Abstract
1-Hydroxy-2-dodecyl-4(1H)quinolone (HDQ) was recently identified as a Toxoplasma gondii inhibitor. We describe here two novel 1-hydroxyquinolones, which displayed 50% inhibitory concentrations 10- and 5-fold lower than that of HDQ. In a mouse model of acute toxoplasmosis, these two compounds and HDQ reduced the percentages of infected peritoneal cells and decreased the parasite loads in lungs and livers. Compound B showed a tendency toward lowering parasite loads in brains in a mouse model of toxoplasmic encephalitis.
Apicomplexan parasites are widespread human pathogens causing diseases such as malaria and toxoplasmosis. Development of drug resistance, particularly in Plasmodium, is a major threat for human health and stock breeding, thus motivating the development of novel drug therapies (3, 7). The mitochondrial physiology of apicomplexan parasites differs in several aspects from the physiology of mammalian mitochondria (10, 15). This feature can be exploited for drug therapy, as shown by treatment with the complex III inhibitor atovaquone (1, 2, 12, 16). 1-Hydroxy-2-dodecyl-4(1H)quinolone (HDQ) was recently shown to effectively inhibit parasite replication of Toxoplasma gondii and Plasmodium falciparum in tissue culture (13). The structural similarity of HDQ and coenzyme Q (6) suggests an inhibition of ubiquinol binding enzymes as a mode of action. Using inhibition kinetics on heterologously expressed enzymes, we recently demonstrated that HDQ is a high-affinity inhibitor of the T. gondii alternative NADH dehydrogenase (TgNDH2-I) (9). HDQ treatment in T. gondii results in a rapid loss of the mitochondrial membrane potential and a subsequent reduction of the cellular ATP concentration (8). HDQ was reported to inhibit the parasitic dihydroorotate dehydrogenase (DHODH) in P. falciparum (4), thus inhibiting the fourth step of the essential de novo pyrimidine synthesis, which uses ubiquinol reduction as an electron sink for dihydroorotate oxidation.
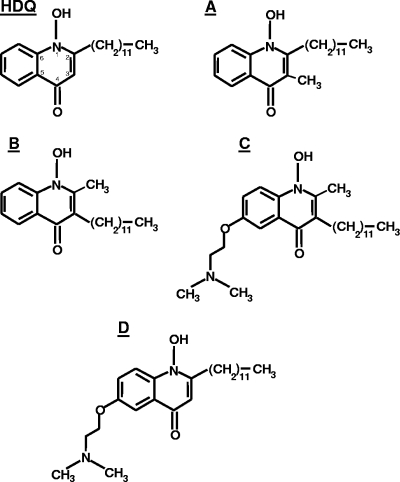
Based on the structure of HDQ, we here describe four novel 1-hydroxyquinolones (compounds A to D) and their anti-Toxoplasma activities and provide the first data on the in vivo efficacy of HDQ, as well as those of compounds A and B, in a mouse model of acute toxoplasmosis. We had previously shown that a long alkyl side chain at C-2 in HDQ of more than five carbons is critical for the anti-Toxoplasma activities of 1-hydroxy-2-alkyl-4(1H)quinolones (13), and it was thus of interest to investigate whether its location at C-2 is strictly required for the antiparasitic effect. In addition, from previous investigations, it was not clear whether the hydrogen at C-3 in HDQ is needed. We therefore prepared the two new 1-hydroxyquinolones, compounds A and B (L. F. Tietze et al., unpublished data). In compound A, the hydrogen at C-3 in HDQ is replaced by a methyl group, and in compound B, the dodecyl and the methyl moiety were swapped (Fig. 1). Furthermore, we synthesized two HDQ derivatives, compounds C and D, containing a N,N-dimethylaminoethoxy group at C-6, in order to improve the water solubility of the otherwise highly hydrophobic substances (Fig. 1).
FIG. 1.
Structures of HDQ and compounds A to D.
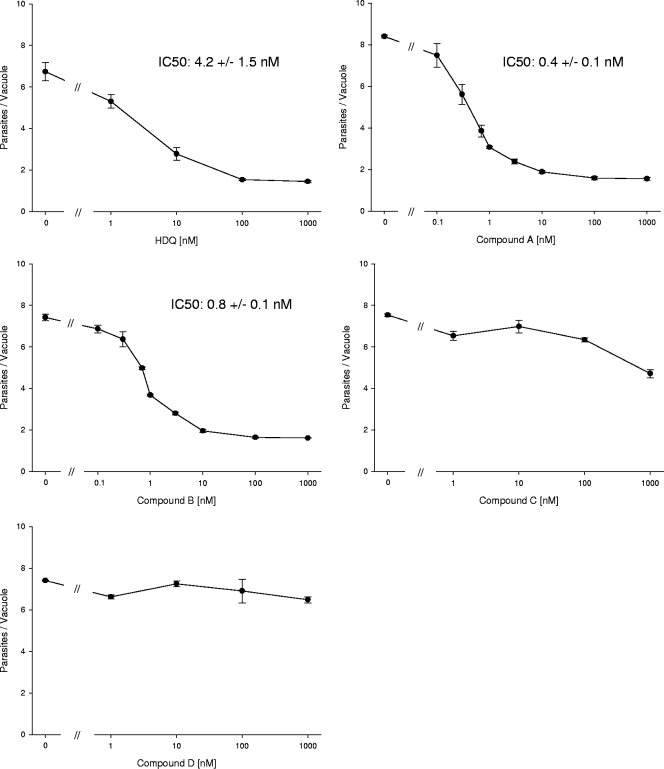
The new compounds A to D were tested for their inhibitory potential against T. gondii replication (type I RH strain) in a growth assay at 24 h postinfection. HDQ was included in parallel assays and yielded a 50% inhibitory concentration (IC50) of 4.2 nM, similar to the value from our previous report (13). Compound A showed a 10-fold-higher bioactivity than HDQ, with an IC50 of 0.4 nM, and compound B was also more active than HDQ, with an IC50 of 0.8 nM. This indicates that the dodecyl side chain can be localized at either C-2 or C-3 of the 1-hydroxyquinolone moiety without losing the antiparasitic activity. It furthermore reveals that a methyl moiety at C-3 further improves the anti-Toxoplasma activity of HDQ. In contrast, compound C displayed a much weaker inhibitory effect, 38% inhibition, at the highest concentrations tested (1 μM), and compound D failed to inhibit parasite replication at this concentration (Fig. 2). A possible explanation for this loss of activity is that the hydrophilic N,N-dimethylaminoethoxy group has some negative effects on the membrane solubility, thereby lowering the effective concentrations of these compounds at the inner mitochondrial membrane where the putative target enzymes are located.
FIG. 2.
IC50 determination. Confluent human foreskin fibroblasts monolayers were infected with RH strain tachyzoites and treated with either the indicated drug concentrations or a DMSO control. After 24 h of drug treatment, the average number of parasites per vacuole was determined by phase-contrast microscopy from at least two independent experiments and given as mean ± standard deviation; IC50s were calculated by using the SigmaPlot software.
To investigate the in vivo efficacies of HDQ and compounds A and B in an acute model of T. gondii infection, female NMRI mice were infected intraperitoneally (i.p.) with 105 green fluorescent protein (GFP)-expressing RH tachyzoites (kindly provided by Dominique Soldati, University of Geneva). Starting at 3 days postinfection, mice were treated for 5 days with 32 mg/kg body weight/day of drug in a volume of 0.2 ml (i.p.). Drugs were dissolved in an ethanol and dimethyl sulfoxide (DMSO) solution (1:1) by using an ultrasonic water bath at 70°C; thereafter, the same volume of phosphate-buffered saline (PBS) was added. PBS-dissolved atovaquone was administered at 100 mg/kg body weight and used as a positive control. Non-drug-treated T. gondii-infected mice received ethanol-DMSO (1:1) or PBS in the same volume and served as negative controls.
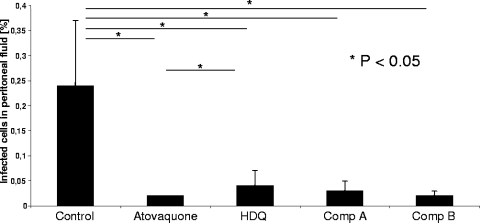
On day 8 postinfection, organs were collected, and peritoneal cells were obtained by peritoneal cavity lavage with 6 ml PBS. Samples were centrifuged at 4°C and 400 × g for 10 min, and the pellet was suspended in 2 ml PBS-5% fetal bovine serum. The cells were fixed in a solution of 3.7% formaldehyde in PBS. Flow cytometry revealed that the percentages of infected peritoneal cells in mice treated with HDQ, compound A, and compound B were significantly lower than the percentage in control-treated mice (Fig. 3); percentages of infected peritoneal cells did not differ significantly between the three drug groups. Atovaquone administered as a positive control also resulted in significantly decreased percentages of infected cells in the peritoneal cavity; percentages were significantly lower than those observed in HDQ-treated mice but similar to those from mice treated with compounds A and B. The parasite loads in lungs and livers were determined by quantitative PCR (qPCR) targeting the T. gondii cryptic gene (11) in 10-μl reaction volumes containing 250 ng of total DNA, 2 μl enzyme mix, 2 mM MgCl2, 1 μM of each oligonucleotide primer (TOX-9, 5′-AggAgAgATATCAggACTgTAg-3′; TOX-10as, 5′-gCgTCgTCTCgTCTAgATCg-3′) (TIB Molbiol, Berlin, Germany), and 0.2 μM of each fluorescent hybridization probe (TOX-HP-1, 5′-GAGTCGGAGAGGGAGAAGATGTT-6-carboxyfluorescein-3′; TOX-HP-2, 5′-CCGGCTTGGCTGCTTTTCCTG-3′) (TIB Molbiol, Berlin, Germany). PCR was performed as follows: 95°C for 10 min, followed by 50 cycles at 95°C for 10 s, 52°C for 20 s, and 72°C for 30 s. After a final extension step at 40°C for 30 s, the samples were cooled and stored at 4°C. With each run, a standard curve was performed using 250 pg to 2.5 pg T. gondii DNA of GFP-expressing tachyzoites; distilled water served as the negative control. Fluorescence was analyzed by LightCycler data analysis software 3.5 (Roche). Crossing points (Cp) were established using the second derivative method (Roche).
FIG. 3.
Flow cytometry analyses. Percentages of infected cells in peritoneal fluid 8 days after infection of NMRI mice i.p. with 105 GFP-expressing RH tachyzoites and treatment. Atovaquone was used as a positive control. The results shown are pooled from three independent experiments; there were at least four mice in each group.
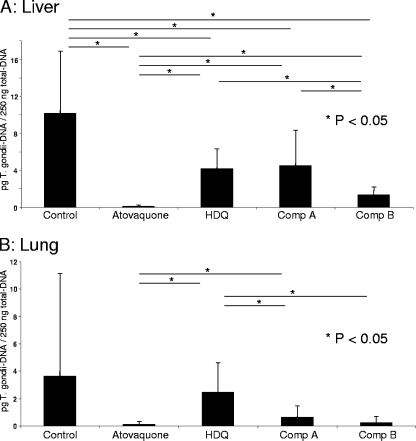
The concentrations of T. gondii DNA in livers of mice treated with HDQ, compound A, and compound B were significantly lower than those in livers of control mice (Fig. 4A). Atovaquone showed an even more pronounced antiparasitic activity. The parasite loads in livers of mice treated with compound B were significantly lower than those in livers of mice treated with compound A or HDQ. In lungs, atovaquone and compound B showed the most pronounced anti-T. gondii effects (Fig. 4B); this difference failed to reach statistical significance due to high standard deviations, but mice treated with atovaquone, compound A, and compound B all had significantly lower parasite concentrations than mice treated with HDQ.
FIG. 4.
Quantitative PCR analysis of liver and lung samples. Four NMRI mice per group were infected i.p. with 105 GFP-expressing RH tachyzoites and treated 3 days after infection daily for 5 days. On day 8 postinfection, organs were collected. The results are given as means ± standard deviations from three independent experiments. P values were determined by an unpaired Student t test.
Finally, to investigate the therapeutic efficacy of these drugs against toxoplasmic encephalitis, IRF-8−/− mice were orally infected with 10 cysts of the type II T. gondii ME49 strain. Two days after infection, mice were treated with sulfadiazine (400 mg/dl) for 28 days to allow cyst development (5, 14). Three days after withdrawal of sulfadiazine, groups of four mice (repeated three times) were treated daily with the respective drug for 5 days. PCR revealed that atovaquone resulted in a significant reduction of the parasite load compared to the case for untreated mice at 39 days after infection (134.0 ± 226.1 [control] versus 0.8 ± 1.3 [atovaquone] pg toxoplasma DNA/250 ng total DNA; P ≤ 0.05); mice treated with compound B showed a tendency toward lower parasite loads in brains (134.0 ± 226.1 [control] versus 6.7 ± 10.8 [compound B] pg toxoplasma DNA/250 ng total DNA; P = 0.067). In addition, numbers of inflammatory foci in brains of mice treated with atovaquone, compounds A, or compound B were significantly reduced compared to those in brains of control mice. Mean inflammatory scores determined as previously described (5) were 1.78, 1.15, 1.31, and 1.25 for control mice and mice treated with atovaquone, compound A, and compound B, respectively (P ≤ 0.05).
Results of the present study reveal for the first time that 1-hydroxy-dodecyl-4(1H)quinolone derivatives possess promising in vivo antitoxoplasmic activity. Treatment with these compounds reduces the parasite load during acute toxoplasmosis, and the severity of toxoplasmic encephalitis was reduced in mice treated with compounds A and B. In vitro, the quinolone derivatives exhibit lower IC50s than atovaquone (1, 12, 13), while in vivo, atovaquone appears to be more efficient under the applied conditions. However, pharmacological characteristics, including serum levels, tissue permeability, and half-life of these compounds, have not been optimized for in vivo use. Therefore, further studies will focus on these pharmacological characteristics of compounds A and B and their impact on efficacy in vivo.
Footnotes
Published ahead of print on 2 November 2009.
REFERENCES
- 1.Araujo, F. G., J. Huskinson, and J. S. Remington. 1991. Remarkable in vitro and in vivo activities of the hydroxynaphthoquinone 566C80 against tachyzoites and tissue cysts of Toxoplasma gondii. Antimicrob. Agents Chemother. 35:293-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baggish, A. L., and D. R. Hill. 2002. Antiparasitic agent atovaquone. Antimicrob. Agents Chemother. 46:1163-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bustamante, C., C. N. Batista, and M. Zalis. 2009. Molecular and biological aspects of antimalarial resistance in Plasmodium falciparum and Plasmodium vivax. Curr. Drug Targets 10:279-290. [DOI] [PubMed] [Google Scholar]
- 4.Dong, C. K., V. Patel, J. C. Yang, J. D. Dvorin, M. T. Duraisingh, J. Clardy, and D. F. Wirth. 2009. Type II NADH dehydrogenase of the respiratory chain of Plasmodium falciparum and its inhibitors. Bioorg. Med. Chem. Lett. 19:972-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunay, I. R., M. M. Heimesaat, F. N. Bushrab, R. H. Müller, H. Stocker, K. Arasteh, M. Kurowski, R. Fitzner, K. Borner, and O. Liesenfeld. 2004. Atovaquone maintenance therapy prevents reactivation of toxoplasmic encephalitis in a murine model of reactivated toxoplasmosis. Antimicrob. Agents Chemother. 48:4848-4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eschemann, A., A. Galkin, W. Oettmeier, U. Brandt, and S. Kerscher. 2005. HDQ (1-hydroxy-2-dodecyl-4(1H)quinolone), a high affinity inhibitor for mitochondrial alternative NADH dehydrogenase. J. Biol. Chem. 280:3138-3142. [DOI] [PubMed] [Google Scholar]
- 7.Fidock, D. A., R. T. Eastman, S. A. Ward, and S. R. Meshnick. 2008. Recent highlights in antimalarial drug resistance and chemotherapy research. Trends Parasitol. 24:537-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin, S. S., U. Groß, and W. Bohne. 2009. Type II NADH dehydrogenase inhibitor 1-hydroxy-2-dodecyl-4(1H)quinolone leads to collapse of mitochondrial inner-membrane potential and ATP depletion in Toxoplasma gondii. Eukaryot. Cell 8:877-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin, S. S., S. Kerscher, A. Saleh, U. Brandt, U. Groß, and W. Bohne. 2008. The Toxoplasma gondii type-II NADH dehydrogenase TgNDH2-I is inhibited by 1-hydroxy-2-alkyl-4(1H)quinolones. Biochim. Biophys. Acta 1777:1455-1462. [DOI] [PubMed] [Google Scholar]
- 10.Mather, M. W., and A. B. Vaidya. 2008. Mitochondria in malaria and related parasites: ancient, diverse and streamlined. J. Bioenerg. Biomembr. 40:425-433. [DOI] [PubMed] [Google Scholar]
- 11.Reischl, U., S. Bretagne, D. Krüger, P. Ernault, and J. M. Costa. 2003. Comparison of two DNA targets for the diagnosis of toxoplasmosis by real-time PCR using fluorescence resonance energy transfer hybridization probes. BMC Infect. Dis. 3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romand, S., M. Pudney, and F. Derouin. 1993. In vitro and in vivo activities of the hydroxynaphthoquinone atovaquone alone or combined with pyrimethamine, sulfadiazine, clarithromycin, or minocycline against Toxoplasma gondii. Antimicrob. Agents Chemother. 37:2371-2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saleh, A., J. Friesen, S. Baumeister, U. Groß, and W. Bohne. 2007. Growth inhibition of Toxoplasma gondii and Plasmodium falciparum by nanomolar concentrations of 1-hydroxy-2-dodecyl-4(1H)quinolone, a high-affinity inhibitor of alternative (type II) NADH dehydrogenases. Antimicrob. Agents Chemother. 51:1217-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schöler, N., K. Krause, O. Kayser, R. H. Müller, K. Borner, H. Hahn, and O. Liesenfeld. 2001. Atovaquone nanosuspensions show excellent therapeutic effect in a new murine model of reactivated toxoplasmosis. Antimicrob. Agents Chemother. 45:1771-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seeber, F., J. Limenitakis, and D. Soldati-Favre. 2008. Apicomplexan mitochondrial metabolism: a story of gains, losses and retentions. Trends Parasitol. 24:468-478. [DOI] [PubMed] [Google Scholar]
- 16.Srivastava, I. K., J. M. Morrisey, E. Darrouzet, F. Daldal, and A. B. Vaidya. 1999. Resistance mutations reveal the atovaquone-binding domain of cytochrome b in malaria parasites. Mol. Microbiol. 33:704-711. [DOI] [PubMed] [Google Scholar]