Abstract
The in vitro and in vivo therapeutic efficacies of teicoplanin-loaded calcium sulfate (TCS; 10% [wt] teicoplanin) were investigated in a rabbit model of chronic methicillin-resistant Staphylococcus aureus (MRSA) osteomyelitis. The in vitro elution characteristics of teicoplanin from TCS pellets were realized by carrying out an evaluation of the release kinetics, recovery rate, and antibacterial activity of the released teicoplanin. Chronic osteomyelitis was induced by inoculating 107 CFU of a MRSA strain into the tibial cavity of rabbits. After 3 weeks, the animals were treated by debridement followed by implantation of TCS pellets in group 1, calcium sulfate (CS) pellets alone in group 2, and intravenous (i.v.) teicoplanin (6 mg/kg of body weight every 12 h for three doses and then every 24 h up to 4 weeks) in group 3. Animals in group 4 were left untreated. After 6 weeks, the efficacy of the osteomyelitis treatment was evaluated by hematological, radiological, microbiological, and histological examinations. In vitro elution studies showed sustained release of teicoplanin at a therapeutic level over a time period of 3 weeks. The released teicoplanin maintained its antibacterial activity. In vivo, the best therapeutic effect was observed in animals treated with TCS pellets, resulting in significantly lower radiological and histological scores, lower positive rates of MRSA culture and bacterial load, and excellent bone regeneration compared with those treated by CS alone or i.v. teicoplanin, without any local or systemic adverse effects. TCS pellets are an effective alternative to i.v. teicoplanin for the treatment of chronic MRSA osteomyelitis, particularly because teicoplanin is delivered locally while the TCS pellets simultaneously promote bone defect repair.
Despite gradual advances in surgical techniques and antimicrobial agents, the treatment of osteomyelitis remains a challenge for orthopedic surgeons. Surgical debridement and prolonged parenteral antibiotic administration constitute the current main therapeutic measures. However, the relapse rate is high, due to residual infection and poor penetration of antibiotics into the bone bed (15). Staphylococcus aureus is the most common osteomyelitis-inducing organism (14). In particular, the increasing emergence of methicillin-resistant S. aureus (MRSA) and other Gram-positive organisms has demanded more effective antibiotics (24). Teicoplanin has a broad-spectrum bactericidal activity against most Gram-positive aerobic and anaerobic organisms, including MRSA and methicillin-resistant coagulase-negative S. aureus. It possesses a long serum half-life, a higher uptake capacity in bone, a higher protein-binding capacity, and a lower propensity to cause nephrotoxicity than vancomycin (2, 8, 28). Furthermore, vancomycin-resistant Enterococcus faecium (VRE) exhibiting a VanB, VanC, VanE, or VanG phenotype also remains sensitive to teicoplanin (5, 12). The efficacy of parenterally administered teicoplanin has been reported (3, 8, 13, 16); however, there are few studies evaluating the use of this antibiotic delivered locally via biodegradable materials against MRSA osteomyelitis.
For the treatment of osteomyelitis, local antibiotic delivery systems have advantages over parenteral administration due to the delivery of high tissue concentrations of antibiotics locally, which potentially avoids systemic toxicity (9). Bone defects, which require reconstruction at the second stage, are inevitable after thorough debridement of osteomyelitis (23). Therefore, biomaterials with favorable osteoconductivity (such as calcium sulfate [CS], hydroxyapatite, tricalcium phosphate, or bioactive glass) impregnated with antibiotics offer attractive alternatives to promote bone defect repair, as well as to deliver local antibiotics within the tissue site. Of these materials, CS is the most commonly used as an antibiotic delivery material for osteomyelitis treatment in experimental and clinical studies (4, 7, 10, 17, 21). The benefit of CS is that implantation provides the opportunity to deliver local antibiotics at high concentrations and to simultaneously promote the bone regeneration process concurrently with the biomaterial's degradation. OsteoSet is a commercially available, sterilized, surgical-grade, alpha hemihydrate CS powder that allows antibiotic loading. Because the material is already sterilized, the potential inactivation of the antibiotics by sterilization procedures is avoided (4, 7).
The purpose of the present study was to investigate the in vitro release of teicoplanin from teicoplanin-loaded CS (TCS) and to assess the anti-infective and osteoconductive efficacies of TCS pellets compared with the efficacy of systemic teicoplanin in the treatment of chronic MRSA osteomyelitis.
MATERIALS AND METHODS
Preparation of drug delivery systems.
Commercially available CS pellets (OsteoSet resorbable mini bead kit; Wright Medical Technology, Inc., Arlington, TN) were made in accordance with the manufacturer's instructions. Briefly, 9.0 g of CS and 3 ml of the diluent were mixed with 1.0 g of teicoplanin (Gruppo Lepetit S.p.A, Localita Volcanello Anagni, Italy). The resultant paste was packed into a silicone mold without compression and allowed to harden for 20 min. The 10% TCS pellets measured an average of 4.7 mm in diameter and 3.5 mm in height and weighed an average of 42.95 mg. CS pellets without teicoplanin were also produced in the same way. All pellets were prepared under sterile conditions on the operation table just before implantation.
Antibiotic release in vitro.
Triplicate samples of 5 pellets were immersed in sterile polyethylene containers containing 10 ml of phosphate-buffered saline (PBS; pH 7.4) and kept in a water bath incubator at 37°C. The PBS solution was completely refreshed every 48 h until no antibiotic could be detected in the eluant. The eluants were frozen in cryovials at −80°C before further analysis. All experiments were performed under aseptic conditions. The teicoplanin level in the sample eluant was determined using high-performance liquid chromatography (HPLC; the detection limit was 0.2 μg/ml) from the assay standard curves of teicoplanin. The HPLC analyses were conducted on a Waters 600 multisolvent delivery system (Agilent Technologies, Palo Alto, CA). A Symmetry C8 HPLC column (3.9 cm by 150 mm; Waters) was used to separate the antibiotics. The mobile phase contained 0.01 mol heptanesulfonic acid (Fisher Scientific UK Ltd.) and acetonitrile (Mallinckrodt, United States) (85/15 [vol/vol]). The absorbency was monitored at 280 nm, and the flow rate was 1.4 ml/min. All samples were assayed in triplicate, and sample dilutions were performed to bring the unknown concentrations into the range of the assay standard curve. A calibration curve was made for each set of measurements (correlation coefficient, >0.99). The total amount of teicoplanin released from the TCS pellets was calculated, and the recovery rate of teicoplanin was obtained by dividing the initially loaded amount by the released amount.
Moreover, for exploring whether the antibacterial activity of teicoplanin would be influenced by the manufacturing process (eluants after 24 h), an evaluation of the antibacterial activity of the teicoplanin released from TCS pellets was conducted for comparison with that of nonprocessed teicoplanin. The MIC for teicoplanin against MRSA (ATCC 43300) was determined using an antibiotic 2-fold tube dilution method standardized by the National Committee for Clinical Laboratory Standards (20).
Bacterial strain.
A standard strain of MRSA (ATCC 43300), provided by the Department of Microbiology laboratory of our hospital, was used to induce osteomyelitis within tibial cavities. For this strain of MRSA, the MIC of teicoplanin was found to be 2 mg by the broth dilution method. The inocula were prepared by subculturing the bacteria in a brain heart infusion (BHI; Oxoid Ltd., Basingstoke, Hampshire, United Kingdom) at 37°C for 24 h, and a stock of aliquots was frozen at −80°C. Preoperatively, a frozen aliquot was thawed and a bacterial suspension containing 1 × 108 CFU/ml in PBS was prepared. A volume of 0.1 ml (1 × 107 CFU) was injected into the medullary cavity of the rabbit tibia to induce osteomyelitis. Postoperatively, the same inoculum was serially diluted and plated on blood agar, and after incubation at 37°C for 24 h, the number of bacteria was confirmed by colony counts.
Operative procedure.
In vivo experiments were performed using 55 adult female and pathogen-free New Zealand White rabbits (mean weight ± standard deviation, 2.76 ± 0.23 kg) according to the guidelines of the local Animal Welfare Committee. Osteomyelitis was induced in the right tibia of rabbits, using the model of Norden et al. (22), by injecting 0.1 ml of 5% sodium morrhuate (Eli Lilly and Company, Indianapolis, IN), 0.1 ml of the MRSA suspension (1 × 107 CFU/ml), and 0.1 ml of sterile PBS in sequence into the medullary cavity. The infection was allowed to progress for 3 weeks, at which time 51 animals diagnosed with chronic osteomyelitis according to the radiographic scoring system of Norden et al. (22) were selected for the remainder of the study. Five were sacrificed for microbiological and histological examinations to confirm chronic osteomyelitis, and the remaining animals were randomly divided into four groups. Animals of group 1 (n = 12) were treated by debridement and implantation of TCS pellets. Animals of group 2 (n = 12) were treated by debridement and implantation of CS pellets alone. Animals of group 3 (n = 12) were treated by debridement and intravenous (i.v.) injections of teicoplanin (6 mg/kg of body weight every 12 h for three doses and then every 24 h up to 4 weeks; the first dose was given immediately after debridement). The debridement was performed for these animals according to their different severities of bone infection in order to ensure the removal of all necrotic tissue. Animals of group 4 (n = 10) were left untreated. Postoperatively, all animals were monitored daily for general conditions, including activity, body temperature, weight, and appearance of the wound. After 6 weeks, the animals were pharmacologically euthanized for autopsy by injecting an i.v. overdose of pentobarbital sodium.
Venous blood analysis.
Before the first operation and weekly thereafter, venous blood samples were taken from the ear vein to determine white blood cell (WBC) count, as well as to assess liver and renal functions as measured by levels of glutamate-pyruvate transaminase, glutamic-oxal(o)acetic transaminase, urea, and creatinine. In addition, at 0 (immediately postoperation), 1, 2, 4, 8, 12, 24, 48, and 72 h and every 2 days thereafter following implantation of TCS pellets, the blood concentration of teicoplanin for group 1 (TCS pellets) was measured using HPLC, and serum samples from group 3 (i.v. teicoplanin) were prepared for the measurement of peak (obtained immediately following drug administration) and trough (just before a scheduled dose) teicoplanin levels after debridement.
Radiology.
Standard radiographs of the right tibiae were taken before and after debridement, as well as at sacrifice. All radiographs were scored based on parameters of the severity score system of osteomyelitis as described by Norden et al. (22), as follows: sequestral bone formation (SBF), periosteal new bone formation (PNBF), destruction of bone (DB), soft tissue calcification (STC), and soft tissue swelling (STW).
Histology.
The bone specimens were fixed in 10% formaldehyde, decalcified in EDTA, dehydrated in a graded series of ethanol dilutions, and embedded in paraffin. Longitudinal sections of 5 μm were stained with hematoxylin and eosin for light microscopy. Sections were assessed in a blind manner by a pathologist according to the scoring system described by Smeltzer et al. (25), including measurements of intraosseous acute inflammation (IAI), intraosseous chronic inflammation (ICI), periosteal inflammation (PI), and bone necrosis (BN).
Microbiology.
The samples collected from tibiae were immediately stored in sterile tubes, inoculated on blood agar, and incubated at 37°C for 48 h. To recover slow-growing microorganisms, specimens were also inoculated in BHI at 37°C for 7 days. Additionally, cancellous bone samples obtained at autopsy from the site of debridement were weighed and homogenized in 10 ml PBS at 10,000 rpm for 5 min using a tissue homogenizer. Serial 10-fold dilutions were prepared in saline, and 10 μl of each dilution was added to blood agar plates and incubated at 37°C for 48 h. Finally, the colony counts were performed and the amount of CFU/g of bone was obtained. For statistical analysis, the negative cultures were conservatively calculated as 2 × 103 CFU/g (the detection limit value). All tests were carried out in triplicate under aseptic conditions. The identification of S. aureus was based on a coagulase tube test and the API Staph system (ATB 32 Staph; bioMerieux, Marcy l'Etoile, France). Resistance to methicillin was further confirmed by detection of the mecA gene using PCR as previously reported (19).
Statistical analysis.
Data were analyzed with Statistical Package for the Social Sciences version 11.0 (SigmaStat; SPSS Inc., Chicago, IL), and the results were reported as means ± standard deviations. A one-way ANOVA test was performed to assess significant differences among body weight, temperature, WBC counts, liver and renal function, and radiographic and histological scores. A chi-square test was used for statistical analysis of MRSA infection rates in bone samples, and nonparametric tests for independent samples (the Mann-Whitney test) were performed for comparison of the CFU count among groups. Significant differences had P values of 0.05 or less.
RESULTS
Teicoplanin release in vitro.
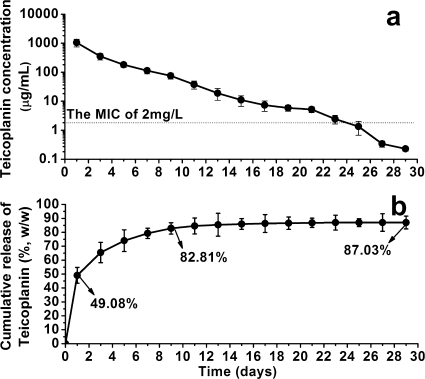
The teicoplanin release from the TCS pellets was measured as a function of immersion time (Fig. 1). During the first 24 h, approximately 49.08% of the initially loaded teicoplanin was released from the TCS pellets, with a mean concentration of 1,053.89 μg/ml. Subsequently, a gradual release period appeared, reaching 82.81% of the initially loaded amount after 9 days, with a mean concentration of 355.68 μg/ml. Thereafter, the cumulative release of teicoplanin maintained a plateau phase up to 29 days, after which it was not detectable (under 0.2 μg/ml). The total amount of teicoplanin released from the TCS pellets (the recovery rate of teicoplanin) was 87.03% (Table 1). Furthermore, the MIC of the released teicoplanin against the standard MRSA strain was identical to that of nonprocessed teicoplanin.
FIG. 1.
Release of teicoplanin from teicoplanin-impregnated calcium sulfate pellets (a) and cumulative percentage (b) as functions of time of immersion in PBS (means ± standard deviations). L, liter; w/w, weight, weight.
TABLE 1.
Pellet weight, initially loaded and actually released teicoplanin per pellet, and recovery rate of teicoplanin after immersion in PBS for 29 daysa
| Parameter | Value |
|---|---|
| Weight per pellet (mg) | 42.95 ± 4.73 |
| TEC ratio per pellet (mg/g) | 100 |
| Amt of TEC initially loaded per pellet (mg) | 4.30 ± 0.19 |
| Amt of TEC actually released per pellet (mg) | 3.74 ± 0.41 |
| Recovery rate of TEC (% [wt/wt]) | 87.03 ± 5.03 |
Values are the mean ± standard deviation unless otherwise indicated. TEC, teicoplanin.
General conditions.
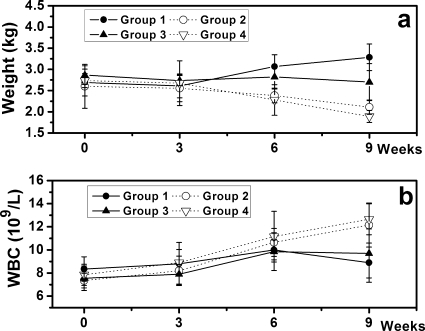
One month after treatment, 1/12 and 2/10 animals in groups 2 (CS pellets) and 4 (untreated), respectively, died because of severe infection as confirmed by autopsy. The remaining animals recovered well, displaying no evident signs of systemic complications during the follow-up period. The body weight of all animals remained consistent between the initial induction of osteomyelitis and tibia debridement. However, at the time of sacrifice, the mean body weight had increased significantly in group 1 (TCS pellets; 3.29 ± 0.32 kg, P = 0.005) and decreased significantly in groups 2 (2.11 ± 0.17 kg, P = 0.005) and 4 (1.89 ± 0.14 kg, P = 0.003). No significant weight changes were observed in group 3 (i.v. teicoplanin; P = 0.146) (Fig. 2a). Although fluctuations followed every operation, body temperatures exhibited no significant differences at the time of sacrifice compared with preoperative levels or compared among groups (data not shown).
FIG. 2.
(a) Changes in body weight of animals at sacrifice compared to preoperative weights (means ± standard deviations) exhibited a significant increase in group 1 and a decrease in groups 2 and 4. (b) Changes in WBC counts of animals showed a significant increase in groups 2 and 4 and no significant changes in groups 1 and 3 compared to preoperative levels (means ± standard deviations). L, liter.
Venous blood analyses.
WBC counts were significantly increased in groups 2 and 4 at sacrifice compared with preoperative levels (P = 0.023 and P = 0.031, respectively). Significant differences were not observed in groups 1 (P = 0.719) or 3 (P = 0.225) (Fig. 2b).
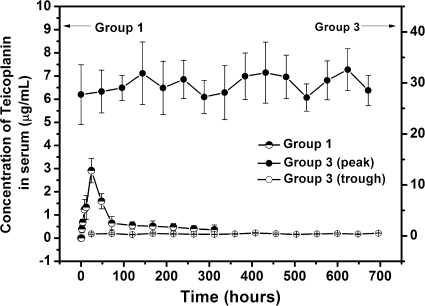
As shown in Fig. 3, the measurements of serum teicoplanin concentrations revealed that the blood drug level in group 1 (TCS pellets) was always low (values ranged from 0.36 ± 0.21 to 2.91 ± 0.53 μg/ml). The drug level reached a peak concentration of 2.91 ± 0.53 μg/ml at 24 h after the implantation of TCS pellets and decreased to below the detection limit (0.2 μg/ml) after 13 days. The mean peak and trough levels of blood drug concentrations in group 3 (i.v. teicoplanin) were 29.69 ± 4.31 μg/ml and 0.38 ± 0.08 μg/ml, respectively, during the 28-day observational period (Fig. 3). The assessment of liver and renal functions showed that there were no significant differences in the four groups compared with preoperative levels (data not shown).
FIG. 3.
Serum concentrations of teicoplanin in groups 1 and 3 (means ± standard deviations).
Radiography.
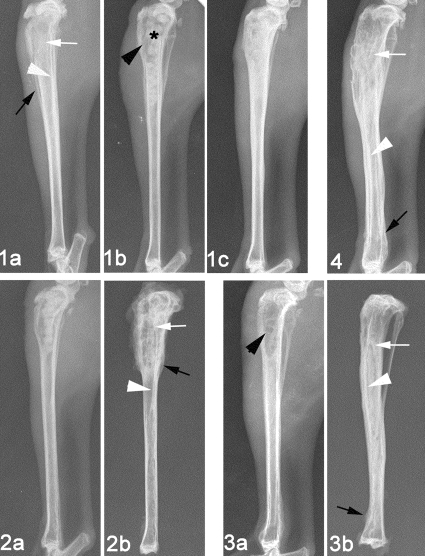
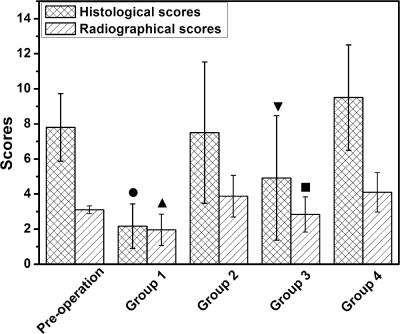
For 51 animals diagnosed with osteomyelitis, all or some signs of bone infection, including SBF, PNBF, DB, STC, and STW, were observed radiographically. Six weeks after treatment, radiographs exhibiting an elimination of bone infection were obtained from group 1 (TCS pellets), with only 2/12 animals demonstrating osteomyelitis radiographically (Fig. 4). Different degrees of infection were observed in groups 2 to 4, with 8/12, 6/12, and 10/10 animals having radiographic scores equal to or greater than 3 for the respective groups, indicating osteomyelitis. The radiographic score in group 1 decreased significantly compared with the preoperative levels (P = 0.039), as well as with those of groups 2 (P < 0.001), 3 (P = 0.040), and 4 (P < 0.001). Significantly lower scores were also observed in group 3 than in groups 2 (P = 0.015) and 4 (P = 0.005) (Fig. 5).
FIG. 4.
Representative radiographs (1, 2, 3, and 4 correlate to group number). Experimental tibial osteomyelitis occurred 3 weeks after induction of MRSA, as evaluated by bone destruction (white arrow), periosteal new bone formation (black arrow), and sequestral bone formation (white arrowhead) (1a). Bone windows (black arrowhead) and implantation of pellets (star) were observed after debridement (1b and 2a). Healing of osteomyelitis was observed 6 weeks after debridement and implantation of TCS in group 1 (1c). Debridement without implantation of pellets is illustrated for group 3 (3a). Osteomyelitis had deteriorated in various degrees 6 weeks after treatment in groups 2, 3, and 4 (2b, 3b, and 4, respectively).
FIG. 5.
Histological and radiographical scoring showed that significantly lower scores occurred in groups 1 (TCS pellets) and 3 (i.v. teicoplanin) than in control groups, as well as between group 1 and 3 (means ± standard deviations). The filled circle and filled upright triangle indicate significant differences (P < 0.05) compared with preoperative scores and scores in groups 2, 3, and 4. The filled inverted triangle and filled square indicate differences (P < 0.05) compared with groups 2 and 4.
Histology.
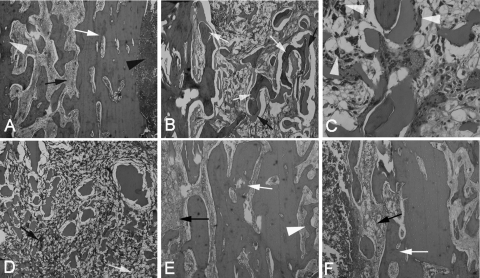
The histological sections are displayed in Fig. 6, illustrating that disease severity varied considerably between animals. Newly formed bone was located intimately around and inside the degrading TCS pellets, accompanied by mild infiltration of inflammatory cells and fibrosis in group 1 (Fig. 6B); only two animals exhibited scores equal to or greater than 4. No obvious new bone formation was observed around the CS pellets in group 2; instead, a large amount of neutrophils and mononuclear cells and significant fibrosis surrounded the pellets, indicating that a serious inflammatory response deterred bone regeneration (Fig. 6D). Various degrees of signs of osteomyelitis, including IAI, ICI, PI and BN, were observed in groups 2, 3, and 4 (Fig. 6E and F), with 8/12, 7/12, and 10/10 animals, respectively, having scores equal to or greater than 4. The histological score in group 1 decreased significantly compared with preoperative levels (P = 0.001), as well as with the scores for groups 2 (P < 0.001), 3 (P = 0.035), and 4 (P < 0.001). Significantly lower scores were also observed in group 3 than in group 2 (P = 0.047) and group 4 (P = 0.001) (Fig. 5).
FIG. 6.
Representative photomicrographs of longitudinal sections of tibiae (hematoxylin and eosin stained). (A) Typical signs of osteomyelitis prior to surgery included destruction of bone (white arrow), reactive hyperostosis (white arrowhead), fibrosis (black arrow), and the presence of pus cells in the medullary cavity (black arrowhead) (×40 magnification). (B) Six weeks after debridement and implantation of TCS pellets, a large amount of new bone formation (white arrows) with the degradation of the pellets (black arrows) occurred in group 1, accompanied by mild fibrosis (×40 magnification). (C) A high-magnification (×200) image from group 1 shows the newly formed bone, accompanied by infiltration by macrophages (white arrowheads), indicating the phagocytic process of the host to dissolve the biodegradable CS. (D) Six weeks after implantation of CS pellets alone in group 2, the pellets were mostly resorbed and surrounded by chronic fibrosis with multinucleated granulocytes (black arrow), proliferated foamy histiocytes (white arrow), and no obvious new bone formation (×40 magnification). (E and F) Moderate to severe inflammation remained in group 3 (E) and group 4 (F) 6 weeks after treatment (×40 magnification).
Microbiology.
The best evidence of eradication of infection was obtained from the microbiological investigations (Table 2). At debridement, 11/12, 10/12, 11/12, and 9/10 animals in groups 1, 2, 3, and 4, respectively, exhibited positive MRSA infection as evidenced by cultivation in BHI bouillon at 37°C for 7 days. No significant differences existed among groups before treatment (χ2 = 0.583 and P = 1.000). Six weeks after treatment, 2/12, 8/12, 6/12, and 10/10 animals in groups 1, 2, 3, and 4, respectively, tested positive for MRSA infection. A significant difference existed among groups (χ2 = 16.159 and P = 0.001), and the positive rate of specimen culture from group 1 (16.67%) was significantly lower than its preoperative value (P = 0.001).
TABLE 2.
Culture results for MRSA in bone samples before and after treatment for groups 1 to 4
| Group (no. per group) | Treatmenta | No. of positive samples/no. of negative samples (%)b |
|
|---|---|---|---|
| Preoperation | Postoperation | ||
| 1 (12) | CS + TEC | 11/1 (91.67) | 2/10 (16.67)* |
| 2 (12) | CS | 10/2 (83.33) | 8/4 (66.67) |
| 3 (12) | TEC, i.v. | 11/1 (91.67) | 6/6 (50) |
| 4 (10) | Untreated | 9/1 (90) | 10/0 (100) |
TEC, teicoplanin.
*, P = 0.001 compared with the preoperative value (chi-square test).
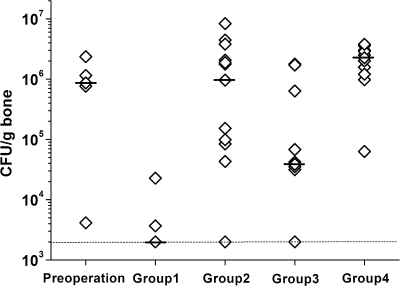
The bacterial load per gram of bone is plotted in Fig. 7. Ten out of 12 animals in group 1 (TCS pellets) did not have quantifiable bacteria using this method. A significantly lower bacterial load (median of 2.0 × 103 CFU/g bone) than at the preoperative level (P < 0.001) and in group 2 (P < 0.001), group 3 (P = 0.010), and group 4 (P < 0.001) was achieved in group 1. Group 3 (i.v. teicoplanin) also had a significantly lower bacterial load (median of 3.9 × 104 CFU/g bone) than group 2 (P = 0.033) and group 4 (P = 0.001). Six out of 12 animals in group 3 had quantifiable bacteria. Furthermore, the bacterial strains cultured from the bone samples at autopsy were confirmed to be identical to those used for induction of osteomyelitis.
FIG. 7.
Quantitative microbiological data are plotted as the number of CFU per gram of bone sample. The individual data (diamonds), the medians (black bars), and the approximate detection limit (dotted line; 2 × 103 CFU/g) are indicated. Group 1 (TCS pellets) had a bacterial load at autopsy that was significantly lower than the preoperative level (P < 0.001) and those of group 2 (CS pellets; P < 0.001), group 3 (i.v. teicoplanin; P = 0.010), and group 4 (untreated; P < 0.001). Additionally, group 3 (i.v. teicoplanin) exhibited a significantly lower CFU count than either group 2 (P = 0.033) or group 4 (P = 0.001).
DISCUSSION
The present study demonstrates the efficacy of CS as a delivery vehicle for teicoplanin in the treatment of chronic MRSA osteomyelitis. The in vitro elution kinetics exhibited an initial rapid release of teicoplanin from TCS pellets, followed by a slowly sustained release up to 29 days. The released teicoplanin was biologically active, as nonprocessed teicoplanin also indicated that the nature of the teicoplanin was not altered as a result of incorporation into the CS pellets. While the teicoplanin concentration in the eluants exceeded the MIC for MRSA over 3 weeks, the level was far lower than the toxic concentration. This level ensures a local effective bactericidal antibiotic concentration while preventing the development of antibiotic resistance, which normally results from a slow, residual drug release at suboptimal concentrations. Additionally, the concentration of released teicoplanin does not inhibit bone regeneration (1).
The primary benefit achieved with local antibiotic delivery vehicles is the ability to obtain high levels of antibiotic locally while maintaining low systemic levels (9). As shown in the present study, the serum concentrations of teicoplanin in animals receiving TCS pellets maintained at low levels for a short period (less than 2 weeks). The levels were much lower than those in animals receiving i.v. teicoplanin and thus minimized the possibility of systemic toxicity. In systemic antibiotic therapy, only a small fraction of the antibiotic will ultimately act at the infected site. Furthermore, high dosages may be required to effectively reach the poorly vascularized area of a bone infection, which can increase the risk of systemic toxicity. Although these side effects were not observed in this study, high costs and low patient compliance continue to make systemic antibiotic treatment undesirable in the clinical setting.
Many orthopedic infections are hospital acquired. The pathogenic organisms of such infections are often multidrug resistant. MRSA has gradually become the most common organism causing bone infection (24). Currently, the most extensively investigated drugs with efficacy against MRSA and available for use clinically are glycopeptide antibiotics (30). Teicoplanin is commonly used in Europe to treat osteomyelitis because of its favorable pharmacokinetics. Previous research has demonstrated the safety and efficacy of teicoplanin for the treatment of MRSA osteomyelitis by i.v. routes (8, 11, 16). However, to date, no data are available for the application of teicoplanin-loaded CS in animal models of osteomyelitis. In the present study, we loaded 10% teicoplanin within CS pellets for local osteomyelitis treatment and compared the effectiveness of TCS to that of standard, systemically administered teicoplanin. The dosing regimen for teicoplanin employed in this study was clinically relevant (Targocid prescribing information; Gruppo Lepetit S.p.A, December 2006) and comparable to dosing regimens used in other studies of experimental rabbit models of MRSA infection (3, 6, 13, 29). As evidenced in the in vivo experiment, the best therapeutic effect was observed in animals treated with TCS pellets, resulting in a significantly lower radiological and histological score and a lower positive rate of MRSA culture and bacterial load than in those treated by CS alone or i.v. teicoplanin, without any local or systemic adverse effects.
Debridement of necrotic and infected tissue is the most important factor in the treatment of chronic osteomyelitis. The goal of surgery is to achieve a viable vascularized environment and eliminate dead bone, which acts as foreign material. However, after all necrotic tissue has been adequately debrided, the remaining tissue bed is still considered contaminated, and a large bone defect is inevitable. Appropriate reconstruction of the resulting bone defect is needed. Impregnation of a selected antibiotic into an osteoconductive CS offers an attractive alternative to promote bone defect repair, as well as to deliver local antibiotics within the tissue site. The porous structure of CS enables teicoplanin to infiltrate adequately and promote the subsequent in-growth of blood vessels. In comparison with traditional polymethylmethacrylate, it can be completely resorbed in human tissue without stimulating an inflammatory response and has a more complete release of the antibiotic contained (10, 18, 21, 26). The pattern of bone formation with the TCS pellets suggested guided bone formation. The CS pellets were found to provide osteoconductive substrates with predictable and consistent rates of resorption and excellent biocompatibility (27). Radiographs and histological micrographs clearly revealed that, with the degradation of CS, the newly formed bone remodelled and restored its original structural integrity regardless of the level of the local antibiotic concentration. Furthermore, the absence of local and systemic adverse effects indicated by normal liver and renal functions, as well as no observed pathological changes at autopsy, support the use of TCS pellets for local osteomyelitis treatment and dead-space management.
The obvious limitation of this study was the lack of determination of the local bone levels of teicoplanin. Therefore, no definite conclusion could be made as to how the local high concentrations of teicoplanin affected the rate of new bone formation, although obvious bone formation occurred in contact with the surface of the pellets and into the porous spaces of the pellets 6 weeks after implantation. Additionally, despite the leaching of an effective concentration (above the MIC) of teicoplanin for approximately 3 weeks in the in vitro elution testing, it was difficult to correlate the measured in vitro concentration of the released teicoplanin with the expected concentrations in vivo, due to various influencing factors, such as the implantation site and amount of pellets (18). While local teicoplanin delivery exhibited positive results, it did not resolve bone infection in all animals; thus, it is worth investigating whether a local teicoplanin delivery system in conjunction with systemic antibiotic therapy would provide an even more efficient treatment for chronic osteomyelitis.
In conclusion, local application of teicoplanin eliminates MRSA infection with fewer propensities to cause adverse systemic effects. CS is an effective teicoplanin carrier material, which potentially provides long-term local delivery of the antibiotic while actively participating in the process of bone repair. Both the effective bactericidal activity against MRSA and the ability to promote site-specific bone regeneration support TCS pellets as a desirable candidate for local MRSA osteomyelitis treatment.
Acknowledgments
All animal experiments were approved by the Animal Ethics Committee of Shanghai Sixth People's Hospital and Shanghai Jiaotong University.
We thank Jun Zhou and Wenjin Tan (Department of Pathology, Shanghai Sixth People's Hospital, Jiaotong University, Shanghai, China) for their assistance with the histological examinations. Additionally, we are grateful to Lili Wan (Department of Pharmacy, Shanghai Sixth People's Hospital, Jiaotong University, Shanghai, China) for her help with the blood samples and to Wright Medical for providing OsteoSet.
There were no funding sources supporting the work.
Footnotes
Published ahead of print on 16 November 2009.
REFERENCES
- 1.Antoci, V., Jr., C. S. Adams, N. J. Hickok, I. M. Shapiro, and J. Parvizi. 2007. Antibiotics for local delivery systems cause skeletal cell toxicity in vitro. Clin. Orthop. Relat. Res. 462:200-206. [DOI] [PubMed] [Google Scholar]
- 2.Brogden, R. N., and D. H. Peters. 1994. Teicoplanin. A reappraisal of its antimicrobial activity, pharmacokinetic properties therapeutic efficacy. Drugs 47:823-854. [DOI] [PubMed] [Google Scholar]
- 3.Cepeda, J. A., T. Whitehouse, B. Cooper, J. Hails, K. Jones, F. Kwaku, L. Taylor, S. Hayman, S. Shaw, C. Kibbler, R. Shulman, M. Singer, and A. P. Wilson. 2004. Linezolid versus teicoplanin in the treatment of Gram-positive infections in the critically ill: a randomized, double-blind, multicentre study. J. Antimicrob. Chemother. 53:345-355. [DOI] [PubMed] [Google Scholar]
- 4.Chang, W., M. Colangeli, S. Colangeli, C. Di Bella, E. Gozzi, and D. Donati. 2007. Adult osteomyelitis: debridement versus debridement plus Osteoset T pellets. Acta Orthop. Belg. 73:238-243. [PubMed] [Google Scholar]
- 5.Courvalin, P. 2006. Vancomycin resistance in gram-positive cocci. Clin. Infect. Dis. 42:S25-S34. [DOI] [PubMed] [Google Scholar]
- 6.Dacquet, V., A. Varlet, R. N. Tandogan, M. M. Tahon, L. Fournier, F. Jehl, H. Monteil, and G. Bascoulergue. 1992. Antibiotic-impregnated plaster of Paris beads. Trials with teicoplanin. Clin. Orthop. Relat. Res. 282:241-249. [PubMed] [Google Scholar]
- 7.Gitelis, S., and G. T. Brebach. 2002. The treatment of chronic osteomyelitis with a biodegradable antibiotic-impregnated implant. J. Orthop. Surg. 10:53-60. [DOI] [PubMed] [Google Scholar]
- 8.Greenberg, R. N. 1990. Treatment of bone, joint, and vascular-access-associated gram-positive bacterial infections with teicoplanin. Antimicrob. Agents Chemother. 34:2392-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanssen, A. D. 2005. Local antibiotic delivery vehicles in the treatment of musculoskeletal infection. Clin. Orthop. Relat. Res. 437:91-96. [DOI] [PubMed] [Google Scholar]
- 10.Heijink, A., M. Yaszemksi, R. Patel, M. Rouse, D. Lewallen, and A. Hanssen. 2006. Local antibiotic delivery with osteoset, DBx, and collagraft. Clin. Orthop. Relat. Res. 451:29-33. [DOI] [PubMed] [Google Scholar]
- 11.Kandemir, O., V. Oztuna, M. Colak, A. Akdag., and H. Camdeviren. 2008. Comparison of the efficacy of tigecycline and teicoplanin in an experimental methicillin-resistant Staphylococcus aureus osteomyelitis model. J. Chemother. 20:53-57. [DOI] [PubMed] [Google Scholar]
- 12.Karmarkar, M. G., E. S. Gershom, and P. R. Mehta. 2004. Enterococcal infections with special reference to phenotypic characterization and drug resistance. Indian J. Med. Res. 119:22-25. [PubMed] [Google Scholar]
- 13.LeFrock, J., and A. Ristuccia. 1999. Teicoplanin in the treatment of bone and joint infections: an open study. J. Infect. Chemother. 5:32-39. [DOI] [PubMed] [Google Scholar]
- 14.Lew, D. P., and F. A. Waldvogel. 2004. Osteomyelitis. Lancet 364:369-379. [DOI] [PubMed] [Google Scholar]
- 15.Mader, J. T., G. C. Landon, and J. Calhoun. 1993. Antimicrobial treatment of osteomyelitis. Clin. Orthop. Relat. Res. 295:87-95. [PubMed] [Google Scholar]
- 16.Marone, P., E. Concia, M. Andreoni, F. Suter, and M. Cruciani. 1990. Treatment of bone and soft tissue infections with teicoplanin. J. Antimicrob. Chemother. 25:435-459. [DOI] [PubMed] [Google Scholar]
- 17.McKee, M. D., L. M. Wild, E. H. Schemitsch, and J. P. Waddell. 2002. The use of an antibiotic-impregnated, osteoconductive, bioabsorbable bone substitute in the treatment of infected long bone defects: early results of a prospective trial. J. Orthop. Trauma 16:622-627. [DOI] [PubMed] [Google Scholar]
- 18.McLaren, A. C. 2004. Alternative materials to acrylic bone cement for delivery of depot antibiotics in orthopaedic infections. Clin. Orthop. Relat. Res. 427:101-106. [DOI] [PubMed] [Google Scholar]
- 19.Murakami, K., W. Minamide, and K. Wada. 1991. Identification of methicillin-resistant strains of staphylococci by polymerase chain reaction. J. Clin. Microbiol. 29:2240-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard M7-A5. NCCLS, Wayne, PA.
- 21.Nelson, C., S. McLaren, R. Skinner, M. Smeltzer, J. R. Thomas, and K. Olsen. 2002. The treatment of experimental osteomyelitis by surgical debridement and the implantation of calcium sulfate tobramycin pellets. J. Orthop. Res. 20:643-647. [DOI] [PubMed] [Google Scholar]
- 22.Norden, C. W., R. L. Myerowitz, and E. Keleti. 1980. Experimental osteomyelitis due to Staphylococcus aureus or Pseudomonas aeruginosa: a radiographic-pathological correlative analysis. Br. J. Exp. Pathol. 61:451-460. [PMC free article] [PubMed] [Google Scholar]
- 23.Parsons, B., and E. Strauss. 2004. Surgical management of chronic osteomyelitis. Am. J. Surg. 188:57-66. [DOI] [PubMed] [Google Scholar]
- 24.Sakoulas, G., and R. C. Moellering, Jr. 2008. Increasing antibiotic resistance among methicillin-resistant Staphylococcus aureus strains. Clin. Infect. Dis. 46:S360-S367. [DOI] [PubMed] [Google Scholar]
- 25.Smeltzer, M. S., J. R. Thomas, S. G. Hickmon, R. A. Skinner, C. L. Nelson, D. Griffith, T. R. Parr, Jr., and R. P. Evans. 1997. Characterization of a rabbit model of staphylococcal osteomyelitis. J. Orthop. Res. 15:414-421. [DOI] [PubMed] [Google Scholar]
- 26.Thomas, M. V., D. A. Puleo, and M. Al-Sabbagh. 2005. Calcium sulfate: a review. J. Long Term Eff. Med. Implants 15:599-607. [DOI] [PubMed] [Google Scholar]
- 27.Turner, T. M., R. M. Urban, S. Gitelis, K. N. Kuo, and G. B. Andersson. 2001. Radiographic and histologic assessment of calcium sulfate in experimental animal models and clinical use as a resorbable bone-graft substitute, a bone-graft expander, and a method for local antibiotic delivery. One institution's experience. J. Bone Joint Surg. Am. 83-A(Suppl. 2):8-18. [DOI] [PubMed] [Google Scholar]
- 28.Wood, M. J. 2000. Comparative safety of teicoplanin and vancomycin. J. Chemother. 12:21-25. [DOI] [PubMed] [Google Scholar]
- 29.Yenice, I., S. Calis, H. Kas, M. Ozalp, M. Ekizoglu, and A. Hincal. 2002. Biodegradable implantable teicoplanin beads for the treatment of bone infections. Int. J. Pharm. 242:271-275. [DOI] [PubMed] [Google Scholar]
- 30.Ziglam, H. M., and R. G. Finch. 2001. Limitations of presently available glycopeptides in the treatment of Gram-positive infection. Clin. Microbiol. Infect. 7(Suppl. 4):53-65. [DOI] [PubMed] [Google Scholar]