Abstract
Activation of estrogen receptor α (ERα) results in both induction and repression of gene transcription; while mechanistic details of estrogen induction are well described, details of repression remain largely unknown. We characterized several ERα-repressed targets and examined in detail the mechanism for estrogen repression of Reprimo (RPRM), a cell cycle inhibitor. Estrogen repression of RPRM is rapid and robust and requires a tripartite interaction between ERα, histone deacetylase 7 (HDAC7), and FoxA1. HDAC7 is the critical HDAC needed for repression of RPRM; it can bind to ERα and represses ERα's transcriptional activity—this repression does not require HDAC7's deacetylase activity. We further show that the chromatin pioneer factor FoxA1, well known for its role in estrogen induction of genes, is recruited to the RPRM promoter, is necessary for repression of RPRM, and interacts with HDAC7. Like other FoxA1 recruitment sites, the RPRM promoter is characterized by H3K4me1/me2. Estrogen treatment causes decreases in H3K4me1/me2 and release of RNA polymerase II (Pol II) from the RPRM proximal promoter. Overall, these data implicate a novel role for HDAC7 and FoxA1 in estrogen repression of RPRM, a mechanism which could potentially be generalized to many more estrogen-repressed genes and hence be important in both normal physiology and pathological processes.
Estrogen is essential for the growth and development of female reproductive tissues and is a known potent mitogen in breast cancer. The pleiotropic effects of 17-β-estradiol (E2), the most potent estrogen, are mediated through the α and β estrogen receptors (ERα and ERβ), which contain an agonist-independent transcriptional activation function (AF-1), a DNA binding domain (DBD), a hinge region, and an agonist-dependent transcriptional activation function (AF-2). ERα can regulate gene expression directly by binding DNA at perfect or imperfect estrogen response elements (EREs) (37) and half-ERE sites or indirectly by tethering to other DNA-bound transcription factors like AP-1, Sp1, and NF-κB (40, 60). ERα coordinates the assembly of chromatin remodeling factors, p160 coactivators (SRC1, SRC2, and SRC3), histone acetyltransferases (HATs) (p300, CBP, and the p300/CBP-associated factor pCAF), histone methyltransferases, histone deacetylases, general transcription factors, the mediator complex, and RNA polymerase II (Pol II) to the promoters of induced genes in an ordered and cyclical fashion (50, 63).
Although ERα has been mostly studied as a transcriptional activator, recent studies have shown that it can also negatively modulate gene expression. For example, gene expression profiling has demonstrated that greater than 50% of ERα target genes are downregulated upon E2 treatment in MCF7 breast cancer cells as well as in breast tumors (5, 8, 16, 53). We (31, 54) and others (1, 3, 19, 21, 52, 58, 59, 65, 70) have shown that critical genes like those coding for CD24, E-cadherin, BASE, interleukin-6 (IL-6), IR, retinoblastoma protein (Rb), ERBB2, vascular endothelial growth factor (VEGF), tumor necrosis factor alpha (TNF-α), and CD36 are repressed by E2. Many of these E2-repressed genes are cell cycle inhibitors like cyclin G2 (CCNG2) (66), proapoptotic genes, or tumor suppressor genes, and thus their repression could be a critical step in augmenting the growth and survival of a tumor and thereby in the development and/or progression of breast cancer. While the mechanisms of E2-mediated induction of genes like the Trefoil factor 1 gene (TFF1/pS2) have been studied in great detail, the mechanisms regulating E2-mediated repression of genes are virtually unknown. One potential mechanism is squelching (i.e., titration of limiting amounts of essential transcription factors by the abundance of an overexpressed transcriptional regulator), which would result in a loss of basal transcription (5). However, active recruitment of repressive complexes—for example, NCoR, (nuclear receptor corepressor), histone deacetylase 1 (HDAC1), and CtBP1 to the CCNG2 promoter (66, 67); NCoR and SMRT (silencing mediator of RAR and TR) to the VEGFR2 promoter (25); and NCoR and TAB2 to the BMP7, ABCG2, and BCL3 promoters (74)—has been shown. Genomewide analysis of ERα binding sites has implicated the involvement of the corepressor NRIP1 (nuclear receptor interacting protein 1) in the E2-mediated repression of genes like BCAS4, IRX4, GUSB, and MUC1, which are repressed at late time points (5). These genes are most likely secondary rather than direct targets of ERα, as they appear to require the E2 induction of NRIP1 for their repression.
Given the paucity of information, it is of particular interest to further explore the mechanism of estrogen repression of a primary ERα target gene. We focused on a target gene which (i) is directly and robustly repressed by estrogen in breast cancer cell lines and (ii) is involved in mediating estrogen's mitogenic effects in breast cancer cells. Our data revealed that the cell cycle inhibitor and tumor suppressor Reprimo (RPRM) gene fits these criteria. Rather unexpectedly, we discovered a unique role for HDAC7 in E2 repression of RPRM. HDAC7, ERα, and FoxA1 are necessary for repression and are recruited to the promoter, ultimately resulting in release of RNA Pol II and decrease of transcription. In summary, we propose a novel model for E2 repression which might also be exploited for repression of other E2-regulated genes and thus play an important role in hormone response.
MATERIALS AND METHODS
Cell culture.
Human breast cancer cells (MCF7, T47D, and ZR-75-1) were maintained in Dulbecco's modified Eagle's medium (DMEM; Invitrogen Life Technologies) supplemented with 5% characterized fetal bovine serum (HyClone), 2 mM glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin. Three days before E2 treatment, the cells were switched to improved minimal essential medium (IMEM) that was supplemented with 5% charcoal-dextran-treated fetal bovine serum (HyClone). The medium was changed on day 2, and the cells were treated with either vehicle or 10 nM E2 (Sigma-Aldrich Corp), unless otherwise specified, 1 μM 4-OH-tamoxifen (4-OH-tam), or 1 μM ICI 182,780 (Imperial Chemical Industries), on day 3 for 4, 8, or 24 h. The serum-free medium (SFM) consisted of phenol-red-free IMEM (Invitrogen), 2 mM glutamine, 10 mM HEPES, 1 μg/ml fibronectin (Invitrogen), trace elements (Biosources), and 1 μg/ml transferrin (Invitrogen). For experiments in which cycloheximide (CHX) was used, CHX at 10 μg/ml was added at the same time as E2 for the entire 4- or 8-h period. For experiments in which actinomycin D (ActD) was used, ActD at 2 μg/ml was added 30 min before E2 treatment and RNA was extracted at the −0.5-, 0-, 4-, 8-, and 24-h time points.
RNA extraction, qRT-PCR, and PCR.
Total RNA was isolated using the RNeasy RNA isolation kit (Qiagen) as recommended by the supplier. Triplicate RNA samples were prepared for each treatment group. RNA was subjected to DNase treatment (Roche) prior to being reverse transcribed using SuperScript II reverse transcriptase (Invitrogen) in accordance with manufacturer's instructions. The SYBR green or TaqMan PCR was then carried out on an ABI PRISM 7700 sequence detector (Applied Biosystems), using the SYBR green master mix (Applied Biosystems) or the TaqMan PCR mix comprised of 300 nM (each) the forward and reverse primers, 100 nM probe, 0.025 U/μl of Taq polymerase (Invitrogen), 1× Rox dye (Invitrogen), 125 μM (each) deoxynucleotide triphosphate (Invitrogen), 5 mM MgCl2 (Invitrogen), and 1× Taq polymerase buffer (Invitrogen). The cycling conditions were 94°C for 1 min, followed by 40 cycles at 94°C for 15 s and 60°C for 30 s. Primer Express 2.0 software (Applied Biosystems) was used to design all of the primers and probes. The sequences of the primers and probes for each of the genes tested are shown in Table S1 in the supplemental material. The fold change for each gene was calculated using the cycle threshold (ΔΔCT) method as described in reference 46, and data are represented as E2 fold change over vehicle, unless otherwise stated. For each sample, real-time quantitative reverse transcription-PCRs (qRT-PCRs) were done in triplicate for each gene of interest and the reference gene (β-actin) to normalize for input cDNA. Methylation-specific PCR (MSP) was performed as described previously (68).
Nuclear run-on assays.
Nuclear run-on assays were performed as described previously (57, 67). Briefly, 6 × 107 MCF7 cells were treated with vehicle or E2 for 0.5 and 7 h. The cells were washed with phosphate-buffered saline (PBS), harvested, and lysed in lysis buffer (0.5% NP-40, 10 mM KCl, 10 mM MgCl2, 10 mM HEPES [pH 7.9], and 0.5 mM β-mercaptoethanol [β-ME]) on ice until the cells had uniformly lysed and the nuclei appeared free of cytoplasmic material (∼1 h). The nuclei were centrifuged at 500 × g, washed with lysis buffer without NP-40, and resuspended in 100 μl storage buffer (50 mM Tris-HCl, 5 mM MgCl2, 0.5 mM β-ME, 40% glycerol) before being frozen at −80°C. A 100-μl amount of transcription buffer (10 mM Tris-HCl [pH 8.0], 0.3 M MgCl2, 5 mM dithiothreitol, 40 U RNase inhibitor [Roche], 1× biotin labeling mix [Roche]) was added to the nuclei, and the reaction mixture was incubated at 30°C for 45 min. RNA was isolated by using Trizol reagent (Invitrogen). A 50-μl amount of streptavidin-conjugated magnetic beads (Invitrogen) resuspended in binding buffer (10 mM Tris-HCl [pH 7.5], 1 mM EDTA, 100 mM NaCl) was added to each sample and incubated for 2 h at room temperature. Beads were washed twice in 500 μl of 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and 15% formamide for 15 min and once in 500 μl of 2× SSC for 5 min and then dissolved in 30 μl of diethyl pyrocarbonate-water. RNA was reverse transcribed, and qPCR reactions were carried out as described above.
siRNA.
MCF7 cells (2 × 105) cells were plated in DMEM, and medium was switched to IMEM the next day. The cells were then transfected with 50 nM small interfering RNA (siRNA) using DharmaFECT1 (Thermo Fisher Scientific). The siRNAs used were as follows: siGENOME nontargeting siRNA 2, ERα, HDACs 1 to 10 (Dharmacon), and FoxA1 (4). NCoR, SMRT, LCoR, and NRIP1 siRNA experiments were performed as described previously (31).
Luc assays.
Dual-luciferase (Luc) reporter assays were performed as previously described (69), with some changes described here. Briefly, MCF7 cells (4 × 105) were plated in 6-well plates in DMEM 1 day before transfection with 200 ng/well ERE-thymidine kinase (Tk)-Luc, 100 ng/well pRL-Null (a Renilla Luc construct for normalizing of transfection efficiency), and SAFB1 (used as a positive control) or various concentrations of HDAC7 or HDAC7 deletion constructs using Lipofectamine 2000. Smaller amounts of Flag-HDAC7(438-912) were transfected to keep the protein level relatively similar to that of Flag-HDAC7, as seen in the Western blot (see Fig S5B in the supplemental material). The amounts used were as follows: 100 ng/well of Flag-HDAC7 or Flag-HDAC7(1-487) or Flag-HDAC7 (H670A) or 10 ng/well of Flag-HDAC7(438-912). For the RPRM promoter reporter assays, MCF7 cells were transfected with 500 ng/well of the RPRM promoter and 100 ng/well of pRL-Null. Medium was changed to IMEM 16 h after transfection, cells were treated with vehicle or E2 for 24 h, and luciferase assays were performed as previously described (30).
Plasmids.
The RPRM promoter was amplified by PCR from bacterial artificial chromosome (BAC) clone 389H5 and primer pairs containing BamHI/NcoI digestion sites: RPRM-BamHI-F1 (GTCGGATCCGATTCATATTTTTGTGCAACCATCA) and RPRM-NcoI-R1 (GTCCCATGGTCATTATGTACAGGCTACGCTCGTC). The 5,566-bp PCR product was digested with BamHI and NcoI and inserted into the pGL3-basic luciferase vector. The HDAC7 constructs have been previously published (14). The Flag-HDAC7 (H670A) construct was cloned by subcloning into the pcDNA3.1-Flag-HDAC7 construct a piece of the mutated construct from a mouse stem cell virus (MSCV)-internal ribosome entry site (IRES)-green fluorescent protein (GFP)-HDAC7(437-912) H657A construct using the NaeI site. The histidine mutated in hHDAC7-H670A is shown in boldface in the sequence 665-RPPGHHADHST-675.
Coimmunoprecipitations.
293T cells were plated in 15-cm dishes, transfected with Flag-HDAC7, and the medium was switched to IMEM. The cells were treated with either vehicle or 10 nM E2 for 16 h. After being washed with ice-cold PBS twice, the cells were collected into 1 ml of ice-cold PBS. They were then centrifuged at 800 × g for 2 min at 4°C, and the cell pellet was resuspended in 600 μl of lysis buffer (150 mM NaCl, 1% NP-40, and 50 mM Tris) and incubated for 20 min on ice. The lysate was centrifuged for 20 min at 14,000 × g at 4°C, and the supernatant containing 1 mg of protein was incubated with the ERα antibody H-184 (Santa Cruz) overnight or with Flag-M2 beads (Sigma-Aldrich) for 1 h. Twenty microliters of protein G-Sepharose was added to the ERα sample, and the incubation was continued for 1 h. The Sepharose and Flag beads were centrifuged and washed 4 times in 1 ml of lysis buffer. The washed beads were boiled with Laemmli buffer and subjected to SDS-PAGE followed by immunoblotting with anti-ERα 6F11 (Novocastra Laboratories) and anti-Flag (M2 F1804; Sigma Aldrich) antibodies. A similar procedure was used for the immunoprecipitation in Ly2 cells, where ERα and HDAC7 were immunoprecipitated with anti-ERα HC-20 (Santa Cruz Biotechnology) and anti-HDAC7 H-273 (Santa Cruz Biotechnology), respectively, and immunoblotted with anti-ERα D-12 and F-10 (Santa Cruz Biotechnology) and anti-HDAC7 H00051564 (Abnova Corporation). Coimmunoprecipitation experiments for HDAC7 and FoxA1, in MCF7 cells, were done using a similar procedure in which HDAC7 was immunoprecipitated with anti-HDAC7 H-273 (Santa Cruz Biotechnology) and ab53101 (Abcam) antibodies and FoxA1 was immunoprecipitated with anti-FoxA1 ab5089 (Abcam) and C20 (Santa Cruz) antibodies. The membranes were immunoblotted with anti-HDAC7 H-273 (Santa Cruz) and anti-FoxA1 ab5089 (Abcam).
ChIP assays.
Chromatin immunoprecipitation (ChIP) assays were performed as described before (64). Briefly, MCF7 cells (3 × 106) were plated in 15-cm dishes in DMEM. After 24 h, the cells were washed with PBS twice and then switched to IMEM. The medium was changed on day 2 of culture, and on day 3 of culture, the cells were treated with either vehicle, 10 nM E2, or 1 μM 4-OH-tam for 45 min. After DNA purification (QIAquick spin kit; Qiagen), the promoter regions were amplified by qRT-PCR using the primers described in Table S2 in the supplemental material. The ChIP antibodies used were ERα antibody H-184 and HC20 (Santa Cruz Biotechnology), FoxA1 antibodies ab5089 (Abcam) and C20 (Santa Cruz Biotechnology), H3K9ac (Jiemin Wong), H3K27me3 antibody (Upstate), H3K4me1 antibody ab8895 (Abcam), H3K4me2 antibody 07-030 (Millipore), H3K4me3 antibody MC315 (Millipore), Pol II antibody 8WG16 (Covance), HDAC7 antibody ab53101 (Abcam), p300 antibody N-15 (Santa Cruz Biotechnology), H3K9me2 antibody ab1220 (Abcam), H3K9me3 antibody ab8898 (Abcam), H4K16ac antibody 07-329 (Upstate), and HA antibody HA.11 (Covance). qPCR was used to calculate enrichment, which is displayed as “% Input” on the y axis or log2 E2 fold change compared to vehicle control. Primers are presented in the supplemental material.
Immunofluorescent staining and confocal microscopy.
293 cells (7.5 × 104) were plated on poly-d-lysine-coated coverslips in DMEM in 24-well plates. One day after plating, cells were washed twice with PBS, fixed in 4% formaldehyde diluted in PBS for 10 min at room temperature, washed twice with PBS, permeabilized with 0.5% Triton X-100 diluted in PBS for 5 min, washed twice with PBS, and then incubated with 1% bovine serum albumin (BSA) in PBS for 1 h. Following this, the cells were incubated with primary antibody (anti-Flag, diluted 1:100 in 1% BSA in PBS) for 1 h at room temperature, washed three times with PBS, incubated with secondary antibody (Alexa 488-conjugated goat anti-mouse IgG, diluted 1:1,000 in 1% BSA in PBS) for 1 h at room temperature in the dark, washed three times with PBS, incubated with the DNA marker TOPRO-3 (diluted 1:80 in PBS) for 30 min at room temperature in the dark, washed three times with PBS, and then mounted with mounting medium. Confocal images were obtained using a Nikon confocal Eclipse E1000 scanning laser microscope (Nikon) with a ×60 objective lens.
RESULTS
RPRM is a primary and direct ERα target gene.
Using previously published gene expression profiling studies (7, 9, 16, 17, 43, 72), we identified 20 candidate genes which were consistently and robustly repressed by estrogen, including the Reprimo (RPRM), ceramide kinase (CERK), kruppel-like factor 6 (KLF6), claudin 4 (CLDN4), EGF-containing fibulin-like extracellular matrix protein 1 (EFEMP1), ectodermal-neural cortex (ENC1), BCL2-interacting killer (BIK), HMG-box transcription factor 1 (HBP1), chemokine (C-X-C motif) receptor 4 (CXCR4), RAP1 GTPase activating protein 1 (RAP1GA1), P-cadherin (CDH3), bone morphogenetic protein 7 (BMP7), MAX dimerization protein 4 (MXD4), mucin 1 (MUC1), N-myc downstream-regulated gene 1 (NDRG1), retinoblastoma-like 2 (RBL2), BCL2-antagonist/killer 1 (BAK), cyclin-dependent kinase inhibitor 1A (p21/CDKN1A), polo-like kinase 2 (PLK2), and dual-specificity phosphatase 1 (DUSP4) genes. To validate this finding, we examined the effect of E2 on expression of these genes in MCF7 cells, using E2 induction of pS2 and E2 repression of CCNG2 as positive controls. We confirmed significant repression of 15 genes (RPRM, KLF6, CLDN4, CERK, EFEMP1, ENCI, BIK, HBP4, CXCR4, RAP1GA1, CDH3, BMP7, MXD4, MUC1, and NDRG1) (Fig. 1A; see Fig. S1 in the supplemental material). As expected, a 100-fold excess of the antiestrogen ICI 182,780 (ICI) prevented the repression of the genes, suggesting that these genes are in fact regulated by ER. Some targets were repressed only at the late time point, such as MXD4, suggesting indirect regulation: for example, through an NRIP1-dependent pathway (5). However, timing of repression needs to be interpreted with caution, since late repression could also simply be a result of a long mRNA half-life. We tested repression of a subset of the genes (RPRM, KLF6, CLDN4, CERK, EFEMP1, and ENC1) in the ERα-positive breast cancer cell lines ZR-75-1 (Fig. 1B; see Fig. S2A in the supplemental material) and T47D (Fig. 1C; also see Fig. S2B in the supplemental material) and found cell-type-specific differences which may be attributed to the absence and/or differential recruitment of coregulators.
FIG. 1.
Identification of estrogen-repressed genes in breast cancer cell lines. qRT-PCR was used to test E2-mediated repression of candidate genes, with pS2 and CCNG2 serving as controls. The relative mRNA expression is depicted as ligand-mediated fold change compared to vehicle control. This fold change is reflected in the color intensity, as shown in the color scale, and E2-mediated repression for each gene shown is significant for at least the time point shown. (The raw relative mRNA expression data are shown in Fig. S1 in the supplemental material.) MCF7 (A), ZR-75-1 (B), and T47D (C) breast cancer cells were treated for 4, 8, or 24 h with either vehicle, E2, or a combination of E2 and ICI 182,780. The data are an average from three replicates.
RPRM showed the most robust repression in both MCF7 and ZR-75-1 breast cancer cells. RPRM has previously been shown to cause cell cycle arrest (55), an effect which we were able to reproduce in MCF7 cells. Knockdown of RPRM with siRNA resulted in significantly increased entry into S phase (Fig. 2A). Based on its robust repression and its potential role in tumorigenesis, we studied the mechanism of its repression in more detail. First, we tested whether repression is mediated via ERα, which is the main functional estrogen receptor in breast cancer cells (38). We examined RPRM repression in the absence and presence of ERα using an siRNA that decreases ERα levels by >90% (Fig. 2B, inset). The E2-mediated repression of RPRM was abolished when ERα was silenced, clearly demonstrating a requirement for ERα in repression (Fig. 2B). Since it has been suggested that E2 can still influence signaling in an ERα-independent manner (24, 49), we tested RPRM repression in an ERα-negative cell line, HCC1143. We chose this less commonly used cell line because RPRM expression was suppressed, due to promoter hypermethylation, in most of the more commonly used ERα-negative breast cancer cell lines (data not shown). E2 failed to repress RPRM in HCC1143 cells, providing additional evidence that ERα is required for the repression (Fig. 2C).
FIG. 2.
RPRM, a cell cycle inhibitor, is a primary and direct ERα target gene. (A to I) qRT-PCR was used to calculate relative RPRM mRNA expression as ligand-mediated fold change compared to the vehicle control. The data are an average of three replicates ± standard error of the mean (SEM). (A) RPRM knockdown increases S-phase entry of breast cancer cells. MCF7 cells were transfected with either nonspecific siRNA (siNS) or RPRM siRNA (siRPRM), and qPCR was used to calculate relative mRNA expression. The data are an average from three replicates ± SEM. siNS or RPRM siRNA-transfected MCF7 cells were treated with either vehicle or E2 for 16 h, and the percentage of cells in each phase of the cell cycle (G0/G1, S, and G2/M) was determined using fluorescence-activated cell sorter (FACS) analysis. The data are an average of three replicates in four independent experiments. (B) MCF7 cells were transfected with either nonspecific siRNA (siNS) or ERα siRNA (siER) followed by either vehicle or E2 treatment for 12 h. The inset shows protein levels of ERα and β-actin as measured by immunoblotting. WB, Western blotting. (C) ERα-negative HCC1143 breast cancer cells were treated with vehicle or E2 for 12 h, and RNA was measured by qPCR. (D) MCF7 cells were treated with E2 for 1, 2, 3, 4, and 12 h. (E) MCF7 cells were treated with different doses of E2, as indicated, for 8 h. The data are represented as relative mRNA expression of the different doses compared to the E2 dose of 10−12 M. (F) MCF7 cells were treated with E2, 4-OH-tamoxifen (tam), and ICI 182,780, for 16 h, and RNA was isolated for qRT-PCR. veh, vehicle. (G) MCF7 cells were treated with either vehicle, E2, or a combination of vehicle or E2 and cycloheximide (CHX) for 4 and 8 h. (H) MCF7 cells were treated with either actinomycin D (ActD) or a combination of ActD and E2 for 4 and 8 h. The data are an average of three replicates ± standard deviations (SD). (I) Nuclear run-on assays were performed in MCF7 cells treated with E2 for 0.5 h and 7 h, as previously described (67). Transcript levels of pS2, CCNG2, and RPRM were determined by qRT-PCR. The error bars represent the SEM from three determinations. In panels B, F, and G, asterisks indicate P < 0.05 by t test.
A time course analysis showed that RPRM was quickly and robustly repressed by E2 as early as 3 h and was reduced to less than 15% of its initial levels by 12 h (Fig. 2D). Maximum RPRM repression was reached at 10−10 M (Fig. 2E), suggesting that the repression occurs at physiological E2 doses. RPRM was also repressed by the selective estrogen receptor modulator (SERM) tamoxifen, but not by the pure antiestrogen ICI 182,780 (Fig. 2F).
Although the rapid repression of RPRM suggested that it is a primary ERα target, we also tested the effect of the translational inhibitor cycloheximide (CHX) on repression (Fig. 2G). E2-mediated repression of RPRM was not affected, suggesting that it does not require the synthesis of an intermediate regulatory protein for its repression. Similarly, some of the other candidate genes previously tested (Fig. 1A), including those coding for KLF6, CLDN4, CERK, EFEMP1, and ENC1, were still repressed by E2 in the presence of CHX, indicating that they are also primary ERα targets (see Fig. S3 in the supplemental material).
To test whether E2 affects the stability of RPRM mRNA, we treated the cells with actinomycin D (ActD). As shown in Fig. 2H, the addition of ActD decreased RPRM mRNA levels, but this was not significantly affected by E2, suggesting that estrogen does not affect the stability of RPRM mRNA. Finally, we performed nuclear run-on assays to directly test whether the addition of estrogen would result in a decrease of newly synthesized RPRM RNA transcripts (Fig. 2I). As a positive control, we used pS2, which showed the expected increase in transcription rate. Transcription for CCNG2 increased initially, but subsequently decreased, as recently reported (67). In contrast, transcription rates for RPRM were already decreased by 16% after 30 min of E2 treatment and were further decreased by 52% after 7 h of treatment. Collectively, these data suggest that RPRM is a primary and direct ERα target gene that is rapidly repressed at the transcriptional level by physiological doses of E2.
ERα is recruited to an E2-responsive promoter region.
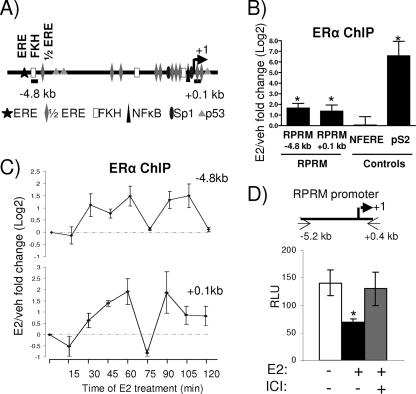
Genome-wide studies using the chromatin immunoprecipitation (ChIP)-on-chip assay, coupled with gene expression array analysis, have shown an overrepresentation of ERα binding sites within 50 kb of the transcriptional start site in upregulated genes. However, this did not hold true for early downregulated genes, suggesting that many E2-repressed genes might not show ERα recruitment or that recruitment is weaker (5, 44). Consistent with this notion, these genomewide studies did not reveal any ERα binding sites within 50 kb of the RPRM transcriptional start site. Intriguingly, the RPRM gene contains multiple half-ERE sites, one palindromic ERE site, and multiple Forkhead (FKH) binding sites, as well as other transcription factor binding sites previously found to play a role in estrogen response (Fig. 3A). To directly test whether ERα could be recruited to these putative sites, we performed ChIP assays, using primers for the ERE/FKH site (−4.8 kb) and the transcriptional start site (+0.1 kb). They showed estrogen-induced recruitment of ERα (Fig. 3B), with minimal recruitment of ERα to a nonfunctional ERE (NFERE), recently described as a negative control for ERα ChIP assays (47). Although the E2-induced recruitment of ERα to the RPRM enhancer was consistent, it was considerably weaker than recruitment to the promoter of pS2, a classical E2-induced gene. Time course ChIP experiments showed that ERα was recruited in a cyclical manner, with two apparent cycles within a 120-min period (Fig. 3C).
FIG. 3.
ERα is recruited to an E2-responsive RPRM promoter. (A) Simplified model showing consensus sites and positions of primers used in subsequent ChIP assays in the RPRM gene. (B) ChIP assays for ERα in MCF7 cells after treatment with vehicle (veh) or E2 for 45 min. The recruitment of ERα to different positions in the RPRM gene as well as to the negative control region, NFERE, and the pS2 promoter is shown. Data are represented as the log2 E2 fold change compared to vehicle control and are an average of four independent experiments ± SEM. A t test analysis was performed in which E2 treatment groups were compared to the vehicle group. *, P < 0.05 (C) ChIP assays for ERα in MCF7 cells after treatment with E2 for various time points, as indicated. The recruitment of ERα to different positions in the RPRM gene is shown. Data are represented as the log2 E2 fold change compared to vehicle control and are an average of two independent experiments. (D) MCF7 cells were transfected with the RPRM promoter and treated with vehicle, E2, or ICI for 24 h, following which luciferease reporter assays were performed. The data represent relative luminescence units (RLU) that are an average of three replicates ± SD and are representative of at least five independent experiments. *, P < 0.05, t test.
To test whether the RPRM promoter, harboring the ERα-binding sites, was indeed repressed by E2, we cloned a 5.6-kb promoter fragment into a luciferase reporter and tested its activity in transient reporter assays (Fig. 3D). These studies showed that E2 was able to repress activity of the promoter and that this repression could be reversed with the antiestrogen ICI 182,780. Collectively, these data suggest that ERα is recruited to the RPRM promoter, which contains a number of EREs, mediating E2 repression of RPRM transcription.
HDAC7 has a unique role in E2 repression of RPRM.
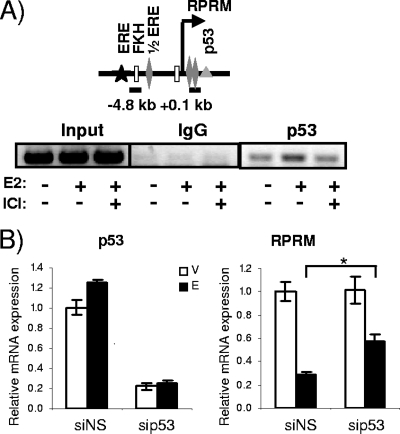
Given the previous evidence for corepressors being involved in estrogen repression (5, 25, 54, 66, 74), we asked whether they would play a role in RPRM repression. However, knockdown of the corepressors NCoR, SMRT, NRIP1, LCoR (ligand-dependent corepressor), SAFB1 (scaffold attachment factor B1), and SAFB2 failed to relieve repression (data not shown). Based on the previously reported role of p53 in regulating ERα activity (45), the lack of RPRM repression in T47D cells which are p53 negative, and finally the putative p53 binding sites in the RPRM promoter, we next asked whether p53 was involved in E2 repression of RPRM. To answer this question, we tested whether p53 was recruited to the promoter and whether it was necessary for repression. ChIP assays revealed p53 binding to the proximal promoter region (Fig. 4A). Knockdown of p53, resulting in ∼80% decrease of RNA levels, had a significant yet minimal effect on repression (Fig. 4B). Hence we conclude that p53 may be involved in E2 repression of RPRM but does not play a major role in this process.
FIG. 4.
Involvement of p53 in E2 repression of RPRM. (A) ChIP assays for p53 and IgG (negative control) in MCF7 cells after treatment with vehicle or E2 for 45 min. The recruitment of p53 to the RPRM +0.1-kb promoter is shown. Data are representative of three independent experiments. (B) MCF7 cells were transfected with nonspecific siRNA (siNS) or siRNA against p53 followed by either a vehicle (V) or an E2 (E) treatment for 12 h. The knockdown of p53 is shown in the left panel, and its effect on RPRM expression is shown in the right panel. qRT-PCR was used to calculate relative mRNA expression as fold change compared to the vehicle control. The data are an average of three replicates ± SEM. *, P < 0.05, t test comparing E2 repression in the presence of siNS and sip53.
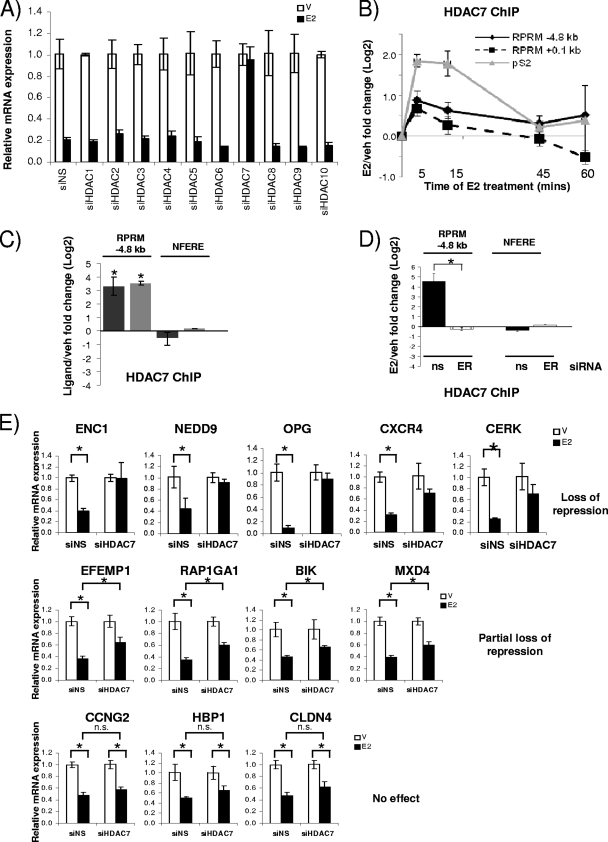
Since the recruitment of HDACs and the deacetylation of histones have been shown for other E2-repressed genes (66), we next examined whether HDACs played a role in RPRM repression. We individually silenced HDACs 1 to 10 using siRNAs, which resulted in 60 to 90% decreases of HDAC RNA levels (see Fig S4A in the supplemental material). Knockdown of HDAC3 and HDAC8 resulted in consistently lower basal activity, suggesting a role for these HDACs as positive regulators of RPRM transcription (Fig. S4B). However, out of the 10 tested HDACs, only HDAC7 affected E2 repression of RPRM—knockdown of HDAC7 resulted in complete relief of repression (Fig. 5A). Subsequent ChIP assays showed that E2 treatment resulted in HDAC7 recruitment to the RPRM promoter (Fig. 5B). Like ERα, it was recruited to both the −4.8-kb ERE and the transcriptional start site. We also observed cycling of HDAC7, although with a shorter cycling time: the maximum recruitment was between 5 and 15 min. Recruitment to the pS2 promoter was used as a control, and HDAC7 recruitment was detected, as previously reported (50).
FIG. 5.
HDAC7 is necessary for repression of RPRM and other E2-repressed genes. (A) RPRM qRT-PCR was performed using RNA from MCF7 cells transfected with nonspecific siRNA (siNS) or siRNA against HDACs 1 to 10 and treated with vehicle (V) or E2 (12 h). (B) HDAC7 ChIP in MCF7 cells treated with vehicle (veh) or E2 for 5, 15, 45, and 60 min. Data are represented as the log2 E2 fold change compared to vehicle control and are an average of two independent experiments ± SEM. (C) HDAC7 ChIP in MCF7 cells treated with vehicle, E2, or 4-OH-tamoxifen for 15 min. Nonfunctional ERE was used as negative control. *, P < 0.05, t test comparing recruitment in the presence of E2 versus vehicle. (D) HDAC7 ChIP in MCF7 cells, transfected with siNS (ns) or ERα (ER) siRNA, followed by 45 min of treatment with vehicle or E2. *, P < 0.05, t test comparing recruitment in the presence of siNS and ERα siRNA. (E) The E2-mediated repression of candidate genes was tested using RNA from MCF7 cells transfected with siNS or HDAC7 siRNA. The data are an average of three replicates ± SD and are representative of two independent experiments. *, P < 0.05, t test.
Given that treatment with the SERM tamoxifen also resulted in repression (Fig. 2F), we asked the question whether tamoxifen treatment would result in recruitment of HDAC7 to the RPRM promoter. ChIP assays were performed in the presence of estradiol or tamoxifen, and HDAC7 recruitment was comparable between the two ligands (Fig. 5C). Finally, we tested whether HDAC7 recruitment was ERα dependent. Therefore, MCF7 cells were transfected with siRNA to ERα and treated with E2, and ChIP assays were performed. These studies showed that loss of ERα totally abolished HDAC7 recruitment to the RPRM promoter (Fig. 5D).
To examine whether the effect of HDAC7 was specific for RPRM repression or might be part of a more general mechanism, we tested whether HDAC7 was required for repression of other E2-repressed genes (n = 12). Intriguingly, knockdown of HDAC7 resulted in complete loss of E2 repression of ENC1, NEDD9, OPG, CXCR4, and CERK (Fig. 5E). There was a partial effect on E2 repression of EFEMP1, RAP1GA1, BIK, and MXD4, while repression of CCNG2, HBP1, and CLDN4 was not affected. Collectively, these data suggest a previously unknown role for HDAC7 in E2 repression of RPRM, which might also be applicable to repression of at least some other E2-regulated genes.
HDAC7 interacts with ERα and can repress ERα's transcriptional activity independent of its deacetylase function.
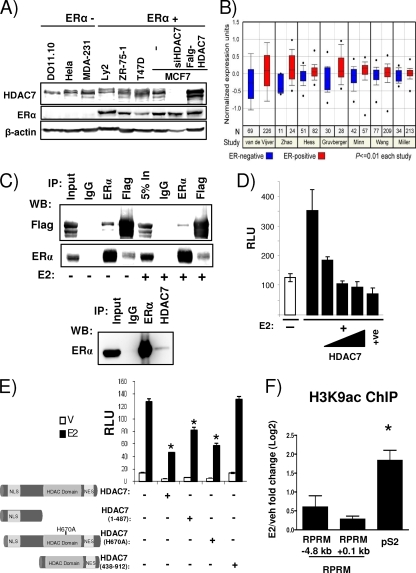
The observed role for HDAC7 in E2-mediated repression prompted us to examine the relationship between HDAC7 and ERα in more detail. First, we determined HDAC7 protein expression in a series of breast cancer cell lines. As controls, we used the thymocyte hybridoma cell line DO11.10 described in previous HDAC7 studies (56) and extracts from cells transfected with HDAC7 siRNA or HDAC7 cDNA. As shown in Fig. 6A, HDAC7 is widely expressed in breast cancer cell lines. In breast tumors, its levels are significantly associated with ERα expression (http://www.oncomine.org/) (Fig. 6B). Coimmunoprecipitations in cells with overexpressed tagged HDAC7 (Fig. 6C, top panel) showed an interaction between HDAC7 and ERα which was not affected by estrogen treatment. Interactions could also be detected with endogenous ERα and HDAC7 proteins (Fig. 6C, bottom panel). Next we asked the question whether the ERα-HDAC7 interaction observed in the coimmunoprecipitation assay would result in decreased ERα activity, as measured in the classical ERE-Tk-reporter assay. Transient transfection of ERE-Tk-Luc, together with increasing concentrations of HDAC7, resulted in a dose-dependent decrease of ERα activity, suggesting that HDAC7 recruitment to ERα caused formation of a repressive complex (Fig. 6D).
FIG. 6.
HDAC7 interacts with ERα and represses its transcriptional induction activity independent of HDAC7's deacetylase activity. (A) Protein expression levels of HDAC7, ERα, and β-actin in cell lines, as indicated, were measured by immunoblotting. (B) Normalized expression data for HDAC7 in ERα-positive and -negative tumors in various studies as listed on the graph obtained from the Oncomine Cancer Profiling Database. (C, upper panel) 293T cells were transfected with ERα and Flag-HDAC7, treated with vehicle or E2 (16 h), lysed, and immunoprecipitated (IP) and immunoblotted as indicated. (C, lower panel) Coimmunoprecipitation of endogenous ERα and HDAC7 in Ly2 breast cancer cells, using antibodies as indicated. Five percent (top panel) or 1% (bottom panel) input was loaded on the gel. (D) MCF7 cells were transfected with ERE-Tk-Luc and increasing amounts of Flag-HDAC7 (0, 50, 100, and 250 ng) or SAFB1 (250 ng [used as a positive control]) (30) and treated with vehicle or E2. The data represent the average of three replicates ± SD and are representative of five independent experiments. (E) MCF7 cells were transfected with ERE-Tk-Luc and pcDNA3.1 or Flag-HDAC7 constructs and treated as indicated. The data represent averages of three replicates ± SD and are representative of three independent experiments. A t test analysis was performed in which the E2 treatment group for each HDAC7 transfection was compared to the E2 treatment group in the absence of HDAC7 transfection. *, P < 0.05. V, vehicle. (F) ChIP assays for H3K9ac were performed in MCF7 cells after treatment with vehicle (veh) or E2 for 45 min. The recruitment to different positions in the RPRM gene and pS2 promoter is shown. Data are represented as the log2 E2 fold change compared to the vehicle control and are an average of two independent experiments ± SEM. *, P < 0.05, t test comparing recruitment in absence and presence of E2.
It was previously shown that the corepressor activity of class II HDAC family members, like HDAC7, on transcription factors, including AR (33), MEF2 (11), and Runx2 (29), can be independent of their deacetylase activity. To test whether HDAC7 deacetylase function was necessary for repression of ERα activity, we overexpressed the N-terminal region (positions 1 to 487) of HDAC7, which lacks the histone deacetylase domain, in transient ERE-Tk reporter assays. We found that the N terminus was sufficient for significant repression (Fig. 6E), suggesting that HDAC7-mediated repression was also independent of its deacetylase activity. To conclusively prove this point, we mutated the histidine at HDAC7 position 670 (H670A), corresponding to H657 in mouse HDAC7, which was previously shown to be necessary for deacetylase activity (10). Overexpression of HDAC7-H670A resulted in repression comparable to that of wild-type HDAC7, thus confirming that the HDAC7-mediated repression of ERα's activity was deacetylase independent. As a control, we transfected a C-terminal protein (positions 439 to 912) that localized to the cytoplasm due to lack of the nuclear localization signal (NLS) (see Fig. S5 in the supplemental material) and, as expected, was unable to confer repression. Consistent with the lack of involvement of HDAC7's deacetylase activity, we were unable to detect E2-mediated changes in histone 3 lysine 9 acetylation (H3K9ac), H4K16 acetylation, or recruitment of p300 (Fig. 6F) (data not shown). Together, these data suggest that HDAC7 can bind to ERα and mediate repression independent of its deacetylase activity.
FoxA1 is necessary for RPRM repression and interacts with HDAC7.
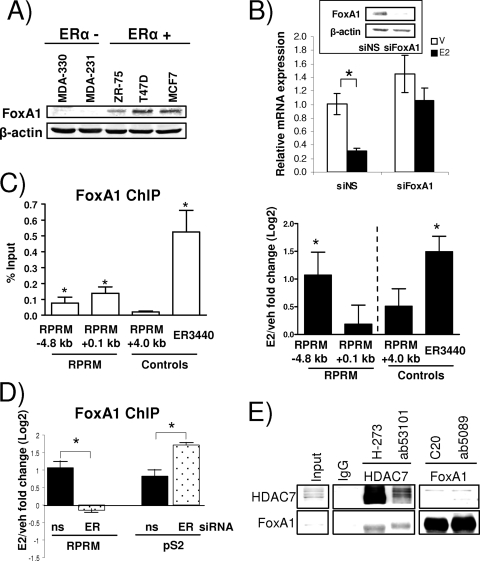
The Forkhead (FKH) family member FoxA1 is a transcription factor known to initiate chromatin remodeling at E2-responsive promoters (4, 12, 41). FoxA1 was originally described as a pioneering factor for chromatin remodeling events associated with activation of genes (4, 41, 47); however, just like induced genes, E2-repressed genes contain significantly more ER/FoxA1-overlapping sites compared to non-E2-regulated genes (47). In addition, FoxA1 has been reported to play a role in regulating basal expression of E2-repressed genes and a significant fraction of its binding sites have a relatively closed chromatin conformation and are characterized by repressive histone marks (3, 47). Given that the functional ERE site at −4.8 kb was adjacent to a sequence (ATGTAAATAC) with high similarity to the consensus FKH FoxA1 binding motif (4) and the presence of additional putative FoxA1 binding sites in the RPRM promoter, we asked whether FoxA1 was involved in RPRM repression.
At first, we measured FoxA1 protein expression in a number of breast cancer cells and observed a strong correlation with ERα positivity (Fig. 7A), as was previously described (2). Knockdown of FoxA1 in MCF7 cells, using siRNA, resulted in loss of E2-mediated repression of RPRM (Fig. 7B), providing strong evidence that FoxA1 activity is necessary for the estrogen repression of RPRM. ChIP assays revealed that FoxA1 was bound to overlapping ERE/FoxA1 site (−4.8 kb), and the proximal promoter region (0.1 kb) (Fig. 7C, left panel), although the estrogen effect was more pronounced at the overlapping ERE/FoxA1 site (right panel). As a positive control, we tested FoxA1 recruitment to ER3440, an ERα/FoxA1-shared binding site recently described (47). Similar results were also obtained using a different FoxA1 antibody in the ChIP assays (see Materials and Methods for details on antibodies). Estrogen-induced recruitment of FoxA1 was ERα dependent—knockdown of ERα by siRNA totally abolished FoxA1 recruitment (Fig. 7D). In contrast, loss of ERα did not affect FoxA1 recruitment to the pS2 promoter, an estrogen-induced gene, suggesting differences in FoxA1 recruitment between estrogen-repressed and -induced genes. Finally, ChIP assays revealed that HDAC7 recruitment was not a prerequisite for FoxA1 recruitment to the RPRM promoter (data not shown).
FIG. 7.
FoxA1 is essential for E2 repression of RPRM. (A) Protein expression levels of FoxA1 and β-actin in ERα-negative and -positive cell lines, as indicated, were measured by immunoblotting. (B) RPRM qRT-PCR using RNA from MCF7 cells that were transfected with siNS or FoxA1 siRNA and treated with vehicle (V) or E2 (12 h). The inset shows protein levels of FoxA1 and β-actin, as measured by a Western blot. The data are an average of three replicates ± SD and are representative of three independent experiments. *, P < 0.05, t test. (C) FoxA1 ChIP in MCF7 cells. (Left panel) Data are represented as percent input and are an average of four independent experiments ± SEM. *, P < 0.05, t test comparing recruitment relative to +4.0-kb RPRM control recruitment. (Right panel) Cells were treated with vehicle (veh) or E2 for 45 min. FoxA1 recruitment is represented as the log2 E2 fold change compared to vehicle control and is an average of three independent experiments ± SEM. *, P < 0.05, t test comparing recruitment in the presence of E2 versus vehicle. (D) FoxA1 ChIP in MCF7 cells, transfected with siNS (ns), or ERα siRNA, followed by 45 min of treatment with vehicle or E2. Data are presented and analyzed as in the right panel in panel C. (E) Endogenous HDAC7 and FoxA1 were coimmunoprecipitated in MCF7 cells, using antibodies as indicated. Five percent input was loaded on the gel.
Given the binding of HDAC7 and FoxA1 to the same RPRM promoter binding sites and their shared requirement for E2 repression, we asked whether the two proteins interact. This was tested in coimmunoprecipitations, using two independent antibodies for both HDAC7 and FoxA1 (Fig. 7E). Performing the immunoprecipitation with the HDAC7 antibodies, we observed strong interactions with FoxA1. The interaction, which was estrogen independent (data not shown), was weaker in the reciprocal coimmunoprecipitation, but could still be detected. The interaction between ERα and HDAC7 was not significantly affected by different amounts of FoxA1 (data not shown), suggesting that HDAC7 and FoxA1 do not compete with each other for binding to ERα.
Ligand-dependent H3K4 demethylation is associated with release of RNA Pol II.
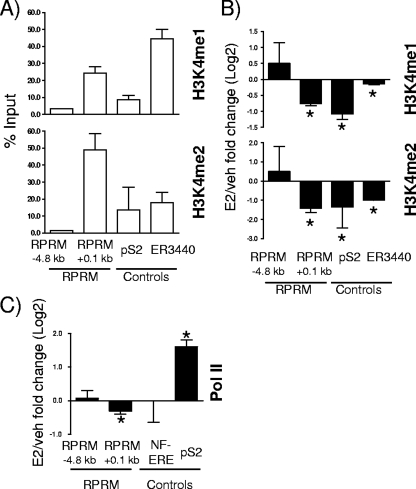
FoxA1 occupies only a very small percentage of its potential recognition motifs (less than 4%) (47), and recent studies have shown that its recruitment is guided by histone H3 lysine 4 (H3K4) methylation, especially H3K4me1/me2 (47). Analysis of these specific histone modifications at the FoxA1 recruitment sites in the RPRM promoter revealed a significant and strong presence of H3K4me1 and H3K4me2 at the proximal ER/FoxA1 binding site at +0.1 kb (Fig. 8A). We again used ER3440, the recently described ERα/FoxA1-shared binding site (47), and pS2 as controls, where we also found H3K4me1/me2 present, as expected. The removal of these marks was recently shown to be necessary for E2 induction of genes (20), and we hence sought to determine if decrease of H3K4me1/me2 recruitment was also associated with E2 repression of target genes. ChIP assays were performed, and significant decreases of H3K4me1/me2 were observed for ER3440 and pS2 and importantly also for the proximal RPRM promoter region (Fig. 8B). Finally, we sought to determine whether this was associated with dissociation of RNA Pol II. In agreement with transcriptional repression of RPRM, RNA Pol II was significantly released upon E2 treatment (Fig. 8C). Together, these results provide compelling evidence for the role of an interplay between FoxA1 and H3K4me1/me2 in regulation of E2-repressed genes, in addition to the previously reported role in gene induction.
FIG. 8.
H3K4me1/2 demethylation and Pol II release at RPRM promoter. (A) ChIP assays for H3K4me1 and H3K4me2 were performed in MCF7 cells. Recruitment to different positions in the RPRM gene, the pS2 promoter, and the ERα binding site ER3440 used as a control (47) is shown. Data are represented as % input and are an average of two independent experiments ± SEM. (B) ChIP assays for H3K4me1 and H3K4me2 were performed in MCF7 cells after treatment with vehicle (veh) or E2 for 45 min. The recruitment to different positions in the RPRM gene, the pS2 promoter, and the ERα binding site ER3440 used as a control (47) is shown. Data are represented as the log2 E2 fold change compared to vehicle control and are an average of two independent experiments ± SEM. *, P < 0.05, t test comparing recruitment in the presence of E2 versus vehicle. (C) ChIP assays for RNA Pol II were performed in MCF7 cells after treatment with vehicle or E2 for 45 min. Data are represented as the log2 E2 fold change compared to vehicle control and are an average of three independent experiments ± SEM. *, P < 0.05, t test comparing recruitment in the presence of E2 versus vehicle.
DISCUSSION
Here we report a novel mechanism for estrogen-mediated repression of gene expression involving an interplay between ERα, HDAC7, and FoxA1. Over the last 2 decades, induction of genes by ERα has been a subject of intense investigation, but recent studies have shown that over half of E2-regulated genes are actually repressed. It is likely that an understanding of E2-mediated repression of transcription will allow a more thorough understanding of how ERα mediates its downstream actions, such as regulation of cell proliferation. Transient repression of cell cycle inhibitors such as CCNG2 and RPRM is likely to play a vital role in mediating estrogen's effects in hormone-responsive cells, thus contributing both to estrogen action in normal physiology and to various pathological processes such as breast cancer. For example, ERα represses ErbB2 expression, and loss of this control may lead to aggressive ErbB2-positive tumors with poor outcome. Surprisingly, despite the long-held knowledge of the inverse correlation between ERα and ErbB2 in breast tumors, few studies have directly investigated how ERα downregulates ErbB2 (28, 52). It is therefore critical that we unravel the full diversity of mechanistic programs for ERα-mediated gene repression.
Currently, there is evidence for three models of estrogen repression, which are not mutually exclusive. In the first model, repression is simply the result of loss of activation, due to squelching of coactivators from promoters or enhancers (5, 32). Second, corepressors can compete with coactivators at the regulatory region of the repressed gene, with competition of PAX2 with the ERα coactivator AIB-1/SRC-3 for binding and regulation of ErbB2 transcription being a recent example (28). The third model involves active recruitment of repressive complexes, including NCoR, SMRT, and HDACs (25, 35, 66, 74). Our data provide strong evidence for “active repression” playing a crucial role for a significant subset of estrogen-repressed genes.
Here we show estrogen-dependent recruitment of ERα, HDAC7, and FoxA1 to the proximal and also more distal promoter in the RPRM gene. We also find a modest role for p53 in RPRM repression, and there might be other factors that play a role in this process that have yet to be identified. Previous studies have shown that HDACs, such as HDAC1 and HDAC4, can interact with ERα and repress its transcriptional activity (34, 42). Moreover, various ERα-associated corepressors, including NCoR, SMRT, NRIP1, LCoR, MTA1, REA, and SAFB1/2 have been shown to recruit HDACs (13, 15, 26, 39, 73). However, this is the first time that a unique role of HDAC7 in estrogen repression has been described.
HDAC7 belongs to the class IIa HDACs, which also include HDAC4, HDAC5, HDAC6, and HDAC9. The members share a bipartite domain organization with significant sequence similarities in the long N-terminal extension and the C-terminal catalytic domains. HDAC7 can bind to a number of DNA-binding transcription factors, including the myocyte enhancer factor MEF2 (11, 22, 23) and the androgen receptor (AR) (33), for which it serves as a corepressor. Its corepressor activity is, at least in part, regulated through interaction with an NCoR/SMRT-containing complex; however, this is not likely to be the case for RPRM repression since NCoR and SMRT knockdown did not affect its repression. HDAC7-mediated repression of RPRM was independent of its deacetylase function, as also reported for its effect on AR (33), MEF2 (11), and Runx2 (29). The deacetylase activity in HDAC7 is known to be relatively weak (61), and it is therefore likely that the protein has additional enzymatic and/or nonenzymatic functions critical in repression, which are yet to be discovered. One possibility is that its recently described Sumo E3 ligase activity (18, 23) might play a role in ERα given that posttranslational modification by sumoylation is primarily associated with transcriptional repression (48). Future studies will address this question by testing whether HDAC7 can mediate sumoylation of ERα, which has previously been shown to be sumoylated (62, 71). Since we identified HDAC7 interaction with FoxA1, a second option, albeit more speculative, is that FoxA1 is also modified by sumoylation, with HDAC7 functioning as its E3 ligase. Clearly, given that HDAC7 is also recruited to pS2, yet pS2 is induced and not repressed, posttranslational modifications of HDAC7 or HDAC7-interacting proteins or recruitment of additional proteins to the complex can be expected.
The presence of FKH consensus sites in the RPRM promoter raised the question whether FoxA1 was involved in RPRM repression. Our results revealed that FoxA1 binds to the promoter and is necessary for repression. This is a novel observation, since to date, FoxA1 has exclusively been described as a licensing factor for chromatin remodeling events associated with E2-induction of genes (4, 41, 47), although there is some evidence for its role in the maintenance of basal expression of estrogen-repressed genes (3, 47). A role for FoxA1 in repression is supported by genomewide ChIP-on-chip studies comparing FoxA1 and ERα cistromes which identified a highly significant overlap of ERα and FoxA1-binding sites in both estrogen-induced and -repressed genes (4, 47). Although FoxA1 in general has mostly been described in induction of transcription, it has been shown to be capable of repression: for example, of AFP promoter activity in hepatoma cells (27).
Recent studies by the Brown laboratory have shown that FoxA1 translates epigenetic signatures into enhancer-driven transcription, and our findings certainly fit this model (47). The binding site of FoxA1 in the RPRM regulatory genomic region is marked by H3K4me1 and -me2, the same epigenetic mark which was previously shown to be critical for FoxA1 recruitment (47). Recent studies have also shown that demethylation of H3K4m1 and H3K4me2 is associated with estrogen response—LSD1 demethylates HeK4me1/m2, which was necessary for estrogen-dependent induction of genes such as the pS2 and GREB1 genes (20). Similarly, a decline in both HeK4me2 and HeK9me has been reported on the prostate-specific antigen (PSA) promoter upon androgen treatment in prostate cancer cells (36, 51). These data support a model in which histone methyltransferases impose inhibitory marks and dismissal of those marks by demethylases is required to permit recruitment of coactivators and other factors necessary for gene induction. Our studies add an additional layer of complexity, showing that decrease of these marks is not only associated with transcriptional activation but also with genes targeted for repression.
Finally, we show that recruitment of ERα, HDAC7, and FoxA1 to the RPRM promoter was associated with dissociation of RNA Pol II from the RPRM promoter and with decreased rates of transcription. Although this was expected, a recent study showed that estrogen-mediated repression of CCNG2 was associated with a transient increase in transcription and recruitment of p300, followed by subsequent recruitment of corepressors (67). This data reflects the existence of diverse mechanisms which might act in a nonredundant fashion to ensure repression of growth-inhibitory signals.
In summary, we have revealed a unique role for HDAC7, which we show is required not only for repression of RPRM, but also for other E2-repressed genes we tested, suggesting that HDAC7-mediated repression may be a common mechanism of repression for a subset of E2-repressed genes. Given the relevance of ERα and its target genes in a number of hormone-dependent diseases, we suggest that this interaction between ERα, HDAC7, and FoxA1 could be interrogated as a novel target for prevention and treatment of such diseases, including breast cancer.
Supplementary Material
Acknowledgments
This work was supported by a Department of Defense Breast Cancer Research Program grant (BC043880) (S. Malik), NIH grants R01 CA097213 and P01030195 (S. Oesterreich), a SPORE pilot grant (CA58183) (S. Oesterreich), and a Nancy Owen Foundation grant (S. Oesterreich).
We thank Herb Kasler (UCSF) for helpful discussions, Jiemin Wong for the H3K9ac antibody, Chad Creighton for help with the Oncomine Cancer Profiling Database, Steven Johnsen (University of Göttingen) for technical support, and Gary Chamness for critical review of the manuscript.
Footnotes
Published ahead of print on 16 November 2009.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.An, J., R. C. Ribeiro, P. Webb, J. A. Gustafsson, P. J. Kushner, J. D. Baxter, and D. C. Leitman. 1999. Estradiol repression of tumor necrosis factor-alpha transcription requires estrogen receptor activation function-2 and is enhanced by coactivators. Proc. Natl. Acad. Sci. U. S. A. 96:15161-15166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badve, S., D. Turbin, M. A. Thorat, A. Morimiya, T. O. Nielsen, C. M. Perou, S. Dunn, D. G. Huntsman, and H. Nakshatri. 2007. FOXA1 expression in breast cancer—correlation with luminal subtype A and survival. Clin. Cancer Res. 13:4415-4421. [DOI] [PubMed] [Google Scholar]
- 3.Bretschneider, N., H. Brand, N. Miller, A. J. Lowery, M. J. Kerin, F. Gannon, and S. Denger. 2008. Estrogen induces repression of the breast cancer and salivary gland expression gene in an estrogen receptor alpha-dependent manner. Cancer Res. 68:106-114. [DOI] [PubMed] [Google Scholar]
- 4.Carroll, J. S., X. S. Liu, A. S. Brodsky, W. Li, C. A. Meyer, A. J. Szary, J. Eeckhoute, W. Shao, E. V. Hestermann, T. R. Geistlinger, E. A. Fox, P. A. Silver, and M. Brown. 2005. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell 122:33-43. [DOI] [PubMed] [Google Scholar]
- 5.Carroll, J. S., C. A. Meyer, J. Song, W. Li, T. R. Geistlinger, J. Eeckhoute, A. S. Brodsky, E. K. Keeton, K. C. Fertuck, G. F. Hall, Q. Wang, S. Bekiranov, V. Sementchenko, E. A. Fox, P. A. Silver, T. R. Gingeras, X. S. Liu, and M. Brown. 2006. Genome-wide analysis of estrogen receptor binding sites. Nat. Genet. 38:1289-1297. [DOI] [PubMed] [Google Scholar]
- 6.Reference deleted.
- 7.Cicatiello, L., C. Scafoglio, L. Altucci, M. Cancemi, G. Natoli, A. Facchiano, G. Iazzetti, R. Calogero, N. Biglia, M. De Bortoli, C. Sfiligoi, P. Sismondi, F. Bresciani, and A. Weisz. 2004. A genomic view of estrogen actions in human breast cancer cells by expression profiling of the hormone-responsive transcriptome. J. Mol. Endocrinol. 32:719-775. [DOI] [PubMed] [Google Scholar]
- 8.Creighton, C. J., K. E. Cordero, J. M. Larios, R. S. Miller, M. D. Johnson, A. M. Chinnaiyan, M. E. Lippman, and J. M. Rae. 2006. Genes regulated by estrogen in breast tumor cells in vitro are similarly regulated in vivo in tumor xenografts and human breast tumors. Genome Biol. 7:R28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunliffe, H. E., M. Ringner, S. Bilke, R. L. Walker, J. M. Cheung, Y. Chen, and P. S. Meltzer. 2003. The gene expression response of breast cancer to growth regulators: patterns and correlation with tumor expression profiles. Cancer Res. 63:7158-7166. [PubMed] [Google Scholar]
- 10.Downes, M., P. Ordentlich, H. Y. Kao, J. G. Alvarez, and R. M. Evans. 2000. Identification of a nuclear domain with deacetylase activity. Proc. Natl. Acad. Sci. U. S. A. 97:10330-10335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dressel, U., P. J. Bailey, S. C. Wang, M. Downes, R. M. Evans, and G. E. Muscat. 2001. A dynamic role for HDAC7 in MEF2-mediated muscle differentiation. J. Biol. Chem. 276:17007-17013. [DOI] [PubMed] [Google Scholar]
- 12.Eeckhoute, J., J. S. Carroll, T. R. Geistlinger, M. I. Torres-Arzayus, and M. Brown. 2006. A cell-type-specific transcriptional network required for estrogen regulation of cyclin D1 and cell cycle progression in breast cancer. Genes Dev. 20:2513-2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandes, I., Y. Bastien, T. Wai, K. Nygard, R. Lin, O. Cormier, H. S. Lee, F. Eng, N. R. Bertos, N. Pelletier, S. Mader, V. K. Han, X. J. Yang, and J. H. White. 2003. Ligand-dependent nuclear receptor corepressor LCoR functions by histone deacetylase-dependent and -independent mechanisms. Mol. Cell 11:139-150. [DOI] [PubMed] [Google Scholar]
- 14.Fischle, W., F. Dequiedt, M. Fillion, M. J. Hendzel, W. Voelter, and E. Verdin. 2001. Human HDAC7 histone deacetylase activity is associated with HDAC3 in vivo. J. Biol. Chem. 276:35826-35835. [DOI] [PubMed] [Google Scholar]
- 15.Fischle, W., F. Dequiedt, M. J. Hendzel, M. G. Guenther, M. A. Lazar, W. Voelter, and E. Verdin. 2002. Enzymatic activity associated with class II HDACs is dependent on a multiprotein complex containing HDAC3 and SMRT/N-CoR. Mol. Cell 9:45-57. [DOI] [PubMed] [Google Scholar]
- 16.Frasor, J., J. M. Danes, B. Komm, K. C. Chang, C. R. Lyttle, and B. S. Katzenellenbogen. 2003. Profiling of estrogen up- and down-regulated gene expression in human breast cancer cells: insights into gene networks and pathways underlying estrogenic control of proliferation and cell phenotype. Endocrinology 144:4562-4574. [DOI] [PubMed] [Google Scholar]
- 17.Frasor, J., F. Stossi, J. M. Danes, B. Komm, C. R. Lyttle, and B. S. Katzenellenbogen. 2004. Selective estrogen receptor modulators: discrimination of agonistic versus antagonistic activities by gene expression profiling in breast cancer cells. Cancer Res. 64:1522-1533. [DOI] [PubMed] [Google Scholar]
- 18.Gao, C., C. C. Ho, E. Reineke, M. Lam, X. Cheng, K. J. Stanya, Y. Liu, S. Chakraborty, H. M. Shih, and H. Y. Kao. 2008. Histone deacetylase 7 promotes PML sumoylation and is essential for PML nuclear body formation. Mol. Cell. Biol. 28:5658-5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia-Arencibia, M., S. Molero, N. Davila, M. C. Carranza, and C. Calle. 2005. 17beta-Estradiol transcriptionally represses human insulin receptor gene expression causing cellular insulin resistance. Leuk. Res. 29:79-87. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Bassets, I., Y. S. Kwon, F. Telese, G. G. Prefontaine, K. R. Hutt, C. S. Cheng, B. G. Ju, K. A. Ohgi, J. Wang, L. Escoubet-Lozach, D. W. Rose, C. K. Glass, X. D. Fu, and M. G. Rosenfeld. 2007. Histone methylation-dependent mechanisms impose ligand dependency for gene activation by nuclear receptors. Cell 128:505-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gottardis, M. M., M. Saceda, P. Garcia-Morales, Y. K. Fung, H. Solomon, P. F. Sholler, M. E. Lippman, and M. B. Martin. 1995. Regulation of retinoblastoma gene expression in hormone-dependent breast cancer. Endocrinology 136:5659-5665. [DOI] [PubMed] [Google Scholar]
- 22.Gregoire, S., L. Xiao, J. Nie, X. Zhang, M. Xu, J. Li, J. Wong, E. Seto, and X. J. Yang. 2007. Histone deacetylase 3 interacts with and deacetylates myocyte enhancer factor 2. Mol. Cell. Biol. 27:1280-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gregoire, S., and X. J. Yang. 2005. Association with class IIa histone deacetylases upregulates the sumoylation of MEF2 transcription factors. Mol. Cell. Biol. 25:2273-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hammes, S. R., and E. R. Levin. 2007. Extranuclear steroid receptors: nature and actions. Endocr. Rev. 28:726-741. [DOI] [PubMed] [Google Scholar]
- 25.Higgins, K. J., S. Liu, M. Abdelrahim, K. Vanderlaag, X. Liu, W. Porter, R. Metz, and S. Safe. 2008. Vascular endothelial growth factor receptor-2 expression is down-regulated by 17beta-estradiol in MCF-7 breast cancer cells by estrogen receptor alpha/Sp proteins. Mol. Endocrinol. 22:388-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang, E. Y., J. Zhang, E. A. Miska, M. G. Guenther, T. Kouzarides, and M. A. Lazar. 2000. Nuclear receptor corepressors partner with class II histone deacetylases in a Sin3-independent repression pathway. Genes Dev. 14:45-54. [PMC free article] [PubMed] [Google Scholar]
- 27.Huang, M. C., K. K. Li, and B. T. Spear. 2002. The mouse alpha-fetoprotein promoter is repressed in HepG2 hepatoma cells by hepatocyte nuclear factor-3 (FOXA). DNA Cell Biol. 21:561-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hurtado, A., K. A. Holmes, T. R. Geistlinger, I. R. Hutcheson, R. I. Nicholson, M. Brown, J. Jiang, W. J. Howat, S. Ali, and J. S. Carroll. 2008. Regulation of ERBB2 by oestrogen receptor-PAX2 determines response to tamoxifen. Nature 456:663-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jensen, E. D., T. M. Schroeder, J. Bailey, R. Gopalakrishnan, and J. J. Westendorf. 2008. Histone deacetylase 7 associates with Runx2 and represses its activity during osteoblast maturation in a deacetylation-independent manner. J. Bone Miner. Res. 23:361-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang, S., R. Meyer, K. Kang, C. K. Osborne, J. Wong, and S. Oesterreich. 2006. Scaffold attachment factor SAFB1 suppresses estrogen receptor alpha-mediated transcription in part via interaction with nuclear receptor corepressor. Mol. Endocrinol. 20:311-320. [DOI] [PubMed] [Google Scholar]
- 31.Kaipparettu, B. A., S. Malik, S. D. Konduri, W. Liu, M. Rokavec, H. van der Kuip, R. Hoppe, S. Hammerich-Hille, P. Fritz, W. Schroth, I. Abele, G. M. Das, S. Oesterreich, and H. Brauch. 2008. Estrogen-mediated downregulation of CD24 in breast cancer cells. Int. J. Cancer 123:66-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalaitzidis, D., and T. D. Gilmore. 2005. Transcription factor cross-talk: the estrogen receptor and NF-kappaB. Trends Endocrinol. Metab. 16:46-52. [DOI] [PubMed] [Google Scholar]
- 33.Karvonen, U., O. A. Janne, and J. J. Palvimo. 2006. Androgen receptor regulates nuclear trafficking and nuclear domain residency of corepressor HDAC7 in a ligand-dependent fashion. Exp. Cell Res. 312:3165-3183. [DOI] [PubMed] [Google Scholar]
- 34.Kawai, H., H. Li, S. Avraham, S. Jiang, and H. K. Avraham. 2003. Overexpression of histone deacetylase HDAC1 modulates breast cancer progression by negative regulation of estrogen receptor alpha. Int. J. Cancer 107:353-358. [DOI] [PubMed] [Google Scholar]
- 35.Kelley, K. M., B. G. Rowan, and M. Ratnam. 2003. Modulation of the folate receptor alpha gene by the estrogen receptor: mechanism and implications in tumor targeting. Cancer Res. 63:2820-2828. [PubMed] [Google Scholar]
- 36.Kim, J., L. Jia, W. D. Tilley, and G. A. Coetzee. 2003. Dynamic methylation of histone H3 at lysine 4 in transcriptional regulation by the androgen receptor. Nucleic Acids Res. 31:6741-6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klinge, C. M. 2001. Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res. 29:2905-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurebayashi, J., T. Otsuki, H. Kunisue, K. Tanaka, S. Yamamoto, and H. Sonoo. 2000. Expression levels of estrogen receptor-alpha, estrogen receptor-beta, coactivators, and corepressors in breast cancer. Clin. Cancer Res. 6:512-518. [PubMed] [Google Scholar]
- 39.Kurtev, V., R. Margueron, K. Kroboth, E. Ogris, V. Cavailles, and C. Seiser. 2004. Transcriptional regulation by the repressor of estrogen receptor activity via recruitment of histone deacetylases. J. Biol. Chem. 279:24834-24843. [DOI] [PubMed] [Google Scholar]
- 40.Kushner, P. J., D. A. Agard, G. L. Greene, T. S. Scanlan, A. K. Shiau, R. M. Uht, and P. Webb. 2000. Estrogen receptor pathways to AP-1. J. Steroid Biochem. Mol. Biol. 74:311-317. [DOI] [PubMed] [Google Scholar]
- 41.Laganiere, J., G. Deblois, C. Lefebvre, A. R. Bataille, F. Robert, and V. Giguere. 2005. Location analysis of estrogen receptor alpha target promoters reveals that FOXA1 defines a domain of the estrogen response. Proc. Natl. Acad. Sci. U. S. A. 102:11651-11656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leong, H., J. R. Sloan, P. D. Nash, and G. L. Greene. 2005. Recruitment of histone deacetylase 4 to the N-terminal region of estrogen receptor alpha. Mol. Endocrinol. 19:2930-2942. [DOI] [PubMed] [Google Scholar]
- 43.Lin, C. Y., A. Strom, V. B. Vega, S. L. Kong, A. L. Yeo, J. S. Thomsen, W. C. Chan, B. Doray, D. K. Bangarusamy, A. Ramasamy, L. A. Vergara, S. Tang, A. Chong, V. B. Bajic, L. D. Miller, J. A. Gustafsson, and E. T. Liu. 2004. Discovery of estrogen receptor alpha target genes and response elements in breast tumor cells. Genome Biol. 5:R66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin, C. Y., V. B. Vega, J. S. Thomsen, T. Zhang, S. L. Kong, M. Xie, K. P. Chiu, L. Lipovich, D. H. Barnett, F. Stossi, A. Yeo, J. George, V. A. Kuznetsov, Y. K. Lee, T. H. Charn, N. Palanisamy, L. D. Miller, E. Cheung, B. S. Katzenellenbogen, Y. Ruan, G. Bourque, C. L. Wei, and E. T. Liu. 2007. Whole-genome cartography of estrogen receptor alpha binding sites. PLoS Genet. 3:e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu, W., S. D. Konduri, S. Bansal, B. K. Nayak, S. A. Rajasekaran, S. M. Karuppayil, A. K. Rajasekaran, and G. M. Das. 2006. Estrogen receptor-alpha binds p53 tumor suppressor protein directly and represses its function. J. Biol. Chem. 281:9837-9840. [DOI] [PubMed] [Google Scholar]
- 46.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 47.Lupien, M., J. Eeckhoute, C. A. Meyer, Q. Wang, Y. Zhang, W. Li, J. S. Carroll, X. S. Liu, and M. Brown. 2008. FoxA1 translates epigenetic signatures into enhancer-driven lineage-specific transcription. Cell 132:958-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lyst, M. J., and I. Stancheva. 2007. A role for SUMO modification in transcriptional repression and activation. Biochem. Soc. Trans. 35:1389-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Madak-Erdogan, Z., K. J. Kieser, S. H. Kim, B. Komm, J. A. Katzenellenbogen, and B. S. Katzenellenbogen. 2008. Nuclear and extranuclear pathway inputs in the regulation of global gene expression by estrogen receptors. Mol. Endocrinol. 22:2116-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Metivier, R., G. Penot, M. R. Hubner, G. Reid, H. Brand, M. Kos, and F. Gannon. 2003. Estrogen receptor-alpha directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell 115:751-763. [DOI] [PubMed] [Google Scholar]
- 51.Metzger, E., M. Wissmann, N. Yin, J. M. Muller, R. Schneider, A. H. Peters, T. Gunther, R. Buettner, and R. Schule. 2005. LSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature 437:436-439. [DOI] [PubMed] [Google Scholar]
- 52.Newman, S. P., N. P. Bates, D. Vernimmen, M. G. Parker, and H. C. Hurst. 2000. Cofactor competition between the ligand-bound oestrogen receptor and an intron 1 enhancer leads to oestrogen repression of ERBB2 expression in breast cancer. Oncogene 19:490-497. [DOI] [PubMed] [Google Scholar]
- 53.Nishidate, T., T. Katagiri, M. L. Lin, Y. Mano, Y. Miki, F. Kasumi, M. Yoshimoto, T. Tsunoda, K. Hirata, and Y. Nakamura. 2004. Genome-wide gene-expression profiles of breast-cancer cells purified with laser microbeam microdissection: identification of genes associated with progression and metastasis. Int. J. Oncol. 25:797-819. [PubMed] [Google Scholar]
- 54.Oesterreich, S., W. Deng, S. Jiang, X. Cui, M. Ivanova, R. Schiff, K. Kang, D. L. Hadsell, J. Behrens, and A. V. Lee. 2003. Estrogen-mediated down-regulation of E-cadherin in breast cancer cells. Cancer Res. 63:5203-5208. [PubMed] [Google Scholar]
- 55.Ohki, R., J. Nemoto, H. Murasawa, E. Oda, J. Inazawa, N. Tanaka, and T. Taniguchi. 2000. Reprimo, a new candidate mediator of the p53-mediated cell cycle arrest at the G2 phase. J. Biol. Chem. 275:22627-22630. [DOI] [PubMed] [Google Scholar]
- 56.Parra, M., T. Mahmoudi, and E. Verdin. 2007. Myosin phosphatase dephosphorylates HDAC7, controls its nucleocytoplasmic shuttling, and inhibits apoptosis in thymocytes. Genes Dev. 21:638-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patrone, G., F. Puppo, R. Cusano, M. Scaranari, I. Ceccherini, A. Puliti, and R. Ravazzolo. 2000. Nuclear run-on assay using biotin labeling, magnetic bead capture and analysis by fluorescence-based RT-PCR. Biotechniques 29:1012-1014, 1016-1017. [DOI] [PubMed] [Google Scholar]
- 58.Perissi, V., N. Menini, E. Cottone, D. Capello, M. Sacco, F. Montaldo, and M. De Bortoli. 2000. AP-2 transcription factors in the regulation of ERBB2 gene transcription by oestrogen. Oncogene 19:280-288. [DOI] [PubMed] [Google Scholar]
- 59.Ray, P., S. K. Ghosh, D. H. Zhang, and A. Ray. 1997. Repression of interleukin-6 gene expression by 17 beta-estradiol: inhibition of the DNA-binding activity of the transcription factors NF-IL6 and NF-kappa B by the estrogen receptor. FEBS Lett. 409:79-85. [DOI] [PubMed] [Google Scholar]
- 60.Safe, S. 2001. Transcriptional activation of genes by 17 beta-estradiol through estrogen receptor-Sp1 interactions. Vitam. Horm. 62:231-252. [DOI] [PubMed] [Google Scholar]
- 61.Schuetz, A., J. Min, A. Allali-Hassani, M. Schapira, M. Shuen, P. Loppnau, R. Mazitschek, N. P. Kwiatkowski, T. A. Lewis, R. L. Maglathin, T. H. McLean, A. Bochkarev, A. N. Plotnikov, M. Vedadi, and C. H. Arrowsmith. 2008. Human HDAC7 harbors a class IIa histone deacetylase-specific zinc binding motif and cryptic deacetylase activity. J. Biol. Chem. 283:11355-11363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sentis, S., M. Le Romancer, C. Bianchin, M. C. Rostan, and L. Corbo. 2005. Sumoylation of the estrogen receptor alpha hinge region regulates its transcriptional activity. Mol. Endocrinol. 19:2671-2684. [DOI] [PubMed] [Google Scholar]
- 63.Shang, Y., X. Hu, J. DiRenzo, M. A. Lazar, and M. Brown. 2000. Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell 103:843-852. [DOI] [PubMed] [Google Scholar]
- 64.Shang, Y., M. Myers, and M. Brown. 2002. Formation of the androgen receptor transcription complex. Mol. Cell 9:601-610. [DOI] [PubMed] [Google Scholar]
- 65.Stoner, M., F. Wang, M. Wormke, T. Nguyen, I. Samudio, C. Vyhlidal, D. Marme, G. Finkenzeller, and S. Safe. 2000. Inhibition of vascular endothelial growth factor expression in HEC1A endometrial cancer cells through interactions of estrogen receptor alpha and Sp3 proteins. J. Biol. Chem. 275:22769-22779. [DOI] [PubMed] [Google Scholar]
- 66.Stossi, F., V. S. Likhite, J. A. Katzenellenbogen, and B. S. Katzenellenbogen. 2006. Estrogen-occupied estrogen receptor represses cyclin G2 gene expression and recruits a repressor complex at the cyclin G2 promoter. J. Biol. Chem. 281:16272-16278. [DOI] [PubMed] [Google Scholar]
- 67.Stossi, F., Z. Madak-Erdogan, and B. S. Katzenellenbogen. 2009. Estrogen receptor alpha represses transcription of early target genes via p300 and CtBP1. Mol. Cell. Biol. 29:1749-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takahashi, T., M. Suzuki, H. Shigematsu, N. Shivapurkar, C. Echebiri, M. Nomura, V. Stastny, M. Augustus, C. W. Wu, I. I. Wistuba, S. J. Meltzer, and A. F. Gazdar. 2005. Aberrant methylation of Reprimo in human malignancies. Int. J. Cancer 115:503-510. [DOI] [PubMed] [Google Scholar]
- 69.Townson, S. M., K. M. Dobrzycka, A. V. Lee, M. Air, W. Deng, K. Kang, S. Jiang, N. Kioka, K. Michaelis, and S. Oesterreich. 2003. SAFB2, a new scaffold attachment factor homolog and estrogen receptor corepressor. J. Biol. Chem. 278:20059-20068. [DOI] [PubMed] [Google Scholar]
- 70.Uray, I. P., Y. Liang, and S. M. Hyder. 2004. Estradiol down-regulates CD36 expression in human breast cancer cells. Cancer Lett. 207:101-107. [DOI] [PubMed] [Google Scholar]
- 71.Vu, E. H., R. J. Kraus, and J. E. Mertz. 2007. Phosphorylation-dependent sumoylation of estrogen-related receptor alpha1. Biochemistry 46:9795-9804. [DOI] [PubMed] [Google Scholar]
- 72.Watanabe, H., A. Suzuki, T. Mizutani, S. Khono, D. B. Lubahn, H. Handa, and T. Iguchi. 2002. Genome-wide analysis of changes in early gene expression induced by oestrogen. Genes Cells 7:497-507. [DOI] [PubMed] [Google Scholar]
- 73.Wei, L. N., M. Farooqui, and X. Hu. 2001. Ligand-dependent formation of retinoid receptors, receptor-interacting protein 140 (RIP140), and histone deacetylase complex is mediated by a novel receptor-interacting motif of RIP140. J. Biol. Chem. 276:16107-16112. [DOI] [PubMed] [Google Scholar]
- 74.Zhu, P., S. H. Baek, E. M. Bourk, K. A. Ohgi, I. Garcia-Bassets, H. Sanjo, S. Akira, P. F. Kotol, C. K. Glass, M. G. Rosenfeld, and D. W. Rose. 2006. Macrophage/cancer cell interactions mediate hormone resistance by a nuclear receptor derepression pathway. Cell 124:615-629. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.