is important in the developing ovary, as deletion of either the upstream p38 kinase lic or p38b itself results in defects in oogenesis (65). Interestingly, ex vivo stimulation of ovaries with anisomycin results in the phosphorylation of both p38 and S6K (Fig. 3B).
We wished to see if the effects of p38 on TORC1 were conserved in mammals. Similar to the results seen in Drosophila S2 cells, stimulation of human A549 cells with either anisomycin or H2O2 increased both phospho-p38 and phospho-S6 (S235/236) (P-S6) (Fig. 3C). Interestingly, the induction of S6 phosphorylation in A549 cells was dependent upon the concentration of H2O2 used (Fig. 3D). Phosphorylation of S6 was seen only with the lower doses of H2O2; higher doses of H2O2 did not induce the phosphorylation of S6 (Fig. 3D). A similar pattern of phosphorylation was observed in another TORC1 target, 4EBP (T37/46) (P-4EBP), suggesting that p38 acts upstream of TORC1 (Fig. 3C and D). Thus, activation of p38 in Drosophila cell lines, human cell lines, and Drosophila ovaries results in the phosphorylation of S6K and its downstream target S6, confirming that p38 signaling likely acts upstream of S6K phosphorylation in the TOR pathway.
Activation of p38 increases cell size in human cells.
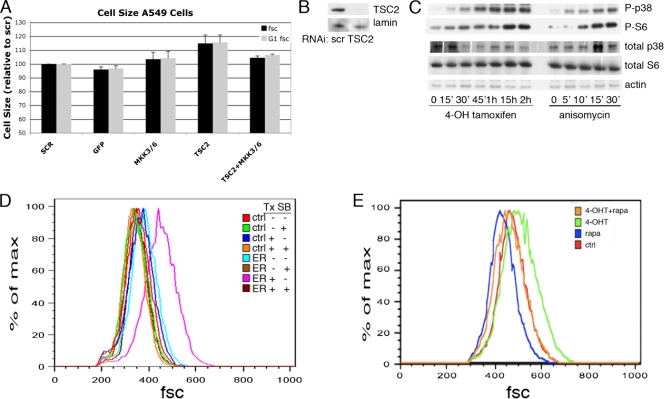
Most of the core components of the TOR signaling pathway are well conserved between humans and Drosophila. To examine whether the p38 pathway can also affect cell size in mammalian cells, we treated A549 cells with RNAi against TSC2 or against the mammalian homologues of Licorne, MKK3 and MKK6. Similar to the results obtained in Drosophila S2 cells, RNAi against MKK3 and MKK6 could prevent the cell size increase induced by TSC2 RNAi (Fig. 4A). The levels of TSC2 protein remaining after siRNA treatment are shown (Fig. 4B). The MEKK3-ER fusion protein contains the kinase domain of MEKK3, the Drosophila Mekk1 homolog and an upstream activator of p38, fused to a modified form of the tamoxifen-responsive ER domain of the estrogen receptor (25). Treatment of these cells with 4-hydroxytamoxifen (4-OHT) activates the p38 pathway and, consistent with the results presented in Fig. 3, induces the phosphorylation of S6 (Fig. 4C) (25, 68). Importantly, treatment of these cells with 4-OHT for 24 h also increased cell size in a p38-dependent manner (Fig. 4D). The tamoxifen-induced cell size increase was dependent upon TOR, as concurrent treatment with rapamycin abolished this effect (Fig. 4E).
FIG. 4.
Activation and inhibition of the p38 pathway alter cell size. (A) A549 cells were treated with the indicated RNAi for 3 days, stained with propidium iodide, and analyzed by flow cytometry. The forward scatter (fsc) of the total population, as well as of the G1-gated population, is shown. Error bars represent SEMs of this representative experiment. (B) Western blot analysis indicating the amount of TSC2 protein remaining following a three-day treatment with TSC2 siRNA. (C) Phosphorylation of p38 and S6 is induced by 4-hydroxytamoxifen treatment of 293 cells stably transfected with MEKK3-ER. 293 cells stably expressing MEKK3-ER were treated with 100 nM 4-hydroxytamoxifen or 10 μg/ml of anisomycin for the indicated amount of time (′, minutes). Total cell lysates were analyzed by immunoblotting with the indicated antibodies. (D, E) Long-term activation of MEKK3-ER by 4-hydroxytamoxifen (4-OHT) treatment increases cell size. (D) Control 293 cells (ctrl) or 293 cells stably expressing MEKK3-ER (ER) were treated with 4-hydroxytamoxifen (Tx) and/or SB202190 (SB) for 24 h as indicated, stained with propidium iodide, and analyzed by flow cytometry. The results for the G1-gated population are shown. (E) 293 cells stably expressing MEKK3-ER were treated with 4-hydroxytamoxifen and/or rapamycin (rapa) as indicated, stained, and analyzed as described for panel D. scr, scrambled siRNA control.
Stresses promote S6 phosphorylation via the TOR pathway.
The TOR pathway responds to external stimuli in the form of growth factors and insulin and to internal stimuli, such as the availability of amino acids. Core components of the TOR pathway, including Rheb, TOR, and S6K, are required for the phosphorylation of S6 in response to all of these stimuli. The pathway through which stresses induce S6 phosphorylation was therefore investigated using RNAi against Rheb and TOR. Consistent with a role for p38 in the activation of the TOR pathway, RNAi against Rheb or TOR was able to prevent the phosphorylation of S6 in response to anisomycin (Fig. 5A). Similarly, treatment of cells with the TOR inhibitor rapamycin was also able to prevent the phosphorylation of S6 in response to anisomycin (Fig. 5C).
FIG. 5.
Amino acid and insulin stimulation of S6 is blocked by p38 pathway inhibition. (A) RNAi against MKK3 and MKK6 can prevent the amino acid-induced phosphorylation of S6 in A549 cells. Cells were treated with the indicated RNAi for two days before being placed in serum-free medium overnight. The cells were then further starved of amino acids for 90 min before being stimulated with either amino acids or 10 μg/ml anisomycin for 20 min. Cells were lysed and analyzed by immunoblotting. “scr” indicates a scrambled RNAi that should not target any known protein. (B) RNAi against MKK3 and MKK6 can prevent the phosphorylation of S6 in response to insulin and EGF in A549 cells. Cells were treated with the indicated RNAi for 48 h, starved overnight of serum (st), and then stimulated for 20 min with 1 μM insulin (Ins) or 100 nM EGF. −, cells were grown in 10% serum without stimulation; P-Akt, Akt phosphorylated on S473; GAPDH, glyceraldehyde-3-phosphate dehydrogenase. (C) The p38 inhibitor SB202190 can prevent the phosphorylation of S6 in response to anisomycin or amino acids in A549 cells. A549 cells were starved as described for panel A. Rapamycin (rapa) or SB202190 (SB) was added 1 h before the stimulation with either amino acids or anisomycin as indicated. (D) Time course of BIRB 796 treatment. A549 cells were treated with 10 μM BIRB 796 or DMSO control for 1 h before being treated with 10 μg/ml anisomycin for the indicated time. (E) Overexpression of dominant negative Rags can prevent the phosphorylation of S6K in response to either anisomycin or amino acids. Dominant negative forms of RagB and RagC were cotransfected into 293 cells along with S6K-EE. Cells were starved and stimulated as described for panel A. S6K was immunoprecipitated using antibodies against the EE tag. Levels of phosphorylated S6K and total S6K present in the immunoprecipitates were determined by Western blotting. (F) Expression levels of overexpressed proteins from total cell lysates used in the experiments whose results are shown in panel E were determined by Western blotting. (G) Constitutively active Rags can induce phosphorylation of TORC1 targets even in the presence of RNAi against MKK3/6. 293 cells were treated with the indicated RNAis and transfected with the indicated Rag constructs. Cells were starved of serum overnight, lysed, and analyzed by immunoblotting. (H) GTP loading of Rheb. 293 cells stably expressing MEKK3-ER were transfected with either empty vector or GFP-Rheb as indicated. Following starvation, cells were stimulated with either 1 μM insulin (Ins) for 30 min or 100 nM 4-hydroxytamoxifen (Tx) for 4 h. The amounts of 32P-radiolabeled GTP and GDP coimmunoprecipitating with GFP-Rheb are indicated. (I) Coimmunoprecipitation between Rheb, mTOR, and Raptor. 293 cells stably expressing MEKK3-ER were transfected as indicated. Immunoprecipitates were analyzed by Western blotting. (J) Phosphorylation of Raptor S863 is not dynamically regulated. HeLa cells were transfected with vectors expressing GFP alone, wild-type HA-Raptor (wt), or S863G HA-Raptor (SG). Cells were starved of serum overnight and then starved of amino acids as indicated. Stimulation with insulin (Ins), anisomycin (an), or amino acids (aa) was performed for 20 min before cells were lysed. Immunoprecipitation was performed with anti-HA antibodies, and immunoprecipitates were analyzed by Western blotting. (K) 293 cells stably transfected with either empty vector (V) or TAP-tagged Raptor (Rap) were lysed, and tandem affinity tag purification was performed. These purified complexes were then incubated with purified, recombinant p38α or p38γ along with [33P]ATP. The proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and visualized by phosphorimager. aa, amino acids; an, anisomycin; V, empty vector; dn, dominant negative Rag; i.p. or I.P., immunoprecipitates.
Interestingly, RNAi against both MKK3 and MKK6 (MKK3/6) was able to prevent the phosphorylation of S6 and 4EBP in response to amino acids (Fig. 5A), insulin, or EGF (Fig. 5B). Consistent with this observation, treatment of cells with the p38 inhibitor SB202190 or BIRB 796 was able to abrogate the phosphorylation of S6 and 4EBP in response to amino acids (Fig. 5C and D). Phosphorylated p38 was not detectible in cells treated with insulin, EGF, or amino acids, suggesting that these stimuli do not directly activate p38 but, rather, that they require basal p38 activity in order to induce the phosphorylation of TORC1 targets. This suggests a role for basal p38 activity in the activation of translation in response to known TOR-activating stimuli, such as growth factors and amino acids. Thus, TOR and Rheb are required for the anisomycin-induced phosphorylation of S6 and 4EBP, and MKK3/6 is required for the phosphorylation of S6 and 4EBP in response to amino acids and growth factors.
Rags are dominant to p38 in the activation of TORC1.
Rags are small GTPases recently described to activate TORC1 in response to amino acids (34, 58). In order to further characterize the level at which p38 is able to influence TORC1 activity, we activated the p38 pathway in the presence of dominant negative Rags (58). In accordance with previous data, we found that Rags are essential for S6K phosphorylation in response to amino acids (Fig. 5E). Similarly, dominant negative Rags are able to prevent the phosphorylation of S6K in response to anisomycin treatment (Fig. 5E). Constitutively activated forms of RagB and RagC can induce the phosphorylation of TORC1 targets even in the absence of amino acids (34, 58). Again, we find that constitutively activated Rags can induce the phosphorylation of TORC1 targets in the presence of siRNA targeting MKK3/6 (Fig. 5G). Taken together, these data suggest that the activation of TORC1 in response to p38 occurs upstream of Rag activation.
We investigated a number of potential mechanisms through which p38 might activate TORC1 downstream of TSC2 but upstream of TOR itself. The GTP loading of Rheb was unchanged upon activation of MEKK3-ER, suggesting that p38 acts downstream of, or in parallel to, Rheb (Fig. 5H). The interaction between Rheb and mTOR or between mTOR and Raptor was similarly unaffected (Fig. 5I). Among all the known components of TORC1, there exists a single conserved p38 phosphorylation site, at S863 on Raptor. Commercially available phosphospecific antibodies raised against this site show that phosphorylation of this site is not modified by anisomycin treatment (Fig. 5J). In addition, p38α, p38γ, and MK2 are not able to significantly phosphorylate purified TORC1 in kinase assays, arguing against a role for direct phosphorylation of TORC1 components by any of these kinases (Fig. 5K and data not shown).
p38 pathway mutants in Drosophila are sensitive to stress and low-nutrient conditions.
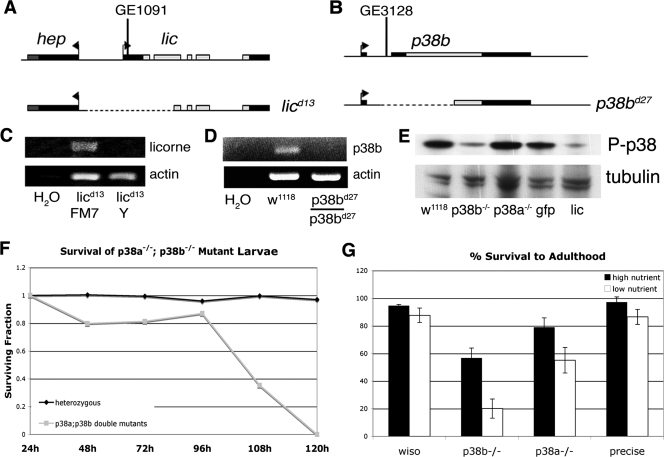
Having identified the p38 pathway as a regulator of growth and cell size in cultured cells, we next sought to examine the contribution of p38 signaling to cell growth in vivo by generating Drosophila strains containing p38 pathway mutations. Mutants with mutations of p38a are sensitive to a range of stresses (16). These flies show sensitivity to high temperature, dry starvation, and hydrogen peroxide but are not sensitive to high salt or bacterial infection (16). Interestingly, a null mutation in mekk1, one of at least four upstream activators of p38, results in sensitivity to both high temperature and high salt, suggesting that osmolarity acts through a kinase other than p38a (32). To further characterize this pathway, we generated null mutations for both p38b and its upstream kinase, lic, by imprecise excision of P elements.
The GenExel P element GE1091 is located within the 5′ untranslated region (UTR) of the lic mRNA. An imprecise excision of this P element produced an allele, licd13, with 1,411 nucleotides removed, including those encoding the initiating methionine and the first 351 nucleotides of the lic coding sequence (Fig. 6A). No lic transcripts are detected in licd13/Y larvae (lic null) (Fig. 6C). In agreement with previous work using a deletion for lic and the neighboring gene, hep, the licd13 allele is lethal (65). licd13/Y (lic null) flies die 96 to 120 h after egg lay, around larval stage L3. The expression of lic cDNA from a transgenic construct rescues this lethality, suggesting that the adjacent gene, hep, is intact, since hep is an essential gene (26).
FIG. 6.
Genetic disruption of licorne and p38b. (A, B) Schematic diagrams showing the genomic regions surrounding licorne (A) and p38b (B). Imprecise excisions from these loci generated null alleles, licd13 and p38bd27, diagrammed below. (C, D) RT-PCR results from larvae of the indicated genotype. (E) Western blot analysis of phospho-p38 levels in larvae of the indicated genotypes harvested 72 h after egg lay. (F) Survival of larvae null for both p38a and p38b. Larvae were counted every 24 h. (G) Fraction of larvae of the indicated genotypes that survived to adulthood. L1 larvae were collected 24 h after egg lay and transferred in groups of 50 to vials containing either high-nutrient or low-nutrient food. The total number of adults eclosing from each vial in the subsequent 14 days were counted. Error bars indicate standard deviations. wiso, wiso; precise, precise excision.
p38b is comprised of two exons, the first of which contains exclusively 5′UTR. An imprecise excision of P-element GE3128 located between these two exons generated a null allele, p38bd27 (Fig. 6B). This 862-bp deletion removes the first exon and half of the second, including the nucleotides coding for the initiating methionine and the first 539 nucleotides of the coding sequence. No p38b transcript is detected in homozygous p38bd27 (p38b null) flies (Fig. 6D).
Unlike the viable p38a null allele, the p38b null allele is semilethal when homozygous (Fig. 6G). Approximately 20% of p38bd27 homozygous larvae die before pupariation, with an additional 20% of homozygous pupae failing to emerge. This suggests a nonredundant role for p38b during development, during both larval and pupal stages. We used an antibody raised against phosphorylated human p38 to investigate the phosphorylation status of p38 in developing larvae. This antibody reacts with human p38α, -β, and -γ and detects phosphorylated p38 in Drosophila S2 cells upon stimulation with known p38-activating stresses (76). This antibody is predicted to detect both phosphorylated p38a and p38b. Interestingly, in the lic null larvae and the p38b null larvae collected 72 h after egg lay, little phosphorylated p38 is detected, whereas larvae homozygous for the p38a null allele have levels of phospho-p38 similar to the levels in w1118 and gfp control larvae (Fig. 6E). p38a and p38b are not completely redundant; while p38a mutants are viable and p38b mutants are semilethal, p38a,b double mutants are lethal and die around the same time as lic null larvae (Fig. 6F).
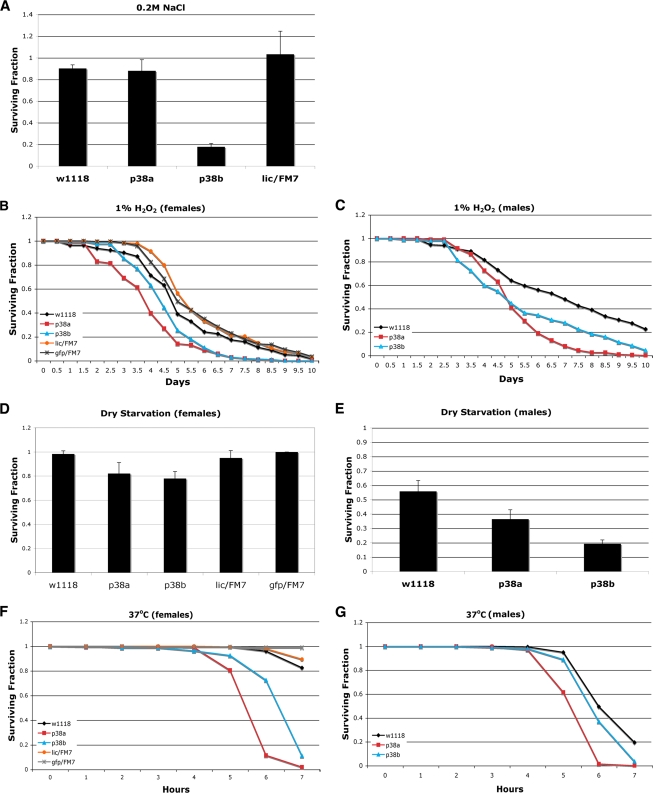
The above-described data suggest that p38a and p38b have both overlapping and distinct functions. Studies of the stress sensitivities of p38 pathway mutants are consistent with this. As mentioned above, p38a mutants and mutants with mutations of the upstream Mekk1 are sensitive to partially overlapping sets of stresses (16, 32). This suggests not only that activation of p38 may occur through different upstream members of this pathway but also that p38a and p38b are nonredundant in these responses. To differentiate between different types of stress, flies null for p38a or p38b or heterozygous for lic were subjected to environmental stresses. Similar to the Mekk1 mutant, p38b mutant embryos die under high-salt conditions (Fig. 7A). Consistent with previous results, the p38a mutant embryos are not sensitive to high salt. Both p38a and p38b mutants are sensitive to H2O2, dry starvation, and high temperatures (Fig. 7B to G). Mutants heterozygous for lic were not sensitive to any of the stresses tested, suggesting that lic is not haploinsufficient under these conditions (Fig. 7).
FIG. 7.
p38b and lic mutants are sensitive to certain stresses. (A) p38bd27 larvae are sensitive to high salt. Larvae of the indicated genotypes were collected 24 h after egg lay and placed on normal food supplemented with 0.2 M NaCl. Surviving adults were counted 12 days later. Error bars indicate standard deviations; n = 225 per genotype. (B, C) p38bd27 adult flies are sensitive to H2O2. Two- to five-day-old females (B) and males (C) of the indicated genotypes were placed in vials containing normal food supplemented with 1% H2O2. Surviving flies were counted every 12 h. n = 200 per genotype. (D, E) p38bd27 and licd13/FM7 adult flies are sensitive to dry starvation. Two- to five-day-old females (D) and males (E) of the indicated genotypes were placed in empty vials. Surviving flies were counted 24 h and 36 h later. Error bars indicate standard deviations; n = 200 to 250 per genotype. (F, G) p38bd27 adult flies are sensitive to high temperatures. Two- to five-day-old females (F) and males (G) of the indicated genotypes were placed in vials containing wet paper towel and placed at 37°C. Surviving flies were counted every 30 min. n = 200 per genotype.
As discussed above, TORC1 responds to amino acids, and the TOR pathway mutants are sensitive to nutritional status. Importantly, p38 pathway mutants are also nutritionally sensitive. Flies null for p38b have a partially lethal phenotype, as approximately half of the larvae null for p38b die during development (Fig. 6G). Interestingly, this lethality can be accentuated by raising the larvae on a low-nutrient medium (Fig. 6G). Under these conditions, only 20% of p38b larvae survive to adulthood. Furthermore, larvae null for p38a, which have a very mild lethality when nutrients are abundant, display a significant developmental lethality when raised on low-nutrient food. Thus, larvae with mutations of either p38a or p38b are hypersensitive to conditions in which nutrients are limited.
Disruption of p38 signaling in Drosophila reduces cell size.
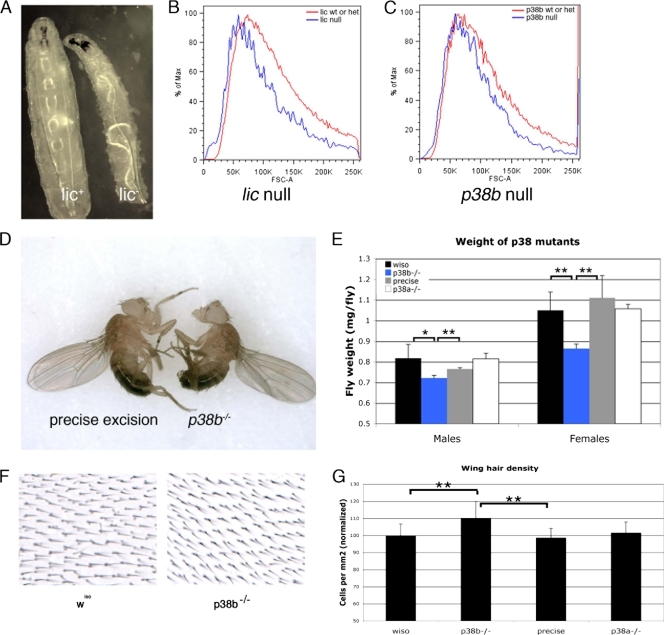
lic mutant larvae are strikingly reduced in size for their developmental stage (Fig. 8A). To investigate whether this body size phenotype reflects a reduction in cell size, we generated mosaic tissue using the FLP-FLP recombination target (FRT) system. We generated mosaic wing discs containing both lic null clones in lic heterozygous tissue and p38b null clones in p38b heterozygous tissue. To reduce any effects due to compensation from p38a, the p38b mosaic wing discs were generated in flies genetically null for p38a. These clones were analyzed by flow cytometry (Fig. 8B). Consistent with the phenotypes seen in lic null larvae, both lic null cells and p38 null cells were approximately 15% smaller than heterozygous cells from the same tissue. No differences in cell cycle profiles were observed (data not shown). Thus, similar to reduction of dTOR signaling, ablation of the p38 signaling pathway has a cell-autonomous effect on wing disc cell size.
FIG. 8.
Genetic disruption of licorne or p38b decreases cell and organism size. (A) Phenotype of licd13 larvae from density-controlled vials 72 h after egg lay. (B, C) Results of flow cytometry analysis of wings discs containing lic null cells (B) and of wing discs containing cells null for both p38a and p38b (C). wt, wild type; het, heterozygous. (D, E) Phenotype of p38bd24/p38bd24 adults. Flies with the indicated genotypes were raised in low-nutrient food at a density of 50 larvae per vial. Adult flies were weighed in groups of 20. Error bars represent the standard deviations; n > 150 per genotype. *, P value of < 0.005 by Student's t test; **, P value of < 0.001 by Student's t test. (F, G) Hair densities on wings from adult flies raised on low-nutrient food. (F) High magnification of a region posterior to the L5 vein of wings with the indicated genotypes. (G) Quantification of the hair densities shown in panel F. The number of hairs in a defined area posterior to L5 was counted. Error bars represent the standard deviations; n = 20. **, P value of < 0.001 by Student's t test; wiso, wiso. Full genotypes are as follows. Panels A to C: licd13/FM7,Kr-GFP and licd13/Y (A); FRT19A,licd13/FRT19A,Ub-GFP; hsp70-FLP (B); hsp70-FLP; FRT40A,p38bd27/FRT40A,GFP; and p38a1/p38a1 (C). Panels D to G: precise excision, p38bd27/p38bd27; p38b, p38bd24/p38bd24; p38a, p38a1/p38a1.
The lethality of lic null larvae precludes the analysis of lic null adult flies, so instead we examined the size of p38b null adults. Significantly, adult flies null for p38b are also small (Fig. 8D and E). Each cell in the wing blade secretes a single hair, or trichome, and the density of these hairs therefore reflects cell size in the wing. The p38b size phenotype appears to be primarily due to a decrease in cell size, as bristle density on the wings of p38b null flies is increased by approximately 10% (Fig. 8F and G). Together, these data are consistent with our findings in the RNAi screen and demonstrate that genetic disruption of either p38b or lic results in cell-autonomous cell size decreases in vivo. Importantly, this phenotype is similar to phenotypes previously observed in TOR pathway mutants. However, we have not been able to assess the activation state of downstream targets of TOR in either p38b or lic mutant embryos, so that the effect of these mutations on cell size seen here could be due in part to TOR-independent mechanisms.
DISCUSSION
The Drosophila S2 cell culture system is particularly amenable to manipulation both by RNAi and by insulin and was therefore chosen for an RNAi screen targeting the TORC1 pathway. Indeed, all three known kinases directly involved in TORC1 signaling downstream of TSC2 (TOR, PDK1, and S6K) were found to be positive regulators of cell size in our screen. There are few described kinases that act as direct negative regulators of TORC1. In addition to its role in phosphorylating TSC2, AMPK has recently been described as phosphorylating and inhibiting Raptor directly (28); AMPK is one of the few non-cell cycle genes identified in our screen as a negative regulator of cell size (see Table S1 in the supplemental material).
TORC1 regulation by p38.
While p38 is necessary for TORC1 activation and p38 activation itself can induce TORC1 activity and the associated cell size changes, the mechanism through which this occurs is not fully understood. Since p38 modulation changes the phosphorylation status of both 4EBP1 and S6K, p38 is likely to act upstream of TOR (Fig. 2, 3, 4, and 5). As the screen itself relied on the use of TSC2 RNAi, the activation of TORC1 by p38 should occur downstream of or in parallel to TSC2. p38 affects cell size though two distinct mechanisms, one of which is S6K dependent and the other of which is MK2 dependent (Fig. 1 and 2). Precisely how MK2 affects cell size remains unexplored; RNAi targeting MK2 does not affect S6K phosphorylation (Fig. 2). MK2 has been described as phosphorylating TSC2, creating a 14-3-3 binding site (38). This is unlikely to be the mechanism through which the p38 pathway was identified in our screen, since TSC2 itself was reduced to undetectable levels in our S2 cells by using RNAi.
The p38 cascade and amino acid sensing.
It is interesting to note that RNAi against MKK3 and MKK6 is able to prevent the phosphorylation of S6 and 4EBP1 in response to amino acids and growth factors (Fig. 5). This is reminiscent of the relationship between growth factors and amino acids; insulin is able to activate TORC1 only when amino acids are present (58). The simplest explanation for this is that amino acids themselves activate p38. In support of this theory, a recent report has shown activation of p38 by amino acids (14). MAP4K3, a kinase activated by amino acids, has homology to other MAP kinases which activate p38, Jun N-terminal protein kinase (JNK), or MEK (22). MAP4K3, however, appears to activate the JNK stress signaling pathway specifically, with little activity toward the p38 cascade (18). We were unable to see a robust, reproducible phosphorylation of p38 in response to either amino acids or insulin (Fig. 3 and 5 and data not shown). Thus, MAP4K3 is likely to activate targets other than p38 in order to induce TORC1 activity in response to amino acids. While p38 phosphorylation is undetectable in unstimulated mammalian cells, some basal level of activity must be present and required for TORC1 activity. Interestingly, in Drosophila systems, basal p38 phosphorylation is detectible (Fig. 3A and B), and in these cells, RNAi against Licorne affects cell size even when the TORC1 pathway is not activated.
To date, much of the evidence linking stresses to TORC1 suggests that stresses inactivate TORC1. For example, hypoxic stress inactivates TORC1 through the phosphorylation of TSC2 by Redd1 (8, 17, 53, 63), energetic stress inactivates TORC1 through the activation of AMPK (15, 28, 31, 63), and the treatment of cells with antibiotics inactivates TORC1 through undefined mechanisms (33). These stresses are all independent of p38. In contrast, UV radiation, which activates stress pathways such as p38 and JNK through the induction of DNA damage, activates TORC1 in a number of cell types (7, 48, 75).
Thus, it appears that, when faced with cellular damage or stress, cells can respond by shutting down cell growth, allowing repair to take place until the cell commits to further growth and division. Alternatively, cells can promote growth and translation, presumably in order to promote the synthesis of stress response proteins and the turnover of damaged molecules. Our data are consistent with the hypothesis that activation of TORC1 in response to stress is dependent on the type, intensity, and duration of the incident stress and on the specific pathways activated by each (Fig. 3). The relationship between p38 phosphorylation and TORC1 activation is not linear. For example, only low doses of H2O2 elicit TORC1 activation (Fig. 3D), suggesting that, beyond a certain damage threshold, increased translation is not a desirable response to oxidative damage. It is therefore possible that under low levels of stress, the appropriate biological response in many cell types involves attempts to combat the incident stress, while high levels of stress or prolonged exposure to stress would induce a response focused on energy conservation.
There are at least two examples of situations in which stresses, via p38, would be predicted to increase growth and translation. First, p38 is a critical component of the immune response to infection, both in antigen-presenting cells and in T cells (1). This response involves p38-dependent increases in both transcription and translation of key cytokines. Increased translation of existing mRNAs occurs at least partially through the stabilization of mRNAs containing 3′ AU-rich elements, although other p38-dependent effects on translational machinery are also observed (2). It is not currently known how increased mRNA stability is achieved or whether there is increased translation of these mRNAs in addition to the effects seen on stability. It is possible that p38 stimulates cytokine production partially through the activation of TORC1, which may contribute to increased translation of cytokine mRNAs. It is noteworthy that the main clinical use of rapamycin is as an immunosuppressant. Rapamycin treatment alters a number of immunological responses, including causing decreased cytokine production by professional antigen-presenting cells (12). Some of the immunosuppressant effects of rapamycin may be due to the inhibition of TORC1 in response to p38 activation.
Numerous growth factors, including known TORC1 activators, such as insulin and EGF, induce the production of reactive oxygen species (ROS). ROS activate p38 (19). As a by-product of mitochondrial respiration, ROS are also produced by metabolically active cells. Interestingly, chemical disruption of mitochondrial energetics (and therefore reduced ROS production) results in the dephosphorylation of TORC1 targets; this can be reversed by simultaneously treating cells with oxidizing compounds (61). Consistently, in hepatic stellate cells, ROS are required for TORC1 activation in response to amino acid treatment (46). Mitochondrial respiration results in the production of ROS; ROS may therefore act as a sensor for mitochondrial capacity. In this context, activation of TORC1 by ROS may be a mechanism through which a cell is able to couple its ability to generate energy with translation rates via p38, only permitting protein synthesis when energy levels are sufficient.
Supplementary Material
Acknowledgments
We thank Julien Colombani, Cedric Polesello, and Mary Wu for helpful discussions and technical advice; Terrence Gilbank, Stephen Murray, and Frances Earl for Drosophila maintenance and transgenic generation; Carolyn Koh and the LRI FACS facility for FACS analysis; and the LRI Equipment Park. We are grateful to Vuk Stambolic and David Sabatini for Rheb and TOR constructs.
This project was funded by Cancer Research UK.
Footnotes
Published ahead of print on 16 November 2009.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Ashwell, J. D. 2006. The many paths to p38 mitogen-activated protein kinase activation in the immune system. Nat. Rev. Immunol. 6:532-540. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee, S., K. Narayanan, T. Mizutani, and S. Makino. 2002. Murine coronavirus replication-induced p38 mitogen-activated protein kinase activation promotes interleukin-6 production and virus replication in cultured cells. J. Virol. 76:5937-5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beugnet, A., A. R. Tee, P. M. Taylor, and C. G. Proud. 2003. Regulation of targets of mTOR (mammalian target of rapamycin) signalling by intracellular amino acid availability. Biochem. J. 372:555-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bilanges, B., R. Argonza-Barrett, M. Kolesnichenko, C. Skinner, M. Nair, M. Chen, and D. Stokoe. 2007. Tuberous sclerosis complex proteins 1 and 2 control serum-dependent translation in a TOP-dependent and -independent manner. Mol. Cell. Biol. 27:5746-5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biondi, R. M., A. Kieloch, R. A. Currie, M. Deak, and D. R. Alessi. 2001. The PIF-binding pocket in PDK1 is essential for activation of S6K and SGK, but not PKB. EMBO J. 20:4380-4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bjorklund, M., M. Taipale, M. Varjosalo, J. Saharinen, J. Lahdenpera, and J. Taipale. 2006. Identification of pathways regulating cell size and cell-cycle progression by RNAi. Nature 439:1009-1013. [DOI] [PubMed] [Google Scholar]
- 7.Brenneisen, P., J. Wenk, M. Wlaschek, T. Krieg, and K. Scharffetter-Kochanek. 2000. Activation of p70 ribosomal protein S6 kinase is an essential step in the DNA damage-dependent signaling pathway responsible for the ultraviolet B-mediated increase in interstitial collagenase (MMP-1) and stromelysin-1 (MMP-3) protein levels in human dermal fibroblasts. J. Biol. Chem. 275:4336-4344. [DOI] [PubMed] [Google Scholar]
- 8.Brugarolas, J., K. Lei, R. L. Hurley, B. D. Manning, J. H. Reiling, E. Hafen, L. A. Witters, L. W. Ellisen, and W. G. Kaelin, Jr. 2004. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 18:2893-2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buerger, C., B. DeVries, and V. Stambolic. 2006. Localization of Rheb to the endomembrane is critical for its signaling function. Biochem. Biophys. Res. Commun. 344:869-880. [DOI] [PubMed] [Google Scholar]
- 10.Burnett, P. E., R. K. Barrow, N. A. Cohen, S. H. Snyder, and D. M. Sabatini. 1998. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc. Natl. Acad. Sci. U. S. A. 95:1432-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byfield, M. P., J. T. Murray, and J. M. Backer. 2005. hVps34 is a nutrient-regulated lipid kinase required for activation of p70 S6 kinase. J. Biol. Chem. 280:33076-33082. [DOI] [PubMed] [Google Scholar]
- 12.Cao, W., S. Manicassamy, H. Tang, S. P. Kasturi, A. Pirani, N. Murthy, and B. Pulendran. 2008. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K-mTOR-p70S6K pathway. Nat. Immunol. 9:1157-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carriere, A., M. Cargnello, L. A. Julien, H. Gao, E. Bonneil, P. Thibault, and P. P. Roux. 2008. Oncogenic MAPK signaling stimulates mTORC1 activity by promoting RSK-mediated Raptor phosphorylation. Curr. Biol. 18:1269-1277. [DOI] [PubMed] [Google Scholar]
- 14.Casas-Terradellas, E., I. Tato, R. Bartrons, F. Ventura, and J. L. Rosa. 2008. ERK and p38 pathways regulate amino acid signalling. Biochim. Biophys. Acta. 1783:2241-2254. [DOI] [PubMed] [Google Scholar]
- 15.Corradetti, M. N., K. Inoki, N. Bardeesy, R. A. DePinho, and K. L. Guan. 2004. Regulation of the TSC pathway by LKB1: evidence of a molecular link between tuberous sclerosis complex and Peutz-Jeghers syndrome. Genes Dev. 18:1533-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Craig, C. R., J. L. Fink, Y. Yagi, Y. T. Ip, and R. L. Cagan. 2004. A Drosophila p38 orthologue is required for environmental stress responses. EMBO Rep. 5:1058-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeYoung, M. P., P. Horak, A. Sofer, D. Sgroi, and L. W. Ellisen. 2008. Hypoxia regulates TSC1/2-mTOR signaling and tumor suppression through REDD1-mediated 14-3-3 shuttling. Genes Dev. 22:239-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diener, K., X. S. Wang, C. Chen, C. F. Meyer, G. Keesler, M. Zukowski, T. H. Tan, and Z. Yao. 1997. Activation of the c-Jun N-terminal kinase pathway by a novel protein kinase related to human germinal center kinase. Proc. Natl. Acad. Sci. U. S. A. 94:9687-9692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dolado, I., A. Swat, N. Ajenjo, G. De Vita, A. Cuadrado, and A. R. Nebreda. 2007. p38alpha MAP kinase as a sensor of reactive oxygen species in tumorigenesis. Cancer Cell 11:191-205. [DOI] [PubMed] [Google Scholar]
- 20.Dong, J., and D. Pan. 2004. Tsc2 is not a critical target of Akt during normal Drosophila development. Genes Dev. 18:2479-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Easton, J. B., and P. J. Houghton. 2006. mTOR and cancer therapy. Oncogene 25:6436-6446. [DOI] [PubMed] [Google Scholar]
- 22.Findlay, G. M., L. Yan, J. Procter, V. Mieulet, and R. F. Lamb. 2007. A MAP4 kinase related to Ste20 is a nutrient-sensitive regulator of mTOR signalling. Biochem. J. 403:13-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fonseca, B. D., E. M. Smith, V. H. Lee, C. MacKintosh, and C. G. Proud. 2007. PRAS40 is a target for mammalian target of rapamycin complex 1 and is required for signaling downstream of this complex. J. Biol. Chem. 282:24514-24524. [DOI] [PubMed] [Google Scholar]
- 24.Garami, A., F. J. Zwartkruis, T. Nobukuni, M. Joaquin, M. Roccio, H. Stocker, S. C. Kozma, E. Hafen, J. L. Bos, and G. Thomas. 2003. Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Mol. Cell 11:1457-1466. [DOI] [PubMed] [Google Scholar]
- 25.Garner, A. P., C. R. Weston, D. E. Todd, K. Balmanno, and S. J. Cook. 2002. Delta MEKK3:ER* activation induces a p38 alpha/beta 2-dependent cell cycle arrest at the G2 checkpoint. Oncogene 21:8089-8104. [DOI] [PubMed] [Google Scholar]
- 26.Glise, B., H. Bourbon, and S. Noselli. 1995. hemipterous encodes a novel Drosophila MAP kinase kinase, required for epithelial cell sheet movement. Cell 83:451-461. [DOI] [PubMed] [Google Scholar]
- 27.Guertin, D. A., and D. M. Sabatini. 2007. Defining the role of mTOR in cancer. Cancer Cell 12:9-22. [DOI] [PubMed] [Google Scholar]
- 28.Gwinn, D. M., D. B. Shackelford, D. F. Egan, M. M. Mihaylova, A. Mery, D. S. Vasquez, B. E. Turk, and R. J. Shaw. 2008. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell 30:214-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inoki, K., Y. Li, T. Xu, and K. L. Guan. 2003. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 17:1829-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inoki, K., Y. Li, T. Zhu, J. Wu, and K. L. Guan. 2002. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol. 4:648-657. [DOI] [PubMed] [Google Scholar]
- 31.Inoki, K., T. Zhu, and K. L. Guan. 2003. TSC2 mediates cellular energy response to control cell growth and survival. Cell 115:577-590. [DOI] [PubMed] [Google Scholar]
- 32.Inoue, H., M. Tateno, K. Fujimura-Kamada, G. Takaesu, T. Adachi-Yamada, J. Ninomiya-Tsuji, K. Irie, Y. Nishida, and K. Matsumoto. 2001. A Drosophila MAPKKK, D-MEKK1, mediates stress responses through activation of p38 MAPK. EMBO J. 20:5421-5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim, D. H., D. D. Sarbassov, S. M. Ali, J. E. King, R. R. Latek, H. Erdjument-Bromage, P. Tempst, and D. M. Sabatini. 2002. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110:163-175. [DOI] [PubMed] [Google Scholar]
- 34.Kim, E., P. Goraksha-Hicks, L. Li, T. P. Neufeld, and K. L. Guan. 2008. Regulation of TORC1 by Rag GTPases in nutrient response. Nat. Cell Biol. 10:935-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kotlyarov, A., Y. Yannoni, S. Fritz, K. Laass, J. B. Telliez, D. Pitman, L. L. Lin, and M. Gaestel. 2002. Distinct cellular functions of MK2. Mol. Cell. Biol. 22:4827-4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kunda, P., A. E. Pelling, T. Liu, and B. Baum. 2008. Moesin controls cortical rigidity, cell rounding, and spindle morphogenesis during mitosis. Curr. Biol. 18:91-101. [DOI] [PubMed] [Google Scholar]
- 37.Lee, D. F., H. P. Kuo, C. T. Chen, J. M. Hsu, C. K. Chou, Y. Wei, H. L. Sun, L. Y. Li, B. Ping, W. C. Huang, X. He, J. Y. Hung, C. C. Lai, Q. Ding, J. L. Su, J. Y. Yang, A. A. Sahin, G. N. Hortobagyi, F. J. Tsai, C. H. Tsai, and M. C. Hung. 2007. IKK beta suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell 130:440-455. [DOI] [PubMed] [Google Scholar]
- 38.Li, Y., K. Inoki, P. Vacratsis, and K. L. Guan. 2003. The p38 and MK2 kinase cascade phosphorylates tuberin, the tuberous sclerosis 2 gene product, and enhances its interaction with 14-3-3. J. Biol. Chem. 278:13663-13671. [DOI] [PubMed] [Google Scholar]
- 39.Li, Y., K. Inoki, H. Vikis, and K. L. Guan. 2006. Measurements of TSC2 GAP activity toward Rheb. Methods Enzymol. 407:46-54. [DOI] [PubMed] [Google Scholar]
- 40.Ma, L., Z. Chen, H. Erdjument-Bromage, P. Tempst, and P. P. Pandolfi. 2005. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell 121:179-193. [DOI] [PubMed] [Google Scholar]
- 41.Miron, M., J. Verdu, P. E. Lachance, M. J. Birnbaum, P. F. Lasko, and N. Sonenberg. 2001. The translational inhibitor 4E-BP is an effector of PI(3)K/Akt signalling and cell growth in Drosophila. Nat. Cell Biol. 3:596-601. [DOI] [PubMed] [Google Scholar]
- 42.Montagne, J., M. J. Stewart, H. Stocker, E. Hafen, S. C. Kozma, and G. Thomas. 1999. Drosophila S6 kinase: a regulator of cell size. Science 285:2126-2129. [DOI] [PubMed] [Google Scholar]
- 43.Nobukuni, T., M. Joaquin, M. Roccio, S. G. Dann, S. Y. Kim, P. Gulati, M. P. Byfield, J. M. Backer, F. Natt, J. L. Bos, F. J. Zwartkruis, and G. Thomas. 2005. Amino acids mediate mTOR/raptor signaling through activation of class 3 phosphatidylinositol 3OH-kinase. Proc. Natl. Acad. Sci. U. S. A. 102:14238-14243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orme, M. H., S. Alrubaie, G. L. Bradley, C. D. Walker, and S. J. Leevers. 2006. Input from Ras is required for maximal PI(3)K signalling in Drosophila. Nat. Cell Biol. 8:1298-1302. [DOI] [PubMed] [Google Scholar]
- 45.Pende, M., S. C. Kozma, M. Jaquet, V. Oorschot, R. Burcelin, Y. Le Marchand-Brustel, J. Klumperman, B. Thorens, and G. Thomas. 2000. Hypoinsulinaemia, glucose intolerance and diminished beta-cell size in S6K1-deficient mice. Nature 408:994-997. [DOI] [PubMed] [Google Scholar]
- 46.Perez de Obanos, M. P., M. J. Lopez-Zabalza, E. Arriazu, T. Modol, J. Prieto, M. T. Herraiz, and M. J. Iraburu. 2007. Reactive oxygen species (ROS) mediate the effects of leucine on translation regulation and type I collagen production in hepatic stellate cells. Biochim. Biophys. Acta 1773:1681-1688. [DOI] [PubMed] [Google Scholar]
- 47.Petersen, J., and P. Nurse. 2007. TOR signalling regulates mitotic commitment through the stress MAP kinase pathway and the Polo and Cdc2 kinases. Nat. Cell Biol. 9:1263-1272. [DOI] [PubMed] [Google Scholar]
- 48.Popowski, M., H. A. Ferguson, A. M. Sion, E. Koller, E. Knudsen, and C. L. Van Den Berg. 2008. Stress and IGF-I differentially control cell fate through mammalian target of rapamycin (mTOR) and retinoblastoma protein (pRB). J. Biol. Chem. 283:28265-28273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Potter, C. J., H. Huang, and T. Xu. 2001. Drosophila Tsc1 functions with Tsc2 to antagonize insulin signaling in regulating cell growth, cell proliferation, and organ size. Cell 105:357-368. [DOI] [PubMed] [Google Scholar]
- 50.Potter, C. J., L. G. Pedraza, and T. Xu. 2002. Akt regulates growth by directly phosphorylating Tsc2. Nat. Cell Biol. 4:658-665. [DOI] [PubMed] [Google Scholar]
- 51.Proud, C. G. 2007. Amino acids and mTOR signalling in anabolic function. Biochem. Soc. Trans. 35:1187-1190. [DOI] [PubMed] [Google Scholar]
- 52.Pullen, N., P. B. Dennis, M. Andjelkovic, A. Dufner, S. C. Kozma, B. A. Hemmings, and G. Thomas. 1998. Phosphorylation and activation of p70s6k by PDK1. Science 279:707-710. [DOI] [PubMed] [Google Scholar]
- 53.Reiling, J. H., and E. Hafen. 2004. The hypoxia-induced paralogs Scylla and Charybdis inhibit growth by down-regulating S6K activity upstream of TSC in Drosophila. Genes Dev. 18:2879-2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rintelen, F., H. Stocker, G. Thomas, and E. Hafen. 2001. PDK1 regulates growth through Akt and S6K in Drosophila. Proc. Natl. Acad. Sci. U. S. A. 98:15020-15025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roccio, M., J. L. Bos, and F. J. Zwartkruis. 2006. Regulation of the small GTPase Rheb by amino acids. Oncogene 25:657-664. [DOI] [PubMed] [Google Scholar]
- 56.Roux, P. P., B. A. Ballif, R. Anjum, S. P. Gygi, and J. Blenis. 2004. Tumor-promoting phorbol esters and activated Ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase. Proc. Natl. Acad. Sci. U. S. A. 101:13489-13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ruvinsky, I., N. Sharon, T. Lerer, H. Cohen, M. Stolovich-Rain, T. Nir, Y. Dor, P. Zisman, and O. Meyuhas. 2005. Ribosomal protein S6 phosphorylation is a determinant of cell size and glucose homeostasis. Genes Dev. 19:2199-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sancak, Y., T. R. Peterson, Y. D. Shaul, R. A. Lindquist, C. C. Thoreen, L. Bar-Peled, and D. M. Sabatini. 2008. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320:1496-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sancak, Y., C. C. Thoreen, T. R. Peterson, R. A. Lindquist, S. A. Kang, E. Spooner, S. A. Carr, and D. M. Sabatini. 2007. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol. Cell 25:903-915. [DOI] [PubMed] [Google Scholar]
- 60.Sarbassov, D. D., S. M. Ali, and D. M. Sabatini. 2005. Growing roles for the mTOR pathway. Curr. Opin. Cell Biol. 17:596-603. [DOI] [PubMed] [Google Scholar]
- 61.Sarbassov, D. D., and D. M. Sabatini. 2005. Redox regulation of the nutrient-sensitive raptor-mTOR pathway and complex. J. Biol. Chem. 280:39505-39509. [DOI] [PubMed] [Google Scholar]
- 62.Smith, E. M., S. G. Finn, A. R. Tee, G. J. Browne, and C. G. Proud. 2005. The tuberous sclerosis protein TSC2 is not required for the regulation of the mammalian target of rapamycin by amino acids and certain cellular stresses. J. Biol. Chem. 280:18717-18727. [DOI] [PubMed] [Google Scholar]
- 63.Sofer, A., K. Lei, C. M. Johannessen, and L. W. Ellisen. 2005. Regulation of mTOR and cell growth in response to energy stress by REDD1. Mol. Cell. Biol. 25:5834-5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stocker, H., T. Radimerski, B. Schindelholz, F. Wittwer, P. Belawat, P. Daram, S. Breuer, G. Thomas, and E. Hafen. 2003. Rheb is an essential regulator of S6K in controlling cell growth in Drosophila. Nat. Cell Biol. 5:559-565. [DOI] [PubMed] [Google Scholar]
- 65.Suzanne, M., K. Irie, B. Glise, F. Agnes, E. Mori, K. Matsumoto, and S. Noselli. 1999. The Drosophila p38 MAPK pathway is required during oogenesis for egg asymmetric development. Genes Dev. 13:1464-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tapon, N., N. Ito, B. J. Dickson, J. E. Treisman, and I. K. Hariharan. 2001. The Drosophila tuberous sclerosis complex gene homologs restrict cell growth and cell proliferation. Cell 105:345-355. [DOI] [PubMed] [Google Scholar]
- 67.Tee, A. R., B. D. Manning, P. P. Roux, L. C. Cantley, and J. Blenis. 2003. Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr. Biol. 13:1259-1268. [DOI] [PubMed] [Google Scholar]
- 68.Todd, D. E., R. M. Densham, S. A. Molton, K. Balmanno, C. Newson, C. R. Weston, A. P. Garner, L. Scott, and S. J. Cook. 2004. ERK1/2 and p38 cooperate to induce a p21CIP1-dependent G1 cell cycle arrest. Oncogene 23:3284-3295. [DOI] [PubMed] [Google Scholar]
- 69.Vander Haar, E., S. I. Lee, S. Bandhakavi, T. J. Griffin, and D. H. Kim. 2007. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat. Cell Biol. 9:316-323. [DOI] [PubMed] [Google Scholar]
- 70.Wang, L., T. E. Harris, R. A. Roth, and J. C. Lawrence, Jr. 2007. PRAS40 regulates mTORC1 kinase activity by functioning as a direct inhibitor of substrate binding. J. Biol. Chem. 282:20036-20044. [DOI] [PubMed] [Google Scholar]
- 71.Weinkove, D., T. P. Neufeld, T. Twardzik, M. D. Waterfield, and S. J. Leevers. 1999. Regulation of imaginal disc cell size, cell number and organ size by Drosophila class I(A) phosphoinositide 3-kinase and its adaptor. Curr. Biol. 9:1019-1029. [DOI] [PubMed] [Google Scholar]
- 72.Wilson, C., C. Bonnet, C. Guy, S. Idziaszczyk, J. Colley, V. Humphreys, J. Maynard, J. R. Sampson, and J. P. Cheadle. 2006. Tsc1 haploinsufficiency without mammalian target of rapamycin activation is sufficient for renal cyst formation in Tsc1+/− mice. Cancer Res. 66:7934-7938. [DOI] [PubMed] [Google Scholar]
- 73.Wu, M. Y., M. Cully, D. Andersen, and S. J. Leevers. 2007. Insulin delays the progression of Drosophila cells through G2/M by activating the dTOR/dRaptor complex. EMBO J. 26:371-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wullschleger, S., R. Loewith, and M. N. Hall. 2006. TOR signaling in growth and metabolism. Cell 124:471-484. [DOI] [PubMed] [Google Scholar]
- 75.Zhang, Y., Z. Dong, M. Nomura, S. Zhong, N. Chen, A. M. Bode, and Z. Dong. 2001. Signal transduction pathways involved in phosphorylation and activation of p70S6K following exposure to UVA irradiation. J. Biol. Chem. 276:20913-20923. [DOI] [PubMed] [Google Scholar]
- 76.Zhuang, Z. H., Y. Zhou, M. C. Yu, N. Silverman, and B. X. Ge. 2006. Regulation of Drosophila p38 activation by specific MAP2 kinase and MAP3 kinase in response to different stimuli. Cell. Signal. 18:441-448. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.