Abstract
ß-l-2′,3′-Didehydro-2′,3′-dideoxy-N4-hydroxycytidine (l-Hyd4C) was demonstrated to be an effective and highly selective inhibitor of hepatitis B virus (HBV) replication in HepG2.2.15 cells (50% effective dose [ED50] = 0.03 μM; 50% cytotoxic dose [CD50] = 2,500 μM). In the present study, we investigated the intracellular pharmacology of tritiated l-Hyd4C in HepG2 cells. l-[3H]Hyd4C was shown to be phosphorylated extensively and rapidly to the 5′-mono-, 5′-di-, and 5′-triphosphate derivatives. Other metabolites deriving from a reduction or removal of the NHOH group of l-Hyd4C could not be detected, although both reactions were described as the primary catabolic pathways of the stereoisomer ß-d-N4-hydroxycytidine in HepG2 cells. Also, the formation of liponucleotide metabolites, such as the 5′-diphosphocholine derivative of l-Hyd4C, as described for some l-deoxycytidine analogues, seems to be unlikely. After incubation of HepG2 cells with 10 μM l-[3H]Hyd4C for 24 h, the 5′-triphosphate accumulated to 19.4 ± 2.7 pmol/106 cells. The predominant peak belonged to 5-diphosphate, with 43.5 ± 4.3 pmol/106 cells. The intracellular half-life of the 5′-triphosphate was estimated to be 29.7 h. This extended half-life probably reflects a generally low affinity of 5′-phosphorylated l-deoxycytidine derivatives for phosphate-degrading enzymes but may additionally be caused by an efficient rephosphorylation of the 5′-diphosphate during a drug-free incubation. The high 5′-triphosphate level and its extended half-life in HepG2 cells are consistent with the potent antiviral activity of l-Hyd4C.
A large number of nucleoside analogues have been described as inhibitors of hepatitis B virus (HBV) and HIV replication. Recently l-nucleoside analogues in particular have gained increasing interest. They are characterized by an opposite configuration from that of the natural d-nucleoside analogues and represent one of the most attractive groups of antiretroviral compounds, including ß-l-2′,3′-dideoxy-3-thiacytidine (3TC) and its 5-fluoro derivative (FTC), ß-l-2′,3′-didehydro-2′,3′-dideoxy-cytidine (l-d4C) and its 5-fluoro derivative (l-d4FC), ß-l-thymidine, ß-l-fluoroarabinosylyluracil (l-FMAU), and ß-l-2′,3′-didehydro-2′,3′-dideoxy-2′-fluoro-cytidine (l-2′Fd4C) (3, 5, 22).
Some of them not only have been found to be more potent than their corresponding d-nucleosides but seem to exhibit lower cytotoxicity and have been proved to be effective and selective agents for the treatment of chronic hepatitis B virus infections (4). However, only long-term therapy with a single nucleoside for several years was shown to be able to completely suppress HBV DNA in serum of patients and to reverse the progression of the disease. The disadvantage connected with such therapy regimens is the development of drug-resistant HBV strains (22). Therefore, the challenge will be to develop more-efficient drugs for shorter treatment regimens and to combine them to reach synergistic or at least additive drug action. This approach has been described not only as being highly efficient for the treatment of HIV infections but also as preventing the development of resistant mutants. Therefore, AIDS therapy is considered a model for future therapy of chronic HBV infections (17).
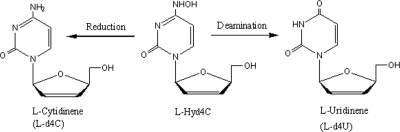
Recently we described a series of new ß-l-N4-hydroxydeoxycytidine and ß-l-5-methyl-deoxycytidine derivatives as inhibitors of HBV replication. Between them, ß-l-2′,3′-didehydro-2′,3′-dideoxy-N4-hydroxycytidine (l-Hyd4C) (Fig. 1) emerged as the most effective in suppression of virus production in HepG2.2.15 cells (50% effective dose [ED50] = 0.03 μM), displaying an extremely low cytotoxicity (50% cytotoxic dose [CD50] for HepG2 cells = 2,500 μM) (12).
FIG. 1.
Structure of l-Hyd4C and possible metabolites formed by reduction (l-d4C) or by deamination (l-d4U).
These encouraging features have prompted us to investigate the cellular pharmacology of l-Hyd4C in a hepatic cell line. This included the activation of this unnatural l-deoxycytidine nucleoside to its 5′-mono-, 5′-di-, and 5′-triphosphate, the search for other metabolites, and the estimation of the intracellular half-lives (t1/2) of the 5′-di- and 5′-triphosphate of l-Hyd4C.
(This work was presented in part at BIT's 5th Anniversary Congress of International Drug Discovery Science and Technology, 7 to 13 November 2007, Xi'an and Beijing, China.)
MATERIALS AND METHODS
Compounds.
The synthesis and characterization of l-Hyd4C and its 5′-triphosphate were described elsewhere (E. Matthes, M. von Janta-Lipinski, H. Will, H. Sirma, and A. Funk, 21 October 2005, European patent application no. PCT/EP2005/011555). l-[3H]Hyd4C (0.2 Ci/mmol) was custom synthesized from l-Hyd4C by catalytic tritium exchange by Moravek Biochemicals Inc. (Brea, CA). High-performance liquid chromatography (HPLC) analysis by methods described below showed 12% of radioactive contamination of the tritiated products which were separated by HPLC.
Acetonitrile (gradient grade), tetrabutylammonium phosphate (monobasic, purissimum), and 3-deazauridine (deazaUR) were obtained from Sigma-Aldrich (Taufkirchen, Germany). Ammonium dihydrogen phosphate (puriss.) and the scintillation reagent Rotiszint eco plus came from Roth (Karlsruhe, Germany).
Cell culture.
The well-established human hepatoblastoma cell line HepG2 was cultured in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum, 1 mM sodium pyruvate, and 1% penicillin G-streptomycin sulfate and maintained at 37°C with a humidified atmosphere of 5% CO2. The medium was changed every 3 days, and the cells were subcultured once per week. Confluent cells were treated with trypsin-EDTA, washed three times with the medium, and seeded in a six-well plate (2.5 × 106 per well). After adherence, the cells were exposed to 2 or 10 μM l-[3H]Hyd4C (440 or 1,000 dpm/pmol, respectively). At the indicated times, medium was removed from the cell layer and the cells were washed with ice-cold phosphate-buffered saline, treated with trypsin-EDTA, washed again, and counted on a hemacytometer. The viability (trpyan blue) was greater than 95%. Cells were spun down at 1,000 × g.
For determination of the intracellular half-life of the 5′-triphosphate of l-Hyd4C, 1.5 × 106 HepG2 cells/well were incubated for 24 h with 10 μM l-[3H]Hyd4C as described above. The cells were then washed three times and incubated in drug-free medium. At the indicated times, cells were harvested and counted as described above.
Extraction of metabolites.
After centrifugation, the cells were extracted twice with 60% methanol-water for 16 h with a total volume of 1.5 ml at −20°C. Extracts were dried in a Speed Vac concentrator and frozen at −80°C until analysis by HPLC.
HPLC analysis.
The extracts were resolved in 60 μl mobile phase, and 50 μl of this material was separated by reverse-phase HPLC (LC-10; Shimadzu, Kyoto, Japan) with a Zorbax 300SB C18 column (250 by 4.6 mm; inside diameter, 5 μm; Agilent, Waldbronn, Germany). Mobile phase A was 10 mM phosphate buffer, pH 4.9, 2 mM tetrabutylammonium phosphate, and 0.4% acetonitrile, and mobile phase B was pure acetonitrile. With a flow rate of 1 ml/min, the linear gradient was 0 to 36% of mobile phase B for 30 min. Fractions of 1 ml (each) were collected, and the radioactivity was measured with a high-salt-capacity scintillation fluid. The l-Hyd4C metabolites were identified by use of the UV spectra (λmax = 235, 271 nm; λmin = 207, 257 nm), using the 5′-triphosphate of l-Hyd4C as a standard (12), and by enzyme digestion of the dried methanol extracts with 1.0 U of alkaline phosphatase (60 μl; 10 mM Tris-HCl, pH 10.8, at 37°C for 12 h).
The half-lives of the 5′-di- and 5′-triphosphate of l-Hyd4C were calculated from 0.693/κ, where κ is the terminal-phase slope of a semilogarithmic plot of the intracellular phosphate concentrations versus time.
CDA.
The susceptibility of l-Hyd4C to cytidine deaminase (CDA) was assessed by using recombinant human CDA (70 U/mg), which was a kind gift of Silvia Vincenzetti, University of Camerino, Camerino, Italy (20). A 0.05-U amount of the enzyme was incubated at 37°C for 30 min in 100 μl containing 100 mM Tris-HCl, pH 7.5, 100 mM KCl, and 100 μM deoxycytidine or l-Hyd4C. The reactions were stopped, and aliquots were separated by HPLC as described above but with a reduced flow rate of 0.7 ml/min.
RESULTS
HPLC analysis of metabolites of l-Hyd4C in HepG2 cells.
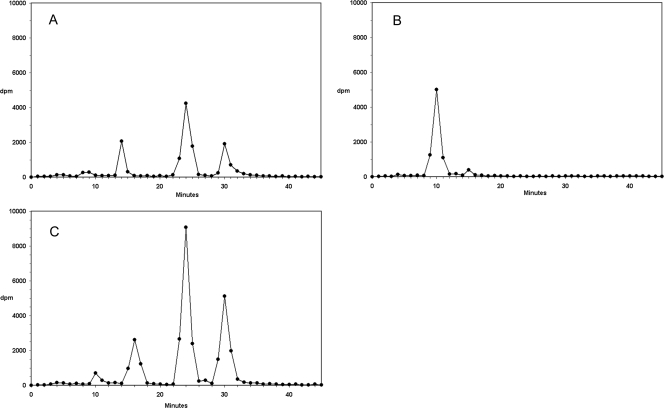
The intracellular metabolites of tritium-labeled l-Hyd4C in HepG2 cells were assessed by reversed-phase HPLC. Figure 2A shows the HPLC radiochromatogram of extracts from HepG2 cells exposed to 2 μM l-[3H]Hyd4C for 24 h. The retention times of the parent nucleoside and 5′-triphosphate were identical to those of unlabeled standards synthesized in our laboratory (12). No radioactive metabolites were detected other than l-[3H]Hyd4C and its 5′-mono-, 5′-di-, and 5′-triphosphate. The retention times were about 10, 14, 24, and 30 min, respectively. Treatment of such cell extracts with alkaline phosphatase revealed that nearly all radioactivities could be recovered as l-[3H]Hyd4C (Fig. 2B).
FIG. 2.
HPLC radiochromatogram of extracts from HepG2 cells: cells treated with 2.0 μM l-[3H]Hyd4C for 24 h (A), cells treated as before but digested with alkaline phosphatase (B), or cells pretreated with 3 μM deazaUR for 24 h, washed, and incubated for a further 24 h with 3 μM deazaUR and 2.0 μM l-[3H]Hyd4C (C).
Cells were treated additionally with deazaUR, an activator of the deoxycytidine kinase, to find out its effect on the phosphate pools of l-Hyd4C. To this aim, cells were preincubated with 3 μM deazaUR for 24 h and then exposed for a second 24-h period to both 3 μM deazaUR and 2 μM l-[3H]Hyd4C. These conditions had only a slight influence on the proliferation of HepG2 cells (11% inhibition of cell growth during 48 h). Figure 2C shows that deazaUR increased strongly the level of phosphorylated metabolites of l-Hyd4C; the 5′-diphosphate peak was about 2-fold higher and the 5′-triphosphate peak about 3.5-fold higher than those found without deazaUR, suggesting that the deoxycytidine kinase, rather than any other kinase, was responsible for the first step of phosphorylation.
Deamination of l-Hyd4C.
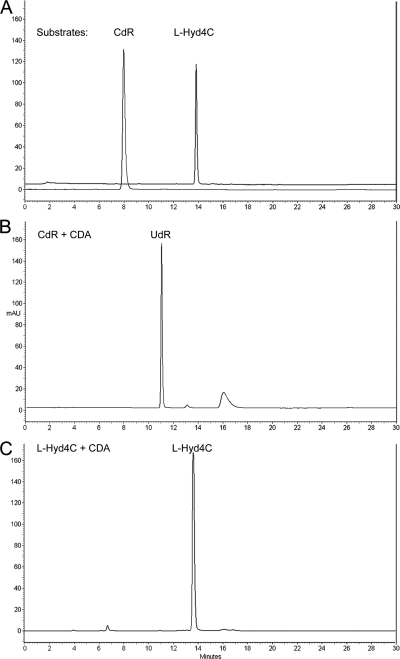
Figure 3C illustrates that l-Hyd4C is resistant against deamination by human recombinant CDA under conditions described in Materials and Methods. Also, more stringent conditions (0.4 U and 2 h) were unable to produce detectable amounts of the deaminated product of l-Hyd4C, l-uridinene (ß-l-2′,3′-didehydro-2′,3′-dideoxyuridine) (Fig. 1). In contrast, deoxycytidine (CdR) was completely cleavaged by the CDA to deoxyuridine (UdR) within 30 min (Fig. 3B).
FIG. 3.
HPLC profile of CdR and l-Hyd4C exposed to human recombinant CDA: separation of CdR and l-Hyd4C (A), complete cleavage of CdR to UdR by CDA (B), and resistance of l-Hyd4C to CDA cleavage (C).
Time course accumulation of phosphorylated metabolites of l-Hyd4C in HepG2 cells.
Table 1 summarizes the intracellular concentrations of phosphorylated metabolites in HepG2 cells exposed to 2 or 10 μM l-[3H]Hyd4C for 6 to 24 h. l-Hyd4C was rapidly phosphorylated, with the 5′-diphosphate as the predominant metabolite. Ten μM l-Hyd4C accumulated to a level of 43.5 ± 4.3 pmol/106 cells and 2 μM l-Hyd4C to a level of 9.8 ± 1.8 pmol/106 cells within 24 h. The pharmacologically active 5′-triphosphate was extensively formed, reaching a concentration of 19.4 ± 2.7 pmol/106 cells for 10 μM and 5.30 ± 1.1 pmol/106 cells for 2 μM l-Hyd4C after 24 h. To examine a possible influence of HBV replication on the phosphorylation of l-Hyd4C, we incubated the HBV-transfected HepG2.2.15 cells with l-[3H]Hyde4C under the conditions described above. The results obtained demonstrate that HBV replication in HepG2.2.15 cells did not influence the level of the phosphorylated products of l-Hyd4C.
TABLE 1.
Time-dependent phosphorylation of l-[3H]Hyd4C in HepG2 cells
| Incubation time (h) | l-Hyd4C concn (μM) | Amt of 5′-phosphorylated metabolite of l-Hyd4C (pmol/106 cells)a |
||
|---|---|---|---|---|
| Monophosphate | Diphosphate | Triphosphate | ||
| 6 | 2 | 0.5 ± 0.1 | 1.3 ± 0.3 | 1.1 ± 0.3 |
| 10 | 4.4 ± 1.2 | 11.5 ± 2.1 | 5.3 ± 1.4 | |
| 12 | 2 | 1.3 ± 0.3 | 4.5 ± 1.2 | 3.1 ± 0.8 |
| 10 | 5.6 ± 1.5 | 22.6 ± 4.1 | 10.4 ± 1.9 | |
| 18 | 2 | 2.3 ± 0.1 | 6.6 ± 1.6 | 3.8 ± 0.8 |
| 10 | 7.3 ± 1.6 | 30.3 ± 4.2 | 15.2 ± 2.7 | |
| 24 | 2 | 4.0 ± 1.0 | 9.8 ± 1.8 | 5.3 ± 1.1 |
| 10 | 11.5 ± 1.8 | 43.5 ± 4.3 | 19.4 ± 2.7 | |
The values are means ± standard deviations of results from three independent experiments.
Rates of decay of phosphorylated metabolites of l-Hyd4C in HepG2 cells.
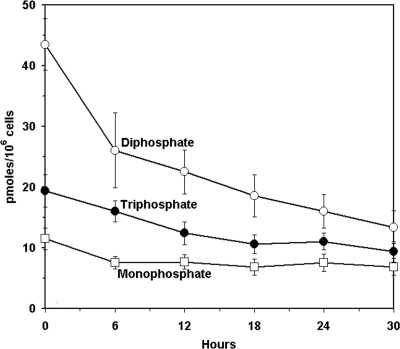
Following exposure to 10 μM l-[3H]Hyd4C for 24 h, cells were washed and incubated in drug-free medium. The intracellular metabolite concentrations were plotted against the indicated times (Fig. 4). The 5′-monophosphate level of l-Hyd4C was 11.5 ± 1.8 pmol/106 cells at the end of incubation time and varied from 6.9 ± 1.4 pmol/106 cells to 7.7 ± 1.2 pmol/106 cells between 6 and 30 h after removal of l-Hyd4C. No clear decay could be seen. The 5′-diphosphate and 5′-triphosphate had extended t1/2 values of 18.6 h and 29.7 h, respectively, as calculated from semilogarithmic plots. This corresponds to about 9.3 pmol 5′-triphosphate/106 cells remaining 30 h after drug removal.
FIG. 4.
Time-dependent decrease of phosphorylated metabolites of l-Hyd4C in HepG2 cells after exposure to 10 μM l-[3H]Hyd4C for 24 h. The concentrations of the 5′-monophosphate, 5′-diphosphate, and 5′-triphosphate of l-Hyd4C are given in pmol/106 cells. The values are means ± standard deviations of results from three independent experiments.
DISCUSSION
The hydroxylation of the 4-NH2 group of the natural ß-d-stereoisomers of deoxycytidine and cytidine was shown many years ago to change remarkably the features of the normal metabolites of nucleic acid synthesis (13, 15).
Recently ß-d-N4-hydroxycytidine (D-HyCR) was discovered as an efficient inhibitor of the bovine viral diarrhea virus, in the hepatitis C virus (HCV) replicon system, and of the severe acute respiratory syndrome-associated coronavirus (SARS-CoV) (1, 19), which initiated new interest in N4-hydroxylated cytidine derivatives.
In contrast to the ß-d-enantiomers, we synthesized ß-l-enantiomeric N4-hydroxydeoxy-cytidine derivatives and investigated them as inhibitors of HBV and HIV replication (12; E. Matthes, unpublished). Between them, a series of strong inhibitors of HBV replication could be found. l-Hyd4C emerged as the most effective one, suppressing HBV replication in HepG2.2.15 cells with an ED50 of 0.03 μM and surprisingly displaying an extremely low cytotoxicity (CD50 in HepG2 cells = 2,500 μM) (12).
Here we describe the cellular pharmacology of this compound in HepG2 cells. With tritium-labeled l-Hyd4C, we found that this analogue is metabolized well to the 5′-mono-, 5′-di-, and 5′-triphosphates, with the 5′-diphosphate having the main peak. No other labeled metabolite could be detected. Digestion of such cell extracts with alkaline phosphatase produced only the labeled nucleoside, suggesting that modifications other than the phosphorylation steps, such as the formation of the 5′-diphosphocholine derivative, seem to be unlikely. Such a liponucleotide metabolite has been detected inside cells for other l-cytidine analogues, such as l-deoxycytidine (l-CdR) (7), 2′,3′-dideoxycytidine (l-ddC), and its 5-fluoro derivative (l-ddFC) (11). This liponucleotide and some 5′-diphosphoethanolamine derivatives too not only are considered to be desired precursors for antiviral l-nucleoside 5′-triphosphates inside cells but might cause an inhibition of lipid biosynthesis, affecting membrane integrity (11).
Additionally, we investigated whether the N4-hydroxylamino group of our l-enantiomeric nucleoside could be deaminated by human CDA to the corresponding uridine derivative, l-uridinene (l-d4U).
This enzymatic reaction was described as one main metabolic pathway of the d-stereoisomer d-HyCR (8). However, we found that l-Hyd4C is completely resistant against deamination, confirming the described strict enantioselectivity of this enzyme (18). The other main pathway described for d-HyCR is a reduction of the NHOH group to the NH2 group, giving d-cytidine. A reduction of l-Hyd4C should produce l-d4C, a compound which is known to display both a relatively high cytotoxicity (CD50 = 20 μM) and strong antiviral activity against HBV and HIV replication (9). The extremely low cytotoxicity of l-Hyd4C and the lack of any effect on HIV replication let us assume that this modification is unlikely (12; Matthes, unpublished). This view is further supported by our radiochromatograms from extracts of HepG2 cells incubated with l-[3H]Hyd4C (Fig. 2), which gave no indication of the formation of l-[3H]d4C, confirming that the phosphorylation is the only modification of l-Hyd4C in HepG2 cells.
We have demonstrated that the level of phosphorylation of l-Hyd4C (2 μM) in HepG2 cells could be increased by a simultaneous incubation of deazaUR. This resulted in a 2-fold-higher concentration of the 5′-diphosphate and a 3-fold-higher concentration of the 5′-triphosphate of l-Hyd4C. DeazaUR inhibits in its 5′-triphosphate form the CTP synthase, with the consequence of depleting the dCTP pool. This lack of dCTP induces a feedback mechanism to increase the phosphorylation rate of CdR and antiviral CdR derivatives, respectively (6). Our finding that deazaUR at nontoxic concentrations increased the phosphorylation of l-Hyd4C too suggests that the deoxycytidine kinase rather than any other kinase could be responsible for the initial phosphorylation step. Furthermore, the presence of 30 μM CdR suppressed competitively the phosphorylation of 2 μM l-Hyd4C in HepG2 cells, supporting further the idea that the deoxycytidine kinase is responsible for the first step of phosphorylation (data not shown).
Human deoxycytidine kinase has been described earlier as having only a low enantioselectivity, which also favors the phosphorylation of a series of l-cytidine derivatives, such as 3TC (2), its 5-fluoro derivative (FTC) (14), l-ddC and its 5-fluoro derivative (l-ddFC), and l-CdR (11, 18). Ten μM l-Hyd4C was converted to 11.5 ± 1.8 pmol monophosphate/106 cells during 24 h.
The pyrimidine nucleoside monophosphate kinase (UMP/CMP kinase) was reported to be responsible for the phosphorylation of the l-enantiomers 3TC- and FTC- 5′-monophosphate (10). Also, l-Hyd4C-5′-monophosphate seems to be a good substrate. Ten μM l-Hyd4C was metabolized during 24 h to 43.5 ± 4.3 pmol 5′-diphosphate/106 cells, which is in the range of l-d4FC (21) but higher than the level described for other l-enantiomeric CdR derivatives, such as FTC (34 pmol/106 HepG2 cells) (14) and 3TC (16).
The following addition of the gamma phosphate is a function not only of the nucleoside diphosphate kinase but also of the pyruvate kinase, creatine kinase, and phosphoglycerate kinase and is considered the rate-limiting step for nucleoside 5′-diphosphates with the l configuration. We found 19.4 ± 2.7 pmol 5′-triphosphate/106 cells after incubation with 10 μM l-Hyd4C over 24 h. Basing on the intracellular water volume of 2.6 μl/106 HepG2 cells (14), the intracellular 5′-triphosphate concentration is equivalent to 7.46 μM, which exceeded by far the concentration of 1 μM required for a 90% inhibition of the HBV DNA polymerase (12).
A further characteristic that contributes to the strong anti-HBV activity of l-Hyd4C is the extended intracellular half-life of the 5′-di- and 5′-triphosphate. The t1/2 values of l-Hyd4C-5′-diphosphate and l-Hyd4C-5′-triphosphate were estimated to be 18.6 and 29.7 h, respectively. Also, other 5′-triphosphates of l-deoxycytidine derivatives are known to have prolonged half-lives, such as 3TC, FTC, l-ddC, l-ddFC, and l-d4FC (14, 16, 21), which probably reflects a low affinity of 5′-phosphorylated l-CdR derivatives for phosphate-degrading enzymes. However, among these l-CdR analogues, l-Hyd4C-5′-triphosphate has the longest t1/2. This may be caused additionally by an efficient rephosphorylation of the diphosphate during a drug-free incubation. Thus, HepG2 cells still contained 3.73 μM triphosphate about 30 h after removal of 10 μM l-Hyd4C, which seems to be sufficient for a complete inhibition of HBV DNA polymerase.
In summary, we found that l-Hyd4C is readily phosphorylated in HepG2 cells to the 5′-triphosphate. Other metabolites could not be detected. The high level of the 5′-triphosphate in cells, its long half-life, and the earlier-described strong inhibition of the HBV replication and its extremely low cytotoxicity recommend l-Hyd4C for further investigations.
Acknowledgments
We thank Christoph Mark from Mark Chemical Laboratories, Worms, Germany, and Vibhuti Klingler-Dabral, BioDeTek, GmbH, Griesheim, Germany, for the synthesis and identification of l-Hyd4C. We also thank Silvia Vincenzetti, University of Camerino, Camerino, Italy, for the recombinant CDA.
Footnotes
Published ahead of print on 16 November 2009.
REFERENCES
- 1.Barnard, D. L., V. D. Hubbard, J. Burton, D. F. Smee, J. D. Morrey, M. J. Otto, and R. S. Sidwell. 2004. Inhibition of severe acute respiratory syndrome-associated corona-virus (SARSCoV) by calpain inhibitors and ß-D-N4-hydroxycytidine. Antivir. Chem. Chemother. 15:15-22. [DOI] [PubMed] [Google Scholar]
- 2.Chang, C. N., S. L. Doong, J. H. Zhou, J. W. Beach, L. S. Jeong, C. K. Chu, C. H. Tsai, Y. C. Cheng, D. Liotta, and R. Schinazi. 1992. Deoxycytidine deaminase-resistant stereoisomer is the active form of (+/−)-2′,3′-dideoxy-3′-thiacytidine in the inhibition of hepatitis B virus replication. J. Biol. Chem. 267:13938-13942. [PubMed] [Google Scholar]
- 3.Chen, S.-H. 2002. Comparative evaluation of L-Fd4C and related nucleoside analogs as promising antiviral agents. Curr. Med. Chem. 9:899-912. [DOI] [PubMed] [Google Scholar]
- 4.Dienstag, J. L. 2008. Hepatitis B infection. N. Engl. J. Med. 359:1486-1500. [DOI] [PubMed] [Google Scholar]
- 5.Férir, G., S. Kaptein, J. Neyts, and E. De Clercq. 2008. Antiviral treatment of chronic hepatitis B virus infections: the past, the present and the future. Rev. Med. Virol. 18:19-34. [DOI] [PubMed] [Google Scholar]
- 6.Gao, W.-Y., D. G. Johns, and H. Mitsuya. 2000. Potentiation of anti-HIV activity of zalcitabine and lamivudine by a CTP synthase inhibitor. Nucleosides Nucleotides Nucleic Acids 19:371-377. [DOI] [PubMed] [Google Scholar]
- 7.Hernandez-Santiago, B., L. Placidi, E. Cretton-Scott, A. Faraj, E. G. Bridges, M. L. Bryant, J. Rodriguez-Orengo, J. L. Imbach, G. Gosselin, C. Pierra, D. Dukhan, and J. P. Sommadossi. 2002. Pharmacology of ß-L-thymidine and ß-L-2′-deoxycytidine in HepG2 cells and primary human hepatocytes: relevance to chemotherapeutic efficacy against hepatitis B virus. Antimicrob. Agents Chemother. 46:1728-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hernandez-Santiago, B. I., T. Beltran, L. Stuyver, C. K. Chu, and R. F. Schinazi. 2004. Metabolism of the anti-hepatitis C virus nucleoside ß-D-N4-hydroxycytidine in different liver cells. Antimicrob. Agents Chemother. 48:4636-4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin, T.-S., M.-Z. Luo, M.-C. Liu, Y.-L. Zhu, E. Gullen, G. E. Dutschman, and Y.-C. Cheng. 1996. Design and synthesis of 2′,3′-dideoxy-2′,3′-didehydro-ß-L-cytidine (ß-L-d4C) and 2′,3′-dideoxy-2′,3′-didehydro-ß-L-5-fluorocytidine (ß-L-Fd4C), two exceptionally potent inhibitors of human hepatitis B virus (HBV) and potent inhibitors of human immunodeficiency virus (HIV) in vitro. J. Med. Chem. 39:1757-1759. [DOI] [PubMed] [Google Scholar]
- 10.Liou, J.-Y., G. E. Dutschman, W. Lam, Z. Jiang, and Y.-C. Cheng. 2002. Characterization of human UMP/CMP kinase and its phosphorylation of D- and L-form deoxycytidine analogue monophosphates. Cancer Res. 62:1624-1631. [PubMed] [Google Scholar]
- 11.Martin, L., A. Faraj, R. F. Schinazi, G. Gosselin, C. Mathe, J. L. Imbach, and J. P. Sommadossi. 1997. Effect of stereoisomerism on the cellular pharmacology of ß-enantiomers of cytidine analogs in HepG2 cells. Biochem. Pharmacol. 53:75-87. [DOI] [PubMed] [Google Scholar]
- 12.Matthes, E., A. Funk, I. Krahn, K. Gaertner, M. von Janta-Lipinski, L. Lin, H. Will, and H. Sirma. 2007. Strong and selective inhibitors of hepatitis B virus replication among novel N4-hydroxy- and 5-methyl-ß-L-deoxycytidine analogues. Antimicrob. Agents Chemother. 51:2523-2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson, D. J., and C. E. Carter. 1966. Inhibition of the biosynthesis of thymidylic acid by 4-N-hydroxy-2′-deoxycytidine in L5178Y leukemic cells. Mol. Pharmacol. 2:248-258. [PubMed] [Google Scholar]
- 14.Paff, M. T., D. R. Averett, K. L. Prus, W. H. Miller, and D. J. Nelson. 1994. Intracellular metabolism of (—) and (+)-cis-5-fluoro-1-[2-hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine in HepG2 derivative 2.2.15 (subclone P5A) cells. Antimicrob. Agents Chemother. 38:1230-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Popowska, E., and C. Janion. 1974. N4-Hydroxycytidine—a new mutagen of a base analogue type. Biochem. Biophys. Res. Commun. 56:459-466. [DOI] [PubMed] [Google Scholar]
- 16.Rahn, J. J., D. M. Kieller, D. L. J. Tyrrell, and W. P. Gati. 1997. Modulation of the metabolism of ß-L-(—)-2′,3′-dideoxy-3′-thiacytidine by thymidine, fludarabine and nitrobenzylthioinosine. Antimicrob. Agents Chemother. 41:918-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sasadeusz, J. J., S. L. Locarnini, and G. Macdonald. 2007. Why do we not yet have combination chemotherapy for chronic hepatitis B? Med. J. Aust. 186:204-206. [DOI] [PubMed] [Google Scholar]
- 18.Shafiee, M., J.-F. Griffon, G. Gosselin, A. Cambi, S. Vincenzetti, A. Vita, S. Eriksson, L.-J. Imbach, and G. Maury. 1998. A comparison of the stereoselectivities of human deoxycytidine kinase and human cytidine deaminase. Biochem. Pharmacol. 56:1237-1242. [DOI] [PubMed] [Google Scholar]
- 19.Stuyver, L. J., T. Whitaker, T. R. McBrayer, B. I. Hernandez-Santiago, S. Lostia, P. M. Tharnish, M. Ramesh, C. K. Chu, R. Jordan, J. Shi, S. Rachakonda, K. A. Watanabe, M. J. Otto, and R. F. Schinazi. 2003. Ribonucleoside analogue that blocks replication of bovine viral diarrhea and hepatitis C viruses in culture. Antimicrob. Agents Chemother. 47:244-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vincenzetti, S., A. Cambi, J. Neuhard, E. Garattini, and A. Vita. 1996. Recombinant human cytidine deaminase: expression, purification, and characterization. Protein Expr. Purif. 8:247-253. [DOI] [PubMed] [Google Scholar]
- 21.Zhu, Y.-L., G. E. Dutschman, S.-H. Liu, E. G. Bridges, and Y.-C. Cheng. 1998. Anti-hepatitis B virus activity and metabolism of 2′,3′-dideoxy-2′,3′-didehydro-ß-L(—)-5-fluorocytidine. Antimicrob. Agents Chemother. 42:1805-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zoulim, F. 2006. Antiviral therapy of chronic hepatitis B. Antiviral. Res. 71:206-215. [DOI] [PubMed] [Google Scholar]