Abstract
The administration of hepatitis C virus (HCV) NS3/4A protease inhibitors to patients with chronic HCV infections has demonstrated that they have dramatic antiviral effects and that compounds acting via this mechanism are likely to form a key component of future anti-HCV therapy. We report here on the preclinical profile of MK-7009, an inhibitor of genotype 1a and 1b proteases at subnanomolar concentrations with modestly shifted potency against genotype 2a and 2b proteases at low nanomolar concentrations. Potent activity was also observed in a cell-based HCV replicon assay in the presence of added human serum (50%). In multiple species evaluated in preclinical studies, the MK-7009 concentrations in the liver were maintained at a significant multiple of the cell-based replicon 50% effective concentration over 12 to 24 h following the administration of moderate oral doses (5 to 10 mg per kg of body weight). MK-7009 also had excellent selectivity against both a range of human proteases and a broad panel of pharmacologically relevant ion channels, receptors, and enzymes. On the basis of this favorable profile, MK-7009 was selected for clinical development and is currently being evaluated in controlled clinical trials with both healthy volunteers and HCV-infected patients.
Chronic infection with hepatitis C virus (HCV) is a major worldwide epidemic, and there are estimates that approximately 130 million to 170 million individuals are infected (17, 51). HCV is a positive-strand RNA virus of the Flaviviridae family and replicates primarily in the liver. While disease progression is typically a slow process that occurs over many years, a significant fraction of patients ultimately develop serious liver disease, including cirrhosis and hepatocellular carcinoma (19). Because of the major advances that have been made in therapy for human immunodeficiency virus (HIV) infection, HCV is currently a leading cause of death in HIV-coinfected patients (42) and is also the most common indication for liver transplantation surgery (1).
HCV shows significant genetic heterogeneity, with six separate genotypes and multiple subtypes having been characterized to date (46). The current standard-of-care therapy for HCV infection involves treatment with a combination of pegylated interferon and ribavirin (10, 26, 31). While the rates of a sustained virologic response (SVR; defined as a viral load below the limit of detection 6 months after the cessation of treatment) are high for genotype 2- and 3-infected patients treated with pegylated interferon and ribavirin (44), the SVR rates in the more prevalent genotype 1-infected population are much lower, constituting 40 to 50% of treated individuals after 48 weeks of therapy. Pegylated interferon and ribavirin therapy is also associated with a number of serious side effects, which limit the number of patients who may be treated (9).
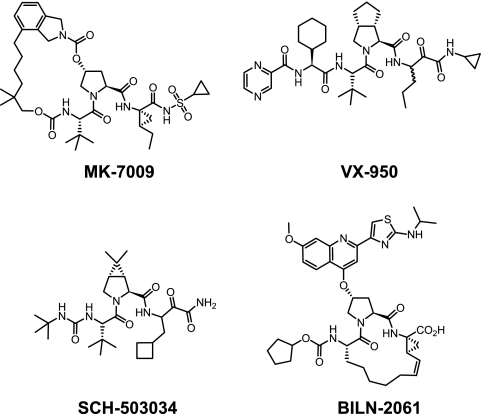
Multiple viral proteins essential for replication have been characterized (4, 27); and a clinical proof of concept has been demonstrated for small-molecule inhibitors that act against several of these, including NS3/4A protease (40, 48), NS5B polymerase (both active site and allosteric inhibitors) (11, 12, 13, 36), NS4A (37), and most recently, NS5A (33). Of these, NS3/4A protease inhibitors have progressed the furthest to date in terms of clinical evaluation and have been demonstrated to achieve highly significant reductions in HCV viral loads in patients (47). The first clinical proof of concept for an HCV direct antiviral inhibitor was shown for BILN-2061 (Fig. 1) (16, 23), a rapidly reversible, P1-P3-constrained macrocyclic compound, although its development was subsequently discontinued as a consequence of the cardiac histology seen in monkeys (41). The clinically most advanced inhibitors acting via NS3/4A inhibition, VX-950 (telaprevir) (18, 35) and SCH-503034 (boceprevir) (43), are both keto-amide compounds which covalently bind to the active-site serine of the protease in a slowly reversible manner. More recently, a number of compounds structurally related to BILN-2061, including ITMN-191 (8), TMC435350 (49), and BI201335 (28), have progressed to the early stages of clinical evaluation.
FIG. 1.
NS3/4A protease inhibitors.
We have previously described an approach to inhibitors of NS3/4A protease at subnanomolar concentrations utilizing a P2-P4 macrocyclic constraint in place of the P1-P3 linker used in the other rapidly reversible potent inhibitors described to date (21). In this report, we describe the preclinical profile of a development compound, MK-7009, a potent and selective NS3/4A protease inhibitor derived from further optimization of the P2-P4 series of macrocycles.
MATERIALS AND METHODS
Compound.
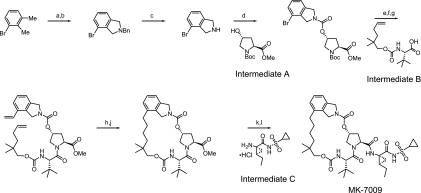
MK-7009, (1R,21S,24S)-21-tert-butyl-N-((1R,2R)-1-{[(cyclopropylsulfonyl)amino]carbonyl}-2-ethylcyclopropyl)-16,16-dimethyl-3,19,22-trioxo-2,18-dioxa-4,20,23-triazatetracyclo[21.2.1.14,70.06,11]heptacosa-6,8,10-triene-24-carboxamide (Fig. 1), was prepared by using the synthetic scheme shown in Fig. 2. The synthesis procedures are available (10a), and a more detailed description of those procedures, along with structure-activity data, leading to MK-7009 will be published elsewhere.
FIG. 2.
Synthetic route and chemical structure of MK-7009. The reagents used are as follows: a, N-bromosuccinimide, carbon tetrachloride; b, benzylamine, potassium carbonate, acetonitrile; c, (i) 1-chloroethyl chloroformate, dichloroethane, (ii) methanol; d, N,N′-carbonyldiimidazole, dimethylformamide, intermediate A (OMe, methoxy; Boc, t-butoxycarbonyl); e, potassium vinyltrifluoroborate, triethylamine, dichloro[1,1-bis(diphenylphosphino)ferrocene]palladium(II) chloride, ethanol; f, HCl, dioxane; g, N-(3-dimethylaminopropyyl)-N′-ethylcarbodiimide, 1-hydroxy-7-azabenzotriazole, dimethylformamide intermediate B; h, Zhan 1B catalyst, 1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidene[2-(i-propoxy)-5-(N,N-dimethylaminosulfonyl)phenyl]methyleneruthenium(II) dichloride (CAS no. 918870-76-5); j, hydrogen, 10% Pd/C, ethyl acetate; k, lithium hydroxide, tetrahydrofuran, methanol, water; l, O-(7-azabenzotriazol-7-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate, intermediate C, diisopropylethylamine, dimethylformamide.
In vitro assays.
Recombinant HCV NS3/4A protease was expressed and purified from Escherichia coli. Inhibition of HCV NS3/4A protease activity in reactions containing MK-7009 or reference compounds VX-950 and SCH503034 was determined in a time-resolved fluorescence assay, as described previously (29). Cell-based HCV replicon assays were conducted with the previously described genotype 1b (con1) stable cell line HB1 (2, 24) in the presence of either 10% fetal bovine serum (FBS) or 50% normal human serum (NHS), as described previously (32). Determinations of the 50% effective concentrations (EC50s) for a panel of mutant replicon cell lines were conducted by a TaqMan-based assay, according to a previously described protocol (5). Cytotoxicity for the HCV replicon cell line was determined by use of a 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt, assay, as described previously (32). The potencies of the compounds against clinical NS3/4A sequences were determined by a recently described transient cell-based phenotype assay and with a panel of NS3/4A sequences cloned from human plasma infected with HCV (25). To assess off-target activities, MK-499 binding (39; J. W. Butcher, D. A. Claremon, T. M. Connolly, D. C. Dean, J. Karczewski, K. S. Koblan, M. J. Kostura, N. J. Liverton, and D. G. Melillo, PCT International Application WO2002/005860) and p450 inhibition assays (22) were carried out as described previously. Broad counterscreening of the activity of MK-7009 against a panel of 169 ion channels, receptors, and enzymes was carried out at MDS Pharma Services (Taipei, Taiwan), in which MK-7009 was evaluated for its activity at a concentration of 10 μM.
Inhibition of the cell-based HCV replicon assay by combinations of MK-7009 and either interferon alfa 2b (Schering-Plough) or MK-0608, a nucleoside analog inhibitor of the HCV RNA-dependent RNA polymerase (34), was determined. MK-7009 and the second inhibitor were placed at various concentrations in a matrix in a 96-well plate format. Inhibition was determined, and the data were modeled by use of the MacSynergy II program (38).
Pharmacokinetic studies.
Pharmacokinetic studies were performed with rats, dogs, rhesus monkeys, and chimpanzees. The housing, maintenance, and care of the chimpanzees (Pan troglodytes) used in the study were in compliance with all relevant guidelines and requirements of Merck Research Laboratories and the New Iberia Research Center (University of Louisiana at Lafayette). The study protocols were reviewed and approved by the institutional animal care and use committees at both sites. For studies in which MK-7009 was dosed intravenously to fasted rats, dogs, and rhesus monkeys, the compound was administered as a bolus (1.0, 0.1, and 0.1 ml/kg of body weight, respectively) in dimethyl sulfoxide. For oral studies with rats, dogs, and rhesus monkeys, the compound was dosed as a solution in polyethylene glycol 400 (2.0 ml/kg). Two HCV-uninfected chimpanzees (weights, approximately 57 and 52 kg) were dosed orally by the voluntary ingestion of MK-7009 in a chocolate milk vehicle (0.67 ml/kg; Nestle brand). For all studies, blood samples were collected in EDTA-containing tubes at the appropriate times, and the plasma was separated by centrifugation and stored at −70°C until analysis. Quantitation of MK-7009 levels was carried out by high-performance liquid chromatography-tandem mass spectroscopy (LC/MS/MS) following protein precipitation. Liver samples from rats were obtained at the termination of the experiment. For the dogs, rhesus monkeys, and chimpanzees, biopsy samples of the liver (∼200 μl) were obtained following sedation of the animals. Tissue samples were homogenized in 4 volumes of deionized water, and quantitation of the drug was carried out by LC/MS/MS after protein precipitation.
RESULTS
In vitro activity and selectivity.
The inhibitory activity of MK-7009 against genotype 1 and 2 HCV NS3/4A proteases was tested. The data (Table 1) demonstrated that MK-7009 inhibits HCV genotype 1a and 1b NS3/4A protease activity at subnanomolar concentrations (Kis = 0.07 and 0.06 nM, respectively) and is only modestly less active against genotype 2a and 2b enzymes (Kis = 1.0 and 1.4 nM, respectively).
TABLE 1.
In vitro data for key NS3/4A protease inhibitorsa
| Protease inhibitor | NS3/4A Ki (nM) |
Replicon EC50 (nM) |
50% cytotoxic concn (nM) | |||||
|---|---|---|---|---|---|---|---|---|
| gt1a | gt1b | gt2a | gt2b | gt1b (10% FBS) | gt1b (50% NHS) | gt2a (10% FBS) | ||
| MK-7009b | 0.07 | 0.06 | 1 | 1.4 | 5 | 13 | 15 | >50,000 |
| BILN-2061 | 0.81 | 0.3 | 7 | 61 | 3 | 17 | 27 | 50,000 |
| VX-950 | 130 | 93 | 54 | 42 | 1,100 | 8,600 | 390 | >50,000 |
| SCH-503034 | 21 | 28 | 25 | 21 | 480 | 1,180 | 170 | >50,000 |
Data are the geometric averages of three or more determinations. gt, genotype.
IC50s for MK-499, P450 (3A4), P450 (2D6), and P450 (2C9) were >10,000 nM.
In a cell-based HCV replicon assay, MK-7009 showed potent activity against the genotype 1b enzyme in the presence of 10% FBS (EC50 = 5 nM), with a modest 2.6-fold shift being observed upon addition of 50% NHS. A very large window with respect to cellular toxicity was observed, with the 50% cytotoxic concentration being greater than 50 μM. In a genotype 2a replicon, the potency of MK-7009 was reduced only modestly (EC50 = 15 nM). By using a panel of genotype 1b replicons harboring mutant NS3 protease sequences conferring resistance to other protease inhibitors, activity shifts ranging from 3- to 275-fold were observed (Table 2), with the D168Y mutation conferring the largest shift. The potencies of MK-7009 against a panel of NS3/4A sequences from plasma from HCV-infected patients, determined by the use of a transient cell-based protease activity assay, ranged approximately 10-fold (EC50 = 2.9 to 27 nM) (Table 3), narrower than the 40-fold and 15-fold distributions previously described for BILN-2061 and VX-950 against these sequence panels, respectively.
TABLE 2.
MK-7009 potencies against a panel of NS3 mutant replicon cell linesa
| Mutant | EC50 (nM) |
|---|---|
| gt1b con1 | 1.6 |
| Q41R | 5.2 |
| F43S | 7.8 |
| R155K | 350 |
| A156T | 200 |
| D168Y | 430 |
Data are the means of three or more determinations, in the presence of 10% FBS. gt, genotype.
TABLE 3.
MK-7009 potencies against NS3/4a patient isolates in an NS3/4a phenotype assaya
| NS3/4A sequence | Genotype | EC50 (nM) |
|---|---|---|
| Con1 | 1b | 4.1 |
| H77 | 1a | 4.5 |
| ps20 | 1b | 2.9 |
| ps30 | 1b | 27 |
| ps31 | 1b | 3.4 |
| ps32 | 1b | 3.8 |
| ps33 | 1b | 9.5 |
| ps36 | 1a | 4.9 |
| ps37 | 1a | 6.5 |
| ps38 | 1a | 6.0 |
| ps40 | 1a | 2.9 |
| ps41 | 1a | 19 |
Data are the geometric averages of three or more determinations.
The selectivity of MK-7009 against a range of human proteases was compared to the selectivity data for VX-950 and SCH-503034 generated under the same conditions (Table 4). In a broader MDS Pharma Services (Panlabs) screen performed to assess potential off-target activities, >10,000-fold selectivity was observed against 169 receptors, enzymes, and ion channels tested. In a preliminary assessment of potential anti-hERG activity, the ability of MK-7009 to interfere with MK-499 binding was measured (50; Butcher et al., PCT International Application WO2002/005860). The results (50% inhibitory concentrations, >10 μM) indicated no significant activity. In a follow-up experiment evaluating the functional activity of MK-7009 at 30 μM in a patch clamp with CHO cells stably expressing hERG, less than 40% inhibition was observed. Furthermore, an assessment of the ability of MK-7009 to reversibly inhibit p450 did not show significant inhibition of the isoforms tested (3A4, 2D6, and 2C9), with the 50% inhibitory concentrations being >10 μM.
TABLE 4.
In vitro selectivity of MK-7009 versus the in vitro selectivities of VX-950 and SCH503034
| Protease | IC50 (nM) |
||
|---|---|---|---|
| MK-7009 | VX-950 | SCH-503034 | |
| Chymotrypsin | 520 | 3,000 | NDa |
| Trypsin | >10,000 | >10,000 | ND |
| Cathepsin Bb | >10,000 | 4,400 | >3,300 |
| Cathepsin Fb | >10,000 | >10,000 | 1,100 |
| Cathepsin Kb | >10,000 | 630 | 40 |
| Cathepsin Lb | >10,000 | 3,500 | 760 |
| Cathepsin Sb | >10,000 | 300 | 120 |
| Cathepsin Vb | >10,000 | 1,350 | 75 |
| Chymasec | >10,000 | 26 | 32 |
| Pancreatic elastase 1c | >10,000 | 30 | >10,000 |
| Neutrophil elastase 2c | >10,000 | 8,000 | >10,000 |
ND, not determined.
Assays (n = 2) were run under the conditions published previously (6).
Assays (n = 3) were run at MDS Pharma Services in the quantitative mode.
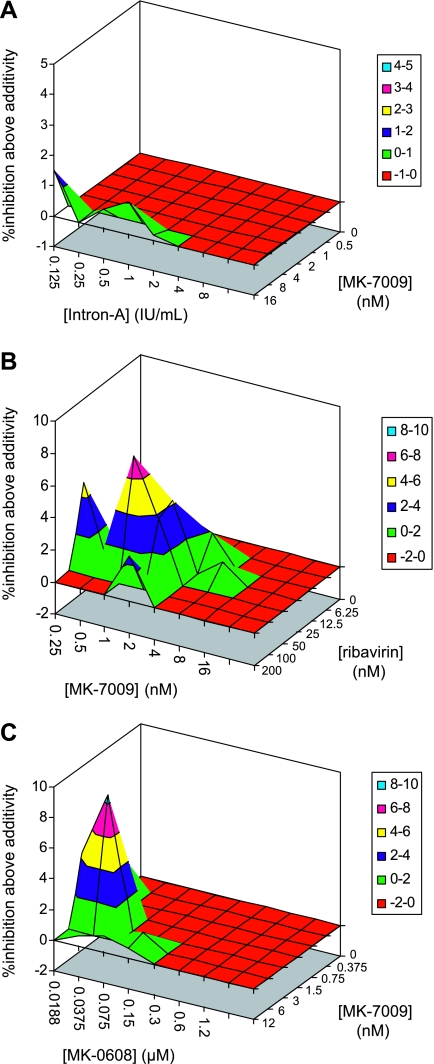
Inhibition of the cell-based replicon assay by combinations of MK-7009 and interferon alfa 2b were assessed by use of the MacSynergy II program, and the results are shown in Fig. 3A. The relatively small volume above the plane of additivity indicates that the combination displays largely additive inhibition. The combination of MK-7009 and ribavirin displayed a region of synergistic inhibition in the replicon assay (Fig. 3B). The combination of MK-7009 and MK-0608, a nucleoside analog inhibitor of the HCV RNA-dependent RNA polymerase (34), also displayed a region of synergistic inhibition (Fig. 3C).
FIG. 3.
Analysis of the inhibition of the cell-based replicon assay by combinations of inhibitors by use of the MacSynergy II program. (A) Combination of MK-7009 and interferon alfa 2b. The data lie primarily in the plane of additivity, indicating additive inhibition by the combination. (B) Combination of MK-7009 and ribavirin. A significant volume above the plane of additivity indicates synergistic inhibition by the combination. (C) Combination of MK-7009 and MK-0608. A significant volume above the plane of additivity indicates synergistic inhibition by the combination.
Pharmacokinetics.
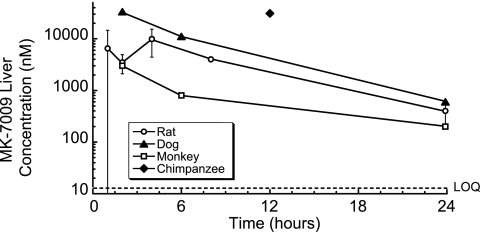
The pharmacokinetic profile of MK-7009 was measured in rats, dogs, rhesus monkeys, and chimpanzees (Table 5). Following intravenous dosing at 2 mg/kg to the rats, dogs, and monkeys, MK-7009 demonstrated moderate to high levels of plasma clearance, a moderate volume of distribution, and a short half-life (54 to 78 min). Intravenous administration was not evaluated in the chimpanzees, and thus, clearance was not determined. The plasma exposure (Fig. 4) in chimpanzees was 5.2 μM·h following a 10-mg/kg oral dose, coupled with a liver concentration of 31 μM (∼2,400 times the genotype 1b replicon EC50) at the single 12-h time point examined. Following oral administration to the rats, dogs, and monkeys, the oral bioavailability (<15%) and plasma exposure were poor. Despite the poor plasma exposure, significant liver concentrations persisted at 24 h postdosing across the species tested in the preclinical studies (200 to 600 nM, 15 to 45 times the genotype 1b replicon EC50 in the presence of 50% NHS) (Fig. 5). The ability of MK-7009 to maintain liver exposure at a significant multiple of the potency against the replicon for extended periods of up to 24 h after the administration of moderate oral doses in multiple species was a key component of the decision to select the compound for clinical development.
TABLE 5.
Pharmacokinetic profiles of MK-7009 in preclinical speciesa
| Species | Intravenous administration |
Oral administration |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Dose (mg/kg) | CLp (ml/min·kg) | V (liters/kg) | t1/2 (min) | AUC0-24 (μM·h) | Dose (mg/kg) | Cmax (μM) | AUC0-24 (μM·h) | Liver level (μM) | |
| Rat | 2 | 74 ± 9 | 1.9 ± 1.6 | 54 ± 42 | 0.6 | 5 | 0-0.1 | 0-0.1 | 0.4 at 24 h |
| Dog | 2 | 11 ± 2 | 0.3 ± 0.1 | 72 ± 0 | 4.1 | 5 | 0.5 ± 0.2 | 1.2 ± 0.4 | 0.6 at 24 h |
| Rhesus | 2 | 18 ± 2 | 0.4 ± 0.1 | 78 ± 12 | 2.5 | 5 | 0.01-0.20 | 0.05-0.2 | 0.2 at 24 h |
| Chimpanzee | 10 | 0.9 | 5.2 | 31 at 12 h | |||||
The studies were performed as described in Materials and Methods. CLp, clearance from plasma; V, volume of distribution; t1/2, half life; Cmax, maximum concentration of drug achieved in plasma; AUC0-24, area under the concentration-time curve from 0 to 24 h. For the chimpanzee study n = 2; for all other studies, n = 3.
FIG. 4.
Concentration of MK-7009 (μM) in the plasma of two chimpanzees following a 10-mg/kg dose administered in chocolate milk vehicle.
FIG. 5.
Concentration of MK-7009 (nM) in liver tissue of species tested in preclinical studies: following a 5-mg/kg oral dose in rats (n = 3), dogs (n = 2), and rhesus monkeys (n = 2) and a 10-mg/kg oral dose in chimpanzees (n = 2). For the dogs, rhesus monkeys, and chimpanzees, individual measurements were within fourfold.
DISCUSSION
HCV NS3/4A protease inhibitors will likely be important cornerstones of anti-HCV therapy as the current standard of care evolves (54). NS3/4A protease inhibitors have previously been shown to demonstrate synergy with agents acting via multiple other mechanisms, including peginterferon alfa 2a (20, 45), NS5B polymerase inhibitors (7, 14), NS4A antagonists (53), and cyclosporine analogs (3, 30). In the short term, they may allow a reduced duration of therapy when they are used in combination with interferon and ribavirin, coupled with improved SVR rates, particularly in difficult-to-treat genotype 1-infected patients. In the longer term, they are likely to form a key component of any combination therapy acting via the direct inhibition of multiple viral proteins and potentially eliminating the need for interferon and/or ribavirin. Safety, tolerability, and dosing convenience are likely to be key features in distinguishing among the NS3/4A protease inhibitors moving forward in clinical development.
MK-7009 demonstrates highly potent inhibition of genotype 1 and 2 NS3/4A proteases, and the potency translates well to cell-based activity against both genotype 1 and genotype 2 replicon cell lines. The range of potency shifts observed with MK-7009 against mutant replicons conferring resistance to other NS3 protease inhibitors is from 3- to 5-fold for Q41R and F43S, which are in close proximity to the modeled binding region of the ethylcyclopropane moiety of MK-7009, to 125- to 270-fold for R155K, A156T, and D168Y, which are anticipated to be closer in space to the macrocyclic portion of MK-7009. The consequences of these shifts in activity against mutant proteases will need to be established through clinical studies. The potency of MK-7009 against panels of NS3/4A sequences generated from the plasma of HCV-infected patients ranged only a modest 10-fold, suggesting that potency against the quasispecies complexity expected clinically will be maintained. The inhibition of the cell-based replicon assay by combinations of MK-7009 and interferon alfa 2b was largely additive, while combinations of MK-7009 and MK-0608 demonstrated significant synergy. If the increased level of inhibition produced by the combination of inhibitors translates to the greater suppression of viral replication in vivo, combination therapy may result in higher SVR rates than therapy with either inhibitor alone.
MK-7009 showed a modest threefold shift in potency upon the addition of 50% NHS to the replicon assay mixture. This shift was relatively small, given the free fraction of 1.7% measured in an equilibrium dialysis-based assay of human plasma protein binding. This could be explained either by the much lower Kd for the target protease versus that for the plasma protein, which allows partitioning to the target protein, or, alternatively, that the assay with 10% FBS already reflects a large degree of the shift due to protein binding. This profile is not unprecedented; for example, the HIV protease inhibitor efavirenz shows a 7- to 10-fold shift in potency on going from 10% FBS to 50% NHS, while the free fraction is <0.5% (15).
The selectivity against human proteases may be an important feature with regard to potential off-target effects of HCV protease inhibitors. MK-7009 demonstrated a large selectivity window against a broad panel of cathepsins, as well as other proteases, such as chymase and elastases. This selectivity profile is in contrast to that seen in the same assays for both VX-950 and SCH-503034, both of which are in advanced clinical studies. While MK-7009 does show modest inhibitory activity against chymotrypsin, the selectivity ratio relative to its potency against NS3/4A is large (8,700-fold). The >10,000-fold selectivity observed for an array of 169 pharmacologically relevant ion channel, receptor, and enzyme targets conducted at MDS Pharma Services (Panlabs) serves to further demonstrate the highly selective nature of MK-7009. The solubility of MK-7009 as the free acid (0.11 mg/ml at pH 7.7) and potassium salt (solubility at >10 mg/ml in water) is sufficient to avoid any confounding effects from precipitation in selectivity assays. The lack of significant activity against the p450 isoforms examined is also clearly an attractive attribute for a drug targeting significant liver exposure.
For any compound intended for use for the treatment of HCV infection, an appropriate drug concentration in the target organ, the liver, will be a critical factor in determining its efficacy. MK-7009 demonstrates a significant exposure in preclinical tests with multiple species which persists over a significant time frame. In particular, the concentrations present represent a significant multiple of the activity (EC50) in the replicon assay measured in the presence of 50% NHS at 24 h in the case of rat (31-fold), dog (46-fold), and rhesus monkey (15-fold) and 12 h in chimpanzee (2,400-fold), for which a sample was not available at 24 h (Fig. 5). The excellent plasma exposure seen with MK-7009 in healthy chimpanzees provided a strong basis for the clinical evaluation of an antiviral effect, and the additivity/synergy observed in combination studies in replicon assays suggests a likely benefit of utilizing MK-7009 in combination with interferon alfa 2b.
On the basis of the profile of MK-7009 in terms of its potency, selectivity, and liver exposure in preclinical studies, the compound was selected for clinical development and is currently being evaluated in controlled clinical trials with both healthy volunteers and HCV-infected patients (52).
Footnotes
Published ahead of print on 19 October 2009.
REFERENCES
- 1.Brown, R. S. 2005. Hepatitis C and liver transplantation. Nature 436:973-978. [DOI] [PubMed] [Google Scholar]
- 2.Carroll, S. S., J. E. Tomassini, M. Bosserman, K. Getty, M. W. Stahlhut, A. B. Eldrup, B. Bhat, D. Hall, A. L. Simcoe, R. LaFemina, C. A. Rutkowski, B. Wolanski, Z. Yang, G. Migliaccio, R. De Francesco, L. C. Kuo, M. MacCoss, and D. B. Olsen. 2003. Inhibition of hepatitis C virus RNA replication by 2′-modified nucleoside analogs. J. Biol. Chem. 278:11979-11984. [DOI] [PubMed] [Google Scholar]
- 3.Coelmont, L., S. Kaptein, J. Paeshuyse, I. Vliegen, J.-M. Dumont, G. Vuagniaux, and J. Neyts. 2009. Debio 025, a cyclophilin binding molecule, is highly efficient in clearing hepatitis C virus (HCV) replicon-containing cells when used alone or in combination with specifically targeted antiviral therapy for HCV (STAT-C) inhibitors. Antimicrob. Agents Chemother. 53:967-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Francesco, R., and G. Migliaccio. 2005. Challenges and successes in developing new therapies for hepatitis C. Nature 436:953-960. [DOI] [PubMed] [Google Scholar]
- 5.Dhanak, D., K. Duffy, V. K. Johnston, J. Lin-Goerke, M. Darcy, A. N. Shaw, B. Gu, C. Silverman, A. T. Gates, M. R. Nonnemacher, D. L. Earnshaw, D. J. Casper, A. Kaura, A. Baker, C. Greenwood, L. L. Gutshall, D. Maley, A. DelVecchio, R. Macarron, G. A. Hofmann, Z. Alnoah, H.-Y. Cheng, G. Chan, S. Khandekar, R. M. Keenan, and R. T. Sarisky. 2002. Identification and biological characterization of heterocyclic inhibitors of the hepatitis C virus RNA-dependent RNA polymerase. J. Biol. Chem. 277:38322-38327. [DOI] [PubMed] [Google Scholar]
- 6.Falgueyret, J.-P., W. C. Black, W. Cromlish, S. Desmarais, S. Lamontagne, C. Mellon, D. Riendeau, S. Rodan, P. Tawa, G. Wesolowski, K. E. Bass, S. Venkatraman, and M. D. Percival. 2004. An activity-based probe for the determination of cysteine cathepsin protease activities in whole cells. Anal. Biochem. 335:218-227. [DOI] [PubMed] [Google Scholar]
- 7.Flint, M., S. Mullen, A. M. Deatly, W. Chen, L. Z. Miller, R. Ralston, C. Broom, E. A. Emini, and A. Y. Howe. 2009. Selection and characterization of hepatitis C virus replicons dually resistant to the polymerase and protease inhibitors HCV-796 and boceprevir (SCH 503034). Antimicrob. Agents Chemother. 53:401-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forestier, N., D. G. Larrey, D. Guyader, P. Marcellin, R. Rouzier, A. A. Patat, W. Z. Bradford, S. Porter, and S. Zeuzem. 2008. Treatment of chronic hepatitis C virus (HCV) genotype 1 patients with the NS3/4A protease inhibitor ITMN-191 leads to rapid reductions in plasma HCV RNA: results of a phase 1b multiple ascending dose (MAD) study. Hepatology 48(Suppl.):1132A. [DOI] [PubMed] [Google Scholar]
- 9.Fried, M. W. 2002. Side effects of therapy of hepatitis C and their management. Hepatology 36:S237-S244. [DOI] [PubMed] [Google Scholar]
- 10.Hadziyannis, S. J., H. Sette, Jr., T. R. Morgan, V. Balan, M. Diago, P. Marcellin, G. Ramadori, H. Bodenheimer, Jr., D. Bernstein, M. Rizzetto, S. Zeuzem, P. J. Pockros, A. Lin, and A. M. Ackrill for the PEGASYS International Study Group. 2004. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann. Intern. Med. 140:346-355. [DOI] [PubMed] [Google Scholar]
- 10a.Holloway, M. K., N. J. Liverton, S. W. Ludmerer, J. A. McCauley, D. B. Olsen, M. T. Rudd, J. P. Vacca, and C. J. McIntyre. December 2008. U.S. patent 7,470,664.
- 11.Jensen, D. M., and A. Ascione. 2008. Future directions in therapy for chronic hepatitis C. Antivir. Ther. 13(Suppl. 1):31-36. [PubMed] [Google Scholar]
- 12.Kneteman, N. M., A. Y. Howe, T. Gao, J. Lewis, D. Pevear, G. Lund, D. Douglas, D. F. Mercer, D. L. Tyrrell, F. Immermann, I. Chaudhary, J. Speth, S. A. Villano, J. O'Connell, and M. Collett. 2009. HCV796: a selective nonstructural protein 5B polymerase inhibitor with potent anti-hepatitis C virus activity in vitro, in mice with chimeric human livers, and in humans infected with hepatitis C virus. Hepatology 49:745-752. [DOI] [PubMed] [Google Scholar]
- 13.Koch, U., and F. Narjes. 2007. Recent progress in the development of inhibitors of the hepatitis C virus RNA-dependent RNA polymerase. Curr. Top. Med. Chem. 7:1302-1329. [DOI] [PubMed] [Google Scholar]
- 14.Koev, G., T. Dekhtyar, L. Han, P. Yan, T. Ng, C. T. Lin, H. Mo, and A. Molla. 2007. Antiviral interactions of an HCV polymerase inhibitor with an HCV protease inhibitor or interferon in vitro. Antivir. Res. 73:78-83. [DOI] [PubMed] [Google Scholar]
- 15.Lai, M.-T., V. Munshi, S. Touch, R. M. Tynebor, T. J. Tucker, P. M. McKenna, T. M. Williams, D. J. DiStefano, D. J. Hazuda, and M. D. Miller. 2009. Antiviral activity of MK-4965, a novel nonnucleoside reverse transcriptase inhibitor. Antimicrob. Agents Chemother. 53:2424-2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamarre, D., P. C. Anderson, M. Bailey, P. Beaulieu, G. Bolger, P. Bonneau, M. Bös, D. R. Cameron, M. Cartier, M. G. Cordingley, A.-M. Faucher, N. Goudreau, S. H. Kawai, G. Kukolj, L. Lagacé, S. R. LaPlante, H. Narjes, M.-A. Poupart, J. Rancourt, R. E. Sentjens, R. St. George, B. Simoneau, G. Steinmann, D. Thibeault, Y. S. Tsantrizos, S. M. Weldon, C.-L. Yong, and M. Llinàs-Brunet. 2003. An NS3 protease inhibitor with antiviral effects in humans infected with hepatitis C virus. Nature 426:186-189. [DOI] [PubMed] [Google Scholar]
- 17.Lavanchy, D. 2009. The global burden of hepatitis C. Liver Int. 29(Suppl. 1):74-81. [DOI] [PubMed] [Google Scholar]
- 18.Lawitz, E., M. Rodriguez-Torres, A. J. Muir, T. L. Kieffer, L. McNair, A. Khunvichai, and J. G. McHutchison. 2008. Antiviral effects and safety of telaprevir, peginterferon alfa-2a, and ribavirin for 28 days in hepatitis C patients. J. Hepatol. 49:163-169. [DOI] [PubMed] [Google Scholar]
- 19.Liang, T. J., and T. Heller. 2004. Pathogenesis of hepatitis C-associated hepatocellular carcinoma. Gastroenterology 127:S62-S71. [DOI] [PubMed] [Google Scholar]
- 20.Lin, K., A. D. Kwong, and C. Lin. 2004. Combination of a hepatitis C virus NS3-NS4A protease inhibitor and alpha interferon synergistically inhibits viral RNA replication and facilitates viral RNA clearance in replicon cells. Antimicrob. Agents Chemother. 48:4784-4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liverton, N. J., M. K. Holloway, J. A. McCauley, M. T. Rudd, J. W. Butcher, S. S. Carroll, J. DiMuzio, C. Fandozzi, K. F. Gilbert, S.-S. Mao, C. J. McIntyre, K. T. Nguyen, J. J. Romano, M. Stahlhut, B.-L. Wan, D. B. Olsen, and J. P. Vacca. 2008. Molecular modeling based approach to potent P2-P4 macrocyclic inhibitors of hepatitis C NS3/4A protease. J. Am. Chem. Soc. 130:4607-4609. [DOI] [PubMed] [Google Scholar]
- 22.Liverton, N. J., R. Bednar, B. Bednar, J. W. Butcher, C. F. Claiborne, D. A. Claremon, M. Cunningham, A. G. DiLella, S. L. Gaul, B. E. Libby, E. A. Lyle, J. J. Lynch, J. A. McCauley, S. D. Mosser, K. T. Nguyen, G. L. Stump, H. Sun, H. Wang, J. Yergey, and K. S. Koblan. 2007. Identification and characterization of 4-methylbenzyl 4-[(pyrimidin-2-ylamino)methyl]piperidine-1-carboxylate, an orally bioavailable, brain penetrant NR2B selective N-methyl-d-aspartate receptor antagonist. J. Med. Chem. 50:807-819. [DOI] [PubMed] [Google Scholar]
- 23.Llinàs-Brunet, M., M. D. Bailey, G. Bolger, C. Brochu, A.-M. Faucher, J. M. Ferland, M. Garneau, E. Ghiro, V. Gorys, C. Grand-Maître, T. Halmos, N. Lapeyre-Paquette, F. Liard, M. Poirier, M. Rhéaume, Y. S. Tsantrizos, and D. Lamarre. 2004. Structure-activity study on a novel series of macrocyclic inhibitors of the hepatitis C virus NS3 protease leading to the discovery of BILN 2061. J. Med. Chem. 47:1605-1608. [DOI] [PubMed] [Google Scholar]
- 24.Lohmann, V., F. Körner, J.-O. Koch, U. Herian, L. Theilmann, and R. Bartenschlager. 1999. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 285:110-113. [DOI] [PubMed] [Google Scholar]
- 25.Ludmerer, S. W., D. J. Graham, M. Patel, K. Gilbert, M. Stahlhut, and D. B. Olsen. 2008. A transient cell-based phenotype assay for hepatitis C NS3/4A protease: application to potency determinations of a novel macrocyclic inhibitor against diverse protease sequences isolated from plasma infected with HCV. J. Virol. Methods 151:301-307. [DOI] [PubMed] [Google Scholar]
- 26.Manns, M. P., J. G. McHutchison, S. C. Gordon, V. K. Rustgi, M. Shiffman, R. Reindollar, Z. D. Goodman, K. Koury, M.-H. Ling, and J. K. Albrecht. 2001. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomized trial. Lancet 358:958-965. [DOI] [PubMed] [Google Scholar]
- 27.Manns, M. P., G. R. Foster, J. K. Rockstroh, S. Zeuzem, F. Zoulim, and M. Houghton. 2007. The way forward in HCV treatment—finding the right path. Nat. Rev. Drug Discov. 6:991-1000. [DOI] [PubMed] [Google Scholar]
- 28.Manns, M. P., M. Bourliere, Y. Benhamou, S. Pol, M. Bonacini, T. Berg, C. Trepo, D. Wright, G. Steinmann, D. B. Huang, J. Mikl, G. Kukolj, and J. O. Stern. 2008. Safety and antiviral activity of BI201335, a new HCV NS3 protease inhibitor, in treatment-naive patients with chronic hepatitis C genotype-1 infection given as monotherapy and in combination with peginterferon alfa 2a and ribavirin. Hepatology 48(Suppl.):1133A. [Google Scholar]
- 29.Mao, S.-S., J. DiMuzio, C. McHale, C. Burlein, D. B. Olsen, and S. S. Carroll. 2008. A time-resolved, internally quenched fluorescence assay to characterize inhibition of hepatitis C virus nonstructural protein 3-4A protease at low enzyme concentrations. Anal. Biochem. 373:1-8. [DOI] [PubMed] [Google Scholar]
- 30.Mathy, J. E., S. Ma, T. Compton, and K. Lin. 2008. Combinations of cyclophilin inhibitor NIM811 with hepatitis C virus NS3-4A protease or NS5B polymerase inhibitors enhance antiviral activity and suppress the emergence of resistance. Antimicrob. Agents Chemother. 52:3267-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McHutchison, J. G., S. C. Gordon, E. R. Schiff, M. L. Shiffman, W. M. Lee, V. K. Rustgi, Z. D. Goodman, M.-H. Ling, S. Cort, and J. K. Albrecht. 1998. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. N. Engl. J. Med. 339:1485-1492. [DOI] [PubMed] [Google Scholar]
- 32.Migliaccio, G., J. E. Tomassini, S. Carroll, L. Tomei, S. Altamura, B. Bhat, L. Bartholomew, M. R. Bosserman, A. Ceccacci, L. F. Colwell, R. Cortese, R. De Francesco, A. B. Eldrup, K. L. Getty, X. S. Hou, R. L. LaFemina, S. W. Ludmerer, M. MacCoss, D. R. McMasters, M. W. Stahlhut, D. B. Olsen, D. J. Hazuda, and O. A. Flores. 2003. Characterization of resistance to non-obligate chain-terminating ribonucleoside analogs that inhibit hepatitis C virus replication in vitro. J. Biol. Chem. 278:49164-49170. [DOI] [PubMed] [Google Scholar]
- 33.Nettles, R., C. Chien, E. Chung, A. Persson, M. Gao, M. Belema, N. A. Meanwell. M. P. DeMicco, T. C. Marbury, R. Goldwater, P. Northup, J. Coumbis, W. K. Kraft, M. R. Charlton, J. C. Lopez-Talavera, and D. Grasela. 2008. BMS-790052 is a first-in-class potent hepatitis C virus (HCV) NS5A inhibitor for patients with chronic HCV infection: results from a proof-of-concept study. Hepatology 48(Suppl.):1025A. [Google Scholar]
- 34.Olsen, D. B., A. B. Eldrup, L. Bartholomew, B. Bhat, M. R. Bosserman, A. Ceccacci, L. F. Colwell, J. F. Fay, O. A. Flores, K. L. Getty, J. A. Grobler, R. L. LaFemina, E. J. Markel, G. Migliaccio, M. Prhavc, M. W. Stahlhut, J. E. Tomassini, M. MacCoss, D. J. Hazuda, and S. S. Carroll. 2004. A 7-deaza-adenosine analog is a potent and selective inhibitor of hepatitis C virus replication with excellent pharmacokinetic properties. Antimicrob. Agents Chemother. 48:3944-3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perni, R. B., S. J. Almquist, R. A. Byrn, G. Chandorkar, P. R. Chaturvedi, L. F. Courtney, C. J. Decker, K. Dinehart, C. A. Gates, S. L. Harbeson, A. Heiser, G. Kalkeri, E. Kolaczkowski, K. Lin, Y.-P. Luong, B. G. Rao, W. P. Taylor, J. A. Thomson, R. D. Tung, Y. Wei, A. D. Kwong, and C. Lin. 2006. Preclinical profile of VX-950, a potent, selective, and orally bioavailable inhibitor of hepatitis C virus NS3-4A serine protease. Antimicrob. Agents Chemother. 50:899-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pockros, P. J., D. Nelson, E. Godofsky, M. Rodriguez-Torres, G. T. Everson, M. W. Fried, R. Ghalib, S. Harrison, L. Nyberg, M. L. Shiffman, I. Najera, A. Chan, and G. Hill. 2008. R1626 plus peginterferon Alfa-2a provides potent suppression of hepatitis C virus RNA and significant antiviral synergy in combination with ribavirin. Hepatology 48:385-397. [DOI] [PubMed] [Google Scholar]
- 37.Pottage, J., E. Lawitz, D. Mazur, D. C. Wyles, H. Vargas, R. Ghalib, R. Gugliotti, M. Donohue, and H. Robison. 2007. Short-term antiviral activity and safety of ACH-806 (GS-9132), an NS4A antagonist, in HCV genotype 1 infected individuals. J. Hepatol. 46:S294-S295. [Google Scholar]
- 38.Prichard, M. N., and C. Shipman. 1990. A three-dimensional model to analyze drug-drug interactions. Antivir. Res. 14:181-206. [DOI] [PubMed] [Google Scholar]
- 39.Raab, C. E., J. W. Butcher, T. M. Connolly, J. Karczewski, N. X. Yu, S. J. Staskiewicz, N. Liverton, D. C. Dean, and D. G. Melillo. 2006. Synthesis of the first sulfur-35-labeled hERG radioligand. Bioorg. Med. Chem. Lett. 16:1692-1695. [DOI] [PubMed] [Google Scholar]
- 40.Reesink, H. W., S. Zeuzem, C. J. Weegink, N. Forestier, A. van Vliet, J. van de Wetering de Rooij, L. McNair, S. Purdy, R. Kauffman, J. Alam, and P. L. Jansen. 2006. Rapid decline of viral RNA in hepatitis C patients treated with VX-950; a phase 1b, placebo-controlled, randomized study. Gastroenterology 131:997-1002. [DOI] [PubMed] [Google Scholar]
- 41.Reiser, M., H. Hinrichsen, Y. Benhamou, H. W. Reesink, H. Wedemeyer, C. Avendano, N. Riba, C.-L. Yong, G. Nehmiz, and G. G. Steinmann. 2005. Antiviral efficacy of NS3-serine protease inhibitor BILN-2061 in patients with chronic genotype 2 and 3 hepatitis C. Hepatology 41:832-835. [DOI] [PubMed] [Google Scholar]
- 42.Salmon-Ceron, D., C. Lewden, P. Morlat, S. Bévilacqua, E. Jougla, F. Bonnet, L. Héripret, D. Costagliola, T. May, and G. J. Chêne. 2005. Liver disease as a major cause of death among HIV infected patients: role of hepatitis C and B viruses and alcohol. J. Hepatol. 42:799-805. [DOI] [PubMed] [Google Scholar]
- 43.Sarrazin, C., R. Rouzier, F. Wagner, N. Forestier, D. Larrey, S. K. Gupta, M. Hussain, A. Shah, D. Cutler, J. Zhang, and S. Zeuzem. 2007. SCH 503034, a novel hepatitis C virus protease inhibitor, plus pegylated interferon alpha-2b for genotype 1 nonresponders. Gastroenterology 132:1270-1278. [DOI] [PubMed] [Google Scholar]
- 44.Shiffman, M. L., F. Suter, B. R. Bacon, D. Nelson, H. Harley, R. Solá, S. D. Shafran, K. Barange, A. Lin, A. Soman, and S. Zeuzem. 2007. Peginterferon alfa-2a and ribavirin for 16 or 24 weeks in HCV genotype 2 or 3. N. Engl. J. Med. 357:124-134. [DOI] [PubMed] [Google Scholar]
- 45.Siewert, S. D., S. W. Andrews, Y. Jiang, V. Serebryany, H. Tan, K. Kossen, P. T. R. Rajagopalan, S. Misialek, S. K. Stevens, A. Stoycheva, J. Hong, S. R. Lim, X. Qin, R. Rieger, K. R. Condroski, H. Zhang, M. G. Do, C. Lemieux, G. P. Hingorani, D. P. Hartley, J. A. Josey, L. Pan, L. Beigelman, and L. M. Blatt. 2008. Preclinical characteristics of the HCV NS3/4A protease inhibitor ITMN-191 (R7227). Antimicrob. Agents Chemother. 52:4432-4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simmonds, P., J. Bukh, C. Combet, G. Deléage, N. Enomoto, S. Feinstone, P. Halfon, G. Inchauspé, C. Kuiken, G. Maertens, M. Mizokami, D. Murphy, H. Okamoto, J.-M. Pawlotsky, F. Penin, E. Sablon, T. Shin-I, L. Stuyver, H. Thiel, S. Viazov, A. Weiner, and A. Widell. 2005. Consensus proposals for a unified system of nomenclature of hepatitis C virus genotypes. Hepatology 42:962-973. [DOI] [PubMed] [Google Scholar]
- 47.Thomson, J. A., and R. B. Perni. 2006. Hepatitis C virus NS3.4A protease inhibitors: countering viral subversion in vitro and showing promise in the clinic. Curr. Opin. Drug Discov. Dev. 9:606-617. [PubMed] [Google Scholar]
- 48.Tsantrizos, Y. S. 2008. Peptidomimetic therapeutic agents targeting the protease enzyme of the human immunodeficiency virus and hepatitis C virus. Acc. Chem. Res. 41:1252-1263. [DOI] [PubMed] [Google Scholar]
- 49.Van't Klooster G. A., I. Vanwelkenhuysen, R. Verloes, K. Marien, P. Van Remoortere, and K. Simmen. 2008. Pharmacokinetics of once-daily regimens of the novel HCV NS3/4A protease inhibitor TMC435350, with and without pegIFN and ribavirin, in HCV-infected individuals. Hepatology 48(Suppl.):1158A. [Google Scholar]
- 50.Wang, J., K. Della Penna, H. Wang, J. Karczewski, T. M. Connolly, K. S. Koblan, P. B. Bennett, and J. J. Salata. 2003. Functional and pharmacological properties of canine ERG potassium channels. Am. J. Physiol. Heart Circ. Physiol. 284:H256-H267. [DOI] [PubMed] [Google Scholar]
- 51.World Health Organization. 1999. Hepatitis C—global prevalence (update). Wkly. Epidemiol. Rec. 74:425-427. [PubMed] [Google Scholar]
- 52.Wright, D. H., J. L. Miller, I. Verlinden, C. Cilissen, J. Valentine, P. Sun, M. De Smet, J. de Hoon, M. Depré, L. Cavens, J. Chodakewitz, and J. A. Wagner. 2008. Safety, tolerability, and pharmacokinetic data following single- and multiple-dose administration of MK-7009, a hepatitis C virus non-structural 3/4a protease inhibitor, to healthy male subjects. Hepatology 48(Suppl.):1165A. [Google Scholar]
- 53.Wyles, D. L., K. A. Kaihara, and R. T. Schooley. 2008. Synergy of an HCV NS4A antagonist in combination with HCV protease and polymerase inhibitors. Antimicrob. Agents Chemother. 52:1862-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zeuzem, S. 2008. Interferon-based therapy for chronic hepatitis C: current and future perspectives. Nat. Clin. Pract. Gastroenterol. Hepatol. 5:610-622. [DOI] [PubMed] [Google Scholar]