Abstract
Microorganisms develop biofilms on indwelling medical devices and are associated with device-related infections, resulting in substantial morbidity and mortality. This study investigated the effect of pretreating hydrogel-coated catheters with Pseudomonas aeruginosa bacteriophages on biofilm formation by P. aeruginosa in an in vitro model. Hydrogel-coated catheters were exposed to a 10 log10 PFU ml−1 lysate of P. aeruginosa phage M4 for 2 h at 37°C prior to bacterial inoculation. The mean viable biofilm count on untreated catheters was 6.87 log10 CFU cm−2 after 24 h. The pretreatment of catheters with phage reduced this value to 4.03 log10 CFU cm−2 (P < 0.001). Phage treatment immediately following bacterial inoculation also reduced biofilm viable counts (4.37 log10 CFU cm−2 reduction; P < 0.001). The regrowth of biofilms on phage-treated catheters occurred between 24 and 48 h, but supplemental treatment with phage at 24 h significantly reduced biofilm regrowth (P < 0.001). Biofilm isolates resistant to phage M4 were recovered from catheters pretreated with phage. The phage susceptibility profiles of these isolates were used to guide the development of a five-phage cocktail from a larger library of P. aeruginosa phages. The pretreatment of catheters with this cocktail reduced the 48-h mean biofilm cell density by 99.9% (from 7.13 to 4.13 log10 CFU cm−2; P < 0.001), but fewer biofilm isolates were resistant to these phages. These results suggest the potential of applying phages, especially phage cocktails, to the surfaces of indwelling medical devices for mitigating biofilm formation by clinically relevant bacteria.
Indwelling medical devices of various kinds may become colonized with microorganisms, resulting in the formation of microbial biofilms (16). Biofilm-associated organisms are tolerant to antimicrobial agents, can evade the host immune system, and can act as a nidus for infection (16). As a result, device-related infections, such as catheter-associated bloodstream infections, cause substantial morbidity and mortality among specific patient populations (9). Attributable mortality rates for healthcare-associated bloodstream infections have been estimated to be 25% (44).
A number of novel strategies have been proposed to more effectively prevent and control device-associated biofilms, either by minimizing microbial attachment to device surfaces or by targeting the biofilm after it has developed. One such strategy is to use bacteriophages (phages) (17). Phages have been used for the treatment of infectious diseases in plants (26), animals (6), and humans (33, 39, 43). The use of phages to control biofilms has potential for several reasons. Phages can replicate at the site of an infection, thereby increasing in numbers where they are most required. During the lytic replication cycle, the infection of a bacterial host cell by a single phage virion will result in the production of dozens or hundreds of progeny phage, depending on the particular phage and host strains. Some phages also have been shown to produce enzymes that degrade the extracellular polymeric substance (EPS) matrix of a biofilm (23, 25). Doolittle et al. (19) showed that progeny phage will propagate radially through a biofilm. At least in theory, a single phage dose should be capable of treating a biofilm infection as progeny phage infect adjacent cells and degrade the biofilm matrix.
Curtin and Donlan (13) demonstrated that a phage that is active against Staphylococcus epidermidis could be incorporated into a hydrogel coating on a catheter and significantly reduce biofilm formation by this organism in an in vitro model system. Based on those studies with S. epidermidis, we have investigated whether phages specific for Pseudomonas aeruginosa also can reduce biofilm formation by this organism in a similar in vitro model. Catheters were treated with a single phage or a combination of phages prior to, immediately following, or 24 h after inoculation with the test P. aeruginosa culture, and the effects of the phage treatments on biofilm formation and maintenance were characterized.
(Portions of this paper were presented as poster no. A-011 at the 2007 American Society for Microbiology General Meeting in Toronto, Canada [20a].)
MATERIALS AND METHODS
Organisms and culture conditions.
P. aeruginosa M4 (Health Protection Agency, Colindale, United Kingdom) was selected as the biofilm-forming organism in this study. Stocks were maintained at −80°C and propagated on Trypticase soy agar (TSA) plates at 37°C unless otherwise indicated.
P. aeruginosa phage M4 (Health Protection Agency, Colindale, United Kingdom) was used for the first round of phage treatment experiments. Phage M4 (35) is a member of the family Myoviridae (1). By using a single-step growth curve (21), this phage was shown to exhibit a latent period (the time from initial infection to lysis) of approximately 35 min and a burst size (number of progeny phage per infected cell) of 95 to 100. Phage M4 stocks were maintained as lyophilized preparations that were stored at 4°C.
Environmental phages were incorporated into the phage cocktail that was used for the second round of biofilm treatment experiments. These phages were isolated from the Snapfinger Creek Wastewater Treatment Plant in Dekalb County, GA. Untreated sewage samples were allowed to settle overnight at 4°C and centrifuged at 9,000 × g for 20 min, after which the supernatant was filter sterilized (0.22-μm pore size; Millipore, Billerica, MA). Twenty milliliters of 2× tryptic soy broth (TSB), 20 ml of the filtered sewage, and 0.4 ml of a log-phase TSB broth culture of one of seven clinical or laboratory strains of P. aeruginosa were combined and incubated at 37°C overnight at 100 rpm in a shaker incubator. Samples that were cleared (indicative of the phage lysis of the bacterial culture) were centrifuged at 4,000 × g for 30 min, filter sterilized (0.22 μm), and stored at 4°C. The titer of the crude phage lysate was determined by plaque assay using the soft-agar overlay method on TSA (2). Ten phage isolates were recovered and used for host susceptibility testing and phage cocktail preparation.
Three of the Colindale Pseudomonas typing phages, M6, F8, and Col11 (35) (Health Protection Agency, Colindale, United Kingdom), also were used for susceptibility testing.
Phages were propagated using the soft-agar overlay method (2). Larger volumes of high-titer phage lysates were prepared as described by Adams (2) using P. aeruginosa M4 as the host strain in TSB (Difco, Becton Dickinson, Sparks, MD) amended with 3 mM MgCl2 (added as MgCl2·6H2O) and 4 mM CaCl2 (added as CaCl2·2H2O). Briefly, 1 ml of an overnight culture of P. aeruginosa M4 (grown at 37°C) was added to 49 ml of TSB containing supplemental MgCl2 and CaCl2, and the culture was incubated at 37°C with shaking at 250 rpm. When the optical density at 550 nm was approximately 0.3 (approximately 109 CFU/ml) using a Microscan turbidity meter (Dade Behring, West Sacramento, CA), M4 phage was added to a final concentration of 109 PFU ml−1. The culture was allowed to stand for 15 min at room temperature and then incubated for 18 h at 37°C with shaking at 100 rpm. Host cell debris was pelleted by centrifugation (4,100 × g for 35 min), and the supernatant containing phage was filter sterilized (0.22-μm pore size; Millipore, Billerica, MA) and stored at 4°C. The titer of the crude phage lysate was determined using the soft-agar overlay method on TSA (2).
In vitro model system for growing biofilms on hydrogel-coated catheters.
Biofilms were grown on Lubri-sil all-silicone 16 French Foley catheters (C. R. Bard, Covington, GA) in a modified drip flow reactor (mDFR) (Biosurface Technologies, Bozeman, MT). These catheters contained a neutral hydrogel coating on external and luminal surfaces. The mDFR has four chambers, each with a sealing lid. The original device was modified to allow the connection of catheter segments to the influent and effluent ports within the device. Following sterilization by ethylene oxide gas, the catheters in the mDFR each were connected by means of silicone tubing to glass vessels containing (i) phage lysate, (ii) bacterial inoculum, (iii) sterile medium, and (iv) an empty waste container. The entire system was assembled and operated in a 35°C incubator.
For experiments examining biofilm formation on non-phage-treated catheters, an overnight culture of P. aeruginosa M4 in 100% TSB was pumped through the catheters at 1 ml min−1 for 2 h using a Masterflex pump (Cole Parmer, Niles, IL) to allow the organisms to attach to the catheter surface. This was followed immediately by pumping sterile 10% (vol/vol) TSB through the catheters at 0.5 ml min−1 for an additional 22 or 46 h depending upon the experimental design used. The mean concentration of the bacterial inoculum ranged from 108 to 109 CFU ml−1 during the 2-h inoculation period.
Phage treatment of the catheter surfaces.
Three types of phage treatments were used: pretreatment, posttreatment, and recharge treatment. Phage pretreatment and posttreatment involved exposing the catheter lumen to a phage lysate for a defined period of time. In both cases, a crude phage lysate (prepared as described above) with a titer of 1.0 × 1010 to 2.2 × 1010 PFU ml−1 was used. For pretreatment experiments, the phage lysate was pumped through the catheter segments for 2 h at 1 ml min−1 prior to exposure to the bacterial inoculum for 2 h, followed by sterile medium for either 22 or 46 h. For posttreatment experiments, catheters were exposed first to bacterial inoculum for 2 h, phage for 2 h, and then sterile medium for 22 or 46 h. For recharge experiments, catheters were pretreated with phage for 2 h, exposed to bacterial inoculum for 2 h, exposed to sterile medium for 22 h, and treated again with phage by pumping the high-titer phage lysate through the catheters for 2 h and then sterile medium for an additional 22 h.
Two rounds of phage treatment experiments were conducted. In the first round, pretreatment, posttreatment, and recharge experiments were conducted using phage M4. In the second round, pretreatment experiments were conducted using a phage cocktail that consisted of equal numbers of each of M4 phage and four environmental phages (ΦE2005-24-39, ΦE2005-40-16, ΦW2005-24-39, and ΦW2005-37-18-03) for a final cocktail titer of 7.0 × 109 PFU ml−1. The phages in this cocktail were selected based on the phage susceptibility profiles of biofilm isolates recovered from the first round of phage treatment experiments.
Two sets of control experiments also were conducted. Catheters were exposed for 2 h to heat-inactivated M4 phage (80°C for 3 h) as a control pretreatment. Also, a conditioning serum film was simulated on the catheter lumen to assess its effect on biofilm formation and phage M4 efficacy. Filter-sterilized whole human serum (complement inactivated at 56°C for 30 min) was instilled into the lumen of phage-pretreated catheter segments in the mDFR and incubated for 2 h at 35°C (13). The experiment continued with the 2-h bacterial inoculation and 22-h sterile medium incubation periods. The catheter segments were sampled and processed to assess biofilm development, and results were compared to results for untreated catheters not exposed to serum.
Recovery and enumeration of biofilm organisms.
Catheters were removed from individual chambers of the mDFR at different time points following bacterial inoculation. In the case of untreated and pretreated catheters, catheter samples were collected after 2, 6, 24, and, in some experiments, 48 h after bacterial inoculation. For posttreatment catheters, samples were collected 4, 6, 24, and 48 h after bacterial inoculation. It was not possible to collect 2-h samples from these systems, since phage posttreatment immediately followed bacterial inoculation.
The ends of the catheter segments were clamped shut with hemostats and cut from the mDFR fittings. All processing was conducted aseptically. Fluid from the catheter lumen was collected to quantify phage in the luminal fluid by the soft-agar overlay method. The catheter was cut into two 1-cm sections. Each section then was cut lengthwise, rinsed gently in phosphate-buffered saline (PBS), and placed into a 50-ml sterile centrifuge tube containing 10 ml PBS. Biofilm organisms were recovered from catheter segments using the following method: tubes containing catheter sections were sonicated at 42 kHz in a water bath sonicator (Branson 2510; Branson, Danbury, CT) for 10 min, vortexed for 30 s, sonicated again for 5 min, vortexed for 30 s, sonicated a third time for 30 s, and then vortexed a final time for 30 s. This recovery method was similar to the method described by Curtin and Donlan (13), which was shown to recover essentially all viable biofilm cells from the catheter surface. The recovered biofilm cells then were quantified by serial dilution spread plating on TSA, and viable counts were determined and expressed as CFU cm−2 of the catheter lumen surface. Organisms recovered from the biofilms were subcultured on TSA and confirmed as P. aeruginosa by the Vitek Legacy GNI+ card (bioMerieux, Durham, NC).
To determine the concentration of phage present on the catheter lumen surface prior to exposure to the bacterial inoculum, a 1.2 × 1010 PFU ml−1 phage M4 lysate in cation-supplemented TSB was pumped at 1 ml min−1 through each of four catheter sections contained in the mDFR for 2 h at 35°C. Catheter sections were clamped and removed from the reactor and processed according to the protocol used to recover biofilm cells, and the phage concentration was determined by plaque assay.
Effect of catheter processing procedure on phage viability and assessment of phage effect on P. aeruginosa during sampling procedure.
The effect of the processing method itself on phage viability was determined by adding 1 ml of a 5 × 1010 PFU ml−1 M4 phage lysate to each of six 9.0-ml Butterfield buffer tubes, exposing three of these tubes to the sonication and vortexing process described above, and enumerating PFU in all six tubes using the soft-agar overlay method (2).
To demonstrate that phage M4 was not causing the further lysis of P. aeruginosa M4 during the biofilm-sampling procedure, a suspension equivalent to a 0.5 McFarland standard (1 × 108 CFU ml−1) of P. aeruginosa M4 was prepared in 5 ml Butterfield buffer at 4°C. A 1-ml aliquot was removed for viable cell count determination, and 1 ml of 1010 PFU ml−1 phage M4 lysate was added to the remaining 4-ml solution. The tube was subjected to the same biofilm removal procedure as that used during mDFR experiments (i.e., sonication and vortexing), followed by chilling on ice for 2 h (which was twice the length of time required for sampling during the biofilm/phage experiments). The number of viable cells then was determined by plate count. Counts of untreated and phage-treated cell suspensions were compared. This experiment was performed in triplicate.
Characterization of phage-resistant biofilm isolates.
Several colonies of biofilm organisms, representing different colony morphologies recovered from M4 phage-treated, cocktail-treated, and untreated catheters, were collected after 2, 24, and 48 h, subcultured, and characterized to assess resistance to phage M4. All isolates were gram-negative rods and were oxidase positive, and they produced a grape-like odor, pyoverdin, and a yellow-green pigment on Pseudomonas Agar F (Difco Laboratories, Becton Dickinson Co., Sparks, MD), which is indicative of P. aeruginosa (7). Isolates exhibiting atypical reactions were reconfirmed using the Vitek GNI+ card. The susceptibility of each isolate from the first round of phage treatment experiments to phage M4 was determined by the spot test (8) and the soft-agar overlay method (2). To determine whether phage resistance was related to a stress response of the organism or a stable characteristic, isolates were subcultured several times during 7 days in TSB and then tested again for susceptibility to phage M4. The biofilm isolates recovered from phage cocktail-treated catheters in the second round of experiments were tested for susceptibility to each of the five phages in that cocktail using the spot test.
Phage-resistant isolates from both rounds of experiments were tested for their ability to adsorb M4 phage using a modification of the methods of Hadas et al. (22) and Karlson (27). Briefly, an overnight culture of each isolate was grown in TSB to an optical density at 550 nm of 0.12 (approximately 3 × 108 CFU ml−1), and 1 ml of this culture was combined with 0.1 ml of phage M4 (concentration, 3 × 108 PFU ml−1) in a glass tube so that the proportion of phage to bacteria was 1:10 (multiplicity of infection [MOI] = 0.1). This tube was held in a 30°C water bath for 4 min to provide time for phage to adsorb to the bacterial cells. An aliquot then was immediately transferred to a tube of TSB containing five drops of chloroform, which would have no effect on unattached phage particles but would kill bacterial cells and prevent plaque formation by any phage particles adsorbed to those cells. Unadsorbed phage were quantified by the soft-agar overlay method, and the number of adsorbed phage was calculated as the difference between the initial phage count prior to the addition of bacteria and the phage count after the 4-min adsorption period.
Additional characterization tests were conducted on the biofilm isolates recovered from catheters treated with the phage cocktail. Certain isolates were exposed to 50 μg/ml mitomycin C (Roche Applied Science, Indianapolis, IN) to test for the presence of temperate phages (42). Isolates also were tested for the presence of integrated Pf4 filamentous phages using PCR (32).
Relative growth rates for certain biofilm isolates were measured by inoculating TSB with a log-phase culture, incubating it at 35°C at 100 rpm, and measuring absorbance at 600 nm hourly for 6 h using a Hach DR 4000 spectrophotometer (Hach Co., Loveland, CO).
Biofilm formation was assessed using a modified crystal violet assay (11, 34). Briefly, microtiter plates were inoculated with an 18-h culture grown in 10% TSB, incubated at 35°C for 10 h, and stained with an aqueous solution of 1% (vol/vol) crystal violet and 3% (vol/vol) glacial acetic acid. After 15 min, plates were washed several times with sterile water to remove excess stain. Biofilm formation was quantified by the addition of 95% ethanol to each well (200 μl each). The A540 of each tube containing the extracted crystal violet was measured using a Hach DR 4000 spectrophotometer.
The stability of biofilm variant colony morphology was determined by subculturing colonies in brain heart infusion broth through 10 serial transfers according to the method of Häussler et al. (24). Reversion to the M4 parent colony morphology was assessed by growth characteristics on Columbia blood agar.
SEM.
Biofilm development on catheter lumen surfaces was visualized by scanning electron microscopy (SEM). Catheter segments were rinsed gently in sterile PBS and then fixed in 5% (vol/vol) glutaraldehyde in 0.67 M cacodylate buffer (pH 6.2) for 1 h and dehydrated through a graded series of 10-min ethanol immersions (30 to 100%). Specimens were immersed overnight in hexamethyldisilazane (Polysciences Inc., Warrington, PA), mounted on aluminum stubs with silver paint (Ted Pella Inc., Redding, CA), coated with gold (Polaron SC7640 sputter coater; Thermo VG Scientific, United Kingdom), and photographed with an ESEM (Philips XL-30; FEI Co., Hillsboro, OR). The entire luminal surface of 1-cm−2 catheter segments was examined, and images were chosen that represented the typical field of view.
Statistical analysis.
All experiments were performed a minimum of three times. Bacterial and phage counts were log10 transformed, and differences in microbial recovery were analyzed using two-tailed t tests (Sigma Stat 3.5; Systat Software Inc., San Jose, CA). P values of <0.05 were considered significant.
RESULTS
Effect of P. aeruginosa M4 phage treatment on biofilm formation on catheter surfaces.
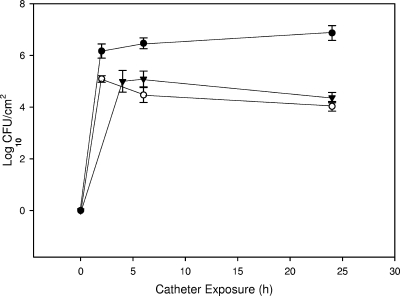
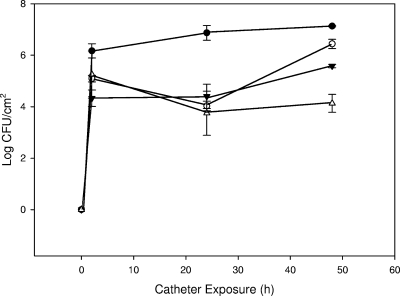
Pretreatment of hydrogel catheters with 10.07 log10 PFU ml−1 P. aeruginosa phage M4 for 2 h at 1 ml min−1 resulted in a mean concentration (n = 4) of 6.16 log10 PFU cm−2 associated with the catheter luminal surface. The method used to recover and quantify phages on the catheter surface was shown to have no significant effect on phage viability when treated and untreated phage lysates were analyzed (P = 0.650). P. aeruginosa M4 attached to untreated hydrogel-coated catheter surfaces and formed biofilms, attaining mean cell densities of 6.87 log10 CFU cm−2 after 24 h of exposure in the catheter model system (Fig. 1). The pretreatment of catheters with phage M4 significantly reduced biofilm formation by P. aeruginosa M4 to 4.03 log10 CFU cm−2 (P < 0.001) after 24 h, a >99.9% reduction. The posttreatment of catheters with phage (i.e., phage treatment immediately following exposure to the bacterial inoculum) also significantly reduced biofilm formation (4.37 log10 CFU cm−2 [P < 0.001]), although to a lesser extent than pretreatment. When catheters were exposed in the mDFR for an additional 24 h (see Fig. 3), biofilm levels on treated catheters increased so that the treatment resulted in overall reductions of less than 1 log10 CFU cm−2 at 48 h, even though this difference between treated and untreated catheters still was statistically significant (P = 0.01 and <0.001 for pretreated and posttreated catheters, respectively). When pretreated catheters were treated a second time with phage (recharged) at 24 h, biofilm cell densities increased but still were significantly (P < 0.001) lower than counts on untreated catheters (5.58 versus 7.13 mean log10 CFU cm−2; 97% reduction) or pretreated catheters that had not been recharged (5.58 versus 6.44 log10 CFU cm−2) at 48 h. The observation that the recharge treatment of catheters significantly reduced biofilm counts compared to catheters that were not recharged suggested that some, but perhaps not all, of the organisms in the biofilm still were susceptible to phage M4 24 h after inoculation. Phage numbers in the luminal fluid in catheters exposed to P. aeruginosa M4 attained 8.13 log10 PFU ml−1 at 2 h, decreased to 6.95 log10 PFU ml−1 at 6 h, and maintained similar levels through 48 h.
FIG. 1.
Effect of P. aeruginosa phage M4 pretreatment and posttreatment of catheter surface on biofilm formation by P. aeruginosa M4 during a 24-h exposure period. Closed circle, untreated; open circle, pretreated; closed triangle, posttreated. Data are means ± standard deviations (n = 3).
FIG. 3.
Effect of P. aeruginosa phage M4 pretreatment, pretreatment and recharge, and the phage cocktail treatment of catheter surface on biofilm formation by P. aeruginosa M4 during a 48-h exposure period. Closed circle, untreated; open circle, pretreated; closed triangle, recharge; open triangle, phage cocktail treatment. Data are means ± standard deviations (n = 3).
Characterization of biofilm isolates from phage-treated catheters.
Eleven biofilm isolates from catheters treated with M4 phage were collected and characterized. Although most exhibited atypical colony morphology, shape, and pigment production, no small-colony variants were observed. All were identified as P. aeruginosa. All except one of the isolates from the 2-, 24-, and 48-h samples were completely resistant to phage M4 upon initial testing. The single exception exhibited only partial resistance (growth of several colonies within the zone of clearing). All isolates lacked the ability to adsorb phage M4, suggesting that these organisms lacked receptors required for phage M4 attachment. All isolates collected from the 2-, 24-, and 48-h time points exhibited complete phage resistance after growth in TSB for 7 days. This suggested that these isolates exhibited stable phenotypes and did not revert to the M4 parent phenotype. Biofilm isolates collected from catheters that were not pretreated with phage also were morphologically diverse, although all were completely susceptible to phage M4.
Effect of serum, heat inactivation of phages, and the sampling procedure on biofilm formation.
Subsequent exposure to human serum following phage pretreatment did not significantly affect the ability of the treatment to reduce attachment and biofilm formation by P. aeruginosa M4; the mean log10 CFU cm−2 reduction compared to results for catheters that were not treated with phage or serum was 2.95 (P = 0.012) after 24 h. The pretreatment of catheters with heat-inactivated phage had no observable effect on bacterial attachment and biofilm formation by P. aeruginosa M4 after 24 h (P = 0.497) (data not shown). To ascertain that there was no further phage lysis of P. aeruginosa cells during the sampling procedure, viable bacterial counts were performed on standardized cell suspensions of P. aeruginosa M4, both before and after the suspensions were exposed to phage M4 and treated according to the normal biofilm-processing procedure. No difference in viable cell count was observed (P ≫ 0.05) (data not shown).
SEM.
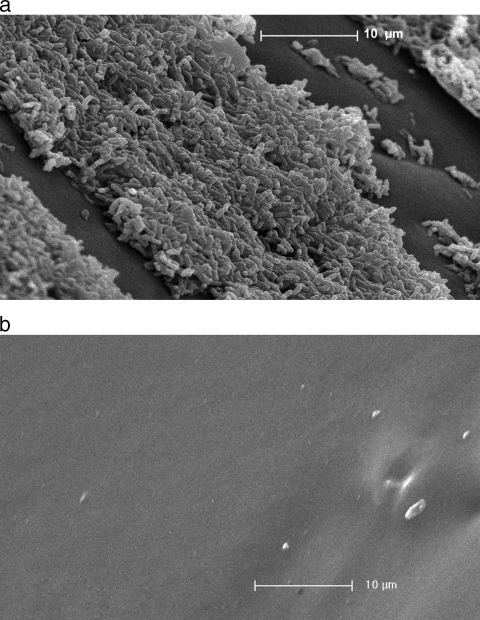
As shown in Fig. 2a, untreated catheters were heavily colonized after 24 h of exposure to P. aeruginosa M4. Exposure to the phage pretreatment reduced biofilm cell densities to the extent that even microcolonies or single cells in representative fields were not visible (Fig. 2b).
FIG. 2.
(a) Scanning electron micrograph of the luminal surface of a section of an untreated Lubri-sil hydrogel-coated all-silicone Foley catheter after biofilm formation by P. aeruginosa M4 for 24 h (2,500× magnification [Magn]). (b) Scanning electron micrograph of the catheter luminal surface pretreated with P. aeruginosa phage M4 and exposed for 24 h to P. aeruginosa M4 (2,500× magnification).
Development and testing of phage cocktail.
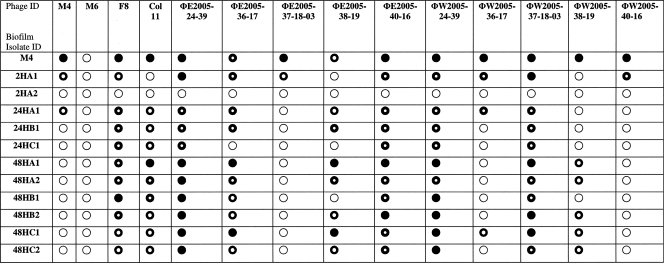
The 11 biofilm isolates described above (designated biofilm variants) that were recovered from the first round of phage M4-treated catheters were tested for their susceptibility to M4, three Colindale typing phages (M6, F8, and Col11), and the 10 environmental phages isolated in this study. The results are shown in Table 1. Phage M4 was effective only against the P. aeruginosa M4 parent strain and 2 of the 11 variants. In contrast, several of the other phages were broadly effective against the phage M4-resistant biofilm variants. Therefore, phages ΦE2005-24-39, ΦE2005-40-16, ΦW2005-24-39, and ΦW2005-37-18-03 were grown to a high titer and combined with phage M4 to formulate a phage cocktail, and this mixture was evaluated for its ability to reduce P. aeruginosa biofilm formation and variant regrowth.
TABLE 1.
Effect of environmental and typing phages on biofilm isolates recovered from P. aeruginosa M4 phage-treated cathetersa
○, no plaque formation (complete resistance);  , partially clear plaque containing bacterial colonies (partial resistance);
, partially clear plaque containing bacterial colonies (partial resistance);  , slightly turbid plaque (partial resistance); •, clear plaque (completely susceptible).
, slightly turbid plaque (partial resistance); •, clear plaque (completely susceptible).
In this second round of experiments, the phage cocktail pretreatment reduced the mean log10 biofilm CFU cm−2 from 7.13 (untreated catheters) to 4.13 (treated catheters) after 48 h (P < 0.001), a 99.9% reduction (Fig. 3). The phage cocktail-treated catheters also contained significantly lower final biofilm counts than catheters that had been recharged at 24 h (4.13 and 5.58 mean log10 CFU cm−2, respectively; P = 0.002). This suggests that a combination of multiple phage strains selected to target resistant bacterial strains within the biofilm can broaden the host range of the phage treatment and more effectively control biofilm formation. However, organisms did colonize phage cocktail-treated catheters and develop biofilms. To determine the basis for this biofilm regrowth in the presence of multiple phage types, representatives of each distinct colony type were collected from biofilm plate counts from each of the three phage cocktail pretreatment studies.
Characterization of biofilm organisms on cocktail-treated catheters.
Five distinct colony morphologies were identified, and each was confirmed as P. aeruginosa. Each variant was tested for its susceptibility to each of the five phages in the cocktail mixture. Four of the isolates were completely susceptible to at least one of the five phages in the cocktail, and one (48C) was completely resistant to all five phages. This isolate produced small (∼2 mm), nonpigmented colonies on TSA after 24 h of incubation at 35°C. Isolate 48C was characterized further to determine the stability of its phenotype and the basis for its phage resistance. The phenotype of this isolate was stable; reversion to the original M4 parent morphology did not occur after 10 serial transfers in brain heart infusion broth. The growth rate of 48C (0.083 h−1) was lower than that of the M4 parent strain (0.106 h−1), but biofilm formation for 48C (A540 = 0.063) was significantly higher (P = 0.017) than that for the M4 parent strain (A540 = 0.027). Isolate 48C readily adsorbed phage M4 (94.4% adsorption) in an adsorption assay, suggesting that the mechanism of resistance toward this phage was not due to an alteration of phage receptors by this strain. The exposure of each of the biofilm isolates to mitomycin C did not result in the induction of any phage having lytic activity against the parent P. aeruginosa M4 or the other, non-phage-resistant variants, suggesting that lysogeny was not the mechanism for resistance to phages in the cocktail mixture. All biofilm isolates, including 48C, were negative for Pf4 prophage DNA.
DISCUSSION
Although there have been several studies published on the effect of phages against biofilms (10, 12, 18, 19, 23, 25, 30, 38), we are unaware of other studies that have used phages to prevent bacterial attachment and initial biofilm formation, rather than attempting to control a mature biofilm. Markoishvili et al. (31) developed a nontoxic polymer film (PhagoBioDerm) that was impregnated with phages, ciprofloxacin, and benzocaine, and it has been used to treat patients with different infectious diseases. Biofilms may be implicated in some of these types of infections; however, an explicit evaluation of PhagoBioDerm against biofilms has not been published.
The results of the present study suggest that the phage pretreatment of catheters can reduce biofilm formation by P. aeruginosa. Treatment with a single phage resulted in a 2.8 log reduction (>99%) after 24 h, which compares favorably to results obtained using catheters that incorporate antimicrobial agents. For example, Raad et al. (36) compared the relative efficacies of several antimicrobial agents in a model system for reducing the colonization of polyurethane central venous catheters by Staphylococcus epidermidis. Reductions in bacterial colonization ranged from 0 to >99% depending on the antibiotic used. Ahearn et al. (3) analyzed adhesion after 2 h by several clinically relevant bacteria onto all-silicone catheters, latex-coated catheters with hydrogel/silver, silicone-coated catheters with hydrogel/silver, and lubricious-coated silicone catheters in an in vitro model. The silver-treated catheters showed the reduced adhesion of all organisms tested, although the percent reductions ranged only from 30 to 95.5%, depending on the organism and type of catheter.
An advantage of phage treatment over treatment with antimicrobial agents is that phages are self replicating in the presence of their host cells and are self limiting in their absence. This ensures that phages are produced continually to infect new bacterial cells as long as the bacteria are able to support their replication, and this is supported by the observation that phage numbers in the luminal fluid were 108 ml−1 at 2 h after addition. The flow of medium following phage pretreatment would be expected to wash out any phage particles that were not associated with the catheter surface. Phage persistence in the catheters for 48 h suggests that progeny phage tend to infect other biofilm cells rather than only planktonic cells. Doolittle et al. (19) observed that the spread of infection by TD4, an Escherichia coli phage, in a flow-cell biofilm resembled plaque formation, in that radial diffusion predominated rather than the downstream flow of progeny phage from lysing cells.
The conditions provided by the in vitro model system in this study differed in several respects from conditions that could be present in vivo. The bacterial inoculum level (108 to 109 CFU/ml) was artificially high to determine whether this treatment could be effective under the most extreme bacterial challenge. A smaller inoculum bacterial cell concentration would be expected to result in a larger effective MOI, which in theory could provide a more significant reduction in bacterial colonization and biofilm formation. Further laboratory studies using different levels of bacterial challenge will be required to define the limits of phage pretreatment technology. It also will be necessary to evaluate this technology in animal model systems and ultimately in human studies to fully assess its clinical relevance. However, the observation that the exposure of phage-treated catheters to human serum did not significantly affect phage efficacy suggests the potential of phage to remain active in the presence of a serum conditioning film under in vivo conditions.
One concern with therapeutic uses of phages is the possibility that organisms in the biofilm will develop resistance to the phages. Our results showed that the strain of P. aeruginosa used in this study contains several subpopulations (variants) with distinct phenotypes, and that phage treatment may select for or induce the expression of those phenotypes that confer resistance to phage M4, allowing biofilms of resistant cells to develop. This was not unexpected, since P. aeruginosa is known to exhibit significant phenotypic variability in biofilms (14, 15, 20). However, we did not observe small-colony variants similar to those reported by Webb et al. (41), nor did we detect the presence of Pf4 prophage genes in variants isolated from phage cocktail-treated catheters. Pf4 was shown to influence the structure and stability of P. aeruginosa PAO1 biofilms (37) and mediate the formation of variants within biofilms.
The resistance of bacteria toward phage may be associated with lysogeny, mutations that affect phage adsorption, restriction modification, or the mechanisms of abortive infection such as the presence of clustered regularly interspaced short palindromic repeats (CRISPRs) in the bacterial genome (4, 5, 17). Lenski and Levin (29) measured bacterial phage resistance rates of 10−8 in a chemostat coinoculated with Escherichia coli and phage T4. Sulakvelidze and Kutter (40) suggested that phage cocktails containing two or more phages could be used to reduce resistance. In the present study, the results obtained when screening the biofilm isolates recovered from phage M4-treated catheters against a collection of phages suggested that it is possible to develop a mixture of phages (phage cocktail) that could increase the effective host range and further reduce biofilm formation. The treatment of catheters with a cocktail of the five best phages from that screening significantly reduced biofilm formation by P. aeruginosa M4 compared to that of treatment with a single phage. However, biofilms on the cocktail-treated catheters also contained several phenotypic subpopulations, and one biofilm isolate (48C) exhibited a stable variant colony morphology and complete resistance to all five phages in the cocktail. This isolate also demonstrated greater biofilm formation potential than the M4 parent strain. The mechanism mediating phage resistance in 48C is unknown.
Through continued screening it might be possible to identify phages with lytic activity against this strain. However, as Lenski has suggested (28), phage have less coevolutionary potential than their bacterial host strains, at least in a closed system, allowing bacterial mutants with no corresponding lytic phage strain to evolve. This suggests that the phage cocktail approach we have described is more effective in applications that provide protection against an initial bacterial challenge rather than applications that require prolonged protection against biofilm formation. However, it is noteworthy that only one of the common phenotypic subpopulations recovered from the phage cocktail experiments was completely resistant to the phages used, whereas all of the variant types recovered from the single-phage treatments were resistant to that phage.
We suggest that the application of phage cocktails to the surfaces of indwelling medical devices, such as intravascular catheters and prosthetic joints, could provide a strategy for the reduction in initial colonization and subsequent biofilm formation by clinically relevant bacteria. However, potential obstacles to the use of this treatment must be carefully considered, including the narrow host range of phage, the resistance of host bacteria to phage, potential for inactivation by the patient's immune system, and the safety of phage preparations in humans (17). Further in vivo studies will be necessary to assess the potential of this approach for the prevention of device-associated infections and to determine whether phage pretreatment can be clinically significant.
Conclusions.
The pretreatment of hydrogel-coated silicone catheters with P. aeruginosa phage M4 significantly reduced attachment and biofilm formation by P. aeruginosa M4 in a laboratory model system. The regrowth of biofilms on phage-treated catheters occurred, but recharge treatment and treatment with a mixture of phages having activity against the biofilm organisms significantly reduced the extent of biofilm formation compared to that of untreated catheters or catheters treated once with a single phage. The efficacy of phage pretreatment was not significantly affected by later exposure to human serum, and viable phages continued to be detected in the luminal fluid during the 48-h time course of the experiment. These results suggest the potential of phage application to the surface of indwelling medical devices for the reduction of biofilm formation by clinically relevant bacteria. Further testing using in vivo model systems is needed to evaluate the therapeutic potential of this technology.
Acknowledgments
We acknowledge Janice Carr for scanning electron micrographs. We also thank Ronny Baxter and Michele Davis of C. R. Bard for providing Foley catheters. We acknowledge Jay Ash and the Snapfinger Creek Water Quality Laboratory staff for assistance in the collection of sewage samples and Tyrone Pitt and Mark Granner (Health Protection Agency, United Kingdom) for phage and bacterial stocks.
This research was supported in part by the appointment of W. Fu and O. Mayer to the Emerging Infectious Diseases Laboratory Fellowship Program administered by the Association of Public Health Laboratories (APHL) and funded by the Centers for Disease Control and Prevention. S. M. Lehman is supported by an American Society for Microbiology/Coordinating Centers for Disease Control and Prevention Postdoctoral Fellowship.
The use of trade names and commercial sources is for identification only and does not imply endorsement by the Public Health Service or the U.S. Department of Health and Human Services.
Footnotes
Published ahead of print on 12 October 2009.
REFERENCES
- 1.Ackermann, H.-W., C. Cartier, S. Slopek, and J.-F. Vieu. 1988. Morphology of Pseudomonas aeruginosa typing phages of the Lindberg set. Ann. Inst. Pasteur Virol. 139:389-404. [DOI] [PubMed] [Google Scholar]
- 2.Adams, M. 1959. Bacteriophages. Interscience Publishers, London, United Kingdom.
- 3.Ahearn, D. G., D. T. Grace, M. J. Jennings, R. N. Borazjani, K. J. Boles, L. J. Rose, R. B. Simmons, and E. N. Ahanotu. 2000. Effects of hydrogel/silver coatings on in vitro adhesion to catheters of bacteria associated with urinary tract infections. Curr. Microbiol. 41:120-125. [DOI] [PubMed] [Google Scholar]
- 4.Allison, G. E., and T. R. Klaenhammer. 1998. Phage resistance mechanisms in lactic acid bacteria. Int. Dairy J. 8:207-226. [Google Scholar]
- 5.Barrangou, R., C. Fremaux, H. Deveau, M. Richards, P. Boyaval, S. Moineau, D. A. Romero, and P. Horvath. 2007. CRISPR provides acquired resistance against viruses in prokaryotes. Science 315:1709-1712. [DOI] [PubMed] [Google Scholar]
- 6.Barrow, P., M. Lovell, and A. Berchieri, Jr. 1998. Use of lytic bacteriophage for control of experimental Escherichia coli septicemia and meningitis in chickens and calves. Clin. Diagn. Lab. Immunol. 5:294-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blondel-Hill, E., D. A. Henry, and D. P. Speert. 2007. Pseudomonas, p. 734-748. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. L. Landry, and M. A. Pfaller (ed.), Manual of clinical microbiology, 9th ed., vol. 1. ASM Press, Washington, DC. [Google Scholar]
- 8.Carlson, K. 2005. Appendix: working with bacteriophages: common techniques and methodological approaches, p. 437-494. In E. Kutter and A. Sulakvelidze (ed.), Bacteriophages: biology and applications. CRC Press, Boca Raton, FL.
- 9.Centers for Disease Control and Prevention. 2002. Guidelines for prevention of intravascular catheter-related infections. MMWR 51(RR-10):1-32. [Google Scholar]
- 10.Cerca, N., R. Oliveira, and J. Azeredo. 2007. Susceptibility of Staphylococcus epidermidis planktonic cells and biofilms to the lytic action of Staphylococcus bacteriophage K. Lett. Appl. Microbiol. 45:313-317. [DOI] [PubMed] [Google Scholar]
- 11.Christensen, G. D., W. A. Simpson, J. J. Younger, L. M. Baddour, F. F. Barrett, D. M. Melton, and E. H. Beachey. 1985. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J. Clin. Microbiol. 22:996-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corbin, B. D., R. J. C. McLean, and G. M. Aron. 2001. Bacteriophage T4 multiplication in a glucose-limited Escherichia coli biofilm. Can. J. Microbiol. 47:680-684. [PubMed] [Google Scholar]
- 13.Curtin, J. J., and R. M. Donlan. 2006. Using bacteriophages to reduce formation of catheter-associated biofilms by Staphylococcus epidermidis. Antimicrob. Agents Chemother. 50:1268-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deretic, V., M. J. Schurr, J. C. Boucher, and D. W. Martin. 1994. Conversion of Pseudomonas aeruginosa to mucoidy in cystic fibrosis: environmental stress and regulation of bacterial virulence by alternative sigma factors. J. Bacteriol. 176:2773-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Déziel, E., Y. Comeau, and R. Villemur. 2001. Initiation and biofilm formation by Pseudomonas aeruginosa 57RP correlates with emergence of hyperpiliated and highly adherent phenotypic variants deficient in swimming, swarming, and twitching motilities. J. Bacteriol. 183:1195-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donlan, R. M., and J. W. Costerton. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donlan, R. M. 2009. Preventing biofilms of clinically relevant organisms using bacteriophage. Trends Microbiol. 17:66-72. [DOI] [PubMed] [Google Scholar]
- 18.Doolittle, M. M., J. J. Cooney, and D. E. Caldwell. 1995. Lytic infection of Escherichia coli biofilms by bacteriophage T4. Can. J. Microbiol. 41:12-18. [DOI] [PubMed] [Google Scholar]
- 19.Doolittle, M. M., J. J. Cooney, and D. E. Caldwell. 1996. Tracing the interaction of bacteriophage with bacterial biofilms using fluorescent and chromogenic probes. J. Indust. Microbiol. 16:331-341. [DOI] [PubMed] [Google Scholar]
- 20.Drenkard, E., and F. M. Ausubel. 2002. Pseudomonas biofilm formation and antibiotic resistance are linked to phenotypic variation. Nature 416:740-743. [DOI] [PubMed] [Google Scholar]
- 20a.Fu, W., T. Forster, O. Mayer, J. J. Curtin, S. M. Lehman, and R. M. Donlan. 2007. Poster no. A-011. Abstr. Am. Soc. Microbiol. Gen. Meet., American Society for Microbiology, Washington, DC.
- 21.Guttman, B., R. Raya, and E. Kutter. 2005. Basic phage biology, p. 29-66. In E. Kutter and A. Sulakvelidze (ed.), Bacteriophages: biology and applications. CRC Press, Boca Raton, FL.
- 22.Hadas, H., M. Einav, I. Fishov, and A. Zaritsky. 1997. Bacteriophage T4 development depends on the physiology of its host Escherichia coli. Microbiology 143:179-185. [DOI] [PubMed] [Google Scholar]
- 23.Hanlon, G. W., S. P. Denyer, C. J. Olliff, and L. J. Ibrahim. 2001. Reduction of exopolysaccharide viscosity as an aid to bacteriophage penetration through Pseudomonas aeruginosa biofilms. Appl. Environ. Microbiol. 67:2746-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Häussler, S., B. Tummler, H. Wessbrodt, M. Rohde, and I. Steinmetz. 1999. Small-colony variants of Pseudomonas aeruginosa in cystic fibrosis. Clin. Infect. Dis. 29:621-625. [DOI] [PubMed] [Google Scholar]
- 25.Hughes, K. A., I. W. Sutherland, and M. V. Jones. 1998. Biofilm susceptibility to bacteriophage attack: the role of phage-borne polysaccharide depolymerase. Microbiology 144:3039-3047. [DOI] [PubMed] [Google Scholar]
- 26.Jones, J. B., L. E. Jackson, B. Balogh, A. Obradovic, F. B. Iriarte, and M. T. Momol. 2007. Bacteriophages for plant disease control. Annu. Rev. Phytopathol. 45:245-262. [DOI] [PubMed] [Google Scholar]
- 27.Karlson, K. 1994. Single-step growth, p. 434-436. In J. D. Karam, J. W. Drake, and K. N. Kreuzer (ed.), Molecular biology of bacteriophage T4. American Society for Microbiology, Washington, DC.
- 28.Lenski, R. E. 1988. Dynamics of interactions between bacteria and virulent bacteriophage, p. 1-44. In K. C. Marshall (ed.), Advances in microbial ecology. Plenum Press, New York, NY.
- 29.Lenski, R. E., and B. R. Levin. 1985. Constraints on the coevolution of bacteria and virulent phage: a model, some experiments, and predictions for natural communities. Am. Nat. 125:585-602. [Google Scholar]
- 30.Lu, T. K., and J. J. Collins. 2007. Dispersing biofilms with engineered enzymatic bacteriophage. Proc. Natl. Acad. Sci. USA 104:11197-11202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Markoishvili, K., G. Tsitlanadze, R. Katsarava, J. G. Morris, Jr., and A. Sulakvelidze. 2002. A novel sustained-release matrix based on biodegradable poly(ester amide)s and impregnated with bacteriophages and an antibiotic shows promise in management of infected venous stasis ulcers and other poorly healing wounds. Int. J. Dermatol. 41:453-458. [DOI] [PubMed] [Google Scholar]
- 32.Mooij, M. J., E. Drenkard, M. A. Llamas, C. M. J. E. Vanderbroucke-Grauls, P. H. M. Savelkoul, F. M. Ausubel, and W. Bitter. 2007. Characterization of the integrated filamentous phage Pf5 and its involvement in small-colony formation. Microbiology 153:1790-1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Flaherty, S. R., R. P. Ross, W. Meaney, G. F. Fitzgerald, M. F. Elbreki, and A. Coffey. 2005. Potential of polyvalent anti-Staphylococcus bacteriophage K for control of antibiotic resistant staphylococci from hospitals. Appl. Environ. Microbiol. 71:1836-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Toole, G. A., and R. Kolter. 1998. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol. Microbiol. 28:449-461. [DOI] [PubMed] [Google Scholar]
- 35.Pitt, T. L. 1988. Epidemiologica typing of Pseudomonas aeruginosa. Eur. J. Clin. Microbiol. Infect. Dis. 7:238-247. [DOI] [PubMed] [Google Scholar]
- 36.Raad, I., R. Darouiche, R. Hachem, M. Sacilowski, and G. P. Bodey. 1995. Antibiotics and prevention of microbial colonization of catheters. Antimicrob. Agents Chemother. 39:2397-2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rice, S. A., C. H. Tan, P. J. Mikkelsen, V. Kung, J. Woo, M. Tay, A. Hauser, D. McDonald, J. S. Webb, and S. Kjelleberg. 2009. The biofilm life cycle and virulence of Pseudomonas aeruginosa are dependent on a filamentous prophage. ISME J. 3:271-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sillankorva, S., R. Oliveira, M. J. Vieira, I. W. Sutherland, and J. Azeredo. 2004. Bacteriophage ΦS1 infection of Pseudomonas fluorescens planktonic cells versus biofilms. Biofouling 20:133-138. [DOI] [PubMed] [Google Scholar]
- 39.Slopek, S., B. Weber-Dabrowska, M. Dabrowski, and A. Kucharewicz-Krukowska. 1987. Results of bacteriophage treatment of supparative bacterial infections in the years 1981-1986. Arch. Immunol. Ther. Exper. 35:569-570. [PubMed] [Google Scholar]
- 40.Sulakvelidze, A., and E. Kutter. 2005. Bacteriophage therapy in humans, p. 381-436. In E. Kutter and A. Sulakvelidze (ed.), Bacteriophages: biology and applications. CRC Press, Boca Raton, FL.
- 41.Webb, J. S., M. Lau, and S. Kjelleberg. 2004. Bacteriophage and phenotypic variation in Pseudomonas aeruginosa biofilm development. J. Bacteriol. 186:8066-8073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Webb, J. S., L. S. Thompson, S. James, T. Charlton, T. Tolker-Nielsen, B. Koch, M. Givskov, and S. Kjelleberg. 2003. Cell death in Pseudomonas aeruginosa biofilm development. J. Bacteriol. 185:4585-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weber-Dabrowska, B., M. Mulczyk, and A. Gorski. 2000. Bacteriophage therapy of bacterial infections: an update of our institution's experience. Arch. Immunol. Ther. Exp. 48:547-551. [PubMed] [Google Scholar]
- 44.Wenzel, R. P., and M. B. Edmond. 1999. The evolving technology of venous access. N. Engl. J. Med. 340:48-50. [DOI] [PubMed] [Google Scholar]