Abstract
We evaluated the efficacy of posaconazole against Candida tropicalis in a systemic infection model with immunosuppressed mice. Posaconazole at 50 mg/kg of body weight/day prolonged the survival of mice and reduced the fungal tissue burden of mice infected with any of the five strains tested, with the exception of one strain that had a high MIC against this drug. Our results demonstrate the efficacy of posaconazole in the treatment of invasive murine infection caused by C. tropicalis.
Candidiasis has become an important cause of nosocomial bloodstream infections, with associated mortality rates as high as 40% (10, 11). Candida tropicalis is the third or fourth most commonly isolated species of Candida (5, 11). Although this species is not considered more virulent than other Candida species, such as C. albicans (7), it has been associated with higher mortality rates in cancer patients (15). It is particularly virulent in neutropenic hosts (10), and current therapies need improvement.
Posaconazole (PSC) is active in vitro, similarly to itraconazole and voriconazole, against most Candida species (3), and it is less likely to be involved in drug-drug interactions (9). PSC is approved for the treatment of several filamentous fungus infections and oropharyngeal candidiasis in immunocompromised patients (14). However, there are no adequate clinical data for making an evidence-based recommendation for treatment of candidiasis infections other than oropharyngeal candidiasis (10).
To improve our knowledge of the part this drug can play in the management of Candida infections, the present study tested the efficacy of PSC in an immunocompromised murine model of disseminated infection by C. tropicalis.
Seventeen clinical strains of C. tropicalis were used in the study (Table 1). Susceptibility was determined using a reference method (2). The minimal fungicidal concentration (MFC) was defined as that producing a 99.9% or greater reduction in the number of CFU/ml (6). A hemocytometer was used to adjust the desired fungal inoculum for both in vitro and in vivo studies.
TABLE 1.
Antifungal activity of posaconazole in vitro against 17 strains of C. tropicalis
| Strain | MIC-2a (μg/ml) | MFCb (μg/ml) |
|---|---|---|
| 8895 | >16 | >16 |
| 8896 | 0.125 | >16 |
| 8897 | 0.06 | >16 |
| 8898 | 0.125 | >16 |
| 9726 | 0.5 | >16 |
| 9727 | <0.03 | >16 |
| 10231 | 0.06 | >16 |
| 10232 | 0.06 | >16 |
| 10333 | 0.06 | >16 |
| 10234 | 0.06 | >16 |
| 10235 | 0.125 | >16 |
| 10236 | 0.06 | >16 |
| 10237 | 1 | >16 |
| 10238 | 0.06 | >16 |
| 10239 | <0.03 | 1 |
| 10240 | <0.03 | >16 |
| 10241 | 0.125 | >16 |
The MIC-2 corresponds to 50% growth inhibition.
The MFC corresponds to a 99.9% or greater reduction in CFU/ml.
Male OF1 mice were used following the procedural standards approved by the Animal Welfare Committee of the Rovira i Virgili University.
For survival and tissue burden studies, mice received 200 mg/kg of body weight of cyclophosphamide intraperitoneally (i.p.) plus 150 mg/kg of 5-fluorouracil intravenously (i.v.) on the same day of infection. PSC was purchased as Noxafil (Schering-Plough Ltd.). Five strains representing different in vitro susceptibility patterns were chosen for the in vivo studies. Mice were challenged with 1 × 105 CFU in 0.2 ml i.v. In previous tests, this inoculum caused mortality rates of 75 to 100% within 12 days of infection (data not shown). Groups of 10 mice were randomly established for survival and tissue burden studies for each treatment and strain. The different groups were treated with PSC at 50 mg/kg given orally (p.o.) once daily (q.d.) (12) or 25 mg/kg given twice daily (b.i.d.). Control animals received no treatment. All treatments began 24 h after challenge, and the therapy lasted for 5 days. For survival studies, mice were checked daily for 15 days. For tissue burden studies mice were killed 1 day after the completion of treatment, the spleen and kidneys were aseptically removed and weighed, and the organs were homogenized in 1 ml of sterile saline. Serial 10-fold dilutions of the homogenates were plated on Sabouraud dextrose agar, incubated at 35°C, and examined daily for 3 days.
Mean survival time was estimated by the Kaplan-Meier method and compared among groups using the log-rank test. Colony counts in tissue burden studies were analyzed using the Mann-Whitney U test. A P value of ≤0.05 was considered statistically significant.
A MIC higher than 16 μg/ml was observed only for strain FMR 8895, while for the other strains PSC showed MICs of ≤1 μg/ml (Table 1). Representative strains with low, intermediate, and high MICs were chosen for in vivo studies. Trailing growth effects and MFCs higher than 16 μg/ml were observed for all the strains tested, with the exception of strain FMR 10239, which was also chosen for the in vivo studies.
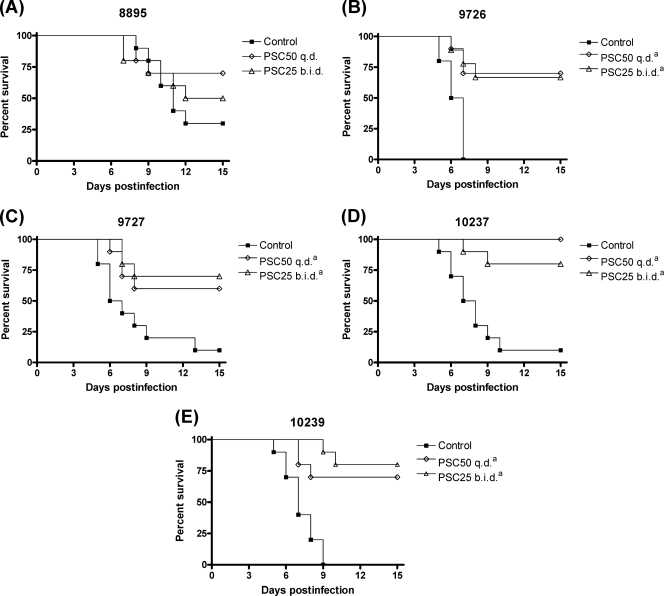
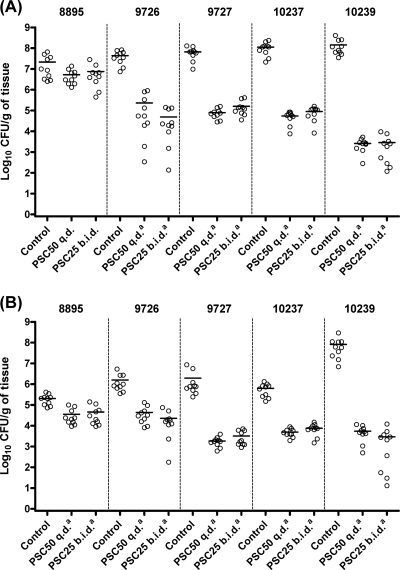
Both PSC regimens significantly prolonged survival (Fig. 1) and reduced fungal tissue burdens in kidneys and spleen (Fig. 2) of mice relative to the control group (P < 0.05) for all the strains with the exception of FMR 8895. Fungal tissue burden reduction was greater for strain FMR 10239 than for the other strains for both organs and for both PSC regimens tested (P < 0.05). No statistical differences were observed in survival or tissue burden between mice treated with PSC at 50 mg/kg q.d. and those treated with PSC at 25 mg/kg b.i.d.
FIG. 1.
Cumulative mortality of mice infected with C. tropicalis FMR 8895 (A), FMR 9726 (B), FMR 9727 (C), FMR 10237 (D), or FMR 10239 (E). PSC50, posaconazole at 50 mg/kg p.o., q.d.; PSC25, posaconazole at 25 mg/kg p.o., b.i.d. The footnote letter a indicates P < 0.05 versus control.
FIG. 2.
Effects of antifungal treatments on tissue burdens of C. tropicalis FMR 8895, FMR 9726, FMR 9727, FMR 10237, and FMR 10239 in kidneys (A) and spleens (B) of mice. PSC50, posaconazole at 50 mg/kg p.o., q.d.; PSC25, posaconazole at 25 mg/kg p.o., b.i.d. The footnote letter a indicates P < 0.05 versus control. Horizontal lines in scatter plots indicate mean values.
In the present study, we selected C. tropicalis isolates with different susceptibility patterns to PSC in order to compare the efficacy of this drug against them in vivo. The high prevalence of trailing growth effects is well known among C. tropicalis isolates in in vitro tests for azole drugs (1). The correct reading of the MICs avoids their misclassification into the resistant category, although the fact that many isolates of this species retain the ability to grow, even at high concentrations of the drug, is a matter for concern.
Dividing daily PSC intakes into various dosages has been shown to increase the absorption of this drug in humans (4, 8). In this study, however, no statistical differences were observed between single and divided dosage regimens.
PSC has been described as a fungistatic against C. tropicalis (13), which agrees with the results of this study, in which a low MFC was observed for only 1 out of 17 strains. The higher activity observed in vitro for this strain (FMR 10239) also corresponded to a higher fungal tissue burden reduction in vivo. Despite the high MFCs in this study, PSC showed efficacy in the treatment of C. tropicalis infection for all the strains tested, with the exception of the strain that showed a high MIC in vitro for this drug (FMR 8895).
Our results suggest that trailing growth effects and high MFCs observed for many strains of C. tropicalis are not predictive of a reduced efficacy of PSC in vivo against this species, and only a correlation between a high MIC and a poor response in vivo was observed for this drug. PSC was effective against most strains of C. tropicalis tested and merits further investigation in order to develop future effective treatments for C. tropicalis disseminated infections.
Acknowledgments
This work was supported by a grant from Fondo de Investigaciones Sanitarias from the Ministerio de Sanidad y Consumo of Spain (PI 050031).
Footnotes
Published ahead of print on 19 October 2009.
REFERENCES
- 1.Arthington-Skaggs, B. A., W. Lee-Yang, M. A. Ciblak, J. P. Frade, M. E. Brandt, R. A. Hajjeh, L. H. Harrison, A. N. Sofair, and D. W. Warnock. 2002. Comparison of visual and spectrophotometric methods of broth microdilution MIC end point determination and evaluation of a sterol quantitation method for in vitro susceptibility testing of fluconazole and itraconazole against trailing and nontrailing Candida isolates. Antimicrob. Agents Chemother. 46:2477-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CLSI. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard, 3rd ed. CLSI document M27-A3. Clinical and Laboratory Standards Institute, Wayne, PA.
- 3.Espinel-Ingroff, A., F. Barchiesi, M. Cuenca-Estrella, M. A. Pfaller, M. Rinaldi, J. L. Rodriguez-Tudela, and P. E. Verweij. 2005. International and multicenter comparison of EUCAST and CLSI M27-A2 broth microdilution methods for testing susceptibilities of Candida spp. to fluconazole, itraconazole, posaconazole, and voriconazole. J. Clin. Microbiol. 43:3884-3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ezzet, F., D. Wexler, R. Courtney, G. Krishna, J. Lim, and M. Laughlin. 2005. Oral bioavailability of posaconazole in fasted healthy subjects: comparison between three regimens and basis for clinical dosage recommendations. Clin. Pharmacokinet. 44:211-220. [DOI] [PubMed] [Google Scholar]
- 5.Hajjeh, R. A., A. N. Sofair, L. H. Harrison, G. M. Lyon, B. A. Arthington-Skaggs, S. A. Mirza, M. Phelan, J. Morgan, W. Lee-Yang, M. A. Ciblak, L. E. Benjamin, L. T. Sanza, S. Huie, S. F. Yeo, M. E. Brandt, and D. W. Warnock. 2004. Incidence of bloodstream infections due to Candida species and in vitro susceptibilities of isolates collected from 1998 to 2000 in a population-based active surveillance program. J. Clin. Microbiol. 42:1519-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isham, N. C., and M. A. Ghannoum. 2007. Voriconazole and caspofungin cidality against non-albicans Candida spp. Infect. Dis. Clin. Pract. 15:250-253. [Google Scholar]
- 7.Kontoyiannis, D. P., I. Vaziri, H. A. Hanna, M. Boktour, J. Thornby, R. Hachem, G. P. Bodey, and I. I. Raad. 2001. Risk factors for Candida tropicalis fungemia in patients with cancer. Clin. Infect. Dis. 33:1676-1681. [DOI] [PubMed] [Google Scholar]
- 8.Krishna, G., A. Moton, L. Ma, M. M. Medlock, and J. McLeod. 2009. Pharmacokinetics and absorption of posaconazole oral suspension under various gastric conditions in healthy volunteers. Antimicrob. Agents Chemother. 53:958-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morris, M. I. 2009. Posaconazole: a new oral antifungal agent with an expanded spectrum of activity. Am. J. Health Syst. Pharm. 66:225-236. [DOI] [PubMed] [Google Scholar]
- 10.Pappas, P. G., C. A. Kauffman, D. Andes, D. K. Benjamin, Jr., T. F. Calandra, J. E. Edwards, Jr., S. G. Filler, J. F. Fisher, B. J. Kullberg, L. Ostrosky-Zeichner, A. C. Reboli, J. H. Rex, T. J. Walsh, and J. D. Sobel. 2009. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 48:503-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfaller, M. A., D. J. Diekema, D. L. Gibbs, V. A. Newell, J. F. Meis, I. M. Gould, W. Fu, A. L. Colombo, and E. Rodriguez-Noriega. 2007. Results from the ARTEMIS DISK Global Antifungal Surveillance study, 1997 to 2005: an 8.5-year analysis of susceptibilities of Candida species and other yeast species to fluconazole and voriconazole determined by CLSI standardized disk diffusion testing. J. Clin. Microbiol. 45:1735-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodríguez, M. M., F. J. Pastor, C. Serena, and J. Guarro. 2009. Posaconazole efficacy in a murine disseminated infection caused by Paecilomyces lilacinus. J. Antimicrob. Chemother. 63:361-364. [DOI] [PubMed] [Google Scholar]
- 13.Sóczó, G., G. Kardos, P. M. McNicholas, E. Balogh, L. Gergely, I. Varga, B. Kelentey, and L. Majoros. 2007. Correlation of posaconazole minimum fungicidal concentration and time kill test against nine Candida species. J. Antimicrob. Chemother. 60:1004-1009. [DOI] [PubMed] [Google Scholar]
- 14.SP Europe. 2008. Noxafil 40 mg/ml oral suspension. Summary of product characteristics. SP Europe, Brussels, Belgium.
- 15.Viscoli, C., C. Girmenia, A. Marinus, L. Collette, P. Martino, B. Vandercam, C. Doyen, B. Lebeau, D. Spence, V. Krcmery, B. de Pauw, and F. Meunier. 1999. Candidemia in cancer patients: a prospective, multicenter surveillance study by the Invasive Fungal Infection Group (IFIG) of the European Organization for Research and Treatment of Cancer (EORTC). Clin. Infect. Dis. 28:1071-1079. [DOI] [PubMed] [Google Scholar]