Abstract
Azithromycin (AZI) is an azalide antibiotic with antimalarial activity that is considered safe in pregnancy. To assess its pharmacokinetic properties when administered as intermittent preventive treatment in pregnancy (IPTp), two 2-g doses were given 24 h apart to 31 pregnant and 29 age-matched nonpregnant Papua New Guinean women. All subjects also received single-dose sulfadoxine-pyrimethamine (SP) (1,500 mg or 75 mg) or chloroquine (450-mg base daily for 3 days). Blood samples were taken at 0, 1, 2, 3, 6, 12, 24, 32, 40, 48, and 72 h and on days 4, 5, 7, 10, and 14 for AZI assay by ultra-high-performance liquid chromatography-tandem mass spectrometry. The treatments were well tolerated. Using population pharmacokinetic modeling, a three-compartment model with zero-order followed by first-order absorption and no lag time provided the best fit. The areas under the plasma concentration-time curve (AUC0-∞) (28.7 and 31.8 mg·h liter−1 for pregnant and nonpregnant subjects, respectively) were consistent with the results of previous studies, but the estimated terminal elimination half-lives (78 and 77 h, respectively) were generally longer. The only significant relationship for a range of potential covariates, including malarial parasitemia, was with pregnancy, which accounted for an 86% increase in the volume of distribution of the central compartment relative to bioavailability without a significant change in the AUC0-∞. These data suggest that AZI can be combined with compounds with longer half-lives, such as SP, in combination IPTp without the need for dose adjustment.
Azithromycin (AZI) is a semisynthetic azalide antibiotic that is structurally related to erythromycin but has a broader spectrum of antibacterial activity and a more favorable pharmacokinetic profile (13, 22). It is widely used in the treatment of respiratory and sexually transmitted infections, including those in HIV-infected patients (32, 34). AZI also inhibits protein synthesis in the plasmodial apicoplast (39, 40) and thus has activity against both Plasmodium falciparum and Plasmodium vivax (5, 12, 16, 27-30, 41). It acts mainly against the progeny of parasites that inherit a nonfunctioning apicoplast after exposure, with the result that its antimalarial effect has a slow onset and is relatively weak. Therefore, AZI is best used in combination with other antimalarial compounds as both treatment (20, 27, 29) and chemoprophylaxis (5, 19), with likely additive or synergistic effects (28, 30, 31).
Malaria in pregnancy can result in adverse outcomes for both mother and fetus (14). Intermittent preventive treatment in pregnancy (IPTp) aims to reduce the burden of malaria by administering treatment doses of antimalarial drugs at predetermined intervals as part of routine antenatal care in areas of endemicity (44). Because AZI is considered safe in pregnancy and could have activity against other clinically significant pathogens (8, 38), it has been suggested as a candidate for IPTp. Although the pharmacokinetics of AZI have been investigated (2, 6, 7, 11, 13, 23-26, 35-37, 45), only one study included pregnant women (36), and most focused on its antibacterial properties. In addition, AZI is likely to be partnered with conventional antimalarial drugs if given as IPTp, and there is evidence that such combinations are safe and well tolerated in studies with chloroquine (CQ) in healthy volunteers (11) and with sulfadoxine-pyrimethamine (SP) in pregnant women (20). Although there does not appear to be a clinically significant pharmacokinetic interaction with CQ (11), AZI interactions with other conventional IPTp treatments are unknown. Therefore, we investigated the pharmacokinetic properties of AZI in combination with CQ or SP in pregnant and nonpregnant women from an area of Papua New Guinea (PNG) with intense transmission of both P. falciparum and P. vivax malaria.
MATERIALS AND METHODS
Study site, sample, and approvals.
The present study was conducted at Alexishafen Health Centre, Madang Province, on the north coast of PNG. The pregnant women were recruited at their first antenatal clinic visit, and the age-matched nonpregnant volunteers were from the same communities as the pregnant participants. Women were eligible if (i) they had not taken any of the study drugs in the previous 28 days, (ii) they had no history of significant allergy to any study drug, (iii) there was no significant comorbidity or clinical evidence of severe malaria, and (v) follow-up was possible for the duration of the study. The study was approved by the Medical Research Advisory Committee of PNG and the Human Ethics Research Committee at the University of Western Australia. Written informed consent was obtained from all participants.
Clinical procedures.
A detailed assessment was performed prior to drug administration, including a side effects questionnaire, point-of-care hemoglobin and blood glucose (HemoCue, Angelholm, Sweden), thick and thin blood films, and (for pregnant participants) estimation of gestational age by fundal height. A 3-ml blood sample was taken for subsequent antimalarial drug assay. All women received 2 g AZI (Zithromax; Pfizer, New York, NY) both at enrolment and 24 h later. Subjects were also randomized to receive single-dose SP (1,500 mg or 75 mg; Fansidar; Roche, Basel, Switzerland) at enrolment (AZI-SP arm) or CQ (Chloroquin; Astra, Sydney, Australia) (450 mg base daily for 3 days; AZI-CQ arm) in accordance with regimens recommended for PNG (15). The administration of all doses was directly observed. The dosing schedule for AZI was chosen as the simplest regimen that would be likely to ensure effective drug concentrations during the first 4 days of treatment (40).
Following the first dose of AZI (day 0), additional blood samples were taken at 1, 2, 3, 6, 12, 24, 32, 40, 48, and 72 h and then on days 4, 5, 7, 10, and 14 for drug assay. The exact timing of each blood sample was recorded. All samples were centrifuged promptly, with red cells and separated plasma stored frozen at −80°C. The side effects questionnaire was readministered at 6 h and then at 1, 2, 3, and 7 days. Hemoglobin, erect and supine heart rates and blood pressure, respiratory rate, temperature, and blood slides were taken on days 1, 2, 3, 7, 14, 28, and 42, and blood glucose was measured on days 1, 2, and 3. After the completion of follow-up, pregnant patients were returned to the usual antenatal care.
Laboratory methods.
Giemsa-stained thick blood smears were examined independently by at least two skilled microscopists who were blinded to pregnancy and treatment status. Each microscopist viewed >100 fields at ×1,000 magnification before a slide was considered negative. Any slide discrepant for positivity/negativity or species identification was referred to a third microscopist.
AZI levels were measured using a validated ultrahigh-performance liquid chromatography-tandem mass spectrometry (UPLC-LCMS-MS) method using a deuterated internal standard. The samples were retained for subsequent SP assay. AZI USP was obtained from APAC Pharmaceutical LLC (Ellicott City, MD) and deuterated AZI from Toronto Research Chemicals (North York, Canada). In brief, following the addition of an internal standard, AZI was extracted from 5 μl of plasma by protein precipitation. After centrifugation, supernatant (5 μl) was injected onto a 2795/Quattro Premier XE UPLC-ESI-MS/MS (Waters Corp, MA) using a Waters BEH C18 1.7-μm, 2.1- by 100-mm column. Gradient elution was performed using mobile phases A (45/55 [vol/vol], comprising 1 g/liter ammonium bicarbonate in 50/50 [vol/vol] methanol-water and acetonitrile) and B (50/50 [vol/vol] methanol-acetonitrile) at 0.4 ml/min. Adduct transitions were monitored using positive electrospray ionization with multiple-reaction monitoring for AZI and d3-AZI and were m/z 749.6 to 591.4 and m/z 752.6 to 594.4, respectively. The method was linear to 1,012 ng/ml (r2 > 0.9997) with a limit of quantification of 2.5 μg/liter AZI. All inter- and intraday coefficients of variation were <10%, and the between-subject variability (BSV) was <5% when matrix effects were investigated at three concentrations.
Population pharmacokinetic analysis.
Concentration-time data sets were analyzed by nonlinear mixed-effect modeling using NONMEM (version 6.2.0; Icon Development Solutions, Ellicott City, MD) with an Intel Visual FORTRAN 10.0 compiler. Linear mamillary model subroutines within NONMEM (ADVAN4 and -12 used with TRANS4 in the PREDPP library), first-order conditional estimation (FOCE) with η-ɛ interaction, and the objective function value (OFV) (a NONMEM-calculated global goodness-of-fit indicator equal to −2 log-likelihood value of data) were used to construct and compare plausible models. Unless otherwise specified, a difference in the OFV of ≥6.63 (χ2 distribution with 1 df; P < 0.01) was considered significant. The R-based model-building aid Xpose 6.0 (http://www.r-project.org/) was used for graphic model diagnosis (18). Secondary pharmacokinetic parameters, including the volume of distribution at steady state (VSS = V1 + V2 + … + Vn), area under the curve (AUC0-∞), and elimination half lives (t1/2), for the nonpregnant and pregnant groups were obtained from post hoc Bayesian prediction in NONMEM using the final model parameters. Macro constants for the three-compartment model were calculated from the modeled parameters using previously published equations (42).
All volume and clearance terms were scaled allometrically using [× (body weight/70)1.0] and [× (body weight/70)0.75], respectively (3), and were expressed relative to bioavailability (/F). Two- and three-compartment models were compared, and then zero- and first-order absorption models with and without a lag time were assessed alone and in combination. The BSV was added to parameters for which it could be estimated reasonably from the available data. Both exponential (proportional) and combined (exponential plus additive) error models were tested for residual unexplained variability (RUV). In developing the final models, we investigated the influence of the covariates pregnancy, treatment type, fundal height, gestational age, malaria status, blood glucose, and hemoglobin on model parameters using Xpose and the generalized additive modeling procedure function, as well as inspection of correlation plots. Covariate relationships found in this way were evaluated within the NONMEM model. Inclusion of the covariate required a decrease of ≥3.84 in the OFV (χ2 df = 2; P < 0.05) and a decrease in the BSV. Correlations among BSV terms and weighted-residuals (WRES) plots were used in model evaluation.
A bootstrap procedure using Perl speaks NONMEM (PSN) (http://psn.sourceforge.net) was used to sample individuals from the original data set with replacement and to generate 1,000 new data sets that were subsequently analyzed using NONMEM. The resulting parameters were then summarized as median and 2.5th and 97.5th percentiles (95% empirical confidence interval [CI]) to facilitate validation of the final model parameter estimates. In addition, a stratified visual predictive check (VPC) was also performed using PSN with 1,000 replicate data sets simulated from the original. The resulting 80% prediction intervals (PI) for AZI were plotted with the observed data to assess the predictive performance of the model.
Statistical analysis.
SigmaStat (version 3.10; Systat Software Inc., Chicago, IL) was used for statistical analysis unless otherwise specified. Data are summarized as mean ± standard deviation (SD) or median and interquartile range (IQR) as appropriate. Student's t test or the Mann-Whitney U test was used for two-sample comparisons. Categorical data were compared using either the Pearson chi-square or Fisher's exact test, and multiple means were compared by repeated-measures analysis of variance (ANOVA). A two-tailed level of significance of 0.05 was used. Drug concentrations at each time point after day 2 were compared to the AUC0-∞ using Pearson correlation.
RESULTS
Patient characteristics.
A total of 31 pregnant and 29 nonpregnant women were recruited between October 2007 and March 2008. All subjects took two AZI doses, but two pregnant patients did not receive either CQ or SP. These women were excluded from initial analyses but were included subsequently if there was no effect of CQ or SP on AZI pharmacokinetic properties in the other subjects. Baseline characteristics of the subjects by pregnancy status and treatment allocation are shown in Table 1. The groups were well matched, except that, consistent with normal physiological changes that occur in pregnancy (4, 17), the pregnant subjects were significantly heavier and had lower hemoglobin than the nonpregnant subjects for each treatment group (P < 0.05). Seven of the pregnant patients were parasitemic at baseline compared with only one of the nonpregnant subjects (P = 0.02).
TABLE 1.
Baseline characteristics of the study participants by pregnancy status and treatment allocation
| Parameter | Valuea |
|||
|---|---|---|---|---|
| Pregnant |
Nonpregnant |
|||
| AZI-CQ (n = 15) | AZI-SP (n = 14) | AZI-CQ (n = 14) | AZI-SP (n = 15) | |
| Age (yr) | 26.9 ± 4.1 | 23.9 ± 5.1 | 25.7 ± 5.8 | 27 ± 6.5 |
| Wt (kg) | 53.5 ± 7.1b | 56.4 ± 7.9a | 51.4 ± 5.4 | 51.9 ± 4.9 |
| Height (cm) | 154 ± 7.4 | 154 ± 7.3 | 154 ± 6.4 | 154 ± 2.8 |
| Axillary temp (°C) | 36.4 ± 0.7 | 36.5 ± 0.6 | 36.7 ± 0.3 | 36.4 ± 0.3 |
| P. falciparum parasitemia | 3 (20) | 3 (21) | 1 (7) | 0 (0) |
| P. vivax parasitemia | 1 (7) | 0 (0) | 0 (0) | 1 (7) |
| Gestational age (wk) | 24 [22-27] | 21 [19-24] | ||
| Gravidity | 3 [2-5] | 2 [1-4] | 1 [0-3] | 2 [0-3] |
| Parity | 2 [1-4] | 1 [0-2] | 0 [0-3] | 1 [0-3] |
| Respiratory rate (/min) | 20 ± 1 | 22 ± 5 | 20 ± 2 | 20 ± 1 |
| Supine pulse rate (/min) | 91 ± 10 | 89 ± 7 | 82 ± 10 | 88 ± 7 |
| Supine MAP (mm Hg)c | 78 ± 7 | 81 ± 10 | 79 ± 9 | 82 ± 7 |
| Hemoglobin (g/dl) | 8.5 ± 1.6b | 8.2 ± 1.2b | 9.3 ± 1.9 | 10 ± 1.3 |
| Blood glucose (mmol/liter) | 5.9 ± 1.6 | 5.7 ± 0.8 | 6.2 ± 1.1 | 5.6 ± 2.7 |
Data are mean ± SD, median [IQR], or number (%).
P < 0.05 versus nonpregnant subjects.
Mean arterial pressure, calculated by adding 1/3 of the pulse pressure (systolic minus diastolic pressure) to the diastolic pressure.
Efficacy, tolerability, and safety.
Three of the seven P. falciparum and one of the two P. vivax cases at baseline received AZI-SP. There was an uncorrected adequate parasitological and clinical response (APCR) of 100% for both treatments. A further eight cases (five of whom were pregnant) became slide positive for P. falciparum and three (two who were pregnant) for P. vivax late in the 42-day follow-up period. All received recommended antimalarial therapy (15). All cases at baseline and during follow-up were asymptomatic.
Both treatments were well tolerated, and no patient required medical attention because of side effects. Table 2 summarizes self-reported symptoms in the first week of follow-up, >90% of which were mild (not influencing usual daily activity) and short-lived (≤2 days). Six patients reported mild pretreatment symptoms (headache, abdominal pain, pruritus, or dizziness), but these resolved subsequently. Posttreatment pruritus was reported only in the AZI-CQ group (P = 0.052). Although not formally assessed, no significant side effects were volunteered at assessments after day 7. No patient developed hypoglycemia (blood glucose < 2.5 mmol/liter) or severe anemia (hemoglobin < 5.0 g/dl). Although postural hypotension (>20 mm Hg systolic or >10 mm Hg diastolic fall after standing) occurred eight times in seven pregnant (four from the AZI-CQ group) and seven times in five nonpregnant (all five in the AZI-CQ group) patients, the differences between groups were not significant and there were no associated symptoms. After completion of the study, one of the study participants had a stillbirth. A medical review of her case notes by three independent physicians concluded that it was unlikely to be the result of the study medication.
TABLE 2.
Side effects reported during the first week after initiation of treatment
| Parameter | Valuea |
|
|---|---|---|
| AZI-CQ (n = 29) | AZI-SP (n = 29) | |
| Fever | 2 (7) | 1 (3) |
| Chills | 2 (7) | 0 (0) |
| Headache | 6 (21) | 4 (14) |
| Nausea | 4 (14) | 7 (24) |
| Vomiting | 2 (7) | 4 (14) |
| Diarrhea | 2 (7) | 2 (7) |
| Abdominal pain | 4 (14) | 3 (10) |
| Rash | 0 (0) | 0 (0) |
| Pruritus | 5 (17) | 0 (0) |
| Anorexia | 1 (3) | 2 (7) |
| Insomnia | 2 (7) | 0 (0) |
| Dizziness | 3 (10) | 1 (3) |
| Bone or joint pain | 1 (3) | 1 (3) |
| Otherb | 5 (17) | 1 (3) |
Data are numbers (%) of patients.
Cough (2), blocked ear (1), “heavy head” (1), and numbness of calf muscles (1) in the AZI-CQ group and cough (1) in the AZI-SP group.
Pharmacokinetic modeling.
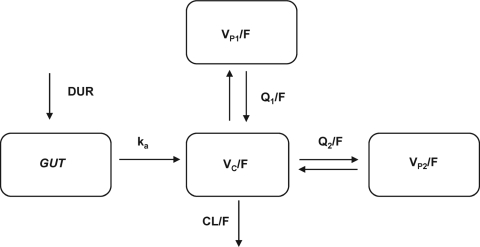
A three-compartment model had a lower OFV than a two-compartment model (8,700.058 versus 8,185.104; P < 0.001 by χ2 test; df = 2) and a more favorable distribution of WRES over time. Zero-order followed by first-order absorption without a lag time provided the lowest OFV and best fit for AZI absorption. The fixed model parameters were DUR (the duration of the zero-order absorption); ka (the first-order absorption rate constant); CL/F (clearance from the central compartment); VC/F, VP1/F, and VP2/F (volumes of distribution of the central, first peripheral, and second peripheral compartments, respectively); and Q1/F and Q2/F (intercompartment clearances for VP1/F and VP2/F, respectively). The model structure is shown in Fig. 1. BSV could be estimated for DUR, CL/F, VC/F, and VP1/F, while a proportional-error model was best for RUV. After testing the various covariates, only pregnancy on VC/F produced a significant decrease in the OFV (χ2 df = 1; P < 0.05) accompanied by a decrease in the BSV of VC/F from 111.0% to 99.6%.
FIG. 1.
Structural model used in the final pharmacokinetic analysis of plasma azithromycin concentrations in the central compartment versus time. GUT, gastrointestinal tract.
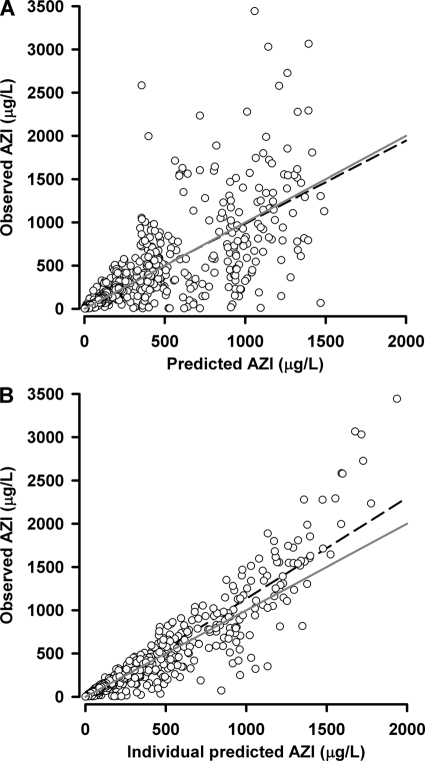
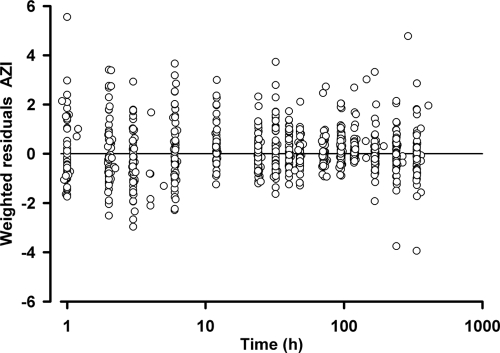
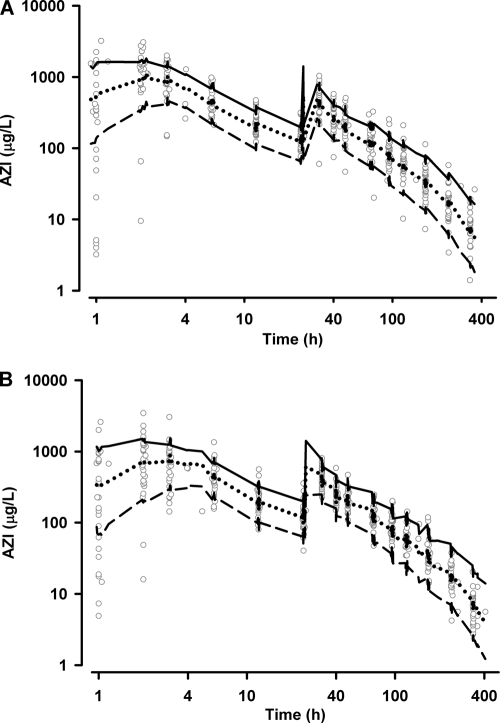
The results of the parameter estimates and their relative standard errors (RSE) are summarized in Table 3 and secondary parameter estimates in Table 4. All drug concentrations after day 2 were strongly correlated with the AUC0-∞ (r > 0.7; P < 0.001), with 96-h levels showing the strongest association (r = 0.78). The bootstrap results (Table 3) demonstrate a robust estimation of both fixed and random parameters with bias < 4% and < 5%, respectively. Goodness-of-fit plots of observed versus population and individual predicted concentrations and WRES versus time are shown in Fig. 2 and 3. The VPC results, stratified for pregnancy status, are presented in Fig. 4 and show reasonable predictive performance of the model while demonstrating some difficulty in capturing postabsorption plasma concentrations peaks.
TABLE 3.
Model building, final parameter estimates, and bootstrap results from the AZI population pharmacokinetic modeling
| Parametera | Value |
||
|---|---|---|---|
| Base model | Final covariate model | Bootstrap (n = 1,000) (median [95% CI]) | |
| OFV | 7,999.870 | 7,993.646 | 7,974.699 [7,756.673-8,201.238] |
| Pharmacokinetic (estimate [% RSE]) | |||
| DUR (h) | 1.66 [10.4] | 1.55 [3.3] | 1.56 [1.21-2.01] |
| ka (h−1) | 0.513 [3.2] | 0.525 [14.8] | 0.524 [0.451-0.623] |
| VC/F (liters) | 504 [13.9] | 384 [17.6] | 371 [235-554] |
| Pregnancy on VC/F (liters) | 330 [69.4] | 318 [48-604] | |
| CL/F (liters h−1) | 158 [3.9] | 158 [6.7] | 158 [145-171] |
| VP1/F (liters) | 4,080 [8.6] | 4,080 [12.5] | 4,045 [3,402-4,870] |
| Q1/F (liters h−1) | 327 [5.7] | 325 [12.7] | 326 [288-368] |
| VP2/F (liters) | 5,070 [5.6] | 5,040 [7.3] | 5,070 [4,262-5,730] |
| Q2/F (liters h−1) | 67.2 [11.5] | 66.4 [12.4] | 67.5 [48.5-84.0] |
| Random (CV % [% RSE]) | |||
| BSV Vc/F | 111.4 [20.9] | 99.6 [35.5] | 99.0 [72.6-127.6] |
| BSV CL/F | 28.3 [24.1] | 28.3 [33.1] | 27.9 [21.6-34.5] |
| BSV VP1/F | 35.8 [27.0] | 35.6 [27.2] | 34.8 [25.7-45.2] |
| BSV DUR | 73.0 [21.4] | 76.9 [22] | 75.5 [55.4-95.6] |
| RUV | |||
| Proportional error (CV % [% RSE]) | 31.3 [9.5] | 31.2 [15.1] | 30.9 [28.1-33.8] |
CV, coefficient of variation.
TABLE 4.
Secondary pharmacokinetic parameters derived from post hoc Bayesian estimates for pregnant and nonpregnant study participants (median [IQR])
| Parameter | Value |
||
|---|---|---|---|
| Pregnant (n = 31) | Nonpregnant (n = 29) | P value | |
| DUR (h) | 1.65 [0.94-2.34] | 1.75 [1.02-2.38] | NSa |
| ka (h−1) | 0.525 [0.525-0.525] | 0.525 [0.525-0.525] | NS |
| VC/F (liters) | 647 [422-995] | 249 [157-363] | <0.001 |
| VP1/F (liters) | 3,620 [2,747-3,951] | 2,909 [2,296-3,586] | NS |
| VP2/F (liters) | 3,888 [3,708-4,104] | 3,672 [3,456-3,888] | 0.034 |
| VSS/F (liters) | 8,355 [7,460-8,973] | 6,875 [6,115-7,526] | 0.002 |
| it1/2αb (h) | 0.88 [0.57-1.36] | 0.39 [0.24-0.56] | <0.001 |
| t1/2βb (h) | 20.7 [18.3-22.8] | 18.8 [15.3-21] | NS |
| t1/2γb (h) | 78.2 [74-82.5] | 77.1 [71.5-84.5] | NS |
| AUC0-∞ (μg h liter−1) | 28,713 [25,913-32,942] | 31,781 [28,736-38,012] | NS |
NS, not significant.
t1/2α, t1/2β, and t1/2γ are the first-distribution, second-distribution, and terminal elimination half-lives respectively.
FIG. 2.
Observed versus model predicted concentrations (A) and individual predicted concentrations (B) for AZI. The solid gray lines are the lines of identity, while the dashed black lines are the linear regression lines of best fit.
FIG. 3.
Weighted residuals versus time after dose (log scale) plot for AZI.
FIG. 4.
Visual predicted check plots showing simulated 10th (short dashed lines), 50th (dotted lines), and 90th (solid lines) percentile concentrations and observed concentration (log scale) data (open circles) versus time (log scale) for nonpregnant (A) and pregnant (B) participants.
DISCUSSION
The present study is the first pharmacokinetic evaluation of AZI in pregnant and nonpregnant women living in a malaria-endemic area. We found that a three-compartment model with a combined absorption process best described the disposition of AZI in our subjects. Both two-compartment (23, 26, 37) and three-compartment (6, 35) models have been found to best describe AZI plasma concentration-time profiles in other contexts. Our ability to differentiate the triexponential elimination of AZI may have been facilitated by the relatively long sampling duration. This may also explain why our estimated terminal elimination half-lives (78 and 77 h for pregnant and nonpregnant participants, respectively) were longer than those in most previous studies (range, 27 to 79 h) (6, 7, 13, 23, 35, 37). The overall drug exposure (AUC0-∞, 28.7 and 31.8 mg·h liter−1 for pregnant and nonpregnant subjects, respectively) was within the range expected from dose-scaled results from previous studies in other contexts (26.5 to 46.4 mg·h liter−1) (2, 7, 11, 13, 23, 24, 26, 35, 37), suggesting that the bioavailability of AZI is not dose dependent.
Both zero-order (23, 37) and first-order (6, 26) absorption have been reported previously for AZI, but neither was appropriate for our data. A combined absorption process in which the drug enters the absorption compartment in a zero-order manner and then is absorbed according to first-order kinetics provided the best model in the present study. This is analogous to the twin processes of (i) gastric emptying of the drug into the small intestine (the zero-order process) and (ii) absorption in the small intestine proportional to the amount present (the first-order process). Despite this more complex model, AZI absorption was still not well characterized in our final model. This has been reported previously (37) but is unlikely to be significant in the treatment of uncomplicated malaria, where exposure of the parasite to therapeutic drug concentrations over several life cycles is more important than that immediately after drug administration.
Plasma AZI concentrations appeared to differ between pregnant and nonpregnant women only in the first 48 h after the first dose. This was confirmed by the population pharmacokinetic modeling, in which pregnancy, the only significant covariate relationship, accounted for an 86% increase in VC/F. Despite significant differences in the secondary parameters VC/F, VP2/F, VSS/F, and t1/2α (first-distribution half-life) between pregnant and nonpregnant subjects, no difference was seen in t1/2γ (terminal elimination half-life) or AUC0-∞. This suggests that the drug elimination and overall exposures were similar in the two groups. A much shorter AZI half-life (12 h) than in the present study was reported previously in pregnant women (36), but the study employed a shorter sampling duration (168 versus 336 h) and included pregnant women at or near term, and the analysis was constrained by relatively sparse sampling.
Because of the need for AZI to be combined with other therapies (12, 41), we included conventional antimalarial drugs currently recommended as part of IPTp in PNG and other countries (10, 20). There were no significant differences in the disposition of AZI between the AZI-CQ and AZI-SP groups, consistent with a study of the interaction of CQ and AZI in healthy volunteers (11). We conclude that AZI dose modification is unnecessary in these combinations. In addition, the lack of an effect of malaria status as a covariate on AZI disposition suggests that, unlike drugs such as quinine (21), the dose may not have to be adjusted when parasitemia is present.
The most common side effects of AZI, especially with higher doses, are nausea and vomiting. These symptoms are thought to be related to the effect of AZI on the motilin receptor in the upper gastrointestinal tract (33). However, with the exception of pruritus, which tended to be associated with AZI-CQ therapy, consistent with known CQ effects (1), there were no differences in the incidences of side effects between the two treatment groups, and most reported adverse effects were mild. The AZI dose regimen in both combination therapy groups in the present study (2.0 g daily for 2 days) was associated with a side effect profile similar to that reported previously after a single 2.0-g dose (35). Use of the sustained-release formulation of AZI should reduce side effects, including nausea and vomiting (9). However, this formulation has a bioavailability of 82.8% relative to conventional AZI, suggesting that a higher dose will be required to achieve the same drug exposure. As well as increasing the cost of AZI treatment, this could mean that side effects are more frequent with higher-dose sustained-release AZI administration.
Although the present study had limited subject numbers, it is encouraging that both regimens achieved a 100% uncorrected APCR. The plasma concentrations of AZI required to achieve cure are unknown, as no efficacy trials have included these data. However, the high correlation between 96-h drug levels and AUC0-∞ in our patients suggests that a day 4 plasma concentration could be an appropriate surrogate for overall AZI exposure in efficacy trials in which serial blood sampling is problematic. It is interesting that prolongation of the in vitro exposure of P. falciparum to 96 h results in substantially increased potency, suggesting that either AZI renders second-generation parasites unable to establish a parasitophorous vacuole upon host cell invasion or the effect on apicoplast protein synthesis inhibits successful development of the progeny of drug-treated parasites (40).
Given the need for relatively prolonged parasite exposure to therapeutic plasma concentrations, it is unlikely that the benefit of “front loading” of AZI used in treating bacterial infections (23, 24) will be relevant in malaria. However, experience with AZI as an antimalarial agent is growing. A Cochrane review of its efficacy is currently under way (43), and promising results are being seen when it is used with SP in IPTp, such as might be given at least twice during pregnancy (20). The present study provides a pharmacokinetic foundation for the further investigation of AZI as an antimalarial agent in pregnancy, particularly in combination IPTp. Further data from the present study should also determine whether AZI influences the disposition of CQ and SP. Although there was a significant increase in AZI VC/F in pregnant women, there was no significant change in the AUC0-∞, and it is therefore likely that no dose adjustments will be required for pregnant women when AZI is given in combination with CQ or SP.
Acknowledgments
We are most grateful to Sr. Valsi Kurian and the staff of Alexishafen Health Centre for their kind cooperation during the study. We also thank Christine Kalopo and Bernard (Ben) Maamu for clinical and/or logistic assistance.
The study was funded by the National Health and Medical Research Council (NHMRC) of Australia (grant 458555) and was supported and endorsed by the MiP consortium, which is funded through a grant from the Bill and Melinda Gates Foundation to the Liverpool School of Tropical Medicine. T.M.E.D. is supported by an NHMRC Practitioner Fellowship.
Footnotes
Published ahead of print on 26 October 2009.
REFERENCES
- 1.Adebayo, R. A., G. G. Sofowora, O. Onayemi, S. J. Udoh, and A. A. Ajayi. 1997. Chloroquine-induced pruritus in malaria fever: contribution of malaria parasitaemia and the effects of prednisolone, niacin, and their combination, compared with antihistamine. Br. J. Clin. Pharmacol. 44:157-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amsden, G. W., and C. L. Gray. 2001. Serum and WBC pharmacokinetics of 1500 mg of azithromycin when given either as a single dose or over a 3 day period in healthy volunteers. J. Antimicrob. Chemother. 47:61-66. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, B. J., and N. H. Holford. 2008. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu. Rev. Pharmacol. Toxicol. 48:303-332. [DOI] [PubMed] [Google Scholar]
- 4.Anderson, G. D. 2005. Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin. Pharmacokinet. 44:989-1008. [DOI] [PubMed] [Google Scholar]
- 5.Anderson, S. L., A. J. Oloo, D. M. Gordon, O. B. Ragama, G. M. Aleman, J. D. German, D. B. Tang, M. W. Dunne, and G. D. Shanks. 1998. Successful double-blinded, randomized, placebo-controlled field trial of azithromycin and doxycycline as prophylaxis for malaria in Western Kenya. Clin. Infect. Dis. 26:146-150. [DOI] [PubMed] [Google Scholar]
- 6.Ballow, C. H., G. W. Amsden, V. S. Highet, and A. Forrest. 1998. Pharmacokinetics of oral azithromycin in serum, urine, polymorphonuclear leucocytes and inflammatory vs non-inflammatory skin blisters in healthy volunteers. Clin. Drug Investig. 15:159-167. [DOI] [PubMed] [Google Scholar]
- 7.Boonleang, J., K. Panrat, C. Tantana, S. Krittathanmakul, and W. Jintapakorn. 2007. Bioavailability and pharmacokinetic comparison between generic and branded azithromycin capsule: a randomized, double-blind, 2-way crossover in healthy male Thai volunteers. Clin. Ther. 29:703-710. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. 2006. Sexually transmitted diseases treatment guidelines, 2006: diseases characterized by urethritis and cervicitis. MMWR Morb. Mortal. Wkly. Rep. 55:35-49. [Google Scholar]
- 9.Chandra, R., P. Liu, J. D. Breen, J. Fisher, C. Xie, R. LaBadie, R. J. Benner, L. J. Benincosa, and A. Sharma. 2007. Clinical pharmacokinetics and gastrointestinal tolerability of a novel extended-release microsphere formulation of azithromycin. Clin. Pharmacokinet. 46:247-259. [DOI] [PubMed] [Google Scholar]
- 10.Chico, R. M., R. Pittrof, B. Greenwood, and D. Chandramohan. 2008. Azithromycin-chloroquine and the intermittent preventive treatment of malaria in pregnancy. Malar. J. 7:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cook, J. A., E. J. Randinitis, C. R. Bramson, and D. L. Wesche. 2006. Lack of a pharmacokinetic interaction between azithromycin and chloroquine. Am. J. Trop. Med. Hyg. 74:407-412. [PubMed] [Google Scholar]
- 12.Dunne, M. W., N. Singh, M. Shukla, N. Valecha, P. C. Bhattacharyya, V. Dev, K. Patel, M. K. Mohapatra, J. Lakhani, R. Benner, C. Lele, and K. Patki. 2005. A multicenter study of azithromycin, alone and in combination with chloroquine, for the treatment of acute uncomplicated Plasmodium falciparum malaria in India. J. Infect. Dis. 191:1582-1588. [DOI] [PubMed] [Google Scholar]
- 13.Foulds, G., R. M. Shepard, and R. B. Johnson. 1990. The pharmacokinetics of azithromycin in human serum and tissues. J. Antimicrob. Chemother. 25(Suppl. A):73-82. [DOI] [PubMed] [Google Scholar]
- 14.Garner, P., and A. M. Gulmezoglu. 2003. Drugs for preventing malaria-related illness in pregnant women and death in the newborn. Cochrane Database Syst. Rev. CD000169. Accessed October 2009. [DOI] [PubMed]
- 15.Gerhardy, C. L., and M. Garrett. 2002. Obstetrics and gynaecology for nurses and midwives, 5th ed. Lutheran School of Nursing, Madang, Papua New Guinea.
- 16.Heppner, D. G., Jr., D. S. Walsh, N. Uthaimongkol, D. B. Tang, S. Tulyayon, B. Permpanich, T. Wimonwattrawatee, N. Chuanak, A. Laoboonchai, P. Sookto, T. G. Brewer, P. McDaniel, C. Eamsila, K. Yongvanitchit, K. Uhl, D. E. Kyle, L. W. Keep, R. E. Miller, and C. Wongsrichanalai. 2005. Randomized, controlled, double-blind trial of daily oral azithromycin in adults for the prophylaxis of Plasmodium vivax malaria in Western Thailand. Am. J. Trop. Med. Hyg. 73:842-849. [PubMed] [Google Scholar]
- 17.Hodge, L. S., and T. S. Tracy. 2007. Alterations in drug disposition during pregnancy: implications for drug therapy. Exp. Opin. Drug Metab. Toxicol. 3:557-571. [DOI] [PubMed] [Google Scholar]
- 18.Jonsson, E. N., and M. O. Karlsson. 1999. Xpose—an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput. Methods Programs Biomed. 58:51-64. [DOI] [PubMed] [Google Scholar]
- 19.Kain, K. C., G. D. Shanks, and J. S. Keystone. 2001. Malaria chemoprophylaxis in the age of drug resistance. I. Currently recommended drug regimens. Clin. Infect. Dis. 33:226-234. [DOI] [PubMed] [Google Scholar]
- 20.Kalilani, L., I. Mofolo, M. Chaponda, S. J. Rogerson, A. P. Alker, J. J. Kwiek, and S. R. Meshnick. 2007. A randomized controlled pilot trial of azithromycin or artesunate added to sulfadoxine-pyrimethamine as treatment for malaria in pregnant women. PLoS One 2:e1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krishna, S., and N. J. White. 1996. Pharmacokinetics of quinine, chloroquine and amodiaquine. Clinical implications. Clin. Pharmacokinet. 30:263-299. [DOI] [PubMed] [Google Scholar]
- 22.Lalak, N. J., and D. L. Morris. 1993. Azithromycin clinical pharmacokinetics. Clin. Pharmacokinet. 25:370-374. [DOI] [PubMed] [Google Scholar]
- 23.Liu, P., H. Allaudeen, R. Chandra, K. Phillips, A. Jungnik, J. D. Breen, and A. Sharma. 2007. Comparative pharmacokinetics of azithromycin in serum and white blood cells of healthy subjects receiving a single-dose extended-release regimen versus a 3-day immediate-release regimen. Antimicrob. Agents Chemother. 51:103-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lucchi, M., B. Damle, A. Fang, P. J. de Caprariis, A. Mussi, S. P. Sanchez, G. Pasqualetti, and M. Del Tacca. 2008. Pharmacokinetics of azithromycin in serum, bronchial washings, alveolar macrophages and lung tissue following a single oral dose of extended or immediate release formulations of azithromycin. J. Antimicrob. Chemother. 61:884-891. [DOI] [PubMed] [Google Scholar]
- 25.Luke, D. R., and G. Foulds. 1997. Disposition of oral azithromycin in humans. Clin. Pharmacol. Ther. 61:641-648. [DOI] [PubMed] [Google Scholar]
- 26.Mazzei, T., C. Surrenti, A. Novelli, A. Crispo, S. Fallani, V. Carla, E. Surrenti, and P. Periti. 1993. Pharmacokinetics of azithromycin in patients with impaired hepatic function. J. Antimicrob. Chemother. 31(Suppl. E):57-63. [DOI] [PubMed] [Google Scholar]
- 27.Miller, R. S., C. Wongsrichanalai, N. Buathong, P. McDaniel, D. S. Walsh, C. Knirsch, and C. Ohrt. 2006. Effective treatment of uncomplicated Plasmodium falciparum malaria with azithromycin-quinine combinations: a randomized, dose-ranging study. Am. J. Trop. Med. Hyg. 74:401-406. [PubMed] [Google Scholar]
- 28.Nakornchai, S., and P. Konthiang. 2006. Activity of azithromycin or erythromycin in combination with antimalarial drugs against multidrug-resistant Plasmodium falciparum in vitro. Acta Trop. 100:185-191. [DOI] [PubMed] [Google Scholar]
- 29.Noedl, H., S. Krudsood, K. Chalermratana, U. Silachamroon, W. Leowattana, N. Tangpukdee, S. Looareesuwan, R. S. Miller, M. Fukuda, K. Jongsakul, S. Sriwichai, J. Rowan, H. Bhattacharyya, C. Ohrt, and C. Knirsch. 2006. Azithromycin combination therapy with artesunate or quinine for the treatment of uncomplicated Plasmodium falciparum malaria in adults: a randomized, phase 2 clinical trial in Thailand. Clin. Infect. Dis. 43:1264-1271. [DOI] [PubMed] [Google Scholar]
- 30.Noedl, H., S. Krudsood, W. Leowattana, N. Tangpukdee, W. Thanachartwet, S. Looareesuwan, R. S. Miller, M. Fukuda, K. Jongsakul, K. Yingyuen, S. Sriwichai, C. Ohrt, and C. Knirsch. 2007. In vitro antimalarial activity of azithromycin, artesunate, and quinine in combination and correlation with clinical outcome. Antimicrob. Agents Chemother. 51:651-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohrt, C., G. D. Willingmyre, P. Lee, C. Knirsch, and W. Milhous. 2002. Assessment of azithromycin in combination with other antimalarial drugs against Plasmodium falciparum in vitro. Antimicrob. Agents Chemother. 46:2518-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oldfield, E. C., III, W. J. Fessel, M. W. Dunne, G. Dickinson, M. R. Wallace, W. Byrne, R. Chung, K. F. Wagner, S. F. Paparello, D. B. Craig, G. Melcher, M. Zajdowicz, R. F. Williams, J. W. Kelly, M. Zelasky, L. B. Heifets, and J. D. Berman. 1998. Once weekly azithromycin therapy for prevention of Mycobacterium avium complex infection in patients with AIDS: a randomized, double-blind, placebo-controlled multicenter trial. Clin. Infect. Dis. 26:611-619. [DOI] [PubMed] [Google Scholar]
- 33.Periti, P., T. Mazzei, E. Mini, and A. Novelli. 1993. Adverse effects of macrolide antibacterials. Drug Saf. 9:346-364. [DOI] [PubMed] [Google Scholar]
- 34.Peters, D. H., H. A. Friedel, and D. McTavish. 1992. Azithromycin. A review of its antimicrobial activity, pharmacokinetic properties and clinical efficacy. Drugs 44:750-799. [DOI] [PubMed] [Google Scholar]
- 35.Pfizer. 2009. Zithromax U.S. physician prescribing information. Pfizer Labs, New York, NY.
- 36.Ramsey, P. S., M. B. Vaules, G. M. Vasdev, W. W. Andrews, and K. D. Ramin. 2003. Maternal and transplacental pharmacokinetics of azithromycin. Am. J. Obstet. Gynecol. 188:714-718. [DOI] [PubMed] [Google Scholar]
- 37.Ripa, S., L. Ferrante, and M. Prenna. 1996. A linear model for the pharmacokinetics of azithromycin in healthy volunteers. Chemotherapy 42:402-409. [DOI] [PubMed] [Google Scholar]
- 38.Sarkar, M., C. Woodland, G. Koren, and A. R. Einarson. 2006. Pregnancy outcome following gestational exposure to azithromycin. BMC Pregnancy Childbirth 6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schlitzer, M. 2007. Malaria chemotherapeutics. Part I: history of antimalarial drug development, currently used therapeutics, and drugs in clinical development. Chem. Med. Chem. 2:944-986. [DOI] [PubMed] [Google Scholar]
- 40.Sidhu, A. B., Q. Sun, L. J. Nkrumah, M. W. Dunne, J. C. Sacchettini, and D. A. Fidock. 2007. In vitro efficacy, resistance selection, and structural modeling studies implicate the malarial parasite apicoplast as the target of azithromycin. J. Biol. Chem. 282:2494-2504. [DOI] [PubMed] [Google Scholar]
- 41.Taylor, W. R., T. L. Richie, D. J. Fryauff, H. Picarima, C. Ohrt, D. Tang, D. Braitman, G. S. Murphy, H. Widjaja, E. Tjitra, A. Ganjar, T. R. Jones, H. Basri, and J. Berman. 1999. Malaria prophylaxis using azithromycin: a double-blind, placebo-controlled trial in Irian Jaya, Indonesia. Clin. Infect. Dis. 28:74-81. [DOI] [PubMed] [Google Scholar]
- 42.Upton, R. N., and G. L. Ludbrook. 2005. Pharmacokinetic-pharmacodynamic modelling of the cardiovascular effects of drugs—method development and application to magnesium in sheep. BMC Pharmacol. 5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Eijk, A. M., and D. J. Terlouw. 2007. Azithromycin for treating uncomplicated malaria. Cochrane Database Syst. Rev. CD006688. Accessed October 2009. [DOI] [PMC free article] [PubMed]
- 44.White, N. J. 2005. Intermittent presumptive treatment for malaria. PLoS Med. 2:e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wildfeuer, A., H. Laufen, M. Leitold, and T. Zimmermann. 1993. Comparison of the pharmacokinetics of three-day and five-day regimens of azithromycin in plasma and urine. J. Antimicrob. Chemother. 31(Suppl. E):51-56. [DOI] [PubMed] [Google Scholar]